Introduction
Endothelial dysfunction, which is the loss of
balance between vasodilator and vasoconstrictor factors in blood
vessels, is a well-accepted precursor of cardiovascular disorder
pathogenesis, occurring at very early stages of diabetes (1-3).
Severe hyperglycemia, as well as functional and structural
alterations in the myocardium and vasculature, are the most typical
characteristics of patients with type 1 diabetes mellitus (T1D)
(4). Hyperglycemia can reduce the
bioavailability of nitric oxide (NO), which is a classical
anti-atherosclerosis substance that impairs endothelium-dependent
vasodilatation in T1D (4). It has
also been demonstrated that hyperglycemia is associated with the
increased production of free radicals and oxidative stress
(5). Increased oxidative stress
appears to be the critical alteration that drives the activation of
many cellular pathways, such as nuclear factor-κB, p38
mitogen-activated protein kinase, NH2-terminal Jun
kinases/stress-activated protein kinases, hexosamines and others
(6). So inhibiting this process may
be beneficial for improving endothelial dysfunction to prevent or
delay the onset of vascular-related T1D complications (7,8).
Reactive oxygen species (ROS), known as free
radicals, serve a pivotal role in the etiology and progression of
endothelial and vascular dysfunctions by creating vascular
oxidative stress (9). The
exacerbation of ROS production or insufficient scavenging impairs
biological processes, including endothelial function in the
vascular system of patients with diabetes mellitus (10,11).
Aside from mitochondria and other enzymes, NADPH oxidase (NOX) is a
prevalent source of ROS. The NOX family represent critical
determinants of the redox state within the vessel wall that
dictate, in part, the pathophysiology of several vascular
phenotypes (12). Previous studies
have demonstrated that decreasing the production of ROS derived
from NOX is helpful for the protection of vascular endothelial
function (13-15).
Astragaloside IV (AS-IV), a representative natural
saponin molecule isolated from Radix Astragali, possesses a variety
of pharmacological properties against inflammation (16), hypertension (17), oxidative stress (18) and fibrosis (19). A previous study reported that AS-IV
significantly inhibited renal oxidative stress and apoptosis in
streptozotocin (STZ)-induced diabetic rats (20). An additional study revealed that
AS-IV is a candidate for podocyte injury protection via the ER
stress pathway in T1D mice (21).
It was also reported that AS-IV treatment ameliorated oxidative
injury to protect retinal capillary endothelial cells, diabetic
gastric mucosa and the developing brain, and to protect against
pulmonary fibrosis (22-26).
However, the precise mechanisms of AS-IV on oxidative
stress-induced injury in diabetic mouse aortas have not yet been
fully elucidated. Consequently, the aim of the present study was to
evaluate the effects of AS-IV in diabetic-induced aortic tissue
injury, and to identify the efficacy as a result of inhibiting
oxidative stress levels.
Materials and methods
Animals
All experimental procedures were conducted in
accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals, and were approved by the Ethics
Committee of Putuo Hospital, Shanghai University of Traditional
Chinese Medicine (Shanghai, China). The dose of AS-IV used in the
present study was selected according to the body surface area
normalization method (27) and
STZ-induced diabetes was generated as previously described
(21). Animals received free access
to water and standard rat chow, and were housed at constant room
temperature (25˚C) and humidity (75%), and were exposed to a 12 h
light and 12 h dark cycle. Male, 6-week-old, C57BL/6J mice (n=40)
were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. After
2 weeks of acclimation, diabetes was induced via the
intraperitoneal injection of freshly prepared STZ (Sigma-Aldrich;
Merck KGaA; dissolved in 0.01 M citrate buffer, pH 4.5) at 100
mg/kg/day for 2 consecutive days. At 1 week post-STZ injection,
fasting blood glucose level was measured to verify the development
of diabetes. Blood samples (~50 µl) were collected from the tail
vein, and mice with blood glucose >350 mg/dl were randomly
separated into diabetes mellitus (DM) and DM+AS-IV (3, 6 and 12
mg/kg) groups (n=8 for each group). Each group was treated with
0.5% carboxymethyl cellulose (CMC; 12 mg/kg) or AS-IV (3, 6 and 12
mg/kg) by daily gavage for 8 weeks. Moreover, mice without STZ
treatment were labelled as the control (n=8), and were also
administered with 0.5% CMC in 12 mg/kg. AS-IV (Shanghai Bogoo
Biological Technology Co., Ltd.; 98% purity) was suspended in 0.5%
CMC, and the three DM+AS-IV group mice were administered with AS-IV
in 3, 6 and 12 mg/kg, respectively. A total of 9 weeks following
the start of STZ treatment, the mice were euthanized with
pentobarbital sodium (120 mg/kg, i.p.). The thoracic aortae were
dissected from the adherent fat and connective tissues for further
analysis.
Isometric tension recordings
The thoracic aortae were dissected and immediately
placed in a petri dish filled with an oxygenated and ice-cold
Krebs-Henseleit solution (119 mM NaCl, 4.7 mM KCl, 2.5 mM
CaCl2, 1 mM MgCl2, 25 mM NaHCO3,
1.2 mM KH2PO4 and 11 mM D-glucose; pH 7.4).
Samples were then sliced into small rings (2 mm in length). Rings
were mounted on a wire myograph system (DMT 620 M; Danish Myo
Technology A/S) for recording isometric tension using Labchart 7.0
software (AD Instruments, Inc.). The organ chamber was filled with
5 ml Krebs solution, 95% O2 and 5% CO2 at
37˚C. Each ring was stretched to an optimal baseline
tension of 3 mN and equilibrated for at least 1 h before being
stimulated with pharmacological agents. The ring was first
contracted by 60 mM KCl. After elution and a further equilibration,
aortae were stimulated with phenylephrine (Phe; 10-6 M;
Sigma-Aldrich; Merck KGaA) followed by acetylcholine (ACh;
10-6 M; Sigma-Aldrich; Merck KGaA) to assess the
function of endothelial cells.
To assess endothelial-dependent vasorelaxation, Phe
(10-6 M) was used to evoke a stable contraction and then
relaxed by accumulative concentrations of ACh (10-9,
10-8.5, 10-8, 10-7.5,
10-7, 10-6.5, 10-6,
10-5.5, 10-5 M) for comparison in aortae
derived from control, DM and DM+AS-IV (3, 6 and 12 mg/kg) mice. To
confirm the role of basal NO production in the reduced
vasorelaxation state, the same experiments were performed in rings
after 30 min at 37˚C exposure to NG-nitro-L-arginine
(L-NAME; 10-4 M; Sigma-Aldrich, Merck KGaA).
Furthermore, to assess the effect of AS-IV to ACh-induced
vasoconstriction, cumulative concentration-response curves were
generated with ACh (10-8, 10-7.5,
10-7, 10-6.5, 10-6,
10-5.5, 10-5, 10-4.5,
10-4 M) that pre-contracted with 60 mM KCl.
Western blot analysis
After the mice had been euthanized, thoracic aortae
were rapidly removed and stored at -80˚C. The aortae were lysed in
RIPA lysis buffer [50 mmol/l Tris (pH, 7.5); 1 mmol/l EDTA; 150
mmol/l NaCl; 20 mmol/l NaF; 0.5% NP-40; 10% glycerol; 1% protease
inhibitor cocktail and 1% phosphatase inhibitor cocktail] via
sonication on ice (3 sec/ml, five times at setting 5 with a 30 sec
break between each sonication). The resultant supernatants were
collected and protein concentration was determined using a BCA
protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal
quantities of protein (20 µg) were separated by 10% SDS-PAGE and
transferred to PVDF membranes (Pierce; Thermo Fisher Scientific,
Inc.). Following blocking with 5% BSA in TBS-Tween-20 for 2 h, the
membrane was incubated with primary antibodies including: p22phox
(1:500; cat. no. sc-20781; Santa Cruz Biotechnology, Inc.); p47phox
(1:500; cat. no. sc-14015; Santa Cruz Biotechnology, Inc.); 67phox
(1:500; cat. no. sc-15342; Santa Cruz Biotechnology, Inc.); NOX2
(1:500; cat. no. sc-130543; Santa Cruz Biotechnology, Inc.); NOX4
(1:500; cat. no. sc-21860; Santa Cruz Biotechnology, Inc.); Rac-1
(1:200; cat. no. sc-95; Santa Cruz Biotechnology, Inc.), eNOS
(1:500; cat. no. ab66127; Abcam); phosphorylated (p1177)
eNOS (1:500; cat. no. ab195944, Abcam) and β-actin (1:1,000; cat.
no. 4970; Cell Signaling Technology, Inc.). After three washes in
TBS-Tween-20, the membranes were incubated with the HRP-labeled
goat anti-rabbit secondary antibody (1:5,000; cat. no. BA1039;
Wuhan Boster Biotech Co. Ltd.) for 1 h at room temperature. Protein
bands were visualized using Immobilon Western Chemiluminescent HRP
Substrate (MilliporeSigma). The ratio of each protein was
normalized to β-actin and analyzed using ImageJ software, version
1.37 (National Institutes of Health).
