Introduction
Sepsis is a systemic inflammatory response triggered
by infection, which is often accompanied by fever, leukopenia and
multiorgan failure (1,2). Although it is common, sepsis is
associated with high mortality rates and often requires patients to
be admitted into intensive care units (1,3). The
incidence rate of sepsis is currently 66-300 per 100,000
individuals in developed countries, and continues to increase
(4,5). Results of a previous study indicated
that almost half of patients with sepsis have clinical
manifestations associated with myocardial dysfunction (6). Sepsis-induced myocardial dysfunction
(SIMD) is a leading cause of the high mortality rates observed in
sepsis (7).
Butorphanol, a US Food and Drug
Administration-approved analgesic agent, is a potent κ-opioid
receptor (KOR) agonist and µ-opioid receptor (MOR) antagonist,
which binds to KORs and modifies their expression (8). It was previously reported that
butorphanol was successfully used to treat patients with moderate
or severe pruritus (9-11).
Comparative studies demonstrated that butorphanol was ~7-fold more
effective compared with morphine sulphate, a full MOR agonist
(12,13). A previous meta-analysis also
reported that butorphanol exerted significant therapeutic effects
by reducing the incidence and severity of opioid-induced cough
(14). In addition, butorphanol was
found to be an optimal drug for prescribing to patients in labor
with chronic hypertension or preeclampsia, as it did not elevate
blood pressure (15). Previous
studies have also shown that butorphanol alleviated myocardial
ischemia/reperfusion-induced injury in rats (16,17).
Recent evidence has suggested a protective role for butorphanol
against sepsis-induced cerebral injury. The aim of the present
study was to determine the potential role of butorphanol in the
inflammation and apoptosis mediated by KOR in lipopolysaccharide
(LPS)-induced cardiomyocytes (18).
LPS is a crucial component of the outer membrane of
Gram-negative bacteria, which plays a significant role in
Gram-negative bacterial infection and induces myocardial damage
(19). The present study used LPS
to induce inflammation and apoptosis in cardiomyocytes in order to
investigate the effects of butorphanol in an in vitro study
of SIMD.
Materials and methods
Cell culture and experimental
grouping
H9C2 cardiomyocytes were purchased from The Cell
Bank of Type Culture Collection of the Chinese Academy of Sciences.
Cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 100 g/ml streptomycin, 100 g/ml penicillin and
10% FBS (Gibco; Thermo Fisher Scientific, Inc.), and maintained in
a humidified atmosphere of 5% CO2 at 37˚C. H9C2 cells
were treated with 1.25, 2.5 or 5 µM butorphanol (1mg/mL,
Butorphanol (Jiangsu Hengrui Medicine Co. Ltd.) for 24 h at 37˚C
for further analysis. Cells were divided into the following four
groups: i) H9C2 group, in which cells were cultured in basic
culture medium; ii) LPS group, in which cells were treated with 10
µg/ml LPS from Escherichia coli O111:B4 (Sigma-Aldrich; Merck KGaA)
for 24 h; iii) LPS + 1.25, 2.5 or 5 µM butorphanol group, in which
cells were pretreated with 1.25, 2.5 or 5 µM butorphanol for 2 h
prior to treatment with 10 g/ml LPS for 24 h (a preliminary
experiment was conducted, which determined that 2 h was the
appropriate pre-conditioning time of butorphanol); and iv) LPS + 5
µM butorphanol + KOR antagonist nor-binaltorphimine (Nor-BNI;
Abcam) group, in which cells were pretreated with 10 µmol/l Nor-BNI
(20) for 30 min prior to the
treatment described for the LPS + 5 µM butorphanol group. After the
experimental treatment, the cells were used for further
analysis.
Cell Counting Kit (CCK)-8 assay
H9C2 cells were digested with Trypsin (cat. no.
R001100; Gibco; Thermo Fisher Scientific, Inc.) and seeded into
96-well plates at a density of 3x105 cells/well.
Following treatment with 10 µg/ml LPS and 1.25, 2.5 or 5 µM
butorphanol for 24 h, the cells were incubated with 10 µl CCK-8
reagent (MedChemExpress) for 4 h at 37˚C. The absorbance of each
well was measured at a wavelength of 450 nm using a microplate
reader. The cell viability was calculated using the formula: Cell
viability (%) = [Adrug -
Ablank/A0drug - Ablank] x 100.
Adrug, absorbance of wells containing cells, CCK8
solution and drug solution; Ablank, absorbance of wells
containing medium and CCK-8 solution, without cells;
A0drug, absorbance of wells containing cells and CCK-8
solution, but no drug solution.
ELISA
After H9C2 cells (1x106 cells) were
seeded into 6-well plates and treated as aforementioned, they were
harvested and centrifuged at 3,000 x g for 5 min. The levels of
tumor necrosis factor (TNF)-α (cat. no. ab181421), IL-1β (cat. no.
ab214025) and IL-6 (cat. no. ab178013) in the H9C2 cell supernatant
were detected using the respective commercial ELISA kits (Abcam)
according to the manufacturer's protocols.
