Introduction
Pulmonary arterial hypertension is characterized by
persistent airflow limitation and is severely harmful to human
health. The pathophysiology of pulmonary arterial hypertension is
characterized by hypoxic pulmonary vasoconstriction and vascular
remodeling (1,2). Pulmonary artery smooth muscle cells
(PASMCs) are not only involved in pulmonary vascular remodeling,
but also act as immune cells that synthesize and secrete
inflammatory factors to promote the development of pulmonary
vascular inflammation. Among these factors, toll-like receptors
(TLRs) are highly expressed in pulmonary macrophages, smooth muscle
cells, epithelial cells and vascular endothelial cells (3). During airway remodeling in asthma,
TLRs mediate nuclear factor (NF)-κB signaling, which is an
important pathway that regulates the synthesis and secretion of
inflammatory factors in bronchial smooth muscle cells (4,5). In
addition, specific mammalian TLRs, such as TLR-2/4, can be
activated by lipopolysaccharides (LPS) to promote the production of
cytokines, chemokines, adhesion molecules and acute-phase proteins
to regulate inflammatory response (6).
Geniposide, an iridoid glycoside extracted from the
fruit of Gardenia jasminoides Ellis, is a popular medicine
for the treatment of acute conjunctivitis, hepatic disorders,
inflammatory diseases and hematuria (7-9).
Evidence to date has identified the anti-tumor, anti-inflammation,
and antioxidant properties of geniposide (10,11).
Several reports have demonstrated that geniposide can protect rat
hepatocytes and hippocampus against oxidative injury (12,13).
Furthermore, geniposide attenuates LPS-induced over-release of
pro-inflammatory cytokines in a mouse model of sepsis (14). However, whether geniposide could
have and anti-inflammatory effect in pulmonary arterial
hypertension remains unclear.
In the present study, the anti-inflammatory role of
geniposide was investigated in relation to α7 nicotine
acetylcholine receptor (α7nAChR), which is a subtype of nAChRs with
critical functions in the cholinergic system (15). α7nAChR is widely distributed in
various neuronal and non-neuronal tissues (16), such as vascular smooth muscle cells
(17), endothelial cells (18), and lung cells (19), and participates in various
physiological and pathological processes, including inflammation
and neurotransmitter release (16,20-22).
α7nAChR was demonstrated to suppress LPS-induced placental
inflammation in rats by inhibiting cytokine release and leukocyte
infiltration (23). The expression
of α7nAChR in normal lung cells and in a series of human lung
cancer cells is ubiquitous and can be promoted by nicotine-derived
nitrosamine ketone (24).
Furthermore, α7nAChR exhibits anti-inflammatory effects in
LPS-induced human airway epithelial cells (25).
The present study explored the effect of geniposide
on LPS-induced injury in PASMCs. While LPS is usually used to
stimulate inflammatory response in certain conditions, such as
sepsis, ARDS and ALI, it has also been used to establish models of
pulmonary hypertension (26,27).
The present study aimed to investigate the involvement of TLR
signaling in vascular remodeling through the study of LPS-induced
inflammatory injury, which is representative of the pulmonary
arterial hypertension phenomenon. It was previously revealed that
geniposide can exert protective effects by regulating the
expression of α7nAChR through the TLR/myeloid differentiation
primary response 88 (MyD88) signaling pathway. The findings
collectively revealed the role of geniposide and α7nAChR activation
on LPS-induced PASMC apoptosis and inflammation, and provided novel
insights into the treatment and management of lung diseases through
treatment using geniposide.
Materials and methods
Materials
LPS, pentobarbital sodium, penicillin and
streptomycin were purchased from Sigma-Aldrich; Merck KGaA.
Dulbecco's modified Eagle medium (DMEM) and fetal bovine serum
(FBS) were purchased from Thermo Fisher Scientific, Inc. Geniposide
was purchased from MedChem Express. PNU282987 (PNU; α7nAChR
agonist) and methyllycaconitine (MLA; α7nAChR inhibitor) were
purchased from Bio-Techne. Primary antibodies against α7nAChR (cat.
no. ab216485; 1:800), Bax (cat. no. ab32503; 1:2,000), Bcl-xL (cat.
no. ab32370; 1:1,000), Bcl-2 (cat. no. ab59348; 1:500),
cytochrome-c (Cyt-c; cat. no. ab76237; 1:200), tumor necrosis
factor-α (TNF-α; cat. no. ab6671; 1:1,200), NF-κB (cat. no.
ab16502; 1:2,000), TLR-2 (cat. no. ab108998; 1:5,000), TLR-4 (cat.
no. ab13556; 1:500), MyD88 (cat. no. ab2068; 1:1,000), and GAPDH
(cat. no. ab181602; 1:10,000) were purchased from Abcam. Secondary
IgG antibody (cat. no. E030130; 1:10,000) was purchased from
EarthOx Life Sciences. ELISA kits for interleukin (IL)-18 (cat. no.
RA20058), TNF-α (cat. no. RA20035), and IL-1β (cat. no. RA20020)
were purchased from Bioswamp Life Science Lab.
Ethics statement
Permission was granted from Wuhan No. 4 Hospital,
Puai Hospital to perform the animal experiments at Wuhan Myhalic
Biotechnology Co., Ltd. (http://www.hlkbio.cn) and the study was approved by
the Animal Ethics Committee of the Model Animal Institute at Wuhan
Myhalic Biotechnology Co., Ltd. (approval no. HLK-20190611-01). All
animal experiments were performed in accordance with the
‘Guidelines for Experimental Animals’ from the Ministry of Science
and Technology (Beijing, China). All dissections were performed
according to recommendations proposed by the European Commission
and all efforts were made to minimize animal suffering.
Cell isolation and culture
Rat PASMCs were isolated as previously described
with some modifications (28).
Three healthy male Sprague-Dawley rats weighing 200 g were obtained
from the Hubei Provincial Center for Disease Control and Prevention
(Hubei, China). Before the experiment, the rats were housed and
maintained at the Model Animal Institute at Wuhan Myhalic
Biotechnology Co., Ltd. After the rats were anesthetized via
intraperitoneal administration of pentobarbital sodium (40 mg/kg),
the pulmonary arteries were obtained, cleaned of connective
tissues, and opened longitudinally in a sterile environment. The
adventitia was carefully removed and the luminal surface was
scraped with forceps to remove endothelial cells. The isolated
tissues were then minced into 1-mm2 pieces and digested
into 0.2% collagenase II (cat. no. 17101015; Gibco; Thermo Fisher
Scientific, Inc.) for 45-60 min. After digestion, the tissues were
centrifuged at 175 x g to obtain smooth muscle cells, which were
then seeded onto culture flasks in DMEM supplemented with 10% FBS,
100 U penicillin, and 100 µg/ml streptomycin (Beijing Solarbio
Science and Technology Co., Ltd.) at 37˚C in a humidified
atmosphere containing 5% CO2 for 2-3 h. The medium was
changed every three days and experiments were performed when the
cells reached 80% confluence at passage 3-6 from primary culture.