ELISA investigation
After mice had been euthanized, thoracic aortae were
rapidly removed and cleaned of adhering connective tissue with PBS.
Total superoxide dismutase (T-SOD) was evaluated using an ELISA kit
(cat. no. A001-1-2; Nanjing Jiancheng Bioengineering Institute)
with hydroxylamine method. In brief, a total of 100 µl SOD sample
was prepared for every 10 mg of tissue, and homogenized on ice.
Samples were centrifuged for 5 min at 12,000 g and 4˚C, and the
supernatant was subsequently extracted for measurement. The
supernatant and SOD detection working solution were mixed at a 1:20
ratio and maintained at 37˚C for 30 min, before chromogenic agent
was added. At each endpoint, red substances were detected at an
absorbance of 550 nm after 10 min at 37˚C. SOD activity unit was
converted into U/mg protein according to the protein concentration
and dilution ratio of the sample.
The malondialdehyde (MDA) was evaluated using an
ELISA kit (cat. no. A003-1-2, Nanjing Jiancheng Bioengineering
Institute) with thiobarbituric acid (TBA) method, as commercially
recommended. Briefly, a total of 100 µl MDA sample was prepared for
every 5 mg of tissue, and homogenized on ice. Samples were
centrifuged for 5 min at 12,000 g and 4˚C, and the supernatant was
subsequently extracted for measurement. The supernatant and MDA
detection working solution were mixed at a ratio of 1:2 and heated
at 95˚C in acidic conditions for 40 min. The samples were cooled to
room temperature (~25˚C) with running water and centrifuged at
4,000 rpm and 25˚C for 10 min. The resulting reaction yields a pink
reactive TBA substance, which was measured at 532 nm. The MDA
content in the sample solution was calculated and converted to
nmol/mg according to the protein concentration.
Fluorescence analysis
As previously described (28), in situ ROS and NO generation
was observed with dihydroethidium (DHE; Sigma-Aldrich; Merck KGaA)
and 4,5-diaminofluorescein diacetate (DAF-2 DA; Sigma-Aldrich;
Merck KGaA) fluorescence probes, respectively. In brief, frozen
thoracic aortas were cut into 4-µm thick slices. DHE (10 µM) was
directly added onto the slices and incubated for 30 min at 37˚C.
Samples were then washed with PBS to remove free DHE molecules. Red
fluorescence was monitored via a Nikon E 600 fluorescence
microscope (Nikon TE2000; Nikon Corporation). Images were obtained
using an excitation wavelength of 520-540 nm and a rhodamine
emission filter, and the fluorescence intensity was analyzed. DAF-2
DA (10 µM) was also added and incubated with different slices using
the same procedure, as previously described. Green fluorescence was
monitored under the same fluorescence microscope fitted with a 40x
PlanFluor objective and a fluorescein isothiocyanate filter set.
The signals were acquired using NIS-Elements software version 3.0
(Nikon Corporation), and the fluorescence intensity was
assessed.
Statistical analysis
Relaxations were expressed as the percentage of
Phe-induced contractions. ACh-induced contractions were expressed
as the percentage of KCl (60 mM)-induced contractions, which were
compared among different groups. Concentration relaxation curves
were analyzed to estimate maximal response (Emax) values
and pD2 (-logEC50) as the negative logarithm of the drug
concentration that produced 50% of the Emax. One-way
ANOVA and subsequent Tukey's post hoc testing was used for
performing statistical comparisons among experimental groups using
GraphPad Prism 5.0 software (GraphPad Software, Inc.). All
experiments were repeated a minimum of three times and results are
presented as the mean ± SEM. P<0.05 was considered to indicate a
statistically significant difference.
Results
AS-IV improves the
endothelium-dependent relaxation (EDR) and contraction (EDC) of
aortae in STZ-induced diabetic mice
Thoracic aortae isolated from STZ-induced diabetic
mice were used to measure the isometric tension. As a known
activator of endothelium-dependent relaxation, acetylcholine
(ACh)-induced EDR were detected after the rings had been
precontracted with phenylephrine (Phe). As presented in
representative traces (Fig. 1A) and
summarized data (Fig. 1B; Table I), the response to accumulative
concentrations of ACh in Phe-precontracted segments of aortae were
significantly reduced in DM mice compared with those in control
mice. In contrast, AS-IV (6 and 12 mg/kg) treatment can improve the
impaired response compared with the DM group. However, after
pre-incubation of aortic segments with L-NAME, a nitric oxide (NO)
synthase inhibitor that can block the production of
endothelium-derived NO, the recovery effect of AS-IV to EDR was
abolished (Fig. 1C).
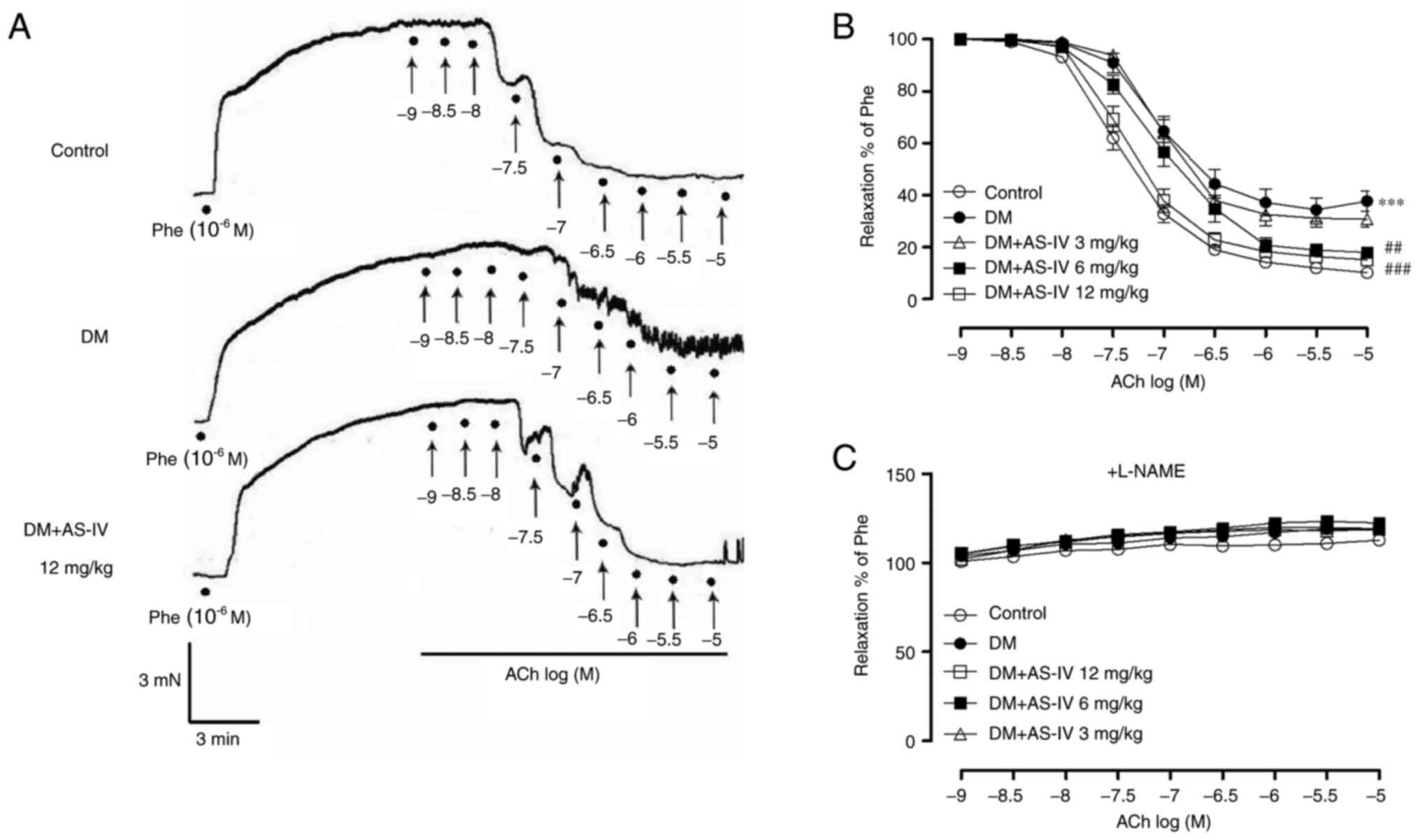 | Figure 1Effect of AS-IV on the EDRs of aortas
obtained from diabetic mice. (A) Representative recording traces
revealed that impaired ACh-induced aortic EDRs from DM mice were
restored following treatment with AS-IV (12 mg/kg). ACh
(10-9, 10-8.5, 10-8,
10-7.5, 10-7, 10-6.5,
10-6, 10-5.5, 10-5 M) was applied
to the organ bath at points indicated by solid black spots. (B)
Summative graphs demonstrated that AS-IV (6 and 12 mg/kg) treatment
improved aortic EDRs in DM mice. (C) EDRs in response to ACh
following 30 min exposure to L-NAME were presented in aortas
obtained from the 5 groups of mice. Data are presented as the mean
± SEM (n=6-8). ***P<0.001 vs. control mice.
