Introduction
Osteoarthritis (OA) is a chronic joint disease
involving articular cartilage erosion, osteophyte formation,
subchondral sclerosis, and morphological changes in the synovium
and articular capsules (1). OA
represents a leading cause of physical disability, affecting the
elderly population (2,3). The known risk factors of OA include
aging, acute trauma, obesity, chronic overload and genetics
(4). Joint-preserving interventions
including lifestyle modification, surgery and pharmaceutical drugs
have been widely recommended (5).
However, no effective therapy is available to actually modify
disease progression. During the course of OA progression,
regeneration of articular cartilage or prevention of further
degeneration represent promising treatment strategies for
preventing OA (6).
In recent years, a novel player, long noncoding RNA
(lncRNA), a class of non-coding RNA products with transcript length
>200 nucleotides, has been discovered to regulate biological
functions (7). As a result of
in-depth research into lncRNAs, it has been found that lncRNAs also
play an important role in the pathogenesis of immune system
diseases, respiratory system diseases, diabetes, heart disease and
other chronic diseases (8). A
number of recent studies provided strong evidence that aberrantly
expressed lncRNAs are critical for chondrocyte dysfunction,
including abnormal apoptosis and inflammatory response (9,10).
Maternally expressed 8, small nucleolar RNA host gene (MEG8) is a
small nucleolar RNA host gene, located at chromosome 14q32.3.
Recently, the regulatory roles of MEG8 have been investigated in
lung and pancreatic cancer, atherosclerosis trophoblast dysfunction
and in liver fibrosis (11-14).
Furthermore, a previous study referred to abnormal MEG8 expression
in patients with OA (15). However,
to the best of our knowledge, the involvement of MEG8 in OA has not
been extensively investigated.
Previous studies have suggested that the PI3K/AKT
signaling pathway may be involved in the regulation of chondrocyte
apoptosis (16,17). Previous studies found that human
C28/I2 chondrocytes treated with IL-1β had significantly increased
expression levels of phosphorylated (p)-PI3K and p-AKT (18). PI3K/AKT is involved in the
inflammatory response and is activated in OA progression (19). Accumulating studies have reported
that inhibition of the PI3K/AKT signaling pathway alleviates the
inflammatory response, reducing the levels of pro-inflammatory
cytokines (20). Therefore, the
inactivation of the PI3K/AKT signaling pathway may help delay OA
progression.
Thus, the aim of the present study was to
investigate the detailed effects of MEG8 silencing or
overexpression on the proliferation, apoptosis and inflammatory
response in IL-1β-treated chondrocytes by regulating the PI3K/AKT
signaling pathway.
Materials and methods
Tissue samples
Articular cartilage tissue samples were collected
from patients with OA (age, 62±5 years) undergoing artificial knee
arthroplasty, and from trauma patients without OA (age, 42±4 years)
who served as the healthy control (n=22 per group). Samples were
collected from The First Affiliated Hospital of Shenzhen
University, Shenzhen Second People's Hospital (Shenzhen, China)
from January 2018 to January 2019. The inclusion criteria were as
follows: i) Patients who were diagnosed as osteoarthritic; ii)
patients who were willing to participate; and iii) patients who
fully understood the experimental protocol. The exclusion criteria
were as follows: i) Patients with other disease complications; ii)
patients who had been treated within the 3 months before admission;
and iii) patients with a history of joint surgery. The clinical
features of patients with OA including sex, disease duration and
Kellgren-Lawrence stage (21) are
presented in Table I. Informed
consent was obtained from all patients and the present study was
approved by the Ethics Committee of The First Affiliated Hospital
of Shenzhen University, Shenzhen Second People's Hospital.
 | Table IBaseline characteristics of patients
with osteoarthritis. |
Table I
Baseline characteristics of patients
with osteoarthritis.
| Clinicopathological
feature | Number of patients,
n |
|---|
| Sex | |
|
Female | 10 |
|
Male | 12 |
| Disease duration
(years) | |
|
<5 | 15 |
|
≥5 | 7 |
| Kellgren-Lawrence
stage | |
|
III | 12 |
|
IV | 10 |
Cell culture, treatment and
transfection
Human C28/I2 chondrocytes were purchased from The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences and cultured in Dulbecco's modified Eagle's
medium/Nutrient Mixture F-12 medium (Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum and 100 U/ml
penicillin-streptomycin solution (Gibco; Thermo Fisher Scientific,
Inc.) in a humidified atmosphere with 5% CO2 at 37˚C.
