Introduction
Prostate cancer (PC) is the most frequent type of
cancer in men worldwide, accounting for 9.5% of morbidity and
mortality among all new cancer cases, and for 4.8% of all
cancer-related deaths (1). Older
men appear to be more susceptible to PC, as it has been reported
that ~99% of patients with PC are aged >50 years (2). Similar to other malignancies, the
timing of diagnosis is crucial for the survival of patients with
PC. Current treatment strategies for PC include surgery,
chemotherapy, radiotherapy and hormonal therapy (3-6);
however, treatment at the molecular level has rarely been reported
in PC. Therefore, the aim of the present study was to improve the
outcome of PC by investigating a molecular treatment approach.
MicroRNAs (miRNAs) are a class of short non-coding
RNAs, which have been found to be implicated in a number of human
malignancies (7,8). miRNAs have been reported to affect
tumor development by binding to the 3' untranslated region (3'UTR)
of target genes (9,10). Accumulating evidence has
demonstrated that miRNAs, including miR-216b-5p, miR-30d,
miR-4638-5p and miR-141, are associated with the tumorigenesis of
PC (11-14).
miR-184 is a single copy gene, evolutionarily
conserved at the nucleotide level, that is located on chromosome
15(15). A strong association
between miR-184 and protection from cancer has been reported by
previous investigations. For example, the upregulation of miR-184
reduced nasopharyngeal carcinoma cell migration and invasion
(16), and it was also shown to
inhibit colorectal cancer cell viability and metastasis by
targeting IGF-1R (17). In
addition, lncRNA UCA1 has been reported to promote oral squamous
cell carcinoma cell proliferation and cisplatin resistance through
miR-184 suppression (18). However,
the role of miR-184 in PC remains unclear.
In the present study, the expression levels of
miR-184 and DLX1 were measured in PC tissues and cell lines.
Functional tests were performed to evaluate the effect of miR-184
overexpression on PC cell lines in vitro. Further
experiments were conducted to confirm whether DLX1 is a direct
target of miR-184, and whether its expression could be reduced by
miR-184 overexpression. Collectively, the results of the present
study may provide a potential novel approach to the molecular
treatment of PC.
Materials and methods
Patient specimens and cell lines
The expression profiles of miR-184 and DLX1 were
downloaded from TCGA, including 52 normal human tissue samples and
499 PC samples. PC cell lines (C4-2, PC-3, Du145 and LNCaP) and a
human prostate epithelial cell line (RWPE-2) were used in the
present study. RWPE-2, C4-2 and Du145 cells were purchased from the
American Type Culture Collection, whereas PC-3 and LNCaP cells were
obtained from the cell bank of Type Culture Collection of the
Chinese Academy of Sciences. All cells were incubated in RPMI-1640
medium supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin-streptomycin at 37˚C with 5% CO2, and
digested by 0.25% trypsin upon each passage.
Cell transfection
miR-184 mimic and pcDNA3.1-DLX1 were used to
upregulate miR-184 and DLX1 expression, respectively. In addition,
to examine the correlation of miR-184 and DLX1, pcDNA3.1-DLX1 (4
µg) and miR-184 mimic (0.1 nmol; designed and synthesized by
Shanghai GenePharma Co., Ltd.) were used to co-transfect Du145
cells. All transfections were conducted using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Reverse
transcription-quantitative PCR (RT-qPCR) analysis was utilized to
verify transfection efficiency at 48 h following transfection.
RT-qPCR
Total RNA was isolated from PC cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and then
reverse-transcribed to cDNA (annealing at 25˚C, extension at 42˚C)
using the GoScript® Reverse Transcription kit (Promega
Corporation) in accordance with the protocol of the manufacturer.
For the measurement of miRNA expression, the GoTaq® qPCR
Master Mix (cat. no. A6001; Promega Corporation) was used with
SYBR®-Green. The program was as follows: Initial
denaturation at 95˚C for 10 min, followed by 40 cycles at 95˚C for
15 sec and at 60˚C for 1 min. The U6 small nuclear RNA was used as
an internal reference for miR-184 expression, while GAPDH was used
as internal control for DLX1 expression. The sequences of the
primers were as follows: miR-184 forward,
5'-AGTGCAGTGGGGTTGGTCTA-3' and reverse,
5'-TCCAAATGGCTCATCTCCGAC-3'; U6 forward, 5'-CTCGCTTCGGCAGCACA-3'
and reverse, 5'-AACGCTTCACGAATTTGCGT-3'; DLX1 forward,
5'-GCTACCCCTACGTCAACTCG-3' and reverse,
5'-CAGATCTTGACCTGGGTCCTC-3'; and GAPDH forward,
5'-GGAGCGAGATCCCTCCAAAAT-3' and reverse,
5'-GGCTGTTGTCATACTTCTCATGG-3'. The relative expression level was
calculated using the 2-ΔΔCq method (19).
Cell Counting Kit-8 (CCK-8) assay
Du145 cells were seeded into 96-well plates at a
density of 1,000 cells/well 24 h after transfection. The cells were
then cultured in a CO2 incubator with 5% CO2
at 37˚C and examined every 24 h. For cell viability, 10 µl CCK-8
reagent was added to each well and incubated for 1.5 h at 37˚C. The
OD value at 450 nm was subsequently determined using an automatic
multi-well spectrophotometer (Bio-Rad Laboratories, Inc.), and a
cell viability curve was drawn. All experiments were performed at
least three times.
