Introduction
Lung ischemia-reperfusion injury (LIRI) is one of
the complications that can potentially arise after lung
transplantation (1). Following
lung transplantation, organ ischemia and subsequent reperfusion is
unavoidable and frequently leads to acute sterile inflammation in a
process known as ischemia reperfusion injury (1,2).
LIRI may also develop into pulmonary infection, acute lung injury
and bronchiolitis obliterans syndrome, thereby reducing the
post-transplant survival rate and increasing risk of mortality
(3).
Sirtuins (SIRT) belong to a protein family that is
responsible for regulating various intracellular events, including
cell proliferation, apoptosis and cell migration (4,5). In
particular, sirtuin 1 (SIRT1) is one of the most studied isoform of
the sirtuin family (6,7). SIRT1 is also known as a
NAD+-dependent histone deacetylase and has been revealed
to be an important modulator of energy metabolism (6,7).
Notably, it has been previously shown that LIRI can be alleviated
by activating SIRT (8-10).
5'AMP-activated protein kinase (AMPK) is an
ubiquitous energy sensor enzyme and is considered to be a
downstream effector of SIRT1, which serves a key role in the
regulation of energy homeostasis and cell survival (4,11,12).
AMPK has been reported to exert SIRT1-dependent anti-inflammatory
activities in sepsis-induced acute lung injury (13).
Over the past decade, there has been growing
interest in the potential application of natural bioactive
components isolated from plants for therapeutic uses. Mangiferin
(MAF) is a C-glucosyl xanthone that is present at high levels in
higher plants such as mango (Mangifera indica L.)
(14,15). It has been demonstrated to possess
numerous pharmacological activities, including antiviral,
anticancer, antioxidative, antiaging, immunomodulatory and
analgesic effects (15-18).
Furthermore, it has been reported that MAF can prevent liver lipid
metabolism disorders by regulating the SIRT1/AMPK pathway (19) whilst also inhibiting pulmonary
fibrosis (20). However, the
effects of MAF on LIRI remains poorly understood. Therefore, the
aim of the present study was to evaluate the effects of MAF on LIRI
using an in vitro hypoxia/reoxygenation (H/R) cell model and
explore its possible mechanism.
Materials and methods
Cell culture and modeling
The human alveolar epithelial cells (A549) were
obtained from American Type Culture Collection (ATCC). They were
routinely cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS) (both from Beijing Solarbio Science &
Technology Co., Ltd.), 100 U/ml penicillin and 100 pg/ml
streptomycin in a humidified incubator with 5% CO2 at
37˚C.
A H/R-A549 cell model was established. Briefly, A549
cells were exposed to anoxia for 24 h (1% O2, 5%
CO2 and 94% N2) at 37˚C followed by 4 h of
reoxygenation (5% CO2 and 95% air) at 37˚C (H/R group)
(21,22).
The cell model with the SIRT1/AMPK signaling pathway
blocked (H/R-A549 + sirtinol) was established. Sirtinol stock
solution (10 mM) (Sigma-Aldrich; Merck KGaA) and MAF
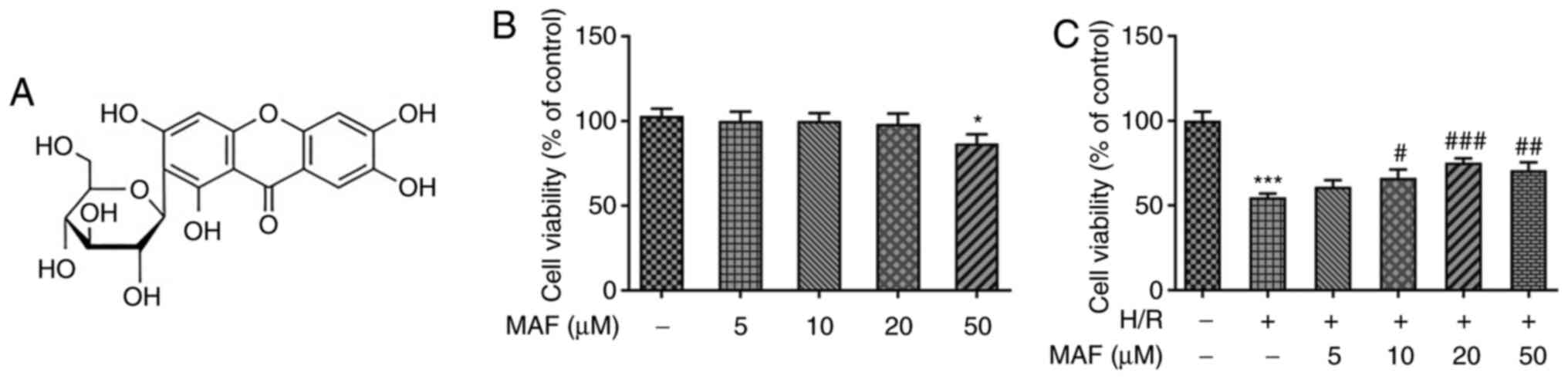
(Sigma-Aldrich; Merck KGaA; Fig.
1A) stock solution (50 mM) were prepared in dimethyl sulfoxide
and stored at -80˚C until further use. H/R-A549 cells were treated
with 15 µM of sirtinol for 72 h at 37˚C (23). Subsequently, the cells were
cultured for another 24 h with 20 µM MAF at 37˚C (H/R + MAF +
sirtinol group). Additionally, H/R-A549 cells were incubated with
20 µM MAF at 37˚C for 24 h (H/R + MAF group).