Western blotting
Total protein was extracted from H9C2 cells using
RIPA lysis buffer (Beyotime Institute of Biotechnology). Protein
concentration was quantified using a BCA assay kit (cat. no.
ab102536; Abcam) according to the manufacturer's protocol. A total
of 50 µg protein was loaded into each lane, separated by SDS-PAGE
on 12% gels and subsequently transferred onto PVDF membranes (EMD
Millipore), which were blocked with 5% skimmed milk at room
temperature for 1 h. The membranes were then incubated with the
following primary antibodies: Anti-phosphorylated (p)-P65 (cat. no.
ab183559; 1:1,000; Abcam), anti-P65 (cat. no. ab32536; 1:1,000;
Abcam), anti-cyclooxygenase 2 (cat. no. ab179800; 1:1,000; Abcam),
anti-inducible nitric oxide synthase (cat. no. ab178945; 1:1,000;
Abcam), anti-Bax (cat. no. ab32503; 1:1,000; Abcam), anti-cleaved
caspase 3 (cat. no. ab32042; 1:1,000; Abcam), anti-cleaved
poly(ADP)-ribose polymerase (PARP1; cat. no. ab32064; 1:1,000;
Abcam), anti-Bcl-2 (cat. no. ab32124; 1:1,000; Abcam) and
anti-GAPDH (cat. no. ab9485; 1:1,000; Abcam). Following incubation
with the primary antibody at 4˚C overnight, the membranes were
incubated with the corresponding HRP-conjugated secondary antibody:
i) rabbit anti-mouse secondary (cat. no. SA5-10317; 1:10,000;
Invitrogen; Thermo Fisher Scientific, Inc.) and ii) goat
anti-rabbit (cat. no. A16104SAMPLE; 1:10,000; Invitrogen; Thermo
Fisher Scientific, Inc.) at room temperature for 2 h. Protein bands
were visualized using enhanced chemiluminescence reagent (Pierce;
Thermo Fisher Scientific, Inc.) and analyzed using ImageJ software
version 1.46r (National Institutes of Health). Protein expression
levels were normalized to GAPDH expression levels.
TUNEL assay
H9C2 cells were harvested, washed with PBS three
times and fixed with 4% paraformaldehyde at room temperature for 20
min. The TUNEL assay was then conducted using an In Situ
Cell Death Detection Kit, Fluorescein (Roche Diagnostics) according
to the manufacturer's protocol. The nuclei were counterstained with
DAPI (0.1 µg/ml) at room temperature in the dark for 5 min.
TUNEL-positive cells were visualized using a fluorescence
microscope (Olympus Corporation) and the percentage of apoptotic
cells was calculated in five randomly selected fields of view. The
apoptotic rate was analyzed using ImageJ 1.52r software and
calculated as follows: Apoptosis rate (%) = (Green fluorescence
intensity/blue fluorescence intensity) x 100.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from H9C2 cells treated with
LPS using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA concentration was measured using a
spectrophotometer. Total RNA was reverse-transcribed into cDNA
using a Reverse Transcription kit (Beijing TransGen Biotech Co.,
Ltd.) according to the manufacturer's protocol. qPCR was performed
using TransScript II Green Two-Step qRT-PCR SuperMix (cat. no.
AQ301-01; TransGen Biotech Co., Ltd.) on a QuantStudio 6 Flex
Real-Time PCR system (Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: Initial denaturation at
94˚C for 30 sec; 40 cycles at 94˚C for 5 sec, annealing at 55˚C for
15 sec and extension at 72˚C for 10 sec. The relative mRNA
expression levels of KOR were quantified using the
2-∆∆Cq method (21) and
normalized to GAPDH. The primers used were as follows: KOR forward,
5'-GATGTG GATGTCATTGAATGCTCCTTGCAG-3' and reverse, 5'-GC
TGTGCTGTGGGAGGTGCTGCCTAGAGCC-3'. GAPDH forward,
5'-AGGCCGGTGCTGAGTATGTC-3' and reverse,
5'-TGCCTGCTTCACCACCTTCT-3'.
