Introduction
Diabetic retinopathy (DR) is a microvascular
complication of diabetes and a major cause of vision loss in
Western countries. The incidence of diabetes worldwide will reach
>366 million by the end of 2030(1). It is reported that nearly all
patients with type 1 diabetes will develop some manifestation of
DR, and that ~50-80% of patients with type 2 diabetes will have DR
within 20-25 years of developing the condition (2). With the growing population of
individuals with diabetes, the incidence of DR will increase
gradually, which poses a major threat to the global population and
a costly burden to health care systems. The exact mechanism of DR
is unclear, and the current treatments for diabetic retinopathy,
such as anti-vascular endothelial growth factors and laser
coagulation, have limited efficacies for patients with DR (3). Wnt signaling is an evolutionally
conserved signaling pathway that serves essential roles in tissue
development and adult homeostasis (4). Dysregulated Wnt signaling plays
pathogenic roles in a variety of human diseases, including DR
(5,6). Upregulated Wnt signaling increases
retinal inflammation and secretion of vascular endothelial growth
factor (VEGF) and results in retinal neovascularization in diabetic
animal models (5,7). Blockage of the Wnt signaling pathway
has anti-angiogenic and anti-inflammatory effects in DR (8,9).
However, the underlying mechanism of aberrant Wnt signaling
activation in DR remains to be determined.
Autophagy is a catabolic process in which cellular
components are degraded by lysosomes (10). Autophagy is essential for retinal
development and vision formation (11). Previous studies have demonstrated
that autophagy is involved in the pathogenesis of DR (12-14).
For example, retinal autophagy is upregulated in diabetic human and
mice, where it has been demonstrated to play dual roles in DR, a
protective role in mild stress [induced by 50 mg/l in
vitro-modified heavily-oxidized glycated LDL (HOG-LDL)] in
cultured human retinal capillary cells and a detrimental role in
severe stress (200 mg/l HOG-LDL) (13). High glucose increases endoplasmic
reticulum stress and induces autophagy in Müller cells (14).
The interplay between autophagy and Wnt signaling
has been previously reported. For example, Kallistatin, which is a
Wnt signaling inhibitor, can induce apoptosis and autophagy in
breast cancer cells, thereby reducing the rates of tumor
angiogenesis and vascular growth (15). A previous study on lung cancer
demonstrated that the tumor suppressor candidate 3 protein could
induce expression of autophagy related proteins, which activates
Wnt/β-catenin signaling in human non-small lung cancer cells
(16). The association between
autophagy and Wnt signaling in cancer field is well established
(17). However, whether autophagy
might regulate Wnt signaling in DR has not yet been
investigated.
The present study aimed to investigate the possible
mechanism of abnormal Wnt signaling in DR and hypothesized that
autophagy may have a regulatory effect on Wnt signaling in DR.
Meanwhile, the investigation of autophagy in DR may shed a light on
how autophagy was changed in DR and the possible function of
autophagy in the retina.
Materials and methods
Animals
Heterozygous BKS.Cg-Dock7m+/+
Leprdb/J mice (stock no. 000642) were purchased from Jackson
Laboratory. Homozygous db/db mice and control mice were obtained by
crossing heterozygous db/+ mice. C57BL/6J [wild-type (WT)] mice
were purchased from the Laboratory Animal Center of Xiamen
University. All animals were housed in a specific-pathogen-free
facility and maintained in 12-h light/dark cycle. All animal
procedures were performed in accordance with the Association for
Research in Vision and Ophthalmology ‘Statement for the Use of
Animals in Ophthalmic and Vision Research’. The animal protocols
were approved by the Xiamen University Experimental Animal Ethics
Committee (approval no. XMULA20190022).
Cell culture
The rat Müller (rMC-1) cell line was a gift from Dr
Vijay Sarthy of Northwestern University (Evanston, IL, USA) and
cultured in Dulbecco's modified Eagle's medium supplemented with
10% fetal calf serum and 1% penicillin streptomycin solution (all
from Gibco; Thermo Fisher Scientific, Inc.) and placed at 37˚C in a
humidified incubator containing 5% CO2.
Induction/inhibition of autophagy in
vitro
rMC-1 cells were plated in a 6-well plate at a
density of 5x105/well. After 24 h, rMC-1 cells were
treated with rapamycin (4 µM) which was dissolved in DMSO (both
from Sigma-Aldrich; Merck KGaA) or with the same amount of DMSO as
a vehicle for 24 h. To suppress the autophagy in rMC-1 cells,
chloroquine (1 µM; Sigma-Aldrich) was used to treat rMC-1 cells for
6 h and DMSO was used as vehicle control.
Induction/inhibition of autophagy in
vivo
An autophagy inhibitor 3-methyladenin (3-MA;
Sigma-Aldrich; Merck KGaA; 25 mg/kg) was dissolved in DMSO and
intraperitoneally injected into db/db mice (5 months old; average
weight, 54 g) every other day for 30 days (18), while the same amount of DMSO was
used as vehicle control in 5-month-old db/db mice with the similar
manner. Similarly, rapamycin (Sigma-Aldrich; Merck KGaA; 25 mg/kg)
was dissolved in DMSO and intraperitoneally injected into C57 mice
(3 months old; average weight, 28 g) once a day for 30 days to
induce autophagy, while DMSO was used as vehicle control (19). At least six mice were used in each
group. Mice were sacrificed using carbon dioxide (30% cage
volume/min) and the retinas were collected for subsequent
experiments and stored in a -80˚C freezer for further use.
Western blotting
The treated rMC-1 cells and retinal tissues were
lysed by radioimmunoprecipitation assay (RPAP) buffer
(Sigma-Aldrich; Merck KGaA) and the total protein concentrations
were measured by bicinchoninic acid assay. Each amount of protein
(25 µg per lane) was resolved by electrophoresis through 12%
Tris-glycine SDS polyacrylamide gel and electrotransferred onto a
polyvinylidene difluoride membrane. The membrane was blocked with
5% non-fat dry milk in Tris-buffered saline with 0.1% Tween
(TBST)-20 for 2 h at room temperature. The membrane was incubated
with the primary antibodies (dilution 1:1,000) at 4˚C overnight.