Cell viability test
Cell viability was measured using MTT assay. A549
and H/R-A549 cells (5x103/well) were each incubated in
96-well plates with various concentrations of MAF (5, 10, 20 and 50
µΜ). Using the RPMI-1640 medium to dilute the drug stock solution,
the cell toxicity of DMSO was negligible due to the use of
≥1,000-fold dilution. After incubation for 24 h, 20 µl MTT (5
mg/ml) was added to each well and cells were incubated for a
further 4 h at 37˚C. A buffer solution (10% SDS, 5% isopropanol and
0.1% HCl) was then added to solubilize the MTT formazan crystals
overnight. The absorbance in each well was measured at 570 nm using
a microplate reader (Thermo Fisher Scientific, Inc.). Untreated
cells were used as a control.
ELISA cytokine assay
A549 and H/R-A549 cells (5x103/well) were
inoculated into 96-well plates and treated with different MAF
concentrations (5, 10 and 20 µΜ) for 24 h at 37˚C. Subsequently,
the cell culture medium was centrifuged at 2,000 x g for 4 min at
4˚C to remove debris and the supernatant was collected for assay.
The levels of IL-6, IL-1β and IL-10 in cells were quantified using
their corresponding ELISA kits (cat. no. E-EL-H0102c, E-EL-H0149c
and E-EL-H0103c; Elabscience Biotechnology Co., Ltd.) according to
the manufacturer's protocols. The absorbance at 450 nm was measured
using a microplate reader (Thermo Fisher Scientific, Inc.).
TUNEL assay
Apoptotic A549 and H/R-A549 cells were visualized
using one-step TUNEL apoptosis detection kit according to the
manufacturer's protocol (cat. no. KGA7071; Nanjing KeyGen Biotech
Co., Ltd.). Briefly, cells (2x106/ml) that were cultured
on cover slips were fixed using 4% neutral-buffered formalin
solution at room temperature for 30 min and incubated with 0.3%
Triton X-100 at room temperature for 5 min. Subsequently, each
sample was supplemented with 50 µl TUNEL detection reagent for 60
min at 37˚C in the dark. The cell nuclei were stained with 5 µg/ml
DAPI at 37˚C in the dark for 5 min. An anti-fade solution (Beijing
Solarbio Science & Technology Co., Ltd.) was dropped onto the
area containing the treated cells and the sections were mounted
onto the slides. Finally, all samples were imaged in three random
fields per coverslip using a fluorescence microscope
(magnification, x100) to view the green fluorescence at 520±20 nm
and blue DAPI at 460 nm.
Western blotting assay
The protein samples from the cells were extracted
using the cell lysis buffer (cat. no. P0013; Beyotime Biotechnology
Inc.) and the protein content was measured using a BCA Protein
Assay Kit (cat. no. P0012S; Beyotime Biotechnology Inc.). The
protein samples (40 µg) were subjected to 15% SDS-PAGE for
separation and transferred onto nitrocellulose membranes. After
blocking in fresh 5% non-fat milk at room temperature for 2 h, the
membranes were incubated overnight at 4˚C with primary antibodies,
followed by incubation with a HRP-conjugated secondary antibody at
room temperature for 2 h. An enhanced chemiluminescence (ECL) kit
(Thermo Fisher Scientific, Inc.) was used to visualize the signals.
The antibodies (Affinity Biosciences) used included: Anti-SIRT1
(cat. no. DF6033; 1:1,000), anti-phosphorylated (p-)-AMPK (cat. no.
AF3423; 1:1,000), anti-AMPK (cat. no. AF6423; 1:1,000), anti-Cox-2
(cat. no. AF7003; 1:1,000), anti-iNOS (cat. no. AF0199; 1:1,000),
anti-Bcl-2 (cat. no. AF6139; 1:1,000), anti-Bax (cat. no. AF0120;
1:1,000), anti-cleaved-caspase-3 (cat. no. AF7022; 1:1,000),
anti-cleaved-caspase-9 (cat. no. AF5240; 1:1,000), anti-GAPDH (cat.
no. AF7021; 1:5,000) and the goat anti-rabbit IgG secondary
antibody (cat. no. S0001; 1:5,000). Protein expression levels were
semi-quantified using Image-Pro Plus software version 6.0 (Media
Cybernetics, Inc.).
Statistical analyses
All data were expressed as the mean ± standard
deviation. statistical analysis was performed with GraphPad Prism
8.0 software (GraphPad Software, Inc.). Differences between the
means of the groups were compared using a one-way ANOVA followed by
Tukey's test. Each experiment was repeated ≥ three times. P<0.05
was considered to indicate a statistically significant
difference.
Results
MAF promotes the viability of H/R-A549
cells
As shown in Fig.
1B, MAF at concentrations of <50 µM, could not exert
toxicity on A549 cells. However. 50 µM MAF was found to be
significantly toxic to cells. According to Fig. 1C, cell viability after H/R
induction was significantly decreased compared with that in the
control group. By contrast, the viability of H/R-A549 cells was
increased significantly after treatment with 20 µM MAF. However,
after treatment with 50 µM MAF, cell viability was markedly
decreased compared with that in the 20 µM MAF treatment group.
Therefore, in the subsequent experiments, the maximum concentration
of MAF used was 20 µM.
MAF inhibits inflammation in H/R-A549
cells
The levels of the inflammatory markers in H/R-A549
cells treated with different concentrations of MAF were measured.
