Introduction
Inflammation, a self-controlled immune process,
occurs in response to different infections and other stimuli, or as
a major component in wound and tissue repair. Orchestrated in a
complex manner, inflammation can induce a dysregulated response,
which underlies a disruption in the homeostasis of other
physiological processes, that at first glance do not seem to be
directly associated with classical inflammation triggers. However,
chronic systemic inflammatory status could be settled, due to an
elicited dysregulated inflammatory response (1,2).
Inflammation is now considered a hallmark feature in various
diseases, including diabetes, asthma, cardiovascular diseases and
cancer (3,4).
Emerging evidence suggests that diet could be an
important player in the onset and progression of inflammation, with
a major impact on the inflammatory disease course.
Dietary components have gained key roles in
promoting good health, with multiple mechanisms being proposed to
reduce inflammation [e.g., concerning the nuclear factor (NF)-κB
pathway] (5). Therefore, various
plant-based diets have been suggested to be beneficial in
alleviating the inflammatory response.
Studies have discovered that plants contain a wide
range of biologically active substances, such as flavonoids,
carotenoids, vitamins, minerals and fiber, which provide,
individually or in a synergistic manner, important nutritional
value and high protection against disease being used as a defense
mechanism against environmental stress and pathogens. Among the
great variety of biological functions, the anti-inflammatory
properties are distinguished as being particularly important;
inflammation standing out as a well-known protective and survival
mechanism (6-10).
Taking into account the deleterious effects of
chronic inflammation as a major component of almost all diseases,
various fruit-derived products (extracts, nutraceutical
supplements, juices) have been studied with an aim to analyze their
anti-inflammatory effects (11) and
to develop a new generation of therapeutic agents, especially
designed for inflammation purposes (8). Based on their capability for producing
secondary metabolites endowed with curative effects, medicinal
plants have real potential in the development of new and potent
drugs (12,13).
Widely studied for its anti-inflammatory and
health-beneficial properties, lingonberry (Vaccinium
vitis-idaea) has arisen as a promising dietary component
(14-18).
St. John's wort (Hypericum perforatum) is endowed with
anti-inflammatory and antiviral properties, potentially used in
inflammatory conditions, including inflammatory bowel disease,
diarrhea, and respiratory infection (19-21).
Thymus vulgaris is rich in phytocompounds with various
pharmacological activities, including an anti-inflammatory effect
(22,23).
Given the wide range of biological properties
attributed to propolis and its complex composition rich in
bioactive compounds, various studies have focused on investigating
the benefits and pharmacological properties of propolis regarding
its antioxidant, antimicrobial, anticancer and anti-inflammatory
effects. The mechanisms proposed for propolis extract have
highlighted pro-inflammatory cytokine suppression or metabolic
reprogramming of lipopolysaccharide (LPS) activity in macrophage
cells, underlying its potential immunomodulatory effect (24).
In view of all these aspects, a novel product has
emerged, which is a dietary supplement containing powders from
lingonberry fruit (Vaccinium vitis-idaea), thyme (Thymus
vulgaris) and Saint John's wort (Hypericum perforatum),
concentrated propolis tincture, ascorbic acid and volatile oils of
thyme and rosemary (Rosmarinus officinalis), being rich in
phytonutrients and bio-active compounds. Compared to marketed
products, our dietary supplement has a complex formula, enriched in
concentrated propolis tincture sprayed on the plant blend (known
for its antibacterial, antiviral, antifungal, anti-inflammatory,
anesthetic, analgesic, antibiotic and regenerator properties), and
a combination of thyme and rosemary volatile oils (25).
Given this context, in the present study, we first
aimed to establish whether the novel dietary supplement extract has
cytotoxicity and, thus, we performed classical biocompatibility
tests (LDH and MTS assays). Subsequently, our study focused on
evaluating its anti-inflammatory properties, in order to extend the
current understanding of the health benefits of the novel dietary
supplemental extract.
Materials and methods
Plant collection and processing
Thyme (Thymus vulgaris) and Saint John's wort
(Hypericum perforatum) were harvested from Hofigal's own
culture (SC Hofigal Export Import SA, Bucharest). The aerial parts
were dried under controlled conditions, at a maximum temperature of
40˚C, and then they were powdered in an industrial mill.
The lingonberry (Vaccinium vitis-idaea)
fruits were purchased from a local supplier and picked from the
spontaneous flora. They also were dried under controlled
conditions, at a maximum temperature of 40˚C, and then were
powdered in an industrial mill. The volatile oils of thyme and
rosemary were obtained by steam distillation in Hofigal's own
industrial plant from fresh plants, harvested from its own
culture.
The concentrated propolis tincture was obtained by a
7-day simple maceration of a previously purified propolis,
purchased from a local supplier, with 96% pharmaceutical ethylic
alcohol, followed by a concentration to a dry mass of 27%.
Obtaining and characterizing the
dietary supplement Sample preparation
For the determination of total polyphenol content
(TPC), the total flavonoid content (TFC) and antioxidant activity,
1 g of the powdered samples was extracted with 50 ml of 50% (v/v)
ethanol under a reflux condenser for 30 min.
Total polyphenol content (TPC)
TPC was measured utilizing the Folin-Ciocalteu's
method, as described in ISO 14502-1:2005(E) (26). Sample extract (1 ml) was mixed with
5 ml of 10% Folin-Ciocalteu's phenol reagent and allowed to stay
for 3 to 5 min at room temperature. After incubation, 4 ml of 7.5%
Na2CO3 was added, and the reaction mixture
was mixed thoroughly and allowed to stay for 1 h at room
temperature. The absorbance was measured at 765 nm against water,
with a Jasco V-530UV-VIS spectrophotometer and total phenolic
content was calculated using a gallic acid standard calibration
curve with concentrations ranging from 1 to 5 µg/ml and expressed
as gallic acid equivalent per gram.
Total flavonoid content (TFC)
TFC was measured using the aluminium chloride
colorimetric method as described in the Romanian Pharmacopoeia
(27). Sample extract (10 ml) was
diluted with methanol in a 25 ml volumetric flask; 5 ml of the
diluted extract was mixed with 5 ml of 100 g/l sodium acetate, 3 ml
of 25 g/l aluminium chloride hexahydrate and 12 ml methanol. The
reaction mixture was mixed thoroughly and incubated for 30 min at
room temperature. The absorbance was measured at 430 nm against a
blank prepared with 5 ml diluted extract and 20 ml methanol, with a
Jasco V-530 UV-VIS spectrophotometer, and TFC was calculated using
a rutin standard calibration curve with concentrations ranging from
20 to 90 µg/ml and expressed as rutin equivalent per gram.
Antioxidant activity
The antioxidant activity was measured using the
CUPRAC assay, as described by Apak et al (28). One milliliter of 10-2 M
copper sulphate was mixed with 1 ml of 7.5x10-3 M
neocuproine, 1 ml of 1 M ammonium acetate buffer with pH 7.0, 0.1
ml sample extract and 1 ml of water. The reaction mixture was mixed
and incubated for 30 min at room temperature. The absorbance was
measured at 450 nm against a blank prepared with water instead of
sample extract, with a Jasco V-530 UV-VIS spectrophotometer, and
the antioxidant activity was calculated using a trolox standard
calibration curve with concentration ranging from 10 to 60 µg/ml
and expressed as trolox equivalent per gram.
Procyanidin content
The procyanidin content was determined using the
acid hydrolysis described in the European Pharmacopoeia (29). Powdered sample (1 g) was extracted
with 30 ml of 70% (v/v) ethanol under a reflux condenser for 30 min
and filtered. The residue was washed with 10.0 ml of 70% (v/v)
ethanol; 15.0 ml of 250 g/l hydrochloric acid was added to the
filtrate and 10.0 ml of water, then it was heated under a reflux
condenser for 80 min. After filtration and washing, the solution
was diluted to 250 ml. Fifty milliliters were evaporated to
approximately 3 ml and transferred to a separating funnel with 15
ml water. After extracting the water phase with butanol (three
times with 15 ml), the combined organic phases were diluted to 100
ml. The absorbance of the solution was measured at 555 nm against
butanol, with a Jasco V-530UV-VIS spectrophotometer and the
procyanidin content was calculated as cyanidin chloride with the
equation [A=absorbance at 555 nm; m=mass of sample (g)]: Content of
procyanidins (mg/g)=(A x 500)/(1,200 x m).