For induction of the OA cellular model, C28/I2 cells were
stimulated with 10 ng/ml IL-1β for 24 h, as previously reported
(22,23). The MEG8 overexpression vector was
generated by inserting its corresponding full-length sequence into
a pcDNA3.1 vector (Thermo Fisher Scientific, Inc.) with a pcDNA3.1
empty vector serving as the negative control (NC). The small
interfering (si)RNA against MEG8 and scrambled siRNA NC (si-NC)
were generated by Chang Jing Bio-Tech, Ltd. The sequences were as
follows: si-MEG8 sense, 5'-GGAAUAGACGAGAUUGGAUTT-3', and antisense,
5'-AUCCAAUCUCGUCUAUUCCTT-3'; si-NC, sense,
5'-UUCUCCGAACGUGUCACGUTT-3', and antisense,
5'-ACGUGACACGUUCGGAGAATT-3'. For cell transfection, the C28/I2
cells were seeded into six-well plates and grown to cell density of
70%, followed by transfection of 50 nM oligonucleotides or 1 µg
vector using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cartilage tissue
samples and C28/I2 cells using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Subsequently, 1 µg
RNA was reverse transcribed into cDNA using a First-strand cDNA
Synthesis Kit (Tiangen Biotech Co., Ltd.) according to the
manufacturer's instructions. To quantify the expression of MEG8,
qPCR was performed using a SYBR® Premix Ex
Taq™ kit (Takara Bio, Inc.). The thermocycling
conditions were as follows: 95˚C for 10 min; 40 cycles of 95˚C for
15 sec; 60˚C for 30 sec. The results are presented as fold-changes
relative to U6 and were calculated using the 2-ΔΔCq
method (24). The primers used were
as follows: MEG8 forward, 5'-CAGTGTTGCCTGGGTCTGA-3' and reverse,
5'-ATCCCCTTGAAAGAGCAGGA-3'; GAPDH forward,
5'-CCACGAAACTACCTTCAACTC-3' and reverse,
5'-TCATACTCCTGCTGCTTGCTGATCC-3'.
Cell viability assay
Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology) was used to detect cell viability according to the
manufacturer's instructions. After IL-1β treatment or transfection,
C28/I2 cells were inoculated onto 96-well plates (1x104
cells/well) and cultured for 48 h. Subsequently, 10 µl CCK-8
solution was added, followed by incubation at 37˚C for 1 h. The
absorbance of each well was analyzed at a wavelength of 450 nm
using a Microplate Reader (BioTek Instruments, Inc.).
Flow cytometry assay
Cell apoptosis was analyzed by flow cytometry. After
IL-1β treatment or transfection, C28/I2 cells were washed with PBS
twice and resuspended in binding buffer, followed by double
staining using an Annexin V-FITC/PI Apoptosis Detection Kit (Thermo
Fisher Scientific, Inc.) in the dark for 15 min. The percentages of
early apoptotic and late apoptotic cells were analyzed by flow
cytometry (BD Biosciences). The lower left quadrants represent
viable cells, the lower right quadrants represent early apoptotic
cells, the upper right quadrants represent late apoptotic cells and
the upper left quadrants represent necrotic cells. The cells in the
lower and upper right quadrants were summed to obtain the total
number of apoptotic cells.
TUNEL staining
Apoptosis was detected by TUNEL staining, according
to the instructions of the TUNEL kit (Beyotime Institute of
Biotechnology). Briefly, slides were permeabilized with 0.2%
Triton-X, fixed with 4% paraformaldehyde and stained with the
reaction mix. The color was developed with 3,3'-diaminobenzidine.
Slides were mounted onto glass slides and cell death was determined
by calculating the number of cells that showed positive TUNEL
staining using a Laser Scanning Confocal Microscope (Leica
Microsystems GmbH).
Caspase-3 activity detection
Caspase-3 activity was determined using the
caspase-3 activity kit (cat. no. BF3100; R&D Systems, Inc.)
according to the manufacturer's instructions. After IL-1β treatment
or transfection, cells (5x106) were resuspended with
cold lysis buffer. Cell lysates were then collected and incubated
in reaction buffer containing substrates for 1 h in the dark at
37˚C. The absorbance was detected at a wavelength of 405 nm.
Enzyme-linked immunosorbent assay
(ELISA)
The inflammatory response was determined by the
secretion of inflammatory cytokines using a commercial ELISA kit
(MilliporeSigma) according to the manufacturer's instructions.
After IL-1β treatment or transfection, the cell medium was
collected and the concentrations of TNF-α and IL-6 in the
supernatant were analyzed. The absorbance was analyzed at a
wavelength of 450 nm.
Western blotting
Following IL-1β treatment or transfection, the total
proteins of C28/I2 cells were extracted using RIPA lysis (Thermo
Fisher Scientific, Inc.) and the protein content was evaluated
using a BCA Protein Assay Kit (Beyotime Institute of
Biotechnology). Proteins (30 µg per lane) were separated by
electrophoresis on 15% SDS-PAGE gels and transferred to
polyvinylidenedifluoride membranes. Afterwards, membranes were
blocked at room temperature for 1 h with 5% skimmed milk powder,
and then incubated with primary antibodies against AKT (cat. no.
#4685), p-AKT (cat. no. #4060), PI3K (cat. no. #4249) and p-PI3K
(cat. no. #17366) (1:1,000 dilution; all from Cell Signaling
Technology, Inc.) overnight at 4˚C. After being washed with
phosphate-buffered saline three times for 5 min, they were
incubated with goat anti-rabbit IgG H&L (HRP) secondary
antibodies (cat. no. #7074; 1:2,000 dilution; Cell Signaling
Technology, Inc.) for 1 h at room temperature according to the
manufacturer's instructions. Protein bands were visualized by
enhanced chemiluminescence (Beyotime Institute of Biotechnology).