##P<0.01 and ###P<0.001 vs. mice with
DM. AS-IV, Astragaloside IV; EDRs, endothelium-dependent
relaxations; DM, diabetes mellitus; ACh, acetylcholine; L-NAME,
NG-nitro-L-arginine; Phe, phenylephrine. |
 | Table IEffect of AS-IV on the
Emax and pD2 values of ACh-induced relaxation and
contraction dose-response curves of the aortas isolated from
control, DM and DM+AS-IV (3, 6, 12 mg/kg) mice. |
Table I
Effect of AS-IV on the
Emax and pD2 values of ACh-induced relaxation and
contraction dose-response curves of the aortas isolated from
control, DM and DM+AS-IV (3, 6, 12 mg/kg) mice.
| | ACh
(relaxation) | ACh
(contraction) |
|---|
| Groups |
Emax | pD2 |
Emax | pD2 |
|---|
| Control | 89.80±1.62 | 7.41±0.06 | 11.54±2.93 | 5.94±0.25 |
| DM |
66.74±4.34b |
7.01±0.09a |
82.24±4.22a |
7.30±0.26a |
| DM+AS-IV 3
mg/kg | 71.23±3.39 | 6.99±0.09 | 73.37±3.63 | 6.92±0.15 |
| DM+AS-IV 6
mg/kg |
85.00±1.67d | 7.02±0.09 |
62.25±6.35c |
6.33±0.27c |
| DM+AS-IV 12
mg/kg |
86.27±1.38e |
7.33±0.07c |
52.19±4.66e |
6.09±0.35c |
Furthermore, the endothelium-dependent contraction
(EDC) in STZ-induced diabetic mice was also detected. As
demonstrated in Fig. 2 and Table I, ACh-induced contractions increased
with accumulative concentration in DM mice compared with the
control. Compared with the DM group, AS-IV (6 and 12 mg/kg)
treatment significantly inhibited the EDC response.
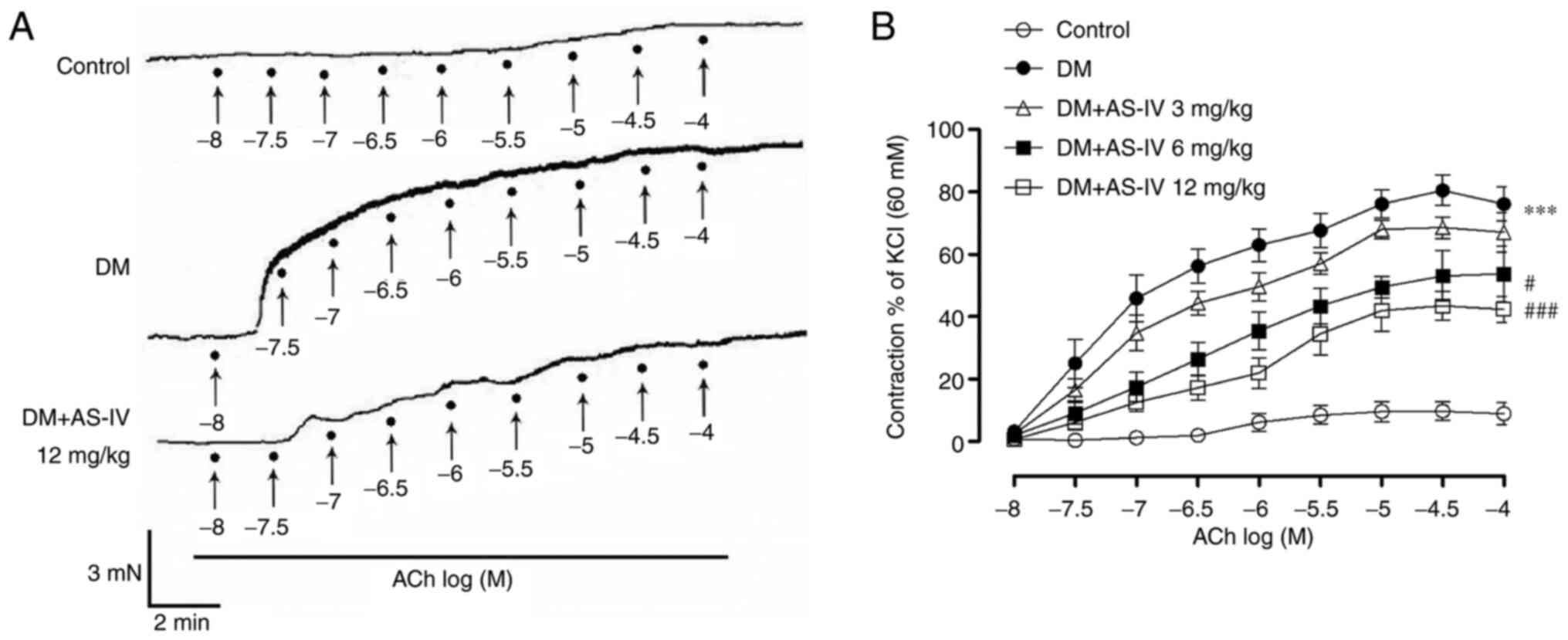 | Figure 2Effect of AS-IV on ACh-induced
vasoconstriction in the aortae of diabetic mice. (A) Representative
recording traces demonstrated that augmented ACh-induced EDCs in
the aortae of DM mice were prevented following AS-IV (12 mg/kg)
treatment. ACh (10-8, 10-7.5,
10-7, 10-6.5, 10-6,
10-5.5, 10-5, 10-4.5,
10-4 M) was applied to the organ bath at points
indicated by solid black spots. (B) Summative graphs revealed that
AS-IV (6 and 12 mg/kg) treatment reduced EDCs in the aortae of DM
mice. Data are presented as the mean ± SEM (n=6-8).