After PASMC isolation, the experimental rats were sacrificed by an
overdose of pentobarbital sodium (120 mg/kg) and death was verified
when heartbeat could not be detected.
Cell treatment
To investigate the effect of geniposide on PASMCs,
cells were pretreated for 2 h with geniposide at various
concentrations (0-20 µM), followed by exposure to LPS (10 mg/l) for
2 h. Both geniposide and LPS were dissolved in ultrapure water.
Then, the PASMCs were randomly divided into the following
experimental groups: i) control; ii) LPS (10 mg/l); iii) LPS (10
mg/l) + geniposide (10 µM); iv) LPS (10 mg/l) + geniposide (10 µM)
+ MLA (10 nM); and v) LPS (10 mg/l) + geniposide (10 µM) + PNU (5
µM). Both MLA and PNU were dissolved in 100 mM DMSO and treatment
time was 48 h at 37˚C.
Identification of PASMCs
The morphology of PASMCs was observed by optical
microscopy. In addition, isolated cells were identified by
immunofluorescence staining of alpha-smooth muscle actin (α-SMA;
cat. no. ab5694; Abcam). For immunofluorescence, cells were seeded
at 1x105 cells/ml onto 1.5-mm glass coverslips coated
with 0.2% gelatin and washed with PBS. The cells were fixed with 4%
paraformaldehyde for 15 min at room temperature and permeabilized
in 0.2% Triton X-100 for 10 min at room temperature. After 1 h of
blocking in 5% bovine serum albumin (cat. no. 10270-106; Gibco;
Thermo Fisher Scientific, Inc.) at room temperature, the cells were
incubated with the primary antibody against α-SMA (1:300 dilution)
for 1 h at room temperature. The cells were then washed and
incubated for 1 h at 37˚C with FITC goat anti-rabbit IgG (H+L)
(1:200; cat. no. SAB43712; Bioswamp Life Science Lab) and
4'-6-diamidino-2-phenylindole as a nuclear counterstain. The
coverslips were washed and imaged using a Zeiss Axioplan II
microscope (Zeiss AG) at x100 magnification.
PASMC viability
After the PASMCs were subjected to various
treatments, they were seeded in a 96-well plate at the density of
1x104 cells per well. Cell viability was determined
using MTT assay (cat. no. PAB180013; Bioswamp Life Science Lab) as
previously reported (29). MTT
reagent (20 µl) was added into each well and incubated for 2-4 h at
37˚C. When the purple precipitate became visible, the medium was
removed and 150 µl DMSO was added to each well. The microplate was
shaken at a low speed for 10 min and the absorbance read at 570 nm
on a microplate reader.
PASMC apoptosis
The apoptosis of PASMCs was measured using an
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
flow cytometry kit (BD Biosciences) according to the manufacturers'
instructions. PASMCs were washed twice with ice-cold PBS and
resuspended in 200 µl of binding buffer at a concentration of
1x106 cells/ml. Annexin V-FITC and PI (10 µl of each)
were added, and the cells were incubated for 30 min at 4˚C in the
dark. Finally, 300 µl of binding buffer was added and the cells
were analyzed by flow cytometry (Cytomics FC 500; Beckman Coulter,
Inc.) within 1 h using CXP Analysis 2.0 software
(Beckman-Coulter).
ELISA
The levels of IL-18, TNF-α and IL-1β secreted in
PASMC culture medium were evaluated by ELISA according to the
manufacturers' instructions.
Western blot
Western blot was performed to determine the
expression of α7nAChR, Bax, Bcl-xL, Bcl-2, Cyt-c, TNF-α, NF-κB,
TLR-2, TLR-4 and MyD88. For total protein extraction, the cells
were washed twice with PBS and lysed with radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology) containing
protease inhibitors at 4˚C. Cell lysate was centrifuged at 12,000 x
g for 15 min at 4˚C and the supernatant was collected. The protein
concentration was determined by a bicinchoninic acid assay kit
(cat. no. PAB180007; Bioswamp Life Science Lab). Proteins (30 µg)
were separated by 10% SDS-PAGE and transferred onto PVDF membranes
(EMD Millipore). The membranes were blocked for 2 h at room
temperature with 5% skimmed milk in Tris-buffered saline (TBS; 20
mmol/l Tris, 500 mmol/l NaCl and 0.05% Tween 20). Subsequently, the
membranes were incubated with primary antibodies against α7nAChR,
Bax, Bcl-xL, Bcl-2, Cyt-c, TNF-α, NF-κB, TLR-2, TLR-4, MyD88, and
GAPDH overnight at 4˚C. GAPDH was selected as an internal
reference. The membranes were then washed with TBS and incubated
with goat secondary antibody for 2 h at room temperature. Enhanced
chemiluminescence reagent (EMD Millipore) was used to detect the
signal on the membrane. Membranes were scanned with Gel Doz EZ
imager (Bio-Rad Laboratories, Inc.). The gray values of the protein
bands were quantified using ImageJ version 1.52a (National
Institute of Health) and relative expression was calculated by
dividing the gray value of each protein of interest with that of
GAPDH.
Statistical analysis
Data were presented as the means ± standard
deviation. Statistical analyses were performed with SPSS 19.0
software package (IBM Corp.) and data were compared using one-way
analysis of variance followed by Tukey's post-hoc test. P<0.01
was considered to indicate a statistically significant
difference.
