Introduction
Cytokines are proteins that regulate the nature,
intensity and duration of the immune response, binding to specific
receptors in target cells (1,2).
Bacterial otitis media is caused by the migration of pathogens from
the nasopharynx to the middle ear, considering that the endotoxin
in the bacterial cell wall component causes the initiation of
inflammation in the middle ear. The endotoxin is a modulator of the
immune response, stimulates macrophages to produce tumor necrosis
factor α (TNF-α) and IL-1β. Keratinocytes produce mediators of
inflammation such as IL-1 α and IL-1β, IL-6 and IL-8(3). Recent findings suggest a link between
processes that occur in the middle ear during otitis and cytokine
levels (4).
IL-6 is involved in regulating the activity of the
tissue factor that leads to the initiation of the coagulation
process. IL-1β regulates thrombomodulin by causing defects in
anticoagulant proteins, thus affecting the activation of protein C,
which is a step in the anticoagulant process that occurs in healthy
individuals. Erythrocytes do not have an IL-1β receptor binding
site, but platelets express the IL-1R1 receptor on their surface
and respond to IL-1β. IL-8 promotes procoagulant activity,
initiating platelet activation. IL-1β, IL-6 and IL-8 produce
changes in the coagulation process by binding to platelets. IL-6
can have the most effects on erythrocytes, due to the presence of a
binding site on them (5,6).
In the present study, IL-1α, IL-6 and IL-8 serum
levels were measured to determine which inflammatory mediators play
a key role in the pathogenesis of chronic suppurative otitis media
(CSOM) and cholesteatoma.
Patients and methods
Patients
The prospective study was performed on a group of 60
patients aged between 9 and 58 years who were diagnosed with CSOM
with and without cholesteatoma, and were hospitalized for surgery
at the Clinical Rehabilitation Hospital, Iasi, Romania. Of these,
28 patients presented with simple CSOM, 21 with CSOM with
cholesteatoma and 11 with cholesteatoma recidivism.
The study was approved by the Ethics Committee of
the ‘Grigore T. Popa’ University of Medicine and Pharmacy (Iasi,
Romania) on 15.07.2017, according to the law of medical research,
no. 206 from 27.05.2004. Informed consent was obtained from all the
patients included in the study.
The inclusion criteria were: Imaging and clinical
diagnosis of CSOM. The exclusion criteria were: Acute or chronic
diseases other than those of the ear, alcohol consumer or smoker,
under any medical treatment, including vitamins.
Patients enrolled were divided into 4 groups: Group
M included healthy individuals, group C included patients with CSOM
with cholesteatoma, group R comprised patients with cholesteatoma
recidivism, and group O included patients with simple CSOM. Group O
included 12 individuals from rural areas and 16 from urban areas,
13 females and 15 males, aged between 16 and 59 years. Group C
included 12 individuals from rural and 9 from urban areas, 10 males
and 11 females aged between 9 and 54 years. Group R was comprised
of 11 individuals, 7 of rural and 4 of urban origin, 5 males and 6
females, aged between 10 and 58 years. The control group comprised
30 clinically healthy individuals, aged between 6 and 54 years; 6
under 18 years and 24 over 18 years. Of these, 13 individuals were
from rural and 17 from urban areas, 18 were female and 12 were
male.
Methods
Blood samples for analysis were taken after 12 h of
fasting and collected into heparin-free tubes and stored on ice at
4˚C. Subsequently, the serum was separated from the cells by
centrifugation at 3,000 x g for 10 min at 4˚C. Serum samples were
stored at -20˚C until they were used.
IL-1α was determined using the MaxDiscovery Human
IL-1α ELISA test kit produced by Bioo Scientific Corporation (cat.
no. 2109). The ELISA technique used specific antibodies and
antigens and different dilutions. IL-6 was determined using the
Human IL-6 ELISA Test Kit produced by Bioo Scientific Corporation
(cat. no. 2107). IL-8 was determined using the Human IL-8 ELISA
Test Kit produced by Bioo Scientific Corporation (cat. no.
2134).