***P<0.001 vs. control mice; #P<0.05
and ###P<0.001 vs. mice with DM. AS-IV, Astragaloside
IV; ACh, acetylcholine; DM, diabetes mellitus; EDCs,
endothelium-dependent contractions. |
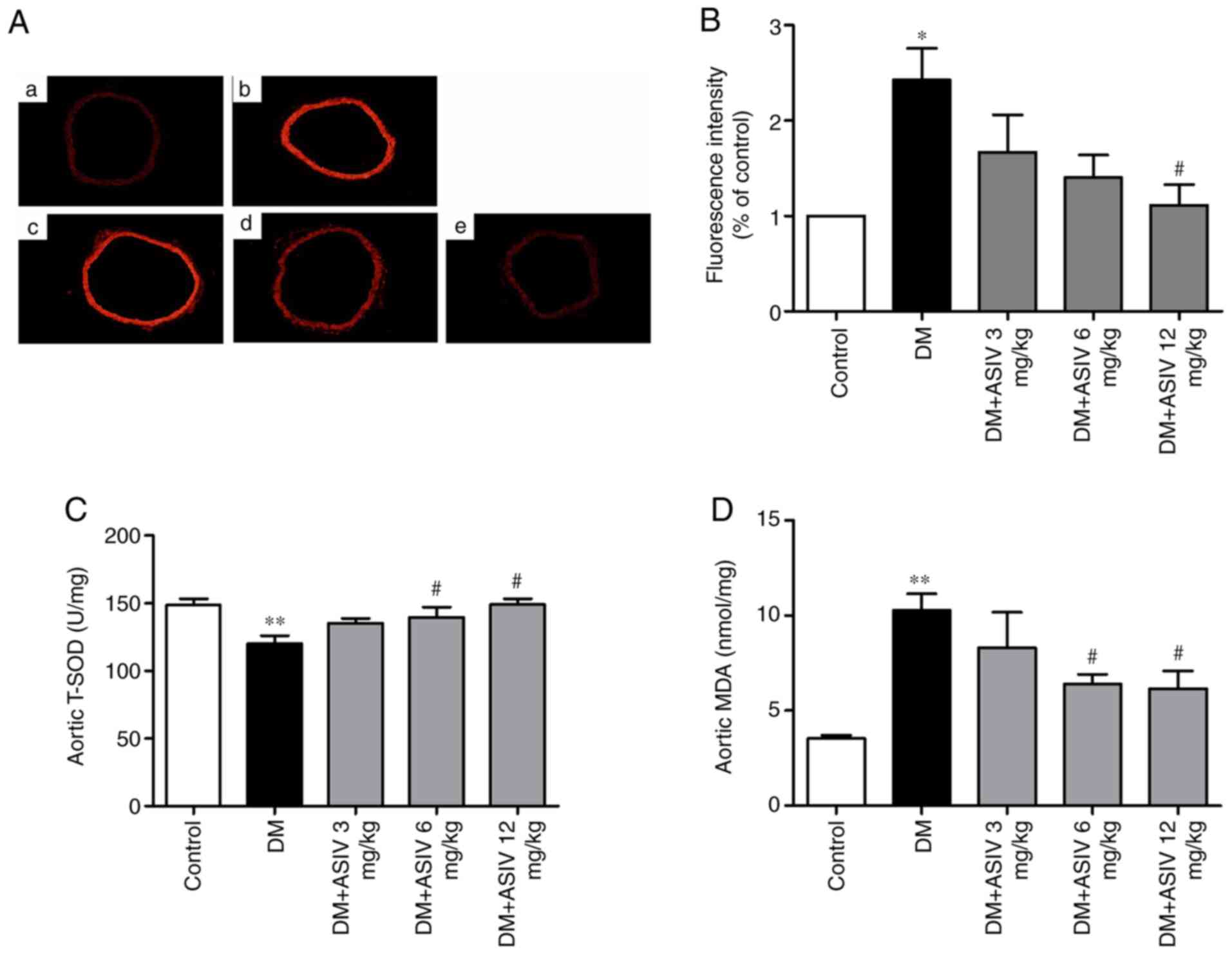
AS-IV increases NO activity in the
aortae of STZ-induced diabetic mice
To characterize and localize NO content within the
vascular wall, green fluorescence was analyzed in sections of aorta
incubated with DAF-2 DA (Fig. 3A).
As presented in Fig. 3B, compared
with the control, fluorescence intensity was greatly inhibited in
the DM group. As demonstrated in Fig.
3C and D, AS-IV-treatment
reversed aortic NO levels in a dose-dependent manner, particularly
at concentrations of 6 and 12 mg/kg. Phosphorylated eNOS
(p1177 eNOS), the active form of eNOS, was also
increased following AS-IV treatment, which was significant at the
12 mg/kg dosage.
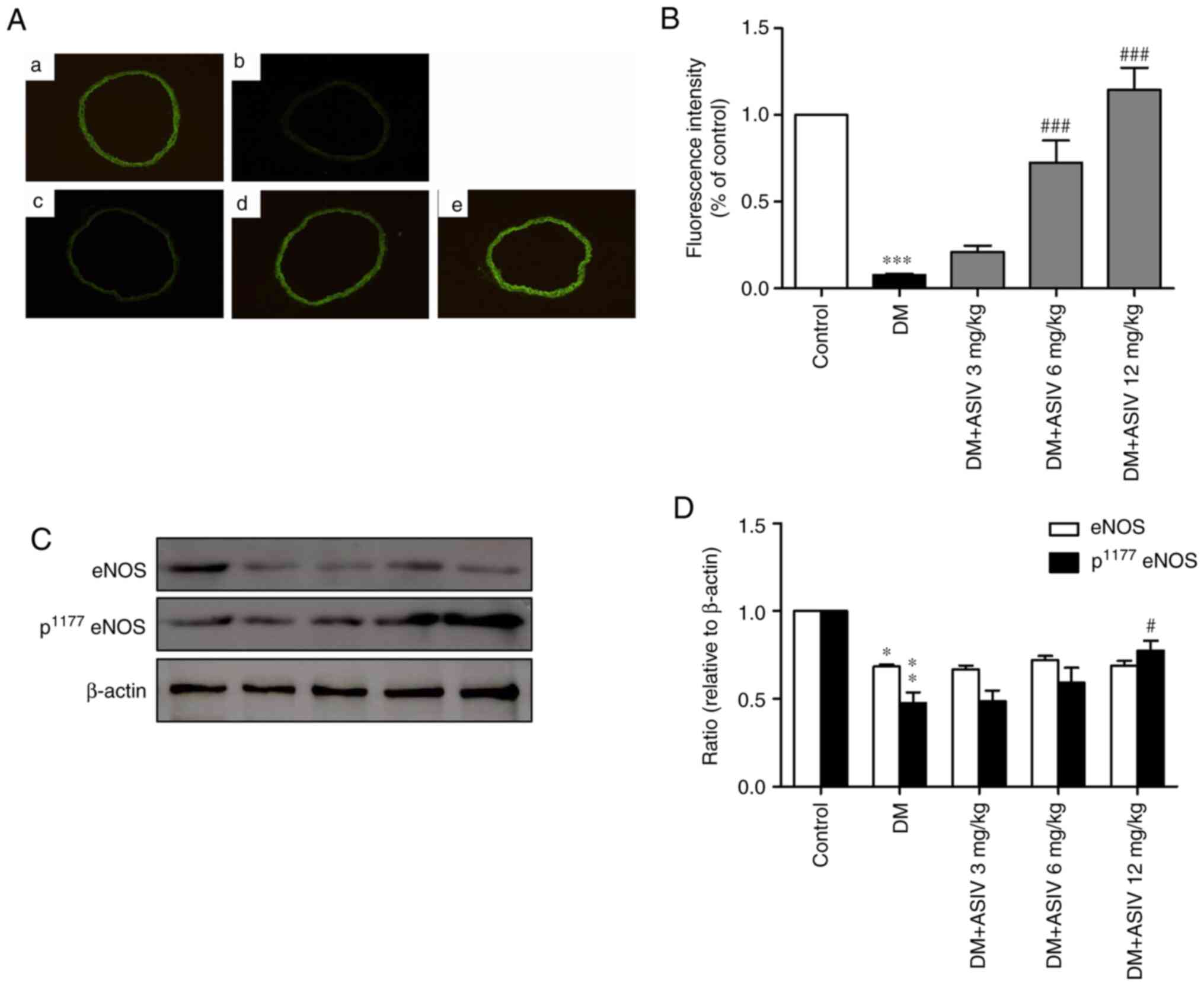 | Figure 3Effect of AS-IV on the activity of NO
in the aortae of diabetic mice. (A) Representative photomicrographs
of 4,5-diaminofluorescein diacetate-stained thoracic aorta sections
from (a) control, (b) DM and (c) 3, (d) 6 and (e) 12 mg/kg DM+AS-IV
groups (magnification, x200). (B) Analysis of fluorescence
intensity of NO from (A). (C) Western blot analysis of eNOS and
p1177 eNOS. (D) Analysis of eNOS and p1177
eNOS from (C). Data are presented as the mean ± SEM (n=4-6).
