Introduction
Bladder cancer (BC) is the predominant malignancy
urinary tract and is ranked as the cancer with the 4th highest
incidence and as the 8th largest estimated cause of death in men in
the United States (1). BC
development and progression is correlated to genetic susceptibility
and environmental exposure to arsenicals, pollutants, cigarette
smoke, insecticides, fungicides and pesticides (2). BC is divided into two main subtypes
based on the assessment of pathophysiology, namely
non-muscle-invasive (NMIBC) and muscle-invasive (MIBC) (3). In Europe, ~75% patients with NMIBC
exhibit high recurrence and low progression rates, whilst patients
with MIBC are associated with high risks for progression and
cancer-specific mortality (4). A
number of studies have previously reported that fibroblast growth
factor receptor 3 mutation, p53 pathway alteration and cyclin
dependent kinase inhibitor 2A promoter hypermethylation are
strongly associated with BC tumor histopathological
characteristics, including histological grade, stage, progression
and recurrence (5-8).
In clinical practice, a combination of cytological and cystoscopic
examination of the urothelium is considered to be the gold standard
for the detection of urothelial neoplasia (9). Additionally, cytogenetic evaluations
via fluorescence in situ hybridization (FISH) assays have
revealed that numerical chromosomal aberrations that occur during
the pathogenesis of BC can be used to assess gains or losses in
centromere number (10-13).
Genetic aberration serves a crucial role in
tumorigenesis (14,15). Using a whole-genome fine-tiling
oligonucleotide array-based comparative genomic hybridization (CGH)
assay, a previous study reported that gains in centromere numbers
of chromosomes 5p, 7q and 19q, in addition to losses in the
centromeres of chromosomes 4q, 9p and 15q, were common among
patients with upper-tract urothelial carcinoma (UTUC) in end-stage
renal disease (ESRD) (16).
Notably, high copy number variants were also observed in high-stage
and -grade tumors of UTUC from these findings (16). In another study, Yamamoto et
al (17) reported that the gain
of chromosomal region 5p15.33, which includes the tubulin
polymerization promoting protein (TPPP) gene, was associated
with the progression of BC. Consistent with this finding, a high
genomic copy number of TPPP was also detected in the early stages
of non-small-cell lung cancer through a high-resolution array CGH
assay (18).
TPPP, also known as p25, was first identified as a
brain-specific protein that is primarily expressed in
oligodendrocytes and the neuropil (19). TPPP has been previously reported to
induce the formation of aberrant microtubule assemblies and was
considered to be a novel marker for Parkinson's disease and
α-synucleinopathy (20). TPPP is a
microtubule-associated protein (MAP) in vertebrates that
polymerizes tubulin into normal or aberrant microtubules, depending
on its concentration and phosphorylation state (21-23).
It also serves as a scaffolding protein to deliver tubulin
heterodimers to nascent protofilaments or block the formation of
mitotic spindles (24,25). Amplification of TPPP may confer a
growth advantage to urothelial cells resulting from abnormalities
in tubulin assembly and spindle formation (17). By contrast, the expression of TPPP
mRNA was reported to be significantly lower in hepatitis C
virus-positive hepatocellular carcinoma tissues compared with that
in adjacent non-cancerous tissues from patients with secondary
metastatic liver malignancies, where low TPPP mRNA expression
indicated a poor prognosis (26).
The present study investigated whether TPPP may
represent a potential target for the evaluation of BC
clinicopathology, serve as an index of risk assessment in patients
with BC and explored the possible in vitro role of TPPP in
cell proliferation, cell migration and invasion in BC using TPPP
RNA interference.
Materials and methods
Tissue specimens
The frozen tissue samples were obtained from 52
patients with urothelial BC (sex, 44 men and 8 women; average age,
65.24±13.35 years; age range, 37-84 years) who received BC
diagnoses at Chang Gung Memorial Hospital, Taiwan, between April
2004 and April 2015. The tissue specimens were frozen at -80˚C
until use. Patients received transurethral resection of bladder
tumor (TUR-BT) and were staged with either CT scan or MRI imaging
study. All patients were diagnosed with urothelial carcinoma. All
tissue samples were retrieved from Biobank and Tissue Bank Core Lab
at Chang Gung Memorial Hospital. Written informed consent was
obtained from all participants before surgical samples were
deposited to Biobank and Tissue Bank Core Lab of Chang Gung
Memorial Hospital. The study was approved by the Human Subject
Research Ethics Committee/Institutional Review Board at Chang Gung
Memorial Hospital at Linkou (approval no. IRB No.103-1999C;
Taoyuan, Taiwan). Each case of urothelial BC was evaluated by
examining the data in the medical records, including tumor size,
pathological type, histological staging and grading, from the
Department of Pathology at Chang Gung Memorial Hospital at Linkou.
The pathological staging and grading were assigned according to the
American Joint Committee on Cancer Cancer Staging Manual (8th
edition) Clinical Practice Guidelines in Bladder Cancer (2017)
(27). Patients who were diagnosed
with urothelial BC and first received TUR-BT were included in this
study. Patients who had known infections or other concurrent severe
and/or uncontrolled medical diseases in this study were excluded.
Pregnant or breastfeeding women were also excluded.
Cell culture
Four human BC epithelial cell lines, namely MGH-U1,
MGH-U1R, MGH-U3, and MGH-U4, were provided by Dr Chi-Wei Lin
(Massachusetts General Hospital, Boston, MA, USA). The
characteristics of these four cell lines were reported in detail in
a previous study (28). Briefly,
MGH-U1 is the subline of T24(29),
which was established from a grade 3, stage B bladder tumor.
MGH-U1R is a doxorubicin-resistant cell line that was derived from
the MGH-U1(30). The MGH-U3 cell
line was established from a grade 1, stage A bladder tumor whereas
the MGH-U4 cell line was established from a stage 0 bladder tumor
with atypia (31). STR analysis was
performed to demonstrate that the MGH-U1 was a subline of T24 (data
not shown). The cells were cultured in RPMI 1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 units/ml
penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.), and 2 mM L-glutamine (Gibco; Thermo Fisher
Scientific, Inc.). SV-HUC-1 cells, a normal human uroepithelial
cell line and MC-SV-HUC T-2, a tumorigenic human urothelial cell
line, were purchased from the American Type Culture Collection and
cultured in Ham's F12 medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 7% FBS, 100 units/ml penicillin and 100 µg/ml
streptomycin and 2 mM L-glutamine. All cell lines were maintained
at 37˚C in a humidified atmosphere with 5% CO2.