The primary antibodies against microtubule-associated protein
1A/1B-light chain 3B (LC3B; cat. no. ab48394), P62 (cat. no.
ab56416) and VEGF (cat. no. ab46154) were obtained from Abcam. The
primary antibodies against Beclin-1 (cat. no. sc-48341) and
β-catenin (cat. no. sc-7199) were purchased from Santa Cruz
Biotechnology, Inc. Primary antibodies against
non-phosphorylated-β-catenin (n-p-β-catenin; cat. no. 4270S) and
β-actin (cat. no. 4970) were purchased from Cell Signaling
Technology, Inc. After three washes with TBST, the membranes were
incubated with 1:5,000 dilution of a HRP-conjugated goat anti-mouse
antibody (Sigma-Aldrich, cat. no. A4416) or an HRP-conjugated
anti-rabbit antibody (Sigma-Aldrich; Merck KGaA; cat. no. A6154) in
TBST containing 1% dry milk for 1 h. After four washes with TBST,
the bands were detected using super ECL detection Reagent (Shanghai
Yeasen Biotechnology Co., Ltd.) and the band intensities were
semi-quantified by densitometry using Quantity One-1D analysis
software (Bio-Rad Laboratories, Inc.).
Statistical analysis
GraphPad Prism version 7 (GraphPad Software, Inc.)
was used for statistical analyses. Data were presented as the means
± standard error of the mean. Comparison between two groups was
performed using Student's t-test. At least three independent
measurements were conducted for each assay. P<0.05 was
considered to indicate a statistically significant difference.
Results
Wnt signaling is activated and
autophagy is stimulated in retinas from db/db mice
Previous studies reported that Wnt signaling is
abnormally activated in diabetic retinas and serves a pathogenic
role in DR (7,20). In the present study, db/db mouse, a
genetic type 2 diabetic animal model, was selected as animal model
of diabetic retinopathy (21). The
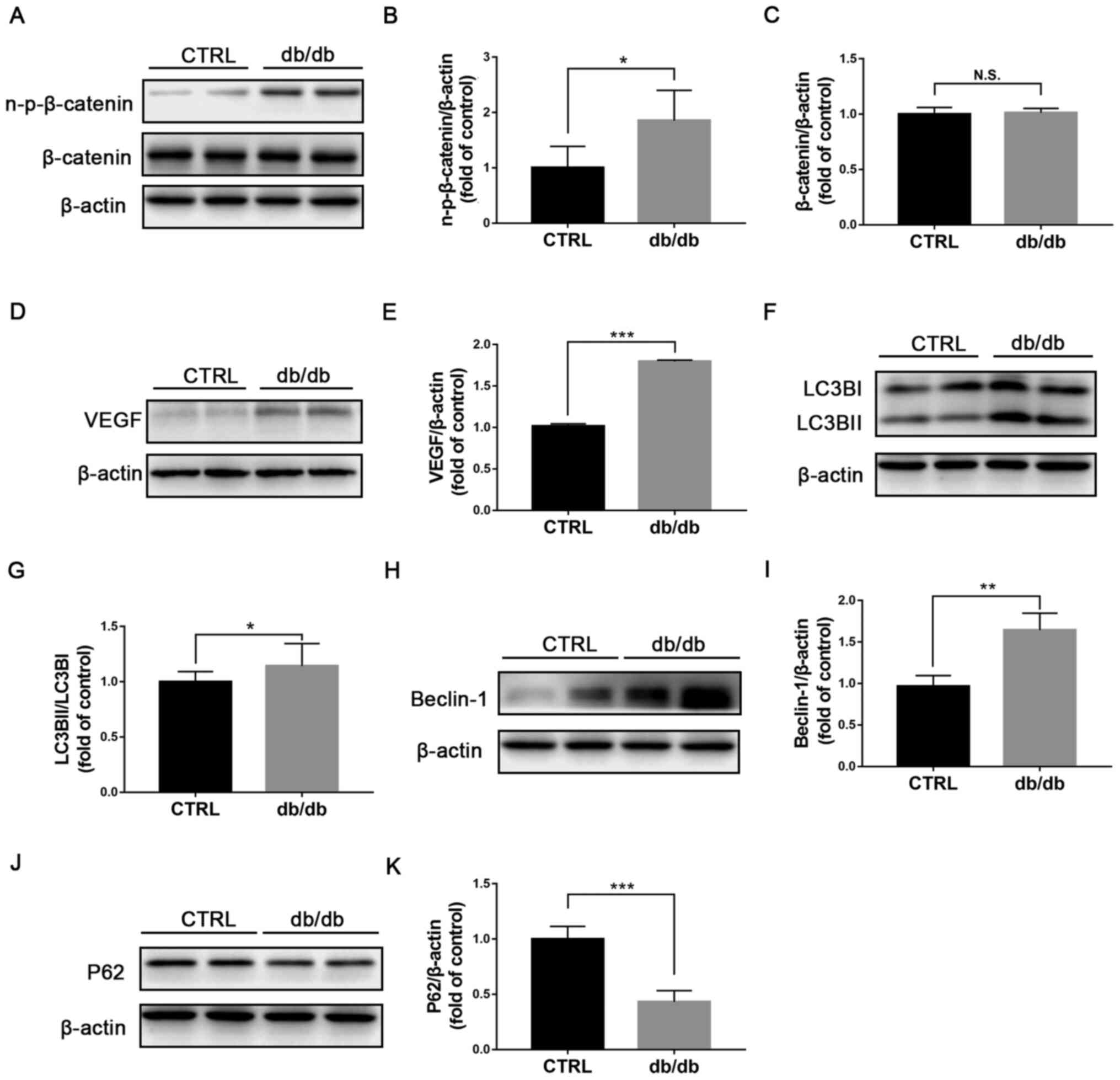
protein expression of non-p-β catenin and β-catenin was evaluated
by western blotting (Fig. 1A) and
non-p-β catenin expression was found to be significantly increased
in db/db retinas (Fig. 1B), while
β-catenin expression was unchanged (Fig. 1C). Protein expression of the target
gene of Wnt signaling VEGF was also significantly upregulated in
db/db retinas compared with those in control retinas (Fig. 1D and E). Furthermore, the expression of the
autophagic proteins LC3BI, LC3BII (Fig. 1F), Beclin-1 (Fig. 1H) and P62 (Fig. 1J) was determined by western
blotting. The ratio LC3BII/LC3BI (Fig.
