Introduction
Inflammation is one of the first lines of defense
against harmful stimuli, such as pathogens, damaged cells, trauma,
bacteria and irritants (1).
Macrophages detect and react to certain pathogens and consequently
regulate the inflammatory response (2). Lipopolysaccharide (LPS) is an
endotoxin derived from the outer membrane of Gram-negative bacteria
and also a powerful mediator of systemic inflammation and a driver
of septic shock (3). LPS is able
to activate macrophages to release several inflammatory cytokines
(4). Activation of the
inflammatory pathway may be induced by pro-inflammatory mediators
and cytokines being secreted, including nitric oxide (NO),
cyclooxygenase-2 (COX-2), tumor necrosis factor-α (TNF-α),
interleukin-1β (IL-1β), IL-6 and prostaglandin (PG)E2(5). Inflammation is one cause of increased
morbidity and mortality in intensive care units, also resulting in
elevated hospital-related costs (6,7).
Nowadays, several anti-inflammatory drugs are available, such as
non-steroidal anti-inflammatory drugs (NSAIDs) (8). However, a previous study suggested
that NSAIDs may induce gastrointestinal tract bleeding (9). Safe and effective strategies to
prevent and treat inflammation and its associated diseases are thus
urgently required.
The freshwater hybrid catfish (Pangasius sp.)
belongs to the freshwater catfish family. It has become one of the
most popular freshwater fish species and has a high demand,
particularly on the European and US markets. Fish contains 2-30%
fat and ~50% of its body weight is discarded as waste during the
fish processing operation (10).
One of the fish processing byproducts is fish oil (FO). FO is a
source of long-chain polyunsaturated fatty acid (e.g. omega-3 fatty
acids), particularly fish oil extracted from marine fish, which is
mainly composed of cis-5,8,11,14,17-eicosapentaenoic acid (EPA) and
cis-4,7,10,13,16,19-docosahexaenoic acid (11). As a component in FO, omega-3 fatty
acids have several benefits, including protection against
atherosclerosis, arrhythmias and chronic obstructive pulmonary
diseases (12). They also reduce
blood pressure, blood glucose and symptoms of asthma and cystic
fibrosis (13-15).
However, a previous study by our group demonstrated that fish oil
from freshwater hybrid catfish contains a high level of
monounsaturated omega-9 fatty acid (MUFA) (16). Furthermore, freshwater hybrid
catfish oil (FFO) was indicated to have anti-diabetic effects by
improving insulin resistance and adipokine imbalance in a rat model
of type 2 diabetes and also suppress pro-inflammatory cytokine
protein expressions in the skeletal muscle tissues of those rats
(17). The omega-9 fatty acid
increased of high-density lipoprotein-cholesterol and decreased
low-density lipoprotein-cholesterol (17). However, the effect of FFO on the
inflammatory condition and the underlying mechanisms have remained
elusive. In the present study, the anti-inflammatory effects of FFO
on RAW264.7 macrophages stimulated by LPS were examined and the
associated mechanism was investigated.
Materials and methods
Chemicals
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were purchased from Gibco; Thermo Fisher
Scientific, Inc. β-nicotinamide adenine dinucleotide phosphate and
LPS were purchased from Merck KGaA. All other chemicals with high
purity were purchased from commercial sources.
Preparation of FFO
FO of freshwater hybrid catfish (Pangasius
gigas x Pangasianodon hypophthalmus) was purchased from
a private company, Me Natural Co., Ltd., which cooperated and
received the adipose tissue from the Center of Excellence in Giant
Catfish and Buk Siam Catfish, Faculty of Fisheries Technology and
Aquatic Resources, Maejo University (Chiang Mai, Thailand). FO was
extracted as previously described, which exhibited a high omega-9
content and biological activity (18). In brief, frozen adipose tissues
were purified by cleaning and steaming at 90˚C for 30 min. The
liquid oil was subsequently filtered through a filter sack and
squeezed using a screw compressor. The squeezed liquid was
centrifuged at 2,268 x g for 10 min at 25˚C to separate the solid
particles from the oil and the supernatant FFO was separated.
Solvent-free extraction was used to obtain FFO. As previously,
adipose tissue was extracted and partially purified as
aforementioned, resulting in FFO at a yield of 300 ml per 1 kg of
adipose tissue.
Determination of fatty acids,
fat-soluble vitamins and heavy metal levels of FFO
The chemical compounds, including the fatty acids
and fat-soluble vitamins, were sent for analysis at a certified lab
with international standardization in the field of information
technology (ISO172025), the Central Laboratory (Thailand) Co. Ltd.,
Chiang Mai Branch, following the TE-CH 260 in-house method of the
Association of Official Analytical Chemists 996.06(19). Heavy metal contamination of FFO was
also detected according to this in-house method.
Cell culture
RAW264.7 cells were purchased from the American Type
Culture Collection. Cells at passage 2-22 were maintained in DMEM
(Thermo Fisher Scientific, Inc) containing 3.7 g/l
NaHCO3 supplemented with 10% FBS (Thermo Fisher
Scientific, Inc) and 1% penicillin/streptomycin in a humidified
atmosphere at 37˚C with 5% CO2 and sub-cultured every
4-5 days using 0.05% trypsin-EDTA in PBS (Thermo Fisher Scientific,
Inc.). Cells were seeded at a density of 1x105
cells/well and cultured in 6-, 12- and 96-well plates for 3 days
until subsequent experimentation. The medium was replaced every 2
days during culture.
Determination of cell viability
The MTT assay was performed to assess the effect of
FFO on cell viability. Cells were incubated with serum-free medium
with FFO at 0, 0.125, 0.25, 0.5, 1 or 2% in 0.5% propylene glycol
(v/v). Subsequently, serum-free medium containing 0.5 mg/ml of MTT
(Thermo Fisher Scientific, Inc.) was added to each well, followed
by incubation at 37˚C for 4 h. The MTT solution was then aspirated
and cells were washed once with ice-cold PBS. The purple formazan
crystals were dissolved in DMSO for 30 min and cell viability was
subsequently analyzed by measuring the absorption at a wavelength
of 570 nm using an M965 AccuReader microplate reader (Metertech,
Inc.). The lysed cells were detected at a wavelength of 680 nm was
used as a reference. Cell viability was calculated as follows: Cell
viability (%) = [(Absorbance value-reference value) x100]/[mean of
(absorbance value-reference value) in untreated cells].
ELISA
RAW264.7 cells were seeded into 12-well plates at a
density of 1x105 cells/ml and incubated for 24 h at 37˚C
in a humidified atmosphere with 5% CO2. The culture
medium was removed and cells were treated with different
concentrations of FFO [0.125-2% in 0.5% propylene glycol (v/v)]
with or without LPS (1 µg/ml) in fresh medium for 24 h at 37˚C in a
humidified atmosphere with 5% CO2. Subsequently, the
cells were homogenized and lysed cells were centrifuged at 2,000 x
g for 10 min at 4˚C. The supernatant was collected and stored at
-80˚C for quantification of IL-6 (cat. no. BIOL-431304), IL-1β
(cat. no. BIOL-432604), TNF-α (cat. no. BIOL-430904), NO (cat. no.