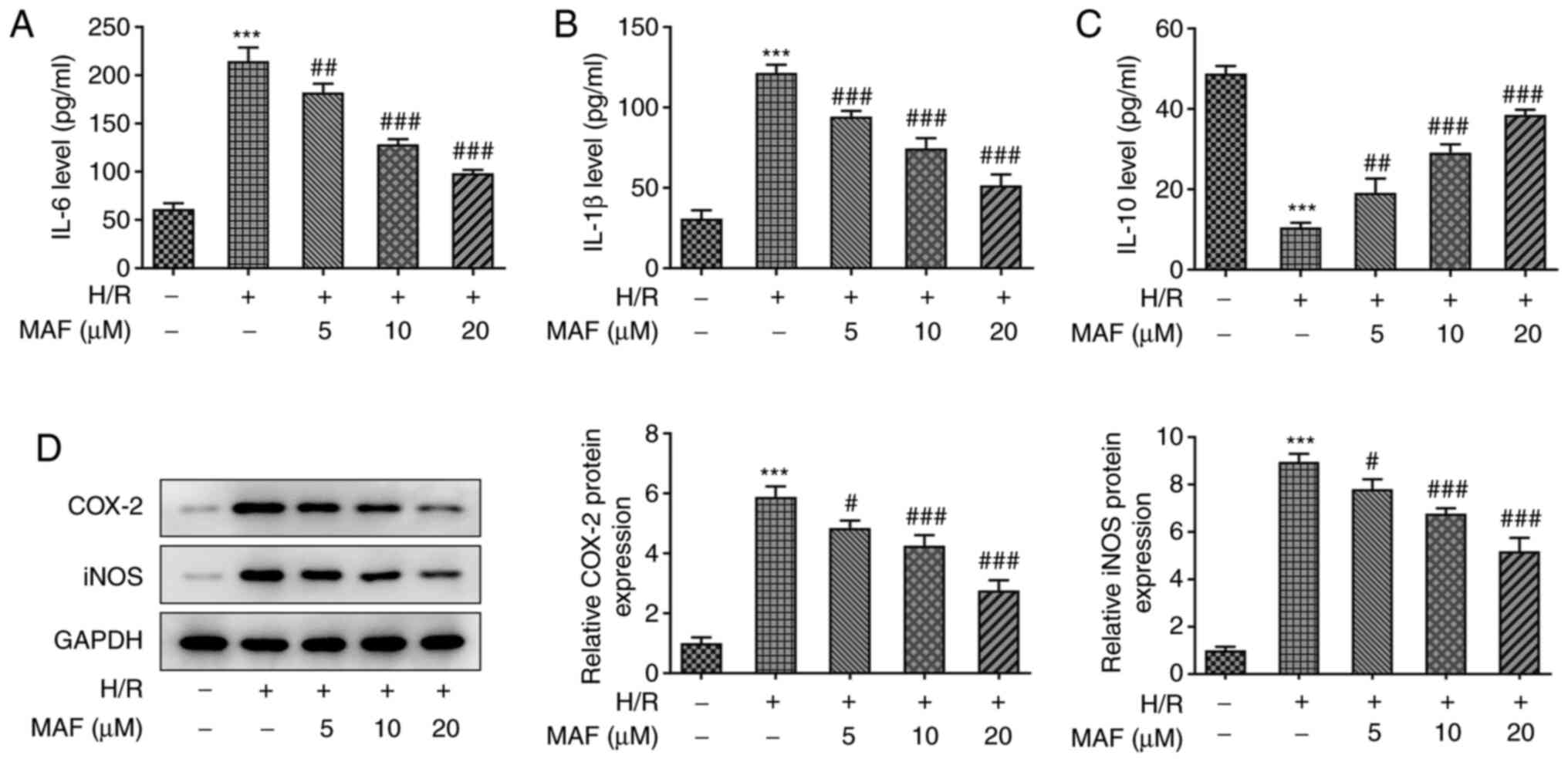
As shown in Fig. 2A-C, ELISA
analysis showed that compared with those in the control group, H/R
significantly upregulated the expression levels of IL-6 and IL-1β,
whilst significantly downregulating the expression level of IL-10.
By contrast, the H/R-induced upregulation of IL-6 and IL-1β and
downregulation of IL-10 were significantly reversed in the
MAF-treated groups in a dose-dependent manner. In addition, the
protein expression levels of Cox-2 and iNOS were also significantly
increased in the H/R induced group compared with those in the
control group, whilst those in the MAF-treated groups were
significantly reversed compared with those in the H/R group. Taken
together, these data suggest that MAF treatment can inhibit the-H/R
induced inflammatory response.