1G) and expression of Beclin-1 (Fig. 1I) were significantly elevated,
whereas P62 expression (Fig. 1K)
was significantly reduced in db/db retinas compared with the
control group, indicating an induction of autophagy in the retina
of db/db mice. Taken together, these findings indicated that Wnt
signaling and autophagy in db/db retinas may be related.
Autophagy inhibition downregulates Wnt
signaling in db/db retinas
To further clarify the relationship between
autophagy and Wnt signaling, db/db mice were treated with 3-MA, a
selective PI3K inhibitor that inhibits autophagy (22). After one-month treatment, the ratio
LC3BII/LC3BI (Fig. 2A and B) and Beclin-1 expression (Fig. 2C and D) were significantly decreased, whereas
P62 expression was significantly increased in the retinas of db/db
mice treated with 3-MA compared with those treated with vehicle
(Fig. 2C and E), suggesting a successful inhibition of
autophagy in db/db retinas. Furthermore, the protein expression of
n-p-β-catenin was significantly decreased in the retinas of
3-MA-treated db/db mice compared with the retinas of
vehicle-treated db/db mice (Fig.
2F and G), and the protein
expression of β-catenin (Fig. 2G
and H) was unchanged. Protein
expression of VEGF was significantly decreased in the retinas of
3-MA-treated db/db mice (Fig. 2I
and J). In addition, the weight
and blood glucose levels of db/db mice were not significantly
changed before or after treatment of 3-MA (Table SI). Taken together, these results
demonstrated that inhibition of autophagy may be accompanied with
inactivation of Wnt signaling, indicating a regulatory effect of
autophagy on Wnt signaling.
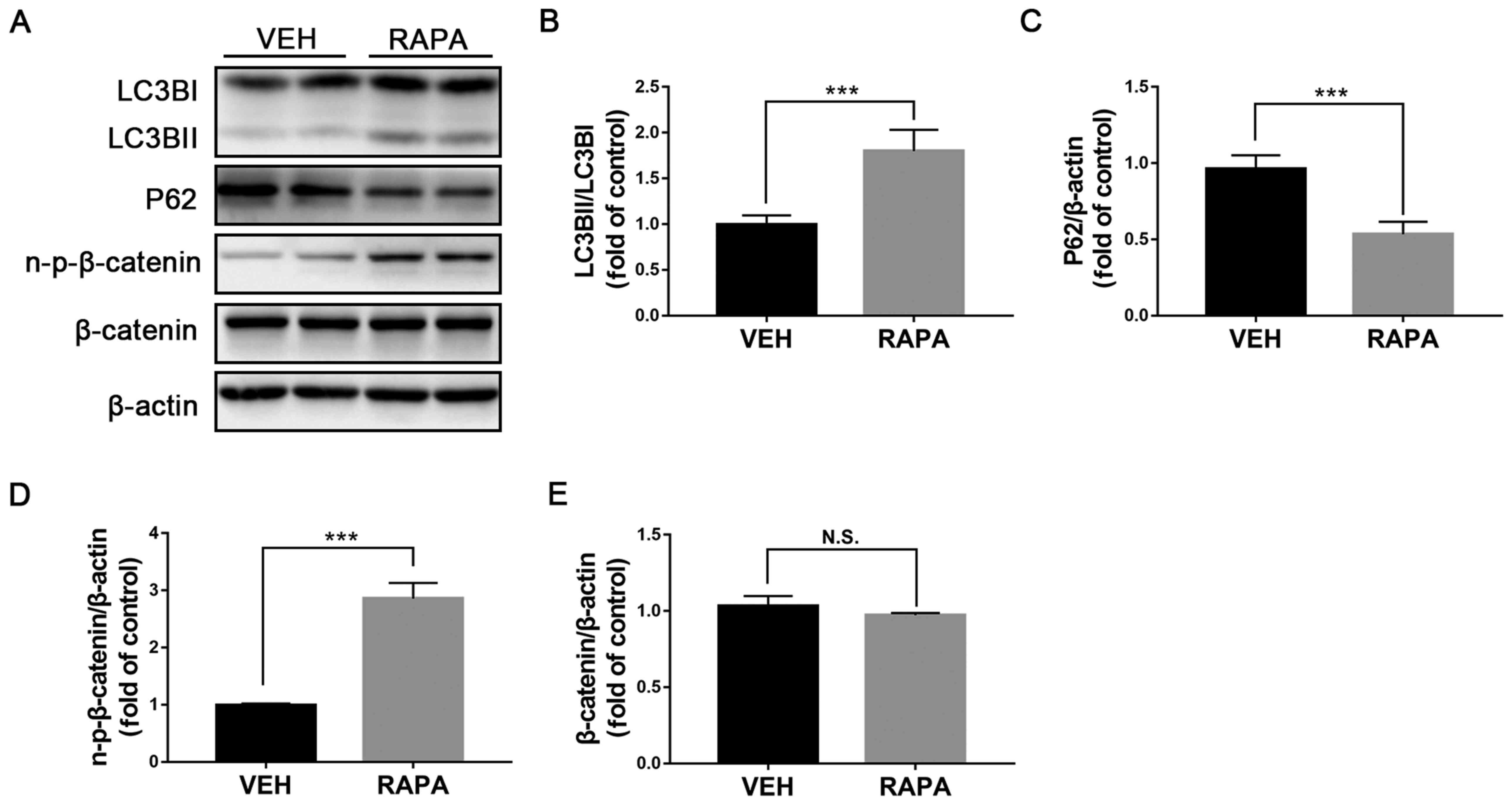
 | Figure 2Inhibition of autophagy downregulates
Wnt signaling in db/db retinas. Representative images of western
blotting for (A) LC3BI/II, (C) Beclin-1, P62, (F) n-p-β-catenin and
β-catenin in db/db mice treated with VEH or 3-MA. (B) Protein
levels of LC3BI/II were quantified by densitometry and LC3BII/LC3BI
was calculated and compared (n=6). Protein levels of (D) Beclin-1,
(E) P62, (G) n-p-β-catenin and (H) β-catenin were quantified by
densitometry and normalized to β-actin levels (n=6). (I and J)
Protein expression of the downstream target gene of Wnt signaling
VEGF was evaluated and quantified in db/db mice treated with VEH or
3-MA (n=6). *P<0.05 and **P<0.01.
