Introduction
Glaucoma is an irreversible ophthalmic disease
leading to blindness, and there are several types of glaucoma,
including congenital glaucoma, primary glaucoma, secondary glaucoma
and mixed glaucoma. High intraocular pressure (IOP) is the primary
cause of acute glaucoma and is accompanied by optic atrophy,
degeneration and necrosis of retinal nerve layers (1-3).
While decreasing the IOP is a key treatment for glaucoma, it is
difficult to stop the progression of glaucoma (4). Several factors are implicated in the
pathogenesis of glaucoma, such as impaired blood circulation,
excitotoxic reactions caused by excessive accumulation of
glutamate, free radical production, oxidative stress and
immunological factors (5).
Therefore, lowering the IOP is insufficient. Emerging evidence has
indicated that proinflammatory factors play a key role in glaucoma
development and progression. Identifying novel and effective
therapeutic interventions to decrease inflammatory responses might
be helpful for treating this disease and ultimately decrease the
rate of blindness.
The nuclear factor κB (NF-κB) signalling pathway is
critical for inflammation and is involved in various autoimmune
diseases (6). The activation of
NF-κB by different stimuli causes the phosphorylation and
ubiquitination of IκB and subsequently activates the transcription
and expression of several downstream genes. By this mechanism, the
NF-κB signalling pathway directly regulates the expression of Th1-
and Th2-type cytokines and the inflammatory response (7,8). In
contrast, inhibition of the NF-κB signalling pathway by Pueraria
and arylsulfonyl indoline-benzamide greatly suppresses the release
of proinflammatory cytokines in mice with glaucoma and thus
attenuates injury (1,9). Accordingly, the NF-κB signalling
pathway plays an important role in glaucoma and may be a potential
target for glaucoma therapy.
Epigallocatechin-3-gallate (EGCG), a major
antioxidant catechin found in green tea (Camellia sinensis),
has key anti-inflammatory (10,11),
antioxidative (12,13), and anticancer (14-16)
effects. It has been reported that EGCG attenuates hypothalamic
inflammation by inhibiting the JAK2/STAT3 signalling pathways in
microglia (17). Additionally, EGCG
has been shown to exert beneficial effects against conditions such
as liver inflammation (18),
arthritis (19) and nephritis
(20). It has been reported that
EGCG can inhibit the phosphorylation and ubiquitination of IκB,
thereby suppressing the activation of the NF-κB signalling pathway
and decreasing the production of inflammatory cytokines (21). However, there have been few studies
on whether EGCG can be used for glaucoma treatment.
The present study investigated the potential
therapeutic effect of EGCG in glaucoma by using a rat glaucoma
model established by high-pressure perfusion. Optic neuropathy was
observed, and the levels of inflammatory-associated factors were
measured in the glaucoma model rats treated with or without EGCG.
Importantly, the mechanism by which EGCG relieves glaucoma was
further explored. Overall, the present study provides a theoretical
basis for glaucoma therapy.
Materials and methods
Establishment of a rat glaucoma model
and drug treatment
Fifteen male Sprague-Dawley rats and 15 female
Sprague-Dawley rats from Hunan SJA Laboratory Animal Co., Ltd. (6-8
weeks old; weight, 220-250 g) were used to establish the glaucoma
model. The animals were housed in the standard animal facility with
12:12 h (light/dark cycle), 40-50% humidity, and 22˚C temperature.
The rats had free access to food and water. No obvious eye disease
was observed before surgery. The rats were randomly divided into
six groups (n=5 in each group): Control group, 1-day group, 3-day
group, 7-day group, 14-day group, and 28-day group. The rats were
anaesthetized via intraperitoneal injection of 2% pentobarbital
sodium (50 mg/kg). Following anaesthesia, a needle was injected
into the corneoscleral margin of the rat eyeball without touching
the lens to prevent cataract formation. Normal saline was infused
into the centre of the anterior chamber for 60 min. No iris or lens
injury was induced during the operation (22). At the indicated time point (day 1,
3, 7, 14 or 28), all the rats in each group were anesthetized with
3% sodium pentobarbital (30 mg/kg) and 3-5 ml of abdominal aortic
blood was collected. Then, eyeballs samples were collected after
rats were sacrificed by cervical dislocation. The samples were used
for assessment of pathological changes and inflammatory
responses.
In order to evaluate the treatment effect of EGCG
(Aladdin; cat. no. E107404-500 mg), 20 Sprague-Dawley rats (10
males and 10 females) were randomly divided into four groups (n=5
in each group): Control group (sham surgery without saline
injection), glaucoma model group (no EGCG in drinking water),
14-day EGCG group (each rat was given 50 mg/kg/day EGCG by gavage
for 14 continuous days immediately after modelling) (4), and 28-day EGCG group (each rat was
given 50 mg/kg/day EGCG by gavage for 28 continuous days
immediately after modelling). All animals in each group were
sacrificed at the indicated time point (day 14 or 28), and samples
were collected and used for assessment of pathological changes and
inflammatory responses.
The animal use protocol was reviewed and approved by
the Institutional Animal Care and Use Committee of Please change it
to The Third Xiangya Hospital of Central South University.
Haematoxylin-eosin (HE) staining
Rat eyeballs were collected from each group at
specific time points and fixed in FAS fixation solution (Wuhan
Servicebio Technology Co., Ltd.; cat. no. G1109) for >24 h at
room temperature. PBS was used to rinse off the fixed solution and
the tissues were embedded in paraffin. Embedded tissues were cut
into 5-µm slices. The sections were stained with haematoxylin for
10 min at room temperature and rinsed by water, followed by eosin
staining for 10 min at room temperature. The stained slices were
washed once with 85% ethanol for 5 min at room temperature, and
then washed once with 95% ethanol for 5 min at room temperature.
Finally, the slices washed twice with 100% ethanol for 10 min each
at room temperature, followed by imaging with light microscopy
(BA400; McAudi Industrial Group Co., Ltd; magnification, x40).
