Introduction
Spinal cord injury (SCI) has been considered one of
the most severe types of central nervous system (CNS) injury that
directly or indirectly results in impairment of motor, sensory and
other functions (1). However,
there is currently a lack of effective therapeutic methods for the
treatment of SCI, including medicine or surgery (2,3). SCI
is characterized by a disruption in the arrangement, type and
number of cells at the injury sites (4). Thus, therapeutic targets should focus
on the retention of neural cells at the injury site (5). Although it has been reported
previously that scaffolds with neural stem cells improved rat
locomotor function, the first choice of therapeutics in a clinical
setting is to promote cell survival or generate additional neural
cells (6).
Insulin-like growth factors (IGFs) serve a crucial
role in the survival and integrity of neurons that promote recovery
in several disease models, such as hypoxia/ischemia brain injury,
multiple sclerosis and Alzheimer's disease (7,8).
IGF-1 regulates a number of important neurophysiological
activities, including neurogenesis, cytoplasmic synthesis and
complex cognitive function (9,10).
Moreover, IGF-1 is a major growth factor for primary neural cells
in the CNS (11). The results of a
previous study demonstrated that, together, IGF-1 and matrix
molecule osteopontin facilitate robust axon regeneration via the
PI3K pathway following optic nerve crush (12).
It has previously been reported that IGF-1
expression is decreased in patients with SCI (13), and that high IGF-1 expression
levels in patients with SCI may contribute to improved functional
recovery (14). Additionally,
IGF-1 with osteopontin promoted mouse hind limb function following
SCI with T10 lateral hemisection (15). Furthermore, IGF promoted the
survival of oligodendrocytes in the spinal cord, and rats with SCI
exhibited a quicker neurological recovery (16).
The results of previous studies have demonstrated
that autophagy is one of the key processes involved in cell
survival, differentiation and homeostasis (17,18).
Autophagy serves an important role in the intracellular catabolic
mechanism underlying the recycling of damaged organelles and
senescent proteins following SCI (19,20).
Previous studies have further confirmed that dysfunction of
autophagy increased neuronal apoptosis following SCI, indicating
that autophagy may be involved in SCI (21,22).
A number of signaling pathways are involved in autophagy, including
the key PI3K/Akt/mTOR signaling pathway (23). Moreover, IGF-1 exerts its function
via the PI3K/Akt/mTOR signaling pathway (24). Although it has previously been
reported that IGF-1 exhibited therapeutic effects on neurological
trauma, few studies have investigated the role and mechanism
underlying IGF-1 in the treatment of SCI (25).
The present study aimed to investigate the
neuroprotective and autophagic effects of IGF-1 on SCI, and to
determine the role of the PI3K/Akt/mTOR signaling pathway in this
process. Investigation into the role of IGF-1 in SCI may lead to
the discovery of novel treatment options for SCI and further
clinical applications of IGF-1.
Materials and methods
SH-SY5Y cell cultures
SH-SY5Y is a human derived neuroblastoma cell line
that is often used to establish neuronal function and
differentiation models in vitro (26). SH-SY5Y cells (cat. no. CL0278) were
obtained from Hunan Fenghui Biotechnology Co., Ltd., and were
evaluated by the short tandem repeat method. Cells were cultured in
DMEM/F12 (Cytiva) supplemented with 10% FBS (Zhejiang Tianhang
Biotechnology, Co., Ltd.), 1% penicillin-streptomycin (Cytiva) and
2 mM L-Glutamine (Beyotime Institute of Biotechnology) at 37˚C and
5% CO2 (27,28). Trypsin (Thermo Fisher Scientific,
Inc.) was prepared for cell separation.
SH-SY5Y cell injury model and IGF-1
interference
Oxidative stress is one of the most important
mechanisms underlying SCI, and H2O2 is used
for simulating oxidative stress in cells in vitro (29). There were three groups,
H2O2 group, IGF-1 group and control group.
Following culture of the three groups of SH-SY5Y cells
(5x104/ml) in 96-well plates for 12 h, supernatants were
removed. In the control group, SH-SY5Y cells were incubated in the
aforementioned neural media (DMEM/F12 with FBS,
penicillin-streptomycin and L-Glutamine) for 6, 12 and 24 h at 37˚C
and 5% CO2. In the H2O2 group,
cells were incubated in neuronal media supplemented with 200 µmol/l
H2O2 for 6, 12 and 24 h at 37˚C and 5%
CO2 (28). In the IGF-1
group, cells were incubated in neuronal media supplemented with 200
µmol/l H2O2 and 100 ng/ml IGF-1
(Sigma-Aldrich; Merck KGaA) for 6, 12 and 24 h at 37˚C and 5%
CO2 (30,31). This concentration of IGF-1 was
selected using preliminary tests (data not shown). At 6 h following
cell injury, cells were washed with PBS twice and collected for
western blot analysis, and at 6, 12 and 24 h, cell samples were
used for the MTT assays.
MTT assay
An MTT assay was performed to examine cell survival
following injury and IGF-1 interference. Cell culture medium was
removed from each well of the 96-well-plate as aforementioned, and
cells were incubated for 4 h at 37˚C in the CO2 (5%)
incubator, followed by the addition of 20 µl MTT reagent (5 mg/ml)
for a further incubation at 37˚C for 4 h. Following the MTT
incubation, 150 µl 20% DMSO was added to each well to remove the
purple formazan crystals. The plate was subsequently shaken for 10
min for solubilization. The absorbance was measured using a
microplate spectrophotometer at a wavelength of 490 nm. The final
optical density was calculated using the average of six wells.
Animal care and groups
In total, 24 healthy adult male Sprague-Dawley rats
(age, 8-12 weeks; weight, 240-280 g) were purchased from Shanghai
Jieyi Biotechnology, Co., Ltd., and used for the SCI model
generation. All rats were housed in a specific-pathogen-free
laboratory under a 12-h light/dark cycle and a stable temperature
of 23-25˚C. All animals were housed individually and had free
access to standard food and water. All experimental procedures were
performed in accordance with the Guiding Opinions on the Ethical
Treatments of Laboratory Animals published by the Ministry of
Science and Technology of the People's Republic of China in
2006(32). All animal protocols
were approved by the Animal Ethics Committee of Capital Medical
University of China (approval no. PYZ2017082).
The rats were assigned into sham (n=8), SCI (n=8)
and SCI + IGF-1 (n=8) groups using a random numbers table. In
total, five rats were randomly selected from each group for
Basso-Beatlie-Bresnahan (BBB) score evaluation, and the remaining
three rats in each group were sacrificed for western blot analysis
at 1 day after SCI modeling.
SCI model and IGF-1 treatment
Rats in the SCI and SCI + IGF-1 groups received an
SCI operation, which was conducted as previously described
(33,34). Briefly, after anesthetization with
4% sodium pentobarbital [50 mg/kg; intraperitoneal (i.p.)
injection], rats were placed on a heating pad to maintain body
temperature. The spine at T9-11 was exposed from the dorsal side
and a laminectomy was performed. A 10-g rod was freely dropped from
a height of 25 mm, which was controlled by an NYU Impactor II (New
York University Medical Center, New York, NY, USA), to impact the
T10 segment of the spinal cord; wagging tail reflex and lower limb
spasms were observed. Laminectomy was performed on the rats in the
sham group without SCI (35). The
wound was sutured layer by layer. Manual bladder emptying was
performed and an i.p. injection of 20 U/kg penicillin was
administered once daily until bladder function was
re-established.
Rats were administered an i.p. injection of 1 ml
IGF-1 (50 g/kg; dissolved in 0.9% NaCl; Sigma-Aldrich; Merck KGaA)
<5 min after SCI operation (36,37).
