Introduction
Graves' disease (GD) is an autoimmune disorder of
the thyroid gland. The annual incidence of GD is 20-50 cases per
100,000 persons (1). Approximately
3% of women and 0.5% of men develop GD during their lifetime
(2). Most patients with GD present
with overt hyperthyroidism along with a variety of symptoms,
including palpitations, tremulousness, heat intolerance, weight
loss, and anxiety (3). The main
pathogenic mechanisms responsible for GD are the stimulation and
secretion of thyroid hormone [thyroxine (T4) and triiodothyronine
(T3)] and the stimulation of thyrocyte growth by
thyroid-stimulating hormone receptor (TSHR) autoantibodies
(TSHR-Ab) (4).
Maternal GD has several effects on the fetus during
pregnancy, including low birth weight and intrauterine growth
retardation (5). Moreover, elevated
TSHR-Ab levels in pregnant women with GD may affect the fetal
thyroid gland through the placenta, resulting in fetal or neonatal
thyroid dysfunction and other adverse effects, either temporarily
or long-term (3). In addition, it
has been reported that neonates with transient thyroid disease from
their euthyroid TSHR-Ab positive mother may suffer from worsening
of their condition during breastfeeding, potentially due to the
presence of TSHR-Ab in breast milk that enters the blood through
the immature neonatal intestinal mucosa (6). However, the relevance of this mode of
transmission and condition aggravation is still controversial, as
only small amounts of TSHR-Ab are thought to be present in breast
milk (7-11).
In the present study, a GD model was established in
mice by repeated injection of TSHR-expressing adenoviruses to
confirm whether TSHR-Ab in mothers can cause neonatal thyroid
disease and the mechanisms underlying this. Excessive serum T4 was
considered as the criterion for hyperthyroidism diagnosis. The
serum T4 levels of both mothers and neonates were measured when the
mice with GD gave birth. Breast milk was collected from the
stomachs of neonatal mice to determine the milk TSHR-Ab levels.
Materials and methods
Animal and grouping
All experimental procedures were carried out
according to the guidelines of the Medicine Laboratory Animal
Center of Anhui Medical University. Male and female BALB/c mice
(age, 6 weeks; weight, 16.6±0.028 g) were obtained from the
Medicine Laboratory Animal Center and maintained in a pathogen-free
environment under a 12 h light/dark cycle, a temperature of 22±1˚C
and a humidity of 45-55%. Animals received free access to food and
drink. The study was approved by the institutional animal care and
use committee of Anhui Medical University (approval reference no.
PJ-YX2019-038).
An adenovirus expressing TSHR A-subunit
(Ad-TSHR-289) and a control adenovirus expressing β-galactosidase
(Ad-β-gal) were obtained from from Dr Chun-Rong Chen (Cedars-Sinai
Medical Center and the University of California Los Angeles, USA).
Beijing Sino Geno Max Co., Ltd. verified the sequences and
amplified these viruses. The TSHR immunization group included 90
female mice that were intramuscularly injected with Ad-TSHR-289
[3.3x108 plaque formation unit (PFU)/50 µl of
phosphate-buffered saline (PBS)]. All mice were injected three
times, once every three weeks. Female mice (90) were injected
intramuscularly with Ad-β-gal (3.3x108 PFU/50 µl of PBS)
and served as the sham immunization group (12). After mice were anesthetized with
sodium pentobarbital (1%; 50 mg/kg intraperitoneally), blood
samples were taken from angular vein of the eyes by capillary after
the third injection and the serum TSHR-Ab levels were measured to
determine the success rate of modeling.
Female mice (15-week-old) in each group were bred
with males (two females and one male per cage). Vaginal plugs were
checked the morning following breeding. The females displaying a
vaginal plug were considered pregnant and this time point was
considered to be pregnancy day 0.5. Pregnant female mice were caged
individually and fed until delivery with free access.
Mother mice, 30 in each group were sacrificed after
breast feeding on days 1, 7 or 21 after delivery by cervical
dislocation. The offspring of the mother mice were sacrificed at
the same time by cervical dislocation. Prior to cervical
dislocation all mice were anesthetized by sodium pentobarbital (1%
and 50 mg/kg intraperitoneally) before blood collection by
retrobulbar plexus.
The milk masses from the gastric sac of the
offspring of the same mother were pooled in a single tube. 100
l/0.05 g of the milk was added to cold PBS, which was mixed and
centrifuged at 1,006.29 x g (4˚C) for 20 min. The supernatant was
taken and diluted with PBS 3 times in series for testing.