Cell migration and invasion
assays
A wound healing assay was conducted to examine cell
migration. Following transfection with specific vectors, cells
suspended in RPMI-1640 medium supplemented with 10% FBS and 1%
antibiotics (penicillin and streptomycin) were seeded into 6-well
plates at a density of 5x105 cells/well overnight, and
then incubated to >90% confluence. Subsequently, a 200-µl
pipette tip was used to create a wound. The existing medium was
then removed and the cells were rinsed with PBS before the addition
of serum-free medium. Images of the wounds were captured 24 h
following incubation using a light microscope and analyzed using
ImageJ version 1.44p software (National Institutes of Health). The
results were quantified using the following formulas: Wound closure
rate=[area (0 h)-area (24 h)]/area (0 h); wound closure (%)=(wound
closure rate in experimental group)/(wound closure rate in control
group) x100.
Cell invasion was assessed using Transwell assay.
Matrigel (BD Biosciences) was diluted in serum-free medium at 1:6
and placed in the upper chamber of transwell chamber at 37˚C for 4
h to polymerize Matrigel into a gel. All cells were seeded into the
upper chambers of Transwell chambers (pore size, 8 µm; Corning
Inc.) at a density of 2x105 cells/well. The upper
chamber was filled with 200 µl serum-free DMEM, while the lower
chamber was filled with 500 µl DMEM supplemented with 10% FBS.
Cells were then cultured for a further 16 h, and cells invading
into the lower chamber were fixed with 4% paraformaldehyde for 30
min at room temperature and stained with 0.1% crystal violet for 20
min at room temperature. The number of invading cells was
subsequently calculated from five random fields of view using a
light microscope.
Bioinformatics analysis
The bioinformatics software PicTar 21.0 (http://pictar.mdc-berlin.de) and TargetScan 7.2
(http://www.targetscan.org/vert_72/)
(20) were used to predict
potential target genes for hsa-miR-184.
Establishment of histogram based on
TCGA database
The present study used prostate cancer expression
data from the TCGA database (https://cancergenome.nih.gov/). Prostate cancer gene
expression profile data and clinical data were downloaded, and the
first group of samples (499 samples, name Tumor) and the second
group (52 samples, named normal), a total of 551 samples, were
selected for analysis. The RNA-Seq data was preprocessed and the
obtained data was used as an input file for subsequent methods. The
method of preprocessing is as follows: First, the raw data was
extracted from the matrix file. RNASeq data obtained from all 551
samples was processed and combined using the Perl programming
language software package (version 5.88; Perl.org), and
the matrix file was extracted to obtain the expression profile of
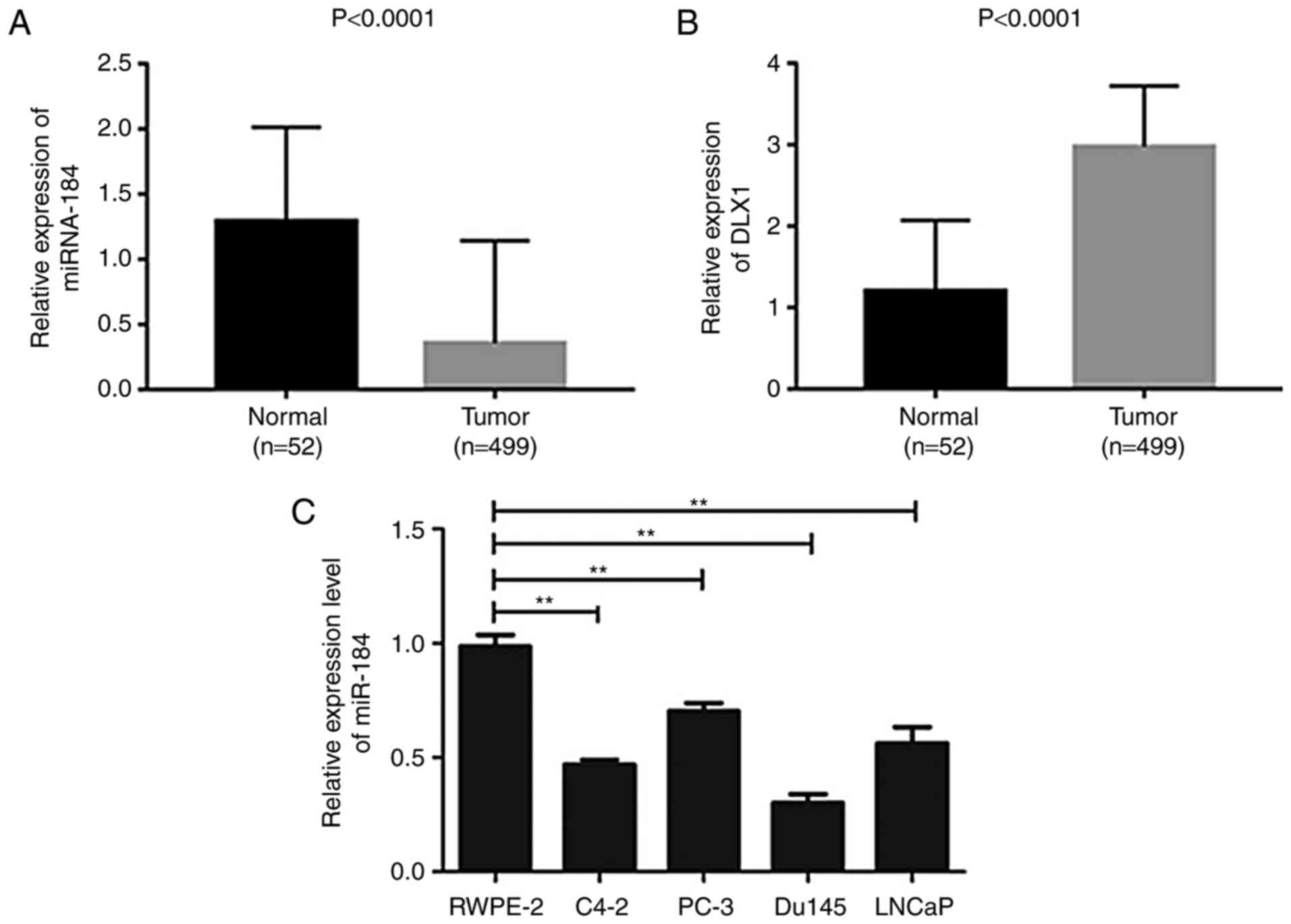
the gene in all samples. Expression maps as shown in Fig. 1A and B were then generated using GraphPad Prism
5.0 (GraphPad Software, Inc.) based on this expression profile
data. Among them, the height of the bar graph indicates the mean
value of the relative expression of miR-184 in the sample, and the
error line represents the maximum relative expression of miR-184 in
the group.