This concentration was selected in preliminary tests (data not
shown). This was repeated once daily in the SCI + IGF-1 group until
animals were sacrificed (36). At
the same time, an i.p. injection of 1 ml 0.9% NaCl was administered
to rats in the sham and SCI groups. All the procedures were
performed by the same unblinded investigator.
Neurological function assessment
The neurological function of the rats was assessed
using the BBB rating scale, which was described in our previous
studies (33,34). The BBB score ranged from 0
(complete paralysis) to 21 (healthy) (38). In total, two blinded, independent
examiners evaluated the BBB score before the operation and again at
1 day, and 1, 2, 3 and 4 weeks following SCI. The average score was
used for subsequent analysis. After 4 weeks, an i.p. injection of
300 mg/kg sodium pentobarbital was used to sacrifice the rats.
Collection of the specimens
Based on the results of previous studies and our
previous research, autophagy is altered and increases quickly
following SCI, reaching a peak on day 1 (20,34).
On day 1 after the initial treatment of IGF-1, rats were
anesthetized with 4% sodium pentobarbital (50 mg/kg; i.p.
injection) followed by an intracardiac perfusion with normal saline
(0.9% NaCl). After SCI, the injury area expanded and the injury
margin was more difficult to clearly distinguish (39). Thus, a 10-mm segment of spinal cord
tissue centered at the injury site was removed. An i.p. injection
of 300 mg/kg sodium pentobarbital was subsequently used to
sacrifice the rats. The spinal cord tissue was immediately frozen
in liquid nitrogen and stored at -80˚C.
Western blot analysis
Protein determination was performed by western blot
analysis. Each specimen was handled individually for the following
procedures. RIPA lysis buffer was used for purification of total
proteins from the spinal cord specimens. The 10-mm spinal cord
tissue and each group of the SH-SY5Y cell samples were dissolved in
ice-cold RIPA lysis buffer containing PMSF (Beyotime Institute of
Biotechnology). Protein concentration was determined by BCA kit
(Beyotime Institute of Biotechnology). Homogenates of spinal cord
specimens and cell samples were centrifuged at 13,500 x g for 30
min at 4˚C to collect the supernatant. A total of 50 µg protein per
lane was separated by SDS-PAGE on 15% gel (80 V for 30 min;
followed by 120 V for 90 min) and transferred onto a PVDF membrane
(Beyotime Institute of Biotechnology). The membranes were blocked
with 5% non-fat milk in TBS with 0.1% Tween-20 for 2 h at 25˚C.
Subsequently, the membranes were incubated with the following
antibodies: GAPDH (1:5,000; cat. no. ab8245; Abcam), anti-LC3I/II
(1:1,500; cat. no. ab52768; Abcam), anti-PI3K (1:1,000; cat. no.
ab191606; Abcam), anti-Akt (1:1,000; cat. no. ab8805; Abcam),
anti-phosphorylated (p)-Akt (1:1,000; cat. no. ab81283; Abcam),
anti-mTOR (1:1,000; cat. no. ab134903; Abcam) and anti-p-mTOR
(1:1,000; cat. no. ab137133; Abcam) at 4˚C overnight. Subsequently,
the membranes were washed three times with TBST (0.2% Tween-20) and
incubated with HRP-conjugated IgG secondary antibody (1:5,000; cat.
no. ab97051; Abcam) at 25˚C for 1 h. Following secondary
incubation, the membranes were washed again, and proteins were
visualized using ECL solution (Beyotime Institute of
Biotechnology). Images were captured using a
ChemiDoc-ItTM TS2 Imager (Analytik Jena GmbH). ImageJ
software version 2 (National Institutes of Health) was used to
measure the density of the scanned protein bands, with GAPDH used
for normalization. All the procedures were performed by the same
blinded investigator.
Statistical analysis
Experimental data are presented as the mean ± SD,
and all data were analyzed using SPSS software version 23.0 (IBM
Corp.). Comparison of BBB score among three groups was performed
using a non-parametric Kruskal-Wallis test followed by Dunn's post
hoc test. Additional comparisons among three groups were performed
using a one-way ANOVA followed by a least significant difference
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
IGF-1 promotes cell survival in
vitro
To simulate the microcirculation in SCI, SH-SY5Y
cells were exposed to 200 µmol/l H2O2 for 6,
12 and 24 h to establish a neural cell injury model in
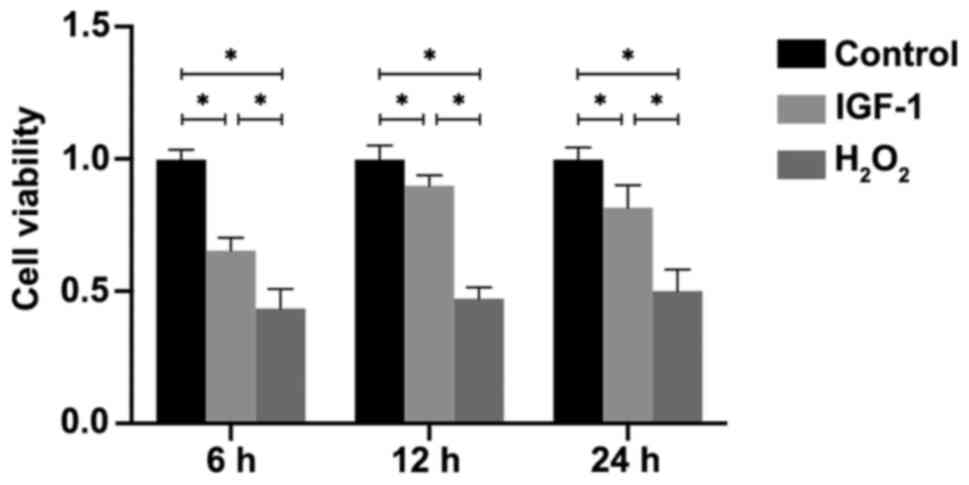
vitro. The MTT assay results indicated that the cell viability
was significantly decreased in the H2O2 and
IGF-1 groups compared with the control group at 6, 12 and 24 h (all
P<0.05; Fig. 1). Furthermore,
viability was significantly higher in the IGF-1 group compared with
the H2O2 group (P<0.05; Fig. 1).