Statistical analysis
Experiments were repeated at least three times and
data are expressed as the mean ± SD. Statistical analysis was
performed using GraphPad Prism software, version 7.0 (GraphPad
Software, Inc.). Statistical differences among different groups
were performed using one-way ANOVA followed by Tukey's post hoc
test for multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
Butorphanol suppresses the release of
inflammatory factors in LPS-induced H9C2 cells
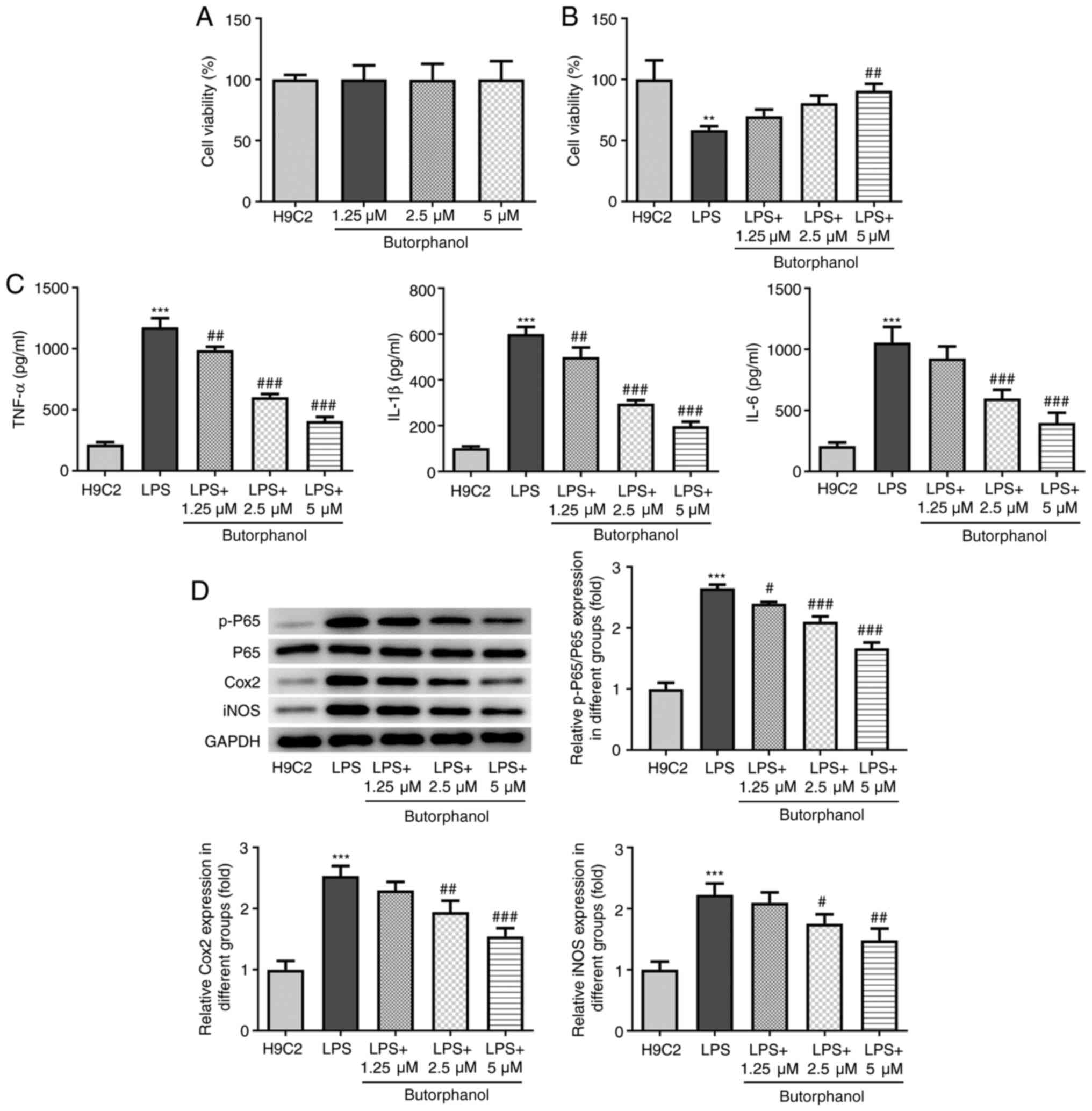
To determine the effects of butorphanol on normal
cardiomyocytes, the viability of H9C2 cells was detected through
CCK-8 assay after butorphanol treatment at different
concentrations. The results of the CCK-8 assay revealed that H9C2
cell viability was not altered, which indicated that butorphanol
did not affect the viability of normal cardiomyocytes (Fig. 1A). H9C2 cells were subsequently
induced with LPS to establish an in vitro sepsis cell model.
As shown in Fig. 1B, LPS
significantly reduced the viability of H9C2 cells, whereas
butorphanol treatment restored the reduced cell viability induced
by LPS in a dose-dependent manner. Inflammation is an important
process involved in sepsis-induced cardiac injury (22); thus, the levels of TNF-α, IL-1β and
IL-6 were detected. As shown in Fig.
1C and D, LPS markedly elevated
the levels of these inflammatory factors and upregulated the
expression levels of inflammation-related proteins containing
p-P65, iNOS and Cox2, whereas treatment with increasing doses of
butorphanol gradually downregulated the levels of all inflammatory
markers.