n-p-β-catenin, non-phosphorylated-β-catenin; N.S., non-significant;
LC3B, microtubule-associated protein 1A/1B-light chain 3; VEGF,
vascular endothelial growth factor; VEH, vehicle; 3-MA,
3-methyladenin; db, diabetic. |
Induction of autophagy activates Wnt
signaling in WT retinas
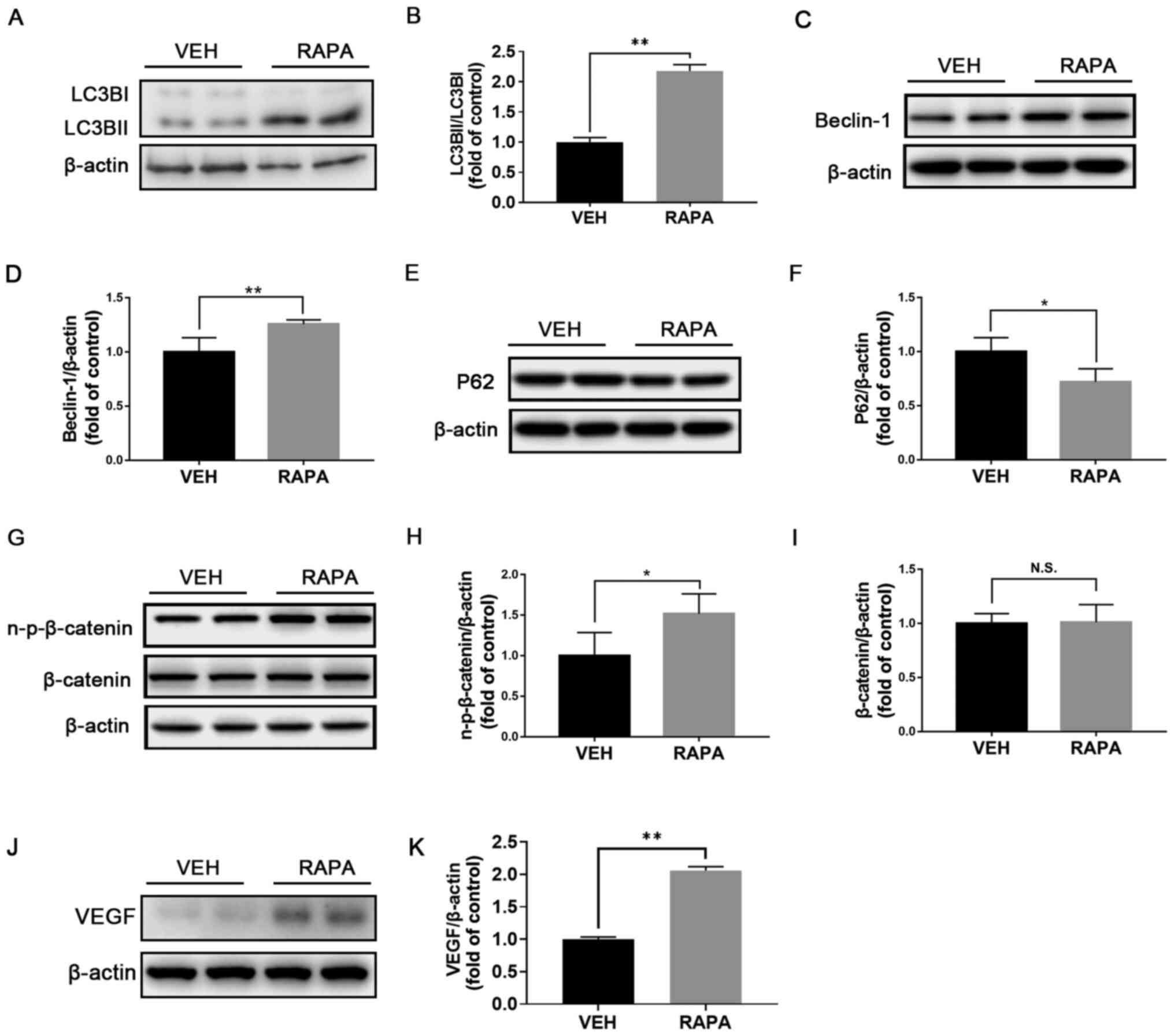
To further investigate the effect of autophagy on
the modulation of Wnt signaling, rapamycin was used to induce
autophagy in normal WT retinas and the expression of proteins
associated with Wnt signaling was evaluated by western blotting.
Following administration of rapamycin, the ratio LC3BII/LC3BI
(Fig. 3A and B) and expression of Beclin-1 (Fig. 3C and D) were significantly increased, while P62
expression was significantly decreased in the retinas (Fig. 3E and F), suggesting the successful induction of
autophagy in the retinas of WT mice. In addition, protein
expression of n-p-β-catenin (Fig.
3G and H) was significantly
increased in the retinas of rapamycin-treated WT retinas compared
with those of vehicle-treated WT mice and the expression of
β-catenin was unchanged (Fig. 3G
and I). Furthermore, protein
expression of VEGF was significantly increased in rapamycin-treated
retinas (Fig. 3J and K). Taken together, these findings
suggested that induction of autophagy may activate Wnt signaling in
the retina.
Induction of autophagy upregulates Wnt
signaling in rMC-1 cells
Müller cells are the primary glial cells that
contribute to the pathological responses in DR (23). A previous study demonstrated that
inactivation of Wnt signaling in Müller cells attenuates
inflammatory responses in diabetic retinas (24). To determine on which cells
autophagy may have an effect, rMC-1 cells were treated with
rapamycin and the expression of autophagic proteins and key
components of Wnt signaling was evaluated. The ratio LC3BII/LC3BI
was significantly increased (Fig.