Assessment of T cell proliferation by
the carboxyfluorescein diacetate succinimidyl ester (CFSE)
method
Rats in each group were sacrificed at specific time
points, and the blood was collected from each rat to assess T
lymphocyte proliferation. The procedure was performed as previously
described (23). Briefly, cells
were isolated by gradient centrifugation and counted with a cell
counter. Cell suspension (1 ml) was added to the bottom of the
tube. CFSE (cat. no. 423801) diluted with PBS was added (final CFSE
concentration, 5 µmol/l), and the tube was rapidly vortexed to
ensure homogeneous dispersal. After labelling with CFSE, the cells
were treated with RPMI-1640 (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 5% foetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) and cultured at 37˚C in a 5%
CO2 incubator for 72 h. After that, the cells were
incubated for 20 min at room temperature with an APC-conjugated
anti-rat CD3 antibody (1 µg per million cells; cat. no. 201413;
BioLegend, Inc.) and analysed within 1 h on a FACSCalibur flow
cytometer (BD Biosciences). Acquired data were analyzed using
FlowJo software (v7.6; FlowJo LLC).
Measurement of intracellular cytokine
levels by flow cytometry
Rats in the control, 1-day, 3-day, 7-day, 14-day and
28-day groups were sacrificed on days 1, 3, 7, 14 and 28,
respectively, and rats from the 14- and 28-day EGCG groups were
sacrificed on days 14 and 28, respectively. Blood was collected
from each rat and used to measure the levels of intracellular IL-4,
IL-6, TNF-α, IL-1β, IL-13 and IFN-γ (custom panel; BioLegend, Inc.)
and the levels of Th1- and Th2-type cytokines (cat. no. 740405;
BioLegend, Inc.) were measured by flow cytometry. The protocol was
performed as previously described (24,25).
Western blot analysis of
inflammation-associated protein expression
The levels of the inflammation-associated proteins
phosphorylated (p)-p65 (cat. no. 3033T), p65 (cat. no. 8242T),
p-IκBα (cat. no. 9246S) and IκBα (cat. no. 4814T) (all from Cell
Signaling Technology, Inc) were assessed by western blotting. The
tissues were lysed in RIPA lysis buffer (Abcam) supplemented with
protease inhibitor. The protein concentration of each sample was
quantified by BCA assay with the BCA assay kit (Bio-Rad
Laboratories, Inc.). Equal amounts of proteins from each sample (20
mg) were loaded to 12% SDS-PAGE gels for electrophoresis and then
transferred onto polyvinylidene difluoride (EMD Millipore)
membranes. The membranes were blocked in 5% dry skimmed milk in
TBST buffer (0.02 M Tris-base, pH 7.6, 0.8% NaCl and 0.1% Tween-20)
for 1 h at room temperature. Specific primary antibodies (1:1,000)
or a β-actin antibody (1:1,000; Cell Signaling Technology, Inc.)
was added, and the membranes were incubated at 4˚C overnight. After
washing three times, the membranes were incubated with the
appropriate HRP-conjugated secondary antibodies (1:3,000; cat. nos.
BA1075 and BA1054; Boster Biological Technology) at room
temperature for 1 h. Finally, the protein bands were detected by an
enhanced chemiluminescence kit (cat. no. 32209; Thermo Fisher
Scientific, Inc.) and then semi-quantified with ImageJ software
(v1.8.0; National Institutes of Health).
Immunofluorescence staining
Rat eyeballs were fixed in FAS fixation solution for
>24 h at room temperature, embedded in paraffin and sectioned
into 5-µm slices. The sections were blocked in blocking buffer
containing 5% BSA in PBS for 30 min at 37˚C. The sections were then
incubated with an anti-class III β-tubulin primary antibody
(1:1,000; cat. no. ab18207; Abcam) overnight at 4˚C (26). After washing three times, the
sections were incubated with secondary antibody (1:2,000; cat. no.
ab150077; Abcam) labelled with fluorescein at room temperature for
1 h in the dark. After washing, anti-fluorescence quenching agent
(cat. no. G1401; Wuhan Servicebio Technology Co., Ltd.) was
applied, and the sections were mounted on glass slides. Finally,
the samples were imaged with a fluorescence microscope
(magnification, x40; TE2000-U; Nikon Corporation).
Statistical analysis
For statistical analyses, one-way ANOVA with Tukey's
post hoc test was performed using SPSS software (v12.0; SPSS Inc.).
The results are presented as the means ± standard deviations of
three independent experiments. P<0.05 was considered to indicate
a statistically significant difference.
Results
Successful establishment of an acute
glaucoma rat model
The eyeballs of the animals were subjected to
high-pressure perfusion to establish an acute glaucoma model
(27). Rat eyeballs were removed
for HE staining on days 1, 3, 7, 14 and 28 after modelling to
observe the neuropathological changes. The results showed that
gradual injury of the optic nerve, manifested as optic lesions and
atrophy, occurred over time after modelling and that degeneration
and necrosis of the retinal nerve layer also occurred (Fig. 1A). In addition, the fluorescence
intensity of the class III-β tubulin protein in the 28-day group
was lower compared with that in the control group (Fig. 1B), indicating that neuronal cells
were damaged in the 28-day group. Flow cytometry revealed that the
levels of intracellular cytokines, including IL-4, IL-6, TNF-α,
IL-1β, IL-13 and IFN-γ, were gradually elevated following
modelling, with the levels of IL-4 and TNF-α reaching the peak at
day 14 and the rest peaking at day 28 (Fig. 1C). Furthermore, the proliferation
rate of T lymphocytes was measured by the CFSE method and found
that the proliferation rate gradually increased with time after
modelling (Fig. 1D). Total Th1 and
Th2 cytokine levels were also assessed by flow cytometry. The
results indicated that the levels of both Th1 and Th2 cytokines
were increased during the early days after remodelling (Fig. 1E). However, the levels returned to
baseline levels by the end of the experiment (Fig. 1E). The ratio of Th1 to Th2 cytokines
was greatly diminished on day 1 and day 3 after remodelling but
gradually recovered to a normal level and was increased by day 28,
indicating an imbalance of Th1/Th2 cytokines developed during the
progression of glaucoma (Fig. 1E).