Dual luciferase reporter assays
A dual-luciferase reporter assay was carried out to
confirm the prediction results. pMIR-DLX1-WT and pMIR-DLX1-MUT were
synthesized by Shanghai GenePharma Co., Ltd. pMIR-DLX1-WT (0.2 µg)
or pMIR-DLX1-MUT (0.2 µg) containing seven mutant nucleotides in
the 3'UTR of DLX were co-transfected into Du145 cells (using the
method described above) seeded in 96 well plates at a density of
1x104, along with miR-184 NC (0.05 nmol) or miR-184
mimic (0.05 nmol). Cells were subsequently incubated for 48 h at
37˚C with 5% CO2 before luciferase assays were
performed. Luciferase activities in Du145 cells were determined
using the Dual-Luciferase® reporter assay system
(Promega Corporation). Firefly luciferase activity was normalized
to Renilla luciferase activity. Each experiment was
performed in triplicate.
Western blot analysis
Protein samples were extracted from the cells using
RIPA lysis buffer (Beyotime Institute of Biotechnology)
supplemented with protease inhibitors. The concentration of protein
was quantified using a bicinchoninic acid assay kit (Beyotime
Institute of Biotechnology). The lysates were then mixed with 5X
loading buffer and boiled at 95˚C for 5 min. SDS-PAGE (12%) was
performed to separate proteins (20 µg in each well) at 100 V for 1
h before being transferred to PVDF membranes at 200 mA for 1.5 h.
The PVDF membrane was then incubated with 5% skimmed milk powder
(diluted in 1X TBST buffer containing 0.1% Tween-20) at room
temperature for 1 h prior to incubation with mouse anti-human DLX1
(1:1,000; cat. no. ab126054; Abcam) or mouse anti-human β-actin
primary antibodies (1:1,000; cat. no. ab179467; Abcam) overnight at
4˚C. The membranes were subsequently rinsed once with PBS prior to
incubation with horseradish peroxidase-conjugated secondary
antibody (1:10,000; cat. no. ab205718; Abcam) for 2 h at room
temperature. The protein bands were visualized using an ECL
chromogenic kit and subsequently quantified using the Quantity One
system (Version number 1708195; Bio-Rad Laboratories Inc.).
Statistical analysis
All data were analyzed using SPSS 18.0 statistical
software (IBM Corp.) and normal distribution was described as mean
± standard deviation. Student's t-test and ANOVA followed by
Dunnett's or Bonferroni's post hoc test was used to compare the
difference between two or multiple groups, respectively.
Correlation between miR-184 and DLX1 was evaluated using Pearson's
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-184 expression is lower and DLX1
expression is higher in PC tissues and cell lines compared with
normal tissues
It was previously reported that DLX1 modulates a
number of signaling pathways during tumor progression by binding to
β-catenin (21). Therefore, RNA-seq
data from PC and normal prostate tissue samples was collected from
the TCGA database to investigate the expression of miR-184 and
DLX1, in order to establish whether there was a correlation between
miR-184 and DLX1 expression and PC tumorigenesis. The expression
level of miR-184 was found to be significantly lower in tumor
tissues from the 499 PC patients compared with normal tissues
(Fig. 1A; P<0.0001). By
contrast, DLX1 expression was significantly higher in PC tissues
compared with that in normal tissues (Fig. 1B; P<0.0001). The expression level
of miR-184 was next measured in human PC cell lines (C4-2, PC-3,
Du145 and LNCaP) and the normal human prostate epithelial cell line
(RWPE-2) using RT-qPCR. miR-184 expression levels were found to be
significantly lower in PC cell lines compared with that in the
RWPE-2 cell line and the Du145 cell line exhibited the lowest level
of expression compared with other PC cell lines (C4-2, PC-3 and
LNCaP; Fig. 1C; P<0.01). To
further investigate the mechanism underlying the potentially
beneficial role of miR-184 in PC, subsequent experiments were
conducted using the Du145 cell line.