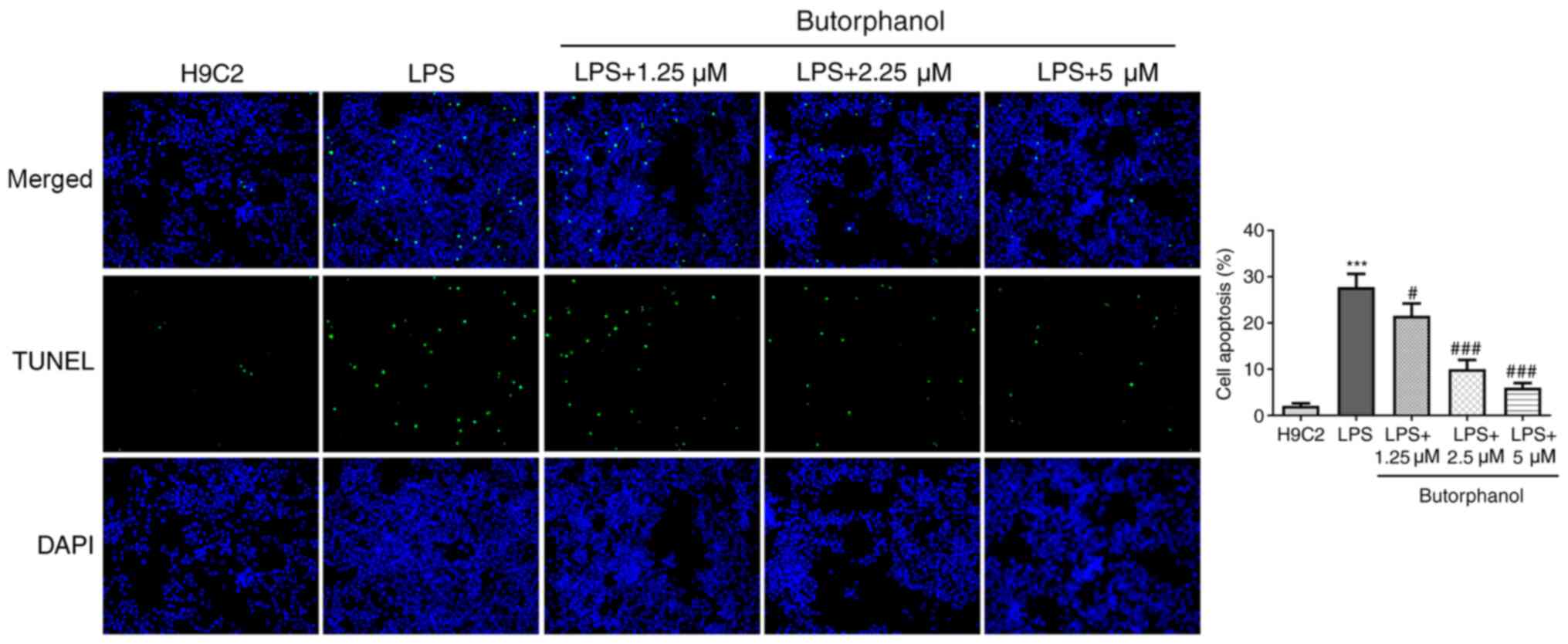
Butorphanol suppresses the apoptosis
of LPS-induced H9C2 cells
Apoptosis is an important process in the occurrence
of SIMD (23); therefore, the
effect of butorphanol on the apoptosis of LPS-induced H9C2 cells
was investigated. As shown in Fig.
2, the apoptosis of H9C2 cells was significantly elevated by
LPS; however, butorphanol treatment reduced the apoptotic rate of
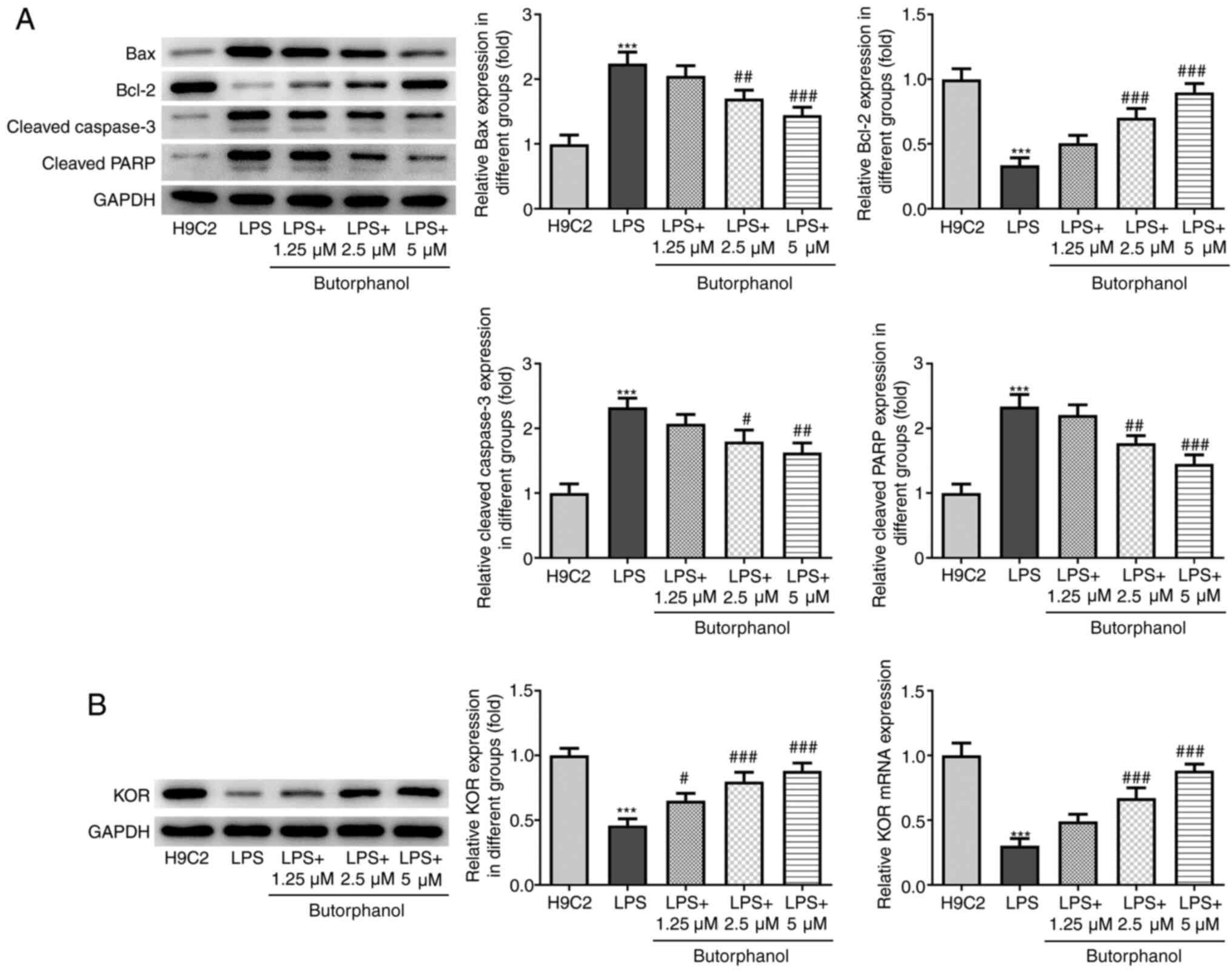
H9C2 cells. Concurrently, the expression levels of proapoptotic
proteins (Bax, cleaved caspase 3 and cleaved PARP) were
upregulated, whereas the expression levels of anti-apoptotic
protein Bcl-2 were downregulated following LPS stimulation. These
trends were all reversed following treatment of cells with
butorphanol (Fig. 3A). These
results suggested that butorphanol may suppress the apoptosis of
LPS-induced H9C2 cells.