Statistical analysis
Data obtained were processed using SPSS database
version 18.0 (SPSS, Inc.) and processed with specific statistical
functions. Data are represented as mean ± standard deviation. The
tests of normality in frequency statistics, Skewness and Kurtosis
(-2<P<2), were used to evaluate the distribution of
continuous variables. The following indicators were used in data
processing: Mean, median, variance, standard deviation, minimum and
maximum values. The Student's t-test, a parametric test that
compares the average values recorded in two groups with normal
distributions was used. The F test (ANOVA) was also used to compare
three or more groups with a normal distribution. Post hoc analysis
was made with Bonferroni test.
Results
The Skewness and Kurtosis tests (-2 <P<2)
suggested that the value series of IL-1α, IL-6 and IL-8 were
homogenous.
IL-1α values
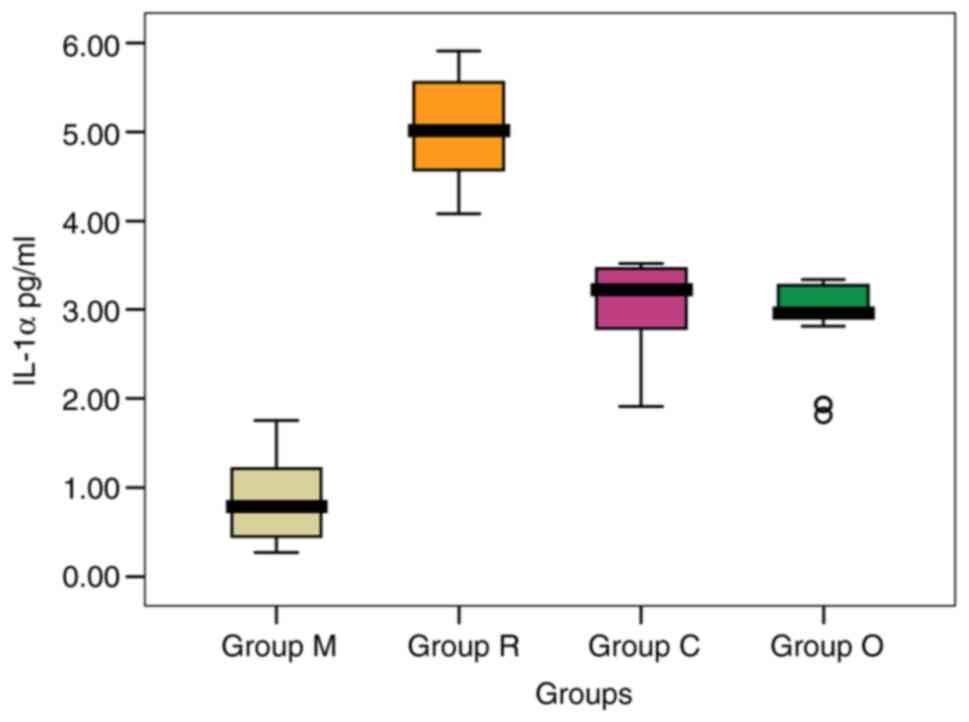
The IL-1α values in group C ranged between 1.91 and
3.52 pg/ml with a mean value of 3.011 pg/ml (Table I). In group O, IL-1α values were
between 1.81 and 3.34 pg/ml. The mean values from the M, C and O
groups were significantly lower compared to that recorded in group
R. [0.815, 3.011 and 2.911 pg/ml (P=0.964) vs. 5.021 pg/ml
(P=0.001)].
 | Table IComparison of IL-1α levels (pg/ml)
between study groups. |
Table I
Comparison of IL-1α levels (pg/ml)
between study groups.