FISH assay
TPPP gene amplification was detected using
dual-color FISH analysis as reported previously (32). For touch imprint cytology smears, a
single 1.0x1.0x0.3 cm fresh tumor sample was cut and the surface
touched against uncharged slides to cover at least 60% of the slide
surface (33). Fresh slides were
aged at room temperature for at least 24 h. BC cells or touch
imprint cytology smears from the bladder tumor samples were then
fixed with fixative solution (methanol: Acetic acid=3:1 v/v) at
room temperature for 30 min. The fixed samples were preserved at
-20˚C for subsequent examination.
For dual-color probe preparation, information in
publicly available databases (https://asia.ensembl.org/index.html), and the BAC
clone for the short arm of chromosome 5 from the Roswell Park
Cancer Institute (RPCI) library RP11 (clone RP11-837K1, chr
5:640447-820395 (180 kb); Invitrogen; Thermo Fisher Scientific,
Inc.), which contained the TPPP gene, was selected in this study.
In preparing DNA probe by nick-translation, the BACs were isolated
using Presto™ Mini Plasmid Kit (cat. no. PDH300; Geneaid Biotech
Ltd.) and labeled with a BIO-PROBE Nick translation DNA labeling
system (cat. no. ENZ-42710; Enzo Life Sciences Inc.), dNTPs (10x A4
dNTP mix; Roche Diagnostics GmBH) and Green-dUTP fluorescent dye
(cat. no. 02N32-050; Abbott Molecular Inc.) at 15˚C for 45 min.
After nick translation, the DNase I was inactivated by heating at
75˚C for 15 min. The probes were then precipitated with 0.3 M
sodium acetate and 70% ethanol at -20˚C for 40 min. The probes were
dissolved in the hybridization solution [50% formamide, 2x SSC (3 M
NaCl, 0.3 M sodium citrate, pH 7), 10% dextran sulfate]. To perform
DNA-FISH, fixed samples were treated with denaturation buffer (70%
formamide, 2x SSC, pH 7) at 58˚C for 1 min, before samples were
washed in PBS and dehydrated through an ethanol gradient (70, 85
and 100% for 2 min each). Samples were heated at 75˚C for 5 min to
separate DNA strands before 12.5 ng/µl of probes in hybridization
buffer were applied and incubated at 37˚C overnight. After
hybridization, the samples were washed in wash buffer I (0.4x SSC,
0.3% Tween-20; 75˚C) and wash buffer II (2x SSC, 0.1% Tween-20;
room temperature) for 2 min each three times and with 1 µg/ml DAPI
at room temperature for 30 min (Sigma-Aldrich; Merck KGaA) for
nuclear staining. In addition, the accuracy and specificity of the
probe were confirmed through hybridization onto commercially
available CGH Metaphase Target Slides (cat. no. 06J96-001; Abbott
Pharmaceutical Co. Ltd.). The hybridization conditions were the
same as for the samples. The results indicated that the probe was
annealed with DNA sequences at 5p15.33 (data not shown). The
fluorescent images were recorded at x630 magnification using a
fluorescence microscope (Leica DM2500; Leica Microsystems GmbH) and
analyzed using FISHView EXPO version 5.5 software (Applied Spectral
Imaging, Ltd.). According to the definition of Yamamoto et
al (17), the criterion for
TPPP gain was defined as a mean copy number of TPPP per nucleus of
>2.2 (cutoff).
Neutrophil-to-lymphocyte ratio
determination
Before TUR-BT, lab experiments including hemogram
and biochemistry exam were performed as part of routine clinical
examination. Neutrophil count, lymphocyte count, red cell
distribution width and mean platelet volume were obtained for each
patient. The neutrophil-to-lymphocyte ratio (NLR) was calculated by
dividing the absolute neutrophil count by the absolute lymphocyte
count. The optimum cutoff value for the NLR was determined to be
≥2.43 after receiver operating characteristic analysis, following a
previously described method (34).
Knockdown of TPPP expression
ON-TARGETplus SMARTpool siRNA of TPPP was
synthesized by Dharmacon (cat. no. L-019695-01-0010; GE Healthcare
Dharmacon, Inc.), where the following four siRNA sequences were
designed: 5'-CCACCGGAAUCACCCGAUA-3', 5'-GGUUGGUGCCCACGAGUUA-3',
5'-CAAAGUGUCUCGCGGAUCA-3' and 5'-GACAAGCAGUCAUCGGAAU-3'. The siRNA
scramble control was synthesized by Biotools Co., Ltd with the
siRNA sequence 5'-UUCUCCGAACGUGUCACGUTT-3'. MGH-U1, MGH-U1R and
MGH-U4 cells lines were transfected with either 30 pmol TPPP siRNAs
or scramble control in medium without antibiotics using the
Lipofectamine® RNAiMAX reagent (Thermo Fisher
Scientific, Inc.) combined, according to the manufacturer's
protocol. After transfection, the cells were used to the further
experiments, such as cell proliferation, in vitro cell
migration and western blotting in the indicated time point.
Protein extraction and western
blotting analysis
The TPPP siRNA (0, 24, 48, 72, 96 h) treated BC
cells (MGH-U1, -U1R, -U4) were lysed in ice-cold modified RIPA
(mRIPA) lysis buffer (50 mM Tris-Cl, pH 7.8; 150 mM NaCl, 5 mM
EDTA, 0.5% Triton X-100 and 0.5% NP-40; 0.1 ml per 106
cells, respectively) on ice for 20 min and 4x sample buffer (cat.
no. 1610747; Bio-Rad Laboratories, Inc.) at 95˚C for 10 min. For
western blotting, 20 µl of cell lysates were separated by 12%
SDS-PAGE and transferred onto a polyvinylidene fluoride membrane
(PVDF) followed by blocking in Tris-buffered saline with 0.05%
Tween-20 (TBST) containing 5% non-fat milk for 1 h, immunoblotting
in primary antibody for 2 h and secondary antibody for 1 h at room
temperature. Blots were visualized using a Immobilon™ Western
Chemiluminescent HRP Substrate (cat. no. WBKLS0500; Merck
Millipore) and analyzed by UVP ChemStudio Plus with software
VisionWorks LS 8.22 (Analytic Jena AG). The primary antibodies used
were anti-TPPP (1:2,000; cat. no. ab92305; Abcam) and
anti-α-tubulin (1:10,000; cat. no. MS-581; Thermo Fisher
Scientific, Inc.). The secondary antibodies were horseradish
peroxidase (HRP) conjugated goat anti-rabbit IgG (1:5,000; cat. no.