Densitometric measurements were performed using ImageJ version 1.48
Software (National Institutes of Health).
Statistical analysis
All experiments were performed three times and data
are expressed as means ± standard deviation. Statistical analysis
was conducted using GraphPad Prism 7 software (GraphPad, Inc.).
Unpaired or paired (for tissue samples) Student's t-tests were used
for comparisons between two groups and one-way analysis of variance
with Tukey's post hoc test was used to compare three or more
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
MEG8 is downregulated in OA cartilage
and IL-1β-induced chondrocytes
To investigate whether MEG8 is involved in OA, MEG8
expression levels in OA and healthy cartilage tissue samples were
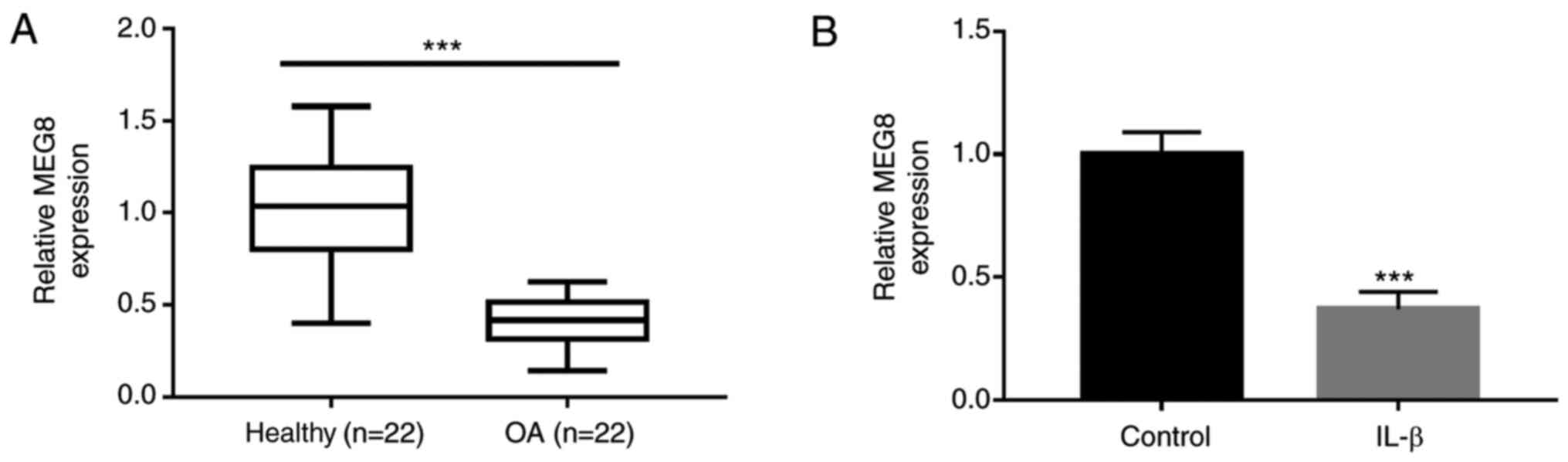
investigated. As shown in Fig. 1A,
the expression level of MEG8 in OA tissue samples was markedly
lower than that in the healthy controls. To establish the cellular
model of OA, C28/I2 chondrocyte cells were stimulated with IL-1β.
The results indicate that IL-1β treatment significantly induced the
downregulated expression of MEG8 (Fig.
1B).
MEG8 mitigates IL-1β-induced
chondrocyte injury
To investigate the biological role of MEG8 in OA,
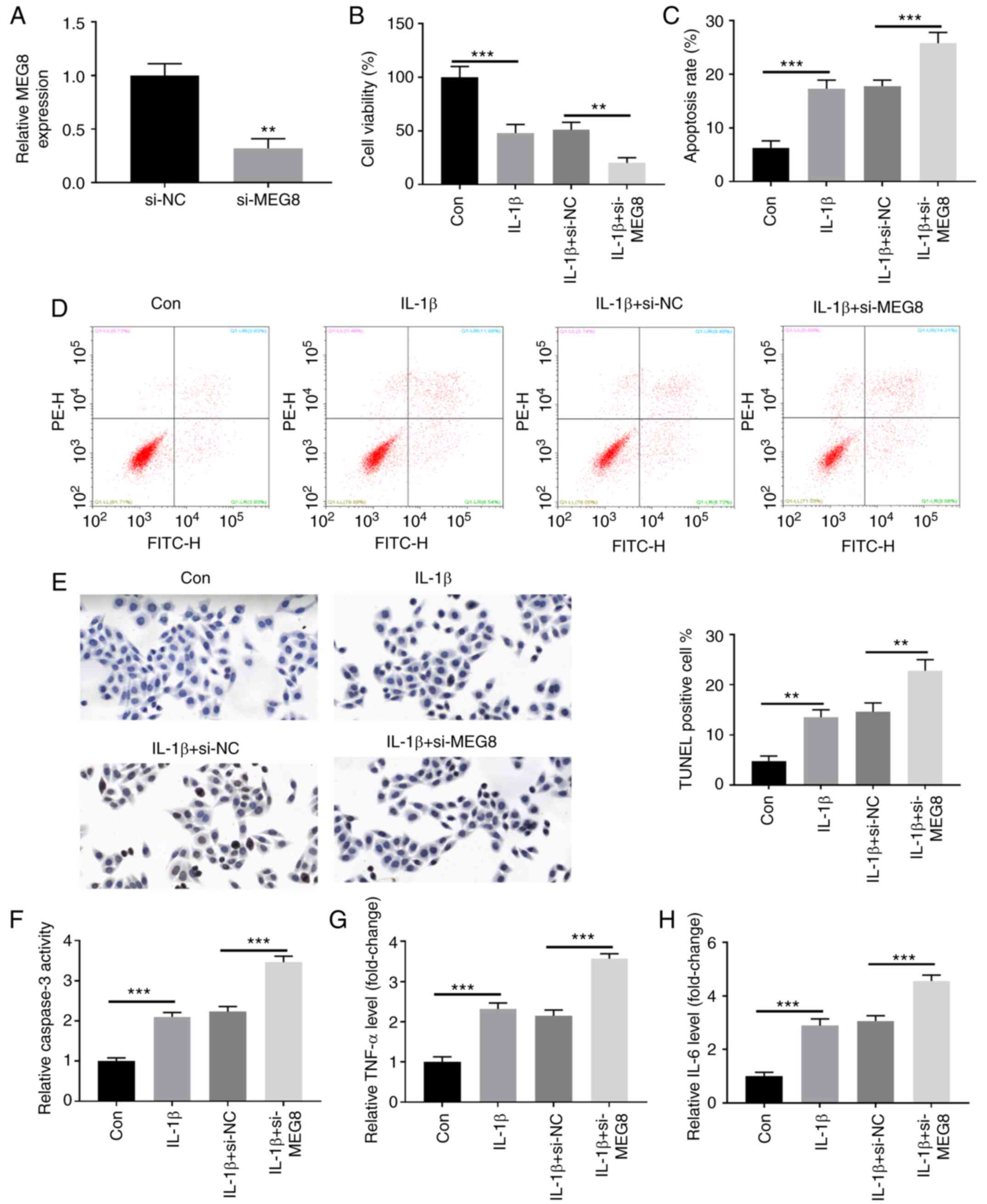
MEG8 expression was suppressed by transfecting C28/I2 cells with
si-MEG8 (Fig. 2A). As demonstrated
by CCK-8 and flow cytometry assays, the IL-1β-induced decrease in
cell viability (Fig. 2B) and
increase in apoptosis (Fig. 2C and
D) in C28/I2 cells were all
promoted by MEG8 knockdown. Apoptosis is indicated by positive
TUNEL staining in apoptotic cell death. Fig. 2E demonstrates the fluorescent signal
of TUNEL staining of C28/I2 cells in the IL-1β-induced OA cell
model. In the untreated group, there was a small number of dead