Blood samples were collected for detecting TSHR-Ab
and T4 and the thyroids were harvested for histological
examination. Milk from the gastric sac of neonatal mice from the
same mother was collected to determine milk TSHR-Ab levels
(Fig. 1).
Preparation of adenoviruses
Both Ad-TSHR-289 and Ad-β-gal were propagated in
293T cells by researchers from Beijing Sino Geno Max Co., Ltd. They
were purified using an ion-exchange chromatographic column
(13), aliquoted, and stored at
-80˚C. The concentration of viral particles was determined using a
standard PFU protocol (14).
Measurement of TSHR-Ab and T4
levels
The levels of TSHR-Ab and T4 in 50 µl of undiluted
sample (serum or milk) were measured. The TSHR-Ab levels were
determined using an Elecsys® Anti-TSHR kit [cat. no.
YZB/GEM 1362-2009; Roche Diagnostics (Shanghai) Co., Ltd.]
according to the manufacturer's instructions and a Cobas e analyzer
(measurement range 0.3-40 IU/l, functional sensitivity 0.9 IU/l;
both supplied by Roche Diagnostics (Shanghai) Co., Ltd.) using a
competitive electrochemiluminescence immunoassay, based on the
inhibitory effect on the binding of the thyroid-stimulating human
monoclonal antibody M22 (labeled with ruthenium) to porcine TSHR
(15).
T4 levels were determined using the iodine thyroxine
radioimmunoassay kit (cat. no. S10930056; Tianjing Jiuding Medical
Bioengineering Co., Ltd.) according to the manufacturer's
instructions and a γ-ray counter [sensitivity of 0.3 µg/dl (1
µg/dl=12.87 nmol/l); intragroup variability of 7.0% and intergroup
variability of 11.2%; Tianjin Jiuding Medical Bioengineering Co.,
Ltd.) in a liquid-phase equilibrium competitive radioimmunoassay,
according to its inhibitory effect on the binding of
125I-labeled T4 to goat anti-T4 antibody. The normal
range of T4 was determined to be the mean T4 level in the sham
group ± three standard deviations. T4 levels higher than the
highest normal level were defined as hyperthyroidism (16).
Histological examination of the
thyroid gland
The dissected thyroids were fixed in 10% buffered
formalin for at least 24 h at 37˚C, dehydrated and embedded in
paraffin. Sections (5 µm) were stained with hematoxylin-eosin
(17). The analysis was performed
using the Olympus Cue-2 image analysis system connected to a
fluorescence microscope (Olympus Corporation; magnification, x200
and x400). The area of each follicle in five random fields was
evaluated from each section. Samples were taken from hyperthyroid
(n=9) and euthyroid mice (n=8).
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(SPSS, Inc.). The TSHR-Ab levels were compared between the two
groups using the Mann-Whitney U rank-sum test. The T4 levels are
presented as the mean ± the standard deviation and were analyzed
using the Student's t-test. P<0.05 was considered to be
statistically significant.
Results
Serum TSHR-Ab in mice after TSHR
immunization
Serum TSHR-Ab was not detectable (<0.3 IU/l) in
the sham group (n=90) after three injections of adenovirus. The
positive rate of serum TSHR-Ab (22.5±1.12 IU/l) in the TSHR group
(n=90) was 99% (89/90), indicating that only one mouse did not
develop autoimmunity to TSHR. After delivery, the serum TSHR-Ab
levels were higher in the TSHR group on days 1, 7 and 21 (n=30 per
time point) compared with the sham group (all P<0.01). The serum
TSHR-Ab levels of the mother mice in the TSHR group were 20.59±1.63
IU/l on day 1, 22.49±1.13 IU/l on day 7 and 23.43±1.10 IU/l on day
21 (n=30 per time point), respectively in the TSHR group. After
delivery, the serum T4 levels of the mother mice in the sham group
were 5.7±0.955, 5.6±0.857 and 5.8±0.826 µg/dl on days 1, 7 and 21
(n=30 per time point), respectively. The serum T4 levels of mother
mice in the TSHR group were higher than in the sham group (all
P<0.05; Fig. 2A). The normal
range of T4 was determined to be the mean T4 level in the sham
group ± three standard deviations. T4 levels higher than the
highest normal level were defined as hyperthyroidism (16). Thus, 22 (73%), 18 (60%) and 23 (77%)
mother mice in the TSHR immunization group were diagnosed with
hyperthyroidism on days 1, 7 and 21, respectively (Table I). These data suggest that TSHR
immunization using Ad-TSHR-289 was an effective way to obtain a GD
model in mice.
 | Table IT4 and TSHR-Ab levels in the mother
mice. |
Table I
T4 and TSHR-Ab levels in the mother
mice.