4A and B) and P62 expression
was significantly decreased in rMC-1 cells treated with rapamycin
compared with those treated with vehicle (Fig. 4A and C), suggesting that autophagy was induced
in rMC-1 cells. Furthermore, protein expression of n-p-β-catenin
was significantly increased in rapamycin-treated rMC-1 cells
(Fig. 4A and D), whereas β-catenin expression was
unchanged (Fig. 4A and E). Taken together, these results
demonstrated that autophagy may activate Wnt signaling in rMC-1
cells.
Inhibition of autophagy downregulates
Wnt signaling in rMC-1 cells
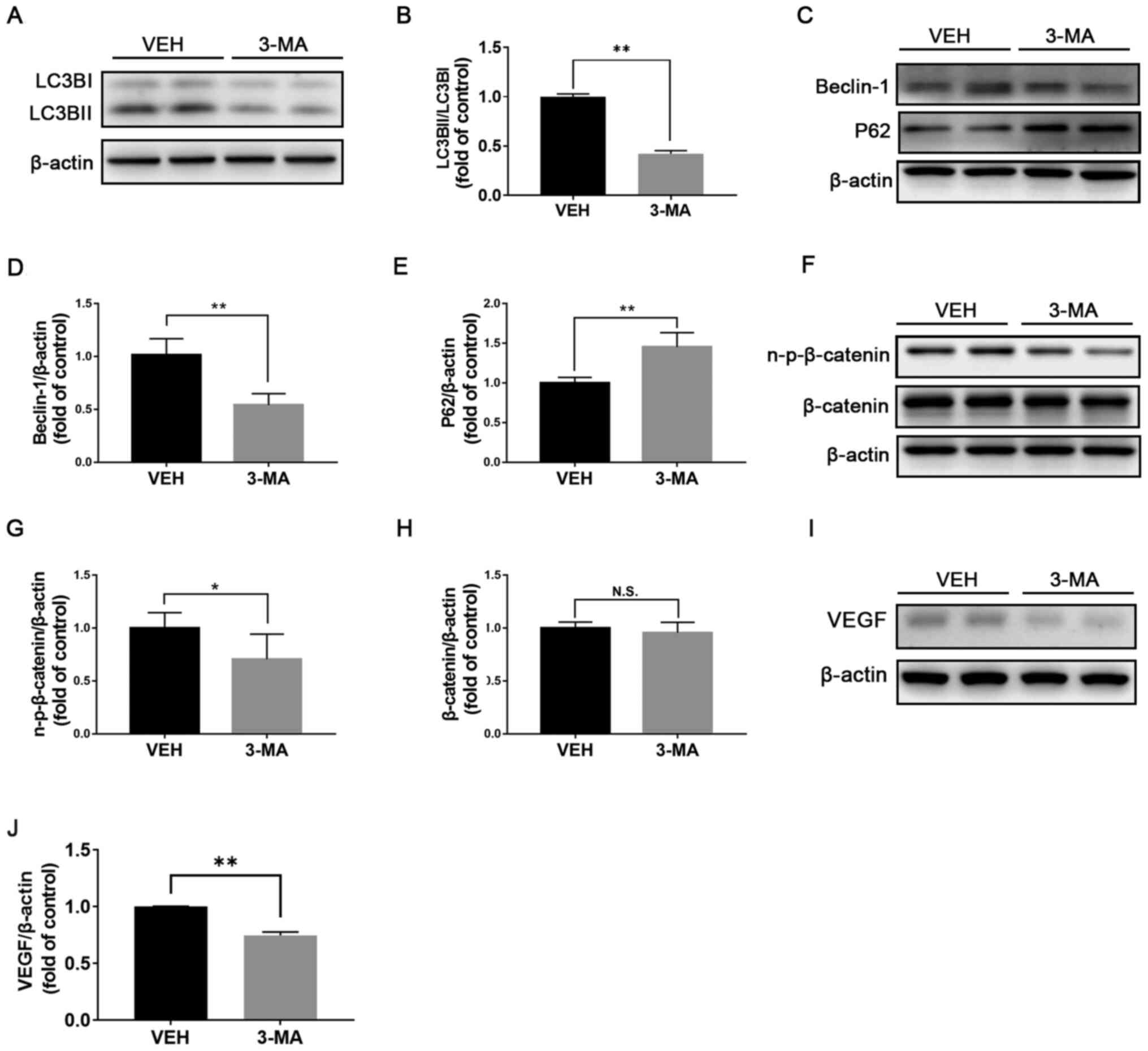
We treated rMC-1 cells with chloroquine, which is a
commonly used autophagy inhibitor (25). Following chloroquine treatment, the
ratio LC3BII/LC3BI was significantly decreased (Fig. 5A and B), whereas expression of P62 was
significantly increased in rMC-1 cells (Fig. 5A and C). The expression of n-p-β-catenin was
significantly decreased in rMC-1 cells treated with chloroquine
compared with those treated with vehicle (Fig. 5A and D), whereas the protein expression of
β-catenin was unchanged (Fig. 5A
and E). Furthermore, the ratio
LC3BII/LC3BI was significantly increased in high glucose-treated
rMC-1 cells compared with control cells (Fig. 5F and G). In addition, the autophagy inhibitor
3-MA inhibited the upregulation of LC3BII/LC3BI in high
glucose-treated rMC-1 cells (Fig.
5H and I), suggesting that
autophagy may play a regulatory role through rMC-1 cells in
diabetic retinas.