Polysaccharides/mucilage
determination
The mucilage content was measured using the method
described by Deshmukh et al (30). Sample (1 g) was mixed with 50 ml of
distilled water and boiled for 1 h at 100˚C. The suspension was
filtered. An equal volume of ethanol was added to the filtrate and
the mixture was kept in a refrigerator for 24 h. The obtained
precipitate was separated by filtration through a quantitative
filter paper and dried in the oven at 50˚C. The content of mucilage
was calculated as the ratio between the dried precipitate and the
mass of sample (30).
Cell line culture and treatment
The monocyte/macrophage cell line, originally
derived from peripheral blood, was purchased from the American Type
Culture Collection (ATCC 9855; ATCC/LGC Standards GmbH). As key
regulators of the immune response, these type of cells are often
used to predict in vitro cytotoxicity and, later on, the
anti-inflammatory response.
The sample and control preparation was performed in
accordance with the international quality standards ISO 10993-12:
2012 ‘Biological evaluation of medical devices-Part 12: Sample
preparation and reference materials’ and ISO 10993-5: 2009
‘Biological evaluation of medical devices-Part 5: Tests for in
vitro cytotoxicity’ (31,32).
The cell cultures were grown in 25 cm2
flasks in complete Iscove's modified Dulbecco's media (IMDM; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 1%
antibiotic-antimycotic mix solution (A5955; Sigma-Aldrich; Merck
KGaA) and 10% fetal bovine serum (FBS) (F7524, non-USA origin,
Sigma-Aldrich; Merck KGaA), 1% HT Supplement 0.1 mM hypoxanthine
and 0.016 mM thymidine (Gibco; Thermo Fisher Scientific, Inc.),
0.1% β-mercaptoetanol (Gibco; Thermo Fisher Scientific, Inc.) and
maintained at 37˚C in a humidified atmosphere (95%) with 5%
CO2.
In order to assess the biocompatibility effect (cell
viability/cytotoxicity) induced in monocyte/macrophage cells by the
novel dietary supplement extract, the cells were seeded in 96-well
plates at a density of 15x103 cells/well. Then, the
novel dietary supplement extract was diluted in cell culture medium
(4,000, 1,300, 400, 130 and 40 µg/ml from stock: 4 g sample/100 ml
solvent ethanol for pharmaceutical use 50%), and incubated with the
cells for 24, 48 and 72 h. The cell culture assays were performed
in triplicates and subsequently, an additional test was performed
with an extensive number of dilutions in order to confirm the
obtained results.
To evaluate the anti-inflammatory effect induced in
monocytes/macrophages by the novel dietary supplement extract, the
cells were seeded in 24-well plates at a density of
1x105 cells/well, pretreated with LPS 50 ng/ml for 1 h,
and then incubated with diluted extract in cell culture medium (40,
66 and 130 µg/ml; selected based on cytotoxicity tests) for 4 and
18 h. In order to evaluate the anti-inflammatory effects of the
extract, we also used a positive control, 50 ng/ml LPS (L4391;
Sigma-Aldrich; Merck KGaA), a negative control of inflammation (40
ng/ml dexamethasone sodium phosphate; E.I.P.I. Co.) and a mix of 50
ng/ml LPS and 40 ng/ml dexamethasone. Cell culture supernatants
were collected and stored at -80˚C until analysis.
Cell viability
Cell viability was measured after cell exposure to
the novel dietary supplement extract by MTS tetrazolium compound
[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,
inner salt] spectrophotometric test. Briefly, after the cells were
seeded in 96-well plates at a density of 15x103
cells/well/200 µl, different dilutions of extract were added to the
culture media and incubated for 24, 48 and 72 h. After each time
interval, 20 µl of CellTiter 96® AQueous One Solution
Reagent was added (CellTiter 96® Aqueous One Solution
Cell Proliferation Assay, G3580; Promega Corporation) into each
well of the 96-well assay plate containing 100 µl of fresh culture
medium. After that, the plates were incubated at 37˚C for 3 h in a
humidified 5% CO2 atmosphere. Then, the absorbance of
the formazan, produced by MTS reduction in metabolic active cells,
was measured at 490 nm using a Microplate Multimode Detector Zenyth
3100 (Anthos Labtec Instruments GmbH). The results were calculated
according to the formula: Viability (%)=100 x [Experimental value
(OD490)-background average (OD490)]/Mean value of the untreated
cells (OD490).
Cytotoxicity assay
The lactate dehydrogenase (LDH) amount released in
the culture medium was assessed as a measure of the cell membrane
integrity using the CytoTox 96® Non-Radioactive
Cytotoxicity Assay (G1780, G1782; Promega Corporation). According
to the manufacturer's instructions, lysis solution was used to
generate a maximum LDH release control. After a 45-min incubation
with lysis solution, the plates were centrifuged for 5 min at 600
rpm; 50 µl of culture supernatants from the same 96-well plates
used for MTS test were transferred to a new 96-well flat clear
bottom plate and 50 µl of the CytoTox 96® Reagent were
added. The plates were incubated at room temperature in darkness,
for 30 min. The reaction was stopped by adding 50 µl of Stop
Solution, and the absorbance was read at 490 nm using Microplate
Multimode Detector Zenyth 3100 (Anthos Labtec Instruments GmbH).
The results were calculated according with the formula:
Cytotoxicity (%)=100 x [Experimental LDH Release (OD490)-background
average (OD490)/Mean value of Maximum LDH Release (OD490).
In vitro anti-inflammatory capacity
assessment by xMAP array
Cell culture supernatants were obtained as mentioned
above and assessed using HCYTOMAG Milliplex™ MAP 10-plex
(Millipore) multiplex magnetic bead-based antibody detection kits
according to the manufacturer's protocols. Briefly, the beads
[interleukin (IL)-1β, IL-4, IL-6, IL-8, IL-10, IL-12p70, fibroblast
growth factor (FGF)2, interferon (INF)γ, tumor necrosis factor
(TNF)α, vascular endothelial growth factor (VEGF)a] provided within
the kits were incubated with buffer, cytokine standards (included
in the kit), or samples in a 96-well plate at 4˚C overnight, with
shaking at 800 rpm. All further incubations with detection
antibodies and streptavidin phycoerythrin conjugate (SAPE) were
performed at room temperature in the dark, with 800 rpm shaking.
Multiplex data acquisition was achieved using the Luminex200
platform (Luminex Corp.) and the analysis was performed using the
xPONENT 4.2 software (Luminex Corp.); the calibration curves were
generated with a 5-parameter logistic fit. Duplicate samples were
used for all specimens, and the average concentrations were used
for statistical analysis.
Selection and validation of
pro-inflammatory cytokines by ELISA
IL-8 and IL-6 were assessed using commercial Legend
Max quantitative assays (BioLegend, Inc.). Standards and samples
(cell culture supernatants) were added to the plates and incubated
at room temperature (RT) for 2 h with 200 rpm shaking. All further
incubations with detection antibody, avidin-HRP solution, substrate
solution and stop solutions were performed according to the
manufacturer's protocols. Samples were analyzed in duplicate, and
the absorbance was read at 450 and 570 nm, using a Sunrise-Basic
Tecan Microplate Reader (Tecan Group Ltd.). The absorbance at 570
nm was subtracted from the absorbance at 450 nm. The data were
calculated with computer-based curve-fitting software (Magellan)
using a 5-parameter logistics curve-fitting algorithm. IL-8 and
IL-6 are expressed in picograms (pg) per ml of sample.
Statistical analysis
All tests were performed in triplicate, and the data
are shown as mean ± standard deviation (SD). The statistical
Student's t-test was performed for biological tests to analyze
significant differences when comparing treated cells with the
controls. The data were processed by one-way analysis of variance
(ANOVA) test using Graph Pad Prism software (version 5; GraphPad
Software, Inc.). The levels of statistical significance were
considered at P<0.05.
Results and Discussion
Dietary supplement composition
The main bio-active compounds determined and
quantified in the novel product are documented in Table I.
 | Table IBioactive compounds in the novel
dietary supplement. |
Table I
Bioactive compounds in the novel
dietary supplement.
| Bio-active
compounds | Quantity |
|---|
| Polyphenols | 146 mg/g gallic
acid equivalents (GAE) |
| Flavonoids | 9.5 mg/g rutin
equivalents |
| Procyanidines | 1.5 mg/g cyanidin
chloride equivalents |
|
Polysaccharides | 105 mg/g expressed
as mucilage |
| Vitamin C | 90 mg/g |
| Trolox
equivalent | 326.5 mg/g |
High cellular viability for 40-130
µg/ml concentrations of the dietary supplement
Our novel dietary supplement extract requires the
simultaneous achievement of two conditions: Higher cell viability
and low cytotoxicity.