AP132P; Chemicon International, Inc.) and HRP conjugated goat
anti-mouse IgG (1:5,000; cat. no. 31430; Thermo Fisher Scientific,
Inc.).
Cell viability measurement
BC cell viability was measured using a Cell Counting
Kit-8 (CCK-8) cell viability assay (Sigma-Aldrich; Merck KGaA)
according to the manufacturer's protocol. Briefly, the cells were
seeded into 96-well plates at a density of 5x103
cells/well. After seeding for 24 h, the cells were transfected with
TPPP siRNAs for 0, 24, 48, 72, and 96 h and incubated at 37˚C in a
humidified atmosphere with 5% CO2. Subsequently, the
cells were incubated with CCK-8 solution (10 µl/well) for 1 h at
37˚C before the absorbance value at 450 nm was measured in each
well using a microplate reader.
In vitro migration assay
BC cell (MGH-U1, -U1R, -U4) migration was assessed
using Transwell assays (24-wells; pore size, 8-µm; Corning, Inc.).
After knockdown of TPPP expression for 48 h, the trypsinized cells
were first suspended in RPMI 1640 medium containing 1% FBS at a
density of 1x105 cells before being added to the upper
chamber of the well. Subsequently, RPMI 1640 medium with 10% FBS
was added to the lower chamber. After incubation for 6 h at 37˚C,
cells that migrated to the lower surface of Transwell were stained
with Giemsa stain solution (Sigma-Aldrich; Merck KGaA) for 1 h at
room temperature, imaged under a light microscope (magnification,
x200) and counted from nine randomly selected fields per well.
Statistical analysis
Statistical analyses were performed using the SPSS
statistical software package (version 22.0; IBM Corp.). A
contingency table was generated for Fisher's exact probability
test. In the in vitro cell assays, data were expressed as
the mean ± standard deviation from three independent measurements
and compared using two-tailed unpaired Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
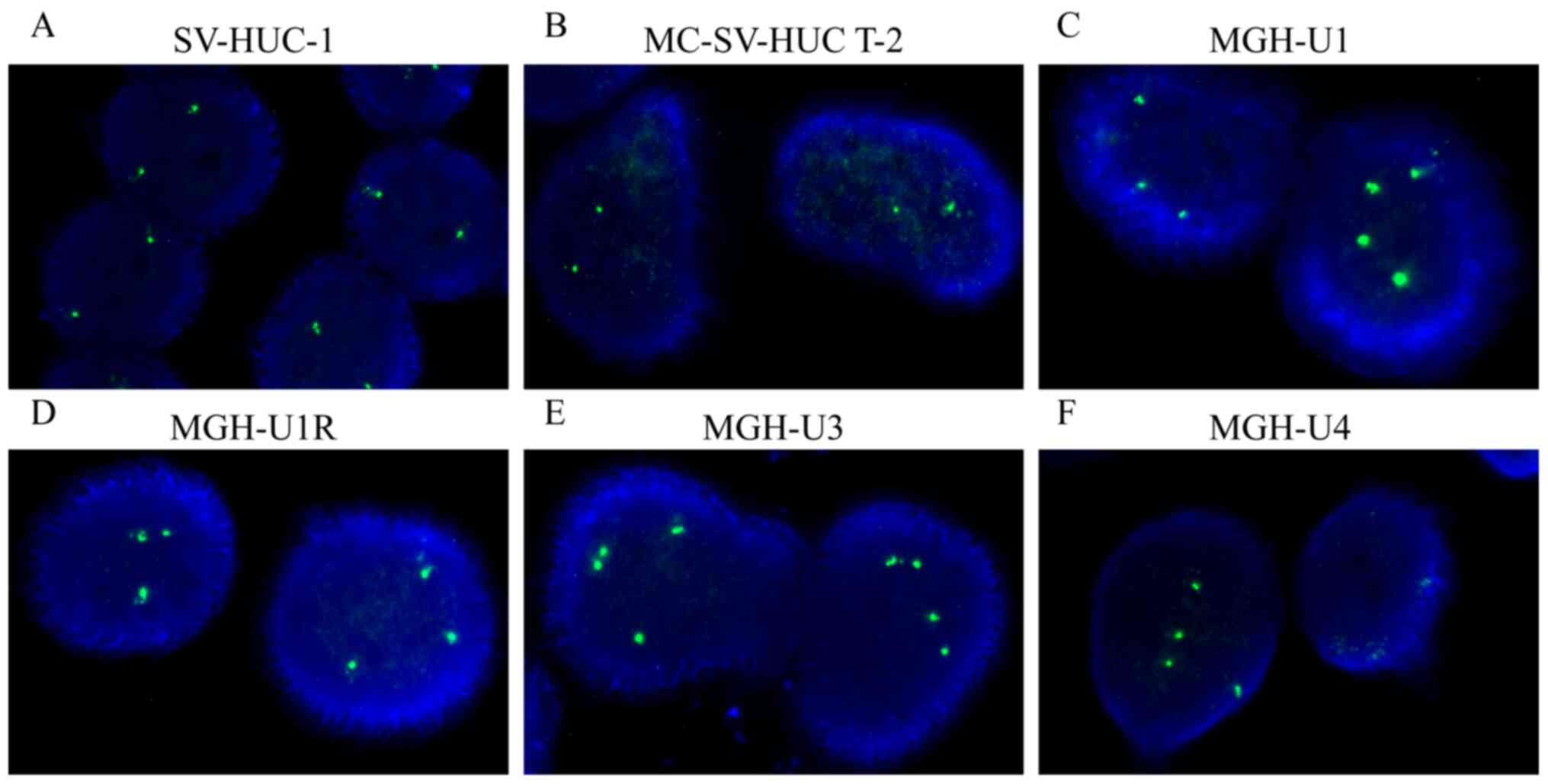
Gain of TPPP copy number in BC
According to the criteria of TPPP gain from a
previous definition (17), the copy
number of TPPP variants was found to be amplified in the four BC
cell lines tested, namely MGH-U1 (primarily three or four copies;
Fig. 1C), MGH-U1R (three copies;
Fig. 1D), MGH-U3 (three copies;
Fig. 1E) and MGH-U4 (four copies;
Fig. 1F) cells. However, TPPP
downregulation occurred in the SV-HUC-1 (two copies; Fig. 1A) and MC-SV-HUC T-2 (two copies;
Fig. 1B) cell lines. Following
quantification, the percentage of cells carrying ≥2.2 copies of
TPPP of the total number of cells counted ranged from 86.0-100.0%
for the four BC cell lines, whilst the percentages of SV-HUC-1 and