Results
Observation and identification of rat
PASMCs
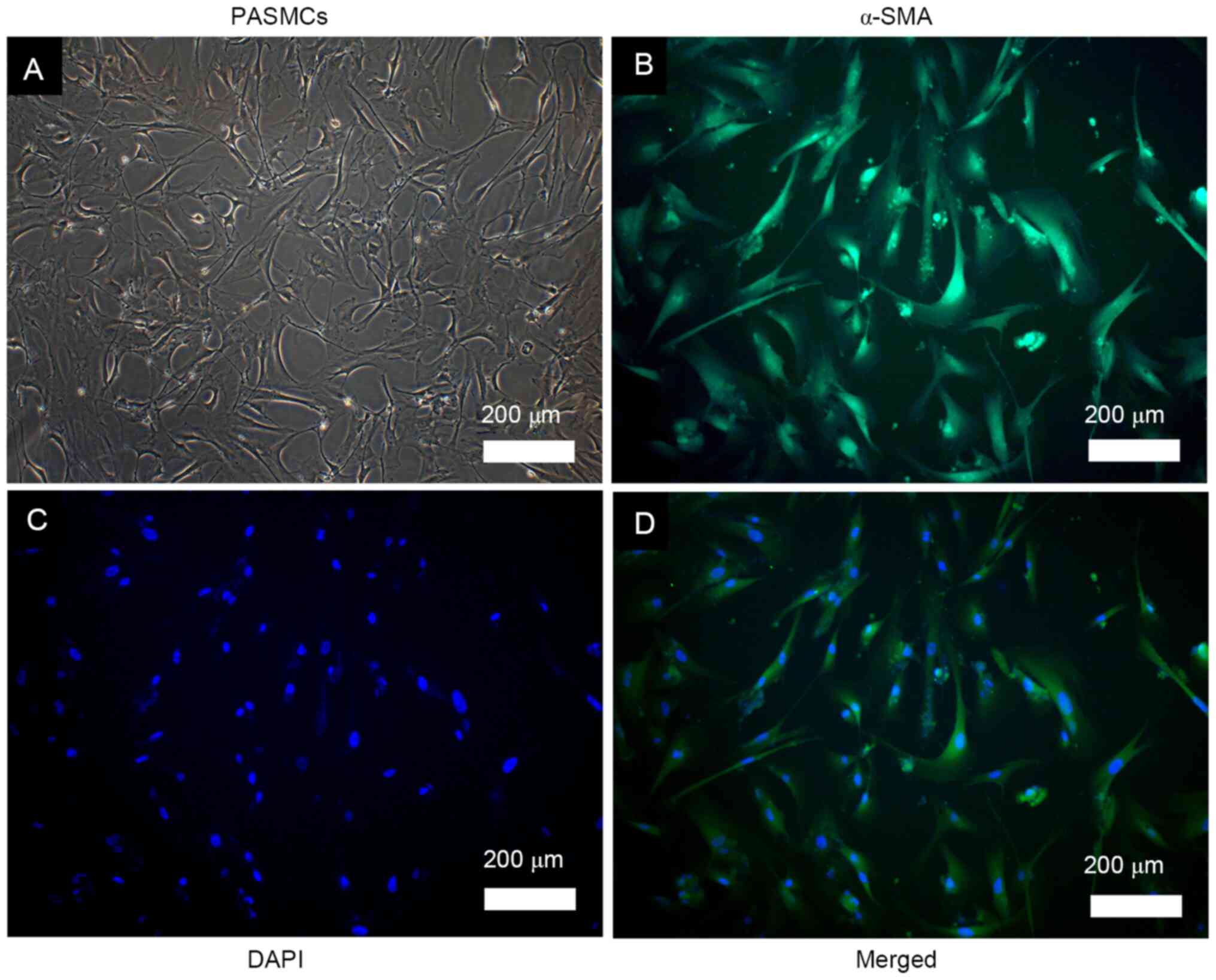
PASMCs were successfully isolated from rat pulmonary
arteries. The cells were elongated and spindle-shaped, exhibiting
the typical hill and valley morphology (Fig. 1A). Positive immunofluorescence
staining was observed for α-SMA, which was homogeneously
distributed into the cytoplasm, as indicated by the green
fluorescence (Fig. 1B-D). These
observations confirmed that the cultured cells were smooth muscle
cells.
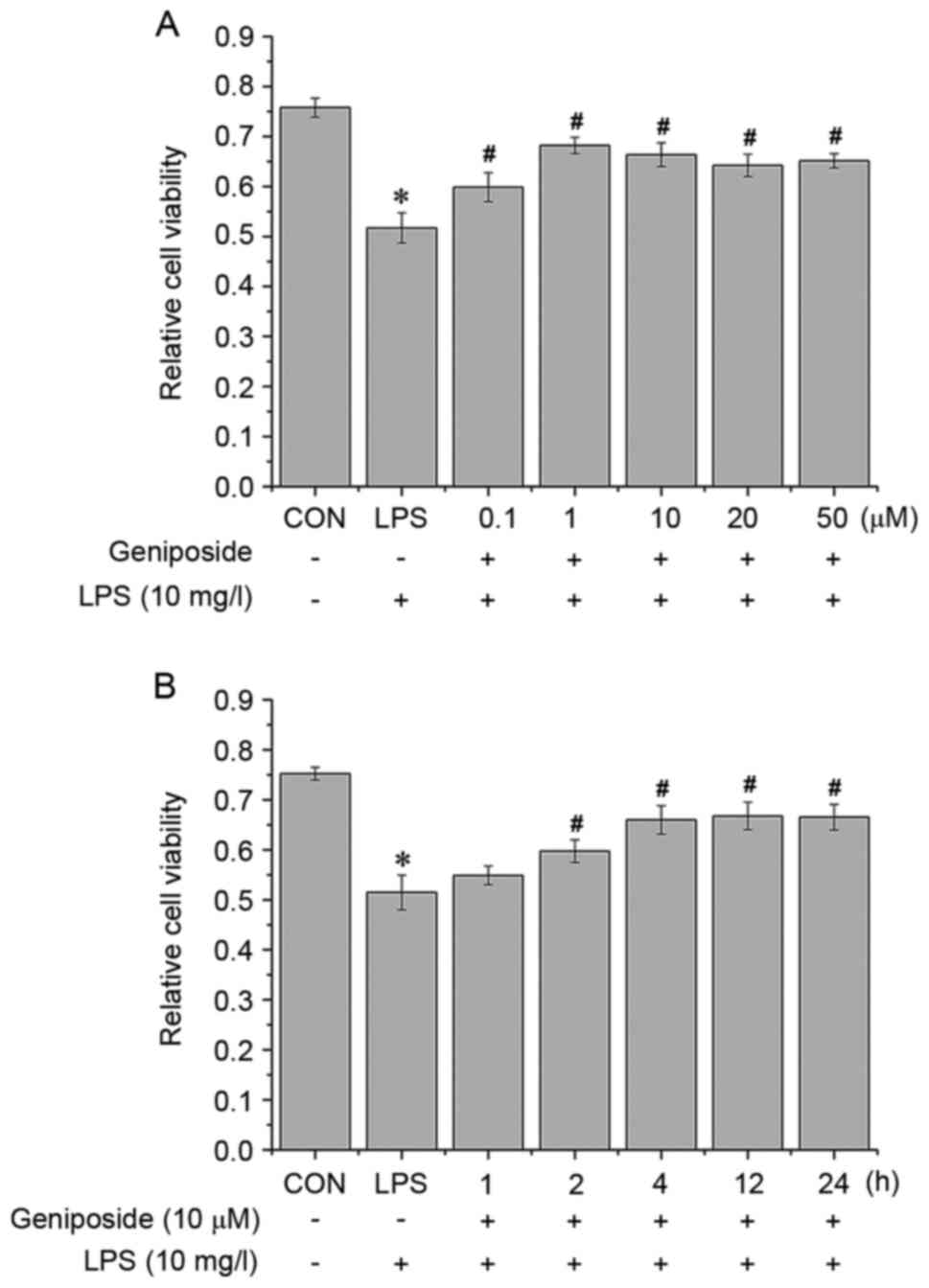
Geniposide improves LPS-induced
decrease in PASMC viability
Prior to experiments involving geniposide, the
optimal duration of LPS treatment was determined in a preliminary
experiment. PASMCs were treated with LPS (10 mg/ml) (17) for 0.5, 1, 2, 4 and 6 h and the cell
viability was measured (results not shown). The results
demonstrated that LPS inhibited cell viability in a time-dependent
manner for up to 2 h; however, the cell viability did not further
decrease thereafter. Subsequently, 2 h treatment was used in
subsequent experiments with LPS stimulation. To determine the
effect of geniposide on LPS-induced cell damage, PASMCs were
pretreated with geniposide at various concentrations for different
durations to determine the best treatment concentration and time
for subsequent experiments. Then, cells were exposed to 10 mg/l LPS
in the presence or absence of geniposide. The results from MTT
assay demonstrated that geniposide increased the viability of
LPS-treated PASMCs. Because there was no significant difference
between 0.1, 1, 10, 20 and 50 µM (Fig.