In order to assess the cellular viability of the
dietary supplement (DS) extract doses ranging from 40 to 1,300
µg/ml, we treated monocyte/macrophage cells for 24, 48 and 72 h.
Untreated cells were used as maximum cell viability control, while
the cells treated with lysis solution were used as minimum cell
viability control; cells treated with different concentrations of
ethanol were also used as extract vehicle controls.
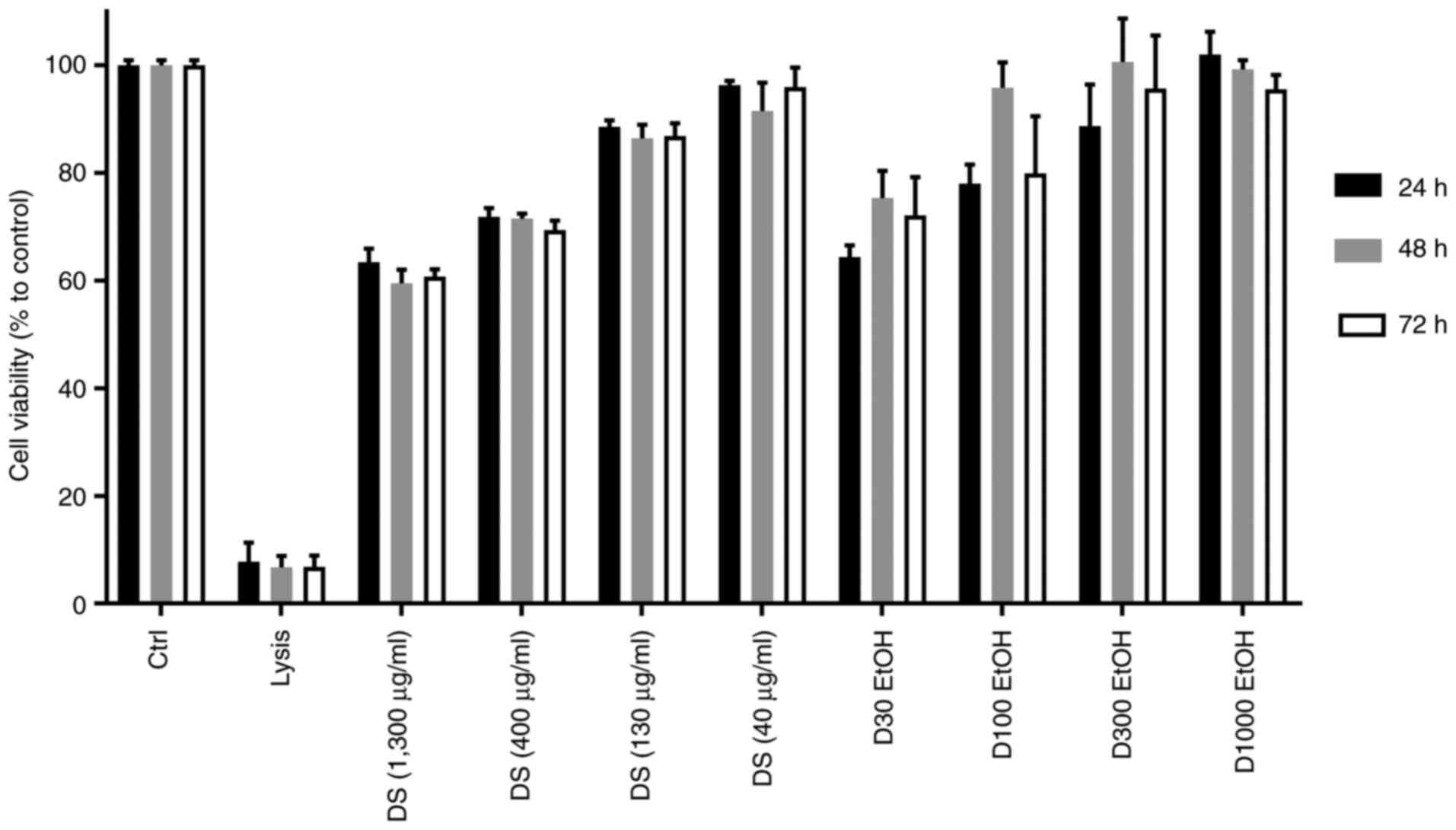
At the highest dose of the novel dietary supplement
extract (1,300 µg/ml), cell viability was decreased after 48 and 72
h exposure from 64 to 60%, the same trend being observed at the
concentration of 400 µg/ml (Fig.
1). After the exposure of monocytes to 40 and 130 µg/ml dietary
supplement extract for 24, 48 and 72 h, it was observed that the
cell viability was not significantly modified, with a mean of 87.3
and 94.6% respectively, compared to the control, suggesting high
biocompatibility for these doses.
We also tested the extract vehicle [ethanol (EtOH)]
and observed that the cell viability in this case was affected at
the higher concentration 1,300 µg/ml (70%), but not at other
concentrations, such as 400 (85%), 130 (95%) and 40 µg/ml
(99%).
Low cytotoxicity registered at 40-130
µg/ml concentrations of dietary supplement
In order to evaluate the possible cytotoxic effects
induced by the novel dietary supplement extract on monocytes, the
cell membrane integrity was evaluated at 24, 48 and 72 h, by
measuring the level of LDH released in the cell culture medium.
Under normal conditions, the LDH enzyme is located in the cytosol,
but when the cell membrane integrity is affected, LDH released into
the cell culture medium is assessed as a marker for membrane
integrity (33).
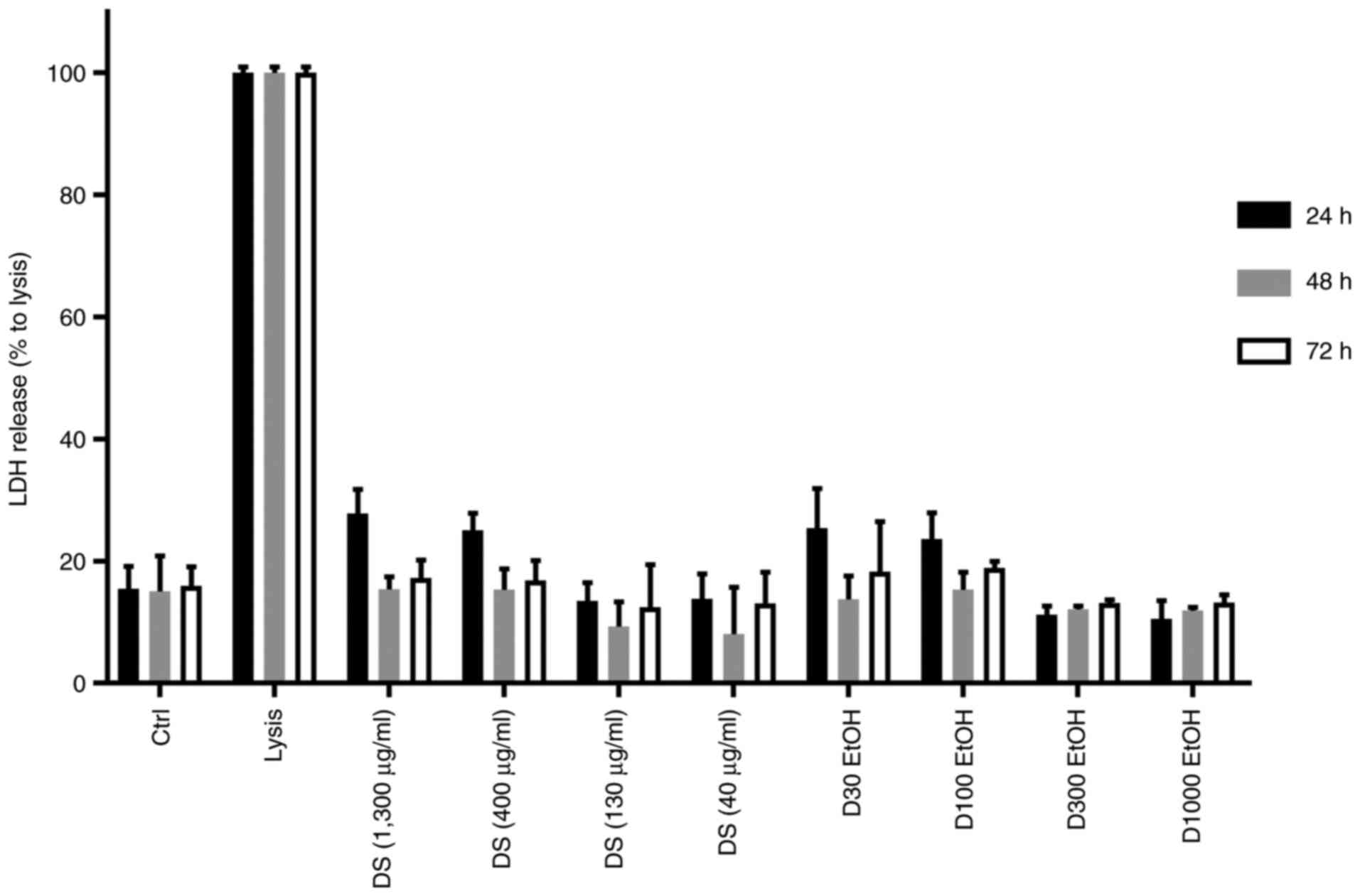
The incubation of monocyte/macrophage cells with our
novel dietary supplement extract induced the release of LDH in the
cell culture medium (Fig. 2). Lysis
solution was added as positive control wells, generating a maximum
LDH release. A higher released LDH level was found in the
supernatants of the monocyte/macrophage cells exposed to the high
concentrations of our extract (400 and 1,300 µg/ml; LDH mean of 19
and 20%, respectively), than from cells treated with 40-130 µg/ml.
