Brain disorders, along with malignant tumors and
cardiovascular disorders, are among the leading causes of morbidity
and mortality worldwide (1). Brain
disorders pose a serious socioeconomic burden, where the Global
Burden of Disease 2017 data demonstrated that ~324.4 million
individuals were affected by brain disorders in Europe, which
accounts for 79.1% of all non-communicable diseases in
2017(1). Although cholinesterase
inhibitors and dopamine-like drugs have shown considerable efficacy
for the treatment of brain disorders, particularly in patients with
degenerative diseases in the central nervous system, side effects
and long-term sequelae remain (2).
Therefore, a more detailed understanding in the mechanism
underlying the progression and pathophysiology of brain disorders
is crucial for developing new therapeutic strategies.
Growth differentiation factor-15 (GDF15), which was
first identified in the human cDNA library that was enriched for
genes associated with macrophage activation using the subtraction
cloning approach, is a distant member of the TGFβ superfamily
(3). GDF15 is highly expressed in
the heart, liver, kidney, intestine, lung, placenta and the
prostate gland (4-6).
In humans, the physiological concentration of GDF15 lies in the
range of 200-1,200 pg/ml, where its levels increase with age
(7). It has been reported that
GDF15 participates in tissue repair by exerting antiapoptotic and
anti-inflammatory effects whilst maintaining vascular endothelial
functions (8,9). There is also accumulating evidence
that GDF15 is involved in the occurrence and development of
cardiovascular diseases, diabetes and cancer (10-12).
In 2017, research successively identified glial cell-derived
neurotrophic factor receptor α-like (GFRAL) as the receptor of
GDF15 (13-16).
Discovery of the GDF15/GFRAL signaling pathway provided a
potentially novel target for the treatment of obesity and metabolic
diseases (16-18).
In recent years, accumulating evidence has also indicated that
GDF15 is associated with a range of brain disorders, including
Alzheimer's disease (AD), cerebral stroke and Parkinson's disease
(PD) (19-21).
Therefore, the aim of the present review is to summarize the
regulatory processes and physiological functions of GDF15, with an
emphasis on its role in brain disorders.
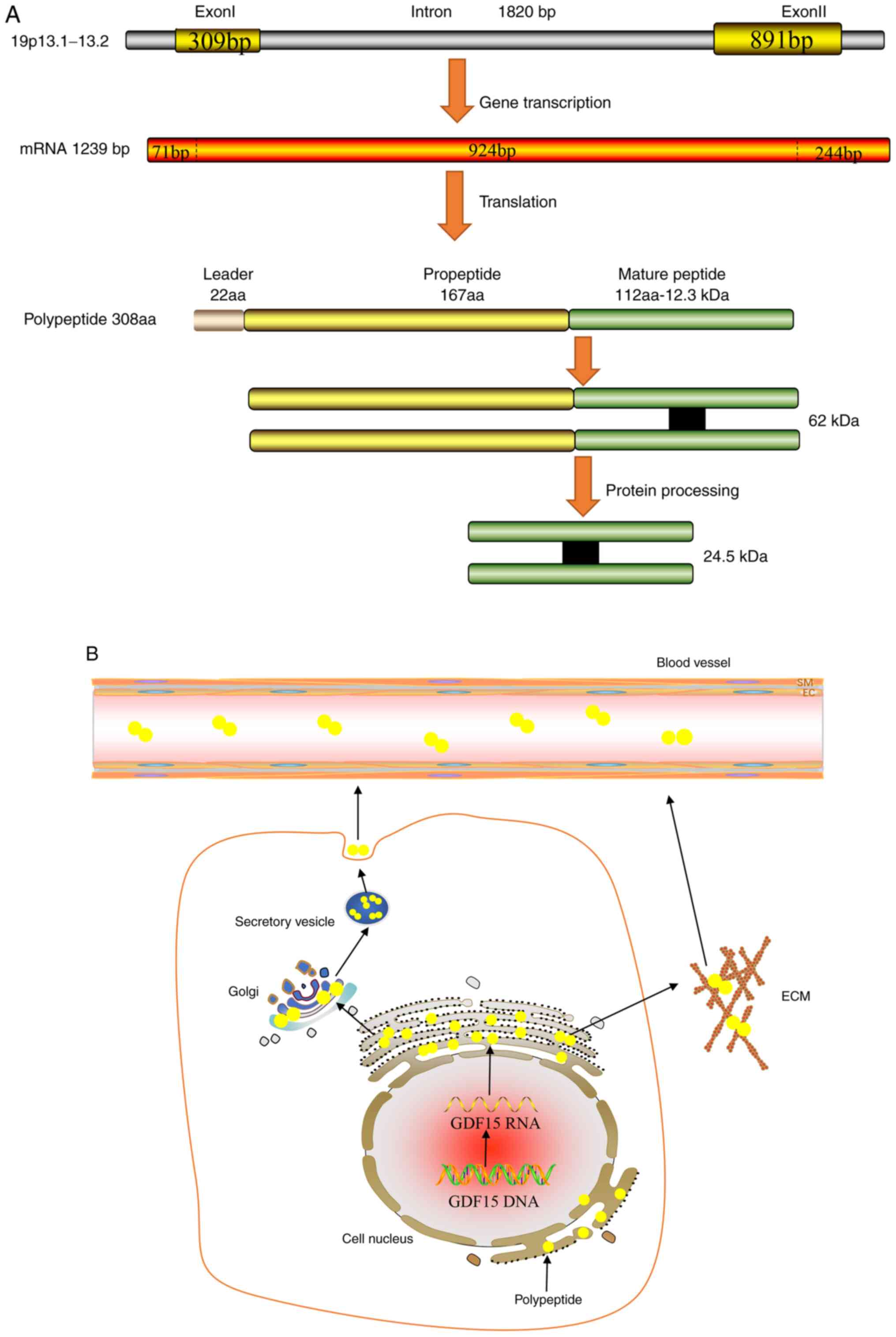
The GDF15 gene can be mapped onto the chromosome
19p13.1-13.2 genomic locus, which contains two exons and one single
intron with an open reading frame of 924 bp (3). Its corresponding mRNA can be
translated into a 308-amino acid polypeptide, which is composed of
the following three parts: Signal peptide, pro-peptide region and a
mature region on the carboxyl terminus (3). The mature domain of 112 amino acids
is first separated from the propeptide region of 167 amino acids by
a furin-like convertase, which is then cleaved by furin/paired
basic amino-acid-cleaving enzyme 4 and MMP-26 (22,23).