These results provide evidence that our animal model of acute
glaucoma was successfully established.
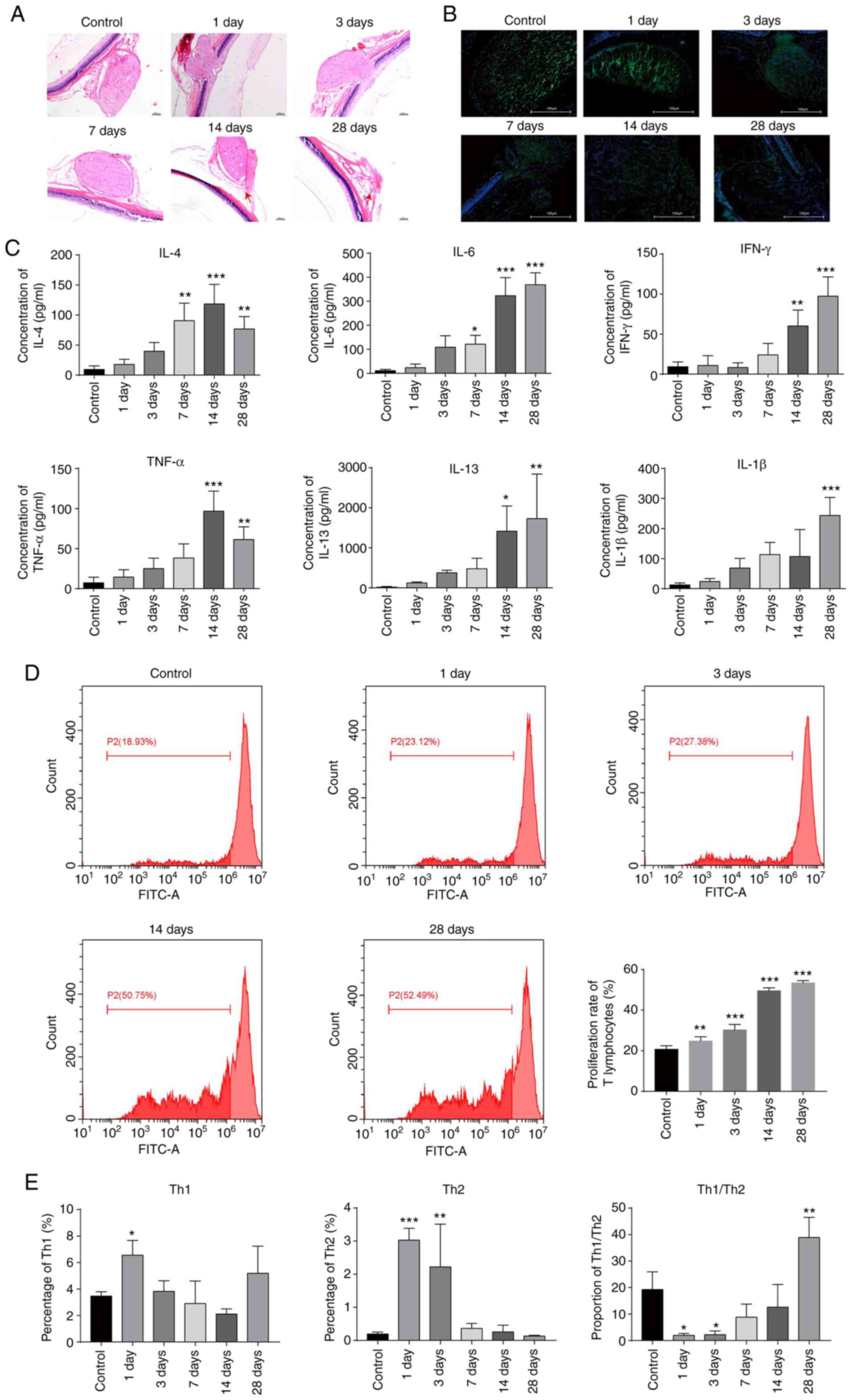 | Figure 1Successful establishment of an acute
glaucoma rat model. (A) Observation of optic neuropathy by
haematoxylin-eosin staining after modelling. Magnification, x40.
(B) Measurement of class III β-tubulin levels by immunofluorescence
staining after modelling. Magnification, x40 (C) Measurement of the
levels of cytokines, including IL-4, IL-6, TNF-α, IL-1β, IL-13 and
IFN-γ, by flow cytometry after modelling. (D) Assessment of T
lymphocyte proliferation by the CFSE method on days 1, 3, 14, and
28 after modelling. The cells were treated with CFSE and analysed
by flow cytometry. (E) The expression levels of Th1 and Th2
cytokines determined by flow cytometry on days 1, 3, 7, 14, and 28
after modelling. *P<0.05; **P<0.01;
***P<0.001. IL, interleukin; TNF, tumour necrosis
factor; IFN, interferon; CFSE, carboxyfluorescein diacetate
succinimidyl ester. |
EGCG suppressed the expression of
inflammatory cytokines in rats with acute glaucoma
In order to study the role of EGCG in acute
glaucoma, animals were fed with EGCG via gavage following
modelling. The HE staining results showed that EGCG greatly
attenuated the optical injury caused by high pressure, with better
effects being observed following 28 days of treatment compared with
after 14 days of treatment (Fig.
2A). In addition, the protein expression of class III-β tubulin
was upregulated in the 28-day EGCG group compared with the model
group, indicating that EGCG could repair the damage to the optic
nerve induced by acute glaucoma (Fig.
2B). The cytokine levels were measured after EGCG treatment.
The levels of cytokines, including IL-4, IL-6, TNF-α, IL-1β, IL-13
and IFN-γ, were significantly increased in the model group compared
with the control group (Fig. 2C).
However, these increases were significantly suppressed following
treatment with EGCG for 14 days, and the levels of these cytokines
returned to baseline levels after 28 days of EGCG treatment
(Fig. 2C). The aforementioned
results indicate that EGCG can suppress inflammatory responses in
glaucoma and thus attenuate optical injury.