*P<0.05, **P<0.01 and
***P<0.001 vs. the control group.
#P<0.05 and ###P<0.001 vs. the DM
group. AS-IV, Astragaloside IV; DM, diabetes mellitus; NO, nitric
oxide; eNOS, endothelial nitric oxide synthase; p,
phosphorylated. |
AS-IV inhibits ROS activity in the
aortae of STZ-induced diabetic mice
ROS activity was detected through red fluorescence,
which was analyzed via DHE incubation (Fig. 4A). Fluorescence intensity calculated
from the aortic rings of mice with DM revealed markedly increased
red staining compared with control mice, which was inhibited
following 12 mg/kg AS-IV treatment (Fig. 4B). The activity of SOD and MDA in
serum was examined to further test ROS levels. As presented in
Fig. 4C and D, serum SOD activity was significantly
decreased and MDA was increased in mice with DM compared with that
in control mice. AS-IV treatment increased SOD activity and
decreased MDA content when compared with mice with DM. The effect
demonstrated by AS-IV was significant at concentrations of 6 and 12
mg/kg.
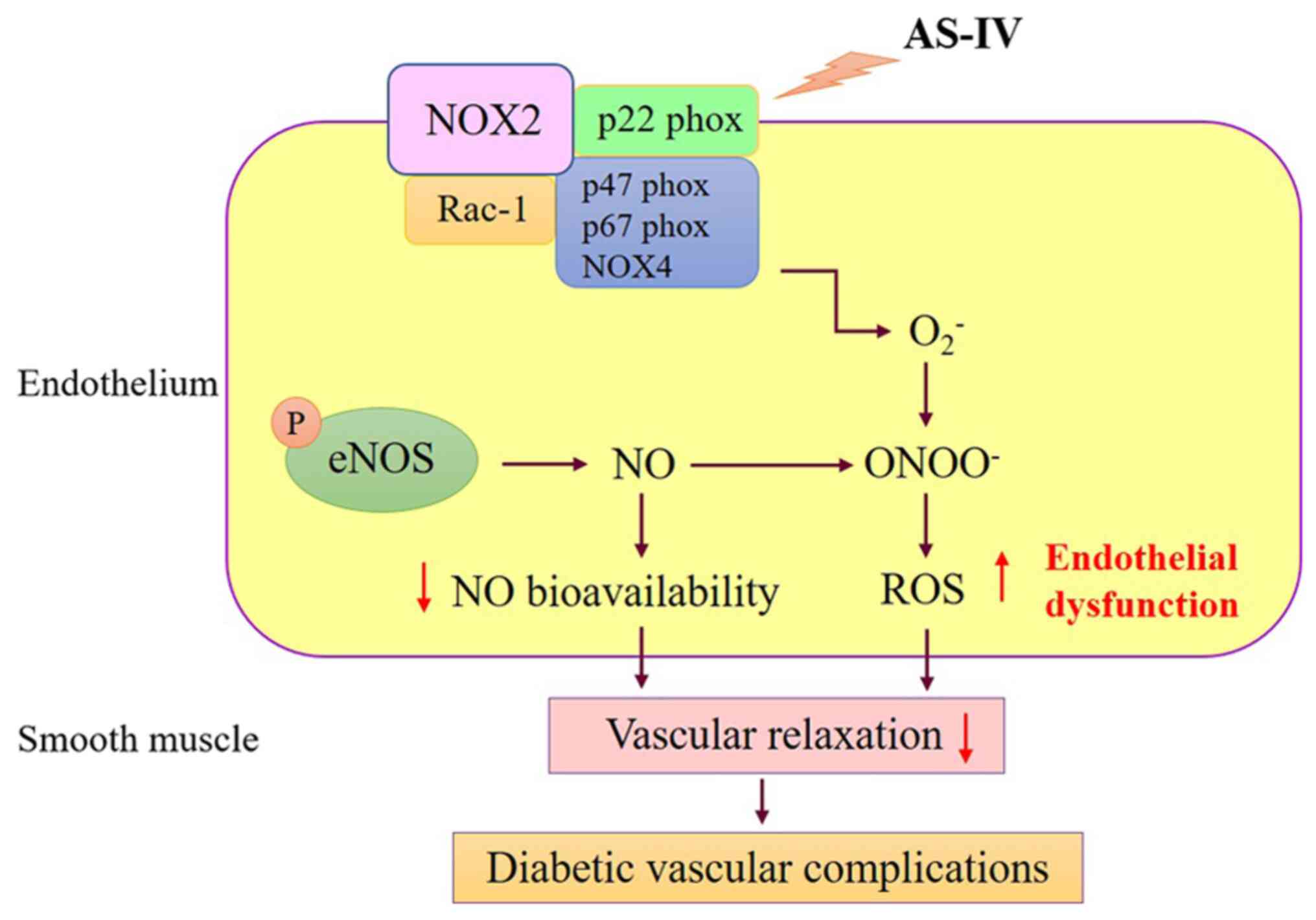
AS-IV ameliorates NADPH oxidase
activity in the aortae of STZ-induced diabetic mice
The expression of NADPH oxidase subunits, including
p22phox, p47phox, p67phox, NOX2, NOX4 and Rac-1, were examined in
the present study (Fig. 5).
Compared with the control group, mice with DM exhibited increased
levels of expression. However, AS-IV treatment decreased the
expression each subunit, with the most significant effect being
demonstrated at the 12 mg/kg dosage. Significant alterations were
observed in p22phox, p47phox, NOX2, NOX4 and Rac-1 expression.