The mature domain of GDF15 contains a highly conserved pattern of
seven cysteine residues, where six of these form intra-chain
disulfide bonds, forming a highly stable cysteine structure that is
resistant to physical and chemical damage, including enzymatic
attacks (24). Unlike other TGFβ
families of proteins, the propeptide is not required for proper
GDF15 folding (25). Although
propeptides may facilitate the detection of improper GDF15 folding,
engineered GDF15 that lacks the pro-peptide domain can still be
secreted in its proper folded form (26). GDF15 is generally secreted as a
dimer that is formed by inter-chain disulphide bonds, which
performs complex biological functions through autocrine or
paracrine pathways (Fig. 1)
(25). In addition, GDF15 can be
secreted as an unprocessed pro-peptide, where it can bind to the
extracellular matrix (ECM) through its 89 amino acids on the
C-terminal domain in a reversible manner in prostate cancer
(27). There, GDF15 can be
released from the ECM into the circulation by locally acting MMPs
or pro-convertases (27).
As a tumor suppressor gene, p53 serves a key role in
controlling cell proliferation, inhibiting malignant cell
proliferation and regulating cell cycle progression (30). p53 was one of the first
transcription factors that was identified to be transcriptional
regulators of GDF15 expression (31). There are ≥ two p53 binding sites in
the GDF15 promoter, one near the transcription start site and
another in the region that lie 851 bp upstream of the transcription
start site (31), where both
binding sites can transactivate the GDF15 promoter (31). It was previously reported that
GDF15 expression was robustly upregulated following the
overexpression or pharmacological induction of p53 in human lung
cancer cell lines, osteosarcoma cell lines and breast cancer cell
lines (32,33). In addition, the DNA intercalator
doxorubicin was found to significantly increase GDF15 expression in
p53 wild-type human breast cancer cell lines, but exerted no
effects on p53-null cells (34).
In vitro, GDF15 expression was found to be markedly
increased in human bronchial epithelial cells and human pulmonary
vascular endothelial cells upon exposure to hyperoxia, whilst p53
knockdown robustly reduced this induction of GDF15 transcription by
hyperoxia (35). Therefore, this
suggests that p53 is an important regulator of the production of
GDF15. Similarly, C-reactive proteins in human aortic endothelial
cells and human maternal expression gene 3 in colon cancer cell
lines were both shown to induce GDF15 expression by recruiting the
p53 protein (36,37). Different members of the p53 tumor
suppressor gene family have been demonstrated to exhibit different
binding affinities for GDF15(38).
The GDF15 promoter region contains two p53-type response elements
(RE), RE1 and RE2, where most 3' quarter-sites (areas bound by the
p53 tetramer) in RE2 exhibit a higher binding affinity for
p53(38). Therefore, GDF15 is
activated by p53 to a greater extent compared with that by other
related family members, including p63 and p73(38).
EGR-1 is a transcription factor that contains three
zinc finger domains and is considered to be an important regulator
of GDF15(39). A number of studies
have previously shown that pharmacological agonists, such as
troglitazone and non-steroidal anti-inflammatory drugs, can
increase EGR-1 expression in human colon cancer cell lines prior to
GDF15 induction, supporting the hypothesis that GDF15 is a
downstream target of EGR-1 (40-42).
Indeed, position -73 to -51 of the GDF15 promoter contains
EGR-1-binding sites (43).
Furthermore, DNA methylation at the -53 site of the GDF15 promoter
site blocks the binding of EGR-1, which subsequently inhibits GDF15
induction in vitro (43).
By contrast, GDF15 transcription by EGR-1 can be restored following
incubation with 5-aza-2'-deoxycytidine (a demethylating reagent)
(43). Accordingly, in HT29 colon
carcinoma cells, activity of the GDF15 promoter and GDF15
expression are markedly increased by the ectopic expression of
EGR-1 in a dose-dependent manner, whereas EGR-1 knockdown using
small interfering (si)RNAs was shown to significantly decrease
silibinin-induced GDF15 expression (44). These observations suggest that
EGR-1 is a direct transcriptional regulator of GDF15.
LncRNAs represents a class of RNAs that cannot be
translated into proteins and are typically >200 nucleotides in
length (45). It has been reported
extensively that lncRNAs can serve key roles in the regulation of
gene expression. Kong et al (46) previously revealed that LINC0113
overexpression decreased the mRNA and protein expression levels of
GDF15 in oral squamous cell carcinoma (OSCC) cell lines, whilst
treatment with the exogenous recombinant human GDF15 protein was
able to restore the migratory and invasive abilities of OSCC cells
that was previously weakened by LINC0113. In addition, a
significant positive correlation was identified between lncRNA
plasmacytoma variant translocation 1 (PVT1) expression and GDF15
expression in hepatocellular carcinoma (HCC) tissues (47). GDF15 knockdown using siRNAs
significantly suppressed the proliferation of HCC cells caused by
lncRNA PVT1 overexpression, suggesting that lncRNA PVT1 may be an
important upstream regulator of GDF15 expression in HCC cells
(47). Another study also revealed
that CCAAT/enhancer-binding protein β (CEBPB) can bind to the
promoter of GDF15 to facilitate GDF15 gene expression in ovarian
cancer cell lines (48). In
addition, GDF15 expression was previously found to be negatively
associated with that of the lncRNA growth arrest-specific 5 (GAS5)
in ovarian cancer tissues (48).
Mechanistically, lncRNA GAS5 was shown to competitively bind to
CEBPB to inhibit the promoting effect of CEBPB on GDF15
transcription (48). It is
expected that additional lncRNAs involved in the regulation of
GDF15 expression will be discovered by future studies.