MC-SV-HUC T-2 cells exhibiting TPPP amplification were found to be
only 9.0 and 3.0%, respectively (Table
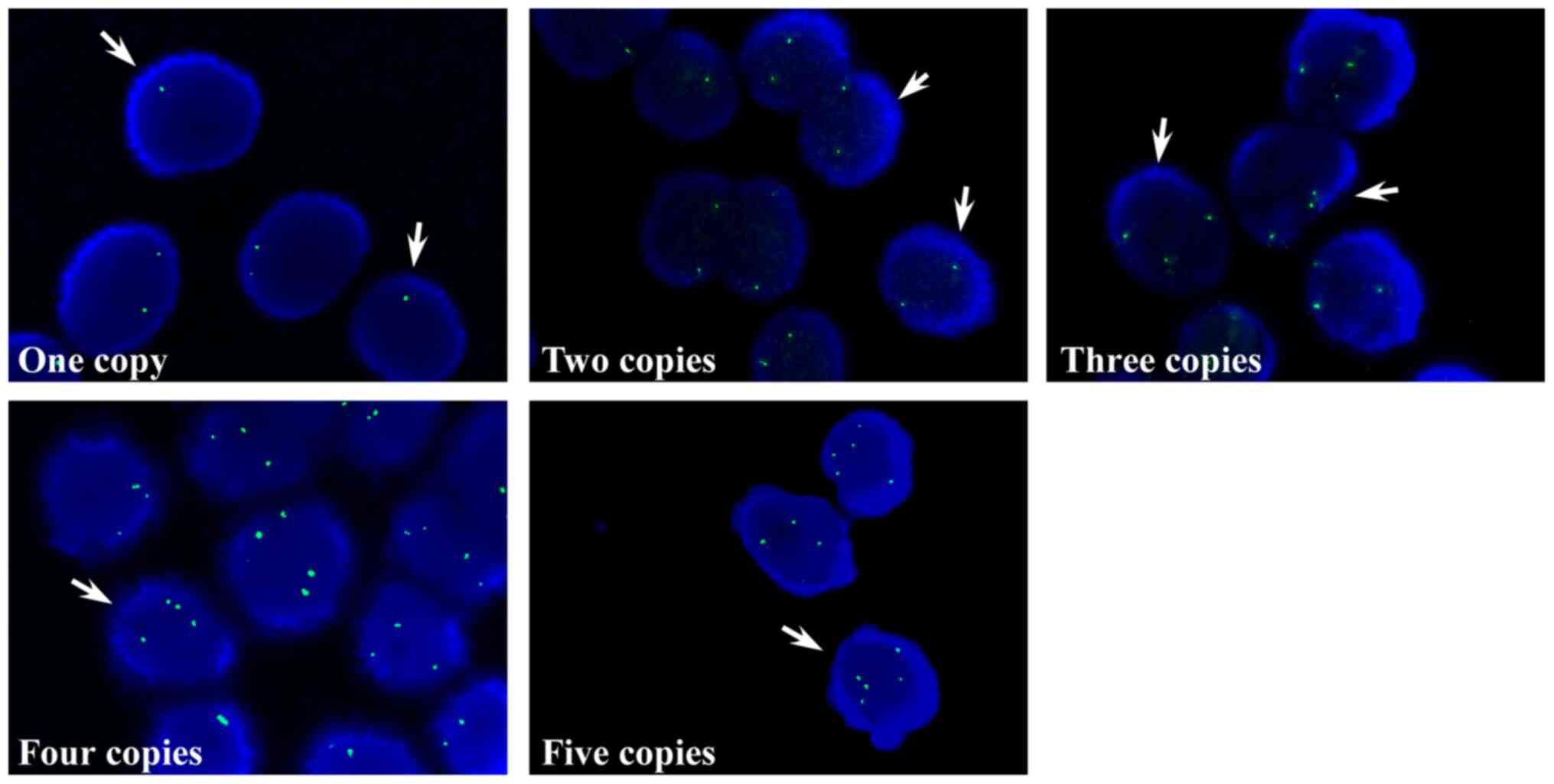
I). Subsequently, the copy numbers of TPPP in the tissue
samples obtained from patients with BC tissues were examined via
touch imprint cytology smears. Consistent with the data of the BC
cell lines, the TPPP copy numbers in the tissue samples exhibited
either one, two and ≥ three spots (Fig.
2). The percentages of patients identified with the respective
TPPP copy numbers were as follows: i) 1.9% with one copy; ii) 55.8%
with two copies; iii) 7.7% with three copies; iv) 26.9% with four
copies; and v) 7.7% with five copies (Table II). FISH results demonstrated that
copy number amplification of TPPP in four human BC cell lines were
higher than in the two normal human uroepithelial cell lines.
 | Table IGenetic copy number data of TPPP in
normal human uroepithelial and bladder cancer cell lines. |
Table I
Genetic copy number data of TPPP in
normal human uroepithelial and bladder cancer cell lines.
| | Percentage of the
alterations in TPPP copy number |
|---|
| Cell lines | Cell number
count | Normal (%) | One copy deletion
(%) | Amplification
(%) |
|---|
| SV-HUC-1 | 432 | 89.0 | 2.0 | 9.0 |
| MC-SV-HUC T-2 | 379 | 96.0 | 1.0 | 3.0 |
| MGH-U1 | 372 | 11.0 | 3.0 | 86.0 |
| MGH-U1R | 438 | 5.0 | 0.2 | 94.8 |
| MGH-U3 | 386 | 0 | 0 | 100.0 |
| MGH-U4 | 452 | 8.0 | 1.0 | 91.0 |
 | Table IIStatistics of the 52 patients with
bladder with various copy numbers of tubulin polymerization
promoting protein. |
Table II
Statistics of the 52 patients with
bladder with various copy numbers of tubulin polymerization
promoting protein.
| | Amplification
(copy) |
|---|
| Copy no. | Normal | One copy
deletion | 3 | 4 | 5 |
|---|
| Patients, n
(%) | 29 (55.8%) | 1 (1.9%) | 4 (7.7%) | 14 (26.9%) | 4 (7.7%) |
Association between the gain of TPPP
and clinicopathological characteristics of patients with BC
Analysis of BC samples with TPPP gain, defined as
the number of TPPP per nucleus >2.2, indicated that TPPP gain
was associated significantly with age (≥60 years; P<0.05),
advanced histological grade (P<0.001) and tumor stage
(P<0.05), histological subtype (papillary vs. non-papillary
urothelial carcinoma; P<0.001) and NLR (P<0.05). However, no
association was revealed between TPPP gain and sex or tumor size
(Table III). These results
suggested that the copy number amplification of TPPP may be
associated with BC progression. FISH results indicated that 34.6%
BC patients had >4 copies of the TPPP gene.