780001) and PGE2 (cat. no. ABBK-KTE70765-96T) concentrations using
commercial kits (BioLegend, Inc.) according to the manufacturer's
protocols.
NO assay
The nitrate/nitrite concentration was determined
using a colorimetric assay kit (Cayman Chemical Co.). In brief,
cells were treated with different concentrations of FFO [0.125-2%
in 0.5% propylene glycol (v/v)] with or without LPS (1 µg/ml) for
24 h at 37˚C in a humidified atmosphere with 5% CO2.
Treated cells were centrifuged at 10,000 x g for 20 min at 4˚C. The
supernatant was subsequently collected to measure the NO
concentration at a wavelength of 540 nm using an M965 AccuReader
microplate reader (Metertech, Inc.).
Hoechst 33342 staining
To confirm the effect of FFO on LPS-induced DNA
damage, RAW264.7 cells were seeded into 8-well cell culture slides
and treated with different concentrations of FFO [0.125-2% in 0.5%
propylene glycol (v/v)] with or without LPS (1 µg/ml) for 24 h at
37˚C in a humidified atmosphere with 5% CO2. Treated
cells were fixed with 4% paraformaldehyde for 10 min at room
temperature and subsequently stained with Hoechst 33342 (5 µg/ml)
for 10 min at room temperature. Cells were washed twice with PBS
and observed under a Nikon Eclipse Ni-U fluorescent microscope
(original magnification, x40; Nikon Corporation).
DNA damage assay
To further determine the protective effect of FFO on
LPS-induced DNA damage, the effect of FFO on DNA impairment was
investigated via ELISA. RAW264.7 cells were seeded into 12-well
plates at a density of 1x105 cells/ml and incubated for
24 h at 37˚C in a humidified atmosphere with 5% CO2. The
culture medium was removed and cells were treated with different
concentrations of FFO [0.125-2% in 0.5% propylene glycol (v/v)]
with or without LPS (1 µg/ml) for 24h at 37˚C in a humidified
atmosphere with 5% CO2. Treated cells were centrifuged
at 10,000 x g for 20 min at 4˚C. The supernatant was collected and
stored at -80˚C for quantification of 8-hydroxy-2'-deoxyguanosine
(8-OHdG; cat. no. AB-EIADNAD), a DNA damage marker, using
commercial kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted and purified from RAW264.7
cells using TRIzol® reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol and reverse
transcribed into cDNA using the SensiFAST™ cDNA synthesis kit
(Bioline). qPCR was subsequently performed using SYBR Real-Time PCR
Master Mix (Bioline) on a CFX Touch real-time PCR system (Bio-Rad
Laboratories, Inc.). PCR amplifications were performed at a 20-µl
volume with the following thermocycling conditions: A polymerase
enzyme activation step at 95˚C for 2 min; followed by 40 cycles of
denaturation at 95˚C for 5 sec, 10 sec of annealing at 60˚C
depending on primers, and 10 sec of elongation at 72˚C. The primer
sequences used for qPCR were purchased from Macrogen, Inc. and used
at a final concentration of 0.4 µM. The primer sequences for mouse
TNF-α, IL-1β, IL-6, COX2, p53, p27, cyclin D2, cyclin E2 and GAPDH
are presented in Table I (20-25).
Gene expression was calculated using the 2-ΔΔCq method
(26) and normalized to GAPDH.
Data were reported as the relative fold change. qPCR amplification
was performed in duplicate for each synthesized cDNA set.
 | Table IPrimer sequences and expected
amplicon sizes for gene amplification. |
Table I
Primer sequences and expected
amplicon sizes for gene amplification.
| cDNA | GenBank accession
no. | Forward primer | Reverse primer | Amplicon size
(bp) |
|---|
| TNF-α | NM013693.3 |
5'-ACCTGGCCTCTCTACCTTGT-3' |
5'-CCCGTAGGGCGATTACAGTC-3' | 161 |
| IL-1β | NM008361.4 |
5'-GCCACCTTTTGACAGTGATGAG-3' |
5'-AGTGATACTGCCTGCCTGAAG-3' | 165 |
| IL-6 | NM031168.2 |
5'-CAACGATGATGCACTTGCAGA-3' |
5'-TCTCTCTGAAGGACTCTGGCT-3' | 201 |
| COX-2 | NM011198.4 |
5'-CCACTTCAAGGGAGTCTGGA-3' |
5'-AGTCATCTGCTACGGGAGGA-3' | 197 |
| Cyclin D2 | NM009829.3 |
5'-ACCTCCCGCAGTGTTCCTATT-3' |
5'-CACAGACCTCTAGCATCCAGG-3' | 93 |
| Cyclin E2 | NM001037134.2 |
5'-TCTGTGCATTCTAGCATCGACTC-3' |
5'-AAGGCACCATCGTCTACACATTC-3' | 149 |
| p27 | NM009875.4 |
5'-GCGGTGCCTTTAATTGGGTCT-3' |
5'-GGCTTCTTGGGCGTCTGCT-3' | 230 |
| p53 | NM011640.3 |
5'-ACCGCCGACCTATCCTTACC-3' |
5'-TCTTCTGTACGGCGGTCTCTC-3' | 118 |
| GAPDH | NM001289726.1 |
5'-TGTGTCCGTCGTGGATCTGA-3' |
5'-TTGCTGTTGAAGTCGCAGGAG-3' | 150 |
Statistical analysis
Statistical analysis was performed using SPSS
version 23 software (IBM Corp.). Values are expressed as the mean ±
standard error of the mean. One-way ANOVA followed by Dunnett's
test was used to compare differences between multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Fatty acids and vitamins in FFO
As presented in Table
II, FFO contained several fatty acids, including saturated,
unsaturated, MUFAs and polyunsaturated fatty acid (PUFAs) at 40.38,
55.80, 46.74 and 12.75 g/100 g of FFO, respectively. Among the
detected MUFAs, the predominant fatty acid was omega-9 (42.27±1.76
g/100 g of FFO). In addition, FFO also contained PUFAs and the
predominant fatty acids were omega-3 (1.17±0.39 g/100 g of FFO) and
omega-6 (10.95±1.46 g/100 g of FFO). In addition, vitamin A was
present at 1.80±0.12 µg/100 g of FFO and vitamin E was present at
0.69±0.06 mg/100 g of FFO.
 | Table IIFatty acid composition and vitamin
content of freshwater hybrid catfish oil. |
Table II
Fatty acid composition and vitamin
content of freshwater hybrid catfish oil.