Similar to lncRNAs, miRNAs are also an important
component of gene transcription regulators, which functions by
pairing with the 3'-untranslated region of target mRNAs (49). Both miR-873-5p and miR-1233-3p have
been shown to exert suppressive effects on GDF15 expression in
melanoma cell lines (50).
However, single-nucleotide polymorphism in miRNA, rs1054564, was
located in the GDF15'UTR complementary to the hsa-miR-1233-3p seed
region, and the presence of this C-allele was discovered to weaken
the binding of hsa-miR-1233-3p to GDF15, thereby enhancing the
expression of the GDF15 protein (50). miR-3189 is a primate-specific miRNA
that is embedded within the introns of GDF15(51). Increased miR-3189 expression
results in elevated GDF15 expression by downregulating p53 in
colorectal cancer cell lines (51). In addition, overexpression of
miR-3189 in HCT116-p53-/- colorectal cancer cells was
shown to upregulate the expression of a subset of p53 targets,
including GDF15 and growth arrest and DNA-damage-inducible 45α
(51).
Hormones and hormone derivatives have also been
demonstrated to lie upstream of GDF15(52). Primary adrenal insufficiency is
accompanied by an increase in GDF15 expression, where
glucocorticoid replacement therapy was shown to effectively reduce
this concentration of GDF15 in a dose-dependent manner (53). In brown adipose tissues,
noradrenergic cAMP-mediated thermogenic activation was found to
increase GDF15 gene expression and subsequent release (54). In addition, metformin was reported
to increase GDF15 levels, but it had no effect on GFRAL
receptor-deficient mice (52).
Zhao et al (55) previously
found that thyroid hormone levels were independently associated
with GDF15 expression, such that T3 treatment promoted GDF15
expression in brown adipose tissues of C57BL/6 mice. Furthermore,
previous in vivo and vitro experiments demonstrated
that testosterone and estradiol treatment reduced GDF15 secretion
through androgen receptor/estrogen receptor-mediated signaling
pathways (56).
Accumulating evidence has suggested that GDF15 is a
potential stimulator of angiogenesis (Table I). After being secreted by
senescent endothelial colony-forming cells generated from adult
human blood in a paracrine manner, GDF15 can promote proliferation,
migration and NO production in non-senescent endothelial
colony-forming cells generated from adult human blood (57). During this process, a number of
signaling pathways are activated by GDF15 in an oxidative
stress-independent manner, including AKT, ERK1/2 and Smad2(57). This improved the function of
vascular progenitor cells, which may serve therapeutic effects on
the damaged vascular system (57).
Similarly, GDF15 can also enhance the expression of cyclins D1 and
E in HUVECs through the PI3K/AKT, JNK and ERK signaling pathways to
promote the proliferation of endothelial cells (58). GDF15 is also involved in the
mechanism underlying ischemia/reperfusion injury. During the
process of cardiac ischemia, GDF15 was shown to stimulate the
angiogenesis of hypoxic HUVECs by inhibiting p53 signaling whilst
upregulating hypoxia-inducible factor 1α expression (59). Furthermore, since the repair of
large bone defects remains to be a major medical challenge, GDF15
was shown to represent a potentially effective solution. Wang et
al (60) found that GDF15 can
promote the formation of functional blood vessels at the site of
artificially-induced angiogenesis, which significantly improved the
healing of critical-sized calvarial defects. Neovascularization is
one of the major characteristics in cancer (61). Following chemotherapy, the
expression of GDF15 was found to be markedly upregulated in HCC
(62). GDF15 can induce Src and
then activate AKT, MAPK and NF-κB downstream in HCC, which promotes
the proliferation, migration and tube formation of surrounding
endothelial cells in vitro (62). By contrast, thalidomide, an agent
with known anti-angiogenic activities, can significantly attenuate
and reverse these effects aforementioned (62). In addition, it was found that GDF15
interacted with connective tissue growth factor (CCN)-2, to inhibit
CCN2-mediated focal adhesion kinase activation, which in turn
decreased avβ3 integrin clustering in HUVECs, to exert antagonistic
effects on angiogenesis (63).
This phenomenon is conducive to understanding the role of GDF15
under various disease conditions further.
Apoptosis is a process in which cell death occurs in
an orderly manner and is crucial for the maintenance of internal
homeostasis (64). Owing to its
reported function as a stress-response protein, GDF15 has been
reported to regulate apoptosis (65). GDF15 is a downstream target of
methylseleninic acid (MSA), where GDF15 knockdown significantly
inhibited the apoptosis of prostate cancer cells mediated by MSA
(66,67). By contrast, GDF15 overexpression
made prostate cancer cells flattened and more spread out and
induced caspase-dependent apoptosis (66,67).
GDF-15 was inducible in human macrophages by oxidized low density
lipoprotein and its mediators in vitro, and GDF15
immunoreactivity was colocalized with apoptosis markers such as
PARP, caspase-3 or apoptosis-inducing factor immunoreactivity,
suggesting that GDF15 may modulate apoptosis process in activated
macrophages (68). In addition,
A549 lung adenocarcinoma cell apoptosis was also induced by GDF15
overexpression through promoting caspase-9 and caspase-3 expression
and inhibition of ERK1/2 and p38 activation, which was mediated in
a TGFβ receptor type II (TGFβRII)-dependent manner (69). Conversely, GDF15 can exert a
protective effect against the apoptosis of HUVECs induced by high
glucose concentrations (70).
Mechanistically, this effect may be caused by GDF15 maintaining the
activity of PI3K/Akt/eNOS pathway and attenuating NF-κB/JNK pathway
(70). In addition, under
conditions of hypoxia and laminar shear stress, GDF15 expression in
the pulmonary microvascular endothelial cells of patients with
pulmonary arterial hypertension was found to be significantly
higher compared with that in normal lung tissues, where the extent
of cell apoptosis was reduced by GDF15 overexpression in an
AKT-dependent manner (71).