IGF-1 activates the PI3K/Akt/mTOR
signaling pathway to reduce autophagy in vitro
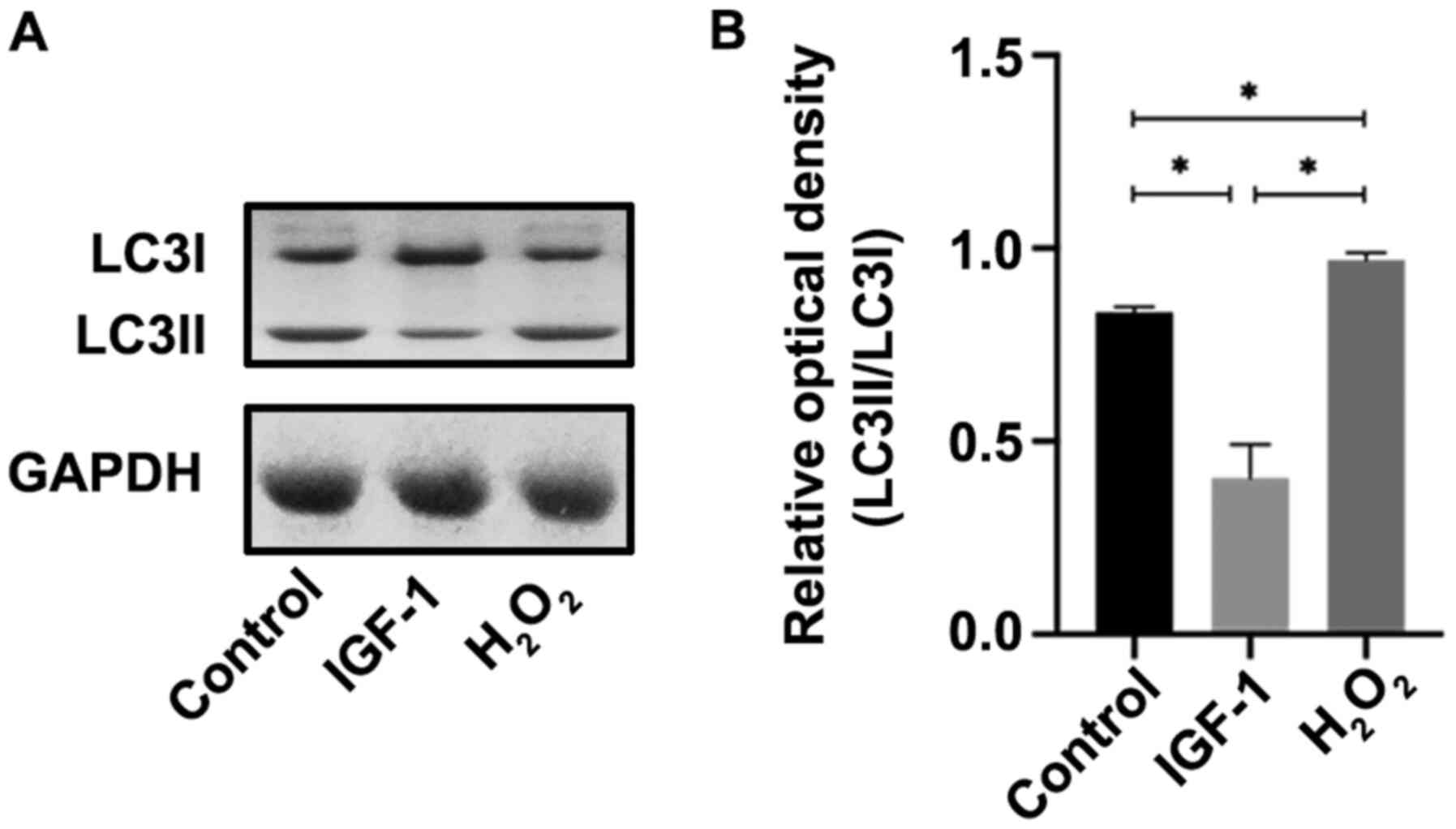
The expression levels of autophagy-associated
proteins were measured to evaluate the effect of IGF-1 on autophagy
and to identify the mechanisms underlying SH-SY5Y cells following
exposure to H2O2 and co-treatment of IGF-1
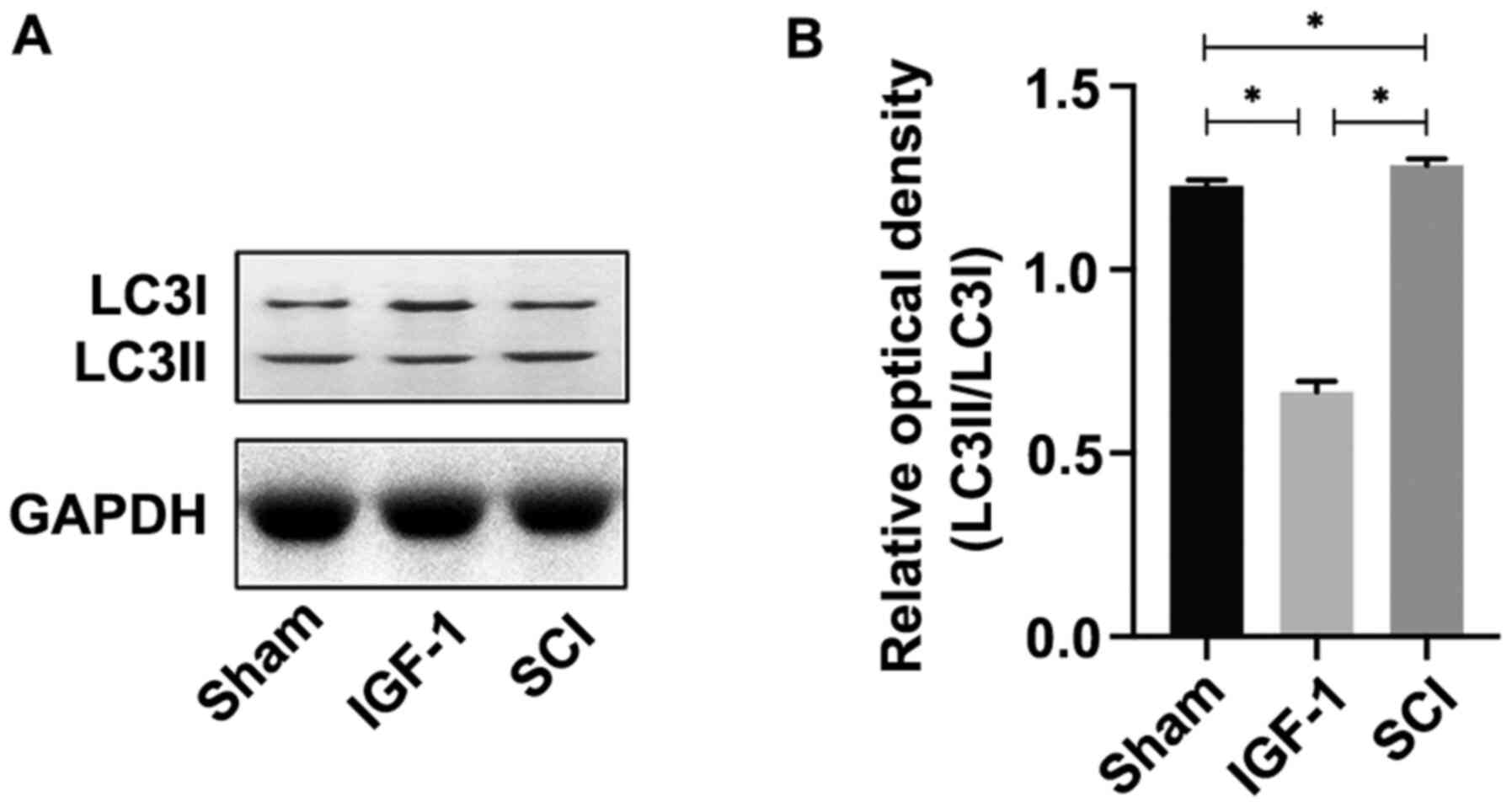
for 6 h. The results demonstrated that LC3II/LC3I expression was
significantly increased in the H2O2 group
compared with the control (P<0.05), whereas it was significantly
decreased in the IGF-1 group compared with the
H2O2 and control groups (both P<0.05;
Fig. 2A and B) at 6 h after cells injury.