 | Table IIIAssociation between the gain of TPPP
and clinicopathological characteristics of patients with bladder
cancer. |
Table III
Association between the gain of TPPP
and clinicopathological characteristics of patients with bladder
cancer.
| | TPPP
gaina | |
|---|
| Category | - | + |
P-valueb |
|---|
| Sex | | | |
|
Male
(n=44) | 27 | 17 | 0.260 |
|
Female
(n=8) | 3 | 5 | |
| Age | | | |
|
≥60 years
(n=38) | 18 | 20 | <0.050 |
|
<60 years
(n=14) | 12 | 2 | |
| Tumor grade | | | |
|
High grade
(n=33) | 11 | 22 | <0.001 |
|
Low grade
(n=19) | 19 | 0 | |
| Tumor stage | | | |
|
0a-I
(n=31) | 22 | 9 | <0.050 |
|
II-IV
(n=21) | 8 | 13 | |
| Tumor
sizec | | | |
|
≥4 cm
(n=14) | 8 | 6 | 1 |
|
<4 cm
(n=31) | 17 | 14 | |
| Histological
subtype | | | |
|
Papillary
urothelial carcinoma (n=22) | 19 | 3 | <0.001 |
|
Non-papillary
urothelial carcinoma (n=30) | 11 | 19 | |
|
Neutrophil-to-lymphocyte
ratioc | | | |
|
≥2.43
(n=26) | 11 | 15 | <0.05 |
|
<2.43
(n=23) | 17 | 6 | |
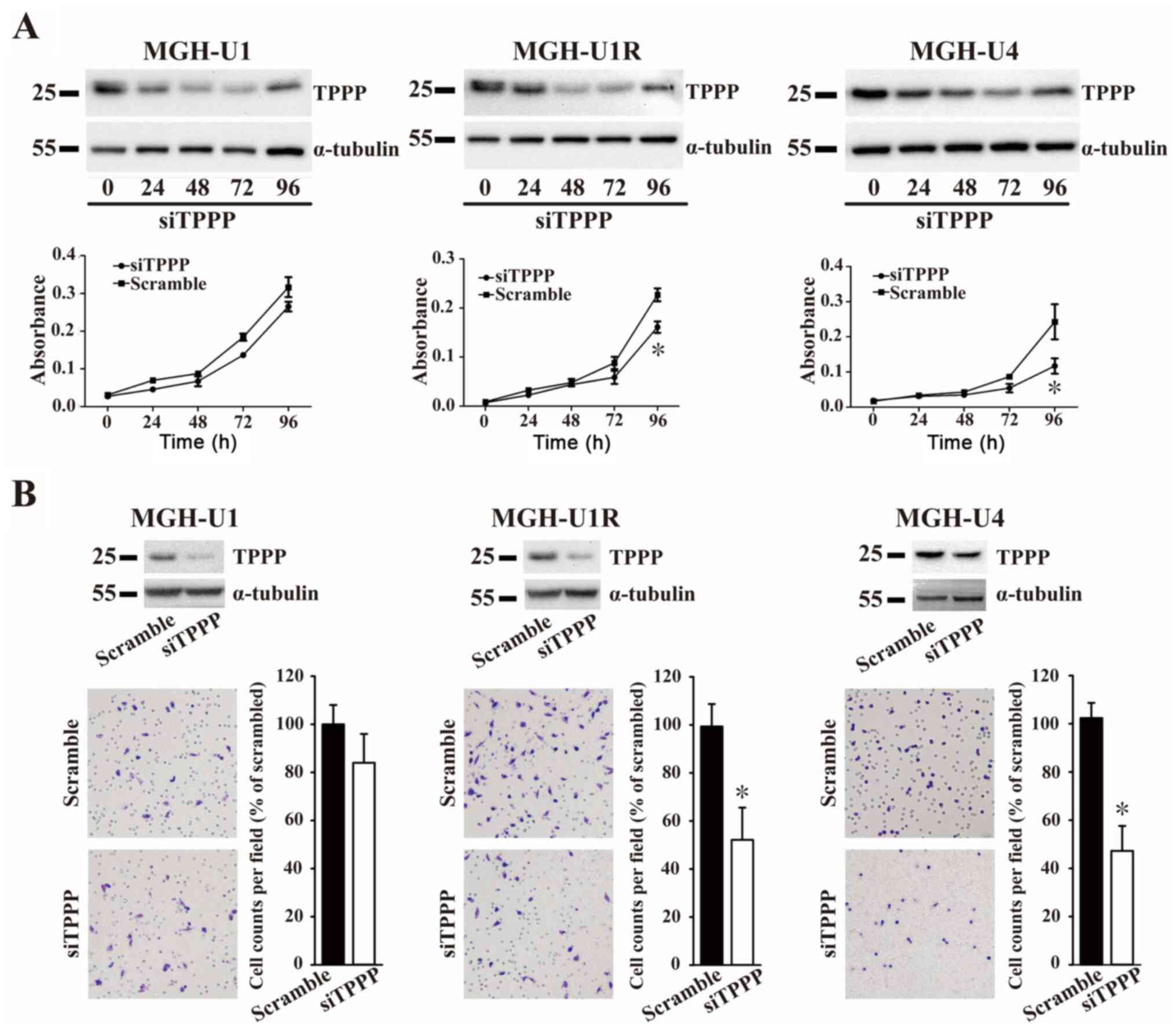
Knockdown of TPPP reduces cell
proliferation and suppresses migration in MGH-U1R and MGH-U4
cells
The effects of TPPP on cell viability and migration
were next determined using CCK-8 and Transwell migration assays.
The expression of TPPP in the MGH-U1, MGH-U1R, and MGH-U4 cells was
measured after siTPPP transfection (Fig. 3A). The results showed that the
expression of TPPP was reduced by TPPP siRNA compared with a
scrambled sequence at 24, 48 and 72 h, while the expression of TPPP
was recovered at 96 h. At 96 h, the growth rates of the MGH-U1R and
MGH-U4 cells after TPPP knockdown were significantly lower compared
with those in the scramble control groups. However, the growth
rates of the MGH-U1 cells did not differ between those transfected
with siTPPP or scrambled control. The role of TPPP in BC cell
migration was subsequently examined. The degree of cell migration
of cells following TPPP knockdown was revealed to be significantly
lower compared with that in the scramble control groups in the
MGH-U1R and MGH-U4 cell lines 72 h after siTPPP transfection
(Fig. 3B). The results of the
present study suggested that TPPP knockdown reduced the levels of
cell proliferation and migration in BC cell lines.