A lower LDH level was released (11 and 10%), after incubation of
cells with low concentrations of the dietary supplement: 130 and 40
µg/ml, respectively, compared to the positive control. In
conclusion, the cell membrane integrity was unaffected at
concentrations of 40-130 µg/ml, this result confirming the high
biocompatibility of the novel dietary supplement extract.
In addition, when we tested the extract vehicle
(EtOH), we noted that the cytotoxicity was higher at 400-1,300
µg/ml (19%). However, at 40 µg/ml (12%) and 130 µg/ml (11%)
concentrations, no cytotoxicity was recorded.
For the first 24 h of treatment, a correlation
between the concentrations of the bioproduct and the LDH release
was noted. The lowest two concentrations (40-130 µg/ml) had similar
LDH release, and similar to their corresponding vehicle-treated
controls, suggesting a non-toxic effect on cell membrane
permeability.
These data were in accordance with the results of
the MTS test and suggest that at 40 and 130 µg/ml concentrations,
the novel dietary supplement extract is safe to use for further
testing.
Inhibition of pro-inflammatory
cytokine (IL-6, IL-8, TNFα) release by the novel dietary supplement
extract
In response to various stimuli, such as LPS,
cytokines or other chemical stimuli, macrophages exert an important
role in the inflammatory response, mediating the secretion of a
cascade of cytokines/chemokines, reactive oxygen/nitrogen species
and growth factors (34,35).
Based on viability and cytotoxicity tests, our study
continued with the inflammatory profile of the dietary
supplement-treated cells, at concentrations ranging between 40 and
130 µg/ml, using xMAP array multiplexing technology.
Since the initial treatment of the monocytes using
the dietary supplement did not generate an inflammatory response,
we treated the cells with LPS, and afterwards applied the treatment
with the dietary supplement at different concentrations. Following
LPS stimulation, the anti-inflammatory response to our dietary
supplement was also analyzed in comparison with dexamethasone and
lingonberry extract (marketed products), widely used as
anti-inflammatory treatments (in clinical practice). Due to the
fact that our dietary supplement has a complex formula, consisting
mainly of lingonberry (50% of all constituents), it was interesting
to investigate the anti-inflammatory effect of lingonberry and the
possible potentiation of this effect through its additional
components.
By using Luminex xMAP array multiplexing technology,
the response to dietary supplement treatment was investigated by
analyzing a 10-plex inflammatory cytokine panel. Dietary supplement
treatment demonstrated a relevant dose-dependent inhibitory pattern
for the most important pro-inflammatory cytokines: IL-6, IL-8,
TNFα, at 4 and 18 h of treatment (Fig.
3).
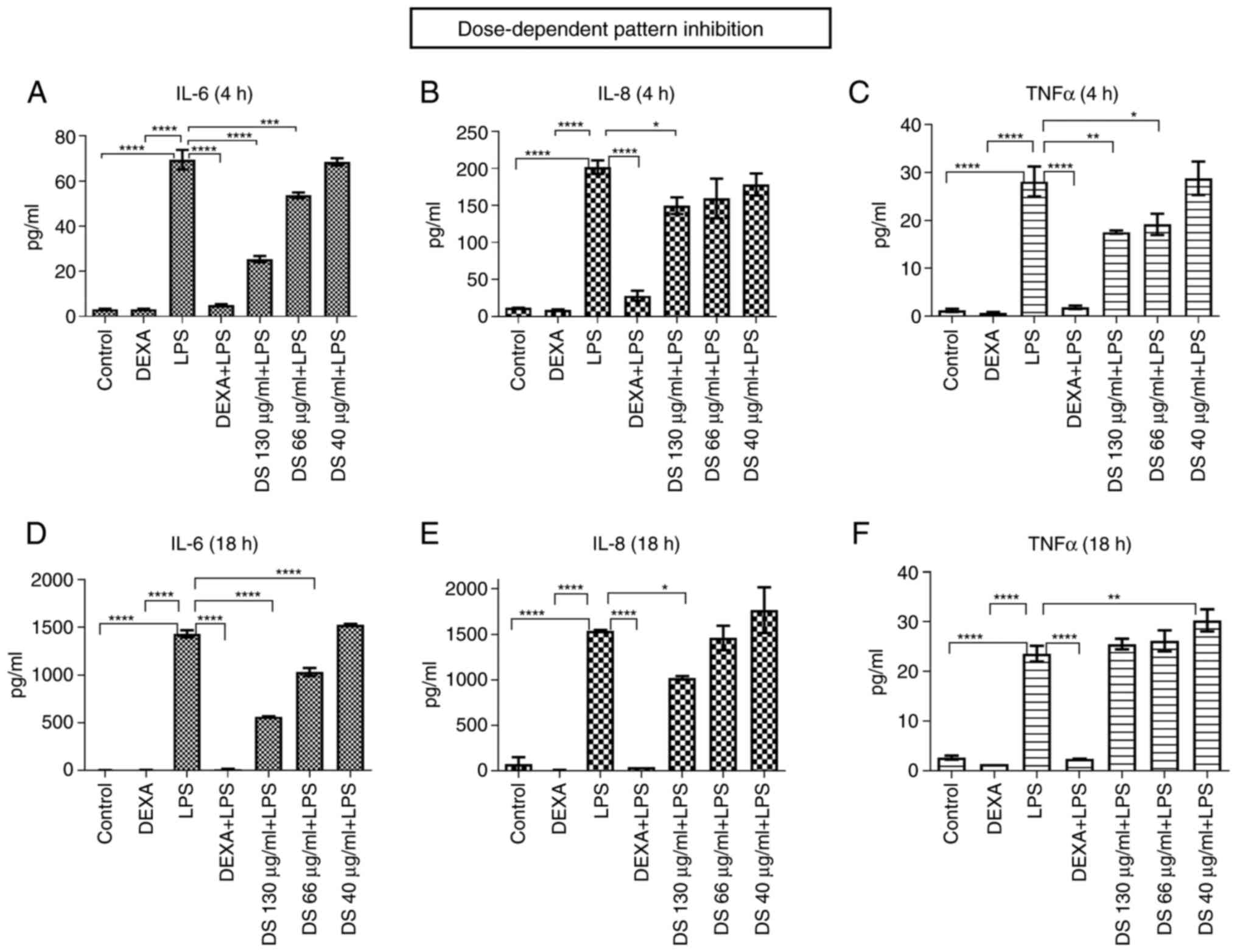 | Figure 3The dose-dependent inhibition pattern
on pro-inflammatory cytokines (IL-6, IL-8, TNFα) released in the
cell culture supernatant, following treatment with the dietary
supplement (DS), at different concentrations (A-F). (A) IL-6, (B)
IL-8 and (C) TNFα at 4 h of treatment; (D) IL-6, (E) IL-8 and (F)
TNFα at 18 h of treatment. Bars represent average of duplicates ±
SD. IL, interleukin; TNF, tumor necrosis factor; LPS,
lipopolysaccharide; DEXA, dexamethasone. *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001. |
The trend registered in cytokine level following
treatment with the new dietary supplement could suggest its
potential anti-inflammatory role. Given the significant amount of
flavonoids contained in our dietary supplement (derived from
lingonberry, thyme, St. John's wort and propolis tincture), its
natural anti-inflammatory effect may be facilitated by the
potential of flavonoids for inhibiting enzymes or transcription
factors, important for mediating the inflammatory response.