cells, whereas the rate of cell death was significantly increased
following MEG8 silencing. In addition, the ELISA results indicated
that MEG8 suppression upregulated caspase-3 activity in
IL-1β-treated cells (Fig. 2F). The
effects of MEG8 downregulation on the production of
pro-inflammatory factors were also evaluated. As shown in Fig. 2G and H, the expression levels of TNF-α and IL-6
were significantly elevated in IL-1β-stimulated C28/I2 cells and
these results were further enhanced by depletion of MEG8
expression.
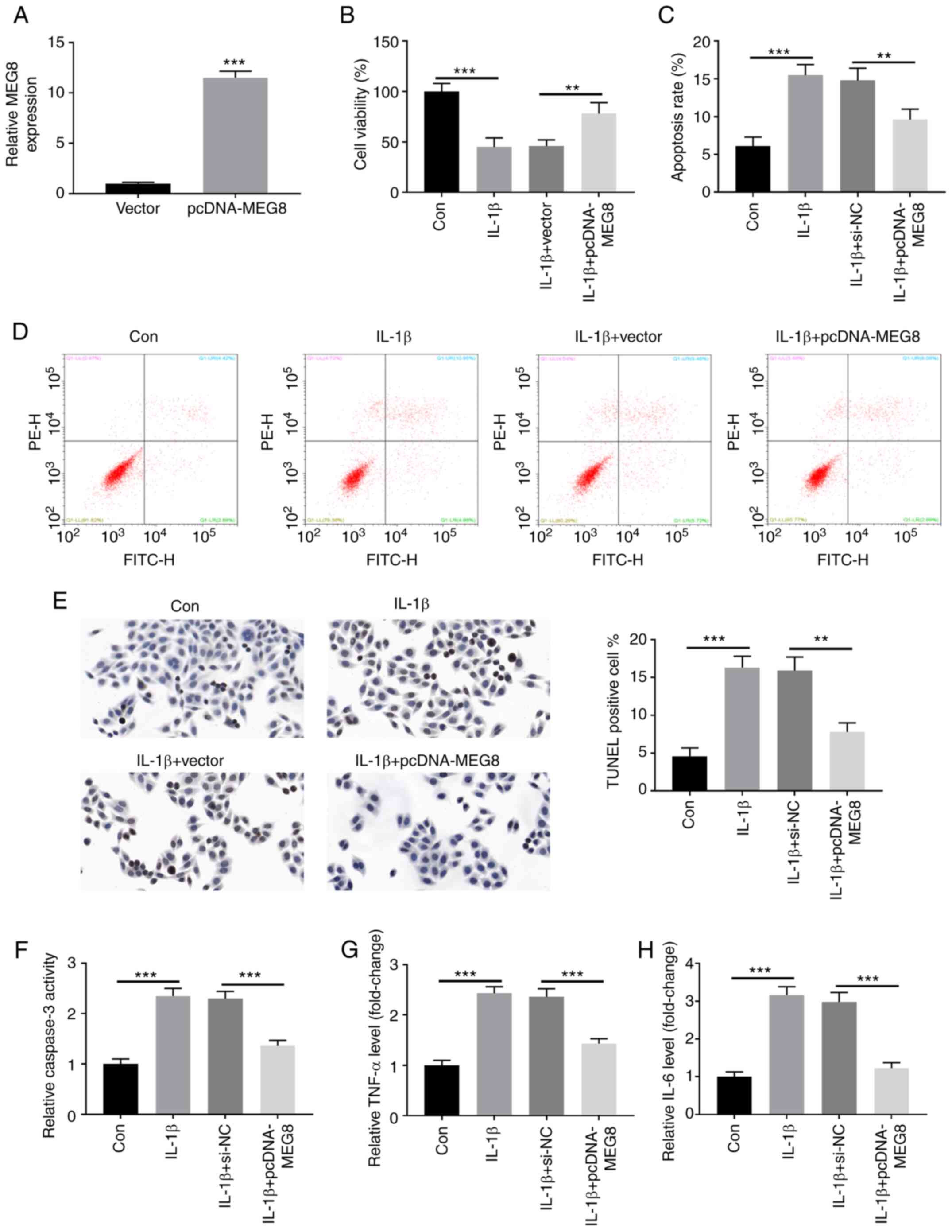
Fig. 3A indicates
that MEG8 was successfully overexpressed in C28/I2 cells by
transfection with MEG8 expression vectors. Furthermore,
overexpression of MEG8 alleviated the IL-1β-induced proliferation
inhibition and apoptosis induction (Fig. 3B-D). In the TUNEL assay, as
expected, MEG8 overexpression inhibited apoptosis induced by IL-1β
(Fig. 3E). Furthermore, the IL-1β
treatment-stimulated increase in caspase-3 activity and production
of TNF-α and IL-6 were reversed by restoration of MEG8 expression
(Fig. 3F-H). Thus, these data
indicated that MEG8 could relieve IL-1β-induced cell apoptosis and
inflammatory injury in C28/I2 cells.
MEG8 inhibits IL-1β-induced activation
of the PI3K/AKT signaling pathway in human chondrocytes
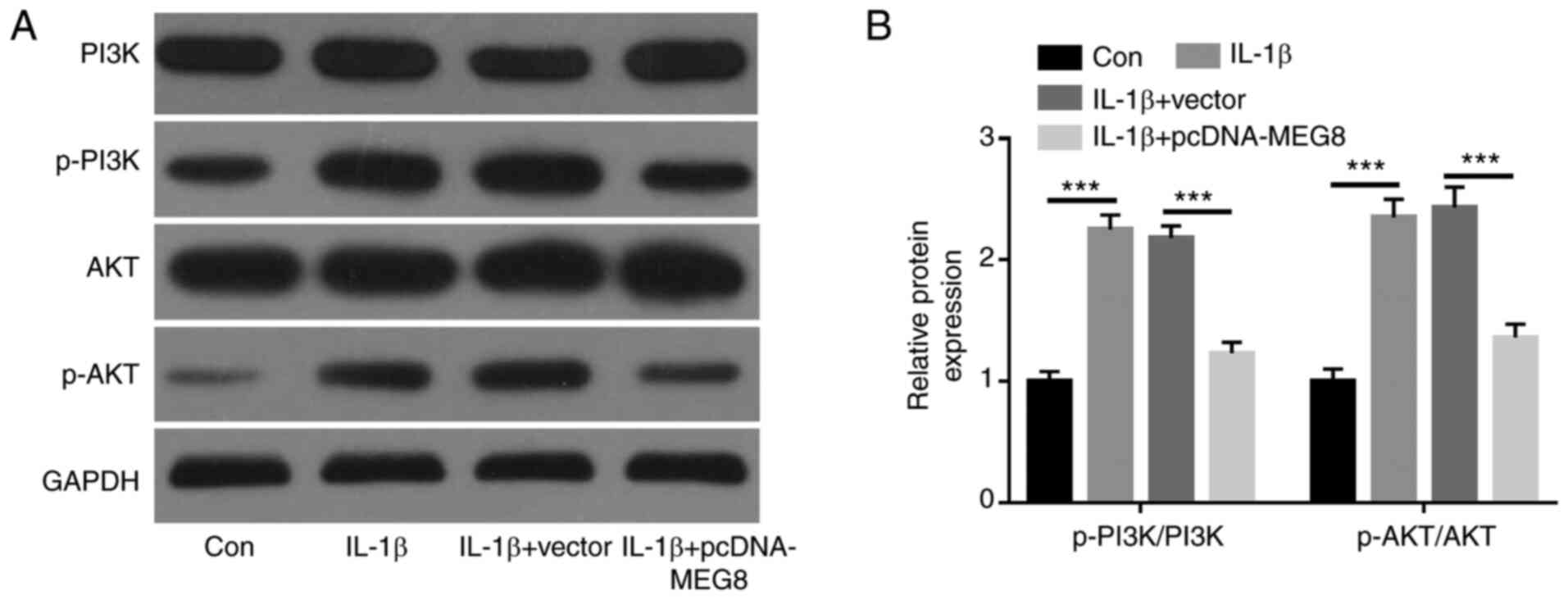
To further investigate the underlying mechanism of
MEG8 in OA, whether MEG8 affected PI3K/AKT signaling
pathway-related protein expression was analyzed. The expression
levels of p-PI3K and p-AKT were markedly increased in C28/I2 cells
by treatment with IL-1β, indicating that IL-1β could activate the
PI3K/AKT signaling pathway in C28/I2 cells. However, the
upregulation of PI3K and AKT phosphorylation induced by IL-1β was
significantly inhibited by ectopic expression of MEG8 in C28/I2