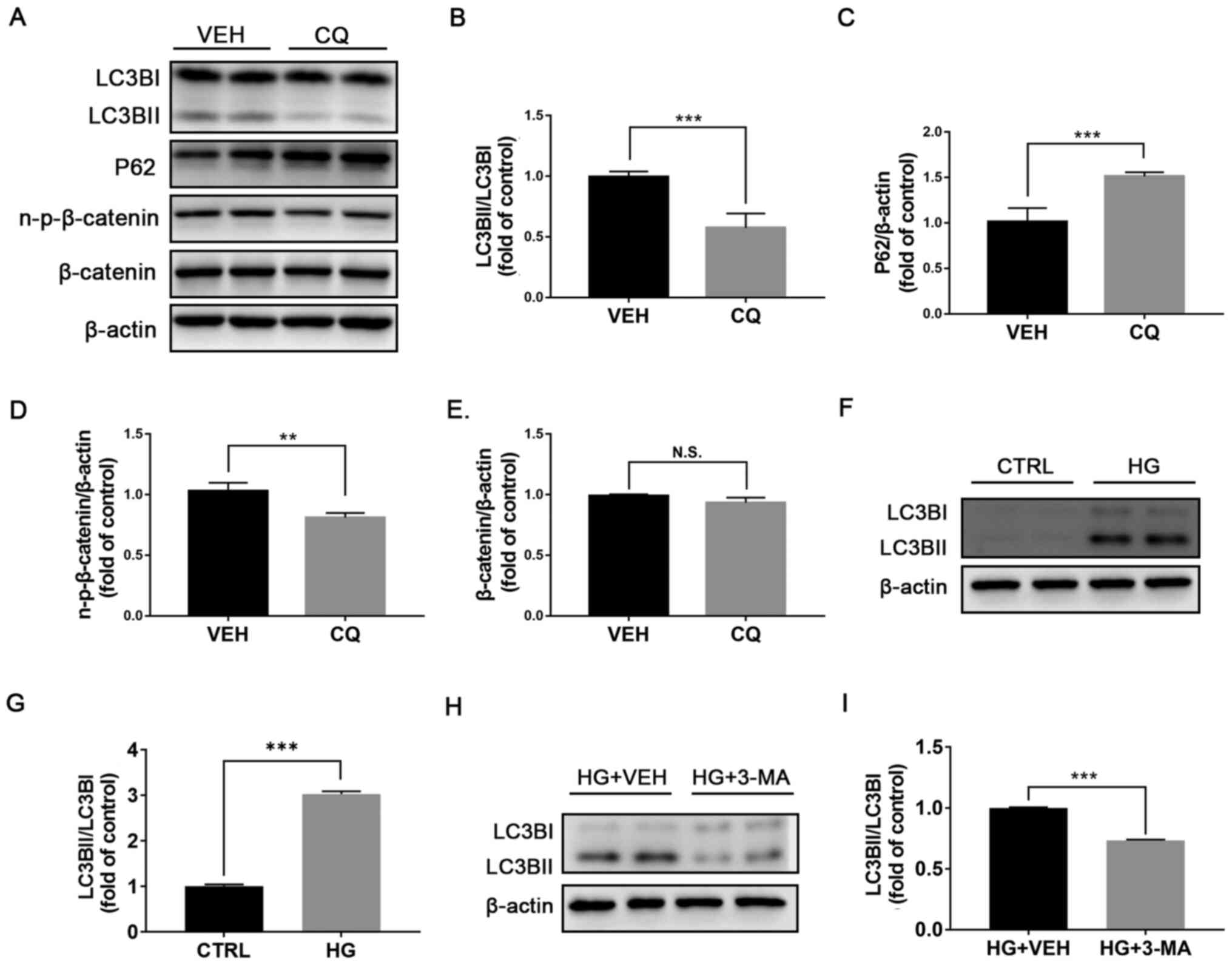 | Figure 5Inhibition of autophagy by CQ
suppresses Wnt signaling in rMC-1 cells. rMC-1 cells were treated
with VEH or CQ (1 µM) for 24 h. (A) Expression of LC3BI and
LC3BI/II, P62, n-p-β-catenin and β-catenin was evaluated by western
blotting. (B) Protein levels of LC3BI/II were quantified by
densitometry and LC3BII/LC3BI was calculated and compared. Protein
levels of (C) P62, (D) n-p-β-catenin and (E) β-catenin were
quantified by densitometry and normalized to β-actin levels. (F amd
G) Expression of LC3BI and LC3BII was evaluated by western blotting
and quantified by densitometry. LC3BII/LC3BI was calculated and
compared between CTRL rMC-1 cells and HG-treated cells. (H and I)
Expression of LC3BI and LC3BII was evaluated by western blotting
and quantified by densitometry. LC3BII/LC3BI was calculated and
compared between rMC-1 cells treated with HG+VEH or HG+3-MA. All
data are representative of three independent experiments.
**P<0.01 and ***P<0.001. n-p-β-catenin,
non-phosphorylated-β-catenin; N.S., non-significant; LC3B,
microtubule-associated protein 1A/1B-light chain 3; VEH, vehicle;
CQ, chloroquine; HG, high glucose; 3-MA, 3-methyladenin; CTRL,
control. |
Discussion
Wnt signaling is involved in the physiological and
pathological processes of a variety of diseases, including DR. The
present study demonstrated that autophagy could positively regulate
Wnt signaling in the retina of diabetic mice. Furthermore,
pharmacological manipulation of autophagy could modulate Wnt
signaling in the retina and retinal cells. To the best of our
knowledge, the present study was the first to report the potential
regulatory role of autophagy on Wnt signaling in the retina of
diabetic mice. This study revealed a novel mechanism of Wnt
signaling upregulation in DR by which autophagy may positively
regulate Wnt signaling, indicating a possible new therapeutic
target for DR via autophagy modulation.
Wnt signaling has been reported to contribute to the
pathogenesis of DR (5); however,
the mechanism of Wnt signaling upregulation in DR remains unknown.
Previous studies demonstrated that autophagy might have a regulator
role in Wnt signaling. For example, a previous study showed that
nutrition starvation, a commonly used condition that induces
autophagy in cells, downregulates Wnt signaling in HEK-293T cells;
while the inhibition of autophagy with 3-MA attenuates the
inhibitory effect of nutrition starvation on Wnt signaling
(17). Furthermore, previous
studies reported that autophagy is induced in the retina of
diabetic human and mice (13,14).
In the present study, a regulatory role of autophagy on Wnt
signaling in the retina of diabetic mice was demonstrated.