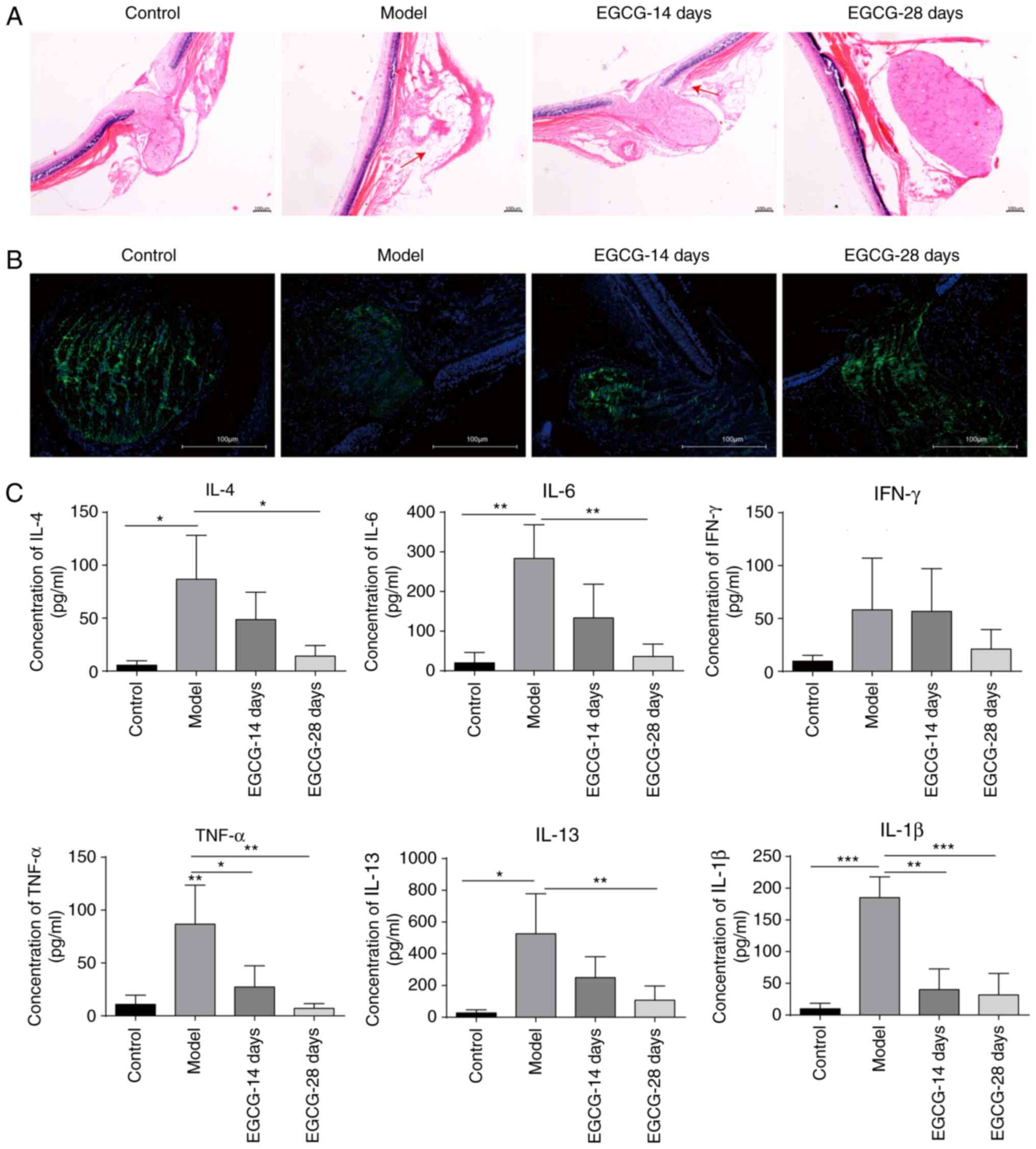 | Figure 2EGCG suppresses the expression of
inflammatory cytokines in rats with acute glaucoma. (A) Observation
of optic neuropathy by haematoxylin-eosin staining after treatment
with EGCG. Magnification, x40. (B) Analysis of class III β-tubulin
levels by immunofluorescence staining in the control group, model
group and groups treated with EGCG for 14 and 28 days.
Magnification, x40. (C) Measurement of the levels of cytokines,
including IL-4, IL-6, TNF-α, IL-1β, IL-13 and IFN-γ, by flow
cytometry after treatment with EGCG. *P<0.05;
**P<0.01; ***P<0.001. EGCG,
epigallocatechin-3-gallate; IL, interleukin; TNF, tumour necrosis
factor; IFN, interferon. |
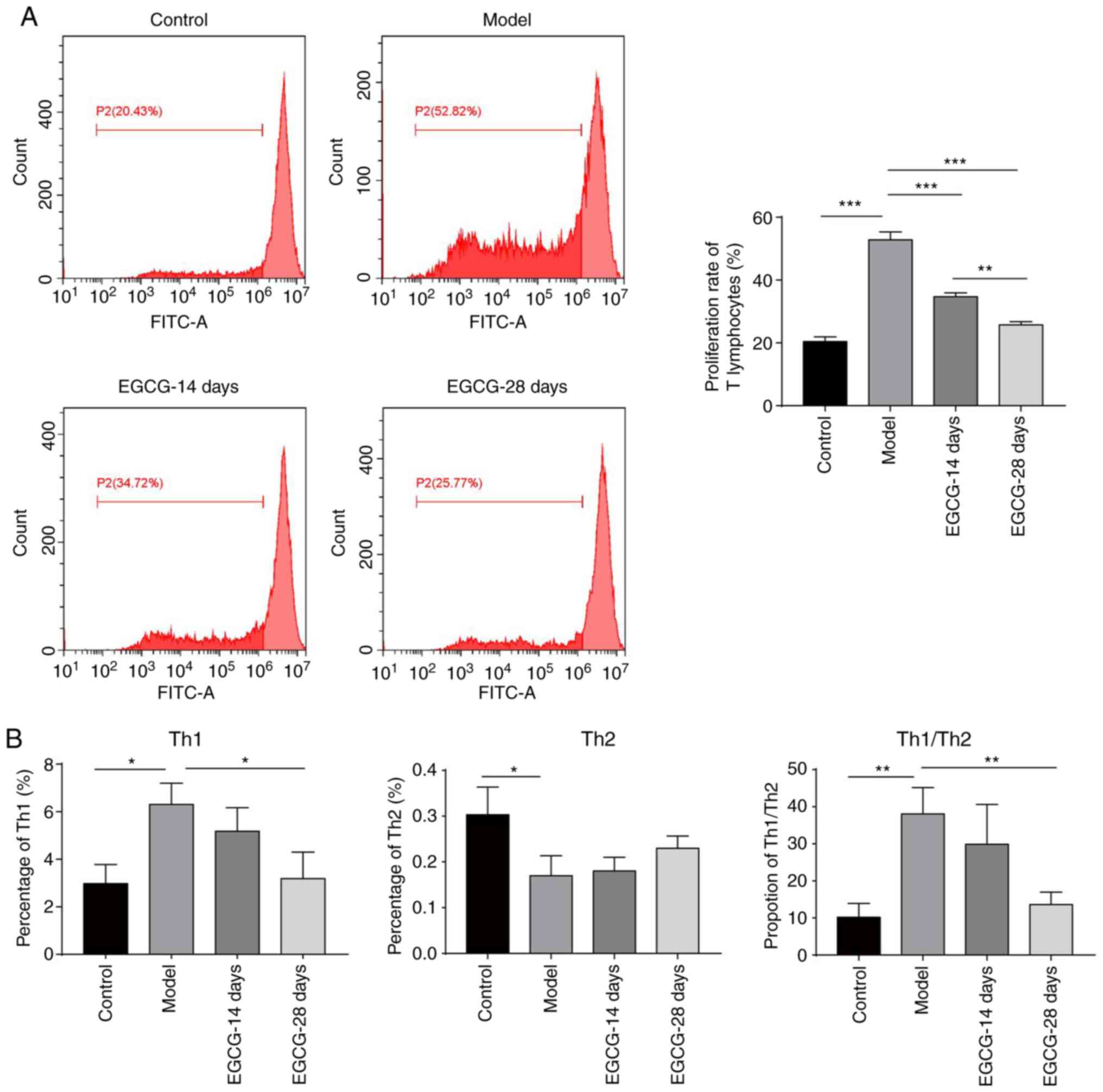
EGCG decreased T cell proliferation
and the Th1/Th2 ratio in the peripheral blood of rats with
glaucoma
The aforementioned results indicate that the
proliferation rate of T lymphocytes was increased and that an
imbalance of Th1/Th2 cells occurred in the glaucoma model group; it
was then assessed whether EGCG regulated this process. As shown in
Fig. 3A, treatment with EGCG for 14
days significantly decreased the T lymphocyte proliferation rate,