| Characteristic | Day 1 after delivery
in the sham group | Day 1 after delivery
in the TSHR immunization group | Day 7 after delivery
in the sham group | Day 7 after delivery
in the TSHR immunization group | Day 21 after delivery
in the sham group | Day 21 after delivery
in the TSHR immunization group |
|---|
| T4 µg/dl | 5.700±0.955 |
10.000±3.011a | 5.600±0.857 |
9.305±2.702a | 5.800±0.826 |
9.400±2.243a |
| TSHR-Ab IU/l | <0.30 |
20.59±1.63b | <0.30 |
22.49±1.13b | <0.30 |
23.43±1.10b |
Higher serum levels of TSHR-Ab and T4
were observed in the offspring of the TSHR group
Serum TSHR-Ab levels were higher in the offspring of
the TSHR group on day 7 (P<0.01) and day 21 (P<0.01) after
delivery, as compared with the offspring in the sham group (TSHR-Ab
<0.3 IU/l at all time points). The serum TSHR-Ab levels of the
offspring of the TSHR group were 19.62±1.59 and 18.69±2.09 IU/l on
days 7 and 21, respectively. The serum T4 levels of the offspring
in the sham group were 5.8±0.561 and 5.7±0.638 µg/dl on days 7 and
21, respectively. The offspring in the TSHR group had higher serum
T4 levels (both P<0.05; Fig.
2B). Considering the mean T4 levels ± three standard deviations
of the sham group as the normal range, the incidence of
hyperthyroidism in the offspring of the TSHR group was 63%
(114/180) and 72% (129/180) on days 7 and 21 after delivery,
respectively. The serum T4 levels of the offspring in the TSHR
group were 9.503±2.61 and 9.123±2.287 µg/dl on days 7 and 21,
respectively (Table II).
 | Table IISerum T4, TSHR-Ab levels and weight of
offspring mice. |
Table II
Serum T4, TSHR-Ab levels and weight of
offspring mice.
| Characteristic | Day 7 after delivery
in the sham group | Day 7 after delivery
in the TSHR immunization group | Day 21 after delivery
in the sham group | Day 21 after delivery
in the TSHR immunization group |
|---|
| N | 192 | 180 | 187 | 180 |