Increasing evidence has revealed that polyphenolic compounds,
including flavonoids, originating from fruits and vegetables may
have anti-inflammatory properties and the potential to hinder the
onset and development of inflammatory diseases (36,37).
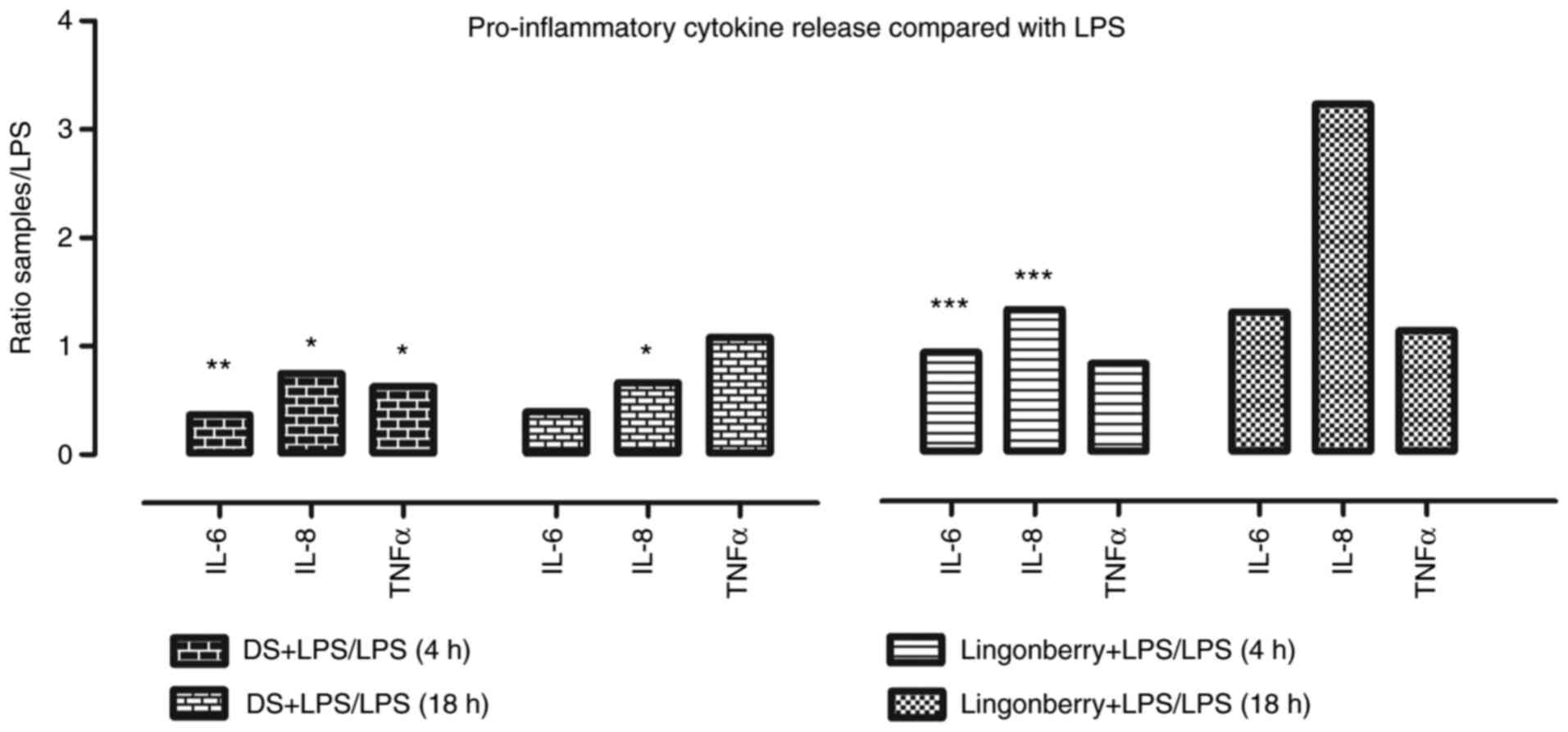
As shown in Fig. 4,
the inhibition of pro-inflammatory cytokines released into the
supernatants of cell samples treated with dietary supplement
(DS)+LPS is depicted, compared to LPS only. After 4 h of treatment,
the fold-change decrease was 0.36 for IL-6 (P=0.005), 0.74 for IL-8
(P=0.03), and 0.62 for TNFα (P=0.04). After 18 h of treatment, the
fold-change decrease was 0.39 for IL-6 (P=0.0009) and 0.66 for IL-8
(P=0.0008), while for TNFα there was no significant decrease
compared to the control.
In the case of the lingonberry extract, known for
its anti-inflammatory properties in the different experimental
models (11-15),
we observed that a fold-change decrease of 0.925 for IL-6 (P=0.30),
0.825 for TNFα (P=0.10), and a fold-change increase of 1.325 for
IL-8 (P=0.01) was registered at 4 h. The fold-change measurements
at 18 h revealed that none of the released cytokine levels was
below the control level.
Our study revealed that the pro-inflammatory
cytokine release following lingonberry treatment was increased in
the case of all analyzed concentrations as compared to our dietary
supplement, which in turn developed a more pronounced
anti-inflammatory activity.
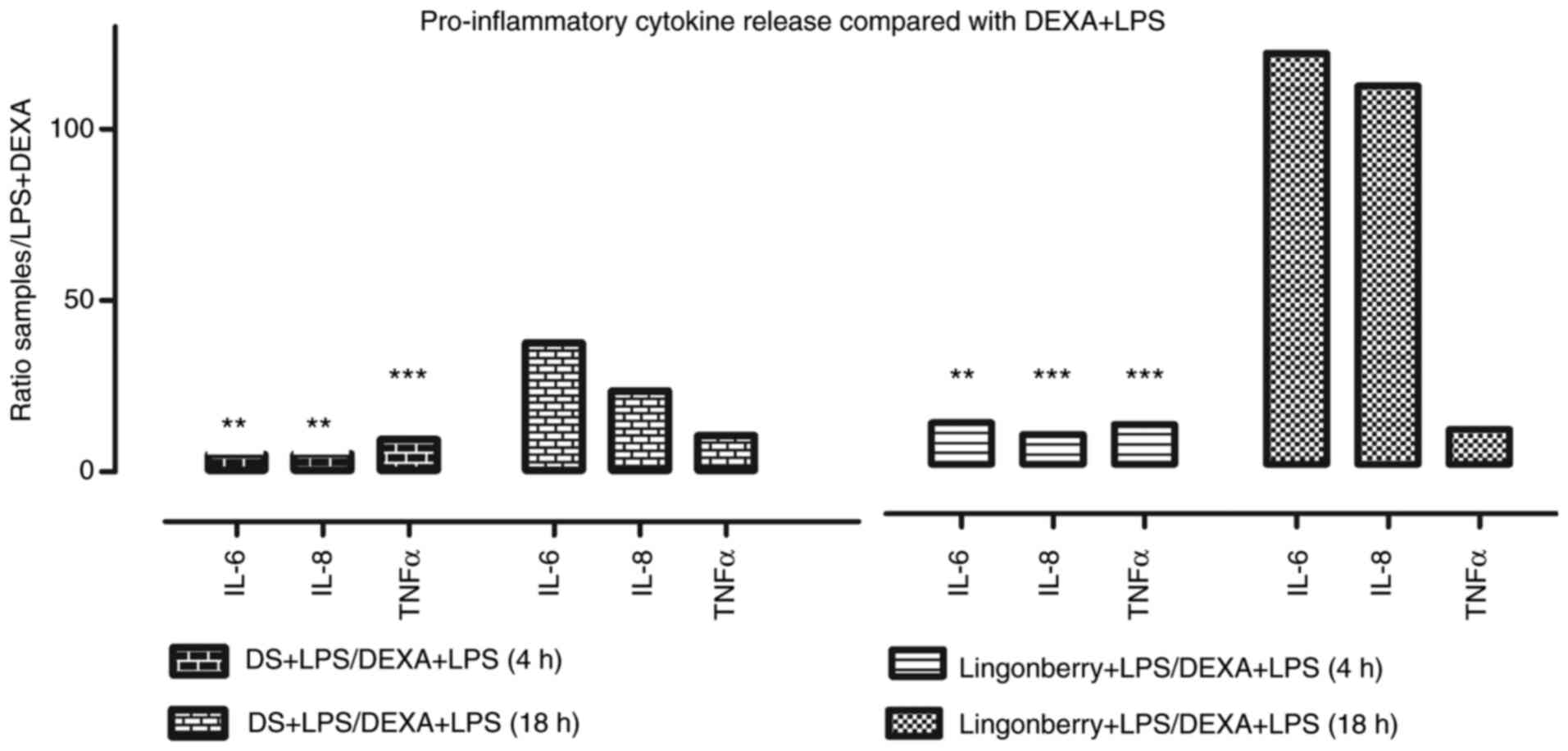
Fig. 5 presents the
pro-inflammatory cytokine profile (IL-6, IL-8 and TNFα) in the
supernatants of cells treated with our dietary supplement,
lingonberry extract and LPS, compared with those treated with LPS
and dexamethasone (DEXA) (anti-inflammatory steroid).