and 28 days of treatment further decreased the rate (Fig. 3A). Furthermore, the increase in the
levels of Th1-type and Th2-type cytokines following modelling were
suppressed by EGCG treatment (Fig.
3B). The Th1/Th2 ratio was also restored by EGCG treatment
(Fig. 3B). Altogether, these data
demonstrate that EGCG can suppress T cell proliferation and restore
the imbalance of Th1/Th2 cytokines during glaucoma.
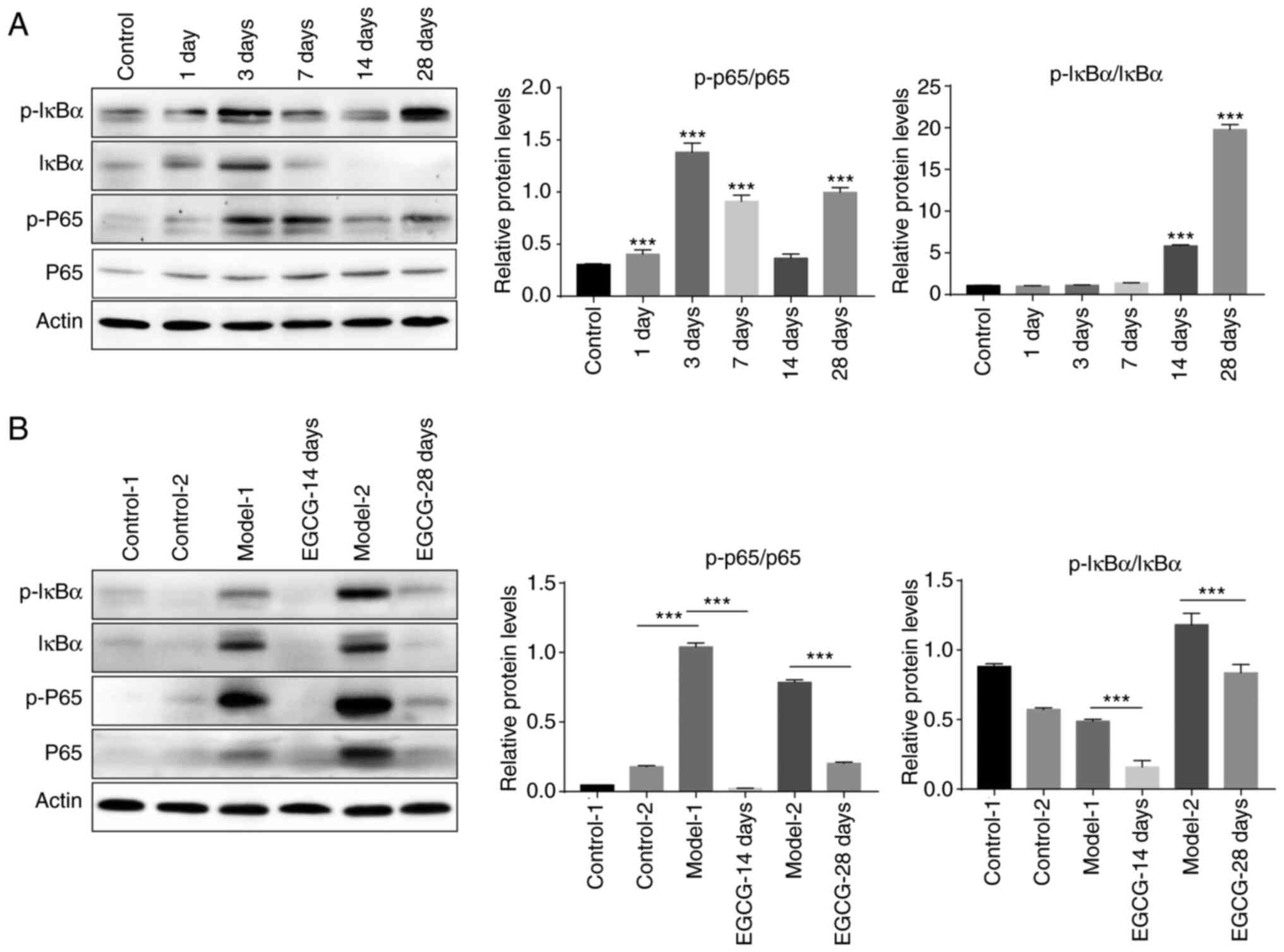
EGCG inhibits the NF-κB signalling
pathway
In order to study the mechanism by which EGCG
suppressed inflammatory responses, the expression levels of
inflammation-associated proteins were measured by western blot
analysis. The results showed that p-IκBα/IκBα and p-p65/p65 levels
gradually increased with time after the establishment of the
glaucoma model (Fig. 4A). Notably,
after treatment with EGCG, the increase in both p-IκBα/IκBα and
p-p65/p65 levels induced by modelling were significantly inhibited
(Fig. 4B). The aforementioned
results indicated that the NF-κB signalling pathway is markedly
activated in the course of glaucoma and that this activation can be
inhibited by EGCG treatment.