| Parameters | Group M | Group R | Group C | Group O |
|---|
| N | 30 | 11 | 21 | 28 |
| Mean | 0.815 | 5.021a,c | 3.011a,d | 2.911a,b,e,f |
| Median | 0.796 | 5.024 | 3.230 | 2.974 |
| Standard
deviation | 0.412 | 0.616 | 0.594 | 0.452 |
| Variance | 0.170 | 0.379 | 0.351 | 0.204 |
| Skewness test | 0.636 | 0.107 | -1.038 | -1.545 |
| Standard error of
skewness | 0.427 | 0.661 | 0.501 | 0.441 |
| Kurtosis | -0.533 | -1.378 | -0.354 | 1.510 |
| Standard error of
kurtosis | 0.833 | 1.279 | 0.972 | 0.858 |
| Minimum | 0.270 | 4.080 | 1.910 | 1.810 |
| Maximum | 1.170 | 5.910 | 3.520 | 3.340 |
| Percentile | | | | |
|
25 | 0.447 | 4.524 | 2.743 | 2.904 |
|
50 | 0.796 | 5.024 | 3.230 | 2.974 |
|
75 | 1.215 | 5.608 | 3.460 | 3.283 |
The mean level recorded in group M was significantly
lower compared to all the other groups analyzed (0.815 pg/ml;
P=0.001) (Fig. 1). The highest mean
value was recorded in patients with cholesteatoma recidivism (group
R, 5.021 pg/ml).
Analyzing the mean values obtained, the patients
with CSOM with cholesteatoma and CSOM without cholesteatoma had
significantly lower IL-1α levels compared to patients with
cholesteatoma recidivism.
In both males and females, the highest mean level of
IL-1α was found in patients with recurrence, and the lowest in
controls. Within the group, no significant differences between
sexes were recorded (P>0.05) (Table
II).
 | Table IIMean IL-1α values (pg/ml) compared by
sex and study group. |
Table II
Mean IL-1α values (pg/ml) compared by
sex and study group.
| Sex | Group M | Group R | Group C | Group O | FANOVA
testa |
|---|
| Male | 0.86±0.42 | 5.07±0.66 | 2.88±0.69 | 2.81±0.50 | 0.001 |
| Female | 0.79±0.42 | 4.97±0.63 | 3.13±0.49 | 3.03±0.37 | 0.001 |
| Student's
t-test | 0.653 | 0.811 | 0.365 | 0.192 | - |
IL-6 serum values
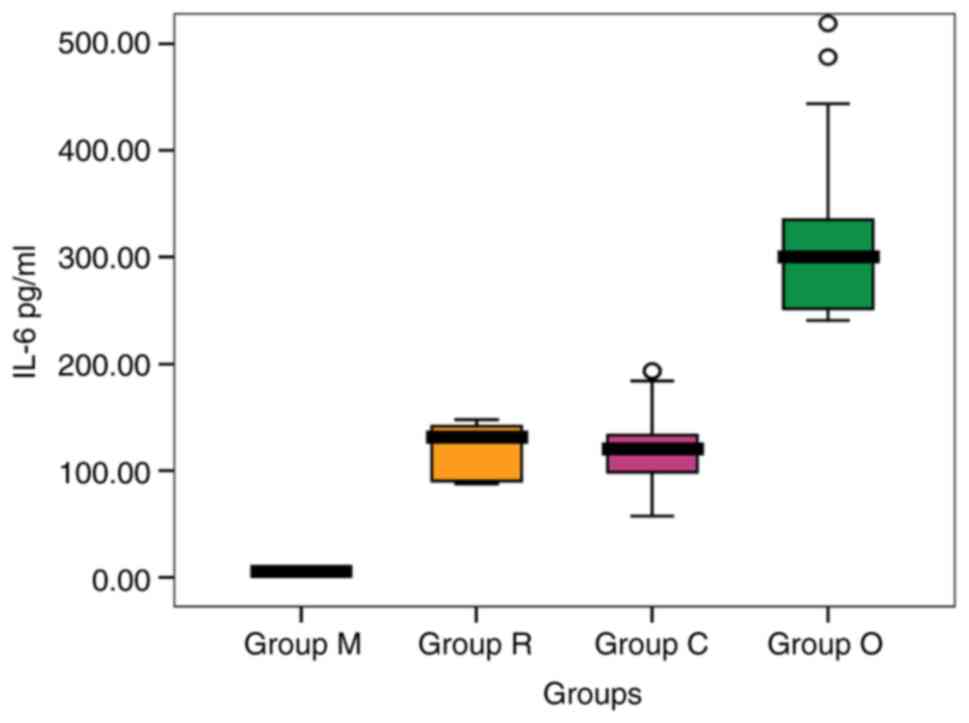
IL-6 serum values recorded the highest mean level in
patients from group O ranging from 57.23 to 193.33 pg/ml, followed
by that recorded in patients from group C with variations in the
range from 240.62 to 519.63 pg/ml. The values were significantly
higher compared to group M [316.59 and 121.23 pg/ml (P=0.001) vs.