In addition to the aforementioned functions, GDF15
can also exert anti-inflammatory and proinflammatory properties
(77,78). GDF15 can inhibit the inflammatory
response induced by lipopolysaccharide (LPS) (79). A previous study demonstrated that
GDF15-knockout mice displayed worsened characteristics following
the induction of LPS-induced renal and cardiac injury, whilst GDF15
overexpression conferred opposite effects (80). GDF15 can inhibit the activation of
the NF-κB pathway to reduce the production of proinflammatory
factors, including moncocyte chemoattractant protein-1 and TNF-α
(81). This in effect prevents
LPS-induced liver injury in mice by blocking the phosphorylation of
TGFβ-activated kinase 1(81). Luan
et al (77) previously
reported that GDF15 improved cardiac and hepatic tolerance to
inflammation in mice by regulating triglyceride metabolism. GDF15
was also found to regulate the response to in human rhinovirus
(HRV) infection and virus-induced lung inflammation (78). In human GDF15 transgenic mice, the
overexpression of GDF15 resulted in enhanced inflammatory responses
to HRV and decreased IFN-λ2/3 mRNA expression (78). In addition, the IFN-λ1/IL-29
protein, which has antiviral activity, was found to be inhibited by
GDF15 in tracheal epithelial cells from human GDF15 transgenic
mice, which promoted HRV replication and the subsequent
inflammatory response (78).
AD is a progressive neurodegenerative disease that
mainly manifests with clinical symptoms of cognitive and behavioral
impairment (82). The pathogenesis
of AD is complex and may involves amyloid β (Aβ) accumulation, tau
protein toxicity, synaptic damage, mitochondrial dysfunction and
oxidative stress (83). GDF15
expression can be detected in the adult rat central and peripheral
nervous systems, where it is mainly secreted into the cerebrospinal
fluid (84,85). It has been demonstrated that GDF15
is closely associated with cognitive impairment, which is a major
characteristic of AD (86). It was
also previously shown that cognitive impairments due to dementia
and AD were associated with higher GDF15 levels, particularly in
the presence of cerebrovascular disease (87). By contrast, GDF15-deficient mice
were shown to exhibit superior fear-associated memory and
sensorimotor gating, which is conducive to cognition (88). Decreased numbers of white matter
and grey matter nerve fibers, coupled with the increased volume of
atrophy, may be important pathophysiological features of AD
(89). Jiang et al
(90,91) revealed that higher GDF15 levels
were negatively correlated with gray matter volume and white matter
integrity. In addition, GDF15 expression likely exhibits a close
association with learning and memory impairments, where the
hippocampus is the region that is typically worst affected by AD
(86,92). GDF15-knockout mice were shown to
display a progressive loss of motor neurons coupled with the
decreased proliferation and migration of adult hippocampal
progenitor cells (93,94). This appears to be a result of the
absence of epidermal growth factor receptor signaling stimulation,
which is normally promoted by GDF15 in a CXC chemokine receptor
4-dependent manner (93,94). Kim et al (95) reported that human recombinant GDF15
can enhance the proliferation and synaptic activity of mouse
hippocampal neural stem cells both in vitro and in
vivo, whereas GDF15 knockdown can reduce the proliferation of
hippocampal neural stem cells in vitro. Within the neural
network, the synapse forms a key component in mediating signal
transmission between neurons, which underlies the mechanism of the
generation and retention of memories (96). It was previously reported that
GDF15 promoted synaptic glutamate release and increased the
miniature excitatory post-synaptic current frequency in the medial
prefrontal cortex of mice (97).
The potential mechanism was mediated through activation of
TGFβRII-mediated ERK1/2 signaling to promote CaV3.1 and
CaV3.3 α subunit expression, which increases T-type
calcium channel activity (97).
This suggests that GDF15 deficiency may impact synaptic function
and accelerate AD progression (97). The production and accumulation of
Aβ is one of the main mechanisms underlying AD development
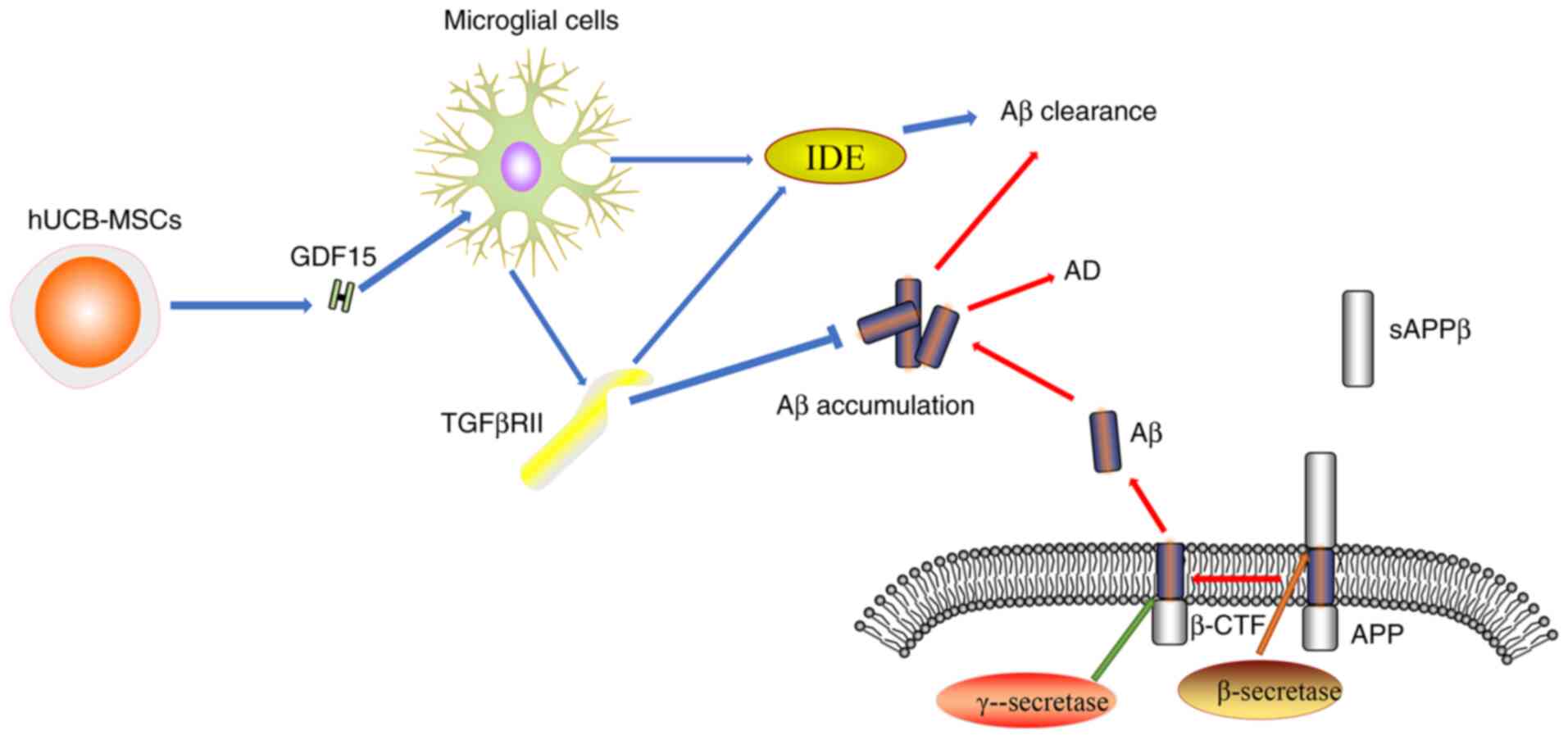
(98). TGFβRII is mainly expressed
in the microglia and neurons, where it participates in
GDF15-associated signaling pathways including Akt/mTOR pathway.