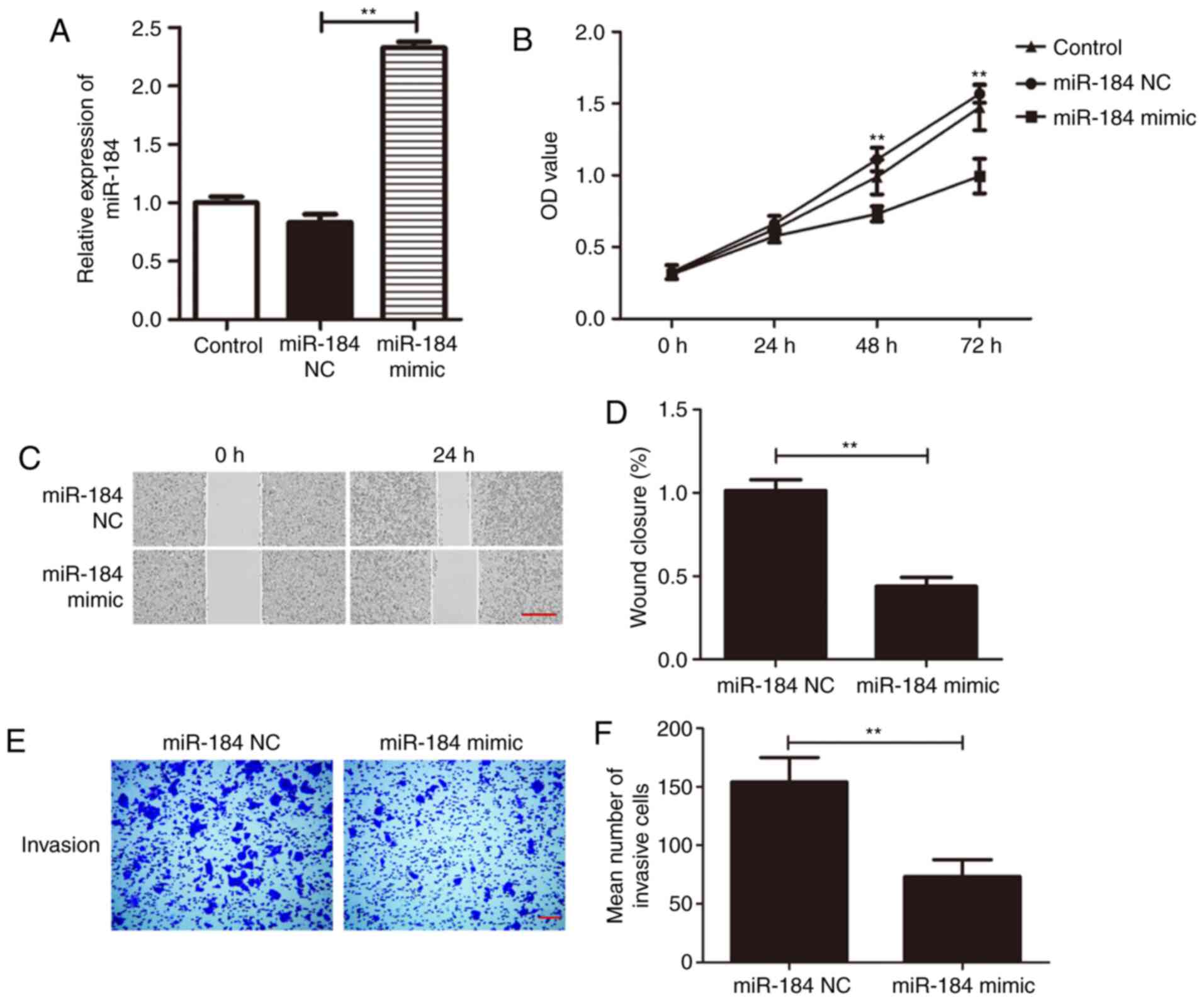
miR-184 overexpression inhibits Du145
cell proliferation, migration and invasion
To investigate the specific effect of miR-184 on
Du145 cells, a miR-184 mimic was used to overexpress miR-184.
miR-184 expression was found to be significantly higher in cells
transfected with miR-184 mimic compared with that in the control
group (Fig. 2A; P<0.01). The
CCK-8 assay was next applied to examine cell viability following
transfection with miR-184 mimic. Du145 cell viability was
significantly increased at 48 and 72 h following miR-184
overexpression (P<0.01; Fig.
2B). In addition, the migratory and invasive abilities of Du145
cells transfected with either miR-184 mimic or miR-184 NC were
examined using wound healing and Transwell assays. Wound closure
rate was significantly reduced in the miR-184 mimic group compared
with the miR-NC group after 24 h of incubation (P<0.01; Fig. 2C and D). The results from the Transwell assay
revealed that the number of invading cells in the miR-184 mimic
group was significantly lower compared with that in the miR-NC
group (P<0.01; Fig. 2E and
F). These findings suggest that
miR-184 overexpression prevents the proliferation, migration and
invasion of PC cells.