cells, implying that MEG8 activated the PI3K/AKT signaling pathway
in IL-1β-treated chondrocytes (Fig.
4A and B).
Discussion
In the present study, the role and regulatory
mechanism of MEG8 in the pathogenesis of OA was investigated. MEG8
expression was shown to be significantly decreased in OA cartilage
and IL-1β-treated chondrocytes. Moreover, functional exploration
demonstrated that MEG8 induced cell proliferation while inhibiting
apoptosis and inflammatory injury in IL-1β-treated chondrocytes.
The present study also indicated that MEG8 overexpression
attenuated IL-1β-induced cell injury via mediation of the PI3K/AKT
signaling pathway. Thus, it was indicated that MEG8 might be an
essential candidate for reversing OA progression.
In recent years, an increasing number of studies
have shown that lncRNAs play pivotal roles in regulating the
biological functions of chondrocytes in OA. For example, Chen et
al (25) indicated that MEG3
promoted proliferation and alleviated apoptosis and extracellular
matrix (ECM) degradation in IL-1β-induced chondrocytes by acting as
a sponge of microRNA (miR)-93. Wang et al (26) showed that FOXD2 adjacent opposite
strand RNA 1 sponged miR-27a-3p to accelerate cell proliferation,
inflammation and ECM degradation in IL-1β and/or TNF-α-treated
chondrocytes by targeting Toll-like receptor 4. Li et al
(27) reported that X-inactive
specific transcript (XIST) was significantly upregulated in OA, and
knockdown of XIST relieved IL-1β-inhibition of chondrocyte
proliferation and promoted IL-1β-induced cell apoptosis by
regulating miR-211-mediated C-X-C motif chemokine receptor 4 and
MAPK signaling pathways. Tian et al (28) demonstrated that small nucleolar RNA
host gene 7 was involved in IL-1β-induced chondrocyte apoptosis and
autophagy by regulating the miR-34a-5p/synoviolin 1 axis. MEG8 has