GDF15 as a soluble paracrine factor can act on microglial cells to
increase the expression level of TGFβRII during AD (12). In addition, GDF15 can enhance the
activity of insulin-degrading enzyme which, together with TGFβRII,
can promote Aβ protein clearance (12). Decreased expression levels of
TGFβRII were found in human and mouse models of AD, which may be
the underlying cause of the increased Aβ accumulation and
neurodegeneration (Fig. 2)
(99,100). In summary, GDF15 appears to be
involved in AD not only by promoting hippocampal neurogenesis and
synaptic activity, but also by enhancing the clearance of the Aβ
protein. Therefore, GDF15 may represent an attractive therapeutic
target for AD.
Cerebral stroke is an acute cerebrovascular disease,
the pathogenesis of which is characterized by inadequate blood flow
to the brain due to the rupture or occlusion of cerebrovascular
vessels, thereby causing brain damage (101). Stroke can be divided into two
categories, namely ischemic and hemorrhagic stroke, where American
Stroke Association data in 2018 indicated that ischemic stroke
accounted for 87% of all cases (102). Since GDF15 levels were found to
be elevated after tissue injury, ischemia or hypoxia, it was
hypothesized that there may be an association between GDF15 and
stroke. Xiang et al (103)
demonstrated that the genotype and allele frequencies of the GDF15
rs1804826G/T polymorphism were associated with the risk of cerebral
stroke within the Chinese population. In a prospective, nested,
case-controlled study of 27,628 initially healthy female
individuals, Brown et al (104) revealed that the GDF15
concentration in patients with cardiovascular events, including
stroke, was higher compared with that in individuals without
cardiovascular events. In addition, a concentration higher than the
90th percentile (>856 pg/ml) was associated with a 2.7-fold
increase in the risk of developing cardiovascular events (104). Previous studies reported that
GDF15 levels may serve as a prognostic marker in patients with a
history of stroke (105,106). Several lines of evidence also
revealed that the level of GDF15 was found to be positively
associated with the severity of ischemic stroke, such that GDF15
can be used as a biomarker for predicting an unfavorable outcome 90
days after the stroke event (107,108). Plasma GDF15 concentration on
admission has been reported to serve as an independent prognostic
biomarker of mortality in patients with ischemic stroke following
acute revascularization therapy (17). A previous study also investigated
the relationship between GDF15 and in 254 patients with
hypertension who suffered from stroke for the first, which found
that GDF15 can be used as an independent predictor of stroke in
individuals without any prior history of stroke (109). In addition, it was reported that
GDF15 mRNA expression was markedly upregulated in the hippocampus
and parietal cortex of mice at 3 and 24 h after middle cerebral
artery occlusion (110). This
suggests that GDF15 can participate in the regulation of
post-lesion responses, further supporting the notion that GDF15
participates in the occurrence and development of cerebral stroke
(110).
The main property of PD is the degeneration and loss
of dopaminergic neurons in the substantia nigra and nigrostriatal
pathway, which is caused by the formation of Lewy bodies as a
result of aberrant α-synuclein deposition (111). PD is characterized by symptoms of
dyskinesia, including tremor, stiffness, slow motion and unstable
posture (111). PD also has a
complex and multifactorial pathological process, which typically
involves the aggregation of α-synuclein, oxidative stress,
mitochondrial dysfunction, iron deposition and neuronal apoptosis
(112). Maetzler et al
(113) previously revealed that
GDF15 exhibited a positive correlation with the age of PD symptom
onset, Hoehn and Yahr scale score and expression of the
neurodegenerative marker Tau. GDF15 was also identified to be an
independent risk factor for Unified Parkinson's Disease Rating
Scale-III score through multiple linear regression analysis, where
subsequent receiver operating characteristic curve analysis
revealed that GDF15 exhibited a sensitivity of 71.15% and a
specificity of 87.50% for the detection of PD (114,115). However, since GDF15 levels
exhibit substantial overlap between patients with PD and healthy
individuals, this marker alone may not be sufficient as a
diagnostic tool. However, these aforementioned findings indicate
collectively that GDF15 expression is closely associated with
PD.