5.81 pg/ml (P=0.001)] (Table
III).
 | Table IIIComparison of IL-6 levels (pg/ml)
between study groups. |
Table III
Comparison of IL-6 levels (pg/ml)
between study groups.
| Parameters | Group M | Group R | Group C | Group O |
|---|
| N | 30 | 11 | 21 | 28 |
| Mean | 5.81 | 117.77a,c | 121.23a,b,d,e | 316.59a,f |
| Median | 6.20 | 131.93 | 121.11 | 300.63 |
| Standard
deviation | 1.63 | 26.01 | 40.25 | 75.84 |
| Variance | 2.64 | 22.09 | 33.20 | 23.96 |
| Skewness test | -0.304 | -0.168 | 0.320 | 1.255 |
| Standard error of
skewness | 0.427 | 0.661 | 0.501 | 0.441 |
| Kurtosis | -1.100 | -2.265 | -0.415 | 1.064 |
| Standard error of
kurtosis | 0.833 | 1.279 | 0.972 | 0.858 |
| Minimum | 3.09 | 87.71 | 57.23 | 240.62 |
| Maximum | 8.06 | 147.38 | 193.33 | 519.63 |
| Percentiles | | | | |
|
25 | 4.32 | 89.40 | 97.01 | 251.71 |
|
50 | 6.20 | 131.93 | 121.11 | 300.63 |
|
75 | 7.17 | 142.18 | 144.66 | 335.46 |
The mean level in group R was slightly lower than in
patients from group C. The serum level obtained was significantly
higher compared to group M [117.77 and 121.23 pg/ml (P=0.551)
vs. 5.81 pg/ml (P=0.001)] (Fig.
2). The highest mean value was recorded in group O (316.59
pg/ml).
In both males and females, the highest mean level of
IL-6 was found in patients from group O and the lowest in group M.
Within the group, only in patients from group O was there a
significantly higher mean value in males than females (P=0.001)
(Table IV).
 | Table IVMean IL-6 values (pg/ml) compared by
sex and study group. |
Table IV
Mean IL-6 values (pg/ml) compared by
sex and study group.
| Sex | Group M | Group R | Group C | Group O | FANOVA
testa |
|---|
| Male | 5.89±1.72 | 123.86±27.91 | 107.43±43.19 | 356.38±80.94 | 0.001 |
| Female | 5.75±1.61 | 110.47±24.40 | 133.78±34.61 | 270.69±32.16 | 0.001 |
| Student's
t-test | 0.817 | 0.424 | 0.138 | 0.001 | - |
IL-8 values
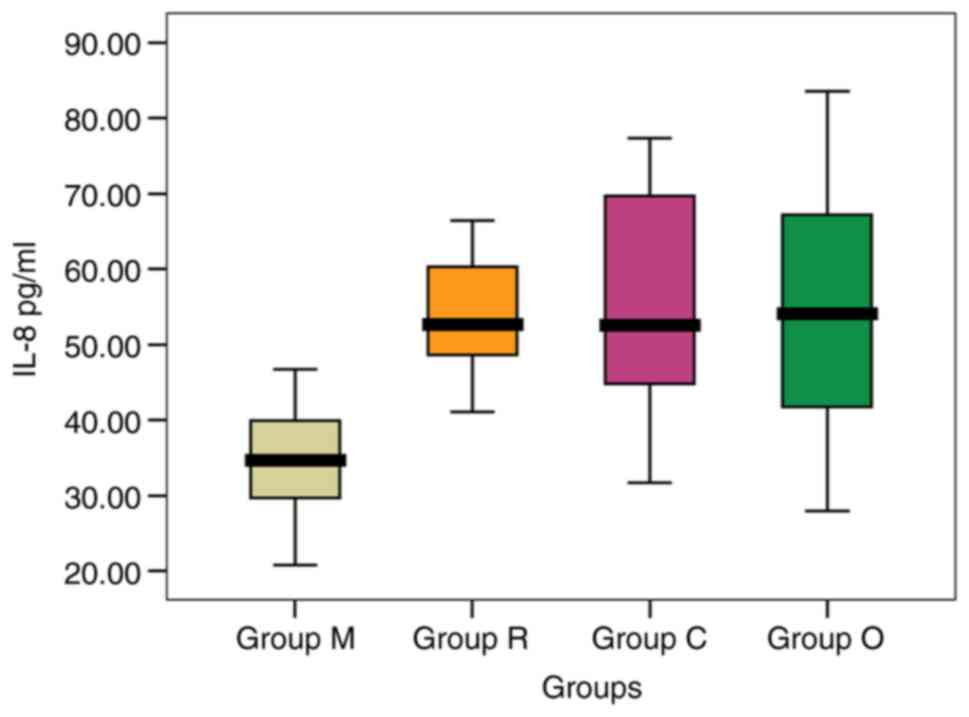
Values obtained after the IL-8 dosing for the groups
are: In group O the IL-8 level varied between 27.95 and 83.58
pg/ml, and in patients from group C the variation was in the range
31.72-77.39 pg/ml, with the lowest mean level being recorded in
patients in group M, significantly lower compared to the levels
recorded in groups C and O [34.31 vs. 53.91 and 54.20 pg/ml
(P=0.001)]. The mean level in patients from group R was slightly