Interestingly, unlike most of studies in cancer field which show a
negative regulatory effect of autophagy on Wnt signaling (17,26),
our findings suggested that autophagy may positively regulate Wnt
signaling in the retina of diabetic mice. A possible mechanism of
autophagy negatively regulating Wnt signaling is that the autophagy
protein P62 could bind to Dishevelled 2 (Dvl2) and promote
LC3-mediated autophagosome recruitment of Dvl2, thus accelerating
Dvl degradation (17). However,
only a few studies demonstrated that autophagy positively regulates
Wnt signaling and described the possible underlying mechanism
(27,28). Further investigation is required to
determine the regulatory mechanisms of autophagy on Wnt signaling
in the retina, especially in the diabetic retina.
Db/db mouse is a commonly used animal model of type
2 diabetes (29). Previous studies
have shown that db/db mice display pathological features of DR,
such as loss of pericytes and retinal capillary degeneration
(30,31). In the present study, the ratio
LC3BII/LC3BI and expression of Beclin-1 were increased, whereas P62
expression was decreased in the retinas of db/db mice, indicating
an activation of autophagy in diabetic retinas. These findings were
consistent with other studies reporting that autophagy is induced
in DR (12-14).
In addition, the expression of a key component of Wnt signaling,
n-p-β-catenin, and of the downstream target gene of Wnt signaling,
VEGF, were increased in the present study, suggesting that Wnt
signaling is activated in db/db retinas. Activation of both
autophagy and Wnt signaling in db/db retinas indicates a possible
relationship between them. Furthermore, Wnt signaling was inhibited
in db/db mice treated with the autophagy inhibitor 3-MA and
activated after intraperitoneal injection of the autophagy
activator rapamycin in normal C57BL/6J mice. These results
suggested that autophagy could positively regulate Wnt signaling in
diabetic retinas, and that modulating autophagy may control Wnt
signaling in DR, which may serve as a potential therapeutic
strategy for DR.
Müller cell, which is a major retinal glial cell
type, covers the whole retina and interacts with many other types
of cell in the retina, and serves therefore a crucial role in
maintaining retinal hemostasis (32). The involvement of Müller cells in
DR has been well studied. For example, Müller cells are activated
in DR and produce inflammatory cytokines, resulting in cell
apoptosis in diabetic retinas (23). Previous studies have reported that
high glucose can promote mitochondrial dysfunction of Müller cells
and induce cells apoptosis, contributing therefore to the
pathogenesis of DR (23,33). Furthermore, other studies have
demonstrated that high glucose could induce autophagy in Müller
cells (14,34). Therefore, the present study used
Müller cells to further investigate the relationship between
autophagy and Wnt signaling. In this study, Wnt signaling was
upregulated in rapamycin-treated Müller cells and inhibited in
chloroquine-treated Müller cells. Interestingly, we found that
chloroquine-induced changes in LC3II expression had a
dose-dependent effect on Müller cells, and low dose of chloroquine
could decrease LC3BII expression. Similar observation was found by
Iwai-Kanai et al (35) in
cardiac-derived myocytes. In addition, in the present study, high
glucose could induce autophagy whereas 3-MA was capable of
reversing the induction of autophagy in Müller cells. These
findings demonstrated that manipulation of autophagy could modulate
Wnt signaling in Müller cells, indicating autophagy may regulate
Wnt signaling in Müller cells.
In the present study, the lack of immunostaining of
autophagic markers in the retina was a limitation. Further
investigation will explore the autophagic changes in specific
retinal cells of diabetic retinas, especially in Müller cells.
In summary, the present study demonstrated that
autophagy could positively regulate Wnt signaling in diabetic
retinas. These findings revealed a potential role for autophagy in
regulating Wnt signaling in DR, and modulation of autophagy may
have therapeutic effects in DR.
Supplementary Material
Weight and blood glucose levels of six
db/db mice before and after treatment with 3-methyladenin
(3-MA).
Acknowledgements
We would like to thank Dr Vijay Sarthy at
Northwestern University (Evanston, IL, USA) for providing the rMC-1
cell line.
Funding
Funding: This study was supported by the Natural Science
Foundation of Fujian Province (grant no. 2019J01017), the National
Science Foundation for Young Scientists of China (grant no.
31807795) and the National Key R&D program of China (grant no.
2018YFA0107302).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
SY, YZ, ZL and QC designed the experiments. SY, YZ,
XW, XL, MW and RZ performed the experiments. SY, YZ and QC analyzed
the data. ZL and QC supervised the experiments. SY, ZL and QC wrote
the manuscript. QC revised the manuscript. SY and QC confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The animal protocols were approved by the Xiamen
University Experimental Animal Ethics Committee (approval no.
XMULA20190022).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053.
2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Klein R, Knudtson MD, Lee KE, Gangnon R
and Klein BE: The Wisconsin Epidemiologic Study of Diabetic
Retinopathy: XXII the twenty-five-year progression of retinopathy
in persons with type 1 diabetes. Ophthalmology. 115:1859–1868.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ting DSW and Wong TY: Proliferative
diabetic retinopathy: Laser or eye injection? Lancet.
389:2165–2166. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen Q and Ma JX: Canonical Wnt signaling
in diabetic retinopathy. Vision Res. 139:47–58. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen Y, Hu Y, Zhou T, Zhou KK, Mott R, Wu
M, Boulton M, Lyons TJ, Gao G and Ma JX: Activation of the Wnt
pathway plays a pathogenic role in diabetic retinopathy in humans
and animal models. Am J Pathol. 175:2676–2685. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu X, Zhang B, McBride JD, Zhou K, Lee K,
Zhou Y, Liu Z and Ma JX: Antiangiogenic and antineuroinflammatory
effects of kallistatin through interactions with the canonical Wnt
pathway. Diabetes. 62:4228–4238. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee K, Hu Y, Ding L, Chen Y, Takahashi Y,
Mott R and Ma JX: Therapeutic potential of a monoclonal antibody
blocking the Wnt pathway in diabetic retinopathy. Diabetes.
61:2948–2957. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Eskelinen EL and Saftig P: Autophagy: A
lysosomal degradation pathway with a central role in health and
disease. Biochim Biophys Acta. 1793:664–673. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Boya P, Esteban-Martínez L, Serrano-Puebla
A, Gómez-Sintes R and Villarejo-Zori B: Autophagy in the eye:
Development, degeneration, and aging. Prog Retin Eye Res.