2A), the middle concentration, 10 µM, was selected as the
treatment dose. Subsequently, pretreatment with 10 µM geniposide
for 2, 4, 12 or 24 h significantly ameliorated the viability of
LPS-treated PASMCs. Because there was no significant difference
between these time points (Fig.
2B), the shortest duration (2 h) was selected as the treatment
time.
Geniposide inhibits LPS-induced
apoptosis and inflammatory factor release in PASMCs
To verify the effect of geniposide on LPS-induced
cell apoptosis, PASMCs were pretreated with 10 µM geniposide for 2
h and exposed to 10 mg/l LPS for 2 h. The results from flow
cytometry demonstrated that PASMC apoptosis was increased after LPS
treatment; however, preincubation with geniposide significantly
decreased the percentage of apoptotic cells due to LPS (Fig. 3A). To further examine PASMC
apoptosis, the apoptosis-related proteins Bax, Bcl-xL, Bcl-2, Cyt-c
and TNF-α were detected by western blotting (Fig. 3B). The protein expression of the
pro-apoptotic factors Bax, Cyt-c and TNF-α in LPS-treated PASMCs
was higher than those in the control group, whereas the protein
expression of the anti-apoptotic protein Bcl-2 and Bcl-xL was
decreased. However, geniposide administration alleviated the
stimulating effect of LPS on apoptosis by downregulating Bax, Cyt-c
and TNF-α expression and upregulating Bcl-2 and Bcl-xL
expression.
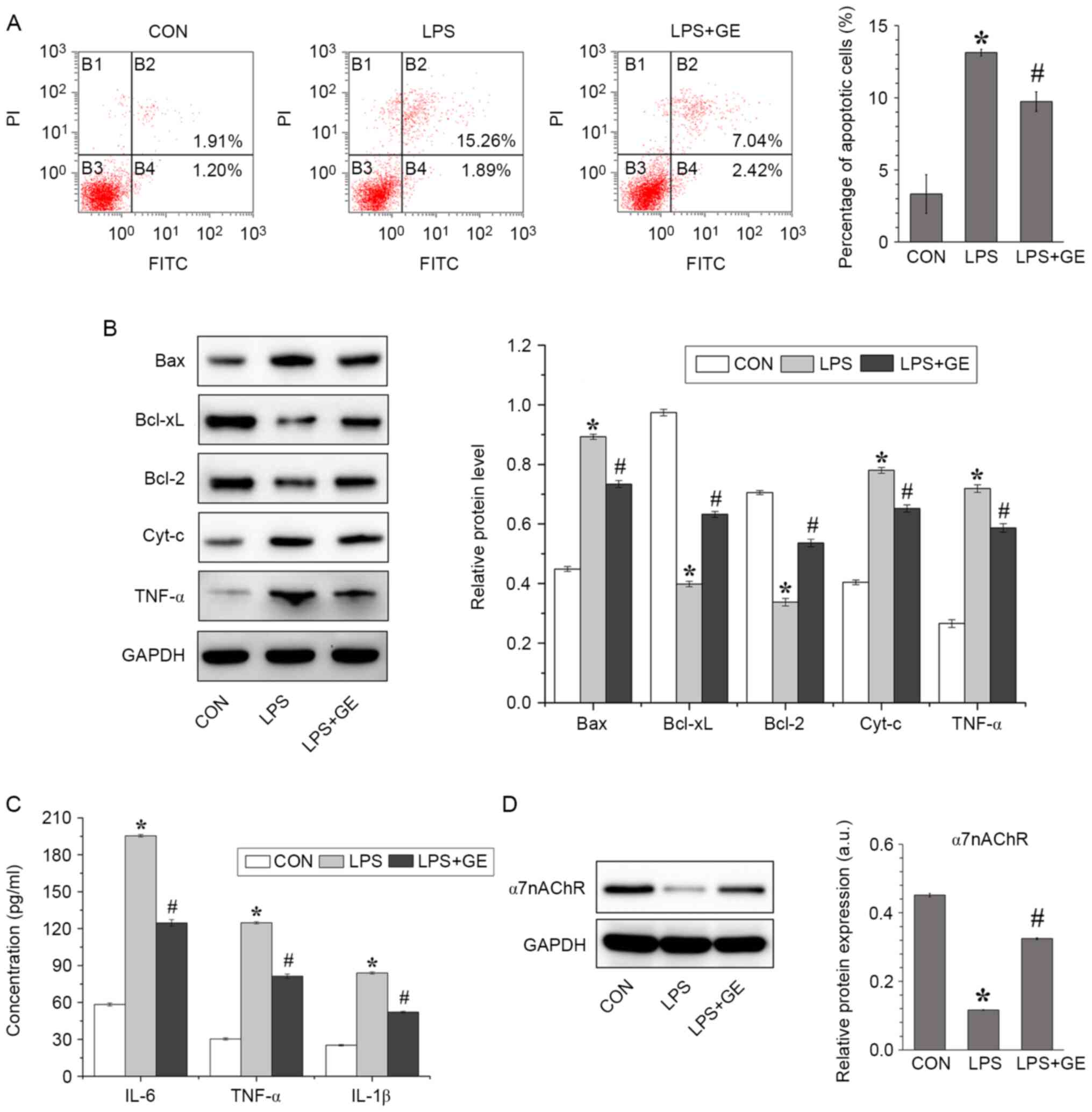 | Figure 3Geniposide inhibited PASMC apoptosis
induced by LPS. (A) Flow cytometric detection and quantification of
PASMC apoptosis after geniposide pretreatment and LPS stimulation.