| T4 µg/dl | 5.834±0.561 |
9.500±2.61a | 5.719±0.638 |
9.100±2.287a |
| TSHR-Ab IU/l | <0.30 |
19.62±1.59b | <0.30 |
18.69±2.09b |
| Weight g | 6.10±1.82 | 3.90±1.34 | 11.20±2.03 | 8.90±1.51 |
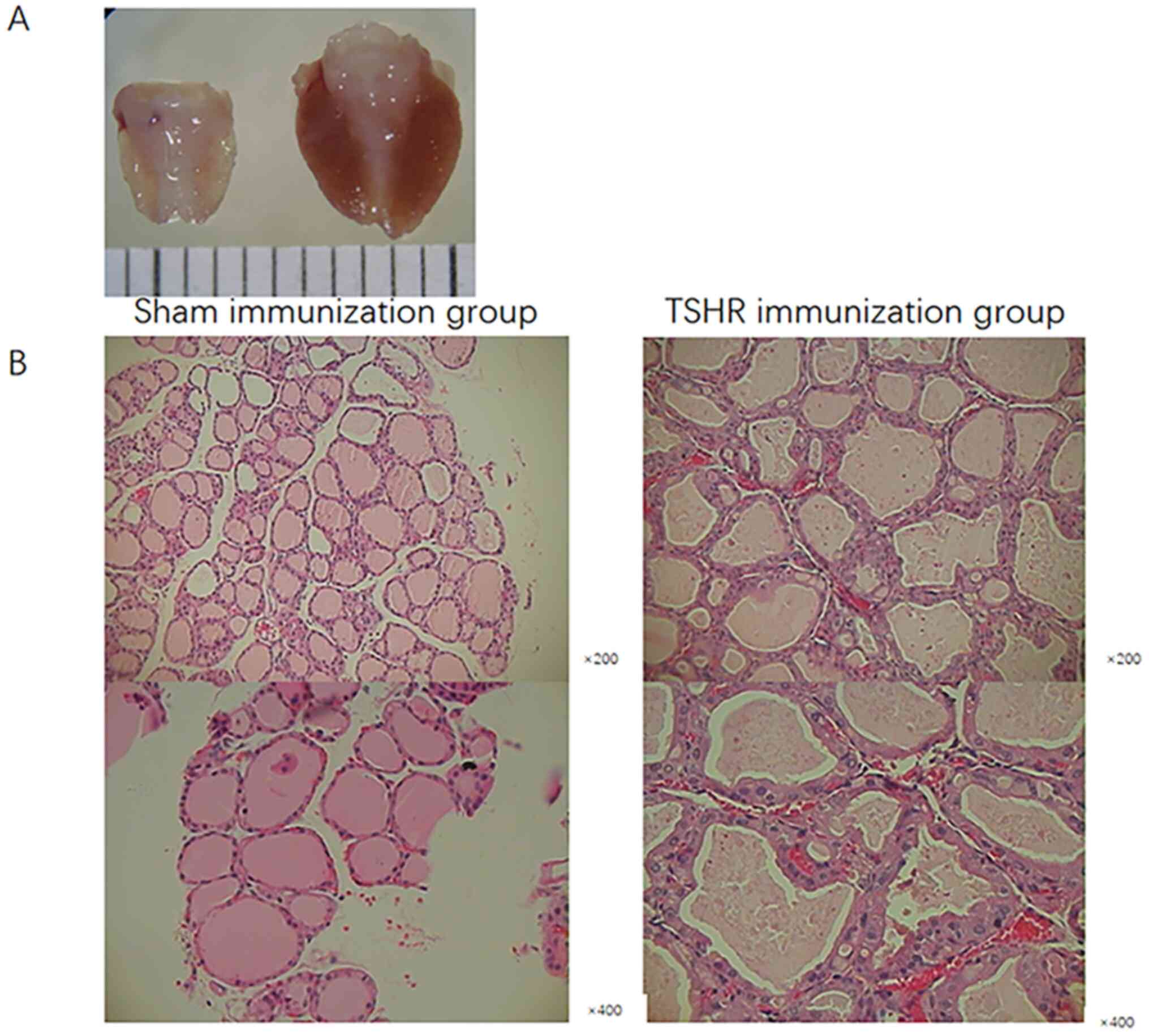
Thyroid appeared larger in the TSHR
mother mice than in the sham mice
The mother mice with hyperthyroidism in the TSHR
immunization group had larger thyroids than sham mice (Fig. 3A). Histological examination revealed
that the thyroids exhibited diffuse enlargement with hypertrophy
and hypercellularity of follicular epithelia, with occasional
protrusion into the follicular lumen, compared with normal thyroids
from the sham group. These changes were consistent with the
clinicopathological features of Graves' hyperthyroidism in humans
(Fig. 3B) (12).
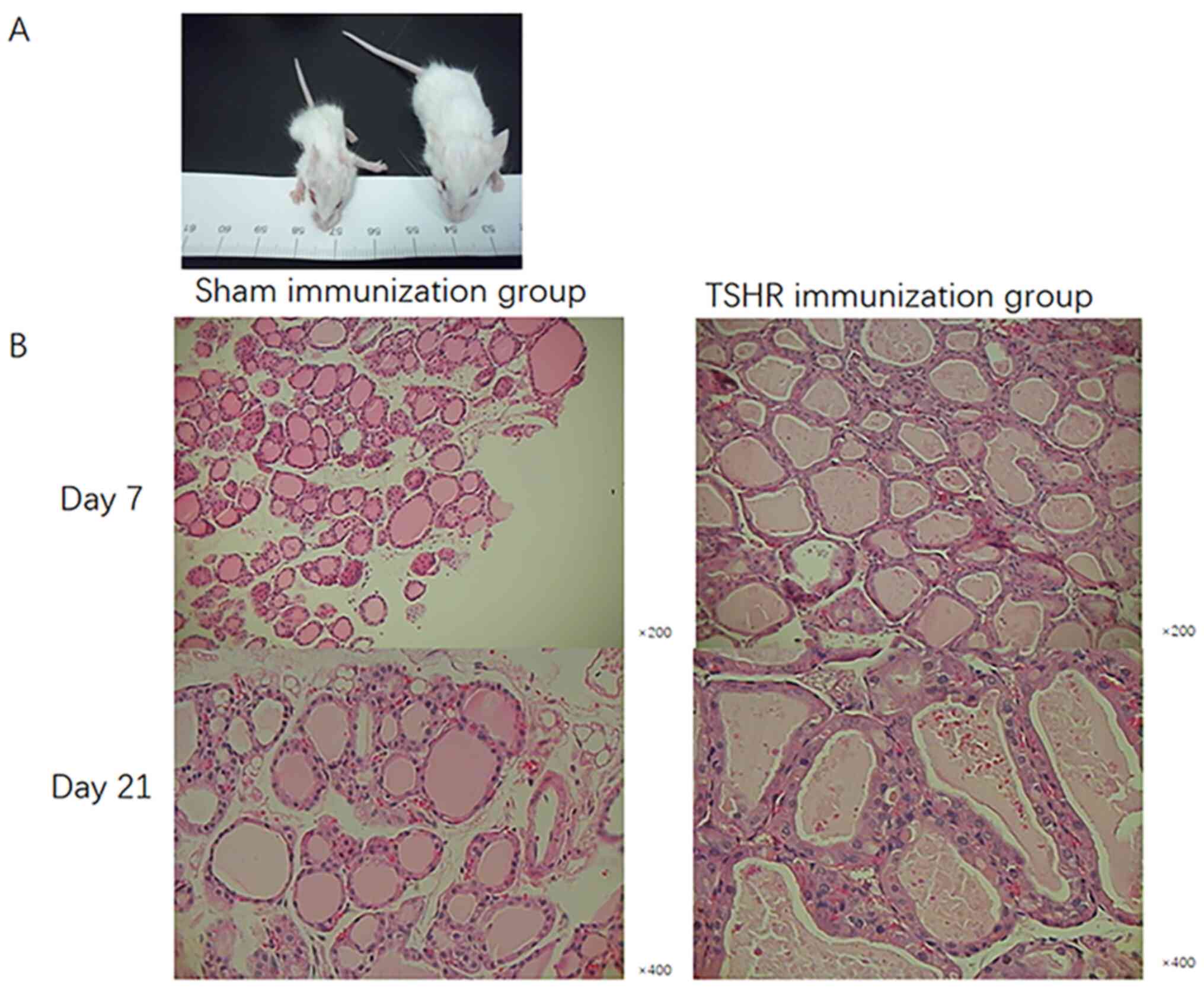
TSHR-immunized mothers gave birth to
smaller newborns with thyroid dysplasia
The average weights of the newborns in the TSHR
group were 3.9±1.34 g on day 7 and 8.9±1.51 g on day 21 after
delivery, which was lower than in the sham group (6.1±1.82 and
11.2±2.03 g, respectively). The newborns born in the TSHR group
were thinner and smaller than those born in the sham group
(Fig. 4A; Table II).
The follicular epithelial cells from the thyroids
were studied in each group. Thyroids were diffusely enlarged,
without interstitial lymphocytic infiltration and nodular
progression in the offspring in the TSHR group compared with those
in the sham group. Thyroid follicles in the goiters also had
cuboidal thyroid epithelial cells with marked intracellular
vacillation, indicating higher secretory activity (18). This showed a difference in the
pathology of follicular epithelial cells of the thyroid gland in
the offspring of the TSHR group in comparison with the sham group
(Fig. 4B).
TSHR-Ab levels in breast milk from the