| Chemical
component | Amount |
|---|
| Saturated fatty
acids, g/100 g | 40.38±2.94 |
| Unsaturated fatty
acids, g/100 g | 55.80±0.64 |
|
Monounsaturated
fatty acids, g/100 g | 46.74±2.24 |
|
Oleic acid,
g/100 g | 42.07±1.79 |
|
Omega-9 | 42.27±1.76 |
|
Polyunsaturated
fatty acids, g/100 g | 12.75±1.04 |
|
Omega-3 | 1.17±0.27 |
|
Omega-6 | 10.95±1.03 |
| Vitamins | |
|
Vitamin A
(retinol), µg/100 g | 1.80±0.12 |
|
Vitamin E
(α-tocopherol), mg/100 g | 0.69±0.06 |
Heavy metal content profiles of
FFO
The concentrations of arsenic, copper, lead,
mercury, tin and zinc in FFO are presented in Table III. The results demonstrated that
FFO contained copper and lead at much lower concentrations, while
arsenic, mercury, tin and zinc were not detected. Moreover, the USA
established a recommended dietary allowance of copper for adults at
0.9 mg/day (27).
 | Table IIIHeavy metal content in freshwater
hybrid catfish oil. |
Table III
Heavy metal content in freshwater
hybrid catfish oil.
| Element
(symbol) | Amount (mg/kg) |
|---|
| Arsenic (As) | Not detected |
| Copper (Cu) | <0.50 |
| Lead (Pb) | <0.050 |
| Mercury (Hg) | Not detected |
| Tin (Sn) | Not detected |
| Zinc (Zn) | Not detected |
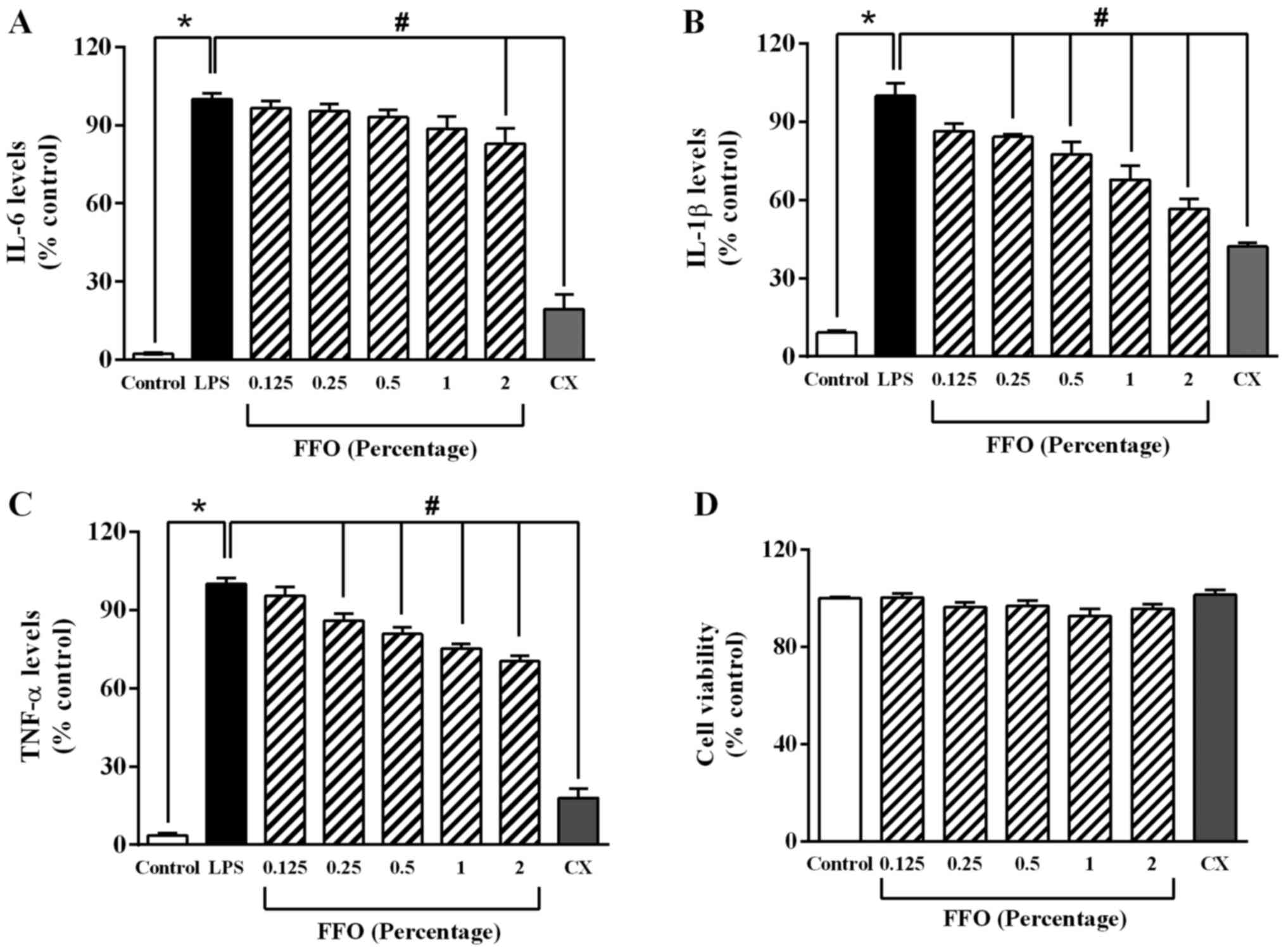
FFO decreases the secretion of
pro-inflammatory cytokines in RAW264.7 cells
To determine the anti-inflammatory effect of FFO in
RAW264.7 cells, the levels of pro-inflammatory cytokines were
detected via ELISA. As presented in Fig. 1A, LPS at 1 µg/ml significantly
increased the levels of pro-inflammatory cytokines, while added FFO
at 2% significantly decreased IL-6 production compared with
LPS-treated cells. Added FFO at 0.25-2% also markedly decreased
TNF-α and IL-1β expression compared with LPS-treated cells
(Fig. 1B and C). Similarly, celecoxib (CX), an NSAID
(28), significantly decreased
IL-1β expression. However, in the absence of LPS, there was no
significant effect of FFO (0.25-2%) and CX on the viability of
RAW264.7 cells compared with the control cells (Fig. 1D). Taken together, these results
suggested that FFO exerts an anti-inflammatory effect without a
cytotoxic effect.