been shown to be implicated in a diverse range of biological
processes, including embryonic development, carcinogenesis and
osteoblast differentiation (11,29,30).
Until recently, little was known about the potential role of MEG8
in OA. However, Fu et al (15) reported that the expression level of
MEG8 was low in OA cartilage when compared with that in healthy
samples.
In the present study, the level of MEG8 expression
was observed to be markedly downregulated in OA cartilage and
IL-1β-treated C28/I2 chondrocytes when compared to healthy tissue
samples or untreated cells. Consistent with previous studies
(31,32), the present study found that cell
viability was inhibited, while apoptosis and pro-inflammatory
cytokine expression were enhanced in IL-1β-stimulated C28/I2 cells.
The inhibition of MEG8 by siRNA could facilitate chondrocyte
apoptosis caused by IL-1β and the production of pro-inflammatory
cytokines, while ameliorating proliferation. However, the
overexpression of MEG8 could partly reverse the acceleration of
cell apoptosis and the inflammatory response, as well as the
anti-proliferative effect caused by IL-1β in chondrocytes.
Consequently, the abovementioned results indicated that MEG8 served
as a suppressor of OA progression by accelerating proliferation and
suppressing apoptosis and inflammatory responses in chondrocytes.
However, the exact molecular mechanism by which MEG8 is implicated
in OA remains unclear.
Compelling evidence has delineated the involvement
of the PI3K/AKT signaling pathway in diverse cell processes, such
as survival, migration, apoptosis, autophagy and differentiation
(33-35).
Several lines of evidence indicate that inhibition of the PI3K/AKT
signaling pathway reduced the apoptosis and inflammatory response
of articular chondrocytes (36,37).