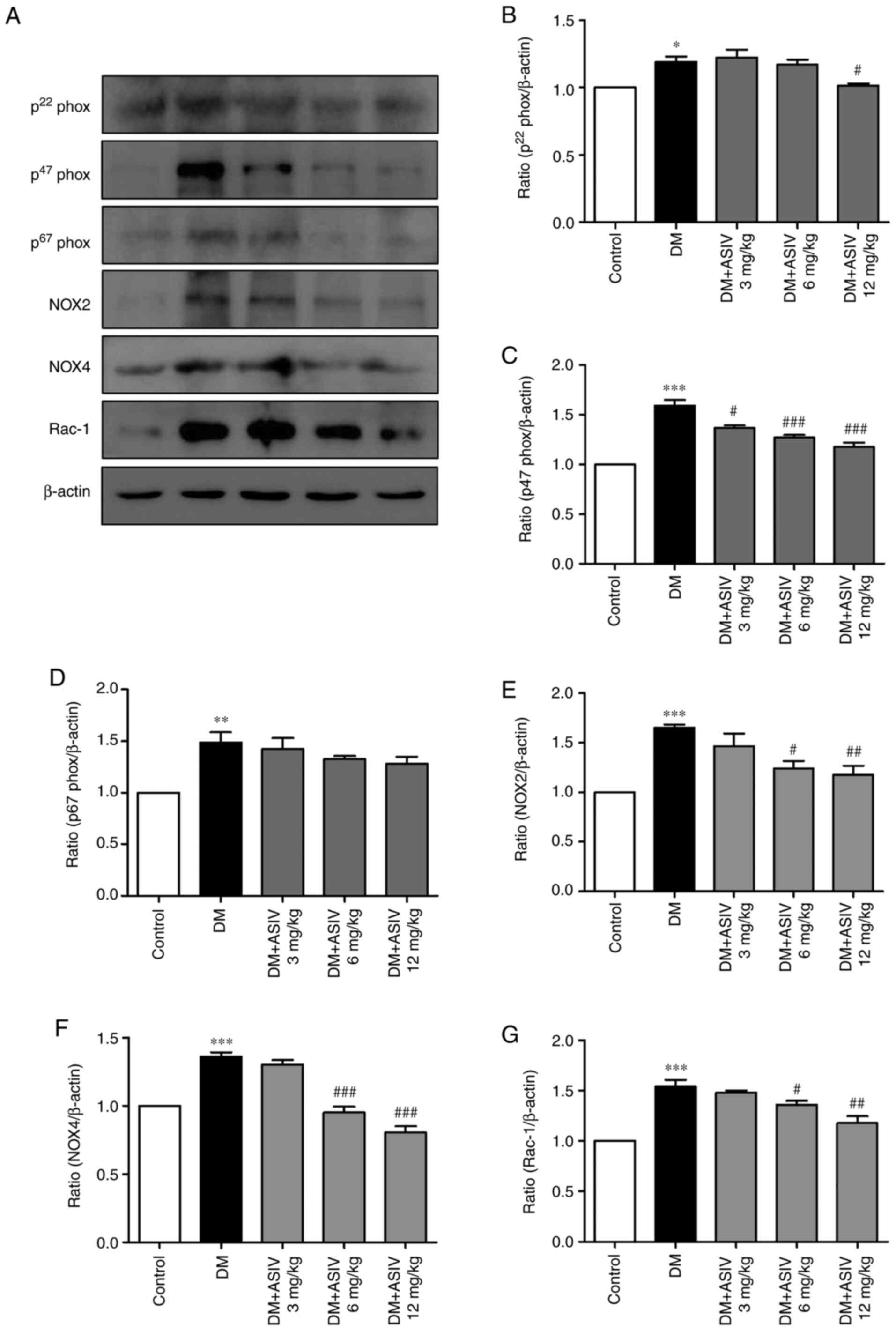 | Figure 5Effect of Astragaloside IV on NADPH
oxidase in the aortae of diabetic mice. (A) Western blot analysis
of various NADPH oxidase subunits, including p22phox, p47phox,
p67phox, NOX2, NOX4 and Rac-1. (B) p22phox, (C) p47phox, (D)
p67phox, (E) NOX2, (F) NOX4 and (G) Rac-1 levels were then
statistically analyzed. Data are presented as the mean ± SEM
(n=4-6). *P<0.05, **P<0.01 and
***P<0.001 vs. the control group.
#P<0.05, ##P<0.01 and
###P<0.001 vs. the DM group. AS-IV, Astragaloside IV;
DM, diabetes mellitus; p22phox, human neutrophil cytochrome
b light chain; p47phox, neutrophil cytosolic factor 1;
p67phox, neutrophil cytosolic factor 2; NOX, NADPH oxidase. |
Discussion
Endothelial dysfunction is characterized by reduced
NO bioavailability, which is considered to be one of the initial
pathological events in the pathogenesis of diabetes-associated
cardiovascular disorders (29,30).
In physiological and pathological conditions, the endothelium
controls the tone of the underlying vascular smooth muscle cells
through NO production (31).
Previous studies have demonstrated that hyperglycemia-induced
damage in endothelial cells is associated with decreased NO
bioavailability in vascular beds (32,33).
Thus, improving endothelial function, particularly NO
bioavailability, is an attractive therapeutic intervention for the
prevention and treatment of cardiovascular complications. In the
present study, impaired vasorelaxation and vasocontraction were
confirmed in the aortic rings of STZ-induced diabetic mice.
However, treatment with AS-IV restored each impairment in a
concentration-dependent manner. Additionally, L-NAME, an inhibitor
of NO synthase, abolished the protective effect of AS-IV, as
demonstrated by higher maximal vasorelaxations in response to ACh,
suggesting that the efficacy of the compound was
endothelium-dependent.
Abnormal NO generation, hyperglycemia, dyslipidemia
and insulin resistance are important causal factors for the
development of DM associated endothelial dysfunction (34). A previous study reported that
reduced endothelial NO production is often associated with the
inhibition of eNOS activity in diabetes-induced vascular
dysfunction (35). In accordance
with the decrease of endothelium-dependent vasodilatation in mice
with DM, the current study revealed that NO bioavailability also
decreased. However, AS-IV treatment increased NO bioavailability in
a concentration-dependent manner. This further confirmed the
potential effect of AS-IV on diabetic-induced aortic tissue injury
by enhancing NO bioavailability. When assessing NO generation, the
current study also revealed that DAF-2DA green fluorescence was
present throughout the arterial wall, including in the media.
DAF-2DA green fluorescence is usually considered as one of the
direct detection methods of intracellular NO (36). However, immunohistochemistry
revealed strong eNOS staining in vessel walls, specifically
localizing to the intima. This method may therefore be used to
identify sites of NO production in intima, vessel walls and other
heterogeneous tissues. DAF fluorescence and chemiluminescence may
produce substantially different results depending on the conditions
and systems under investigation (36,37). A
previous study demonstrated that the different relaxing capacities
of NO for smooth muscle cell contractions initiated by
depolarization (high K+) or by α1 adrenoceptor
stimulation is not dependent on the source of NO (endogenous or
exogenous), but depends primarily on the pathways of
(Ca2+)i mobilization during the contraction
of murine aortas (38). DAF
fluorescence may therefore not be an independent indicator of NO.
Since NO biosynthesis in vascular endothelial cells is
undisputable, positive DAF-2DA fluorescence in vessel walls may be
regarded as an internal positive control (37). The results of the current study are
congruent with those of a previous study in which DAF-2DA green
fluorescence was indicative of NO activity (28).
Improvement of endothelial NO production and the
prevention of vascular oxidative stress are other reasonable
therapeutic strategies for the treatment of DM, targeting diabetic
endothelial progenitor cells (39).
ROS, which include hydrogen peroxide (H2O2),
superoxide anion (O2-), hydroxyl radicals
(OH) and peroxynitrite anion (ONOO-), play important
signaling roles under physiological conditions at moderate
concentrations (40). However,
during excessive or sustained ROS production, where production
exceeds the available antioxidant defenses, oxidative stress occurs
(41). The diabetic production of
excessive endothelial ROS is the main reason for the pathogenesis
of vascular complications (11,42).
The current study demonstrated that ROS generation increased in
mice with DM. Additionally, levels of MDA, a marker of ROS-induced
lipid peroxidation, also significantly increased, which was
accompanied by decreased SOD activities in aortic tissue. These
data indicated the presence of oxidative stress in the aortas of
STZ-induced diabetic mice.
Oxidative stress due to exaggerated NOX-induced ROS
formation and decreased NO bioavailability is a hallmark of
diabetic vasculopathy. Although the suppression of ROS and
restoration of NO bioactivity are two strategies employed by
antioxidants, each are closely associated as eNOS uncoupling can
lead to the production of superoxide anions rather than NO,
resulting in the increased production of vascular ROS (43,44).
Additionally, ROS as well as peroxynitrite, a potent oxidant
produced by the interaction of superoxide anions with NO, can
further induce eNOS uncoupling, reduce endothelium-derived NO
required for vascular relaxation and modify endothelial cell
molecules via oxidation (40,45).
Superoxide quenches NO and impairs the endothelial-derived
vasodilator system. Therefore, it is reasonable to speculate that
the generation of NOX-derived ROS may be associated with decreased
NO bioavailability and vascular dysfunction (46).