MAF inhibits apoptosis in H/R-A549
cells
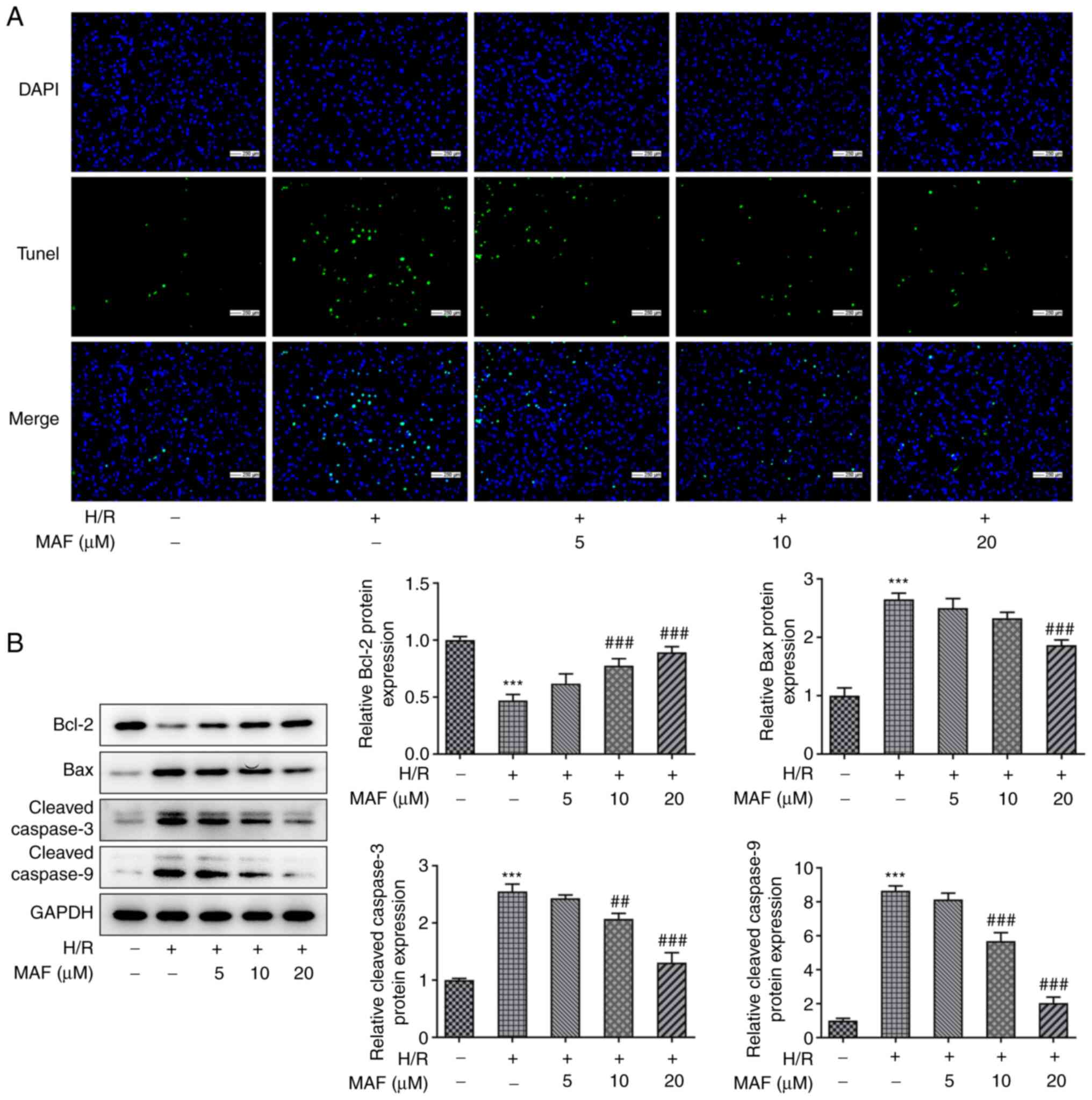
Nuclear DNA fragmentation is an important
biochemical event during apoptosis in many cell types and was
therefore measured by TUNEL assay in the present study. The TUNEL
results showed that A549 cell apoptosis was markedly increased
after H/R induction (Fig. 3A). By
contrast, after the addition of MAF, the H/R-induced cell apoptosis
was reduced (Fig. 3A). In
addition, compared with those in the control group, the expression
levels of Bax, cleaved-caspase-3 and cleaved-caspase 9 protein were
significantly increased in the H/R group, whilst those of Bcl-2
were reduced (Fig. 3B). Notably,
the expression level of Bcl-2 was significantly increased by 10 and
20 µM MAF treatment compared with that in the H/R group (Fig. 3B). In addition, the expression
levels of Bax, cleaved-caspase-3 and cleaved-caspase-9 were
significantly decreased in 20 µM MAF treatment group compared with
those in the H/R group (Fig. 3B).
These results suggest that MAF can inhibit H/R-induced apoptosis in
A549 cells.
MAF activates the SIRT1/AMPK signaling
pathway
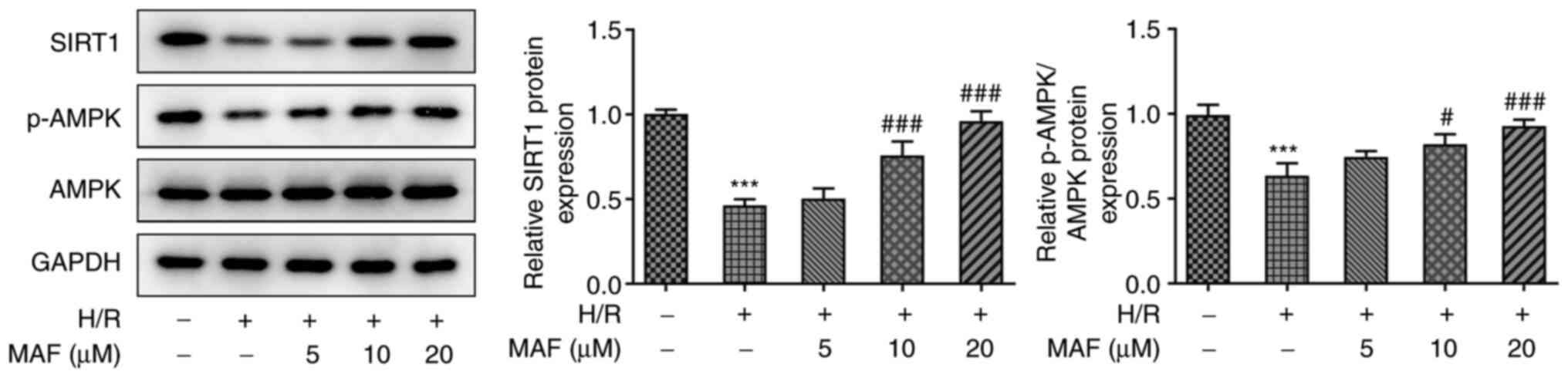
To verify the hypothesis that MAF may serve a role
in the SIRT1/AMPK signaling pathway, the protein levels of SIRT1
and ratio of p-AMPK/AMPK were evaluated in H/R cells by western
blotting assay. As shown in Fig.