The neurotoxin 6-hydroxydopamine (6-OHDA) can be
taken up preferentially by dopaminergic and noradrenergic
transporters, leading to the degeneration of catecholaminergic
neurons (116). Strelau et
al (84) demonstrated that
unilateral injections of GDF15 into the medial forebrain bundle
immediately above the substantia nigra prior to 6-OHDA
administration were able to confer protection against complete
lesion formation induced by 6-OHDA, which prevented the loss of the
dopaminergic neurons. Consistently, Machado et al (117,118) further demonstrated that
endogenous GDF15 may promote the survival of dopaminergic neurons
by regulating the inflammatory response after 6-OHDA-induced brain
injury. GDF15 released by astrocytes exerted a protective effect on
vulnerable nigral neurons during PD and on induced pluripotent stem
cell-derived dopaminergic neurons subjected to
1-methyl-4-phenylpyridinium toxicity, which may explain the
selective degeneration or protection of dopaminergic neurons in PD
because GDF15 is expressed 230-fold higher in the neighboring
ventral tegmental area astrocytes than the substantia nigra pars
compacta (20,119). Mitochondrial dysfunction is
another possible mechanism that has been proposed to serve a role
in PD (112). The HT22
hippocampal cell line is considered to be a suitable model for
studying PD. GDF15 overexpression was shown to reverse the effects
of oxygen consumption, cell viability and mitochondrial membrane
potential caused by oligomycin in HT22 cells, where further study
revealed that GDF15 may regulate mitochondrial membrane potential
and oxygen consumption through the PI3K/AKT signaling pathway
(120). Furthermore, GDF15 was
reported to be a more sensitive measure for diagnosing
mitochondrial dysfunction compared with that of lactate stress test
in Japanese patients with PD (121). Collectively, these findings
suggest that GDF15 may promote the survival of dopaminergic neurons
and exerts a protective effect by preserving normal mitochondrial
function.
GDF15 is widely expressed in brain tissues and has
been found to be involved in the pathophysiological processes
underlying a number of brain disorders, particularly in AD,
cerebral stroke and PD. Although progress has been made over the
past decade, several unresolved problems remain. The specific
signaling pathways mediated by GDF15 in AD have not been fully
elucidated. In addition, the reference range and sensitivity of
GDF15 as a biomarker for the prognosis and risk stratification of
related diseases have yet to be determined. Furthermore, it remains
unknown if there are other GDF15 receptors involved in mediating
the pathophysiological processes of brain disorders in addition to
the GFRAL receptor. Whether the plasma concentration of GDF15 can
be artificially regulated to treat brain disorders is also a
question that require further study. Therefore, addressing these
questions aforementioned may provide further clues for the
prevention and treatment of these brain disorders.
Not applicable.
Funding: The authors acknowledge the financial supports from the
Natural Science Foundation of Hunan Province, (grant nos.
2019JJ40249 and 2018JJ3455), Outstanding Young Aid Program for
Education Department of Human Province (grant no. 18B274), the
Major Project of Social Science Achievement Review Committee in
Hunan Province (grant no. XSP20ZDI013), Key Project of Hunan
Provincial Department of Education (grant no. 20A427).
Not applicable.
WWJ, ZZZ, PPH, XPO contributed to data gathering,
manuscript drafting, critical revision of the manuscript. LPJ, JZC,
XTZ, MH and YKZ revised the manuscript. All authors read and
approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Raggi A and Leonardi M: Burden of brain
disorders in Europe in 2017 and comparison with other
non-communicable disease groups. J Neurol Neurosurg Psychiatry.
91:104–105. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bautista-Aguilera OM, Ismaili L, Iriepa I,
Diez-Iriepa D, Chabchoub F, Marco-Contelles J and Pérez M: Tacrines
as therapeutic agents for alzheimer's disease. V. recent
developments. Chem Rec. 21:162–174. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bootcov MR, Bauskin AR, Valenzuela SM,
Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor
K, et al: MIC-1, a novel macrophage inhibitory cytokine, is a
divergent member of the TGF-beta superfamily. Proc Natl Acad Sci
USA. 94:11514–11519. 1997.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yokoyama-Kobayashi M, Saeki M, Sekine S
and Kato S: Human cDNA encoding a novel TGF-beta superfamily
protein highly expressed in placenta. J Biochem. 122:622–626.
1997.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bottner M, Laaff M, Schechinger B, Rappold
G, Unsicker K and Suter-Crazzolara C: Characterization of the rat,
mouse, and human genes of growth/differentiation
factor-15/macrophage inhibiting cytokine-1 (GDF-15/MIC-1). Gene.
237:105–111. 1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Koopmann J, Buckhaults P, Brown DA,
Zahurak ML, Sato N, Fukushima N, Sokoll LJ, Chan DW, Yeo CJ, Hruban
RH, et al: Serum macrophage inhibitory cytokine 1 as a marker of
pancreatic and other periampullary cancers. Clin Cancer Res.
10:2386–2392. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wiklund FE, Bennet AM, Magnusson PK,
Eriksson UK, Lindmark F, Wu L, Yaghoutyfam N, Marquis CP, Stattin
P, Pedersen NL, et al: Macrophage inhibitory cytokine-1
(MIC-1/GDF15): A new marker of all-cause mortality. Aging Cell.
9:1057–1064. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Conte M, Martucci M, Chiariello A,
Franceschi C and Salvioli S: Mitochondria, immunosenescence and
inflammaging: A role for mitokines? Semin Immunopathol. 42:607–617.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rochette L, Zeller M, Cottin Y and Vergely
C: Insights into mechanisms of GDF15 and receptor GFRAL:
Therapeutic targets. Trends Endocrinol Metab. 31:939–951.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kang YE, Kim JM, Lim MA, Lee SE, Yi S, Kim
JT, Oh C, Liu L, Jin Y, Jung SN, et al: Growth differentiation
factor 15 is a cancer cell-induced mitokine that primes thyroid
cancer cells for invasiveness. Thyroid. 31:772–786. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nakayasu ES, Syed F, Tersey SA, Gritsenko
MA, Mitchell HD, Chan CY, Dirice E, Turatsinze JV, Cui Y, Kulkarni
RN, et al: Comprehensive proteomics analysis of stressed human
islets identifies GDF15 as a target for type 1 diabetes
intervention. Cell Metab. 31:363–374.e6. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang Y, Zhen C, Wang R and Wang G:
Growth-differentiation factor-15 predicts adverse cardiac events in
patients with acute coronary syndrome: A meta-analysis. Am J Emerg
Med. 37:1346–1352. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang L, Chang CC, Sun Z, Madsen D, Zhu H,
Padkjær SB, Wu X, Huang T, Hultman K, Paulsen SJ, et al: GFRAL is
the receptor for GDF15 and is required for the anti-obesity effects
of the ligand. Nat Med. 23:1158–1166. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mullican SE, Lin-Schmidt X, Chin CN,
Chavez JA, Furman JL, Armstrong AA, Beck SC, South VJ, Dinh TQ,
Cash-Mason TD, et al: GFRAL is the receptor for GDF15 and the
ligand promotes weight loss in mice and nonhuman primates. Nat Med.