Discussion
A multicolor FISH-based urine assay has been
developed for BC screening in clinical practice, which has been
documented to confer higher sensitivity compared with urine
cytology (35). Previous studies
have revealed that copy number alterations detected via FISH assays
can aid in the identification and localization of genes associated
with cancer (32,36,37).
Initially, the present study demonstrated that the occurrence of
TPPP copy number amplification in the four human BC epithelial cell
lines was higher compared with that in the two normal human
uroepithelial cell lines tested. In addition, the occurrence of
copy number amplification of TPPP was also demonstrated to be
associated with age, histological type, NLR, advanced histological
grade and pathological stage of the patients with BC. FISH analysis
subsequently revealed that tissue samples obtained from 34.6% of
the patients with BC contained ≥ four copies of the TPPP gene.
The present study confirmed that the copy number of
TPPP was higher in BC cells compared with that in normal
uroepithelial cells. Accumulating evidence has highlighted the role
of inflammation as a critical component in processes associated
with tumor progression, including angiogenesis, cell proliferation
and metastasis (38,39). Neutrophil and lymphocyte counts
serve crucial roles in systemic inflammation. The neutrophil count
increases in response to tumor growth and proangiogenic factors,
regulated by the upregulation of mediators such as NF-κB and
vascular endothelial growth factor (40-42).
Therefore, a high NLR has been considered to be a potential
prognostic factor in various cancer types, including pancreatic
cancer, gastric cancer and BC (34,42,43).
Previous studies have indicated that preoperative NLR served a
vital role in predicting the recurrence of high-grade bladder
tumors consisting of the superficial transitional cell type and in
distinguishing between MIBC and NMIBC (34,37).
The present study indicated that gain of TPPP copy numbers is
associated with higher NLR in patients with BC. Therefore, the
potential interaction between TPPP and NLR in the pathogenesis of
BC warrants further future study.
Transitional cell carcinomas (TCCs) represent
>90% of all BCs, where most BCs exhibit papillary features,
particularly in the noninvasive types (44). Polesel et al (45) previously reported that 57.7%
non-papillary TCCs were muscle invasive, whilst 85.4% papillary
TCCs were low-grade superficial tumors of BC. The results of the
present study indicated that 62% non-papillary TCCs were muscle
invasive, while 91% papillary TCCs were low-grade tumors. Results
from the present study were similar to those reported by Polesel
et al Low-grade papillary urothelial carcinoma is
characterized by an orderly overall appearance, minimal variability
in the cellular architecture and the lack of significant atypia in
terms of cytological and mitotic activity (44). Additionally, the present study
revealed that the gain of TPPP copy numbers was strongly associated
with non-papillary urothelial carcinomas, suggesting that
amplification of TPPP promoted the aberrance of tubulin assemblies
in BCs.
Chromosomal locus 5p15.33 amplification has been
previously reported to be a predictor for disease progression in BC
(17). Yamamoto et al
(17) examined the number of
aberrations in the 5p15.33 locus via CGH array and FISH, but did
not directly focus on the evaluation of TPPP genetic variants
(17). Notably, the present study
demonstrated that in patients with BC, the gain of TPPP occurrence
associated strongly with histological type, though this association
was not observed in the report by Yamamoto et al (17) Furthermore, another previous study
also revealed that gains at the chromosome loci 5p, 7 and 19q and
losses at loci 4q, 9p and 15q were prevalent in UTUC samples of
patients with ESRD (16).
Therefore, genetic alterations are likely to serve an important
role in the development of urothelial carcinoma from the
observations of the present study.
Metastasis is the cause of the majority of
mortalities associated with cancer (46). Microtubules regulate cell shape
maintenance, cell migration and cell division via the activity of
MAPs (47). In addition, agents
targeting the microtubule system have been identified to confer
among the most effective anticancer effects, which have also been
recognized to exhibit potent anti-mitotic and anti-proliferative
properties (48). As TPPP is a MAP,
the present study demonstrated that knockdown of TPPP attenuated
cell migration and proliferation in MGH-U1R and MGH-U4 cells, which
may have resulted from disrupted microtubule dynamics. However,
previous studies have reported that TPPP overexpression also
reduced cell proliferation and migration in U2SO osteosarcoma cells
(49,50). Downregulation of LIM-kinase 2 has
been reported to sensitize neuroblastoma cell lines BE(2)-C and SHEP, to chemotherapeutic drugs,
enhancing mitotic arrest and cell apoptosis via reducing TPPP and
acetylated-tubulin expression levels (51). Chen et al (52) demonstrated that TPPP promoted the
migration, invasion and angiogenesis via the p38/MAPK and PI3K/AKT
signaling pathways in pancreatic cancer. Although results from the
present study also supported these aforementioned findings (data
not shown), the underlying mechanisms of TPPP in the regulation of
BC metastasis require further exploration.
In summary, the present study highlighted the
crucial role of TPPP in cell viability, cell migration and BC
progression, suggesting TPPP to be a novel therapeutic target for
BC treatment. In addition, it emphasized the possibility of
expanding preoperative urinary cytology, the current gold standard,
to the clinical examination of TPPP copy numbers for the detection
of urothelial neoplasia.
Acknowledgements
The authors thank Dr Chi-Wei Lin (Massachusetts
General Hospital, Boston MA, USA) for providing the four human
bladder cancer cell lines, MGH-U1, -U1R, -U3 and -U4.
Funding
Funding: The present study was supported by grants from The
Chang Gung Memorial Hospital Research Foundation (grant nos.