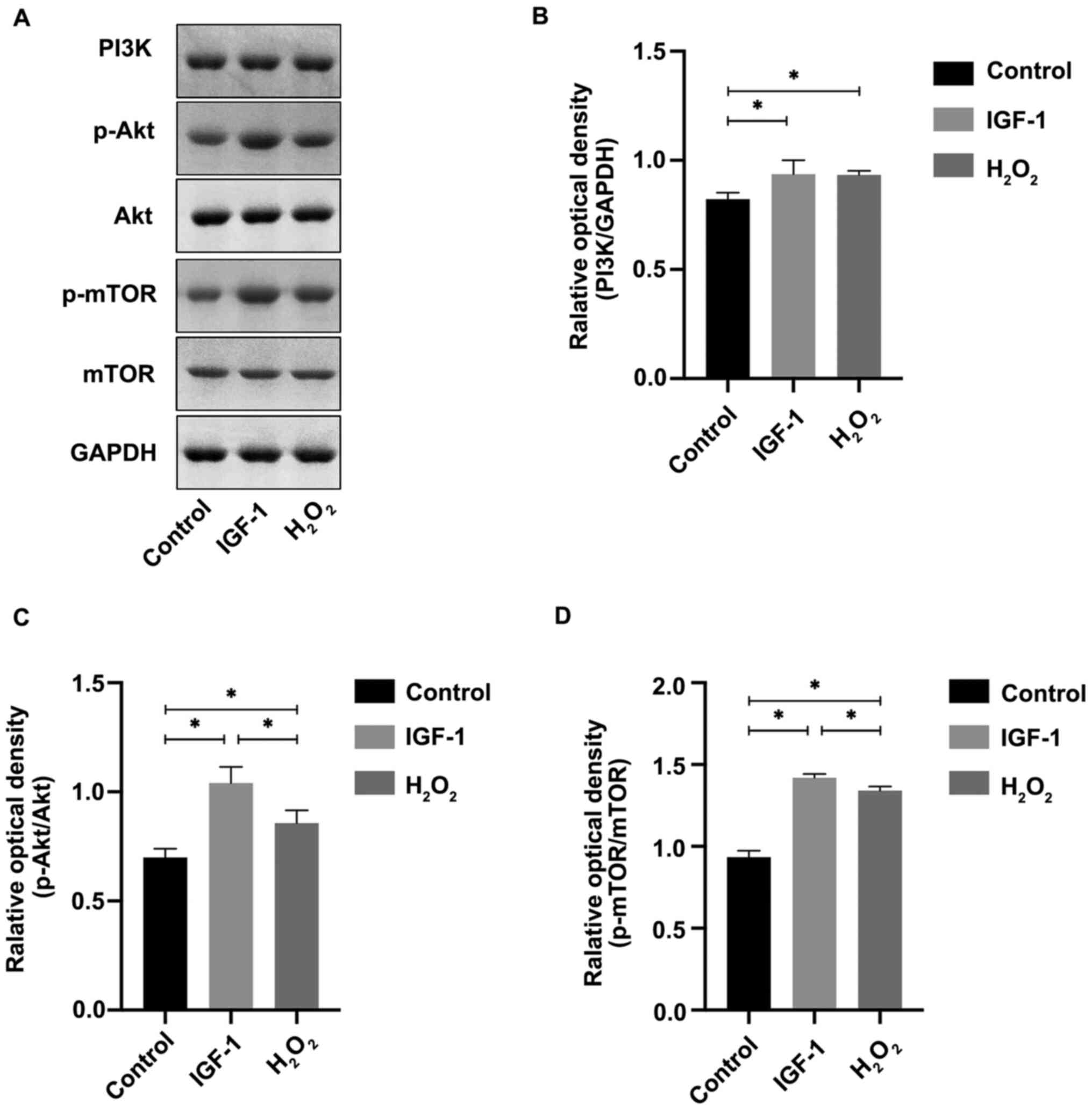
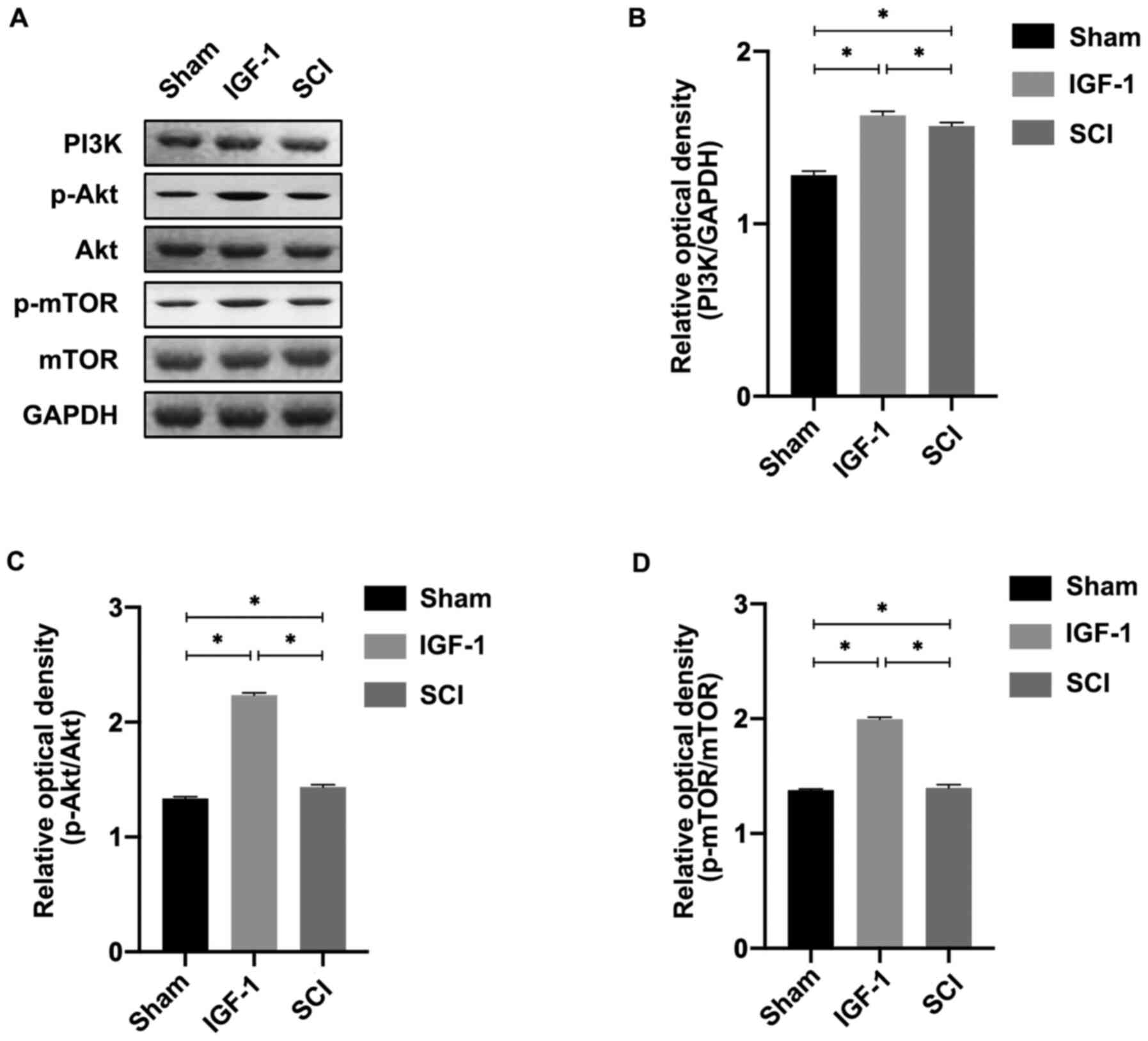
To study the role of the PI3K/Akt/mTOR signaling
pathway in IGF-1-induced inhibition of autophagy, activation of the
pathway was determined using western blotting. As presented in
Fig. 3, PI3K expression was
increased significantly in the IGF-1 group compared with the
control (P<0.05). In the same trend as PI3K expression,
p-Akt/Akt and p-mTOR/mTOR expression was increased in the IGF-1
group compared with both the H2O2 and control
groups (P<0.05).
Overall, these data suggested that IGF-1 may
activate the PI3K/Akt/mTOR signaling pathway, which resulted in
downregulation of autophagy.