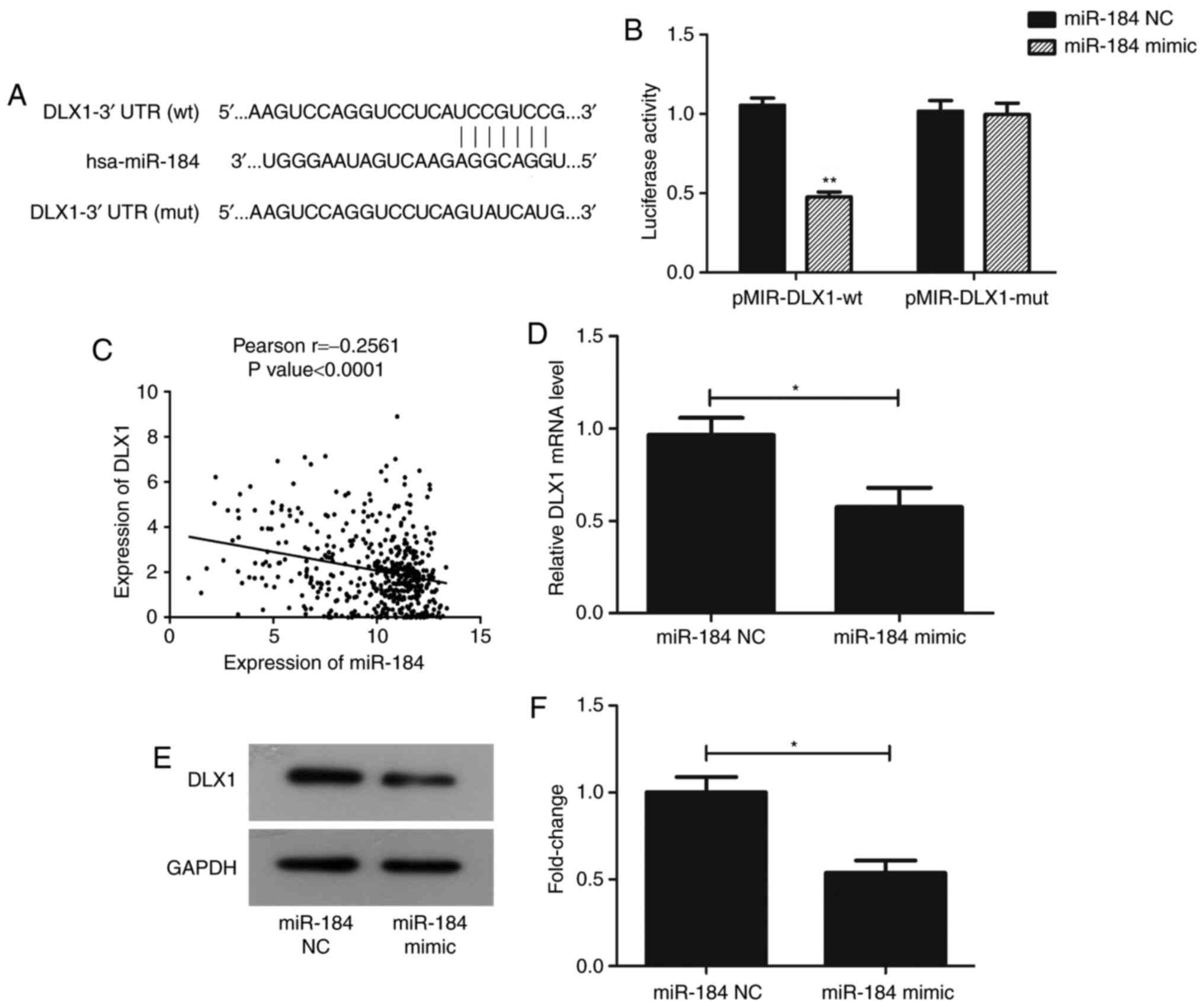
DLX1 is a target gene of miR-184
TargetScan 7.2 was used to confirm whether DLX1 is
directly targeted by miR-184. DLX1 has a seven-nucleotide
miR-184-binding site in its 3'UTR (Fig.
3A). Luciferase activity was significantly repressed in PC
cells co-transfected with pMIR-DLX1-WT and miR-184 mimic
(P<0.01), but not in those co-transfected with pMIR-DLX1-mut and
miR-184 mimic (Fig. 3B). In
addition, Pearson's analysis revealed that the expression levels of
miR-184 and DLX1 were negatively correlated using the clinical data
obtained from TCGA (P<0.0001; Fig.
3C). RT-qPCR and western blotting data also revealed that,
compared with GAPDH, the expression of DLX1 was reduced at the mRNA
and protein levels following transfection with miR-184 mimic
(Fig. 3D-F; P<0.05).