NOX is the major source of ROS in the vasculature,
which exerts effects under physiological and pathological
conditions. NOX is also considered to be the trigger of development
of diabetic endothelial dysfunction (47). The NO family is composed of
catalytic subunits (NOX1, 2, 3, 4 and 5) and a docking subunit,
p22phox, both of which are present in the cell membrane. Regulatory
subunits, including p47phox, p67phox or homologs, are located in
the cytosol (46). The activation
of Rac-1, a key event in NOX activation, regulates the
translocation and assembly of NOX subunits in the plasma membrane
(48). Although NOXs are
differentially expressed in the vascular wall and have expression
profiles that vary in different disease states, it is accepted that
the expression of NOXs increase in response to certain stimuli such
as hyperglycemia, which eventually results in the disruption of
vascular homeostasis (49). NOX4 is
the major source of ROS in endothelial cells. Therefore, increased
NOX4 expression is associated with the early progression of
vascular disease (50,51). Increased endothelial NOX2 levels
contributes to angiotensin II-induced endothelial dysfunction,
vascular remodeling and hypertension (52). Other NOX organizers, such as NOX1
and p47phox, are mediators of diabetes-induced vascular dysfunction
in mice (53). Considering the
potentially important role of NOX in the pathogenesis of
cardiovascular complications in patients with diabetes, the
application of NOX inhibitors may serve as a viable therapeutic
target. The current study revealed that AS-IV inhibited the
expression of p22phox, p47phox, NOX2, NOX4 and Rac-1, which
indicated that AS-IV inhibited endothelial dysfunction through the
oxidative stress pathway (Fig.
6).
In summary, the present study demonstrated that
AS-IV inhibited endothelial dysfunction in the aortas of
STZ-induced diabetic mice, which was associated with the
attenuation of NADPH oxidase expression, the attenuation of ROS
production and the promotion of NO production.
Acknowledgements
Not applicable.
Funding
Funding: The current study was supported by the Innovation
Program of Talent Project of Putuo District (grant no. 2020360A),
the National Natural Science Foundation of China (grant no.
81403235), the Key Medical Discipline Project of Shanghai Municipal
Health Bureau (grant no. ZK2019A12) and the Independent Innovation
Research Fund of Putuo District Science and Technology Committee
(grant no. 2012PTKW006).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW and HW participated in the design and
coordination, and guaranteed the integrity of the entire study. YZ,
XDM, ALC, SC and ZJL carried out the experiments. YMW and WP
performed statistical analysis and edited the manuscript. LW was
involved in drafting the manuscript, evaluated the statistical
analysis and critically revised the manuscript. LW and HW confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All experimental procedures involving animals were
conducted in accordance with the National Institutes of Health
Guide for the Care and Use of Laboratory Animals and were approved
by the Ethics Committee of Putuo Hospital, Shanghai University of
Traditional Chinese Medicine.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Akalin S, Berntorp K, Ceriello A, Das AK,
Kilpatrick ES, Koblik T, Munichoodappa CS, Pan CY, Rosenthall W,
Shestakova M, et al: Intensive glucose therapy and clinical
implications of recent data: A consensus statement from the global
task force on glycaemic control. Int J Clin Pract. 63:1421–1425.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Preis SR, Pencina MJ, Hwang SJ, D'Agostino
RB Sr, Savage PJ, Levy D and Fox CS: Trends in cardiovascular
disease risk factors in individuals with and without diabetes
mellitus in the Framingham heart study. Circulation. 120:212–220.
2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Castro-Correia C, Maia ML, Norberto S,
Costa-Santos C, Barroso MF, Carvalho A, Fontoura M, Domingues V and
Calhau C: Can antioxidative status be involved in type 1 diabetes?
J Clin Med Res. 9:998–1001. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
McCarthy O, Moser O, Eckstein ML, Bain SC,
Pitt J and Bracken R: Supplementary nitric oxide donors and
exercise as potential means to improve vascular health in people
with type 1 diabetes: Yes to no? Nutrients. 11(1571)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Giugliano D, Ceriello A and Paolisso G:
Diabetes mellitus, hypertension, and cardiovascular disease: Which
role for oxidative stress? Metabolism. 44:363–368. 1995.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Evans JL, Goldfine ID, Maddux BA and
Grodsky GM: Are oxidative stress-activated signaling pathways
mediators of insulin resistance and beta-cell dysfunction?
Diabetes. 52:1–8. 2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gori T and Münzel T: Oxidative stress and
endothelial dysfunction: Therapeutic implications. Ann Med.
43:259–272. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kurosaki Y, Imoto A, Kawakami F, Yokoba M,
Takenaka T, Ichikawa T, Katagiri M and Ishii N: Oxidative stress
increases megalin expression in the renal proximal tubules during
the normoalbuminuric stage of diabetes mellitus. Am J Physiol Renal
Physiol. 314:F462–F470. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Salisbury D and Bronas U: Reactive oxygen
and nitrogen species: Impact on endothelial dysfunction. Nurs Res.
64:53–66. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sáez T, Salsoso R, Leiva A, Toledo F, de
Vos P, Faas M and Sobrevia L: Human umbilical vein
endothelium-derived exosomes play a role in foetoplacental
endothelial dysfunction in gestational diabetes mellitus. Biochim
Biophys Acta Mol Basis Dis. 1864:499–508. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ambasta RK, Kohli H and Kumar P: Multiple
therapeutic effect of endothelial progenitor cell regulated by
drugs in diabetes and diabetes related disorder. J Transl Med.
15(185)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Burtenshaw D, Hakimjavadi R, Redmond EM
and Cahill PA: Nox, reactive oxygen species and regulation of
vascular cell fate. Antioxidants (Basel). 6(90)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dong J, Wong SL, Lau CW, Lee HK, Ng CF,
Zhang L, Yao X, Chen ZY, Vanhoutte PM and Huang Y: Calcitriol
protects renovascular function in hypertension by down-regulating
angiotensin II type 1 receptors and reducing oxidative stress. Eur
Heart J. 33:2980–2990. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Buday A, Orsy P, Godó M, Mózes M, Kökény
G, Lacza Z, Koller A, Ungvári Z, Gross ML, Benyó Z and Hamar P:
Elevated systemic TGF-beta impairs aortic vasomotor function
through activation of NADPH oxidase-driven superoxide production
and leads to hypertension, myocardial remodeling, and increased
plaque formation in apoE(-/-) mice. Am J Physiol Heart Circ
Physiol. 299:H386–H395. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang X, Zhao S, Su M, Sun L, Zhang S, Wang
D, Liu Z, Yuan Y, Liu Y and Li Y: Geraniol improves endothelial
function by inhibiting NOX-2 derived oxidative stress in high fat
diet fed mice. Biochem Biophys Res Commun. 474:182–187.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li J, Huang L, Wang S, Yao Y and Zhang Z:
Astragaloside IV attenuates inflammatory reaction via activating
immune function of regulatory T-cells inhibited by HMGB1 in mice.
Pharm Biol. 54:3217–3225. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang N, Wang XH, Mao SL and Zhao F:
Astragaloside IV improves metabolic syndrome and endothelium
dysfunction in fructose-fed rats. Molecules. 16:3896–3907.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo H, Cao A, Chu S, Wang Y, Zang Y, Mao
X, Wang H, Wang Y, Liu C, Zhang X and Peng W: Astragaloside IV
attenuates podocyte apoptosis mediated by endoplasmic reticulum
stress through upregulating sarco/endoplasmic reticulum
Ca2+-ATPase 2 expression in diabetic nephropathy. Front
Pharmacol. 7(500)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang L, Chi YF, Yuan ZT, Zhou WC, Yin PH,
Zhang XM, Peng W and Cai H: Astragaloside IV inhibits renal
tubulointerstitial fibrosis by blocking TGF-β/Smad signaling
pathway in vivo and in vitro. Exp Biol Med (Maywood).