(B) α7nAChR protein expression was assessed by western blotting.
(C) Evaluation of the secretion of inflammatory cytokines IL-18,
TNF-α, and IL-1β in culture medium by ELISA. (D) Bax, Bcl-xL,
Bcl-2, Cyt-c and TNF-α protein expression was assessed by western
blotting. All values were expressed as the means ± standard
deviation (n=3). *P<0.01 vs. CON.
#P<0.01 vs. LPS. CON, control; LPS,
lipopolysaccharides; GE: Geniposide; PASMCs, pulmonary arterial
smooth muscle cells; Cyt-c, cytochrome c; TNF-α, tumor necrosis
factor-α; IL, interleukin; PI, propidium iodide; FITC, fluorescein
isothiocyanate. |
Subsequently, the levels of IL-18, TNF-α and IL-1β
secreted in the cell culture medium were detected by ELISA
(Fig. 3C). Compared with the
non-treated cells, cells treated with LPS presented significantly
higher levels of cytokines; however, geniposide pretreatment
significantly decreased the levels of IL-18, TNF-α and IL-1β
released by LPS-treated PASMCs. In addition, the protein expression
of α7nAChR was evaluated in PASMCs (Fig. 3D). The results demonstrated that LPS
significantly downregulated the protein expression of α7nAChR in
PASMCs compared with non-treated cells; however, this decrease in
α7nAChR expression was reversed by geniposide treatment.
Activation of α7nAChR enhances the
effect of geniposide on LPS-induced injury
To verify the role of α7nAChR in geniposide
treatment against PASMC damage, LPS-treated PASMCs were cultured
with the α7nAChR agonist PNU282987 or the α7nAChR antagonist MLA.
The apoptotic rate of LPS-treated PASMCs pretreated with geniposide
and PNU282987 was significantly decreased compared with that of
LPS-treated cells treated with geniposide only. Furthermore, the
decrease in PASMC apoptotic rate following geniposide treatment was
eliminated by the α7nAChR antagonist MLA (Fig. 4A). The activation of α7nAChR also
increased the viability of LPS-treated PASMCs that were pretreated
with geniposide, whereas inhibition of α7nAChR had the opposite
effect (Fig. 4B).
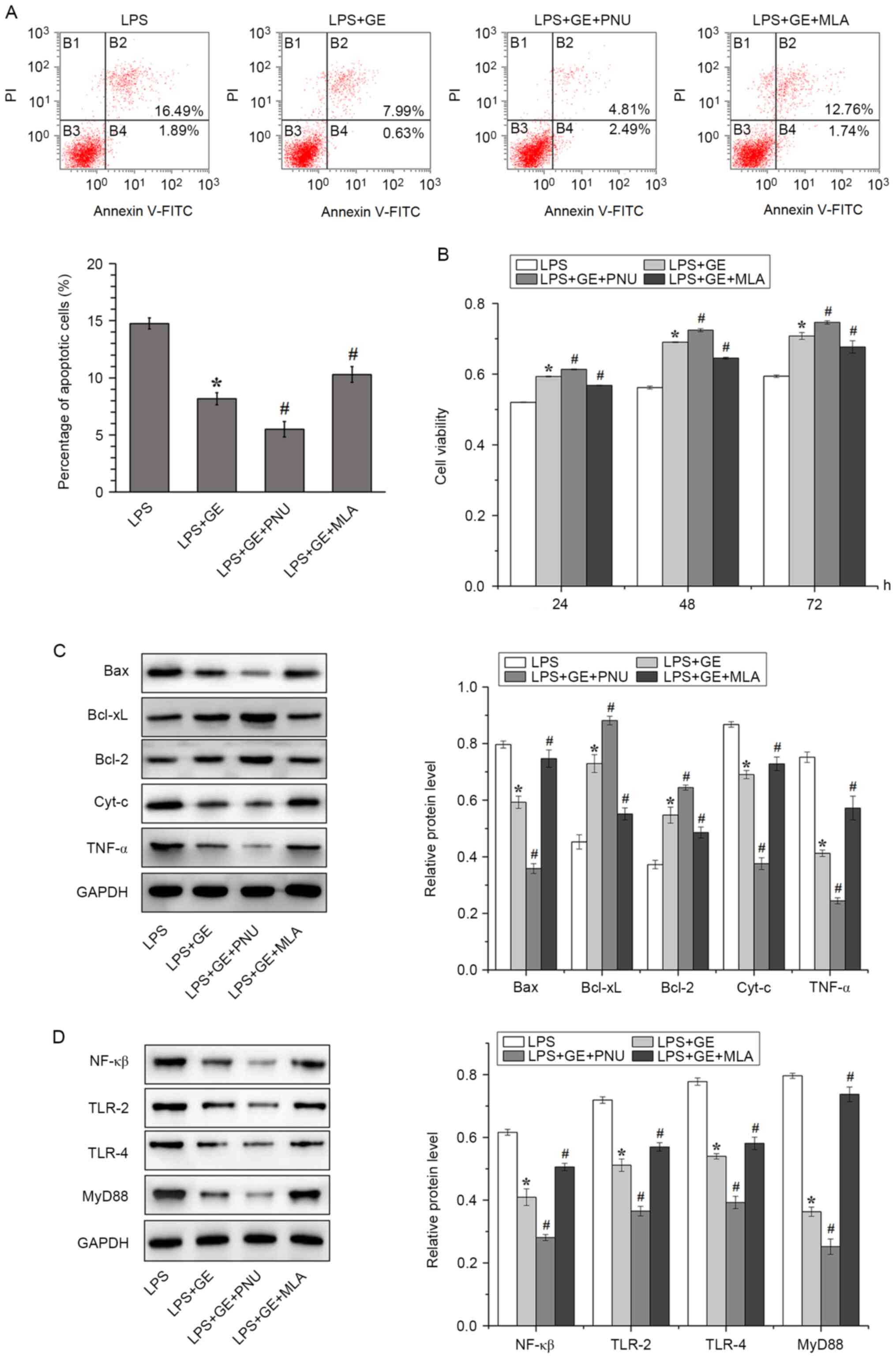 | Figure 4Involvement of α7nAChR in the
protective effect of geniposide against LPS-induced PASMC injury.