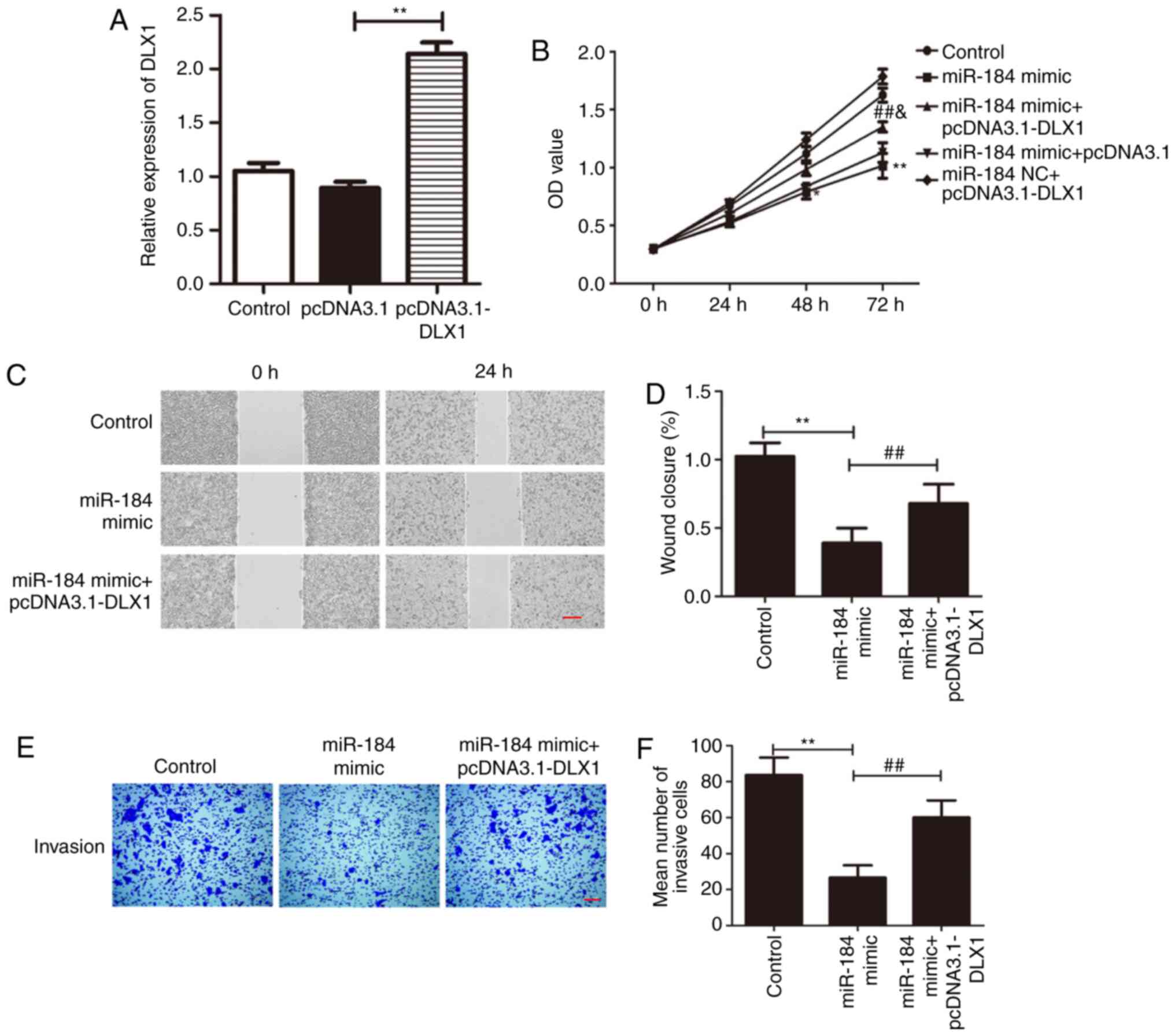
The effects of miR-184 overexpression
on Du145 cells are reversed by DLX1 overexpression
To further elucidate the molecular mechanisms
underlying the anti-proliferative and anti-migratory effects of
miR-184, the role of DLX1 was further studied. Given the negative
correlation between miR-184 expression and DLX1 expression, it was
hypothesized that the anti-proliferative and anti-migratory effects
of miR-184 mimic in Du145 cells might be reversed by overexpression
of DLX1. RT-qPCR showed that DLX1 expression was significantly
increased in Du145 cells transfected with pcDNA3.1-DLX when
compared with cells transfected with empty vector (Fig. 4A; P<0.01). It was subsequently
found that the overexpression of DLX1 reversed the effects of
miR-184 mimic on Du145 cells. The results of the CCK-8 assay
suggested that the anti-proliferative effects of miR-184 mimic
overexpression in Du145 cells were partially reversed following
co-transfection with plasmids expressing DLX1 (P<0.05; F=0.37;
Fig. 4B). Similarly, the wound
healing and Transwell assays showed similar patterns. Although the
overexpression of DLX1 did not restore the migration and
invasiveness of Du145 cells to levels comparable to the control
group, it partially alleviated the inhibitory effects of miR-184
overexpression on Du145 cells (P<0.01; Fig. 4C-F). Taken together, these findings
indicate that DLX1 is a direct target gene of miR-184, and its
overexpression can partially reverse the effects of miR-184
overexpression.
Discussion
Previous studies have demonstrated that miRNAs can
modulate a diverse number of cellular functions in several
diseases, rendering it meaningful to investigate the regulatory
network of miRNAs (22). Indeed, a
variety of biological functions have been found to be associated
with the aberrant expression of miRNAs, including cell apoptosis,
proliferation, differentiation and immune regulation, such as
pathogen recognition (23-26).
In particular, dysregulated miR-184 has been reported to be
associated with a number of malignancies (16-18).
The aim of the present study was to investigate the role of miR-184
in PC.