239:1310–1324. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Motomura K, Fujiwara Y, Kiyota N,
Tsurushima K, Takeya M, Nohara T, Nagai R and Ikeda T:
Astragalosides isolated from the root of astragalus radix inhibit
the formation of advanced glycation end products. J Agric Food
Chem. 57:7666–7672. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Guo H, Wang Y, Zhang X, Zang Y, Zhang Y,
Wang L, Wang H, Wang Y, Cao A and Peng W: Astragaloside IV protects
against podocyte injury via SERCA2-dependent ER stress reduction
and AMPKα-regulated autophagy induction in streptozotocin-induced
diabetic nephropathy. Sci Rep. 7(6852)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Qiao Y, Fan CL and Tang MK: Astragaloside
IV protects rat retinal capillary endothelial cells against high
glucose-induced oxidative injury. Drug Des Devel Ther.
11:3567–3577. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang N, Siu F and Zhang Y: Effect of
astragaloside IV on diabetic gastric mucosa in vivo and in vitro.
Am J Transl Res. 9:4902–4913. 2017.PubMed/NCBI
|
|
24
|
Yu WN, Sun LF and Yang H: Inhibitory
effects of astragaloside IV on bleomycin-induced pulmonary fibrosis
in rats via attenuation of oxidative stress and inflammation.
Inflammation. 39:1835–1841. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun J, Chen XL, Zheng JY, Zhou JW and Ma
ZL: Astragaloside IV protects new born rats from anesthesia-induced
apoptosis in the developing brain. Exp Ther Med. 12:1829–1835.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gu DM, Lu PH, Zhang K, Wang X, Sun M, Chen
GQ and Wang Q: EGFR mediates astragaloside IV-induced Nrf2
activation to protect cortical neurons against in vitro
ischemia/reperfusion damages. Biochem Biophys Res Commun.
457:391–397. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Reagan-Shaw S, Nihal M and Ahmad N: Dose
translation from animal to human studies revisited. FASEB J.
22:659–661. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chu S, Wang L, Mao XD and Peng W:
Improvement of huangqi decoction on endothelial dysfunction in 5/6
nephrectomized rats. Cell Physiol Biochem. 40:1354–1366.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kawano N, Emoto M, Mori K, Yamazaki Y,
Urata H, Tsuchikura S, Motoyama K, Morioka T, Fukumoto S, Shoji T,
et al: Association of endothelial and vascular smooth muscle
dysfunction with cardiovascular risk factors, vascular
complications, and subclinical carotid atherosclerosis in type 2
diabetic patients. J Atheroscler Thromb. 19:276–284.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Islam MZ, Van Dao C, Miyamoto A and
Shiraishi M: Rho-kinase and the nitric oxide pathway modulate
basilar arterial reactivity to acetylcholine and angiotensin II in
streptozotocin-induced diabetic mice. Naunyn Schmiedebergs Arch
Pharmacol. 390:929–938. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guo Z, Zhang Y, Liu C, Youn JY and Cai HL:
Toll-like Receptor 2 (TLR2) deficiency abrogates diabetic and obese
phenotypes while restoring endothelial function via inhibition of
NOX1. Diabetes: Jun 14, 2021 (Epub ahead of print). doi:
10.2337/db20-0591.
|
|
32
|
Varghese JF, Patel R and Yadav UCS: Novel
insights in the metabolic syndrome-induced oxidative stress and
inflammation-mediated atherosclerosis. Curr Cardiol Rev. 14:4–14.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Versari D, Daghini E, Virdis A, Ghiadoni L
and Taddei S: Endothelial dysfunction as a target for prevention of
cardiovascular disease. Diabetes Care. 32 (Suppl 2):S314–S321.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tangvarasittichai S: Oxidative stress,
insulin resistance, dyslipidemia and type 2 diabetes mellitus.
World J Diabetes. 6:456–480. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cicek FA, Kandilci HB and Turan B: Role of
ROCK upregulation in endothelial and smooth muscle vascular
functions in diabetic rat aorta. Cardiovasc Diabetol.
12(51)2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Schwendemann J, Sehringer B, Noethling C,
Zahradnik HP and Schaefer WR: Nitric oxide detection by DAF
(diaminofluorescein) fluorescence in human myometrial tissue.
Gynecol Endocrinol. 24:306–311. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Planchet E and Kaiser WM: Nitric oxide
(NO) detection by DAF fluorescence and chemiluminescence: A
comparison using abiotic and biotic NO sources. J Exp Bot.
57:3043–3055. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Van Hove CE, Van der Donckt C, Herman AG,
Bult H and Fransen P: Vasodilator efficacy of nitric oxide depends
on mechanisms of intracellular calcium mobilization in mouse aortic
smooth muscle cells. Br J Pharmacol. 158:920–930. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cao W, Cui J, Li S, Zhang D, Guo Y, Li Q,
Luan Y and Liu X: Crocetin restores diabetic endothelial progenitor
cell dysfunction by enhancing NO bioavailability via regulation of
PI3K/AKT-eNOS and ROS pathways. Life Sci. 181:9–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Magenta A, Greco S, Capogrossi MC, Gaetano
C and Martelli F: Nitric oxide, oxidative stress, and p66Shc
interplay in diabetic endothelial dysfunction. Biomed Res Int.
2014(193095)2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Förstermann U, Xia N and Li H: Roles of
vascular oxidative stress and nitric oxide in the pathogenesis of
atherosclerosis. Circ Res. 120:713–735. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ding X, Zhang M, Gu R, Xu G and Wu H:
Activated microglia induce the production of reactive oxygen
species and promote apoptosis of co-cultured retinal microvascular
pericytes. Graefes Arch Clin Exp Ophthalmol. 255:777–788.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Thum T, Fraccarollo D, Schultheiss M,
Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G and Bauersachs J:
Endothelial nitric oxide synthase uncoupling impairs endothelial
progenitor cell mobilization and function in diabetes. Diabetes.
56:666–674. 2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hohenstein B, Hugo CP, Hausknecht B,
Boehmer KP, Riess RH and Schmieder RE: Analysis of NO-synthase
expression and clinical risk factors in human diabetic nephropathy.
Nephrol Dial Transplant. 23:1346–1354. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang BQ, Hu SJ, Qiu LH, Zhu JH, Xie XJ,
Sun J, Zhu ZH, Xia Q and Bian K: Effects of Astragalus
membranaceus and its main components on the acute phase
endothelial dysfunction induced by homocysteine. Vascul Pharmacol.
46:278–285. 2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Holterman CE, Read NC and Kennedy CR: Nox
and renal disease. Clin Sci (Lond). 128:465–481. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tian R, Ding Y, Peng YY and Lu N:
Myeloperoxidase amplified high glucose-induced endothelial
dysfunction in vasculature: Role of NADPH oxidase and hypochlorous
acid. Biochem Biophys Res Commun. 484:572–578. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Drummond GR, Selemidis S, Griendling KK
and Sobey CG: Combating oxidative stress in vascular disease: NADPH
oxidases as therapeutic targets. Nat Rev Drug Discov. 10:453–471.
2011.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Konior A, Schramm A, Czesnikiewicz-Guzik M
and Guzik TJ: NADPH oxidases in vascular pathology. Antioxid Redox
Signal. 20:2794–2814. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhao QD, Viswanadhapalli S, Williams P,
Shi Q, Tan C, Yi X, Bhandari B and Abboud HE: NADPH oxidase 4
induces cardiac fibrosis and hypertrophy through activating
Akt/mTOR and NFκB signaling pathways. Circulation. 131:643–655.
2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lee HY, Zeeshan HMA, Kim HR and Chae HJ:
Nox4 regulates the eNOS uncoupling process in aging endothelial
cells. Free Radic Biol Med. 113:26–35. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Murdoch CE, Alom-Ruiz SP, Wang M, Zhang M,
Walker S, Yu B, Brewer A and Shah AM: Role of endothelial Nox2
NADPH oxidase in angiotensin II-induced hypertension and vasomotor
dysfunction. Basic Res Cardiol. 106:527–538. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Rezende F, Moll F, Walter M, Helfinger V,
Hahner F, Janetzko P, Ringel C, Weigert A, Fleming I, Weissmann N,
et al: The NADPH organizers NoxO1 and p47phox are both mediators of
diabetes-induced vascular dysfunction in mice. Redox Biol.
15:12–21. 2018.PubMed/NCBI View Article : Google Scholar
|