4, compared with those in the control group, the protein
expression of SIRT1 and ratio of p-AMPK/AMPK were significantly
deceased in the H/R-induced group. By contrast, compared with the
H/R-induced group, the protein levels of SIRT1 and ratio
p-AMPK/AMPK were significantly increased in the 10 and 20 µM MAF
treatment groups (Fig. 4). Since a
concentration of 20 µM MAF resulted in a highly significant
increase in SIRT1 and p-AMPK/AMPK levels, 20 µM MAF was used
subsequently to explore the effects of MAF on the SIRT1/AMPK
signaling pathway.
MAF inhibits H/R injury in cells
through the SIRT1/AMPK signaling pathway
To understand whether the SIRT1/AMPK signaling
pathway affected H/R-induced inflammation and apoptosis, the SIRT1
inhibitor sirtinol was used to intercept the SIRT1/AMPK signaling
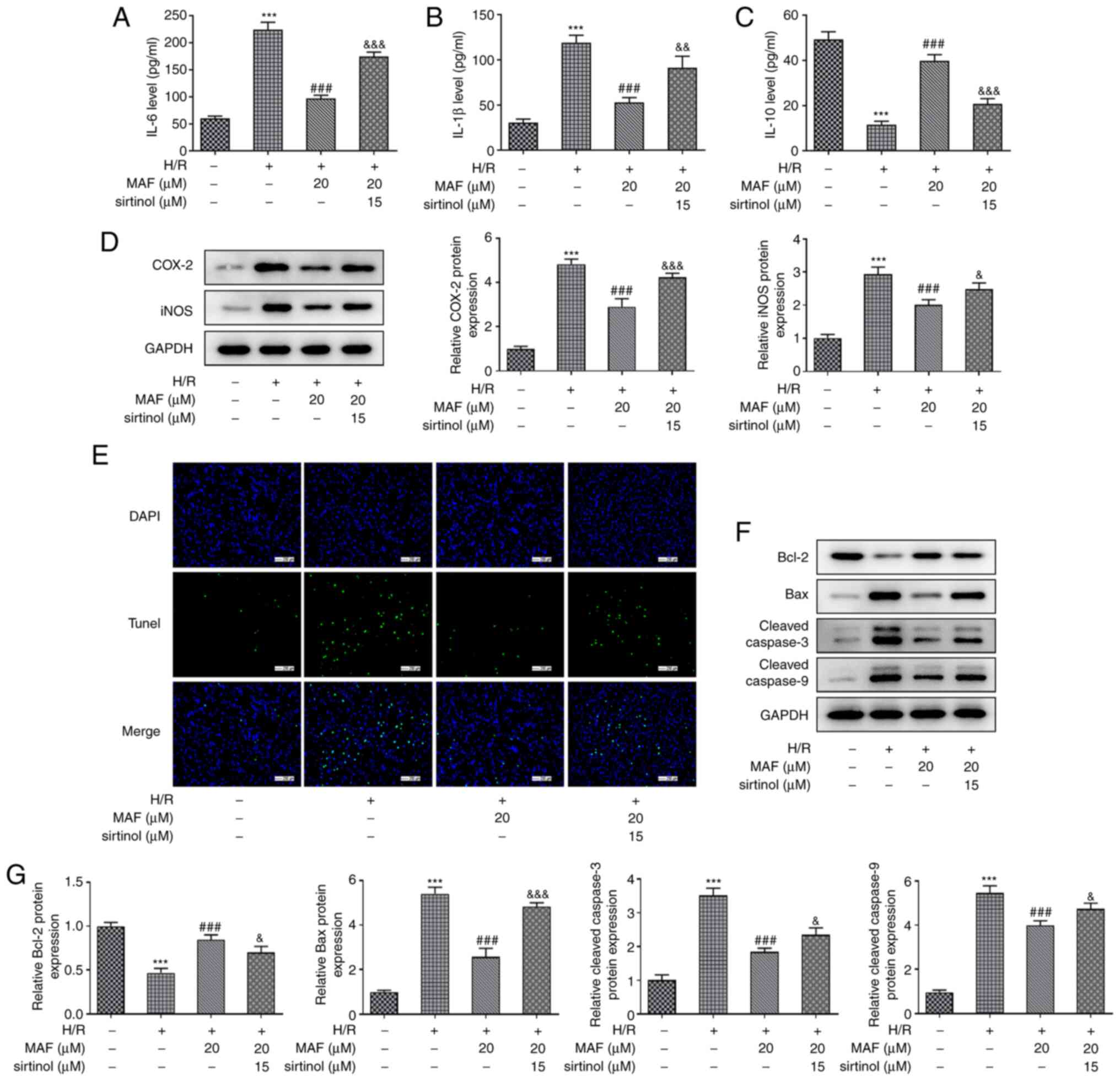
pathway following MAF treatment. As shown in Fig. 5A-D, after blocking the SIRT1/AMPK
signaling pathway, the protective effects of 20 µM MAF against
inflammation were reversed. Compared with those in the MAF
treatment group (H/R + MAF), the pathway inhibition group (H/R +
MAF + sirtinol) significantly upregulated the expression levels of
IL-6 and IL-1β, whilst significantly downregulating the expression
level of IL-10 (Fig. 5A-C). In
addition, the protein expression levels of Cox-2 and iNOS were also
significantly increased in the H/R + MAF + sirtinol group compared
with those in the H/R + MAF group (Fig. 5D). After inhibiting the SIRT1/AMPK
signaling pathway, the rate of apoptosis in MAF treatment was
markedly increased compared with that in the H/R + MAF group
(Fig. 5E). Compared with those in
the MAF treatment group, the protein expression levels of Bax,
cleaved-caspase-3 and cleaved-caspase-9 were significantly
increased after the addition of sirtinol, whereas Bcl-2 expression
was significantly reduced (Fig.