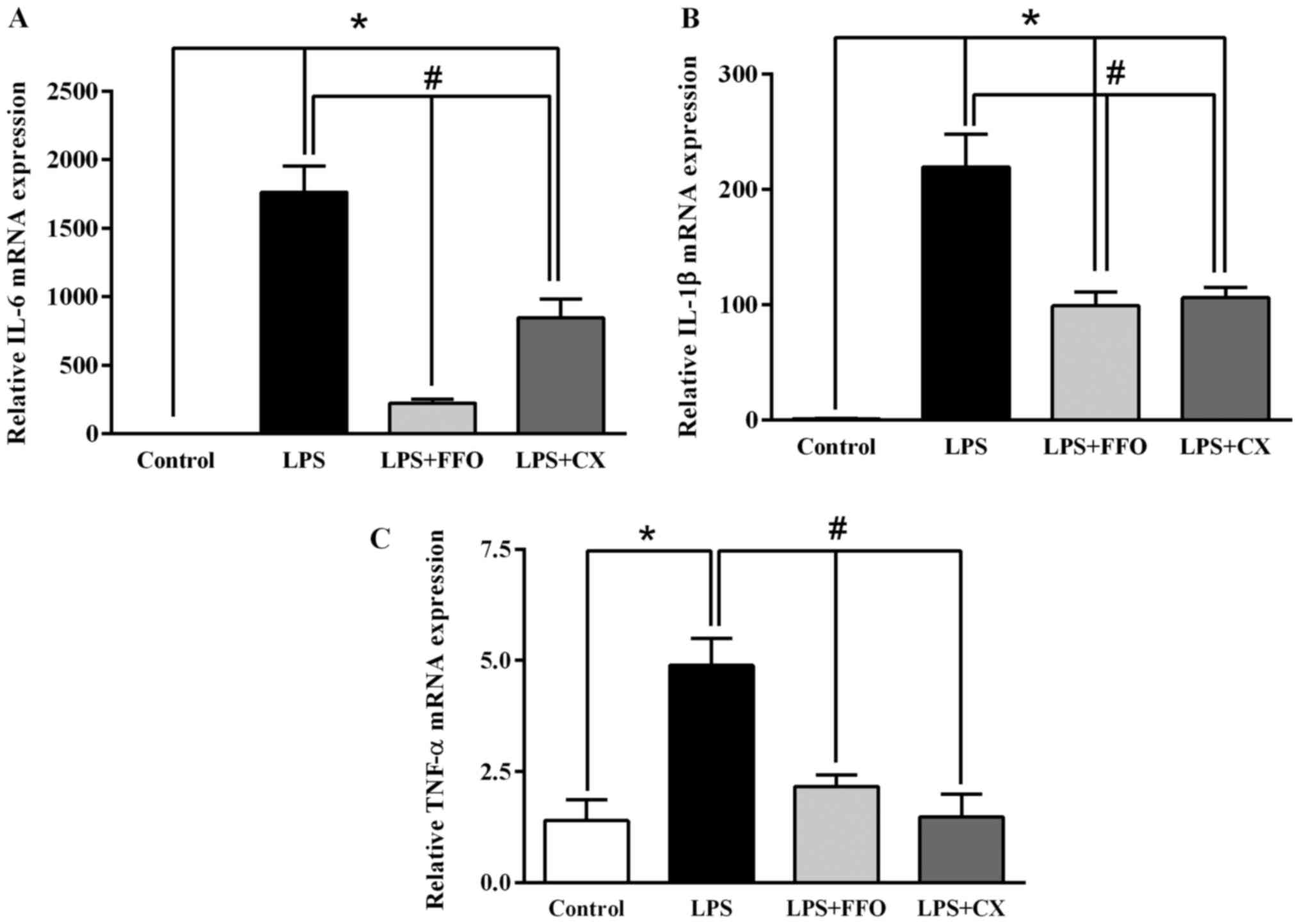
FFO decreases the mRNA expression
levels of pro-inflammatory cytokines in RAW264.7 cells
To confirm the inhibitory effect of FFO on
inflammation, RT-qPCR analysis was performed to detect the mRNA
expression levels of the pro-inflammatory cytokines IL-6, IL-1β and
TNF-α in RAW264.7 cells. A single dose (2%) was selected, as it
significantly decreased all inflammatory cytokines (Fig. 1). The results demonstrated that the
expression levels of the pro-inflammatory cytokines significantly
decreased following additional treatment with FFO and CX compared
with LPS-treated cells (Fig. 2).
Collectively, these results suggested that FFO inhibits the
synthesis of pro-inflammatory cytokines in activated
macrophages.
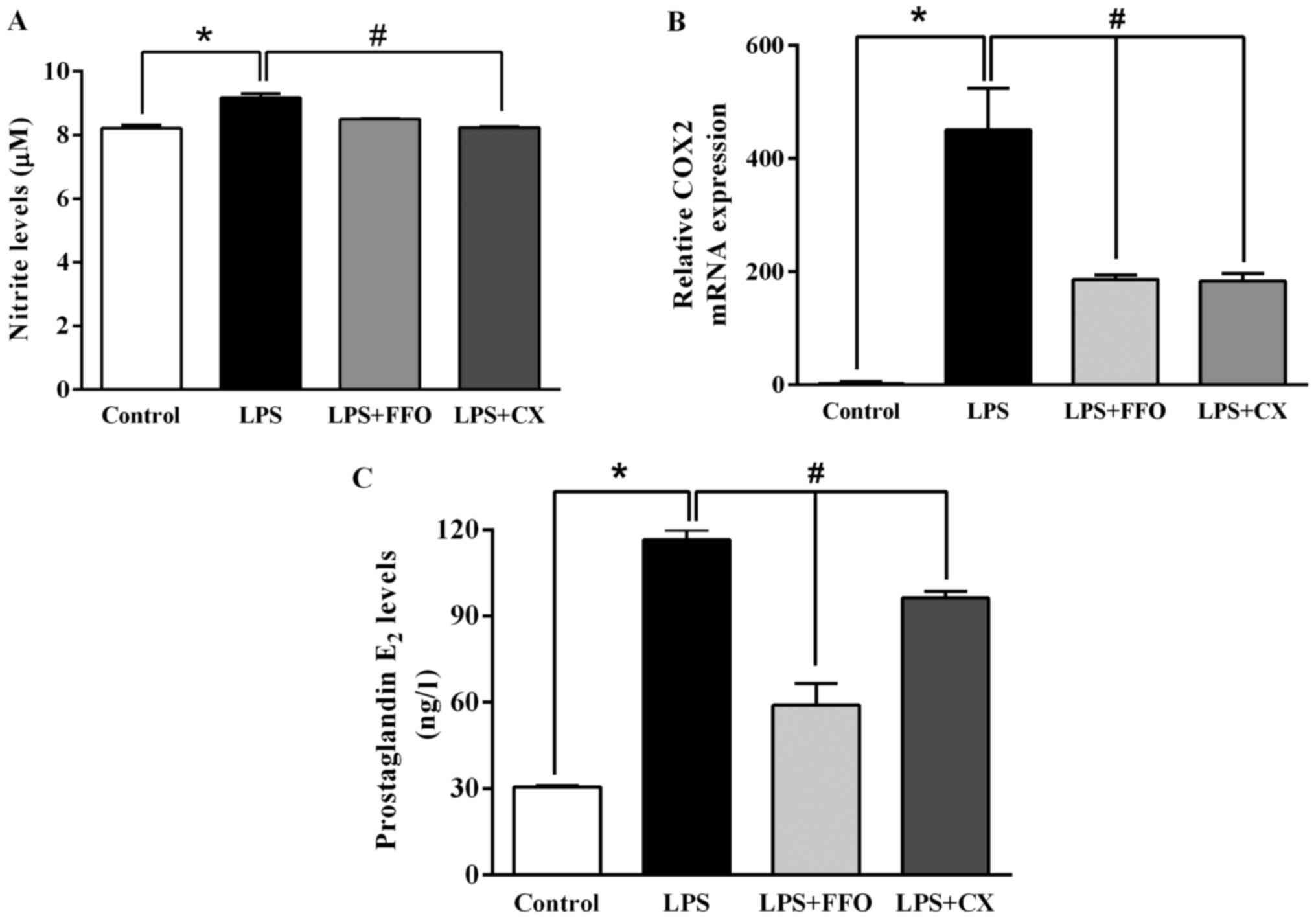
FFO suppresses molecules involved in
inflammatory signaling
The present study further investigated the molecular
mechanisms by which FFO reduces inflammation and thus, signalling
molecules of the inflammatory pathway were assessed. As presented
in Fig. 3A, LPS significantly
enhanced NO production compared with the control. However, the
addition of FFO had no effect on NO production compared with
LPS-treated cells. Furthermore, the effects of FFO on the
production of PGE2, a principal mediator of inflammation, and
COX-2, a prostaglandin-endoperoxide synthase (29), were investigated in the present
study. The results demonstrated that FFO significantly decreased
COX-2 mRNA expression, which in turn decreased PGE2 production
(Fig. 3B and C). Taken together, these results
suggested that FFO exerts anti-inflammatory effects on
LPS-stimulated RAW264.7 cells.