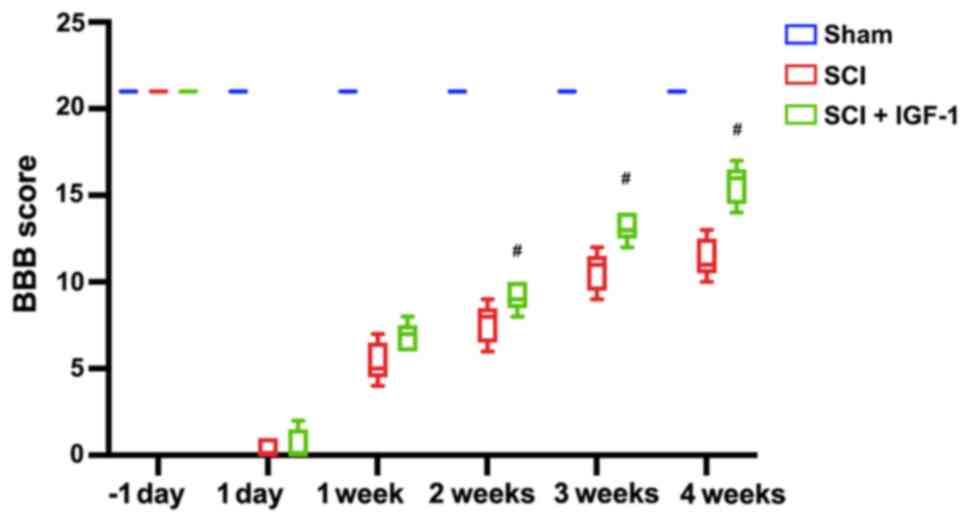
Neurological functional recovery
In the SCI model rats, lower hindlimb function was
evaluated using the BBB scale 1 day before SCI, and at 1 day and 1,
2, 3 and 4 weeks after SCI. Following SCI, the locomotor function
score of rats was zero in the SCI + IGF-1 and SCI groups at 1 day
after SCI, which gradually improved over time in these groups
(Fig. 4). Although there was no
significant difference between the SCI + IGF-1 and SCI groups at 1
day and 1 week (P>0.05), rats in the SCI + IGF-1 group exhibited
significantly improved neurological functional recovery compared
with the SCI group at 2, 3 and 4 weeks (P<0.05; Fig. 4).
IGF-1 inhibits autophagy via the
PI3K/Akt/mTOR signaling pathway following SCI in vivo
LC3II and LC3I expression levels were evaluated by
western blot analysis on day 1 after SCI. The expression level of
LC3II/LC3I was significantly lower in the SCI + IGF-1 sham group
compared with sham group, whereas it was significantly higher in
the SCI group compared with the sham group (P<0.05; Fig. 5).
To evaluate the activation of the PI3K/Akt/mTOR
signaling pathway, the relative expression level of PI3K and the
ratios of p-Akt/Akt and p-mTOR/mTOR expression were analyzed.
Following SCI, the relative expression levels of PI3K, p-Akt and
p-mTOR were higher compared with the expression levels in the sham
group (all P<0.05). However, the relative expression levels of
p-Akt/Akt and p-mTOR/mTOR in the SCI + IGF-1 group were
significantly higher compared with the expression levels in the
untreated SCI group (all P<0.05; Fig. 6).
Discussion
IGF-1 is an inherent molecule of the insulin family
that has numerous physiological functions in the human body, such
as regulating growth, insulin sensitivity and cardiovascular
activity (40). Moreover, IGF-1
regulates various neurological activities, such as neuronal
survival, energy metabolism and plasticity (41). In addition, IGF-1 is a potent
growth factor for different types of nerve cells (42). A higher expression level of IGF-1
in patients with SCI may improve functional recovery (14).
A significant decrease in serum IGF-1 levels was
observed in patients with SCI compared with physiological range
(13,43), and Ferbert et al (43) reported that patients with higher
IGF-1 levels exhibited improved neurological recovery at 12 weeks
following SCI. The results of another study indicated that the
IGF-1 concentration in the plasma and injured spinal cord of SD
rats was significantly decreased compared with the control group,
and the administration of exogenous IGF-1 reduced cell damage after
SCI (44). The results of the
present study are consistent with these previous data; the
neurological function of SCI rats receiving treatment with IGF-1
recovered to a greater extent compared with untreated SCI rats.
However, the role of IGF-1 and the underlying mechanisms are yet to
be fully elucidated.
It has been reported that autophagy serves an
important role in the process of cell death (45,46).
The present study was conducted to examine the inhibitory effect of
IGF-1 on autophagy as a therapy for SCI and aimed to elucidate the
potential underlying mechanisms. The results of the present study
demonstrated that IGF-1 promoted functional recovery in SCI rats
and increased the number of surviving neural cells following injury
in vitro. In vivo and in vitro results
demonstrated that IGF-1 inhibited autophagy, with higher expression
levels of p-Akt and p-mTOR. These findings indicated that IGF-1 may
inhibit autophagy via the PI3K/Akt/mTOR signaling pathway.
However, the detailed mechanisms underlying
autophagy in cell death and survival remain a matter of debate
(47-50).
Autophagy involves a series of complex processes, such as the
delivery of cytoplasmic cargo sequestered inside double-membrane
vesicles to the lysosome, which is a dynamic equilibrium and
changes rapidly from one stage to the other. The process is hard to
monitored in real-time accurately. The intensity, timing and
regulating mechanisms underlying autophagy may have protective
effects or may aggravate damage in cells (20,51).
At present, it is recognized that autophagy is increased following
SCI (52,53). However, it has been demonstrated by
various studies that the same interference may lead to different
outcomes (18). On the one hand, a
number of studies reported that the therapeutic effects of various
methods, like rapamycin, simvastatin, lithium, were based on
increased autophagy, which protected a larger number of neural
cells from death (20,34,54,55).
On the other hand, the results of previous studies have indicated
that increased autophagy was the cause of neural cell death, as it
has been observed that inhibition of autophagy promoted
neurological functional recovery (20,56,57).
The present study demonstrated that IGF-1 treatment
improved neurological function following SCI. IGF-1 is involved in
neural cell proliferation, clearance of abnormal cells,
inflammation, myelination, neurogenesis and plasticity (24,58-61).
Moreover, the results of the present study demonstrated that IGF-1
may improve neurological function via the PI3K/Akt/mTOR signaling
pathway and inhibition of autophagy.
In addition to the aforementioned functions of
IGF-1, it has been reported that antagonism of the IGF-1 receptor
inhibits rather than induces autophagy (62). A potential factor that may affect
the function of the IGF-1 signaling pathway in autophagy includes
the existence of different types of PI3K proteins, which function
in different manners (63). The
PI3K family comprises kinases that are key to the regulation of
autophagy, and they are divided into three classes. Class I PI3K
proteins have been largely described and activate the Akt/mTOR
signaling pathway that inhibits autophagy (64). Class II PI3K proteins are involved
in a wide variety of biological activities. For example, PI3K-C2a
participates in glucose transportation, secretion of insulin,
release of neurosecretory granules, endocytosis and contraction of
muscle cells; PI3K-C2b is active in cell migration and potassium
channel activation; and both PI3K-C2a and PI3K-C2b are involved in
cell proliferation and survival (65). Moreover, PI3K-C2 is involved in
autophagy (66). It has previously
been reported that both the Class II and Class III PI3K proteins
enhance autophagy (67). In
addition, Class I proteins contribute to the formation of
phosphatidylinositol-3-phosphate (PI3P), which is the key secondary
messenger in autophagy and is further affected and regulated by the
Class II and Class III PI3Ks (68).