TSHR group were not significantly different from that of the sham
group
Offspring mice were sacrificed and breast milk was
collected from their stomachs for the determination of TSHR-Ab
level. The levels of TSHR-Ab in breast milk were lower than 0.3
IU/l in all mice of the sham group. In the TSHR group, 9 out of 30
mother mice had detectable TSHR-Ab levels in their breast milk on
day 1 after delivery (1.1, 1.2, 1.4, 2.1, 2.4, 2.9, 3.5, 5.3 and
6.5 IU/l). The proportion of mother mice with breast milk TSHR-Ab
levels >0.3 IU/l declined to 4/30 on day 7 after delivery (1.5,
1.8, 2.3 and 3.4 IU/l). However, no significant difference was
observed between the two groups on days 1 (P=0.055) or 7 (P=0.230).
The levels of TSHR-Ab in breast milk were all lower than 0.3 IU/l
in the TSHR group on day 21.
Discussion
Maternal autoimmune thyroid disorders may affect
fetal and neonatal thyroid function through placental transfer of
TSHR-Ab (15). Additionally,
anti-thyroid drugs can affect fetuses and neonates through both the
placenta and breast milk. However, few reports focus on TSHR-Ab in
breast milk (19). The hypothesis
that transmission and aggravation of thyroid conditions can occur
through breast milk is controversial, as only small amounts of
TSHR-Ab are thought to be present in breast milk (1,8-11).
Whether TSHR-Ab can affect neonates through breast milk was
unclear, therefore, a GD animal model of pregnancy needed to be
established to investigate the dynamics of TSHR-Ab in both the
mother and the fetus/neonate.
In a previous study, a GD mouse model was
successfully established in syngeneic AKR/N mice by repeated
injections with fibroblasts stably transfected with cDNAs of the
human TSHR and major histocompatibility complex class II (20). Elevated TSHR-Ab and serum T4 levels
and diffuse goiter and thyrocyte hyperplasia were induced in ~20%
of mice using this method (20).