5F). Altogether, these results suggest that MAF can activate
the SIRT1/AMPK signaling pathway to inhibit H/R-induced A549 cell
injury.
Discussion
The success of lung transplantation was limited by
the high rates of primary graft dysfunction due to
ischemia-reperfusion injury, which is characterized by potent
inflammation, alveolar damage and vascular permeability (1,24).
Ischemia-reperfusion injury that occurs following lung
transplantation typically activates the innate immune system to
induce inflammation (1,9). In turn, this inflammation can enhance
acute allograft rejection, impair transplant tolerance and
accelerate the progression of chronic rejection (25). A number of reports in cellular and
animal models have shown that the levels of inflammatory factors,
including IL-6 and IL-1β, were significantly increased after lung
injury, whereas those of immunosuppressive factors, including
IL-10, were decreased (26,27).
Therefore, it is necessary to reduce the occurrence of inflammation
to mitigate the damage induced by LIRI.
In recent years, the potential application of
naturally occurring components of plants for clinical uses has
attracted much attention (28-30).
MAF is a natural constituent of foods and traditional herbal
medicines, such as Mangifera indica, Anemarrhena
asphodeloides and Coffea pseudozanguebariae, that
exhibits almost no adverse effects or toxicity (31). In addition, it possesses numerous
pharmacological activities, such as anti-inflammatory,
immunomodulatory and antioxidative effects (15). In the present study, MAF exerted
little to no toxicity on A549 cells but mediated protective effects
on cells after H/R injury, which was similar to previous reports
that MAF attenuated myocardial ischemia-reperfusion injury and lung
injury (32,33). When the concentration of MAF used
was <20 µM, H/R injury was protected by MAF in a dose-dependent
manner.
A previous study showed that MAF could improve cell
viability after H/R injury, which clarified the protective effects
of MAF against myocardial injury (32). In addition, on inflammation, Wang
et al found that MAF pretreatment significantly inhibits
ischemia-reperfusion-induced elevated expression levels of TNF-α
and IL-1β (34). Similarly, in the
present study, the increased levels of IL-6 and IL-1β induced by
H/R were markedly inhibited after MAF pretreatment. Previous
studies have demonstrated that MAF can suppress apoptosis by
reducing the protein expression levels of cleaved-caspase-3,
caspase-9 and Bax whilst increasing those of Bcl-2 (35-37).
Likewise, the apoptosis data found in the present study showed that
MAF downregulated the expression levels of Bax, cleaved-caspase-3
and cleaved-caspase-9 protein and increased the expression of Bcl-2
to reduce apoptosis, consistent with the previously reported data
aforementioned. Therefore, based on these data, the present study
suggests that MAF exerted anti-inflammatory and anti-apoptotic
effects on A549 cells.
Mechanistically, MAF exerts anti-inflammatory
effects by activating the SIRT1 signaling pathway (38,39).
Various studies have revealed that SIRT1 is a key component in a
number of stress-related pathways, including cell apoptosis,
cellular senescence and angiogenesis (40,41).
In addition, SIRT1 has been proposed to be an attractive
therapeutic target for myocardial ischemia-reperfusion injury
(42,43). SIRT1 activation can inhibit the
cardiac inflammatory response following ischemia-reperfusion
(44). Once activated, SIRT1 can
mediate a wide range of downstream signaling pathways, such as
AMPK, peroxisome proliferator-activated receptor γ coactivator 1α
and endothelial nitric oxide synthase (39,42,45).
In the present study, the protein expression of SIRT1 and AMPK was
increased in a dose-dependent manner after MAF treatment. After the
addition of sirtinol, a SIRT1 inhibitor, the anti-inflammatory and
anti-apoptotic effects of MAF were reversed. Therefore, MAF can
activate the SIRT1/AMPK signaling pathway. Taken together,
activation of the SIRT1/AMPK signaling pathway confer protective
effects against ischemia-reperfusion injury.
In the present study, the effects of MAF were
evaluated on an in vitro A549 cell model of LIRI using a
cellular H/R model. The results showed that MAF could induce the
activation of the SIRT1/AMPK signaling pathway, where it protected
A549 cell injury induced by H/R by inhibiting inflammation and
apoptosis. Application of MAF therefore provides a promising
strategy for the treatment of LIRI. However, the fact that the
present study only used in vitro and not in vivo
models and examined the effects of MAF concentration on
inflammation and apoptosis are limitations of the present study. In
addition, further elucidation in clinical samples is required.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and JH conceived and designed the study, acquired
and interpreted the data and revised it for critically important
intellectual content. XC and JH confirm the authenticity of all raw
data in the present study. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Laubach VE and Sharma AK: Mechanisms of
lung ischemia-reperfusion injury. Curr Opin Organ Transplant.