Different types of cells, such as neurons,
astrocytes and oligodendrocytes in the spinal cord produce
different responses to harmful stimuli and varying activation of
the same signaling pathway, including autophagy. Neurons would
suffer apoptosis after damage, while the astrocytes would
proliferate and secrete neurotrophic factors (69). The results of a previous study
demonstrated that the expression of p-Akt was increased at 6 h
after contusion rat SCI in neurons, reaching a peak at 3 days,
before returning to baseline. However, the increase in the
expression of p-Akt was initiated at 3 days and continued until 7
days after SCI in astrocytes (70). The characteristics of autophagy
vary amongst different neural cells involved in SCI. A higher level
of autophagy was found in neurons at 3 h following SCI (26), whereas Hou et al (71) observed an increased level of
autophagy in astrocytes at 3 days after SCI. Moreover, no increase
of autophagy was observed in astrocytes at 4 days after SCI with
enhanced autophagy, but the glial scar was reduced at 2 weeks after
SCI (72,73). The results of a previous study
revealed that changes in neuronal autophagy are associated with the
neuronal subtype, and neurons from the spinal cord may be affected
more easily by the inhibition of autophagic flux compared with
brain neurons (53). Previous
studies have also demonstrated that the accumulation of
autophagosomes in oligodendrocytes was observed at later timepoints
compared with neurons, suggesting that the reaction to damage is
not the same between these two cells (21,74,75).
Thus, it was suggested that autophagy is a dynamic process, which
exerts positive or negative effects in early or late stages
(20,76). Fang et al (76) reported that neurological function
was improved when autophagy was stimulated at an early stage after
SCI, compared with poor neurological function when autophagy was
inhibited at the early stage. However, neurological function was
not improved when autophagy was stimulated at a late stage compared
with improved neurological function when autophagy was inhibited at
the same stage following SCI.
IGF-1 has been applied in the clinical setting for
the treatment of growth failure in pediatric patients with severe
primary IGF-1 deficiency and in patients with growth hormone (GH)
gene deletion who have developed neutralizing antibodies to GH
(77,78). IGF-1 is safe and positively effects
body composition, and IGF-1 treatment for SCI in the clinical
setting is easily accessible, which has been approved by The Food
and Drug Administration (FDA) (34). Based on previous studies and the
results of the present study, IGF-1 has potential as a clinical
treatment of SCI. However, determining the optimal timing for IGF-1
treatment requires further investigation.
The present study is the first to report the effect
of IGF-1 on autophagy in treating SCI involving the PI3K/Akt/mTOR
signaling pathway. However, the current study presents preliminary
evidence lacking deep exploration, thus, further investigation is
required. In future studies, the duration of the BBB scoring
experiment will be extended to 2 months. Additionally, inhibitors
of PI3K, Akt, mTOR and the IGF-1 receptor will be used to confirm
the impact of IGF-1 and mechanisms underlying this protein in the
PI3K/Akt/mTOR signaling pathway. Although the BBB scale is widely
used to evaluate neurological function, it is a subjective tool.
More objective techniques involve measurement of the neurological
regeneration, including immunofluorescence staining of regenerated
nerve fibers, examination of the myelinated axon density,
electrophysiological tests and diffusion tensor imaging (33,79).
In conclusion, the present study demonstrated that
IGF-1 promoted functional recovery in rats after SCI and exerted
neuroprotective effects. Furthermore, the IGF-1-induced inhibition
of autophagy may be associated with activation of the PI3K/Akt/mTOR
signaling pathway. However, whether the regulation of autophagy by
IGF-1 is associated with the activation of different PI3K signaling
pathways in different neural cells at varying injury stages remains
to be elucidated and requires further investigation.
Acknowledgements
The authors would like to thank Dr Fang Wang from
Xi'an Jiaotong University of China (Xi'an China) for technical
support.
Funding
Funding: This work was supported by The Beijing Excellent Talent
Training Funding (grant no. 2017000021469G215), The Natural Science
Foundation of Capital Medical University of China (grant nos.
PYZ2017082; PYZ2018081) and China Rehabilitation Research Center
Foundation (grant no. 2018zx-Q3).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DZha and YY conceived the project. DZha, DZhu, CL
and SD performed the animal and cell experiments. DZha, YY and JZ
integrated and analyzed the experimental data. DZha, YY and BL
conceived and designed the study. DZha drafted the manuscript. JZ,
LW, SM and WC performed the statistical analysis. BL and DZha
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed in
accordance with the Guidelines for the Care and Use of Laboratory
Animals (Institute for Laboratory Animal Research, National Academy
of Science). The protocols were approved by the Animal Ethics
Committee of Capital Medical University of China (Beijing, China;
approval no. PYZ2017082).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Casili G, Campolo M, Lanza M, Filippone A,
Scuderi S, Messina S, Ardizzone A, Esposito E and Paterniti I: Role
of ABT888, a Novel Poly(ADP-Ribose) Polymerase (PARP) Inhibitor in
Countering Autophagy and Apoptotic Processes Associated to Spinal
Cord Injury. Mol Neurobiol. 57:4394–4407. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Duncan GJ, Manesh SB, Hilton BJ, Assinck
P, Plemel JR and Tetzlaff W: The fate and function of
oligodendrocyte progenitor cells after traumatic spinal cord
injury. Glia. 68:227–245. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Imai T, Katoh H, Suyama K, Kuroiwa M,
Yanagisawa S and Watanabe M: Amiloride promotes oligodendrocyte
survival and remyelination after spinal cord injury in rats. J Clin
Med. 7(7)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhou K, Sansur CA, Xu H and Jia X: The
Temporal Pattern, Flux, and Function of Autophagy in Spinal Cord
Injury. Int J Mol Sci. 18(466)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Silva NA, Sousa N, Reis RL and Salgado AJ:
From basics to clinical: A comprehensive review on spinal cord
injury. Prog Neurobiol. 114:25–57. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ham TR, Pukale DD, Hamrangsekachaee M and
Leipzig ND: Subcutaneous priming of protein-functionalized chitosan
scaffolds improves function following spinal cord injury. Mater Sci
Eng C Mater Biol Appl 110: 10.1016/j.msec.2020.110656, 2020.
|
|
7
|
Aleman A and Torres-Alemán I: Circulating
insulin-like growth factor I and cognitive function:
Neuromodulation throughout the lifespan. Prog Neurobiol.
89:256–265. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Russo VC, Gluckman PD, Feldman EL and
Werther GA: The insulin-like growth factor system and its
pleiotropic functions in brain. Endocr Rev. 26:916–943.
2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu W, D'Ercole JA and Ye P: Blunting type
1 insulin-like growth factor receptor expression exacerbates
neuronal apoptosis following hypoxic/ischemic injury. BMC Neurosci.
12(64)2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
O'Donnell SL, Frederick TJ, Krady JK,
Vannucci SJ and Wood TL: IGF-I and microglia/macrophage
proliferation in the ischemic mouse brain. Glia. 39:85–97.
2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang TS, Wang YH and Lien IN: Suppression
of the hypothalamus-pituitary somatotrope axis in men with spinal
cord injuries. Metabolism. 44:1116–1120. 1995.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Petrova V and Eva R: The virtuous cycle of
axon growth: Axonal transport of growth-promoting machinery as an
intrinsic determinant of axon regeneration. Dev Neurobiol.
78:898–925. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bauman WA, Kirshblum SC, Morrison NG,
Cirnigliaro CM, Zhang RL and Spungen AM: Effect of low-dose
baclofen administration on plasma insulin-like growth factor-I in
persons with spinal cord injury. J Clin Pharmacol. 46:476–482.
2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Moghaddam A, Sperl A, Heller R, Kunzmann
K, Graeser V, Akbar M, Gerner HJ and Biglari B: Elevated serum
insulin-like growth factor 1 levels in patients with neurological
remission after traumatic spinal cord injury. PLoS One.
11(e0159764)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu Y, Wang X, Li W, Zhang Q, Li Y, Zhang
Z, Zhu J, Chen B, Williams PR, Zhang Y, et al: A sensitized IGF1
treatment restores corticospinal axon-dependent functions. Neuron.
95:817–833.e4. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fu C-F, Liu Y, Li X, Shen B, Zhang S-K,
Song Z-M and Zhang X: Inhibitory effect of IGF-1 gene on
motoneurons apoptosis in anterior horn after acute spinal cord
injury in adult rats. 32nd edition. J Jilin Univeristy (Medicine).