55:206–245. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rosa MD, Distefano G, Gagliano C, Rusciano
D and Malaguarnera L: Autophagy in Diabetic Retinopathy. Curr
Neuropharmacol. 14:810–825. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fu D, Yu JY, Yang S, Wu M, Hammad SM,
Connell AR, Du M, Chen J and Lyons TJ: Survival or death: A dual
role for autophagy in stress-induced pericyte loss in diabetic
retinopathy. Diabetologia. 59:2251–2261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lopes de Faria JM, Duarte DA, Montemurro
C, Papadimitriou A, Consonni SR and Lopes de Faria JB: Defective
Autophagy in Diabetic Retinopathy. Invest Ophthalmol Vis Sci.
57:4356–4366. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li P, Guo Y, Bledsoe G, Yang Z, Chao L and
Chao J: Kallistatin induces breast cancer cell apoptosis and
autophagy by modulating Wnt signaling and microRNA synthesis. Exp
Cell Res. 340:305–314. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Peng Y, Cao J, Yao XY, Wang JX, Zhong MZ,
Gan PP and Li JH: TUSC3 induces autophagy in human non-small cell
lung cancer cells through Wnt/β-catenin signaling. Oncotarget.
8:52960–52974. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gao C, Cao W, Bao L, Zuo W, Xie G, Cai T,
Fu W, Zhang J, Wu W, Zhang X, et al: Autophagy negatively regulates
Wnt signalling by promoting Dishevelled degradation. Nat Cell Biol.
12:781–790. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Liu H, Lei H, Shi Y, Wang JJ, Chen N, Li
ZH, Chen YF, Ye QF and Yang Y: Autophagy inhibitor 3-methyladenine
alleviates overload-exercise-induced cardiac injury in rats. Acta
Pharmacol Sin. 38:990–997. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
James MH, Quinn RK, Ong LK, Levi EM, Smith
DW, Dickson PW and Dayas CV: Rapamycin reduces motivated responding
for cocaine and alters GluA1 expression in the ventral but not
dorsal striatum. Eur J Pharmacol. 784:147–154. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li X, Shan J, Chang W, Kim I, Bao J, Lee
HJ, Zhang X, Samuel VT, Shulman GI, Liu D, et al: Chemical and
genetic evidence for the involvement of Wnt antagonist Dickkopf2 in
regulation of glucose metabolism. Proc Natl Acad Sci USA.
109:11402–11407. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen Q, Qiu F, Zhou K, Matlock HG,
Takahashi Y, Rajala RVS, Yang Y, Moran E and Ma JX: Pathogenic Role
of microRNA-21 in Diabetic Retinopathy Through Downregulation of
PPARα. Diabetes. 66:1671–1682. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Seglen PO and Gordon PB: 3-Methyladenine:
Specific inhibitor of autophagic/lysosomal protein degradation in
isolated rat hepatocytes. Proc Natl Acad Sci USA. 79:1889–1892.
1982.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Coughlin BA, Feenstra DJ and Mohr S:
Müller cells and diabetic retinopathy. Vision Res. 139:93–100.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhou KK, Benyajati S, Le Y, Cheng R, Zhang
W and Ma JX: Interruption of Wnt signaling in Müller cells
ameliorates ischemia-induced retinal neovascularization. PLoS One.
9(e108454)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr
M, Hijlkema KJ, Coppes RP, Engedal N, Mari M and Reggiori F:
Chloroquine inhibits autophagic flux by decreasing
autophagosome-lysosome fusion. Autophagy. 14:1435–1455.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cicchini M, Chakrabarti R, Kongara S,
Price S, Nahar R, Lozy F, Zhong H, Vazquez A, Kang Y and Karantza
V: Autophagy regulator BECN1 suppresses mammary tumorigenesis
driven by WNT1 activation and following parity. Autophagy.
10:2036–2052. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fan Q, Yang L, Zhang X, Ma Y, Li Y, Dong
L, Zong Z, Hua X, Su D, Li H, et al: Autophagy promotes metastasis
and glycolysis by upregulating MCT1 expression and Wnt/β-catenin
signaling pathway activation in hepatocellular carcinoma cells. J
Exp Clin Cancer Res. 37(9)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ríos JA, Godoy JA and Inestrosa NC: Wnt3a
ligand facilitates autophagy in hippocampal neurons by modulating a
novel GSK-3β-AMPK axis. Cell Commun Signal. 16(15)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Burke SJ, Batdorf HM, Burk DH, Noland RC,
Eder AE, Boulos MS, Karlstad MD and Collier JJ: db/db mice exhibit
features of human type 2 diabetes that are not present in
weight-matched C57BL/6J mice fed a Western diet. J Diabetes Res.
2017(8503754)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bogdanov P, Corraliza L, Villena JA,
Carvalho AR, Garcia-Arumí J, Ramos D, Ruberte J, Simó R and
Hernández C: The db/db mouse: A useful model for the study of
diabetic retinal neurodegeneration. PLoS One.
9(e97302)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Midena E, Segato T, Radin S, di Giorgio G,
Meneghini F, Piermarocchi S and Belloni AS: Studies on the retina
of the diabetic db/db mouse. I. Endothelial cell-pericyte ratio.
Ophthalmic Res. 21:106–111. 1989.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bringmann A, Pannicke T, Grosche J,
Francke M, Wiedemann P, Skatchkov SN, Osborne NN and Reichenbach A:
Müller cells in the healthy and diseased retina. Prog Retin Eye
Res. 25:397–424. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tien T, Zhang J, Muto T, Kim D, Sarthy VP
and Roy S: High Glucose Induces Mitochondrial Dysfunction in
Retinal Müller Cells: Implications for Diabetic Retinopathy. Invest
Ophthalmol Vis Sci. 58:2915–2921. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ao H, Li H, Zhao X, Liu B and Lu L: TXNIP
positively regulates the autophagy and apoptosis in the rat müller
cell of diabetic retinopathy. Life Sci. 267(118988)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Iwai-Kanai E, Yuan H, Huang C, Sayen MR,
Perry-Garza CN, Kim L and Gottlieb RA: A method to measure cardiac
autophagic flux in vivo. Autophagy. 4:322–329. 2008.PubMed/NCBI View Article : Google Scholar
|