Accumulating evidence supports an association
between miR-184 expression and the promotion or prevention of tumor
development. miR-184 may impair cancer progression, as reported by
Su et al (27), who found
that the overexpression of miR-184 exerted an inhibitory effect on
renal cell carcinoma cell proliferation. Feng et al
(28) suggested that miR-184
expression was decreased in glioblastoma (GBM) tissues and
suppressed the development of GBM by binding to stanniocalcin-2;
similarly, an inhibitory effect was also reported in breast cancer
(29). By contrast, miR-184 was
found to promote cell proliferation in tongue squamous cell
carcinoma by targeting SOX7(30).
These findings collectively raise the possibility that miR-184
serves a dual function in tumorigenesis, namely as a tumor
suppressor as well as tumor promoter, although it may be
hypothesized that this largely depends on the tumor type and a
diverse number of potential target genes. In the present study, the
results were consistent with previous investigations (25,26),
supporting the hypothesis of an inhibitory role of miR-184 in
PC.
DLX1 is a binding protein of β-catenin, which can
increase cancer cell viability and migration by activating
β-catenin/TCF signaling (21). In
fact, abnormal activation of β-catenin/TCF signaling is considered
to be a signature of PC (31).
Therefore, DLX1 appears to be a potential carcinogenic factor. DLX1
has been reported to promote ovarian cancer progression by
interacting with Forkhead box protein M1, and to also contribute to
the development of oral clefts (32,33).
DLX1 has also been implicated in PC (34,35).
In the present study, the results of the dual luciferase reporter
assay and the analysis of cell proliferation, migration and
invasion in vitro indicated that DLX1 is a downstream target
gene of miR-184. In addition, the overexpression of DLX1 partially
reversed the inhibitory effects of miR-184 overexpression on
PC.
In conclusion, miR-184 and DLX1 exhibited opposite
trends in PC tissues; namely, miR-184 expression was suppressed,
whereas DLX1 expression was increased. Although further mechanistic
studies are required, including chromatin immunoprecipitation,
immunohistochemical analyses, use of animal models and recording of
the clinical characteristics of patients with PC during follow-up,
the findings of the present study suggest, to a certain extent,
that miR-184 can affect PC progression by suppressing DLX1
expression. Therefore, miR-184 may be of value as a potential
treatment target of PC in the future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
GGT and CX analyzed the data and wrote the
manuscript. WKZ and CYW revised the manuscript. CYW, GGT, CX and
WKZ collected the data and designed the study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cancer Stat Facts: Prostate Cancer in
2018; Available from: https://seer.cancer.gov/statfacts/html/prost.html.
|
|
2
|
World Cancer Report 2014. World Health
Organization. 2014. pp. Chapter 5.11.
|
|
3
|
Caini S, Gandini S, Dudas M, Bremer V,
Severi E and Gherasim A: Sexually transmitted infections and
prostate cancer risk: A systematic review and meta-analysis. Cancer
Epidemiol. 38:329–338. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Miller DC, Hafez KS, Stewart A, Montie JE
and Wei JT: Prostate carcinoma presentation, diagnosis, and
staging: An update form the national cancer data base. Cancer.
98:1169–1178. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Redman JM, Gulley JL and Madan RA:
Combining immunotherapies for the treatment of prostate cancer.
Urol Oncol. 35:694–700. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jang TL, Kim IY, Scardino PT and Eastham
JA: Reply to effectiveness of radical prostatectomy with adjuvant
radiotherapy versus radiotherapy plus androgen deprivation therapy
for men with advanced prostate cancer: Do we have certainties
today? Cancer. 125:2318–2320. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 4:143–159. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pillai RS, Bhattacharyya SN and Filipowicz
W: Repression of protein synthesis by miRNAs: How many mechanisms?
Trends Cell Biol. 17:118–126. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
O'Hara SP, Mott JL, Splinter PL, Gores GJ
and LaRusso NF: MicroRNAs: Key modulators of posttranscriptional
gene expression. Gastroenterology. 136:17–25. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang XJ, Reyes JL, Chua NH and Gaasterland
T: Prediction and identification of arabidopsis thaliana microRNAs
and their mRNA targets. Genome Biol. 5(R65)2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
He J, Sun M, Geng H and Tian S: Long
non-coding RNA Linc00518 promotes paclitaxel resistance of the
human prostate cancer by sequestering miR-216b-5p. Biol Cell.
111:39–50. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liang YK, Han ZD, Lu JM, Liu ZZ, Zhuo YJ,
Zhu XJ, Chen JX, Ye JH, Liang YX, He HC and Zhong WD:
Downregulation of ARID4A and ARID4B promote tumor progression and
directly regulated by microRNA-30d in patient with prostate cancer.
J Cell Biochem. 119:7245–7255. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jiang W, Gao Y, Wang Z, Gong C, Hu C, Ding
X, Qiang L, Gao S and Ren F: Co-delivery of miR-4638-5p and
docetaxel based on redox-sensitive polypeptide micelles as an
improved strategy for the treatment of castration-resistant
prostate cancer. Mol Pharm. 16:437–447. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Khorasani M, Teimoori-Toolabi L, Farivar
TN, Asgari M, Abolhasani M, Shahrokh H, Afgar A, Kalantari E,
Peymani A and Mahdian R: Aberrant expression of miR-141 and nuclear
receptor small heterodimer partner in clinical samples of prostate
cancer. Cancer Biomark. 22:19–28. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Aboobaker AA, Tomancak P, Patel N, Rubin
GM and Lai EC: Drosophila microRNAs exhibit diverse spatial
expression patterns during embryonic development. Proc Natl Acad
Sci USA. 102:18017–18022. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu X, Ding X, Ding Z and Jia P: Total
flavonoids from leaves of carya cathayensis ameliorate renal
fibrosis via the miR-21/Smad7 signaling pathway. Cell Physiol
Biochem. 49:1551–1563. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu G, Liu J, Wu Z, Wu X and Yao X:
MicroRNA-184 inhibits cell proliferation and metastasis in human
colorectal cancer by directly targeting IGF-1R. Oncol Lett.