lower than that in patients from groups O and C [53.74 vs. 54.20
and 53.91 pg/ml (P=0.944)], but significantly higher compared to
the healthy subjects [53.74 vs. 34.31 pg/ml (P=0.001)] (Table V).
 | Table VComparison of IL-8 levels (pg/ml)
between study groups. |
Table V
Comparison of IL-8 levels (pg/ml)
between study groups.
| Parameters | Group M | Group R | Group C | Group O |
|---|
| N | 30 | 11 | 21 | 28 |
| Mean | 34.31 | 53.74a,c | 53.91a,b,d,e | 54.20a,b,f,g |
| Median | 34.79 | 52.72 | 52.65 | 54.17 |
| Standard
deviation | 7.28 | 8.60 | 14.64 | 16.99 |
| Variance | 21.21 | 16.00 | 27.16 | 31.37 |
| Skewness test | -0.171 | 0.154 | 0.203 | 0.117 |
| Standard error of
skewness | 0.427 | 0.661 | 0.501 | 0.441 |
| Kurtosis | -0.882 | -1.030 | -1.123 | -1.058 |
| Standard error of
kurtosis | 0.833 | 1.279 | 0.972 | 0.858 |
| Minimum | 20.76 | 41.10 | 31.72 | 27.95 |
| Maximum | 46.68 | 66.45 | 77.39 | 83.58 |
| Percentiles | | | | |
|
25 | 29.44 | 47.74 | 42.19 | 41.75 |
|
50 | 34.79 | 52.72 | 52.65 | 54.17 |
|
75 | 40.28 | 60.84 | 70.04 | 67.63 |
In males, the highest mean level of IL-8 was found
in patients from group O, while in females, the highest mean level
of IL-8 was found in patients from group C, and in both sexes the
lowest level average IL-8 was observed in group M (Fig. 3). Within the group, no significant
differences between sexes were registered (P>0.05) (Table VI).
 | Table VIMean IL-8 values (pg/ml) compared by
sex and study group. |
Table VI
Mean IL-8 values (pg/ml) compared by
sex and study group.
| Sex | Group M | Group R | Group C | Group O | FANOVA
testa |
|---|
| Male | 35.34±7.80 | 56.15±11.33 | 50.18±13.45 | 58.33±19.45 | 0.002 |
| Female | 33.62±7.06 | 50.85±2.32 | 57.31±15.46 | 49.44±12.76 | 0.001 |
| Student's
t-test | 0.537 | 0.335 | 0.275 | 0.172 | - |
Discussion
The study by Skotnicka and Hassmann (7) investigated the levels of IL-1β, IL-8
and IL-10 in the middle ear effusions of 38 patients with otitis
media. While there was a strong statistical correlation between
IL-1β and IL-6 levels and also between IL-6 and IL-10 levels, no
direct correlation could be established between IL-1β and IL-10
levels. It was also not possible to establish a correlation between
age, hearing loss, number of episodes of acute otitis media and
cytokine levels (7).