Subsequently, other models were successfully established using the
following approaches: i) TSHR cDNA vaccination using a eukaryotic
expression plasmid (the DNA-TSHR model); ii) immunization with a
recombinant adenovirus coding TSHR (the Ad-TSHR model) (21,22);
iii) immunization with dendritic cells (DCs) infected with Ad-TSHR
(the DC-TSHR model) (23); and iv)
a model involving combined DNA-TSHR and in vivo
electroporation (24). According to
a study by Chen et al (12),
the Ad-TSHR A-subunit is more effective at inducing GD than both
the Ad-TSHR and Ad-TSHR-D1NET (12). Therefore, GD animal models were
established in the present study via immunization with Ad-TSHR-289.
TSHR-Ab levels were found to be the highest in the early stage of
the study, i.e. day 1 after virus delivery, and could be detected
in a majority of Ad-TSHR-289-treated mice (89/90), which was
significantly different from the sham mice (0/90). These results
suggested that the Ad-TSHR-289 induced model of GD was successfully
established. In the study by Chen et al (12), the incidence of hyperthyroidism in
Ad-TSHR-289-injected BALB/c mice ranged from 65 to 80% (12). In the present study, the
TSHR-Ab-positive rate in the Ad-TSHR-289-treated group was 99%,
which was higher than that in Chen's study. As the virus strains of
Ad-TSHR-289 and Ad-β-gal are from Chen's laboratory, it is possible
that pregnancy may directly affect the establishment of the GD
model. During breeding and pregnancy, all BALB/c mice immunized
with Ad-TSHR-289 conceived naturally and gave birth to offspring
without any perceived difficulties. This may be related to the role
of Th2 cytokines that predominate during pregnancy (25). Though the maternal immune system
undergoes immune suppression to protect the fetus, hyperthyroidism
may recur during the postpartum period as immune status reverts to
a Th1 state. Similarly to other autoimmune diseases, GD generally,
though not always (26),
ameliorates during pregnancy (27)
due to TSHR-Ab decline (28).
Additionally, pregnancy and delivery can act as triggers for the
onset/recurrence of hyperthyroidism in women with GD (29).
Fetal thyrotoxicosis is a rare disease resulting
from the transfer of thyroid-stimulating immunoglobulins from the
mother to the fetus through the placenta during the second half of
pregnancy (20-30th week) (30).
These autoantibodies bind to the TSHRs and increase the secretion
of thyroid hormones (T4). An improvement in GD is always associated
with a reduction in the levels of maternal serum TSHR-Ab during
pregnancy. A mother may be euthyroid due to past treatment but
still have persistent and active TSHR-Ab (31), affecting fetal thyroid function
through transplacental transfer. The results of the present study
suggested that the serum T4 levels of mice with GD on days 1, 7 and
21 were higher than those in the Ad-β-gal injected mice. This was
consistent with the serum T4 levels in the offspring of the mice
with GD. TSHR-Ab could cross the placenta, like all immunoglobulin
G (IgG) antibodies, to appear in the fetal circulation, at least
during the time period investigated in this study. These data
suggested that GD model was successfully established in the present
study and can produce TSHR-Ab that entered the fetal mouse through
the placenta.
Neonatal thyroid disease has been reported to
develop due to TSHR-Ab in breast milk, possibly because neonates
have an immature intestinal mucosa that allows for passage of
macromolecules (6). Differing from
humans, IgGs from breast milk in many animal species, including
rodents, bovines, cats and ferrets, are transported across the
intestinal epithelium into the neonatal circulation (32). In the present study TSHR-Ab levels
were assessed in breast milk on day 1, 7 and 21. Antibody levels
measured from breast milk gradually reduced from day 1 to day 21.
The levels of thyroid autoantibodies in the mother with GD could
affect the offspring's thyroid function through breast milk.
Moreover, both maternal TSHR-Ab and T4 may cross the placental
barrier and contribute to the development of GD in their offspring.
There is a possibility that the timing of breastfeeding and
sacrifice could affect the levels of TSHR-Ab in the stomach of the
offspring. It was considered that the effect of trans-placental
diffusion of stimulating antibodies may be more prevalent than the
effect of lactation in the development of GD in the offspring mice.
However, additional studies are required to examine this
hypothesis.
Compared to the control immunized group, GD mothers
gave birth to smaller newborns with pathological thyroid changes
typical of GD and higher serum levels of TSHR-Ab and T4. The
TSHR-Ab levels in breast milk from the GD mice declined with time.