14:3215–3222. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fang Z, Zhao J, Xie W, Sun Q, Wang H and
Qiao B: LncRNA UCA1 promotes proliferation and cisplatin resistance
of oral squamous cell carcinoma by suppressing miR-184 expression.
Cancer Med. 6:2897–2908. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qadir M and Faheem A: mRNA: A diagnostic
and therapeutic tool for pancreatic cnacer. Crit Rev Eukaryot Gene
Expr. 27:197–204. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liang M, Sun Y, Yang HL, Zhang B, Wen J
and Shi BK: DLX1, a binding protein of beta-catenin, promoted the
growth and migration of prostate cancer cells. Exp Cell Res.
363:26–32. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li JY, Wei X, Sun Q, Zhao XQ, Zheng CY,
Bai CX, Du J, Zhang Z, Zhu LG and Jia YS: MicroRNA-449b-5p promotes
the progression of osteoporosis by inhibiting osteogenic
differentiation of BMSCs via targeting Satb2. Eur Rev Med Pharmacol
Sci. 23:6394–6403. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gironella M, Seux M, Xie MJ, Cano C,
Tomasini R, Gommeaux J, Garcia S, Nowak J, Yeung ML, Jeang KT, et
al: Tumor protein 53-induced nuclear protein 1 expression is
repressed by miR-155, and its restoration inhibits pancreatic tumor
development. Proc Natl Acad Sci USA. 104:16170–16175.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Akao Y, Nakagawa Y and Naoe T: MicroRNAs
143 and 145 are possible common onco-microRNAs in human cancers.
Oncol Rep. 16:845–850. 2006.PubMed/NCBI
|
|
25
|
Neilson JR, Zheng GX, Burge CB and Sharp
PA: Dynamic regulation of miRNA expression in ordered stages of
cellular development. Genes Dev. 21:578–589. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen XM, Splinter PL, O'Hara SP and
LaRusso NF: A cellular micro-RNA, let-7i, regulates Toll-like
receptor 4 expression and contributes to cholangiocyte immune
responses against cryptosporidium parvum infection. J Biol Chem.
282:28929–28938. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Su Z, Chen D, Li Y, Zhang E, Yu Z, Chen T,
Jiang Z, Ni L, Yang S, Gui Y, et al: microRNA-184 functions as
tumor suppressor in renal cell carcinoma. Exp Ther Med. 9:961–966.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Feng L, Ma J, Ji H, Liu Y and Hu W:
MiR-184 retarded the proliferation, invasiveness and migration of
glioblastoma cells by repressing stanniocalcin-2. Pathol Oncol Res.
24:853–860. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Phua YW, Nguyen A, Roden DL, Elsworth B,
Deng N, Nikolic I, Yang J, Mcfarland A, Russell R, Kaplan W, et al:
MicroRNA profiling of the pubertal mouse mammary gland identifies
miR-184 as a candidate breast tumour suppressor gene. Breast Cancer
Res. 17(83)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chen D, Li J, Li S, Han P, Li N, Wang Y
and Du S: miR-184 promotes cell proliferation in tongue squamous
cell carcinoma by targeting SOX7. Oncol Lett. 16:2221–2228.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Schneider JA and Logan SK: Revisiting the
role of Wnt/β-catenin signaling in prostate cancer. Mol Cell
Endocrinol. 462:3–8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chan DW, Hui WW, Wang JJ, Yung MMH, Hui
LMN, Qin Y, Liang RR, Leung THY, Xu D, Chan KKL, et al: DLX1 acts
as a crucial target of FOXM1 to promote ovarian cancer
aggressiveness by enhancing TGF-β/SMAD4 signaling. Oncogene.
36:1404–1416. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sabóia TM, Reis MF, Martins AM, Romanos
HF, Tannure PN, Granjeiro JM, Vieira AR, Antunes LS, Küchler EC and
Costa MC: DLX1 and MMP3 contribute to oral clefts with and without
positive family history of cancer. Arch Oral Biol. 60:223–228.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Leyten GH, Hessels D, Smit FP, Jannink SA,
de Jong H, Melchers WJ, Cornel EB, de Reijke TM, Vergunst H, Kil P,
et al: Identification of a candidate gene panel for the early
diagnosis of prostate cancer. Clin Cancer Res. 21:3061–3070.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rhie SK, Guo Y, Tak YG, Yao L, Shen H,
Coetzee GA, Laird PW and Farnham PJ: Identification of activated
enhancers and linked transcription factors in breast, prostate, and
kidney tumors by tracing enhancer networks using epigenetic traits.
Epigenetics Chromatin. 9(50)2016.PubMed/NCBI View Article : Google Scholar
|