Butorphanol upregulates the expression
of KOR in LPS-induced H9C2 cells
Butorphanol is known to act as a partial agonist of
KOR in the G-protein activation signaling pathway (24). The results of western blot analysis
revealed that LPS stimulation downregulated the expression levels
of KOR in H9C2 cells, whereas the expression levels of KOR were
upregulated following butorphanol treatment (Fig. 3B). Of note, treatment with 5 µM
butorphanol upregulated the expression levels of KOR to the
greatest extent. Additionally, 1.25 and 2.5 µm butorphanol
significantly increased the expression level of KOR compared with
LPS group. These results suggested that butorphanol may upregulate
the expression of KOR in LPS-induced H9C2 cells.
Butorphanol suppresses the release of
inflammatory factors and the apoptosis of LPS-induced H9C2 cells by
activating KOR
It was subsequently hypothesized that butorphanol
may exert its anti-inflammatory and anti-apoptotic effects by
activating KOR. The LPS-induced increases in secretory levels of
inflammatory factors and expression levels of inflammation-related
proteins were reduced following butorphanol treatment, whereas
further addition of Nor-BNI, an inhibitor of KOR, reversed this
trend (Fig. 4A and B). Western blot analysis also revealed
that butorphanol treatment downregulated the expression levels of
proapoptotic proteins; Bax, cleaved caspase 3 and cleaved PARP;
however, these trends were subsequently reversed by the addition of
Nor-BNI (Fig. 4C). There was no
significant difference in the expression levels of the
anti-apoptotic protein Bcl-2 between the butorphanol and Nor-BNI
groups. Taken together, these findings indicated that butorphanol
may suppress the release of inflammatory factors and the apoptosis
of LPS-induced H9C2 cells by upregulating KOR expression.
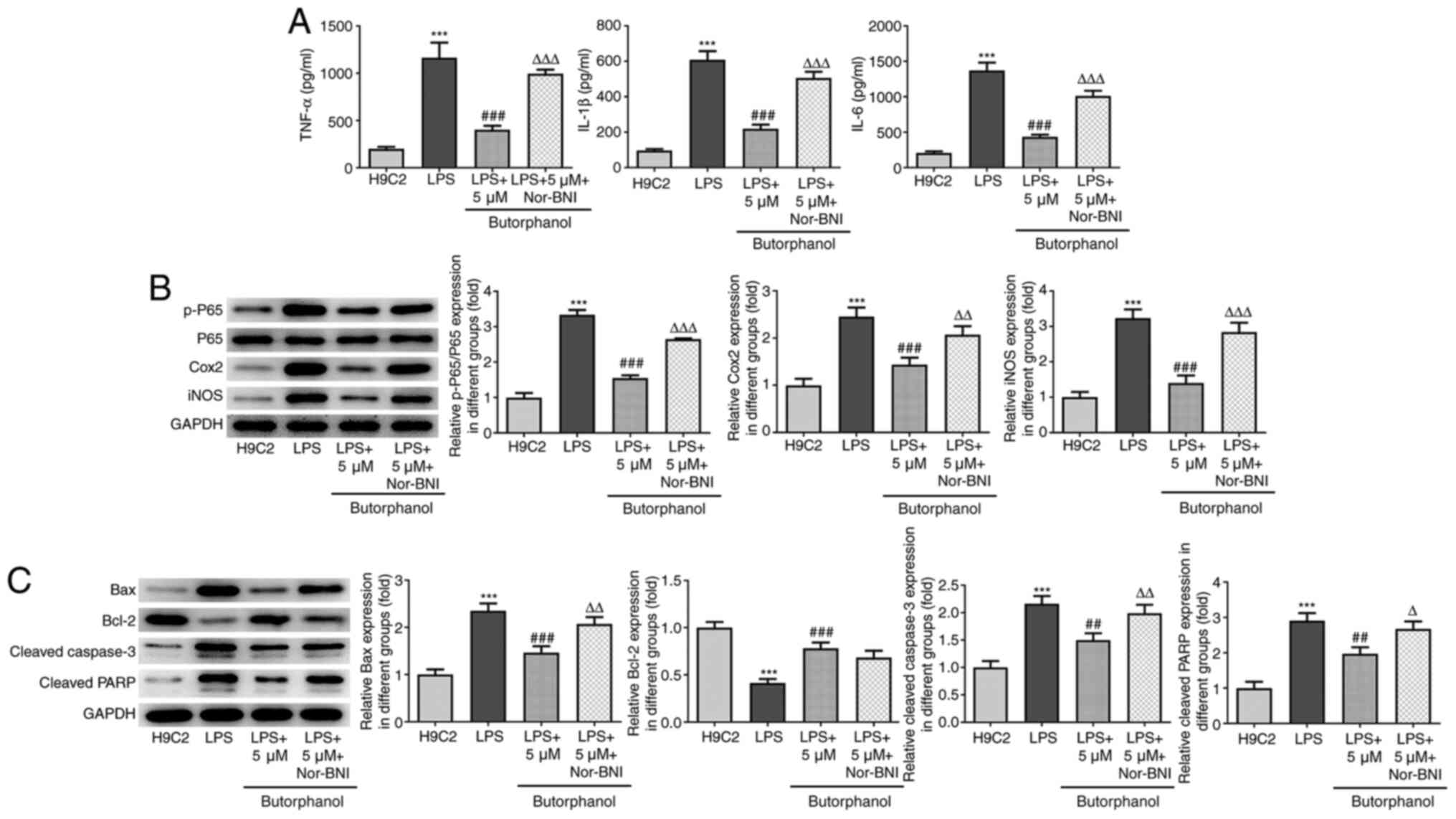 | Figure 4Butorphanol suppresses the release of
inflammatory factors and apoptosis of LPS-induced cardiomyocytes by
activating KOR. (A) The effect of Nor-BNI on the levels of
inflammatory factors in LPS-induced H9C2 cells treated with 5 µM
butorphanol was evaluated by ELISA. (B) The effect of Nor-BNI on
the protein expression levels of NF-κB P65, Cox2 and iNOS was
evaluated by western blotting. (C) The effect of Nor-BNI on the
levels of apoptosis-related proteins was evaluated by western
blotting. ***P<0.001 vs. H9C2 group;
##P<0.01, ###P<0.001 vs. LPS group;
∆P<0.05, ∆∆P<0.01,
∆∆∆P<0.001 vs. LPS + 5 µm butorphanol group. All
statistical analyses were performed using one-way ANOVA followed by
Tukey's test. LPS, lipopolysaccharide; KOR, κ-opioid receptor;
Nor-BNI, nor-binaltorphimine; Cox2 cyclooxygenase 2; iNOS,
inducible nitric oxide synthase; p-, phosphorylated; PARP,
poly(ADP)-ribose polymerase. |