IL-1 stimulates the release of other cytokines,
arachidonic acid metabolism, cyclooxygenase and lipoxygenase
pathways. IL-1 is synthesized by activated macrophages, where IL-1
production is stimulated by lipopolysaccharides and leukotrienes.
IL-1β is produced and released extracellularly by inflammatory
cells, such as macrophages and monocytes. IL-1α is located
predominantly intracellularly or on the surface of these cells
(3).
In the current study, the patients with CSOM with
and without cholesteatoma had significantly lower IL-1α levels
compared to patients with cholesteatoma recidivism. However, the
lowest value of this cytokine were found in the healthy group. In
both males and females, the highest mean level of IL-1α was found
in patients with cholesteatoma recidivism, and the lowest in the
healthy group. It can be stated that a higher systemic inflammatory
status is maintained with the chronicity of the disease,
particularly in the group of patients with cholesteatoma
recidivism.
Recent findings demonstrated the association between
TNF-α and IL-1α and the degree of bone destruction in individuals
diagnosed with CSOM with cholesteatoma, suggesting the necessity of
complementary therapy to reduce TNF-α and IL-1α in this category of
patients (8).
Kuczkowski et al (9) found high levels of IL-1α and IL-6 in
cholesteatoma compared to granulation tissue, the highest values
being recorded in cases with significant lesions of the ossicles in
the middle ear, thus justifying the aggressiveness of cholesteatoma
(9). IL-1 stimulates bone
resorption by increasing the number of osteoclast precursor cells
(10) and the proliferation of
keratinocytes (11), which are part
of the cholesteatoma, causing both an increase in size and an
increased rate of relapse.
IL-6 is an acute phase cytokine and can induce CRP
production. It is also frequently used as a marker of bacterial
infection (12). IL-6 appears to
play an important role in inflammation in otitis media. Elevated
concentrations of IL-1, IL-6 and TNF-α have been correlated with
otitis media in children (13).
Kerschner et al stated that IL-6 regulates mucin secretion
in the epithelial cells of the middle ear, a process involved in
the pathogenesis of serous and mucous otitis media (14).
IL-6 induces osteoclast formation. High
concentrations of IL-6 have been correlated with bone chain erosion
and the presence of large amounts of granular tissue
intraoperatively (10).
IL-6 serum values obtained in the present study were
statistically significant higher compared to the control group, the
highest being in patients with CSOM without cholesteatoma. The mean
level recorded in patients with recurrence was slightly lower
compared to that recorded in patients with CSOM with cholesteatoma,
without statistical significance, but significantly higher compared
to the control. In both males and females, the highest mean level
of IL-6 was found in patients with CSOM, and the lowest in
individuals without otic impairment.
Similar results with elevated serum IL-6 levels were
obtained in a recent study showing that IL-6, metalloproteinase-2
and metalloproteinase-9 are related to the degree of destruction in
the middle ear and the severity of the disease (15).
The results were similar to the study conducted by
Nofal and Kwatly (16) which
obtained a higher serum level of IL-6 in acute otitis media and
CSOM, compared to a control group of healthy individuals. Authors
of that study argue that IL-6 and CRP may be factors in predicting
streptococcal otitis media (16).
Liu et al (17) observed an association between IL-6
and p-STAT3 expression: Increased expression of p-STAT3 in the
cholesteatoma epithelium causes an increase in IL-6, leading to
activation of IL-6/JAK/STAT3 signaling in cholesteatoma epithelial
hyperplasia (17).
Certain authors consider IL-8 responsible for the
formation of serous otitis media by: Accumulation of leukocytes in
the middle ear, and in situ activation of leukocytes
followed by tissue damage. IL-8 promotes the production of
collagenases, proteins involved in the process of bone lysis
(10). It is a secondary cytokine
that intervenes in inflammation in the middle ear, being a
chemotaxic agent for neutrophils (3). IL-8 also increases the production of
adhesion molecules for the attachment and migration of neutrophils
that occur during the acute inflammatory response. As IL-8 can
cause the release of lysosomal enzymes, it can initiate tissue
damage leading to chronic inflammation in the ear (18-20).