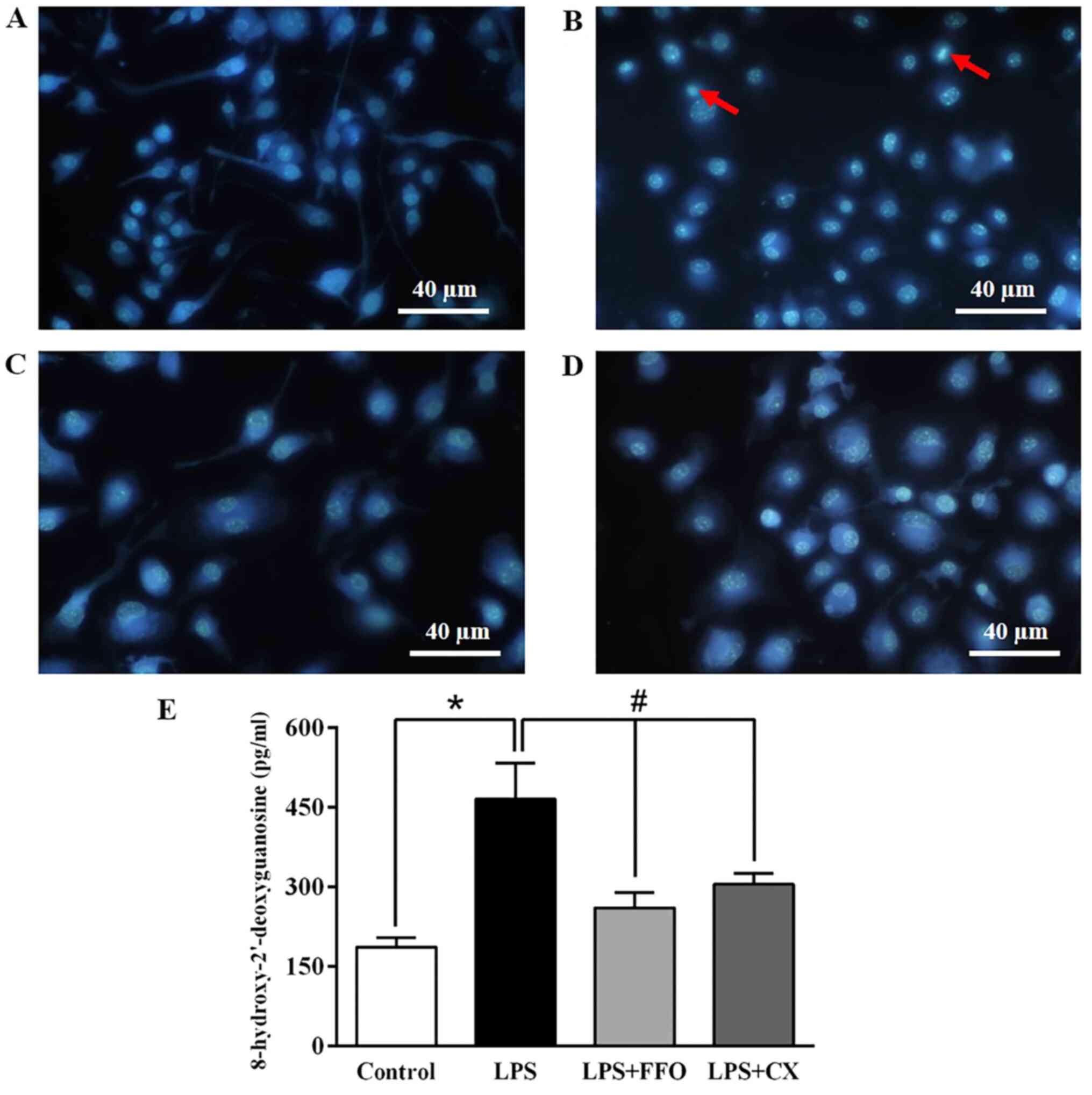
FFO prevents apoptosis and DNA
damage
To validate the inhibitory effect of FFO on cell
apoptosis, RAW264.7 cells treated with FFO, with or without LPS for
24 h, were stained with Hoechst 33342. Microscopic observation
demonstrated that treatment with LPS increased the rate of cell
apoptosis featuring nuclear fragmentation, chromatin condensation
and apoptotic body formation compared with the control cells
(Fig. 4A and B). However, these features were reduced
in FFO- and CX-treated cells (Fig.
4C and D).
In addition, it was investigated whether FFO is able
to prevent DNA damage. As presented in Fig. 4E, treatment with LPS markedly
increased the production of the 8-OHdG adduct, an oxidative
stress-induced DNA damage marker (30), compared with the control cells. It
was observed that the production of 8-OHdG induced by LPS
significantly decreased in cells co-treated with FFO. Similarly, CX
also significantly decreased 8-OHdG levels. Collectively, these
results suggested that FFO has a cytoprotective effect.