Discussion
Sepsis can cause multiorgan failure, and SIMD is the
most complex and multifactorial type of organ failure (25). Myocardial dysfunction commonly
occurs as a result of sepsis, affecting 64% of all patients with
sepsis (25). However, to the best
of our knowledge, the pathophysiology of SIMD has not been fully
elucidated and SIMD requires further study. Butorphanol is widely
used in the clinical setting. A previous study reported the
beneficial therapeutic effects of butorphanol, such as improving
pathology abnormalities and inflammation in a rat model of sepsis
with brain injury (18). In
addition, the transnasal application of butorphanol was able to
alleviate pain in patients with acute migraines (26). However, current research mainly
focuses on investigating the analgesic effects of butorphanol,
whereas its effects on diseases such as SIMD remain poorly
understood. Therefore, the present study aimed to investigate the
effects of butorphanol on SIMD and elucidate the potential
underlying mechanism.
Several previous studies investigating the
underlying mechanisms of sepsis have confirmed that LPS (10 mg/kg),
when injected intravenously into rats with septic shock, caused
myocardial contraction and myocardial damage (27-29).
Similarly, the present study used LPS to establish a cell model of
sepsis, as SIMD is a major complication following the occurrence of
septic shock (30). SIMD is a
severe complication caused by inflammation and it has been
suggested that the activation of inflammasomes may be involved in
myocardial tissue damage (31). In
the present study, the release of inflammatory cytokines was
increased following LPS stimulation of H9C2 cells, whereas
butorphanol suppressed the release of inflammatory factors. A
previous study also reported that the levels of inflammatory
cytokines were increased in sepsis-induced myocardial injury in
rats (23). Apoptosis was found to
play an important role in the occurrence of SIMD (23). Similar to its effects on
inflammation, butorphanol treatment decreased the levels of
apoptosis in LPS-induced H9C2 cells.
As a member of the G protein-coupled receptor
superfamily, KOR is the product of a single gene, KOR1, in species
including pigs and humans (32-34).
KOR internalization has been reported to be induced by butorphanol,
which is a crucial step in the receptor signaling transduction
cascade, which can bring about pharmacological changes that are
associated with the efficacy and side effects of opioids (35). Previous studies have reported that
the opioid receptor comprises the MOR, δ-opioid receptor and KOR,
and is not the product of a distinct gene; butorphanol shares a
binding site with these three major opioid receptors, with an
affinity ratio of 1:4:25, respectively (36,37).