Hence, to investigate the underlying mechanism of MEG8 in OA
chondrocytes, the present study focused on the PI3K/AKT signaling
pathway. MEG8 overexpression reversed IL-1β-induced activation of
the PI3K/AKT signaling pathway. These findings suggested that
PI3K/AKT signaling was a critical target for OA and that MEG8 may
harness its potential therapeutic effect by inhibiting this
signaling pathway.
Thus, the present results showed that downregulation
of MEG8 induced by IL-1β aggravated OA progression via activation
of the PI3K/AKT signaling pathway. Due to the small sample size and
the lack of an in vivo assay, further investigation of the
MEG8/PI3K/AKT axis in OA is essential.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS, JT and WX designed the study; MG, HY, WX, LJ,
XH, HS and WY performed the experiments, analyzed the data and
prepared the manuscript. WX, MG, JT and HY reviewed the manuscript.
All authors confirmed the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethic
committee of The First Affiliated Hospital of Shenzhen University,
Shenzhen Second People's Hospital. Written informed consent was
obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pereira D, Ramos E and Branco J:
Osteoarthritis. Acta Med Port. 28:99–106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Neogi T and Zhang Y: Epidemiology of
osteoarthritis. Rheum Dis Clin North Am. 39:1–19. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vina ER and Kwoh CK: Epidemiology of
osteoarthritis: Literature update. Curr Opin Rheumatol. 30:160–167.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
He Y, Li Z, Alexander PG, Ocasio-Nieves
BD, Yocum L, Lin H and Tuan RS: Pathogenesis of osteoarthritis:
Risk factors, regulatory pathways in chondrocytes, and experimental
models. Biology (Basel). 9(194)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Glyn-Jones S, Palmer AJ, Agricola R, Price
AJ, Vincent TL, Weinans H and Carr AJ: Osteoarthritis. Lancet.
386:376–387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liao J and Lin Y: Stem cells and cartilage
tissue engineering. Curr Stem Cell Res Ther. 13(489)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sanchez Calle A, Kawamura Y, Yamamoto Y,
Takeshita F and Ochiya T: Emerging roles of long non-coding RNA in
cancer. Cancer Sci. 109:2093–2100. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mishra S, Verma SS, Rai V, Awasthee N,
Chava S, Hui KM, Kumar AP, Challagundla KB, Sethi G and Gupta SC:
Long non-coding RNAs are emerging targets of phytochemicals for
cancer and other chronic diseases. Cel Mol Life Sci. 76:1947–1966.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liang Z and Ren C: Emodin attenuates
apoptosis and inflammation induced by LPS through up-regulating
lncRNA TUG1 in murine chondrogenic ATDC5 cells. Biomed
Pharmacother. 103:897–902. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pan L, Liu D, Zhao L, Wang L, Xin M and Li
X: Long noncoding RNA MALAT1 alleviates lipopolysaccharide-induced
inflammatory injury by upregulating microRNA-19b in murine
chondrogenic ATDC5 cells. J Cell Biochem. 119:10165–10175.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Terashima M, Ishimura A, Wanna-Udom S and
Suzuki T: MEG8 long noncoding RNA contributes to epigenetic
progression of the epithelial-mesenchymal transition of lung and
pancreatic cancer cells. J Biol Chem. 293:18016–18030.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang B, Dong Y and Zhao Z: lncRNA MEG8
regulates vascular smooth muscle cell proliferation, migration and
apoptosis by targeting PPARα. Biochem Biophys Res Commun.
510:171–176. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sheng F, Sun N, Ji Y, Ma Y, Ding H, Zhang
Q, Yang F and Li W: Aberrant expression of imprinted lncRNA MEG8
causes trophoblast dysfunction and abortion. J Cell Biochem.
120:17378–17390. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen T, Lin H, Chen X, Li G, Zhao Y, Zheng
L, Shi Z, Zhang K, Hong W and Han T: lncRNA Meg8 suppresses
activation of hepatic stellate cells and epithelial-mesenchymal
transition of hepatocytes via the Notch pathway. Biochem Biophys
Res Commun. 521:921–927. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fu M, Huang G, Zhang Z, Liu J, Zhang Z,
Huang Z, Yu B and Meng F: Expression profile of long noncoding RNAs
in cartilage from knee osteoarthritis patients. Osteoarthritis
Cartilage. 23:423–432. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li H, Xie S, Li H, Zhang R and Zhang H:
lncRNA MALAT1 mediates proliferation of LPS treated-articular
chondrocytes by targeting the miR-146a-PI3K/Akt/mTOR axis. Life
Sciences. 254(116801)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fan Z, Liu Y, Shi Z, Deng K and Zhang H,
Li Q, Cao S, Li S and Zhang H: MiR-155 promotes
interleukin-1β-induced chondrocyte apoptosis and catabolic activity
by targeting PIK3R1-mediated PI3K/Akt pathway. J Cell Mol Med.
24:8441–8451. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Y, Shen S, Li Z, Li W and Weng X:
MIR-140-5p affects chondrocyte proliferation, apoptosis, and
inflammation by targeting HMGB1 in osteoarthritis. Inflamm Res.