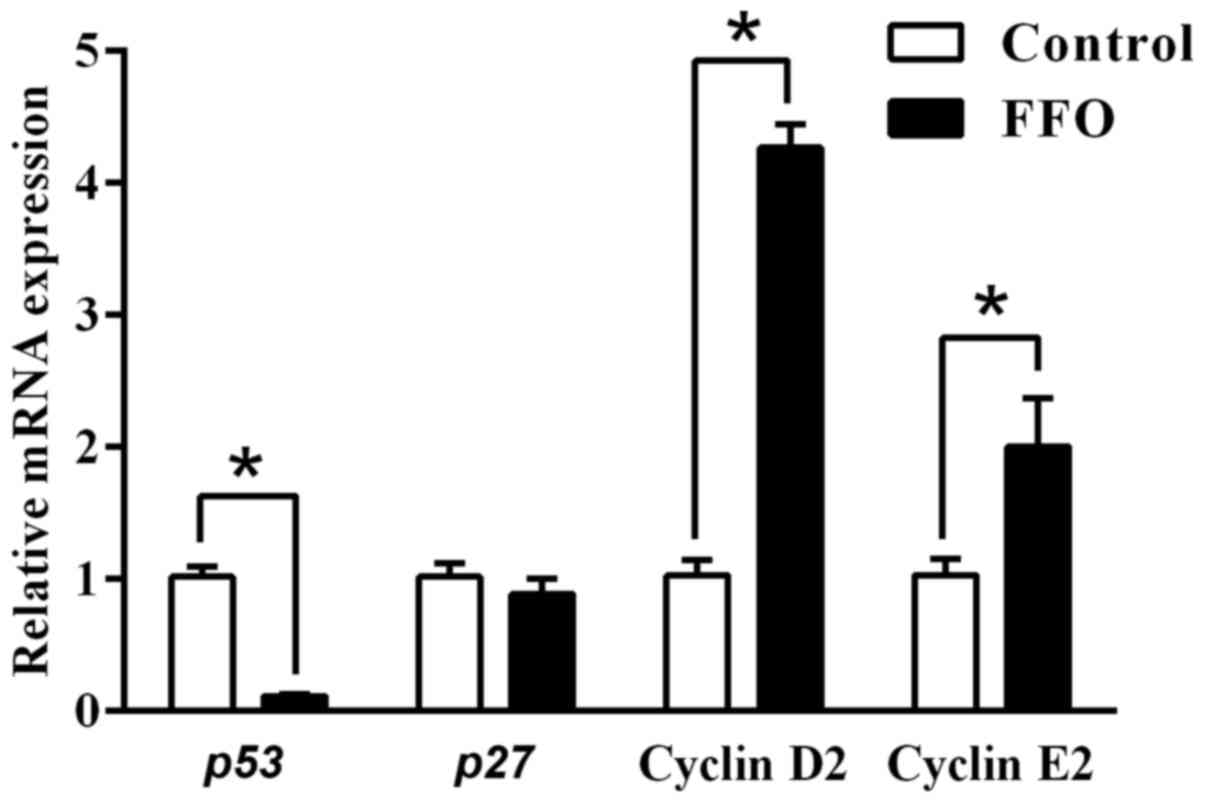
FFO enhances immune response by
modulating cell cycle regulators
A previous study reported that programmed cell death
serves an important role in the regulation of inflammation
(31). Thus, the current study
also investigated the effect of FFO on cell cycle regulators. To
identify the effect of FFO that are responsible for enhancing the
immune response, the present study also investigated the expression
of cell proliferation markers in RAW264.7 cells. As presented in
Fig. 5, the gene expression of the
cell cycle inhibitors p27 and p53 (32-35)
decreased in FFO-treated cells compared with the control.
Furthermore, gene expression of the cell cycle inducers cyclin D2
and cyclin E2 increased in RAW264.7 cells treated with FFO. Taken
together, these results suggested that FFO improves inflammatory
status by modulating cell cycle regulators.
Discussion
To the best of our knowledge, the present study was
the first to demonstrate that FFO rich in omega-9 exerts
anti-inflammatory effects in vitro by decreasing the
expression and secretion of pro-inflammatory cytokines and
mediators, preventing DNA damage via reduction of apoptotic body
formation and 8-OHdG, and also promotes an immune response. A
previous study demonstrated that the activation of tissue
macrophages releases various pro-inflammatory cytokines, including
TNF-α, IL-1 and IL-6, resulting in autoimmune and inflammatory
diseases. In addition, n-3 polyunsaturated fatty acids (PUFAs)
serve anti-inflammatory effects by reducing the production of
TNF-α, IL-1β, IL-6 and tissue factors by stimulated monocytes
(36). Thus, inhibiting the
synthesis of these cytokines may prove useful for the treatment of
autoimmune and inflammatory diseases. The results of the present
study demonstrated that FFO markedly decreased the production of
IL-6, IL-1β and TNF-α and mRNA expression levels in RAW264.7 cells,
similar to NSAIDs. These results suggested that FFO exerts an
anti-inflammatory effect by downregulating pro-inflammatory
cytokines at both the transcriptional and translational levels,
without any cell toxicity. Similarly, oleic acid, one of the most
representative monounsaturated omega-9 fatty acids, was reported to
mediate anti-inflammatory effects by inhibiting reactive oxygen
species, p38 MAPK and Akt signaling pathways/IKK/NF-κB in BV2 cells
(37).
Macrophages are associated with acute and chronic
inflammatory responses by stimulating NO generation, resulting in
an increment of macrophage activity (38). NO and PGE2 production are critical
immune-regulatory biomarkers for chronic inflammatory diseases,
such as hepatic dysfunction and pulmonary disease (39). The results of the present study
demonstrated that FFO decreased PGE2 and its synthase enzyme COX-2,
but not the NO level, similar to the action of NSAIDs. Previous
studies have reported that natural products, including coumarin,
Indonesian cassia extract and Halocynthia aurantium or
docosahexaenoic acid-omega-3, decrease PGE2 and NO expression
levels, which suggests that they have potential as
anti-inflammatory agents (40-42).
Conversely, it has been demonstrated that omega 3 increases the
production of PGE2(43). The
increment of the PGE2 concentration may be inhibited by the NF-κB
signaling pathway and EP4 receptor, resulting in anti-inflammatory
effects (44). There are
controversial data on the effect of PGE2 in inflammation. The
results of the present study demonstrated that FFO contains several
fatty acids, including omega-3, -6 and -9. Consistently, previous
studies have demonstrated that omega-3 fatty acids decrease PGE2 by
decreasing the catalytic monomer of COX-1 dimer by arachidonic acid
and inhibiting COX-1 oxygenation (45,46).
In addition, omega-9 exerts anti-inflammatory effects in
inflammation via a PPAR-γ expression-dependent mechanism (47).
It is well-known that there is a close association
between inflammation and DNA damage (48). NO generated by inflammatory
cytokine stimulation is sufficient to induce oxidative DNA damage
(49). The results of the present
study demonstrated that LPS induced DNA damage by nuclear
fragmentation, chromatin condensation and apoptotic body formation,
the effects of which were reversed following treatment with FFO and
NSAIDs. Consistently, n-3 polyunsaturated fatty acids attenuate
oxidative stress-induced DNA damage in vascular endothelial cells
through upregulation of nuclear factor-mediated antioxidant
response and the decrease in intracellular reactive oxygen species
(50). In addition, the present
study demonstrated that the expression of cell cycle regulators,
including cyclin D2 and cyclin E2, increased following treatment
with FFO, while p53 expression was inhibited. A previous study
reported that cyclin D2 deficiency suppresses immune activity
(51). On the other hand,
hyperactive cyclin D2 expression promotes autoimmune disease or
allograft rejection (52). Other
natural products merely promote immune responses by regulating cell
cycle regulators. For instance, A. asphodeloides enhances
the immune response of RAW264.7 cells by extending the cell cycle
S-phase, suppressing p27 and increasing cyclin D2 and cyclin E2
gene expression (53).
In conclusion, the results of the present study
demonstrated that FFO improved inflammation by suppressing the mRNA
expression and secretion of pro-inflammatory cytokines and their
mediators, and inhibiting apoptotic body formation and DNA damage.