KOR activation was reported to elicit anti-inflammatory and
suppress pro-apoptotic effects (38). As regards the mechanism of
regulation, it was previously revealed that KOR activation was
involved in regulating the STAT3-OPA1 pathway to facilitate
mitochondrial fusion and inhibit cell apoptosis (39). In the present study, butorphanol
treatment upregulated the expression levels of KOR in LPS-induced
H9C2 cells, suggesting that KOR was activated by butorphanol.
Following pretreatment of butorphanol-treated LPS-induced H9C2
cells with the KOR inhibitor Nor-BNI, the inhibitory effects of
butorphanol were reversed, indicating that butorphanol may exert
inhibitory effects over inflammation and apoptosis in LPS-induced
H9C2 cells by activating KOR. Further investigation is required to
determine whether butorphanol exerts the same effects on
inflammation and apoptosis in a KOR-dependent manner. Additionally,
the lack of in vivo validation of the findings of the
present study is a potential limitation that can be addressed in
future studies.
In conclusion, the present study provided evidence
to suggest that butorphanol may alleviate LPS-induced inflammation
and apoptosis of cardiomyocytes by activating KOR, which may help
researchers more comprehensively understand the therapeutic effects
of butorphanol and its underlying mechanism of action.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WQT, LL, BJH and MZZ made substantial contributions
to the conception and design of the study, performed the
experiments, interpreted the data and drafted and revised the
manuscript for important intellectual content. WQT and MZZ confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kaukonen KM, Bailey M, Pilcher D, Cooper
DJ and Bellomo R: Systemic inflammatory response syndrome criteria
in defining severe sepsis. N Engl J Med. 372:1629–1638.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Delano MJ and Ward PA: Sepsis-induced
immune dysfunction: Can immune therapies reduce mortality? J Clin
Invest. 126:23–31. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Gaieski DF, Edwards JM, Kallan MJ and Carr
BG: Benchmarking the incidence and mortality of severe sepsis in
the United States. Crit Care Med. 41:1167–1174. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dombrovskiy VY, Martin AA, Sunderram J and
Paz HL: Rapid increase in hospitalization and mortality rates for
severe sepsis in the United States: A trend analysis from 1993 to
2003. Crit Care Med. 35:1244–1250. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Keeley A, Hine P and Nsutebu E: The
recognition and management of sepsis and septic shock: A guide for
non-intensivists. Postgrad Med J. 93:626–634. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lv X and Wang H: Pathophysiology of
sepsis-induced myocardial dysfunction. Mil Med Res.
3(30)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yao Y, Sun F and Lei M: miR-25 inhibits
sepsis-induced cardiomyocyte apoptosis by targetting PTEN. Biosci
Rep. 38(38)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hua J, Miao S, Shi M, Tu Q, Wang X, Liu S,
Wang G and Gan J: Effect of butorphanol on etomidate-induced
myoclonus: A systematic review and meta-analysis. Drug Des Devel
Ther. 13:1213–1220. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dawn AG and Yosipovitch G: Butorphanol for
treatment of intractable pruritus. J Am Acad Dermatol. 54:527–531.
2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bailey AG, Valley RD, Freid EB and Calhoun
P: Epidural morphine combined with epidural or intravenous
butorphanol for postoperative analgesia in pediatric patients.
Anesth Analg. 79:340–344. 1994.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gunter JB, McAuliffe J, Gregg T, Weidner
N, Varughese AM and Sweeney DM: Continuous epidural butorphanol
relieves pruritus associated with epidural morphine infusions in
children. Paediatr Anaesth. 10:167–172. 2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Heel RC, Brogden RN, Speight TM and Avery
GS: Butorphanol: A review of its pharmacological properties and
therapeutic efficacy. Drugs. 16:473–505. 1978.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hoskin PJ and Hanks GW: Opioid
agonist-antagonist drugs in acute and chronic pain states. Drugs.
41:326–344. 1991.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang J, Miao S, Tu Q, Shi M, Zou L, Liu S
and Wang G: Effect of butorphanol on opioid-induced cough: A
meta-analysis of randomized controlled trials. Drug Des Devel Ther.
12:3263–3268. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Garrity K, Jang A and Wagner S:
Butorphanol use in laboring patients with preeclampsia or chronic
hypertension. Pregnancy Hypertens. 6:288–290. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang H, Wang JL, Ren HW, He WF and Sun M:
Butorphanol protects on myocardial ischemia/reperfusion injury in
rats through MAPK signaling pathway. Eur Rev Med Pharmacol Sci.
23:10541–10548. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang LH, Li J, Gu JP, Qu MX, Yu J and
Wang ZY: Butorphanol attenuates myocardial ischemia reperfusion
injury through inhibiting mitochondria-mediated apoptosis in mice.
Eur Rev Med Pharmacol Sci. 22:1819–1824. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Meng J, Jiang SJ, Jiang D and Zhao Y:
Butorphanol attenuates inflammation via targeting NF-κB in septic
rats with brain injury. Eur Rev Med Pharmacol Sci. 23 (Suppl
3):161–170. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baradaran Rahim V, Khammar MT, Rakhshandeh
H, Samzadeh-Kermani A, Hosseini A and Askari VR: Crocin protects
cardiomyocytes against LPS-Induced inflammation. Pharmacol Rep.