4:568–570. 2006.(In Chinese).
|
|
17
|
Mizushima N: Autophagy: Process and
function. Genes Dev. 21:2861–2873. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rao S, Tortola L, Perlot T, Wirnsberger G,
Novatchkova M, Nitsch R, Sykacek P, Frank L, Schramek D, Komnenovic
V, et al: A dual role for autophagy in a murine model of lung
cancer. Nat Commun. 5(3056)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang D, Zhu D, Wang F, Zhu JC, Zhai X,
Yuan Y and Li CX: Therapeutic effect of regulating autophagy in
spinal cord injury: A network meta-analysis of direct and indirect
comparisons. Neural Regen Res. 15:1120–1132. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu S, Sarkar C, Dinizo M, Faden AI, Koh
EY, Lipinski MM and Wu J: Disrupted autophagy after spinal cord
injury is associated with ER stress and neuronal cell death. Cell
Death Dis. 6(e1582)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Silva R, Mesquita AR, Bessa J, Sousa JC,
Sotiropoulos I, Leão P, Almeida OF and Sousa N: Lithium blocks
stress-induced changes in depressive-like behavior and hippocampal
cell fate: The role of glycogen-synthase-kinase-3beta.
Neuroscience. 152:656–669. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Periyasamy-Thandavan S, Jiang M,
Schoenlein P and Dong Z: Autophagy: Molecular machinery,
regulation, and implications for renal pathophysiology. Am J
Physiol Renal Physiol. 297:F244–F256. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang W, Liu Y, Wu M, Zhu X, Wang T, He K,
Li P and Wu X: PI3K inhibition protects mice from NAFLD by
down-regulating CMKLR1 and NLRP3 in Kupffer cells. J Physiol
Biochem. 73:583–594. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kazanis I, Giannakopoulou M, Philippidis H
and Stylianopoulou F: Alterations in IGF-I, BDNF and NT-3 levels
following experimental brain trauma and the effect of IGF-I
administration. Exp Neurol. 186:221–234. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu M, Li B, Ma X, Huang C, Wu R, Zhu W,
Li X, Liang Z, Deng F, Zhu J, et al: Folic acid protected neural
cells against aluminum-maltolate-induced apoptosis by preventing
miR-19 downregulation. Neurochem Res. 41:2110–2118. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Park KW, Lin C-Y, Benveniste EN and Lee
Y-S: Mitochondrial STAT3 is negatively regulated by SOCS3 and
upregulated after spinal cord injury. Exp Neurol. 284 (Pt
A):98–105. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang D, Zhai X, Wang F, Li XH and He XJ:
Study of the neural protective effect of lithium on enhancement of
autophagy in vitro. Zhongguo Gu Shang. 32:952–956. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
29
|
Zhaohui C and Shuihua W: Protective
effects of SIRT6 against inflammation, oxidative stress, and cell
apoptosis in spinal cord injury. Inflammation. 43:1751–1758.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang Y, Wang W, Li D, Li M, Wang P, Wen J,
Liang M, Su B and Yin Y: IGF-1 alleviates NMDA-induced
excitotoxicity in cultured hippocampal neurons against autophagy
via the NR2B/PI3K-AKT-mTOR pathway. J Cell Physiol. 229:1618–1629.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang D, Shen S, Cai M, Jin L, Lu J, Xu K,
Zhang J, Feng G, Hu Y, Zheng K, et al: Role of mTOR complex in
IGF-1 induced neural differentiation of DPSCs. J Mol Histol.
50:273–283. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ministry of Science and Technology of the
People's Republic of China: Guidance to Ethical Treatment of
Animals for Experiments. http://www.most.gov.cn/xxgk/xinxifenlei/fdzdgknr/fgzc/gfxwj/gfxwj2010before/201712/t20171222_137025.html.
Accessed August 19, 2021 (In Chinese).
|
|
33
|
Zhang D, Li XH, Zhai X and He XJ:
Feasibility of 3.0 T diffusion-weighted nuclear magnetic resonance
imaging in the evaluation of functional recovery of rats with
complete spinal cord injury. Neural Regen Res. 10:412–418.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang D, Wang F, Zhai X, Li XH and He XJ:
Lithium promotes recovery of neurological function after spinal
cord injury by inducing autophagy. Neural Regen Res. 13:2191–2199.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Barros AGC, Cristante AF, Santos GBD,
Natalino RJM, Ferreira RJR and Barros-Filho TEP: Evaluation of the
effects of erythropoietin and interleukin-6 in rats submitted to
acute spinal cord injury. Clinics (São Paulo).
74(e674)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhou L, Fan MD, Jiang J and Xue W: Effect
of IGF-1 on cognitive function and apoptosis of hippocampal tissue
neurons in rats with delayed neuropsychologic sequelae after carbon
monoxide poisoning. J Brain Nerv Dis. 27:661–666. 2019.
|
|
37
|
Wang S and Gu K: Insulin-like growth
factor 1 inhibits autophagy of human colorectal carcinoma
drug-resistant cells via the protein kinase B/mammalian target of
rapamycin signaling pathway. Mol Med Rep. 17:2952–2956.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Turtle JD, Henwood MK, Strain MM, Huang
YJ, Miranda RC and Grau JW: Engaging pain fibers after a spinal
cord injury fosters hemorrhage and expands the area of secondary
injury. Exp Neurol. 311:115–124. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Frysak Z, Schovanek J, Iacobone M and
Karasek D: Insulin-like Growth Factors in a clinical setting:
Review of IGF-I. Biomed Pap Med Fac Univ Palacky Olomouc Czech
Repub. 159:347–351. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
de la Monte SM, Tong M, Cohen AC, Sheedy
D, Harper C and Wands JR: Insulin and insulin-like growth factor
resistance in alcoholic neurodegeneration. Alcohol Clin Exp Res.
32:1630–1644. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Madathil SK, Carlson SW, Brelsfoard JM, Ye
P, D'Ercole AJ and Saatman KE: Astrocyte-specific overexpression of
insulin-like growth factor-1 protects hippocampal neurons and
reduces behavioral deficits following traumatic brain injury in
mice. PLoS One. 8(e67204)2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ferbert T, Child C, Graeser V, Swing T,
Akbar M, Heller R, Biglari B and Moghaddam A: Tracking spinal cord
injury: Differences in cytokine expression of IGF-1, TGF-B1, and
sCD95I can be measured in blood samples and correspond to
neurological remission in a 12-week follow-up. J Neurotrauma.
34:607–614. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Muresanu DF, Sharma A, Lafuente JV,
Patnaik R, Tian ZR, Nyberg F and Sharma HS: Nanowired delivery of
growth hormone attenuates pathophysiology of spinal cord injury and
enhances insulin-like growth factor-1 concentration in the plasma
and the spinal cord. Mol Neurobiol. 52:837–845. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Galluzzi L, Morselli E, Vicencio JM, Kepp
O, Joza N, Tajeddine N and Kroemer G: Life, death and burial:
Multifaceted impact of autophagy. Biochem Soc Trans. 36:786–790.
2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hayashi-Nishino M, Fujita N, Noda T,
Yamaguchi A, Yoshimori T and Yamamoto A: A subdomain of the
endoplasmic reticulum forms a cradle for autophagosome formation.
Nat Cell Biol. 11:1433–1437. 2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kourtis N and Tavernarakis N: Autophagy
and cell death in model organisms. Cell Death Differ. 16:21–30.
2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kroemer G and Levine B: Autophagic cell
death: The story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Levine B and Yuan J: Autophagy in cell
death: An innocent convict? J Clin Invest. 115:2679–2688.
2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wu YT, Tan HL, Huang Q, Ong CN and Shen
HM: Activation of the PI3K-Akt-mTOR signaling pathway promotes
necrotic cell death via suppression of autophagy. Autophagy.