CMRPG3D1101-03, CMRPG3H0551 and the Ministry of Science and
Technology (MOST 107-2314-B-182A-018-MY3).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YHC and STP provided the initial concept and
arranged the study design. PHL and CCC executed the major
experiments and wrote the majority of the manuscript. WHW made
substantial intellectual contributions to the study design and
technical support. KJY, CYL and CKC performed clinical specimen
collection and clinicopathological analysis. CHH performed western
blot assay. THC, IHS and HCK performed data analysis and figure
preparation. CKC and STP edited the final manuscript. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Human Subject
Research Ethics Committee/Institutional Review Board at Chang Gung
Memorial Hospital at Linkou (approval no. IRB No.103-1999C;
Taoyuan, Taiwan). All tissue samples were retrieved from Biobank
and Tissue Bank Core Lab at Chang Gung Memorial Hospital. Written
informed consent was obtained from all participants before surgical
samples were deposited to Biobank and Tissue Bank Core Lab of Chang
Gung Memorial Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Polo A, Crispo A, Cerino P, Falzone L,
Candido S, Giudice A, De Petro G, Ciliberto G, Montella M, Budillon
A and Costantini S: Environment and bladder cancer: Molecular
analysis by interaction networks. Oncotarget. 8:65240–65252.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Goebell PJ and Knowles MA: Bladder cancer
or bladder cancers? Genetically distinct malignant conditions of
the urothelium. Urol Oncol. 28:409–428. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sanguedolce F, Bufo P, Carrieri G and
Cormio L: Predictive markers in bladder cancer: Do we have
molecular markers ready for clinical use? Crit Rev Clin Lab Sci.
51:291–304. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hernández S, López-Knowles E, Lloreta J,
Kogevinas M, Amorós A, Tardón A, Carrato A, Serra C, Malats N and
Real FX: Prospective study of FGFR3 mutations as a prognostic
factor in nonmuscle invasive urothelial bladder carcinomas. J Clin
Oncol. 24:3664–3671. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lamy A, Gobet F, Laurent M, Blanchard F,
Varin C, Moulin C, Andreou A, Frebourg T and Pfister C: Molecular
profiling of bladder tumors based on the detection of FGFR3 and
TP53 mutations. J Urol. 176:2686–2689. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lopez-Knowles E, Hernández S, Kogevinas M,
Lloreta J, Amorós A, Tardón A, Carrato A, Kishore S, Serra C,
Malats N, et al: The p53 pathway and outcome among patients with
T1G3 bladder tumors. Clin Cancer Res. 12:6029–6036. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dominguez G, Carballido J, Silva J, Silva
JM, García JM, Menéndez J, Provencio M, España P and Bonilla F:
p14ARF promoter hypermethylation in plasma DNA as an indicator of
disease recurrence in bladder cancer patients. Clin Cancer Res.
8:980–985. 2002.PubMed/NCBI
|
|
9
|
Brown FM: Urine cytology. It is still the
gold standard for screening? Urol Clin North Am. 27:25–37.
2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chuang KL, Chuang HC, Ng KF, Chang YH, Wu
CT, Chuang CK, Liao SK and Pang ST: Urinary fluorescence in situ
hybridization assay for detecting urothelial carcinoma in Taiwanese
patients. BJU Int. 105:1413–1416. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mischinger J, Guttenberg LP, Hennenlotter
J, Gakis G, Aufderklamm S, Rausch S, Neumann E, Bedke J, Kruck S,
Schwentner C, et al: Comparison of different concepts for
interpretation of chromosomal aberrations in urothelial cells
detected by fluorescence in situ hybridization. J Cancer Res Clin
Oncol. 143:677–685. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Molitor M, Junker K, Eltze E, Toma M,
Denzinger S, Siegert S, Knuechel R and Gaisa NT: Comparison of
structural genetics of non-schistosoma-associated squamous cell
carcinoma of the urinary bladder. Int J Clin Exp Pathol.
8:8143–8158. 2015.PubMed/NCBI
|
|
13
|
Moktefi A, Pouessel D, Liu J, Sirab N,
Maille P, Soyeux P, Bergman CC, Auriault ML, Vordos D, Taille A, et
al: Reappraisal of HER2 status in the spectrum of advanced
urothelial carcinoma: A need of guidelines for treatment
eligibility. Mod Pathol. 31:1270–1281. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lengauer C, Kinzler KW and Vogelstein B:
Genetic instabilities in human cancers. Nature. 396:643–649.
1998.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wu CF, Pang ST, Shee JJ, Chang PL, Chuang
CK, Chen CS, Liao SK and Weng WH: Identification of genetic
alterations in upper urinary tract urothelial carcinoma in
end-stage renal disease patients. Genes Chromosomes Cancer.
49:928–934. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamamoto Y, Chochi Y, Matsuyama H, Eguchi
S, Kawauchi S, Furuya T, Oga A, Kang JJ, Naito K and Sasaki K: Gain
of 5p15.33 is associated with progression of bladder cancer.
Oncology. 72:132–138. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kang JU, Koo SH, Kwon KC, Park JW and Kim
JM: Gain at chromosomal region 5p15.33, containing TERT, is the
most frequent genetic event in early stages of non-small cell lung
cancer. Cancer Genet Cytogenetics. 182:1–11. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Takahashi M, Tomizawa K, Fujita SC, Sato
K, Uchida T and Imahori K: A brain-specific protein p25 is
localized and associated with oligodendrocytes, neuropil, and
fiber-like structures of the CA3 hippocampal region in the rat
brain. J Neurochem. 60:228–235. 1993.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kovács GG, László L, Kovács J, Jensen PH,
Lindersson E, Botond G, Molnár T, Perczel A, Hudecz F, Mezo G, et
al: Natively unfolded tubulin polymerization promoting protein
TPPP/p25 is a common marker of alpha-synucleinopathies. Neurobiol
Dis. 17:155–162. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hlavanda E, Kovacs J, Olah J, Orosz F,
Medzihradszky KF and Ovadi J: Brain-specific p25 protein binds to
tubulin and microtubules and induces aberrant microtubule
assemblies at substoichiometric concentrations. Biochemistry.
41:8657–8664. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Acevedo K, Li R, Soo P, Suryadinata R,
Sarcevic B, Valova VA, Graham ME, Robinson PJ and Bernard O: The
phosphorylation of p25/TPPP by LIM kinase 1 inhibits its ability to
assemble microtubules. Exp Cell Res. 313:4091–4106. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hlavanda E, Klement E, Kókai E, Kovács J,
Vincze O, Tökési N, Orosz F, Medzihradszky KF, Dombrádi V and Ovádi
J: Phosphorylation blocks the activity of tubulin
polymerization-promoting protein (TPPP): Identification of sites
targeted by different kinases. J Biol Chem. 282:29531–29539.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tirian L, Hlavanda E, Oláh J, Horváth I,
Orosz F, Szabó B, Kovács J, Szabad J and Ovádi J: TPPP/p25 promotes
tubulin assemblies and blocks mitotic spindle formation. Proc Natl
Acad Sci USA. 100:13976–13981. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zotter A, Bodor A, Olah J, Hlavanda E,
Orosz F, Perczel A and Ovádi J: Disordered TPPP/p25 binds GTP and
displays Mg2+-dependent GTPase activity. FEBS Lett. 585:803–808.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Inokawa Y, Sonohara F, Kanda M, Hayashi M,
Nishikawa Y, Sugimoto H, Kodera Y and Nomoto S: Correlation between
poor prognosis and lower TPPP gene expression in hepatocellular
carcinoma. Anticancer Res. 36:4639–4645. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Edge SB and Edge SB: AJCC Cancer Staging
Manual, 8th edition. Springer, 2017.
|
|
28
|
Chuang CK, Pang ST, Chuang TJ and Liao SK:
Profiling of matrix metalloproteinases and tissue inhibitors of
metalloproteinases proteins in bladder urothelial carcinoma. Oncol
Lett. 1:691–695. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kato T, Ishikawa K and Nemoto R:
Morphologic characterization of two established cell lines, T24 and
MGH-U1, derived from human bladder carcinoma (author's transl).