(A) Flow cytometric detection and quantification of PASMC apoptosis
after geniposide pretreatment, LPS stimulation and/or PNU/MLA
administration. (B) PASMC viability assessed by MTT after
geniposide pretreatment, LPS stimulation and/or PNU/MLA
administration. Western blotting and quantification of the
expression of proteins associated with (C) apoptosis and (D)
MyD88/NF-κB signaling pathway. All values were expressed as the
means ± standard deviation (n=3). *P<0.01 vs. LPS.
#P<0.01 vs. LPS+GE. LPS, lipopolysaccharides; GE,
geniposide; PNU, PNU282987; MLA, methyllycaconitine; Cyt-c,
cytochrome c; TNF-α, tumor necrosis factor-α; PI, propidium iodide;
FITC, fluorescein isothiocyanate; TLR, toll-like-receptor; NF-κB,
nuclear factor-κB; PASMCs, pulmonary arterial smooth muscle
cells. |
The involvement of α7nAChR in apoptosis and
inflammation was subsequently examined. The activation of α7nAChR
downregulated the pro-apoptotic proteins Bax, Cyt-c and TNFα and
upregulated the anti-apoptotic proteins Bcl-xL and Bcl-2, whereas
inhibition of α7nAChR had the opposite effect (Fig. 4C). To assess whether activation of
α7nAChR could decrease inflammation via the MyD88/NF-κB signaling
pathway, the protein levels of NF-κB, TLR-2, TLR-4 and MyD88 were
detected in LPS-treated PASMCs (Fig.
4D). In LPS-treated cells, geniposide decreased the protein
expression of NF-κB, TLR-2, TLR-4 and MyD88. The treatment with PNU
further downregulated the expression of these proteins, whereas
treatment with MLA exerted the opposite effect.
Discussion
Geniposide is the major iridoid glycoside
constituent of gardenia herbs and has been used as a component of
traditional medical formulations because of its anti-inflammatory
and antioxidant properties (30,31).
It has been reported that geniposide can alter the NF-κB signaling
pathway by controlling the overproduction of pro-inflammatory
mediators in asthmatic lung tissues (32); however, its effects on pulmonary
artery endothelial inflammation remain unclear. The present study
demonstrated that geniposide attenuated LPS-induced PASMC injury
and that activation of α7nAChR could enhance the protective effect
of geniposide by inhibiting the stimulation of the MyD88/NF-κB
signaling pathway.
Vascular inflammation and remodeling are important
pathological features of chronic obstructive pulmonary disease and
PASMCs are the main effectors of pulmonary vascular remodeling
(33,34). Under stimulation induced by LPS,
inflammation and oxidative stresses are activated and a complex
network of antioxidants, including antioxidant enzymes and
stress-response proteins, such as heme oxygenase-1, is triggered
(35). The overall effects of
geniposide on inflammation are primarily exerted by regulating the
synthesis of various cytokines. In particular, TNF-α and IL-1β play
critical roles in alveolar macrophages to induce the production of
a large number of secondary inflammatory cytokines, such as IL-6
and IL-8. This subsequently results in the participation of
neutrophils and CD8+ T lymphocytes in the inflammatory
response involved in chronic obstructive pulmonary disease
(36). Regarding apoptosis, the
pro-apoptotic protein Bax can initiate cell death pathways, whereas
Cyt-c can lead to apoptotic cell dismantling by mediating the
allosteric activation of apoptosis-protease activating factor 1 to
trigger caspase pathways (37). In
the present study, the concentrations of the pro-inflammatory
factors IL-18, TNF-α and IL-1β and the expression of the
pro-apoptosis proteins Bax, Cyt-c and TNF-α were significantly
elevated following cell treatment with LPS; however, geniposide
mitigated these effects and exerted protection against LPS-induced
injury in PASMCs.
Nicotinic acetylcholine receptors are
neurotransmitter-gated ion channels of pentameric structure
composed of α and β subunits (38).
The activation of α7nAChR, which is a nicotinic acetylcholine
receptor, has been implicated in the treatment of various diseases,
including sepsis, atherosclerosis, and oxazolone-induced colitis
(39-41).
Administration of α7nAChR agonists can also suppress cytokine
release and attenuate tissue damage during inflammation (42). Deficiency or impairment of α7nAChR
signaling or the cholinergic anti-inflammatory pathway results in
the overproduction of cytokines and enhanced tissue damage
(42). In the present study,
activation of α7nAChR by PNU enhanced the protective effect of
geniposide on LPS-treated PASMCs by regulating the TLR-4/NF-κB
signal pathway. Passive smoking and intratracheal instillation of
LPS may cause lung injury similar to chronic obstructive pulmonary
disease via the TLR-4/NF-κB signaling pathway (36). In lung tissues, upregulation of
TLR-4 may activate NF-κB and induce the expression of inflammatory
mediators. Furthermore, in macrophages from patients with chronic
obstructive pulmonary disease, the TLR-4/MyD88 pathway is
activated, and downstream inflammatory cytokines, such as TNF-α and
IL-6, are upregulated (43). MyD88
is an adaptor molecule that is engaged by all TLRs, except for
TLR-3. It triggers signaling via the MyD88-dependent pathway, which
activates NF-κB to release cytokines, such as TNF-α and
IL-1(44). In the present study,
the α7nAChR agonist PNU significant inhibited TLR-4/MyD88 signaling
in LPS-treated PASMCs pretreated with geniposide, indicating that
inflammation was further alleviated upon activation of α7nAChR.