Discussion
Glaucoma caused by high IOP is an optic nerve
disease that can ultimately result in blindness. Currently,
treatments for this disease include drugs (28) and surgery (29,30) to
control IOP and limit optic nerve damage. However, prognosis
remains to be improved, due to the various unwanted side effects of
these treatments (31). In the
present study, through the use of an animal model, it was
demonstrated that EGCG significantly ameliorates optic injury in
the course of glaucoma by suppressing inflammatory responses and
restoring the balance of Th1 and Th2 cytokines via the NF-κB
pathway. The present study provides an avenue for the development
of therapeutic strategies for glaucoma.
High IOP is a key risk factor for glaucoma. Thus,
many studies have established animal models of glaucoma by
increasing the IOP (32,33). It has been reported that IOP can be
increased by injecting saline into the eyes. For example, Zhong
(34) successfully generated an
ocular hypertension rat model by injecting hypertonic saline into
the episcleral veins once weekly for two weeks. The success rate of
this method was >80%, and IOP was elevated by 81% for 24 weeks.
Similarly, Morrison et al (35) established an experimental rat model
of acute glaucoma through injecting hypertonic saline into the
aqueous outflow pathway, and the resulting inflammatory response
led to increased IOP one week later. In the present study, acute
glaucoma was modelled in rats by high-pressure infusion of saline.
After infusion, optic nerve damage begins to develop, such as optic
atrophy, degeneration and necrosis of the retinal nerve layer. In
addition, a decrease in class III-β tubulin protein expression was
observed, further indicating damage to optic nerves. Accompanying
these pathological changes were marked increases in the levels of
proinflammatory Th1/Th2 cytokines. These results indicate that the
animal model was successfully established; thus, this approach for
increasing IOP could be used as an alternative method for modelling
acute glaucoma in animals.
EGCG is the main ingredient of green tea and has
been shown to have a neuroprotective effect (36,37).
For instance, Kian et al (38) found that EGCG may be effective in
protecting neuronal cells against apoptosis and may increase
neuronal survival time following nerve transection. Furthermore,
Shen et al (4) observed that
EGCG exerts a neuroprotective effect on retinal ganglion cells
(RGCs) in chronic glaucoma. Consistently, the present study
indicated that EGCG attenuates optic nerve injury induced by high
IOP, as indicated by decreased optic atrophy, optic nerve
degeneration and necrosis. Taken together, the present study
results and those of previous studies indicate that EGCG has a
conserved neuroprotective role. It might be interesting to examine
whether EGCG can be applied for the treatment of other neurological
diseases, such as Alzheimer's disease and Parkinson's disease.
Inflammation significantly contributes to the
development and progression of glaucoma, and substantial changes in
the levels of cytokines, such as IFN-γ, TNF-α, IL-1 and IL-6, have
been reported (39,40). In patients with glaucoma, the
expression of IL-4, IL-6, TNF-α, IL-1β, IL-13 and IFN-γ is
upregulated, and this upregulation exacerbates the disease. The
present study found that EGCG inhibits the increase in IL-4, IL-6,
TNF-α, IL-1β, IL-13 and IFN-γ expression levels in vivo,
suggesting that it suppresses inflammatory responses. In addition,
other studies have shown that the T cell response plays a role in
neurodegeneration (41,42). Indeed, T cell proliferation has been
observed in glaucoma. T cell proliferation in the context of
glaucoma could induce the production of proinflammatory cytokines
and thus promote inflammation. The present study data showed that
EGCG greatly decreases the proliferation rate of T cells. This
might be one of the mechanisms by which the inflammatory responses
are inhibited by EGCG in glaucoma. In addition, the Th1/Th2 ratio
reflects inflammation in vivo and is associated with the
progression of glaucoma. The finding that the Th1/Th2 ratio was
restored by EGCG in the present glaucoma model further suggests
that EGCG serves a neuroprotective role in glaucoma. This finding
is consistent with growing evidence indicating that an imbalance in
Th1/Th2 cytokine production contributes to glaucomatous optic
neuropathy (43).
Activation of the NF-κB pathway plays a crucial role
in inflammation, as it can promote the transcription and expression
of several proinflammatory cytokines, which may further facilitate
T-cell communication (44). The
present study confirmed that the NF-κB pathway was activated in a
rat model. Notably, EGCG treatment remarkably suppressed NF-κB
pathway activation, indicating that EGCG inhibits inflammation by
inhibiting the NF-κB signalling pathway. Future studies are
required to determine whether other mechanisms are involved in the
neuroprotective role of EGCG in glaucoma. Additionally, it remains
to be further explored whether the neuroprotective effect of EGCG
is dose-dependent.