21:246–252. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Saito M, Chen-Yoshikawa TF, Suetsugu K,
Okabe R, Takahagi A, Masuda S and Date H: Pirfenidone alleviates
lung ischemia-reperfusion injury in a rat model. J Thorac
Cardiovasc Surg. 158:289–296. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shah RJ and Diamond JM: Primary graft
dysfunction (PGD) following lung transplantation. Semin Respir Crit
Care Med. 39:148–154. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
You J, Cheng J, Yu B, Duan C and Peng J:
Baicalin, a Chinese herbal medicine, inhibits the proliferation and
migration of human non-small cell lung carcinoma (NSCLC) Cells,
A549 and H1299, by activating the SIRT1/AMPK signaling pathway. Med
Sci Monit. 24:2126–2133. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jin MS, Hyun CL, Park IA, Kim JY, Chung
YR, Im SA, Lee KH, Moon HG and Ryu HS: SIRT1 induces tumor invasion
by targeting epithelial mesenchymal transition-related pathway and
is a prognostic marker in triple negative breast cancer. Tumour
Biol. 37:4743–4753. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Suzuki K, Hayashi R, Ichikawa T, Imanishi
S, Yamada T, Inomata M, Miwa T, Matsui S, Usui I, Urakaze M, et al:
SRT1720, a SIRT1 activator, promotes tumor cell migration, and lung
metastasis of breast cancer in mice. Oncol Rep. 27:1726–1732.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lovaas JD, Zhu L, Chiao CY, Byles V,
Faller DV and Dai Y: SIRT1 enhances matrix metalloproteinase-2
expression and tumor cell invasion in prostate cancer cells.
Prostate. 73:522–530. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu Z, Meng Y, Miao Y, Yu L and Yu Q:
Propofol reduces renal ischemia/reperfusion-induced acute lung
injury by stimulating sirtuin 1 and inhibiting pyroptosis. Aging
(Albany NY). 13:865–876. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang C, Yang W, He Z, He H, Yang X, Lu Y
and Li H: Kaempferol improves lung ischemia-reperfusion injury via
antiinflammation and antioxidative stress regulated by
SIRT1/HMGB1/NF-κB axis. Front Pharmacol. 10(1635)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fu C, Hao S, Xu X, Zhou J, Liu Z, Lu H,
Wang L, Jin W and Li S: Activation of SIRT1 ameliorates LPS-induced
lung injury in mice via decreasing endothelial tight junction
permeability. Acta Pharmacol Sin. 40:630–641. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Salomone F, Barbagallo I, Godos J, Lembo
V, Currenti W, Cinà D, Avola R, D'Orazio N, Morisco F, Galvano F
and Li Volti G: Silibinin restores NAD+ levels and
induces the SIRT1/AMPK pathway in non-alcoholic fatty liver.
Nutrients. 9(1086)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mulchandani N, Yang WL, Khan MM, Zhang F,
Marambaud P, Nicastro J, Coppa GF and Wang P: Stimulation of brain
AMP-activated protein kinase attenuates inflammation and acute lung
injury in sepsis. Mol Med. 21:637–644. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li X, Jamal M, Guo P, Jin Z, Zheng F, Song
X, Zhan J and Wu H: Irisin alleviates pulmonary epithelial barrier
dysfunction in sepsis-induced acute lung injury via activation of
AMPK/SIRT1 pathways. Biomed Pharmacother.
118(109363)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ajila CM, Rao LJ and Rao UJ:
Characterization of bioactive compounds from raw and ripe Mangifera
indica L. peel extracts. Food Chem Toxicol. 48:3406–3411.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Du S, Liu H, Lei T, Xie X, Wang H, He X,
Tong R and Wang Y: Mangiferin: An effective therapeutic agent
against several disorders (Review). Mol Med Rep. 18:4775–4786.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dar A, Faizi S, Naqvi S, Roome T,
Zikr-ur-Rehman S, Ali M, Firdous S and Moin ST: Analgesic and
antioxidant activity of mangiferin and its derivatives: The
structure activity relationship. Biol Pharm Bull. 28:596–600.
2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Duang XY, Wang Q, Zhou XD and Huang DM:
Mangiferin: A possible strategy for periodontal disease to therapy.
Med Hypotheses. 76:486–488. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Imran M, Arshad MS, Butt MS, Kwon JH,
Arshad MU and Sultan MT: Mangiferin: A natural miracle bioactive
compound against lifestyle related disorders. Lipids Health Dis.
16(84)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li J, Liu M, Yu H, Wang W, Han L, Chen Q,
Ruan J, Wen S, Zhang Y and Wang T: Mangiferin improves hepatic
lipid metabolism mainly through its metabolite-norathyriol by
modulating SIRT-1/AMPK/SREBP-1c signaling. Front Pharmacol.
9(201)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jia L, Sun P, Gao H, Shen J, Gao Y, Meng
C, Fu S, Yao H and Zhang G: Mangiferin attenuates Bleomycin-induced
pulmonary fibrosis in mice through inhibiting TLR4/p65 and
TGF-β1/Smad2/3 pathway. J Pharm Pharmacol. 71:1017–1028.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liao WI, Wu SY, Wu GC, Pao HP, Tang SE,
Huang KL and Chu SJ: Ac2-26, an Annexin A1 peptide, attenuates
ischemia-reperfusion-induced acute lung injury. Int J Mol Sci.
18(1771)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hung KY, Liao WI, Pao HP, Wu SY, Huang KL
and Chu SJ: Targeting F-box protein Fbxo3 attenuates lung injury
induced by ischemia-reperfusion in rats. Front Pharmacol.