5:824–834. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Authophagy. 12:1–222.
2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang H, Wu Y, Han W, Li J, Xu K, Li Z,
Wang Q, Xu K, Liu Y, Xie L, et al: Hydrogen Sulfide Ameliorates
Blood-Spinal Cord Barrier Disruption and Improves Functional
Recovery by Inhibiting Endoplasmic Reticulum Stress-Dependent
Autophagy. Front Pharmacol. 9(858)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wu J and Lipinski MM: Autophagy in
neurotrauma: Good, bad, or dysregulated. Cells. 8(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Bai L, Mei X, Shen Z, Bi Y, Yuan Y, Guo Z,
Wang H, Zhao H, Zhou Z, Wang C, et al: Netrin-1 improves functional
recovery through autophagy regulation by activating the AMPK/mTOR
signaling pathway in rats with spinal cord injury. Sci Rep.
7(42288)2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Li J, Wang Q, Cai H, He Z, Wang H, Chen J,
Zheng Z, Yin J, Liao Z, Xu H, et al: FGF1 improves functional
recovery through inducing PRDX1 to regulate autophagy and anti-ROS
after spinal cord injury. J Cell Mol Med. 22:2727–2738.
2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Li Z, Liu F, Zhang L, Cao Y, Shao Y, Wang
X, Jiang X and Chen Z: Neuroserpin restores autophagy and promotes
functional recovery after acute spinal cord injury in rats. Mol Med
Rep. 17:2957–2963. 2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wang P, Lin C, Wu S, Huang K, Wang Y, Bao
X, Zhang F, Huang Z and Teng H: Inhibition of autophagy is involved
in the protective effects of ginsenoside Rb1 on spinal cord injury.
Cell Mol Neurobiol. 38:679–690. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bianchi VE, Locatelli V and Rizzi L:
Neurotrophic and neuroregenerative effects of GH/IGF1. Int J Mol
Sci. 18(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Marín-Aguilar F, Castejón-Vega B,
Alcocer-Gómez E, Lendines-Cordero D, Cooper MA, de la Cruz P,
Andújar-Pulido E, Pérez-Alegre M, Muntané J, Pérez-Pulido AJ, et
al: NLRP3 Inflammasome Inhibition by MCC950 in Aged Mice Improves
Health via Enhanced Autophagy and PPARα Activity. J Gerontol A Biol
Sci Med Sci. 75:1457–1464. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Marín-Aguilar F, Lechuga-Vieco AV,
Alcocer-Gómez E, Castejón-Vega B, Lucas J, Garrido C,
Peralta-Garcia A, Pérez-Pulido AJ, Varela-López A, Quiles JL, et
al: NLRP3 inflammasome suppression improves longevity and prevents
cardiac aging in male mice. Aging Cell. 19(e13050)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zhang HY, Wang ZG, Wu FZ, Kong XX, Yang J,
Lin BB, Zhu SP, Lin L, Gan CS, Fu XB, et al: Regulation of
autophagy and ubiquitinated protein accumulation by bFGF promotes
functional recovery and neural protection in a rat model of spinal
cord injury. Mol Neurobiol. 48:452–464. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Renna M, Bento CF, Fleming A, Menzies FM,
Siddiqi FH, Ravikumar B, Puri C, Garcia-Arencibia M, Sadiq O,
Corrochano S, et al: IGF-1 receptor antagonism inhibits autophagy.
Hum Mol Genet. 22:4528–4544. 2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Jean S and Kiger AA: Classes of
phosphoinositide 3-kinases at a glance. J Cell Sci. 127:923–928.
2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Gulluni F, De Santis MC, Margaria JP,
Martini M and Hirsch E: Class II PI3K functions in cell biology and
disease. Trends Cell Biol. 29:339–359. 2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Alliouachene S, Bilanges B, Chicanne G,
Anderson KE, Pearce W, Ali K, Valet C, Posor Y, Low PC, Chaussade
C, et al: Inactivation of the class II PI3K-C2β potentiates insulin
signaling and sensitivity. Cell Rep. 13:1881–1894. 2015.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Valet C, Chicanne G, Severac C, Chaussade
C, Whitehead MA, Cabou C, Gratacap MP, Gaits-Iacovoni F,
Vanhaesebroeck B, Payrastre B, et al: Essential role of class II
PI3K-C2α in platelet membrane morphology. Blood. 126:1128–1137.
2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Yu X, Long YC and Shen HM: Differential
regulatory functions of three classes of phosphatidylinositol and
phosphoinositide 3-kinases in autophagy. Autophagy. 11:1711–1728.
2015.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Vanhaesebroeck B, Guillermet-Guibert J,
Graupera M and Bilanges B: The emerging mechanisms of
isoform-specific PI3K signalling. Nat Rev Mol Cell Biol.
11:329–341. 2010.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Okada S, Hara M, Kobayakawa K, Matsumoto Y
and Nakashima Y: Astrocyte reactivity and astrogliosis after spinal
cord injury. Neurosci Res. 126:39–43. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Chen CH, Sung CS, Huang SY, Feng CW, Hung
HC, Yang SN, Chen NF, Tai MH, Wen ZH and Chen WF: The role of the
PI3K/Akt/mTOR pathway in glial scar formation following spinal cord
injury. Exp Neurol. 278:27–41. 2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Hou H, Zhang L, Zhang L and Tang P: Acute
spinal cord injury in rats should target activated autophagy. J
Neurosurg Spine. 20:568–577. 2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Goldshmit Y, Kanner S, Zacs M, Frisca F,
Pinto AR, Currie PD and Pinkas-Kramarski R: Rapamycin increases
neuronal survival, reduces inflammation and astrocyte proliferation
after spinal cord injury. Mol Cell Neurosci. 68:82–91.
2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Latacz A, Russell JA, Ocłon E, Zubel-łojek
J and Pierzchała-Koziec K: mTOR pathway - novel modulator of
astrocyte activity. Folia Biol (Krakow). 63:95–105. 2015.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Muñoz-Galdeano T, Reigada D, Del Águila Á,
Velez I, Caballero-López MJ, Maza RM and Nieto-Díaz M: Cell
specific changes of autophagy in a mouse model of contusive spinal
cord injury. Front Cell Neurosci. 12(164)2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Sarkar C, Zhao Z, Aungst S, Sabirzhanov B,
Faden AI and Lipinski MM: Impaired autophagy flux is associated
with neuronal cell death after traumatic brain injury. Autophagy.
10:2208–2222. 2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Fang B, Li XQ, Bao NR, Tan WF, Chen FS, Pi
XL, Zhang Y and Ma H: Role of autophagy in the bimodal stage after
spinal cord ischemia reperfusion injury in rats. Neuroscience.
328:107–116. 2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Woelfle J, Chia DJ, Massart-Schlesinger
MB, Moyano P and Rotwein P: Molecular physiology, pathology, and
regulation of the growth hormone/insulin-like growth factor-I
system. Pediatr Nephrol. 20:295–302. 2005.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Ranke MB, Wölfle J, Schnabel D and
Bettendorf M: Treatment of dwarfism with recombinant human
insulin-like growth factor-1. Dtsch Arztebl Int. 106:703–709.
2009.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Mukhamedshina YO, Gilazieva ZE, Arkhipova
SS, Galieva LR, Garanina EE, Shulman AA, Yafarova GG, Chelyshev YA,
Shamsutdinova NV and Rizvanov AA: Electrophysiological,
Morphological, and Ultrastructural Features of the Injured Spinal
Cord Tissue after Transplantation of Human Umbilical Cord Blood
Mononuclear Cells Genetically Modified with the VEGF and GDNF
Genes. Neural Plasticity. 2017(9857918)2017.PubMed/NCBI View Article : Google Scholar
|