In conclusion, the present study results demonstrate
that EGCG attenuates optic nerve injury during glaucoma by
suppressing the activation of the NF-κB signalling pathway and
inflammatory responses. This study indicates that EGCG could serve
as a promising adjuvant intervention for glaucoma treatment in the
future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WHZ and YNC were responsible for the conception and
design, and revision of the manuscript. WHZ prepared the draft of
the manuscript and performed the major experiments that contributed
to the acquisition, analysis and interpretation of data. YC and LMG
assisted in the experiments and analyzed the data. WHZ and YNC
confirmed the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The animal use protocol has been reviewed and
approved by the Institutional Animal Care and Use Committee
(IACUC), The Third Xiangya Hospital of Central South University
[approval no. LLSC (LA) 2018-038].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lei X and Zhao Y: Neovascular glaucoma
regulation by arylsulfonyl indoline-benzamide (ASIB) through
targeting NF-κB signalling pathway. 3 Biotech.
9(211)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Quigley HA: Neuronal death in glaucoma.
Prog Retin Eye Res. 18:39–57. 1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Foster PJ: The epidemiology of primary
angle closure and associated glaucomatous optic neuropathy. Semin
Ophthalmol. 17:50–58. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shen C, Chen L, Jiang L and Lai TY:
Neuroprotective effect of epigallocatechin-3-gallate in a mouse
model of chronic glaucoma. Neurosci Lett. 600:132–136.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wong M, Huang P, Li W, Li Y, Zhang SS and
Zhang C: T-helper1/T-helper2 cytokine imbalance in the iris of
patients with glaucoma. PLoS One. 10(e0122184)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang J and Zhao Q: Kaempferitrin inhibits
proliferation, induces apoptosis, and ameliorates inflammation in
human rheumatoid arthritis fibroblast-like synoviocytes. Phytother
Res. 33:1726–1735. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Niu Y, Dong Q and Li R: Matrine regulates
Th1/Th2 cytokine responses in rheumatoid arthritis by attenuating
the NF-kappaB signaling. Cell Biol Int. 41:611–621. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu B, Lu R, Li H, Zhou Y, Zhang P, Bai L,
Chen D, Chen J, Li J, Yu P, et al: Zhen-wu-tang ameliorates
membranous nephropathy rats through inhibiting NF-κB pathway and
NLRP3 inflammasome. Phytomedicine. 59(152913)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wei HY, Zhang YJ and Zhao SZ: Puerarin
regulates neovascular glaucoma through pigment epitheliumderived
growth factorinduced NF-κB signaling pathway. Mol Med Rep.
17:7866–7874. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kar AK, Singh A, Dhiman N, Purohit MP,
Jagdale P, Kamthan M, Singh D, Kumar M, Ghosh D and Patnaik S:
Polymer-assisted in situ synthesis of silver nanoparticles with
epigallocatechin gallate (EGCG) impregnated wound patch potentiate
controlled inflammatory responses for brisk wound healing. Int J
Nanomedicine. 14:9837–9854. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nguyen T, Payan B, Zambrano A, Du Y,
Bondesson M and Mohan C: Epigallocatechin-3-gallate suppresses
neutrophil migration speed in a transgenic zebrafish model
accompanied by reduced inflammatory mediators. J Inflamm Res.
12:231–239. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yelins'ka AM, Liashenko LI and Kostenko
VO: Quercetin potentiates antiradical properties of
epigallocatechin-3-gallate in periodontium of rats under systemic
and local administration of lipopolisaccharide of salmonella typhi.
Wiad Lek. 72:1499–1503. 2019.PubMed/NCBI
|
|
13
|
Wang J, Jia R, Celi P, Ding X, Bai S, Zeng
Q, Mao X, Xu S and Zhang K: Green tea polyphenol
epigallocatechin-3-gallate improves the antioxidant capacity of
eggs. Food Funct. 11:534–543. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sharifi-Rad M, Pezzani R, Redaelli M,
Zorzan M, Imran M, Ahmed Khalil A, Salehi B, Sharopov F, Cho WC and
Sharifi-Rad J: preclinical pharmacological activities of
epigallocatechin-3-gallate in signaling pathways: An update on
cancer. Molecules. 25(467)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Md Nesran ZN, Shafie NH, Ishak AH, Mohd
Esa N, Ismail A and Md Tohid SF: Induction of endoplasmic reticulum
stress pathway by green tea epigallocatechin-3-Gallate (EGCG) in
colorectal cancer cells: Activation of PERK/p-eIF2α/ATF4 and IRE1α.
Biomed Res Int. 2019(3480569)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen J, Chen L, Lu T, Xie Y, Li C, Jia Z
and Cao J: ERα36 is an effective target of
epigallocatechin-3-gallate in hepatocellular carcinoma. Int J Clin
Exp Pathol. 12:3222–3234. 2019.PubMed/NCBI
|
|
17
|
Mao L, Hochstetter D, Yao L, Zhao Y, Zhou
J, Wang Y and Xu P: Green Tea Polyphenol (-)-Epigallocatechin
Gallate (EGCG) attenuates neuroinflammation in palmitic
acid-stimulated BV-2 microglia and high-fat diet-induced obese
mice. Int J Mol Sci. 20(5081)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang D, Gao Q, Wang T, Kan Z, Li X, Hu L,
Peng CY, Qian F, Wang Y and Granato D: Green tea polyphenols and
epigallocatechin-3-gallate protect against perfluorodecanoic acid
induced liver damage and inflammation in mice by inhibiting NLRP3
inflammasome activation. Food Res Int. 127(108628)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Karatas A, Dagli AF, Orhan C, Gencoglu H,
Ozgen M, Sahin N, Sahin K and Koca SS: Epigallocatechin 3-gallate
attenuates arthritis by regulating Nrf2, HO-1, and cytokine levels
in an experimental arthritis model. Biotechnol Appl Biochem.