In order to investigate the beneficial
anti-inflammatory effect of our dietary supplement, we compared its
inflammatory profile with that of a lingonberry-based marketed
product, and to dexamethasone, a well-known anti-inflammatory
treatment in clinical practice.
When compared to LPS and dexamethasone (negative
control), at which the anti-inflammatory effect was maximum, that
was registered at 4 h, in the case of the lingonberry marketed
product, a higher fold-change release in IL-6 (12.93) (P=0.003) was
noted compared to only 5.09 (P=0.002) in the case of our product.
As for the IL-8 fold-change release, we found an increase of 9.47
(P=0.0005) (lingonberry marketed product) vs. 5.34 (P=0.005) (our
dietary supplement), and for TNFα the data showed 12.48 (P=0.0003)
vs. 9.44 (P=0.0004) fold-change. At 18 h, the anti-inflammatory
release for IL-6 was 3.3 times lower in the case of our product
compared to the lingonberry marketed product, while for IL-8 it was
4.84 times lower, and 1.04 times lower in the case of TNFα
(Fig. 5).
There are various studies in the literature which
have focused on the anti-inflammatory effects of lingonberry under
different medical conditions. Madduma Hewage et al
investigated the role of lingonberry supplementation in the
inhibition of inflammatory cytokine expression, which prevents
high-fat diet (HFD)-induced kidney injury. They found a significant
elevation in renal TNFα, IL-6, and MCP-1 mRNA expression, and a
significant elevation in inflammatory cytokines (TNFα and MCP-1) in
the plasma of mice fed an HFD. Lingonberry supplementation reduced
these inflammatory cytokine levels in the circulation. Several
lines of evidence from their study suggest that NF-κB activation
may play an important role in HFD-induced renal inflammatory
response (38). Kowalska et
al found that lingonberry fruit extract exhibited a high
anti-inflammatory potential in a macrophage (RAW 264.7) cell
culture by downregulating the expression of proinflammatory
mediators (IL-6, TNFα, IL-1β, MCP-1, COX-2, iNOS) (39). It is worth mentioning that
lingonberry (Vaccinium vitis-idaea) and cranberry (European
Vaccinium oxycoccos or North American Vaccinium
macrocarpon) are both members of the Vaccinium family, and the
specific characteristic that contributes the most to their
differentiation is their polyphenol and phospholipid contents
(40). An interesting study
conducted by Kylli et al highlighted the beneficial
properties of lingonberry phenolics, which inhibited IL-6 and TNFα
production at a concentration of 100 µg/ml in murine macrophages
and human promonocytes stimulated by LPS (41). The potential health benefits of
lingonberries are attributed to their diverse polyphenol
composition, such as flavonoids, phenolic acids, anthocyanins and
procyanidins, as well as organic acids and different vitamins (A,
B1, B2, B3 and C) (42).
As revealed by our study, the treatment of cells
with the new dietary supplement following LPS treatment led to a
significant inflammatory suppression, which was enhanced as the
concentration of the analyzed product increased, with the best-case
scenario at 130 µg/ml concentration. Our study showed that the
inflammatory suppression occurred at all analyzed concentrations
for both lingonberry and our dietary supplement, compared to the
control, but in case of the dietary supplement, the
anti-inflammatory effect was more pronounced.
Given these observations, we can conclude that the
potentiated anti-inflammatory effect may be the result of the
complex formula of our dietary supplement, as well as by the
addition of propolis, whose effects in reducing the inflammatory
response are well known. The main proposed mechanisms underlying
the anti-inflammatory effects of propolis are, among others, the
decrease in inflammatory cytokine concentration and
immunosuppressive activity (43).
Selection and validation of
pro-inflammatory cytokines IL-6 and IL-8
Considering the xMAP analysis results, our study
continued with the selection and validation of pro-inflammatory
cytokines IL-6, IL-8 and TNFα, which exhibited the most prominent
activity. Pro-inflammatory cytokine expression was assessed using
cell culture supernatant samples harvested at 4 and 18 h, under the
same experimental condition, as used in the xMAP analysis.
As for the TNFα level, its expression was
insignificant, so that it should be further validated. TNFα was not
detectable by the method chosen for any of the samples tested,
including the LPS-treated cells.
Following 18 h of treatment with the dietary
supplement (DS), a decrease in IL-6 release was observed [0.22
fold-change decrease compared to the positive control (LPS)], while
in the case of lingonberry extract treatment, IL-6 release was 1
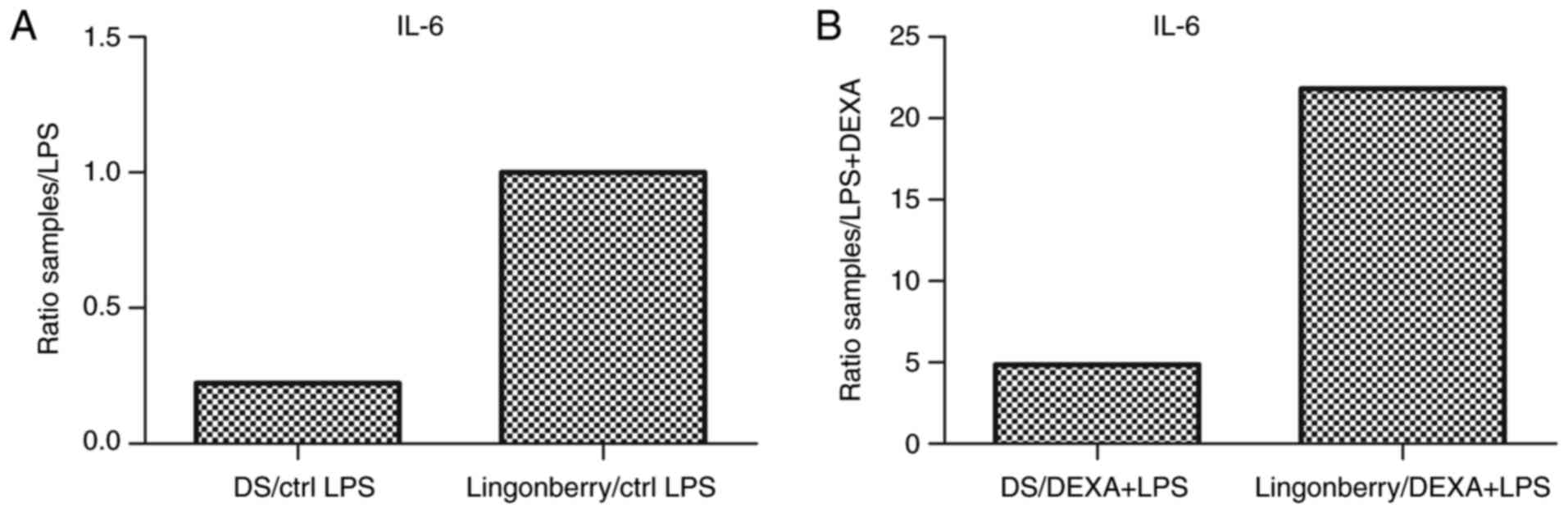
fold-change increased compared to the positive control (Fig. 6A). For IL-6, a ratio of 4.87 was
observed in the case of the dietary supplement treatment compared
to the negative control (DEXA+LPS), and 21.8 in case of lingonberry
extract treatment (Fig. 6B).