71:1228–1234. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cui Y, Feng N, Gu X, Fu F, Li J, Guo H,
Liu Y, Zhang S, Li J, Wang Y, et al: κ-Opioid receptor stimulation
reduces palmitate-induced apoptosis via Akt/eNOS signaling pathway.
Lipids Health Dis. 18(52)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kenneth JL and Thomas DS: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liang W, Li J, Bai C, Chen Y, Li Y, Huang
G and Wang X: Interleukin-5 deletion promotes sepsis-induced M1
macrophage differentiation, deteriorates cardiac dysfunction, and
exacerbates cardiac injury via the NF-κB p65 pathway in mice.
Biofactors. 46:1006–1017. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang C, Xia W, Liu X, Lin J and Wu A: Role
of TXNIP/NLRP3 in sepsis-induced myocardial dysfunction. Int J Mol
Med. 44:417–426. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ji J, Lin W, Vrudhula A, Xi J, Yeliseev A,
Grothusen JR, Bu W and Liu R: Molecular interaction between
butorphanol and κ-opioid receptor. Anesth Analg. 131:935–942.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Pulido JN, Afessa B, Masaki M, Yuasa T,
Gillespie S, Herasevich V, Brown DR and Oh JK: Clinical spectrum,
frequency, and significance of myocardial dysfunction in severe
sepsis and septic shock. Mayo Clin Proc. 87:620–628.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hoffert MJ, Couch JR, Diamond S, Elkind
AH, Goldstein J, Kohlerman NJ III, Saper JR and Solomon S:
Transnasal butorphanol in the treatment of acute migraine.
Headache. 35:65–69. 1995.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Afulukwe IF, Cohen RI, Zeballos GA, Iqbal
M and Scharf SM: Selective NOS inhibition restores myocardial
contractility in endotoxemic rats; however, myocardial NO content
does not correlate with myocardial dysfunction. Am J Respir Crit
Care Med. 162:21–26. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cohen RI, Wilson D and Liu SF: Nitric
oxide modifies the sarcoplasmic reticular calcium release channel
in endotoxemia by both guanosine-3',5' (cyclic) phosphate-dependent
and independent pathways. Crit Care Med. 34:173–181.
2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chagnon F, Bentourkia M, Lecomte R,
Lessard M and Lesur O: Endotoxin-induced heart dysfunction in rats:
Assessment of myocardial perfusion and permeability and the role of
fluid resuscitation. Crit Care Med. 34:127–133. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Walley KR: Sepsis-induced myocardial
dysfunction. Curr Opin Crit Care. 24:292–299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Su Q, Li L, Sun Y, Yang H, Ye Z and Zhao
J: Effects of the TLR4/Myd88/NF-κB signaling pathway on NLRP3
inflammasome in coronary microembolization-induced myocardial
injury. Cell Physiol Biochem. 47:1497–1508. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Commiskey S, Fan LW, Ho IK and Rockhold
RW: Butorphanol: Effects of a prototypical agonist-antagonist
analgesic on kappa-opioid receptors. J Pharmacol Sci. 98:109–116.
2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xie GX, Meng F, Mansour A, Thompson RC,
Hoversten MT, Goldstein A, Watson SJ and Akil H: Primary structure
and functional expression of a guinea pig kappa opioid (dynorphin)
receptor. Proc Natl Acad Sci USA. 91:3779–3783. 1994.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yasuda K, Espinosa R III, Takeda J, Le
Beau MM and Bell GI: Localization of the kappa opioid receptor gene
to human chromosome band 8q11.2. Genomics. 19:596–597.
1994.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu J, Chen F, Wang S, Akins NS, Hossain
MI, Zhou Y, Huang J, Ji J, Xi J, Lin W, et al: Kappa opioid
receptors internalization is protective against oxygen-glucose
deprivation through β-arrestin activation and Akt-mediated
signaling pathway. Neurochem Int. 137(104748)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Simonin F, Slowe S, Becker JA, Matthes HW,
Filliol D, Chluba J, Kitchen I and Kieffer BL: Analysis of
[3H]bremazocine binding in single and combinatorial opioid receptor
knockout mice. Eur J Pharmacol. 414:189–195. 2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chang KJ, Hazum E and Cuatrecasas P: Novel
opiate binding sites selective for benzomorphan drugs. Proc Natl
Acad Sci USA. 78:4141–4145. 1981.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chéret J, Gherardini J, Soeberdt M, Hundt
JE, Abels C, Bertolini M and Paus R: Non-neuronal kappa-opioid
receptor activation enhances epidermal keratinocyte proliferation,
and modulates mast cell functions in human skin ex vivo. J
Dermatol. 47:917–921. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang K, Liu Z, Zhao M, Zhang F, Wang K,
Feng N, Fu F, Li J, Li J, Liu Y, et al: κ-opioid receptor
activation promotes mitochondrial fusion and enhances myocardial
resistance to ischemia and reperfusion injury via STAT3-OPA1
pathway. Eur J Pharmacol. 874(172987)2020.PubMed/NCBI View Article : Google Scholar
|