10(583)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kang H, Lee Y, Bae M, Park YK and Lee JY:
Astaxanthin inhibits alcohol-induced inflammation and oxidative
stress in macrophages in a sirtuin 1-dependent manner. J Nutr
Biochem. 85(108477)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yabuki H, Watanabe T, Oishi H, Katahira M,
Kanehira M and Okada Y: Muse cells and ischemia-reperfusion lung
injury. Adv Exp Med Biol. 1103:293–303. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kreisel D and Goldstein DR: Innate
immunity and organ transplantation: Focus on lung transplantation.
Transpl Int. 26:2–10. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rognlien AGW, Wollen EJ, Atneosen-Asegg M,
Suganthan R, Bjoras M and Saugstad OD: Neonatal Ogg1/Mutyh knockout
mice have altered inflammatory gene response compared to wildtype
mice in the brain and lung after hypoxia-reoxygenation. J Perinat
Med. 47:114–124. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu PL, Chong IW, Lee YC, Tsai JR, Wang
HM, Hsieh CC, Kuo HF, Liu WL, Chen YH and Chen HL:
Anti-inflammatory effects of resveratrol on
Hypoxia/Reoxygenation-induced alveolar epithelial cell dysfunction.
J Agric Food Chem. 63:9480–9487. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xie W, Zhou P, Sun Y, Meng X, Dai Z, Sun G
and Sun X: Protective effects and target network analysis of
ginsenoside Rg1 in cerebral ischemia and reperfusion injury: A
comprehensive overview of experimental studies. Cells.
7(270)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sun Z and Wang X: Protective effects of
polydatin on multiple organ ischemia-reperfusion injury. Bioorg
Chem. 94(103485)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ye J, Lu S, Wang M, Ge W, Liu H, Qi Y, Fu
J, Zhang Q, Zhang B, Sun G and Sun X: Hydroxysafflor yellow a
protects against myocardial ischemia/reperfusion injury via
suppressing NLRP3 inflammasome and activating autophagy. Front
Pharmacol. 11(1170)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Luczkiewicz P, Kokotkiewicz A, Dampc A and
Luczkiewicz M: Mangiferin: A promising therapeutic agent for
rheumatoid arthritis treatment. Med Hypotheses. 83:570–574.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu K, Wang F, Wang S, Li WN and Ye Q:
Mangiferin attenuates myocardial ischemia-reperfusion injury via
MAPK/Nrf-2/HO-1/NF-κ B in vitro and in vivo. Oxid Med Cell Longev.
2019(7285434)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li N, Xiong R, He R, Liu B, Wang B and
Geng Q: Mangiferin mitigates lipopolysaccharide-induced lung injury
by inhibiting NLRP3 inflammasome activation. J Inflamm Res.
14:2289–2300. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang B, Wan J, Gong X, Kuang G, Cheng X
and Min S: Mangiferin attenuates renal ischemia-reperfusion injury
by inhibiting inflammation and inducing adenosine production. Int
Immunopharmacol. 25:148–154. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Du M, Wen G, Jin J, Chen Y, Cao J and Xu
A: Mangiferin prevents the growth of gastric carcinoma by blocking
the PI3K-Akt signalling pathway. Anticancer Drugs. 29:167–175.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xia G, Li X, Zhu X, Yin X, Ding H and Qiao
Y: Mangiferin protects osteoblast against oxidative damage by
modulation of ERK5/Nrf2 signaling. Biochem Biophys Res Commun.
491:807–813. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jiang T, Han F, Gao G and Liu M:
Mangiferin exert cardioprotective and anti-apoptotic effects in
heart failure induced rats. Life Sci. 249(117476)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen L, Li S, Zhu J, You A, Huang X, Yi X
and Xue M: Mangiferin prevents myocardial infarction-induced
apoptosis and heart failure in mice by activating the Sirt1/FoxO3a
pathway. J Cell Mol Med. 25:2944–2955. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen M, Wang Z, Zhou W, Lu C, Ji T, Yang
W, Jin Z, Tian Y, Lei W, Wu S, et al: SIRT1/PGC-1α signaling
activation by mangiferin attenuates cerebral hypoxia/reoxygenation
injury in neuroblastoma cells. Eur J Pharmacol.
907(174236)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Michan S and Sinclair D: Sirtuins in
mammals: Insights into their biological function. Biochem J.
404:1–13. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu S, Xu J, Fang C, Shi C, Zhang X, Yu B
and Yin Y: Over-expression of heat shock protein 70 protects mice
against lung ischemia/reperfusion injury through SIRT1/AMPK/eNOS
pathway. Am J Transl Res. 8:4394–4404. 2016.PubMed/NCBI
|
|
42
|
Li D, Wang X, Huang Q, Li S, Zhou Y and Li
Z: Cardioprotection of CAPE-oNO2 against myocardial
ischemia/reperfusion induced ROS generation via regulating the
SIRT1/eNOS/NF-κB pathway in vivo and in vitro. Redox Biol.
15:62–73. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ding S, Liu D, Wang L, Wang G and Zhu Y:
Inhibiting microRNA-29a protects myocardial ischemia-reperfusion
injury by targeting SIRT1 and suppressing oxidative stress and
NLRP3-mediated pyroptosis pathway. J Pharmacol Exp Ther.
372:128–135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Han Y, Sun W, Ren D, Zhang J, He Z,
Fedorova J, Sun X, Han F and Li J: SIRT1 agonism modulates cardiac
NLRP3 inflammasome through pyruvate dehydrogenase during ischemia
and reperfusion. Redox Biol. 34(101538)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lempiainen J, Finckenberg P, Levijoki J
and Mervaala E: AMPK activator AICAR ameliorates ischaemia
reperfusion injury in the rat kidney. Br J Pharmacol.
166:1905–1915. 2012.PubMed/NCBI View Article : Google Scholar
|