In the present study, the mean level of IL-8 in
patients with cholesteatoma recurrence was slightly lower than that
in patients with CSOM with or without cholesteatoma, but
significantly higher compared to the control group.
In males, the highest mean level of IL-8 was found
in patients with CSOM, while in females, the highest mean level of
IL-8 was found in patients with CSOM with cholesteatoma, in both
cases there are higher values compared to the control.
Elevated levels of IL-8 have also been identified in
middle ear fluid in otitis serosa, with higher values in children
than in adults (21). In
experiments performed in guinea pigs, TNF-α and IL-1β reached peak
values before IL-8, suggesting that it is produced by inflammatory
cells accumulated by the influence of TNF-α and IL-1β (3,22).
The most recent study found a reduction in IL-8 and
A20 (protein that is encoded by the TNFAIP3 gene) expression
and an almost complete decrease in IL-1β, IL-6 and TNFα expression
in cholesteatoma stem cells exposed to both lipopolysaccharides and
the antagonist lipopolysaccharide toll-like receptor 4 (TLR4) from
R. sphaeroides, compared to those treated with
lipopolysaccharides only and untreated subjects. This could become
a specific local treatment strategy that acts on TLR4-mediated
signaling in cholesteatoma stem cells. It is therefore suggested
that local administration of post-surgical drugs could reduce the
cholesteatoma recidivism (23).
The current study has some limitations. Firstly, the
study enrolled a relatively small number of patients, making it
difficult to generalize the results. Secondly, children and adults
were included together in the analysis and we consider that in the
future differentiated studies on pediatric and adult populations
are needed, which would allow a more accurate quantification of the
inflammation parameters.
In conclusion, high serum levels of Il-1α, Il-6 and
IL-8 were recorded in all otitis media groups compared to the
healthy group. IL-1α had the highest value in patients with
cholesteatoma recidivism. IL-6 and IL-8 had the highest value in
patients with CSOM.
Acknowledgements
Not applicable.
Funding
Funding: The present study was financially supported by the
‘Grigore T. Popa’ University of Medicine and Pharmacy.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RS, CF and LMR acquired experimental data and
created the database. MCB, MMB and BMD analyzed the data and
drafted the manuscript. BMC and MDC designed the study. RS, CF, MDC
and LMR supervised data analysis. RS, CF and LMR checked and
approved the authenticity of the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the ‘Grigore T. Popa’ University of Medicine and Pharmacy (Iasi,
Romania) on 09.06.2015, according to the law of medical research,
no. 206 from 27.05.2004. Informed consent was obtained from all the
patients included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dinarello CA: Historical insights into
cytokines. Eur J Immunol. 37 (Suppl 1):S34–S45. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Turner MD, Nedjai B, Hurst T and
Pennington DJ: Cytokines and chemokines: At the crossroads of cell
signalling and inflammatory disease. Biochim Biophys Acta.
1843:2563–2582. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Juhn SK, Jung MK, Hoffman MD, Drew BR,
Preciado DA, Sausen NJ, Jung TT, Kim BH, Park SY, Lin J, et al: The
role of inflammatory mediators in the pathogenesis of otitis media
and sequelae. Clin Exp Otorhinolaryngol. 1:117–138. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Skovbjerg S, Roos K, Nowrouzian F, Lindh
M, Holm SE, Adlerberth I, Olofsson S and Wold AE: High cytokine
levels in perforated acute otitis media exudates containing live
bacteria. Clin Biol Infect. 16:1382–1388. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Regnault V, de Maistre E, Carteaux JP,
Gruel Y, Nguyen P, Tardy B and Lecompte T: Platelet activation
induced by human antibodies to interleukin-8. Blood. 101:1419–1421.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bester J and Pretorius E: Effects of
IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot
viscoelasticity. Sci Rep. 6(32188)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Skotnicka B and Hassmann E:
Proinflammatory and immunoregulatory cytokines in the middle ear
effusions. Int J Pediatr Otorhinolaryngol. 72:13–17.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Artono Surarto B, Purnami N, Hutahaen F
and Mahardhika MR: The association of IL-1 alpha level and TNF
alpha expressions on bone destruction in chronic suppurative otitis
media and cholesteatoma. Indian J Otolaryngol Head Neck Surg.