FFO also enhanced the immune response by modulating cell cycle
regulators. Thus, FFO may be used as a natural anti-inflammatory
supplement. Moreover, future in vivo studies and clinical
trials are required to elucidate whether FFO has an overall
anti-inflammatory effect in autoimmune or inflammatory
diseases.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BT performed the experiments, collected and analysed
the data, and wrote the first draft of the manuscript. AO designed
the experiments, collected and analysed the data and wrote the
manuscript. NL and KM provided, analyzed and interpreted the data.
DA designed and verified the experiments, analysed the data, and
wrote and provided critical feedback for the manuscript. DA and AO
confirm the authenticity of all the raw data. All authors read and
approved the final version of the study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pereira DM, Correia-da-Silva G, Valentão
P, Teixeira N and Andrade PB: Anti-inflammatory effect of
unsaturated fatty acids and Ergosta-7,22-dien-3-ol from
Marthasterias glacialis: Prevention of CHOP-mediated
ER-stress and NF-κB activation. PLoS One. 9(e88341)2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lee HJ, Shin JS, Lee KG, Park SC, Jang YP,
Nam JH and Lee KT: Ethanol extract of Potentilla supina
Linne suppresses LPS-induced inflammatory responses through NF-κB
and AP-1 inactivation in macrophages and in endotoxic mice.
Phytother Res. 31:475–487. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Bennett JE, Dolin R and Blaser MJ:
Mandell, Douglas, and Bennett's Principles and Practice of
Infectious Diseases. 8th edition. Bennett JE, Dolin R and Blaser MJ
(eds). Elsevier/Saunders, Philadelphia, PA, p27, 2015.
|
|
4
|
Oh YC, Cho WK, Oh JH, Im GY, Jeong YH,
Yang MC and Ma JY: Fermentation by Lactobacillus enhances
anti-inflammatory effect of Oyaksungisan on LPS-stimulated RAW
264.7 mouse macrophage cells. BMC Complement Altern Med.
12(17)2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fard MT, Arulselvan P, Karthivashan G,
Adam SK and Fakurazi S: Bioactive extract from Moringa
oleifera inhibits the pro-inflammatory mediators in
lipopolysaccharide stimulated macrophages. Pharmacogn Mag. 11
(Suppl 4):S556–S563. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Adrie C, Alberti C, Chaix-Couturier C,
Azoulay E, De Lassence A, Cohen Y, Meshaka P, Cheval C, Thuong M,
Troché G, et al: Epidemiology and economic evaluation of severe
sepsis in France: Age, severity, infection site, and place of
acquisition (community, hospital, or intensive care unit) as
determinants of workload and cost. J Crit Care. 20:46–58.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
O'Brien DJ and Gould IM: Maximizing the
impact of antimicrobial stewardship: The role of diagnostics,
national and international efforts. Curr Opin Infect Dis.
26:352–358. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Parvizi J and Kim GK: High Yield
Orthopaedics. Saunders/Elsevier, Philadelphia, PA, pp325-326,
2010.
|
|
9
|
Dhikav V, Singh S, Pande S, Chawla A and
Anand KS: Non-steroidal drug-induced gastrointestinal toxicity:
Mechanisms and management. JIACM. 4:315–322. 2003.
|
|
10
|
Wangcharoen W, Mengumphan K and
Amornlerdpison D: Fatty acid composition, physical properties,
acute oral toxicity and antioxidant activity of crude lipids from
adipose tissue of some commercialized freshwater catfish. Warasan
Khana Witthayasat Maha Witthayalai Chiang Mai. 42:626–636.
2015.
|
|
11
|
Khoddami A, Ariffin A, Bakar J and Mohd
Ghazali H: Fatty acid profile of the oil extracted from fish waste
(head, intestine and liver) (Sardinella lemuru). World Appl
Sci J. 7:127–131. 2009.
|
|
12
|
Gammone MA, Riccioni G, Parrinello G and
D'Orazio N: Omega-3 polyunsaturated fatty acids: Benefits and
endpoints in sport. Nutrients. 11(46)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim JS and Park JW: Mince from seafood
processing by-product and surimi as food ingredients. In:
Maximising the Value of Marine By-Products. Shahidi F (ed).
Woodhead Publishing, Sawston, pp196-228, 2007.
|
|
14
|
Kim SK and Mendis E: Bioactive compounds
from marine processing byproducts - A review. Food Res Int.
39:383–393. 2006.
|
|
15
|
Tawfik M: Proximate composition and fatty
acids profiles in most common available fish species in Saudi
market. Asian J Clin Nutr. 1:50–57. 2009.
|
|
16
|
Bussarin T, Kriangsak M, Narissara L and
Doungporn A: Comparison of fatty acid profiles of freshwater hybrid
catfish. In: Proceedings of MJU Annual Conference. The Office of
Agricultural Research and Extension Maejo, Maejo University.
pp60-61. 2018.
|
|
17
|
Keapai W, Apichai S, Amornlerdpison D and
Lailerd N: Evaluation of fish oil-rich in MUFAs for anti-diabetic
and anti-inflammation potential in experimental type 2 diabetic
rats. Korean J Physiol Pharmacol. 20:581–593. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Amornlerdpison D, Rattanaphot T, Tongsiri
S, Srimaroeng C and Mengumphan K: Effect of omega-9-rich fish oil
on antioxidant enzymes and relative immune gene expressions in
Nile tilapia (Oreochromis niloticus). J Sci Technol.
41:1287–1293. 2019.
|
|
19
|
AOAC Official Method of Analysis: Official
Method 996.06 Fat (Total, Saturated, and Unsaturated) in Foods.
AOAC International Chapter 41. Oils and Fats: 20-24. 2002.
|
|
20
|
Li Y, Hao N, Zou S, Meng T, Tao H, Ming P,
Li M, Ding H, Li J, Feng S, et al: Immune regulation of RAW264.7
cells in vitro by flavonoids from Astragalus complanatus via
activating the NF-κB signalling pathway. J Immunol Res.
2018(7948068)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yen TL, Chang CC, Chung CL, Ko WC, Yang CH
and Hsieh CY: Neuroprotective effects of platonin, a therapeutic
immunomodulating medicine, on traumatic brain injury in mice after
controlled corticalimpact. Int J Mol Sci. 19(1100)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Teratake Y, Kuga C, Hasegawa Y, Sato Y,
Kitahashi M, Fujimura L, Watanabe-Takano H, Sakamoto A, Arima M,
Tokuhisa T, et al: Transcriptional repression of p27 is essential
for murine embryonic development. Sci Rep. 6(26244)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao H, Bauzon F, Fu H, Lu Z, Cui J,
Nakayama K, Nakayama KI, Locker J and Zhu L: Skp2 deletion unmasks
a p27 safeguard that blocks tumorigenesis in the absence of pRb and
p53 tumor suppressors. Cancer Cell. 24:645–659. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tokumoto M, Fujiwara Y, Shimada A,
Hasegawa T, Seko Y, Nagase H and Satoh M: Tokumoto1 M, Fujiwara Y,
Shimada A, Hasegawa T, Seko Y, Nagase H, Satoh M: Cadmium toxicity
is caused by accumulation of p53 through the down-regulation of
Ube2d family genes in vitro and in vivo. J Toxicol Sci. 36:191–200.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen YG, Zhang Y, Deng LQ, Chen H, Zhang
YJ, Zhou NJ, Yuan K, Yu LZ, Xiong ZH, Gui XM, et al: Control of
methicillin-resistant Staphylococcus aureus pneumonia
utilizing TLR2 agonist Pam3CSK4. PLoS One.