69:63–73. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xie L, Xie H, Chen C, Tao Z, Zhang C and
Cai L: Inhibiting the PI3K/AKT/NF-κB signal pathway with nobiletin
for attenuating the development of osteoarthritis: In vitro and in
vivo studies. Food Funct. 10:2161–2175. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu M, Hu R, Wang J, An Y, Lu L, Long C and
Yan L: Salidroside Suppresses IL-1β-induced apoptosis in
chondrocytes via phosphatidylinositol 3-kinases (PI3K)/Akt
signaling inhibition. Med Sci Monit. 25:5833–5840. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Luyten FP, Bierma-Zeinstra S, Dell'Accio
F, Kraus VB, Nakata K, Sekiya I, Arden NK and Lohmander LS: Toward
classification criteria for early osteoarthritis of the knee. Semin
Arthritis Rheum. 47:457–463. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lei J, Fu Y, Zhuang Y, Zhang K and Lu D:
lncRNA SNHG1 alleviates IL-1β-induced osteoarthritis by inhibiting
miR-16-5p-mediated p38 MAPK and NF-κB signaling pathways. Biosci
Rep. 39(BSR20191523)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lu X, Yu Y, Yin F, Yang C, Li B, Lin J and
Yu H: Knockdown of PVT1 inhibits IL-1β-induced injury in
chondrocytes by regulating miR-27b-3p/TRAF3 axis. Int
Immunopharmacol. 79(106052)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen K, Zhu H, Zheng MQ and Dong QR:
lncRNA MEG3 inhibits the degradation of the extracellular matrix of
chondrocytes in osteoarthritis via targeting miR-93/TGFBR2 axis.
Cartilage: Jun 28, 2019 (Epub ahead of print).
|
|
26
|
Wang Y, Cao L, Wang Q, Huang J and Xu S:
lncRNA FOXD2-AS1 induces chondrocyte proliferation through sponging
miR-27a-3p in osteoarthritis. Artif Cells Nanomed Biotechnol.
47:1241–1247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li L, Lv G, Wang B and Kuang L: The role
of lncRNA XIST/miR-211 axis in modulating the proliferation and
apoptosis of osteoarthritis chondrocytes through CXCR4 and MAPK
signaling. Biochem Biophys Res Commun. 503:2555–2562.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tian F, Wang J, Zhang Z and Yang J: lncRNA
SNHG7/miR-34a-5p/SYVN1 axis plays a vital role in proliferation,
apoptosis and autophagy in osteoarthritis. Biol Res.
53(9)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gu T, He H, Han Z, Zeng T, Huang Z, Liu Q,
Gu N, Chen Y, Sugimoto K, Jiang H and Wu Q: Expression of macro
non-coding RNAs Meg8 and Irm in mouse embryonic development. Acta
Histochem. 114:392–399. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zheng Y, Li X, Huang Y, Jia L and Li W:
Time series clustering of mRNA and lncRNA expression during
osteogenic differentiation of periodontal ligament stem cells.
PeerJ. 6(e5214)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhou X, Jiang L, Fan G, Yang H, Wu L,
Huang Y, Xu N and Li J: Role of the ciRS-7/miR-7 axis in the
regulation of proliferation, apoptosis and inflammation of
chondrocytes induced by IL-1β. Int Immunopharmacol. 71:233–240.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu M, Zhong S, Kong R, Shao H, Wang C,
Piao H, Lv W, Chu X and Zhao Y: Paeonol alleviates
interleukin-1β-induced inflammatory responses in chondrocytes
during osteoarthritis. Biomed Pharmacother. 95:914–921.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chai C, Song LJ, Han SY, Li XQ and Li M:
MicroRNA-21 promotes glioma cell proliferation and inhibits
senescence and apoptosis by targeting SPRY1 via the PTEN/PI3K/AKT
signaling pathway. CNS Neurosci Ther. 24:369–380. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang Q, Lai S, Hou X, Cao W, Zhang Y and
Zhang Z: Protective effects of PI3K/Akt signal pathway induced cell
autophagy in rat knee joint cartilage injury. Am J Transl Res.
10:762–770. 2018.PubMed/NCBI
|
|
35
|
Wu R, Ruan J, Sun Y, Liu M, Sha Z, Fan C
and Wu Q: Long non-coding RNA HIF1A-AS2 facilitates adipose-derived
stem cells (ASCs) osteogenic differentiation through miR-665/IL6
axis via PI3K/Akt signaling pathway. Stem Cell Res Ther.
9(348)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xue JF, Shi ZM, Zou J and Li XL:
Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of
articular chondrocytes and attenuates inflammatory response in rats
with osteoarthritis. Biomed Pharmacother. 89:1252–1261.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sun J, Song X, Su L and Cao S: Long
non-coding RNA LncHIFCAR promotes osteoarthritis development via
positively regulating HIF-1α and activating the PI3K/AKT/mTOR
pathway. Int J Clin Exp Pathol. 11:3000–3009. 2018.PubMed/NCBI
|