72:1–7. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kuczkowski J, Sakowicz-Burkiewicz M,
Iżycka-Świeszewska E, Mikaszewski B and Pawełczyk T: Expression of
tumor necrosis factor-α, interleukin-1α, interleukin-6 and
interleukin-10 in chronic otitis media with bone osteolysis. ORL J
Otorhinolaryngol Relat Spec. 73:93–9. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Alves AL and Ribeiro FAQ: The role of
cytokines in acquired middle ear cholesteatoma: Literature review.
Rev Bras Otorrinol. 70:813–818. 2004.
|
|
11
|
Didierjean L, Salomon D, Mèrot Y,
Siegenthaler G, Shaw A, Dayer JM and Saurat JH: Localization and
characterization of the interleukin 1 immunoreactive pool (IL-1
alpha and beta forms) in normal human epidermis. J Invest Dermatol.
92:809–816. 1989.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kishimoto T: The biology of interleukin-6.
Blood. 74:1–10. 1989.PubMed/NCBI
|
|
13
|
Yellon RF, Doyle WJ, Whiteside TL, Diven
WF, March AR and Fireman P: Cytokines, immunoglobulins, and
bacterial pathogens in middle ear effusions. Arch Otolaryngol Head
Neck Surg. 121:865–869. 1995.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kerschner JE, Meyer TK, Yang C and Burrows
A: Middle ear epithelial mucin production in response to
interleukin-6 exposure in vitro. Cytokine. 26:30–36.
2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu Y, Tang X, Shao W and Lu Y: Effect of
CT manifestations of cholesteatoma on MMP-2, MMP-9 and IL-6 in the
serum of patients. Exp Ther Med. 17:4441–4446. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nofal K and Kwatly K: Serum interleukin-6
and C-reactive protein in bacterial otitis media patients in
Damascus city. Int J Chem Farm. 7:403–408. 2015.
|
|
17
|
Liu W, Xie S, Chen X, Rao X, Ren H, Hu B,
Yin T, Xiang Y and Ren J: Activation of the IL-6/JAK/STAT3
signaling pathway in human middle ear cholesteatoma epithelium. Int
J Clin Exp Pathol. 7:709–715. 2014.PubMed/NCBI
|
|
18
|
Schröder JM: The neutrophil-activating
peptide 1/interleukin 8, a novel neutrophil chemotactic cytokine.
Arch Immunol Ther Exp (Warsz). 40:23–31. 1992.PubMed/NCBI
|
|
19
|
Takeuchi K, Maesako K, Yuta A and Sakakura
Y: Interleukin-8 gene expression in middle ear effusions. Ann Otol
Rhinol Laryngol. 103:404–407. 1994.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Butnaru C, Serban R, Martu C, Lungu A,
Doroftei EA, Cobzeanu B and Cozma S: Otitis media complications.
In: Proceeding of National ENT Head and Neck Surgery Conference.
Berteșteanu SVG and Grigore R (eds). Filodiritto Publ., Arad,
pp102-106, 2018.
|
|
21
|
Hotomi M, Samukawa T and Yamanaka N:
Interleukin-8 in otitis media with effusion. Acta Otolaryngol.
114:406–409. 1994.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sato K, Liebeler CL, Quartey MK, Le CT and
Giebink GS: Middle ear fluid cytokine and inflammatory cell
kinetics in the chinchilla otitis media model. Infect Immun.
67:1943–1946. 1999.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schürmann M, Greiner JFW, Volland-Thurn V,
Oppel F, Kaltschmidt C, Sudhoff H and Kaltschmidt B: Stem
cell-induced inflammation in cholesteatoma is inhibited by the TLR4
antagonist LPS-RS. Cells. 9(199)2020.PubMed/NCBI View Article : Google Scholar
|