11(e0149233)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Institute of Medicine (US) Panel on
Micronutrients: Dietary Reference Intakes for Vitamin A, Vitamin K,
Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese,
Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies
Press, Washington, DC, 2001.
|
|
28
|
Shin S: Safety of celecoxib versus
traditional nonsteroidal anti-inflammatory drugs in older patients
with arthritis. J Pain Res. 11:3211–3219. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Park JY, Pillinger MH and Abramson SB:
Prostaglandin E2 synthesis and secretion: The role of PGE2
synthases. Clin Immunol. 119:229–240. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Valavanidis A, Vlachogianni T and Fiotakis
C: 8-Hydroxy-2'-deoxyguanosine (8-OHdG): A critical biomarker of
oxidative stress and carcinogenesis. J Environ Sci Health Part C
Environ Carcinog Ecotoxicol Rev. 27:120–139. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yuan L, Zhang Y, Xia J, Liu B, Zhang Q,
Liu J, Luo L, Peng Z, Song Z and Zhu R: Resveratrol induces cell
cycle arrest via a p53-independent pathway in A549 cells. Mol Med
Rep. 11:2459–2464. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yuan L, Zhang Y, Xia J, Liu B, Zhang Q,
Liu J, Luo L, Peng Z, Song Z, Zhu R, Zhu R, et al: Resveratrol
induces cell cycle arrest via a p53-independent pathway in A549
cells. Mol Med Rep. 11:2459–2464. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Shaw PH: The role of p53 in cell cycle
regulation. Pathol Res Pract. 192:669–675. 1996.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Donehower LA: Phosphatases reverse
p53-mediated cell cycle checkpoints. Proc Natl Acad Sci USA.
111:7172–7173. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Møller MB: P27 in cell cycle control and
cancer. Leuk Lymphoma. 39:19–27. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Priante G, Bordin L, Musacchio E, Clari G
and Baggio B: Fatty acids and cytokine mRNA expression in human
osteoblastic cells: A specific effect of arachidonic acid. Clin Sci
(Lond). 102:403–409. 2002.PubMed/NCBI
|
|
37
|
Oh YT, Lee JY, Lee J, Kim H, Yoon KS, Choe
W and Kang I: Oleic acid reduces lipopolysaccharide-induced
expression of iNOS and COX-2 in BV2 murine microglial cells:
Possible involvement of reactive oxygen species, p38 MAPK, and
IKK/NF-kappaB signaling pathways. Neurosci Lett. 464:93–97.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Olefsky JM and Glass CK: Macrophages,
inflammation, and insulin resistance. Annu Rev Physiol. 72:219–246.
2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ansar W and Ghosh S: Inflammation and
inflammatory diseases, markers, and mediators: Role of CRP in some
inflammatory diseases. In: Biology of C Reactive Protein in Health
and Disease. pp67-107, 2016.
|
|
40
|
Kondreddy VK and Kamatham AN: Celecoxib, a
COX-2 inhibitor, synergistically potentiates the anti-inflammatory
activity of docosahexaenoic acid in macrophage cell line.
Immunopharmacol Immunotoxicol. 38:153–161. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Monmai C, Go SH, Shin IS, You SG, Lee H,
Kang SB and Park WJ: Immune-enhancement and anti-inflammatory
activities of fatty acids extracted from Halocynthia
aurantium tunic in RAW264.7 cells. Mar Drugs.
16(16)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sandhiutami NM, Moordiani M, Laksmitawati
DR, Fauziah N, Maesaroh M and Widowati W: In vitro assesment of
anti-inflammatory activities of coumarin and Indonesian cassia
extract in RAW264.7 murine macrophage cell line. Iran J Basic Med
Sci. 20:99–106. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Denkins Y, Kempf D, Ferniz M, Nileshwar S
and Marchetti D: Role of omega-3 polyunsaturated fatty acids on
cyclooxygenase-2 metabolism in brain-metastatic melanoma. J Lipid
Res. 46:1278–1284. 2005.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Liu Y, Chen LY, Sokolowska M, Eberlein M,
Alsaaty S, Martinez-Anton A, Logun C, Qi HY and Shelhamer JH: The
fish oil ingredient, docosahexaenoic acid, activates cytosolic
phospholipase A2 via GPR120 receptor to produce prostaglandin E2
and plays an anti-inflammatory role in macrophages. Immunology.
143:81–95. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wada M, DeLong CJ, Hong YH, Rieke CJ, Song
I, Sidhu RS, Yuan C, Warnock M, Schmaier AH, Yokoyama C, et al:
Enzymes and receptors of prostaglandin pathways with arachidonic
acid-derived versus eicosapentaenoic acid-derived substrates and
products. J Biol Chem. 282:22254–22266. 2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yuan C, Sidhu RS, Kuklev DV, Kado Y, Wada
M, Song I and Smith WL: Cyclooxygenase allosterism, fatty
acid-mediated cross-talk between monomers of cyclooxygenase
homodimers. J Biol Chem. 284:10046–10055. 2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Medeiros-de-Moraes IM,
Gonçalves-de-Albuquerque CF, Kurz AR, Oliveira FM, de Abreu VH,
Torres RC, Carvalho VF, Estato V, Bozza PT, Sperandio M, et al:
Omega-9 oleic acid, the main compound of olive oil, mitigates
inflammation during experimental sepsis. Oxid Med Cell Longev.
2018(6053492)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kawanishi S, Ohnishi S, Ma N, Hiraku Y and
Murata M: Crosstalk between DNA damage and inflammation in the
multiple steps of carcinogenesis. Int J Mol Sci.
18(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jaiswal M, LaRusso NF, Burgart LJ and
Gores GJ: Inflammatory cytokines induce DNA damage and inhibit DNA
repair in cholangiocarcinoma cells by a nitric oxide-dependent
mechanism. Cancer Res. 60:184–190. 2000.PubMed/NCBI
|
|
50
|
Sakai C, Ishida M, Ohba H, Yamashita H,
Uchida H, Yoshizumi M and Ishida T: Fish oil omega-3
polyunsaturated fatty acids attenuate oxidative stress-induced DNA
damage in vascular endothelial cells. PLoS One.
12(e0187934)2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Chunder N, Wang L, Chen C, Hancock WW and
Wells AD: Cyclin-dependent kinase 2 controls peripheral immune
tolerance. J Immunol. 189:5659–5666. 2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Laphanuwat P and Jirawatnotai S:
Immunomodulatory roles of cell cycle regulators. Front Cell Dev
Biol. 7(23)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ji KY, Kim KM, Kim YH, Im AR, Lee JY, Park
B, Na M and Chae S: The enhancing immune response and
anti-inflammatory effects of Anemarrhena asphodeloides
extract in RAW 264.7 cells. Phytomedicine.
59(152789)2019.PubMed/NCBI View Article : Google Scholar
|