23:1150–1157. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hsu JY, Crawley S, Chen M, Ayupova DA,
Lindhout DA, Higbee J, Kutach A, Joo W, Gao Z, Fu D, et al:
Non-homeostatic body weight regulation through a
brainstem-restricted receptor for GDF15. Nature. 550:255–259.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Emmerson PJ, Wang F, Du Y, Liu Q, Pickard
RT, Gonciarz MD, Coskun T, Hamang MJ, Sindelar DK, Ballman KK, et
al: The metabolic effects of GDF15 are mediated by the orphan
receptor GFRAL. Nat Med. 23:1215–1219. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Borner T, Shaulson ED, Ghidewon MY,
Barnett AB, Horn CC, Doyle RP, Grill HJ, Hayes MR and De Jonghe BC:
GDF15 induces anorexia through Nausea and Emesis. Cell Metab.
31:351–362.e5. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Klaus S, Igual Gil C and Ost M: Regulation
of diurnal energy balance by mitokines. Cell Mol Life Sci.
78:3369–3384. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim DH, Lee D, Lim H, Choi SJ, Oh W, Yang
YS, Chang JH and Jeon HB: Effect of growth differentiation
factor-15 secreted by human umbilical cord blood-derived
mesenchymal stem cells on amyloid beta levels in in vitro and in
vivo models of Alzheimer's disease. Biochem Biophys Res Commun.
504:933–940. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kostuk EW, Cai J and Iacovitti L:
Subregional differences in astrocytes underlie selective
neurodegeneration or protection in Parkinson's disease models in
culture. Glia. 67:1542–1557. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Breniere C, Meloux A, Pedard M, Marie C,
Thouant P, Vergely C and Béjot Y: Growth differentiation factor-15
(GDF-15) is associated with mortality in ischemic stroke patients
treated with acute revascularization therapy. Front Neurol.
10(611)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li S, Wang Y, Cao B, Wu Y, Ji L, Li YX,
Liu M, Zhao Y, Qiao J, Wang H, et al: Maturation of growth
differentiation factor 15 in human placental trophoblast cells
depends on the interaction with Matrix Metalloproteinase-26. J Clin
Endocrinol Metab. 99:E2277–E2287. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Couture F, Sabbagh R, Kwiatkowska A,
Desjardins R, Guay SP, Bouchard L and Day R: PACE4 undergoes an
oncogenic alternative splicing switch in cancer. Cancer Res.
77:6863–6879. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fairlie WD, Russell PK, Wu WM, Moore AG,
Zhang HP, Brown PK, Bauskin AR and Breit SN: Epitope mapping of the
transforming growth factor-beta superfamily protein, macrophage
inhibitory cytokine-1 (MIC-1): Identification of at least five
distinct epitope specificities. Biochemistry. 40:65–73.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bauskin AR, Zhang HP, Fairlie WD, He XY,
Russell PK, Moore AG, Brown DA, Stanley KK and Breit SN: The
propeptide of macrophage inhibitory cytokine (MIC-1), a TGF-beta
superfamily member, acts as a quality control determinant for
correctly folded MIC-1. EMBO J. 19:2212–2220. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bauskin AR, Jiang L, Luo XW, Wu L, Brown
DA and Breit SN: The TGF-beta superfamily cytokine MIC-1/GDF15:
secretory mechanisms facilitate creation of latent stromal stores.
J Interferon Cytokine Res. 30:389–397. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bauskin AR, Brown DA, Junankar S, Rasiah
KK, Eggleton S, Hunter M, Liu T, Smith D, Kuffner T, Pankhurst GJ,
et al: The propeptide mediates formation of stromal stores of
PROMIC-1: Role in determining prostate cancer outcome. Cancer Res.
65:2330–2336. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tsai VWW, Husaini Y, Sainsbury A, Brown DA
and Breit SN: The MIC-1/GDF15-GFRAL pathway in energy homeostasis:
Implications for obesity, cachexia, and other associated diseases.
Cell Metab. 28:353–368. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wollert KC, Kempf T and Wallentin L:
Growth differentiation factor 15 as a biomarker in cardiovascular
disease. Clin Chem. 63:140–151. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Demir O, Barros EP, Offutt TL, Rosenfeld M
and Amaro RE: An integrated view of p53 dynamics, function, and
reactivation. Curr Opin Struct Biol. 67:187–194. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tan M, Wang Y, Guan K and Sun Y:
PTGF-beta, a type beta transforming growth factor (TGF-beta)
superfamily member, is a p53 target gene that inhibits tumor cell
growth via TGF-beta signaling pathway. Proc Natl Acad Sci USA.
97:109–114. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kannan K, Amariglio N, Rechavi G and Givol
D: Profile of gene expression regulated by induced p53: Connection
to the TGF-beta family. FEBS Lett. 470:77–82. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li PX, Wong J, Ayed A, Ngo D, Brade AM,
Arrowsmith C, Austin RC and Klamut HJ: Placental transforming
growth factor-beta is a downstream mediator of the growth arrest
and apoptotic response of tumor cells to DNA damage and p53
overexpression. J Biol Chem. 275:20127–20135. 2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang H, Filipovic Z, Brown D, Breit SN and
Vassilev LT: Macrophage inhibitory cytokine-1: A novel biomarker
for p53 pathway activation. Mol Cancer Ther. 2:1023–1029.