67:317–322. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Chen J, Liu J, Lei Y and Liu M: Potential
ameliorative effects of epigallocatechin-3-gallate against
cigarette smoke exposure induced renal and hepatic deficits.
Ecotoxicol Environ Saf. 191(110202)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Singh BN, Shankar S and Srivastava RK:
Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms,
perspectives and clinical applications. Biochem Pharmacol.
82:1807–1821. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liang L, He L, Zhu M, Chen B and Xiao C:
Protective effects of carnosic acid on retinal ganglion cells in
acute ocular hypertension rats. Int Ophthalmol. 40:1869–1878.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Azarsiz E, Karaca N, Ergun B, Durmuscan M,
Kutukculer N and Aksu G: In vitro T lymphocyte proliferation by
carboxyfluorescein diacetate succinimidyl ester method is helpful
in diagnosing and managing primary immunodeficiencies. J Clin Lab
Anal. 32(e22216)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yin Y, Mitson-Salazar A and Prussin C:
Detection of intracellular cytokines by flow cytometry. Curr Protoc
Immunol. 110:6.24.1–6.24.18. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Foster B, Prussin C, Liu F, Whitmire JK
and Whitton JL: Detection of intracellular cytokines by flow
cytometry. Curr Protoc Immunol Chapter. 6(Unit 6 24)2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li W, Ma N, Liu MX, Ye BJ, Li YJ, Hu HY
and Tang YH: C1q/TNF-related protein-9 attenuates retinal
inflammation and protects blood-retinal barrier in db/db mice. Eur
J Pharmacol. 853:289–298. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Paula AP, Paula JS, Silva MJ, Rocha EM, De
Moraes CG and Rodrigues ML: Effects of swimming goggles wearing on
intraocular pressure, ocular perfusion pressure, and ocular pulse
amplitude. J Glaucoma. 25:860–864. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sanchez-Sanchez C, Puerto B,
Lopez-Caballero C and Contreras I: Unilateral acute iris
depigmentation and transillumination after glaucoma surgery with
mitomycin application and intracameral moxifloxacin. Am J
Ophthalmol Case Rep. 18(100639)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Otarola F, Virgili G, Shah A, Hu K, Bunce
C and Gazzard G: Ab interno trabecular bypass surgery with Schlemm
s canal microstent (Hydrus) for open angle glaucoma. Cochrane
Database Syst Rev. 3(CD012740)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dada T, Midha N, Shah P, Sidhu T, Angmo D
and Sihota R: Innovations in glaucoma surgery from Dr. Rajendra
Prasad Centre for Ophthalmic Sciences. Indian J Ophthalmol.
65:103–108. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Weinreb RN, Aung T and Medeiros FA: The
pathophysiology and treatment of glaucoma: A review. JAMA.
311:1901–1911. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hong S, Kim CY, Lee WS, Shim J, Yeom HY
and Seong GJ: Ocular hypotensive effects of topically administered
agmatine in a chronic ocular hypertensive rat model. Exp Eye Res.
90:97–103. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yu S, Tanabe T and Yoshimura N: A rat
model of glaucoma induced by episcleral vein ligation. Exp Eye Res.
83:758–770. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhong L: A modified chronic ocular
hypertension rat model for retinal ganglion cell neuroprotection.
Front Med. 7:367–377. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Morrison JC, Johnson EC and Cepurna WO:
Hypertonic saline injection model of experimental glaucoma in rats.
Methods Mol Biol. 1695:11–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou J, Mao L, Xu P and Wang Y: Effects of
(-)-Epigallocatechin Gallate (EGCG) on energy expenditure and
microglia-mediated hypothalamic inflammation in mice fed a high-fat
diet. Nutrients. 10(1681)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu D, Perkins JT and Hennig B: EGCG
prevents PCB-126-induced endothelial cell inflammation via
epigenetic modifications of NF-κB target genes in human endothelial
cells. J Nutr Biochem. 28:164–170. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kian K, Khalatbary AR, Ahmadvand H,
Karimpour Malekshah A and Shams Z: Neuroprotective effects of
(-)-epigallocatechin-3-gallate (EGCG) against peripheral nerve
transection-induced apoptosis. Nutr Neurosci. 22:578–586.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Husain S, Abdul Y, Webster C, Chatterjee
S, Kesarwani P and Mehrotra S: Interferon-gamma
(IFN-gamma)-mediated retinal ganglion cell death in human
tyrosinase T cell receptor transgenic mouse. PLoS One.
9(e89392)2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Borkenstein A, Faschinger C, Maier R,
Weger M, Theisl A, Demel U, Graninger W, Irene H and Mossböck G:
Measurement of tumor necrosis factor-alpha, interleukin-6, Fas
ligand, interleukin-1a, and interleukin-1β in the aqueous humor of
patients with open angle glaucoma using multiplex bead analysis.
Mol Vis. 19:2306–2311. 2013.PubMed/NCBI
|
|
41
|
Gramlich OW, Ding QJ, Zhu W, Cook A,
Anderson MG and Kuehn MH: Adoptive transfer of immune cells from
glaucomatous mice provokes retinal ganglion cell loss in
recipients. Acta Neuropathol Commun. 3(56)2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wax MB, Tezel G, Yang J, Peng G, Patil RV,
Agarwal N, Sappington RM and Calkins DJ: Induced autoimmunity to
heat shock proteins elicits glaucomatous loss of retinal ganglion
cell neurons via activated T-cell-derived fas-ligand. J Neurosci.
28:12085–12096. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Huang P, Zhang SS and Zhang C: The two
sides of cytokine signaling and glaucomatous optic neuropathy. J
Ocul Biol Dis Infor. 2:78–83. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen H, Cho KS, Vu THK, Shen CH, Kaur M,
Chen G, Mathew R, McHam ML, Fazelat A, Lashkari K, et al: Commensal
microflora-induced T cell responses mediate progressive
neurodegeneration in glaucoma. Nat Commun. 9(3209)2018.PubMed/NCBI View Article : Google Scholar
|