Therefore, the dietary supplement exerted a more distinct
inhibitory effect on the release of the pro-inflammatory cytokine
IL-6, compared to the lingonberry extract.
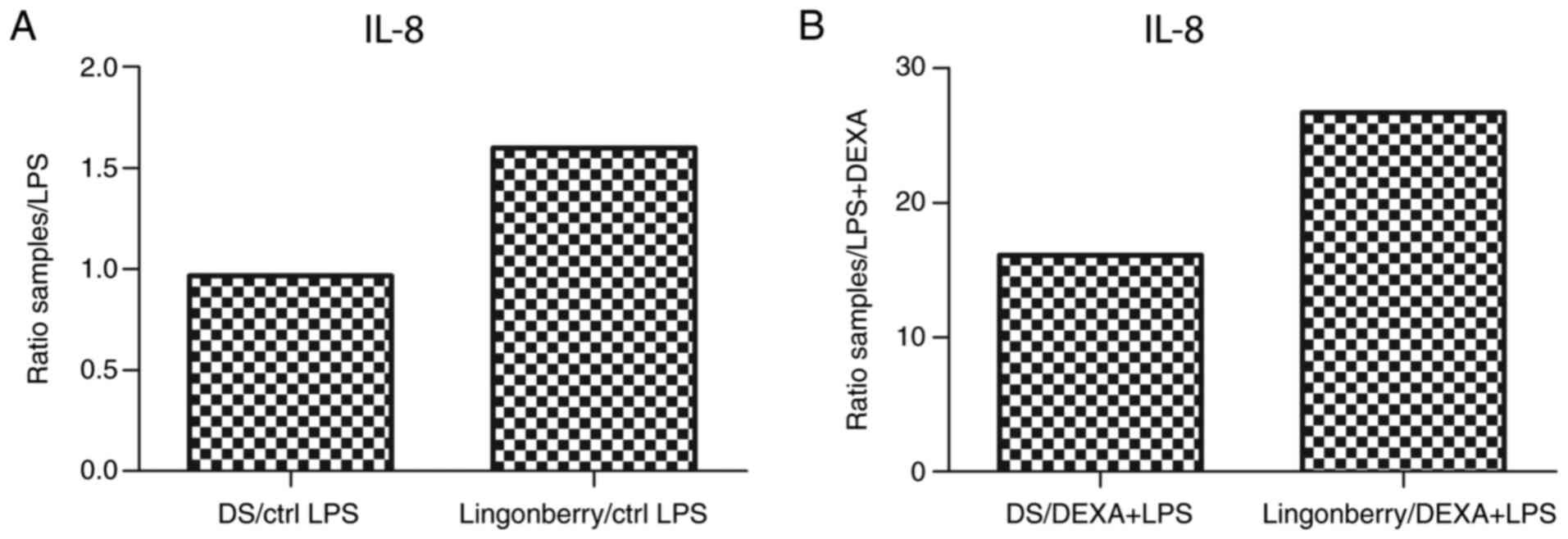
As for IL-8, a decreased level in the supernatant at
18 h of treatment with dietary supplement (DS) was observed, with a
ratio of 0.96 compared to the positive control (LPS), while for the
lingonberry extract the ratio was 1.6. (Fig. 7A). When comparing the amount of IL-8
released in the culture medium to the negative control (DEXA+LPS),
a ratio of 16.11 was found in the case of the dietary supplement
treatment, and 26.6 in the case of lingonberry extract treatment
(Fig. 7B). Consequently, the novel
dietary supplement extract recorded a notable inhibitory effect on
the release of the pro-inflammatory cytokine IL-8, compared to
lingonberry extract.
Regarding the expression levels of IL-6 and IL-8
released at 4 h of treatment, the results recorded were below the
detection limit.
Not all studies in the literature have reported a
decline in inflammatory marker expression. A study conducted by
Zhao et al observed a significant decrease following
treatment with Brazilian green propolis for TNFα, while for IL-1β
and IL-6 a significant increase was recorded. It was hypothesized
that propolis, through its components, actively stimulated the
release of a cascade of cytokines, including IL-1β, and the
subsequent pro-inflammatory action induced by IL-1β release was
probably hampered by the anti-inflammatory effects of IL-6. As a
result, the combined action of propolis components on the
inflammatory process was favorable (44).
Based on the present data, the precise mechanisms
underlying the novel lingonberry-based dietary supplement effects
are still to be elucidated; therefore, further studies are needed
in order to reveal the beneficial effects for each of the active
compounds included in our dietary supplement.
To conclude, in the present study, we investigated
the potential role as an inflammation suppressor for a novel
dietary supplement containing powders from lingonberry fruits,
thyme and Saint John's wort, concentrated propolis tincture,
ascorbic acid and volatile oils of thyme and rosemary. The results
of our biological studies revealed that the treatment with our
dietary supplement at concentrations of 40-130 µg/ml recorded a
cell viability of over 85% and did not exhibit cytotoxicity.
Moreover, the treatment with the novel dietary supplement induced a
significant inflammatory suppression in monocyte/macrophage cells,
highlighting an inhibition of pro-inflammatory cytokine (IL-6,
IL-8) release, at the same concentrations. In addition, the
anti-inflammatory response was more prominent for our dietary
supplement compared to lingonberry extract (from marketed
products), being a potential successful candidate as an
inflammation suppressant in various diseases in which the
inflammatory component is important. Nevertheless, further studies
are needed in order to elucidate the beneficial role of our novel
dietary supplement, unravelling potential therapeutic agents of new
generation, especially designed for inflammation purposes.
Acknowledgements
We would like to thank Dr Ana-Maria Enciu for her
help during the revision of the manuscript. Professional editing,
linguistic and technical assistance was performed by Irina Radu,
Individual Service Provider.
Funding
Funding: The present study was partially funded by Ministry of
Research and Inovation grant (COP A 1.2.3., ID:P_40_197/2016, Ctr.
52/2016, PN 19.29.01.04 and PN 19.29.02.02).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IDP, EC and SM equally contributed to the conception
and design of the study, and they were involved in the
interpretation of the results and in the writing of the manuscript.
CL and MN were involved in the novel dietary supplement description
and characterization. CT critically revised the manuscript. All
authors read and approved the final manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Franceschi C and Campisi J: Chronic
inflammation (inflammaging) and its potential contribution to
age-associated diseases. J Gerontol A Biol Sci Med Sci. 69 (Suppl
1):S4–S9. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kotas ME and Medzhitov R: Homeostasis,
inflammation, and disease susceptibility. Cell. 160:816–827.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Singh N, Baby D, Rajguru JP, Patil BP,
Thakkannavar SS and Pujari VB: Inflammation and Cancer. Ann Afr
Med. 18:121–126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alfaddagh A, Martin SS, Leucker TM, Michos
ED, Blaha MJ, Lowenstein CJ, Jones SR and Toth PP: Inflammation and
cardiovascular disease: From mechanisms to therapeutics. Am J Prev
Med. 4(100130)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Peng C, Ouyang Y, Lu N and Li N: The NF-κB
signaling pathway, the microbiota, and gastrointestinal
tumorigenesis: Recent advances. Front Immunol.
11(1387)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu RH: Health-promoting components of
fruits and vegetables in the diet. Adv Nutr. 4:384S–392S.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Slavin JL and Lloyd B: Health benefits of
fruits and vegetables. Adv Nutr. 3:506–516. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu CH, Abrams ND, Carrick DM, Chander P,
Dwyer J, Hamlet MRJ, Macchiarini F, PrabhuDas M, Shen GL, Tandon P
and Vedamony MM: Biomarkers of chronic inflammation in disease
development and prevention: Challenges and opportunities. Nat
Immunol. 18:1175–1180. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Nunes CDR, Barreto Arantes M, Menezes de
Faria Pereira S, Leandro da Cruz L, de Souza Passos M, Pereira de
Moraes L, Vieira IJC and Barros de Oliveira D: Plants as sources of
anti-inflammatory agents. Molecules. 25(3726)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Boda D, Negrei C, Arsene A, Caruntu C,
Lupuleasa D and Ion RM: Spectral and photochemical properties of
hyperbranched nanostructures based on gardiquimod and
tpps4. Farmacia. 63:218–23. 2015.