In summary, the findings from the present study
demonstrated that geniposide may attenuate LPS-induced PASMC
injury, and that activation of α7nAChR could further contribute to
the protective effects of geniposide. The mechanism responsible for
these effects may involve the inhibition of TLR-4/MyD88 signaling
and downregulation of NF-κB expression. These results suggested
that the curative potential of geniposide may be associated with
the modulation of inflammatory response, offering novel insights
into the management of lung diseases. However, further
investigation should be carried out to determine the therapeutic
effects of geniposide before using it in clinical practice.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Wuhan Municipal Health
and Family Planning Commission (grants nos. WX18D11 and WZ17B09)
and the Health Commission of Hubei Province Scientific Research
Project (grant no. WJ2019F165).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Conceptualization: SYS, XQT and YHX; Development and
design of methodology, and creation of models: SYS, LQR, HFZ and
YHX; Conducting research and the investigation process,
specifically performing the experiments and data collection: SYS,
LQR, HDC, HFZ, DFZ; Application of statistical, mathematical,
computational or other formal techniques to analyze or synthesize
study data: SYS, BZ and YHX; Provision of study materials,
reagents, materials, patients, laboratory samples, animals,
instrumentation, computing resources, or other analysis tools: XQT
and YHX; Preparation, creation and/or presentation of the published
work, specifically visualization/data presentation: SYS;
Supervision: YHX; Project administration: XQT and YHX; Funding
acquisition: XQT and YHX; Writing-original draft: SYS;
Writing-review and editing: SYS, XQT and YHX. SYS and YHX confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Permission was granted from Wuhan Fourth Hospital,
Puai Hospital to perform the animal experiments at Wuhan Myhalic
Biotechnology Co., Ltd. (http://www.hlkbio.cn) and the study was approved by
the Animal Ethics Committee of the Model Animal Institute at Wuhan
Myhalic Biotechnology Co., Ltd. (approval no. HLK-20190611-01). All
animal experiments were performed in accordance with the
‘Guidelines for Experimental Animals’ from the Ministry of Science
and Technology (Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bailly K, Ridley AJ, Hall SM and Haworth
SG: RhoA activation by hypoxia in pulmonary arterial smooth muscle
cells is age and site specific. Circ Res. 94:1383–1391.
2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pugliese SC, Poth JM, Fini MA, Olschewski
A, El Kasmi KC and Stenmark KR: The role of inflammation in hypoxic
pulmonary hypertension: From cellular mechanisms to clinical
phenotypes. Am J Physiol Lung Cell Mol Physiol. 308:L229–L252.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Knobloch J, Chikosi SJ, Yanik S, Rupp J,
Jungck D and Koch A: A systemic defect in Toll-like receptor 4
signaling increases lipopolysaccharide-induced suppression of
IL-2-dependent T-cell proliferation in COPD. Am J Physiol Lung Cell
Mol Physiol. 310:L24–L39. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yi B, Cui J, Ning JN, Wang GS, Qian GS and
Lu KZ: Over-expression of PKGIα inhibits hypoxia-induced
proliferation, Akt activation, and phenotype modulation of human
PASMCs: The role of phenotype modulation of PASMCs in pulmonary
vascular remodeling. Gene. 492:354–360. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bauer EM, Shapiro R, Zheng H, Ahmad F,
Ishizawar D, Comhair SA, Erzurum SC, Billiar TR and Bauer PM: High
mobility group box 1 contributes to the pathogenesis of
experimental pulmonary hypertension via activation of Toll-like
receptor 4. Mol Med. 18:1509–1518. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Molteni M, Gemma S and Rossetti C: The
role of Toll-Like Receptor 4 in infectious and noninfectious
inflammation. Mediators Inflamm. 2016(6978936)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Koo HJ, Lim KH, Jung HJ and Park EH:
Anti-inflammatory evaluation of Gardenia extract, geniposide
and genipin. J Ethnopharmacol. 103:496–500. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kang JJ, Wang HW, Liu TY, Chen YC and Ueng
TH: Modulation of cytochrome P-450-dependent monooxygenases,
glutathione and glutathione S-transferase in rat liver by
geniposide from Gardenia jasminoides. Food Chem Toxicol.
35:957–965. 1997.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang SW, Lai CY and Wang CJ: Inhibitory
effect of geniposide on aflatoxin B1-induced DNA repair synthesis
in primary cultured rat hepatocytes. Cancer Lett. 65:133–137.
1992.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Koo HJ, Lee S, Shin KH, Kim BC, Lim CJ and
Park EH: Geniposide, an anti-angiogenic compound from the fruits of
Gardenia jasminoides. Planta Med. 70:467–469.
2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim CY and Kim J: Preparative isolation
and purification of geniposide from Gardenia fruits by
centrifugal partition chromatography. Phytochem Anal. 18:115–117.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Lee P, Lee J, Choi SY, Lee SE, Lee S and
Son D: Geniposide from Gardenia jasminoides attenuates
neuronal cell death in oxygen and glucose deprivation-exposed rat
hippocampal slice culture. Biol Pharm Bull. 29:174–176.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu J, Zheng X, Yin F, Hu Y, Guo L, Deng
X, Chen G, Jiajia J and Zhang H: Neurotrophic property of
geniposide for inducing the neuronal differentiation of PC12 cells.
Int J Dev Neurosci. 24:419–424. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zheng X, Yang D, Liu X, Wang N, Li B, Cao
H, Lu Y, Wei G, Zhou H and Zheng J: Identification of a new
anti-LPS agent, geniposide, from Gardenia jasminoides Ellis,
and its ability of direct binding and neutralization of
lipopolysaccharide in vitro and in vivo. Int Immunopharmacol.
10:1209–1219. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kurzen H, Wessler I, Kirkpatrick CJ,
Kawashima K and Grando SA: The non-neuronal cholinergic system of
human skin. Horm Metab Res. 39:125–135. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Taly A and Charon S: α7 nicotinic
acetylcholine receptors: A therapeutic target in the structure era.
Curr Drug Targets. 13:695–706. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li DJ, Huang F, Ni M, Fu H, Zhang LS and
Shen FM: α7 Nicotinic acetylcholine receptor relieves angiotensin
II-Induced senescence in vascular smooth muscle cells by raising
nicotinamide adenine Dinucleotide-Dependent SIRT1 activity.
Arterioscler Thromb Vasc Biol. 36:1566–1576. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li DJ, Zhao T, Xin RJ, Wang YY, Fei YB and
Shen FM: Activation of α7 nicotinic acetylcholine receptor protects
against oxidant stress damage through reducing vascular
peroxidase-1 in a JNK signaling-dependent manner in endothelial
cells. Cell Physiol Biochem. 33:468–478. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Deng Y, Guo SL, Wei B, Gao XC, Zhou YC and
Li JQ: Activation of nicotinic acetylcholine α7 receptor attenuates
progression of Monocrotaline-Induced pulmonary hypertension in rats
by downregulating the NLRP3 inflammasome. Front Pharmacol.