The model mimics neonatal GD in humans. However, because the mouse
mothers had stimulating TSHR-Ab in their sera when the pups were
in utero, it is unclear whether any contribution was made to
hyperthyroidism in the pups from breast milk TSHR-Ab.
In conclusion, an animal model of GD was
successfully established by immunization with Ad-TSHR-289. Higher
levels of thyroid auto-antibodies may result in neonatal thyroid
disease. The levels of thyroid auto-antibodies in the mother with
GD could affect the offspring's thyroid function through the
placenta and breast milk.
Acknowledgements
The authors would like to thank Dr Chun-Rong Chen
(Cedars-Sinai Medical Center and the University of California Los
Angeles, USA) who provided the adenovirus expressing TSHR A-subunit
(Ad-TSHR-289) and a control adenovirus expressing β-galactosidase
(Ad-β-gal).
Funding
Funding: The study was supported by the Clinicaln Research
Cultivation Project of the Second Affiliated Hosptial of Anhui
Medical University (grant no. 2020LCYB07) and the Natural Science
Foundation of Anhui Province Universities (grant no.
KJ2020A0182).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY, QQL and DDW contributed to the study design,
data collection, statistical analysis, data interpretation and
manuscript preparation. SMY contributed to the data collection and
performed statistical analysis. LQY and DYL contributed to the data
collection, statistical analysis and manuscript preparation. YY and
SMY confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
animal care and use committee of Anhui Medical University (approval
reference no. PJ-YX2019-038).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Azizi F, Amouzegar A, Mehran L, Alamdari
S, Subekti I, Vaidya B, Poppe K, Sarvghadi F, San Luis T Jr and
Akamizu T: Management of hyperthyroidism during pregnancy in Asia.
Endocr J. 61:751–758. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nyström HF, Jansson S and Berg G:
Incidence rate and clinical features of hyperthyroidism in a
long-term iodine sufficient area of Sweden (Gothenburg) 2003-2005.
Clin Endocrinol (Oxf). 78:768–776. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Burch HB and Cooper DS: Management of
graves disease: A review. JAMA. 314:2544–2554. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Marinò M, Latrofa F, Menconi F, Chiovato L
and Vitti P: Role of genetic and non-genetic factors in the
etiology of Graves' disease. J Endocrinol Invest. 38:283–294.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Paunkovic N and Paunkovic J: The
diagnostic criteria of Graves' disease and especially the
thyrotropin receptor antibody; our own experience. Hell J Nucl Med.
10:89–94. 2007.PubMed/NCBI
|
|
6
|
Törnhage CJ and Grankvist K: Acquired
neonatal thyroid disease due to TSH receptor antibodies in breast
milk. J Pediatr Endocrinol Metab. 19:787–794. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Azizi F, Bahrainian M, Khamseh ME and
Khoshniat M: Intellectual development and thyroid function in
children who were breast-fed by thyrotoxic mothers taking
methimazole. J Pediatr Endocrinol Metab. 16:1239–1243.
2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mandel SJ and Cooper DS: The use of
antithyroid drugs in pregnancy and lactation. J Clin Endocrinol
Metab. 86:2354–2359. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Momotani N, Yamashita R, Makino F, Noh JY,
Ishikawa N and Ito K: Thyroid function in wholly breast-feeding
infants whose mothers take high doses of propylthiouracil. Clin
Endocrinol (Oxf). 53:177–181. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Speller E and Brodribb W: Breastfeeding
and thyroid disease: A literature review. Breastfeed Rev. 20:41–47.
2012.PubMed/NCBI
|
|
11
|
Stagnaro-Green A, Abalovich M, Alexander
E, Azizi F, Mestman J, Negro R, Nixon A, Pearce EN, Soldin OP,
Sullivan S, et al: Guidelines of the American thyroid association
for the diagnosis and management of thyroid disease during
pregnancy and postpartum. Thyroid. 21:1081–1125. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen CR, Pichurin P, Nagayama Y, Latrofa
F, Rapoport B and McLachlan SM: The thyrotropin receptor
autoantigen in Graves disease is the culprit as well as the victim.
J Clin Invest. 111:1897–1904. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Green AP, Huang JJ, Scott MO, Kierstead
TD, Beaupré I, Gao GP and Wilson JM: A new scalable method for the
purification of recombinant adenovirus vectors. Hum Gene Ther.
13:1921–1934. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yakimovich A, Andriasyan V, Witte R, Wang
IH, Prasad V, Suomalainen M and Greber UF: Plaque2.0-A
high-throughput analysis framework to score virus-cell transmission
and clonal cell expansion. PLoS One. 10(e0138760)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Smith BR, Bolton J, Young S, Collyer A,
Weeden A, Bradbury J, Weightman D, Perros P, Sanders J and
Furmaniak J: A new assay for thyrotropin receptor autoantibodies.