|
|
11
|
Joseph SV, Edirisinghe I and
Burton-Freeman BM: Fruit polyphenols: A review of anti-inflammatory
effects in humans. Crit Rev Food Sci Nutr. 56:419–444.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li Y, Kong D, Fu Y, Sussman MR and Wu H:
The effect of developmental and environmental factors on secondary
metabolites in medicinal plants. Plant Physiol Biochem. 148:80–89.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zaynab M, Fatima M, Abbas S, Sharif Y,
Umair M, Zafar MH and Bahadar K: Role of secondary metabolites in
plant defense against pathogens. Microb Pathog. 124:198–202.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ryyti R, Hamalainen M, Peltola R and
Moilanen E: Beneficial effects of lingonberry (Vaccinium
vitis-idaea L.) supplementation on metabolic and inflammatory
adverse effects induced by high-fat diet in a mouse model of
obesity. PLoS One. 15(e0232605)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Drozdz P, Seziene V, Wojcik J and
Pyrzynska K: Evaluation of bioactive compounds, minerals and
antioxidant activity of lingonberry (Vaccinium vitis-idaea
L.) fruits. Molecules. 23(53)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kowalska K and Olejnik A: Current evidence
on the health-beneficial effects of berry fruits in the prevention
and treatment of metabolic syndrome. Curr Opin Clin Nutr Metab
Care. 19:446–452. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mane C, Loonis M, Juhel C, Dufour C and
Malien-Aubert C: Food grade lingonberry extract: Polyphenolic
composition and in vivo protective effect against oxidative stress.
J Agric Food Chem. 59:3330–3339. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kivimäki AS, Siltari A, Ehlers PI, Korpela
R and Vapaatalo H: Lingonberry juice negates the effects of a high
salt diet on vascular function and low-grade inflammation. J Funct
Foods. 7:238–245. 2014.
|
|
19
|
Huang N, Rizshsky L, Hauck C, Nikolau BJ,
Murphy PA and Birt DF: Identification of anti-inflammatory
constituents in Hypericum perforatum and Hypericum gentianoides
extracts using RAW 264.7 mouse macrophages. Phytochemistry.
72:2015–2023. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Birt DF, Widrlechner MP, Hammer KD,
Hillwig ML, Wei J, Kraus GA, Murphy PA, McCoy J, Wurtele ES,
Neighbors JD, et al: Hypericum in infection: Identification of
anti-viral and anti-inflammatory constituents. Pharm Biol.
47:774–782. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hammer KD, Hillwig ML, Solco AK, Dixon PM,
Delate K, Murphy PA, Wurtele ES and Birt DF: Inhibition of
prostaglandin E(2) production by anti-inflammatory hypericum
perforatum extracts and constituents in RAW264.7 mouse macrophage
cells. J Agric Food Chem. 55:7323–7331. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Aazza S, Lyoussi B, Megias C,
Cortés-Giraldo I, Vioque J, Figueiredo AC and Miguel MG:
Anti-oxidant, anti-inflammatory and anti-proliferative activities
of Moroccan commercial essential oils. Nat Prod Commun. 9:587–594.
2014.PubMed/NCBI
|
|
23
|
Oliveira JR, de Jesus Viegas D, Martins
APR, Carvalho CAT, Soares CP, Camargo SEA, Jorge AOC and de
Oliveira LD: Thymus vulgaris L. extract has antimicrobial
and anti-inflammatory effects in the absence of cytotoxicity and
genotoxicity. Arch Oral Biol. 82:271–279. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Alqarni AM, Niwasabutra K, Sahlan M,
Fearnley H, Fearnley J, Ferro VA and Watson DG: Propolis exerts an
anti-inflammatory effect on PMA-differentiated THP-1 cells via
inhibition of purine nucleoside phosphorylase. Metabolites.
9(75)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shamilov AA, Bubenchikova VN, Chernikov
MV, Pozdnyakov DI and Garsiya ER: Vaccinium vitis-idaea L: Chemical
contents, pharmacological activities. Pharm Sci. 26:344–362.
2020.
|
|
26
|
International Organization for
Standardization (ISO): Determination of substances characteristic
of green and black tea. Part 1: Content of total polyphenols in
tea. Colorimetric method using Folin-Ciocalteu reagent. ISO
14502-1:2005. ISO, Geneva, 2005. https://www.iso.org/standard/31356.html. Accessed
March, 2005.
|
|
27
|
Romanian Pharmacopoeia Commission National
Medicines Agency: Cynarae folium Monography. In: Romanian
Pharmacopoeia. 10th edition. Medical Publishing House, Bucharest,
pp334-335, 2008.
|
|
28
|
Apak R, Guclu K, Ozyurek M, Karademir SE
and Altun M: Total antioxidant capacity assay of human serum using
copper(II)-neocuproine as chromogenic oxidant: The CUPRAC method.
Free Radic Res. 39:949–961. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
European Directorate for the Quality of
Medicines & HealthCare: Crataegi fructus Monography. In:
European Pharmacopoeia. 9th edition. EDQM, Strasbourg, pp1384-1385,
2016.
|
|
30
|
Deshmukh SS, Katare YS, Shyale SS, Bhujbal
SS, Kadam SD, Landge DA, Shah DV and Pawar JB: Isolation and
evaluation of mucilage of adansonia digitata linn as a suspending
agent. J Pharm (Cairo). 2013(379750)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
International Organization for
Standardization (ISO): Biological evaluation of medical devices.
Part 12: Sample preparation and reference materials. ISO
10993-10912, 2012. ISO, Geneva 2012. https://www.iso.org/standard/75769.html.
|
|
32
|
International Organization for
Standardization (ISO): Biological evaluation of medical devices.
Part 5: Tests for in vitro cytotoxicity. ISO 10993-10995, 2009.
ISO, Geneva, 2009. https://www.iso.org/standard/36406.html.
|
|
33
|
Chan FK, Moriwaki K and De Rosa MJ:
Detection of necrosis by release of lactate dehydrogenase activity.
Methods Mol Biol. 979:65–70. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286.
2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dinkova-Kostova AT, Cheah J, Samouilov A,
Zweier JL, Bozak RE, Hicks RJ and Talalay P: Phenolic Michael
reaction acceptors: Combined direct and indirect antioxidant
defenses against electrophiles and oxidants. Med Chem. 3:261–268.
2007.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Maleki SJ, Crespo JF and Cabanillas B:
Anti-inflammatory effects of flavonoids. Food Chem.
299(125124)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zălaru C, Crişan C, Călinescu I, Moldovan
Z, Tarcomnicu I, Litescu S, Tatia R, Moldovan L, Boda D and Iovu M:
Polyphenols in Coreopsis tinctoria Nutt. fruits and the plant
extracts antioxidant capacity evaluation. Cent Eur J Chem.
12:858–867. 2014.
|
|
38
|
Madduma Hewage S, Prashar S, Debnath SC, O
K and Siow YL: Inhibition of inflammatory cytokine expression
prevents high-fat diet-induced kidney injury: Role of lingonberry
supplementation. Front Med (Lausanne). 7(80)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kowalska K, Olejnik A, Zielińska-Wasielica
J and Olkowicz M: Inhibitory effects of lingonberry (Vaccinium
vitis-idaea L.) fruit extract on obesity-induced inflammation
in 3T3-L1 adipocytes and RAW 264.7 macrophages. J Funct Foods.
54:371–380. 2019.
|
|
40
|
Hurkova K, Uttl L, Rubert J, Navratilova
K, Kocourek V, Stranska-Zachariasova M, Paprstein F and Hajslova J:
Cranberries versus lingonberries: A challenging authentication of
similar Vaccinium fruit. Food Chem. 284:162–170. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kylli P, Nohynek L, Puupponen-Pimia R,
Westerlund-Wikström B, Leppänen T, Welling J, Moilanen E and
Heinonen M: Lingonberry (Vaccinium vitis-idaea) and European
cranberry (Vaccinium microcarpon) proanthocyanidins:
Isolation, identification, and bioactivities. J Agric Food Chem.
59:3373–3384. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ek S, Kartimo H, Mattila S and Tolonen A:
Characterization of phenolic compounds from lingonberry
(Vaccinium vitis-idaea). J Agric Food Chem. 54:9834–9842.
2006.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Braakhuis A: Evidence on the Health
benefits of supplemental propolis. Nutrients.
11(2705)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhao L, Pu L, Wei J, Li J, Wu J, Xin Z,
Gao W and Guo C: Brazilian green propolis improves antioxidant
function in patients with type 2 diabetes mellitus. Int J Environ
Res Public Health. 13(498)2016.PubMed/NCBI View Article : Google Scholar
|