Nihon Hinyokika Gakkai Zasshi. 69(40)1978.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
30
|
Floyd JW, Lin CW and Prout GR Jr:
Multi-drug resistance of a doxorubicin-resistant bladder cancer
cell line. J Urol. 144:169–171. 1990.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lin CW, Lin JC and Prout GR: Establishment
and characterization of four human bladder tumor cell lines and
sublines with different degrees of malignancy. Cancer Res.
45:5070–5079. 1985.PubMed/NCBI
|
|
32
|
Weng WH, Chen YT, Yu KJ, Chang YH, Chuang
CK and Pang ST: Genetic alterations of HER genes in chromophobe
renal cell carcinoma. Oncol Lett. 11:2111–2116. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dogan S, Becker JC, Rekhtman N, Tang LH,
Nafa K, Ladanyi M and Klimstra DS: Use of touch imprint cytology as
a simple method to enrich tumor cells for molecular analysis.
Cancer Cytopathol. 121:354–360. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ozyalvacli ME, Ozyalvacli G, Kocaaslan R,
Cecen K, Uyeturk U, Kemahlı E and Gucuk A: Neutrophil-lymphocyte
ratio as a predictor of recurrence and progression in patients with
high-grade pT1 bladder cancer. Can Urol Assoc J. 9:E126–E131.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sarosdy MF, Kahn PR, Ziffer MD, Love WR,
Barkin J, Abara EO, Jansz K, Bridge JA, Johansson SL, Persons DL
and Gibson JS: Use of a multitarget fluorescence in situ
hybridization assay to diagnose bladder cancer in patients with
hematuria. J Urology. 176:44–47. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Pan A, Zhou Y, Mu K, Liu Y, Sun F, Li P
and Li L: Detection of gene copy number alterations in DCIS and
invasive breast cancer by QM-FISH. Am J Transl Res. 8:4994–5004.
2016.PubMed/NCBI
|
|
37
|
Silva MP, Barros-Silva JD, Vieira J,
Lisboa S, Torres L, Correia C, Vieira-Coimbra M, Martins AT,
Jerónimo C, Henrique R, et al: NCOA2 is a candidate target gene of
8q gain associated with clinically aggressive prostate cancer.
Genes Chromosomes Cancer. 55:365–374. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Colotta F, Allavena P, Sica A, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer: Links to genetic instability. Carcinogenesis.
30:1073–1081. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444.
2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Azab B, Bhatt VR, Phookan J, Murukutla S,
Kohn N, Terjanian T and Widmann WD: Usefulness of the
neutrophil-to-lymphocyte ratio in predicting short- and long-term
mortality in breast cancer patients. Ann Surg Oncol. 19:217–224.
2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Halazun KJ, Hardy MA, Rana AA, Woodland DC
IV, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS Jr and
Emond JC: Negative impact of neutrophil-lymphocyte ratio on outcome
after liver transplantation for hepatocellular carcinoma. Ann Surg.
250:141–151. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jung MR, Park YK, Jeong O, Seon JW, Ryu
SY, Kim DY and Kim YJ: Elevated preoperative neutrophil to
lymphocyte ratio predicts poor survival following resection in late
stage gastric cancer. J Surg Oncol. 104:504–510. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim
YT and Lee K: Pre-treatment neutrophil to lymphocyte ratio is
elevated in epithelial ovarian cancer and predicts survival after
treatment. Cancer Immunol Iimmunother. 58:15–23. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Eble JN SG, Epstein JI and Sesterhenn IAD
(eds): World health organization classification of tumors. Tumors
of the urinary system and genital male organs. IARC Press, Lyon.,
2004.
|
|
45
|
Polesel J, Bosetti C, di Maso M, Montella
M, Libra M, Garbeglio A, Zucchetto A, Turati F, Talamini R, La
Vecchia C and Serraino D: Duration and intensity of tobacco smoking
and the risk of papillary and non-papillary transitional cell
carcinoma of the bladder. Cancer Causes Control. 25:1151–1158.
2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Schroeder A, Heller DA, Winslow MM,
Dahlman JE, Pratt GW, Langer R, Jacks T and Anderson DG: Treating
metastatic cancer with nanotechnology. Nat Rev Cancer. 12:39–50.
2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kaverina I and Straube A: Regulation of
cell migration by dynamic microtubules. In: Seminars in cell &
developmental biology Elsevier, pp968-974, 2011.
|
|
48
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265.
2004.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Schofield AV, Steel R and Bernard O:
Rho-associated coiled-coil kinase (ROCK) protein controls
microtubule dynamics in a novel signaling pathway that regulates
cell migration. J Biol Chemistry. 287:43620–43629. 2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Schofield AV, Gamell C, Suryadinata R,
Sarcevic B and Bernard O: Tubulin polymerization promoting protein
1 (Tppp1) phosphorylation by Rho-associated coiled-coil kinase
(rock) and cyclin-dependent kinase 1 (Cdk1) inhibits microtubule
dynamics to increase cell proliferation. J Biol Chem.
288:7907–7917. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gamell C, Schofield AV, Suryadinata R,
Sarcevic B and Bernard O: LIMK2 mediates resistance to
chemotherapeutic drugs in neuroblastoma cells through regulation of
drug-induced cell cycle arrest. PLoS One. 8(e72850)2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chen Q, Yang C, Chen L, Zhang JJ, Ge WL,
Yuan H, Meng LD, Huang XM, Shen P, Miao Y and Jiang KR: YY1 targets
tubulin polymerisation-promoting protein to inhibit migration,
invasion and angiogenesis in pancreatic cancer via p38/MAPK and
PI3K/AKT pathways. Br J Cancer. 121:912–921. 2019.PubMed/NCBI View Article : Google Scholar
|