Thyroid. 14:830–835. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xia N, Ye X, Hu X, Song S, Xu H, Niu M,
Wang H and Wang J: Simultaneous induction of Graves'
hyperthyroidism and Graves' ophthalmopathy by TSHR genetic
immunization in BALB/c mice. PLoS One. 12(e0174260)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu L, Xun L, Yang J, Xu L, Tian Z, Gao S,
Zhang Y, Hou P and Shi B: Induction of murine neonatal tolerance
against Graves' disease using recombinant adenovirus expressing the
TSH receptor A-subunit. Endocrinology. 152:1165–1171.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Holder AT, Aston R, Rest JR, Hill DJ,
Patel N and Ivanyi J: Monoclonal antibodies can enhance the
biological activity of thyrotropin. Endocrinology. 120:567–573.
1987.PubMed/NCBI View Article : Google Scholar
|
|
19
|
van Trotsenburg ASP: Management of
neonates born to mothers with thyroid dysfunction, and points for
attention during pregnancy. Best Pract Res Clin Endocrinol Metab.
34(101437)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shimojo N, Kohno Y, Yamaguchi K, Kikuoka
S, Hoshioka A, Niimi H, Hirai A, Tamura Y, Saito Y, Kohn LD and
Tahara K: Induction of Graves-like disease in mice by immunization
with fibroblasts transfected with the thyrotropin receptor and a
class II molecule. Proc Natl Acad Sci USA. 93:11074–11079.
1996.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Costagliola S, Many MC, Denef JF, Pohlenz
J, Refetoff S and Vassart G: Genetic immunization of outbred mice
with thyrotropin receptor cDNA provides a model of Graves' disease.
J Clin Invest. 105:803–811. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Holthoff HP, Goebel S, Li Z, Faßbender J,
Reimann A, Zeibig S, Lohse MJ, Münch G and Ungerer M: Prolonged TSH
receptor A subunit immunization of female mice leads to a long-term
model of Graves' disease, tachycardia, and cardiac hypertrophy.
Endocrinology. 156:1577–1589. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kita-Furuyama M, Nagayama Y, Pichurin P,
McLachlan SM, Rapoport B and Eguchi K: Dendritic cells infected
with adenovirus expressing the thyrotrophin receptor induce Graves'
hyperthyroidism in BALB/c mice. Clin Exp Immunol. 131:234–240.
2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kaneda T, Honda A, Hakozaki A, Fuse T,
Muto A and Yoshida T: An improved Graves' disease model established
by using in vivo electroporation exhibited long-term immunity to
hyperthyroidism in BALB/c mice. Endocrinology. 148:2335–2344.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Piccinni MP and Romagnani S: Regulation of
fetal allograft survival by hormone-controlled Th1- and Th2-type
cytokines. Immunol Res. 15:141–150. 1996.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Di Bari F, Perelli S, Scilipoti A,
Wasniewska M, Vita R, Vermiglio F, Benvenga S and Moleti M:
Stress-Triggered Graves' Disease with multiple exacebations in a
pregnant woman with levels of thyrotropin receptor antibodies and
no complicated delivery: A case report. SN Compr Clin Med.
2:355–360. 2020.
|
|
27
|
Weetman AP: Immunity, thyroid function and
pregnancy: Molecular mechanisms. Nat Rev Endocrinol. 6:311–318.
2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bucci I, Giuliani C and Napolitano G:
Thyroid-stimulating hormone receptor antibodies in pregnancy:
Clinical relevance. Front Endocrinol (Lausanne).
8(137)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vita R, Lapa D, Vita G, Trimarchi F and
Benvenga S: A patient with stress-related onset and exacerbations
of Graves' disease. Nat Clin Pract Endocrinol Metab. 5:55–61.
2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Batra CM: Fetal and neonatal
thyrotoxicosis. Indian J Endocrinol Metab. 17 (Suppl 1):S50–S54.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Laurberg P, Wallin G, Tallstedt L,
Abraham-Nordling M, Lundell G and Tørring O: TSH-receptor
autoimmunity in Graves' disease after therapy with anti-thyroid
drugs, surgery, or radioiodine: A 5-year prospective randomized
study. Eur J Endocrinol. 158:69–75. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Van de Perre P: Transfer of antibody via
mother's milk. Vaccine. 21:3374–3376. 2003.PubMed/NCBI View Article : Google Scholar
|