10(128)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kelso ML and Oestreich JH: Traumatic brain
injury: Central and peripheral role of alpha7 nicotinic
acetylcholine receptors. Curr Drug Targets. 13:631–636.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Colon-Saez JO and Yakel JL: A mutation in
the extracellular domain of the α7 nAChR reduces calcium
permeability. Pflugers Arch. 466:1571–1579. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stegemann A, Sindrilaru A, Eckes B, del
Rey A, Heinick A, Schulte JS, Müller FU, Grando SA, Fiebich BL,
Scharffetter-Kochanek K, et al: Tropisetron suppresses collagen
synthesis in skin fibroblasts via α7 nicotinic acetylcholine
receptor and attenuates fibrosis in a scleroderma mouse model.
Arthritis Rheum. 65:792–804. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bao J, Liu Y, Yang J, Gao Q, Shi SQ,
Garfield RE and Liu H: Nicotine inhibits LPS-induced cytokine
production and leukocyte infiltration in rat placenta. Placenta.
39:77–83. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Plummer HK III, Dhar M and Schuller HM:
Expression of the alpha7 nicotinic acetylcholine receptor in human
lung cells. Respir Res. 6(29)2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li Q, Zhou XD, Kolosov VP and Perelman JM:
Nicotine reduces TNF-α expression through a α7 nAChR/MyD88/NF-κB
pathway in HBE16 airway epithelial cells. Cell Physiol Biochem.
27:605–612. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pandolfi R, Barreira B, Moreno E,
Lara-Acedo V, Morales-Cano D, Martínez-Ramas A, de Olaiz Navarro B,
Herrero R, Lorente JÁ, Cogolludo Á, et al: Role of acid
sphingomyelinase and IL-6 as mediators of endotoxin-induced
pulmonary vascular dysfunction. Thorax. 72:460–471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chou HC, Lin W and Chen CM: Human
mesenchymal stem cells attenuate pulmonary hypertension induced by
prenatal lipopolysaccharide treatment in rats. Clin Exp Pharmacol
Physiol. 43:906–914. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nelson MT and Quayle JM: Physiological
roles and properties of potassium channels in arterial smooth
muscle. Am J Physiol. 268:C799–C822. 1995.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Green DE, Kang BY, Murphy TC and Hart CM:
Peroxisome proliferator-activated receptor gamma (PPARү) regulates
thrombospondin-1 and Nox4 expression in hypoxia-induced human
pulmonary artery smooth muscle cell proliferation. Pulm Circ.
2:483–491. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Koo HJ, Song YS, Kim HJ, Lee YH, Hong SM,
Kim SJ, Kim BC, Jin C, Lim CJ and Park EH: Antiinflammatory effects
of genipin, an active principle of Gardenia. Eur J
Pharmacol. 495:201–208. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lee JH, Lee DU and Jeong CS: Gardenia
jasminoides Ellis ethanol extract and its constituents reduce
the risks of gastritis and reverse gastric lesions in rats. Food
Chem Toxicol. 47:1127–1131. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Deng Y, Guan M, Xie X, Yang X, Xiang H, Li
H, Zou L, Wei J, Wang D and Deng X: Geniposide inhibits airway
inflammation and hyperresponsiveness in a mouse model of asthma.
Int Immunopharmacol. 17:561–567. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Postma DS and Timens W: Remodeling in
asthma and chronic obstructive pulmonary disease. Proc Am Thorac
Soc. 3:434–439. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang P, Han X, Mo B, Huang G and Wang C:
LPS enhances TLR4 expression and IFN-γ production via the
TLR4/IRAK/NF-κB signaling pathway in rat pulmonary arterial smooth
muscle cells. Mol Med Rep. 16:3111–3116. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Huang XL, Ling YL, Ling YQ, Zhou JL, Liu Y
and Wang QH: Heme oxygenase-1 in cholecystokinin-octapeptipe
attenuated injury of pulmonary artery smooth muscle cells induced
by lipopolysaccharide and its signal transduction mechanism. World
J Gastroenterol. 10:1789–1794. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Meng Y, Yu CH, Li T, Li W, Cai SX and Li
X: Expression and significance of Toll-like receptor-4 in rats lung
established by passive smoking or associated with intratracheal
instillation of lipopolysaccharide. Zhonghua Yi Xue Za Zhi.
93:2230–2234. 2013.PubMed/NCBI(In Chinese).
|
|
37
|
Suzuki M, Youle RJ and Tjandra N:
Structure of Bax: Coregulation of dimer formation and intracellular
localization. Cell. 103:645–654. 2000.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Unwin N: Nicotinic acetylcholine receptor
and the structural basis of neuromuscular transmission: Insights
from Torpedo postsynaptic membranes. Q Rev Biophys. 46:283–322.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kim TH, Kim SJ and Lee SM: Stimulation of
the α7 nicotinic acetylcholine receptor protects against sepsis by
inhibiting Toll-like receptor via phosphoinositide 3-kinase
activation. J Infect Dis. 209:1668–1677. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hashimoto T, Ichiki T, Watanabe A,
Hurt-Camejo E, Michaëlsson E, Ikeda J, Inoue E, Matsuura H, Tokunou
T, Kitamoto S and Sunagawa K: Stimulation of α7 nicotinic
acetylcholine receptor by AR-R17779 suppresses atherosclerosis and
aortic aneurysm formation in apolipoprotein E-deficient mice.
Vascul Pharmacol. 61:49–55. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Galitovskiy V, Qian J, Chernyavsky AI,
Marchenko S, Gindi V, Edwards RA and Grando SA: Cytokine-induced
alterations of α7 nicotinic receptor in colonic CD4 T cells mediate
dichotomous response to nicotine in murine models of Th1/Th17-
versus Th2-mediated colitis. J Immunol. 187:2677–2687.
2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Parrish WR, Rosas-Ballina M,
Gallowitsch-Puerta M, Ochani M, Ochani K, Yang LH, Hudson L, Lin X,
Patel N, Johnson SM, et al: Modulation of TNF release by choline
requires alpha7 subunit nicotinic acetylcholine receptor-mediated
signaling. Mol Med. 14:567–574. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zeng X, Liu X, Bao H, Zhang Y, Wang X, Shi
K and Pang Q: Effects of sulforaphane on Toll-like receptor
4/myeloid differentiation factor 88 pathway of monocyte-derived
macrophages from patients with chronic obstructive pulmonary
disease. Zhonghua Jie He He Hu Xi Za Zhi. 37:250–254.
2014.PubMed/NCBI(In Chinese).
|
|
44
|
Kaisho T and Akira S: Toll-like receptor
function and signaling. J Allergy Clin Immunol. 117:979–987; quiz
988. 2006.PubMed/NCBI View Article : Google Scholar
|