2003.PubMed/NCBI
|
|
35
|
Tiwari KK, Moorthy B and Lingappan K: Role
of GDF15 (growth and differentiation factor 15) in pulmonary oxygen
toxicity. Toxicol In Vitro. 29:1369–1376. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kim Y, Noren Hooten N and Evans MK: CRP
stimulates GDF15 expression in endothelial cells through p53.
Mediators Inflamm. 2018(8278039)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhou Y, Zhong Y, Wang Y, Zhang X, Batista
DL, Gejman R, Ansell PJ, Zhao J, Weng C and Klibanski A: Activation
of p53 by MEG3 non-coding RNA. J Biol Chem. 282:24731–24742.
2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Osada M, Park HL, Park MJ, Liu JW, Wu G,
Trink B and Sidransky D: A p53-type response element in the GDF15
promoter confers high specificity for p53 activation. Biochem
Biophys Res Commun. 354:913–918. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pagel JI and Deindl E: Disease progression
mediated by egr-1 associated signaling in response to oxidative
stress. Int J Mol Sci. 13:13104–13117. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Baek SJ, Kim JS, Nixon JB, DiAugustine RP
and Eling TE: Expression of NAG-1, a transforming growth
factor-beta superfamily member, by troglitazone requires the early
growth response gene EGR-1. J Biol Chem. 279:6883–6892.
2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Baek SJ, Kim JS, Moore SM, Lee SH,
Martinez J and Eling TE: Cyclooxygenase inhibitors induce the
expression of the tumor suppressor gene EGR-1, which results in the
upregulation of NAG-1, an antitumorigenic protein. Mol Pharmacol.
67:356–364. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chintharlapalli S, Papineni S, Baek SJ,
Liu S and Safe S:
1,1-Bis(3'-indolyl)-1-(p-substitutedphenyl)methanes are peroxisome
proliferator-activated receptor gamma agonists but decrease HCT-116
colon cancer cell survival through receptor-independent activation
of early growth response-1 and nonsteroidal anti-inflammatory
drug-activated gene-1. Mol Pharmacol. 68:1782–1792. 2005.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kadowaki M, Yoshioka H, Kamitani H,
Watanabe T, Wade PA and Eling TE: DNA methylation-mediated
silencing of nonsteroidal anti-inflammatory drug-activated gene
(NAG-1/GDF15) in glioma cell lines. Int J Cancer. 130:267–277.
2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Woo SM, Min KJ, Kim S, Park JW, Kim DE,
Chun KS, Kim YH, Lee TJ, Kim SH, Choi YH, et al: Silibinin induces
apoptosis of HT29 colon carcinoma cells through early growth
response-1 (EGR-1)-mediated non-steroidal anti-inflammatory
drug-activated gene-1 (NAG-1) upregulation. Chem Biol Interact.
211:36–43. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Statello L, Guo CJ, Chen LL and Huarte M:
Gene regulation by long non-coding RNAs and its biological
functions. Nat Rev Mol Cell Biol. 22:96–118. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kong J, Sun W, Zhu W, Liu C, Zhang H and
Wang H: Long noncoding RNA LINC01133 inhibits oral squamous cell
carcinoma metastasis through a feedback regulation loop with GDF15.
J Surg Oncol. 118:1326–1334. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Xiong X, Yuan J, Zhang N, Zheng Y, Liu J
and Yang M: Silencing of lncRNA PVT1 by miR-214 inhibits the
oncogenic GDF15 signaling and suppresses hepatocarcinogenesis.
Biochem Biophys Res Commun. 521:478–484. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Guo LL and Wang SF: Downregulated long
noncoding RNA GAS5 fails to function as decoy of CEBPB, resulting
in increased GDF15 expression and rapid ovarian cancer cell
proliferation. Cancer Biother Radiopharm. 34:537–546.
2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Liu B, Li J and Cairns MJ: Identifying
miRNAs, targets and functions. Brief Bioinform. 15:1–19.
2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Teng MS, Hsu LA, Juan SH, Lin WC, Lee MC,
Su CW, Wu S and Ko YL: A GDF15 3' UTR variant, rs1054564, results
in allele-specific translational repression of GDF15 by
hsa-miR-1233-3p. PLoS One. 12(e0183187)2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jones MF, Li XL, Subramanian M, Shabalina
SA, Hara T, Zhu Y, Huang J, Yang Y, Wakefield LM, Prasanth KV and
Lal A: Growth differentiation factor-15 encodes a novel microRNA
3189 that functions as a potent regulator of cell death. Cell Death
Differ. 22:1641–1653. 2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Coll AP, Chen M, Taskar P, Rimmington D,
Patel S, Tadross JA, Cimino I, Yang M, Welsh P, Virtue S, et al:
GDF15 mediates the effects of metformin on body weight and energy
balance. Nature. 578:444–448. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Melvin A, Chantzichristos D, Kyle CJ,
Mackenzie SD, Walker BR, Johannsson G, Stimson RH and O'Rahilly S:
GDF15 is elevated in conditions of glucocorticoid deficiency and is
modulated by glucocorticoid replacement. J Clin Endocrinol Metab.
105:1427–1434. 2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Campderros L, Moure R, Cairo M,
Gavaldà-Navarro A, Quesada-López T, Cereijo R, Giralt M, Villarroya
J and Villarroya F: Brown Adipocytes Secrete GDF15 in response to
thermogenic activation. Obesity (Silver Spring). 27:1606–1616.
2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhao J, Li M, Chen Y, Zhang S, Ying H,
Song Z, Lu Y, Li X, Xiong X and Jiang J: Elevated serum growth
differentiation Factor 15 levels in hyperthyroid patients. Front
Endocrinol (Lausanne). 9(793)2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liu H, Dai W, Cui Y, Lyu Y and Li Y:
Potential associations of circulating growth differentiation
factor-15 with sex hormones in male patients with coronary artery
disease. Biomed Pharmacother. 114(108792)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ha G, De Torres F, Arouche N, Benzoubir N,
Ferratge S, Hatem E, Anginot A and Uzan G: GDF15 secreted by
senescent endothelial cells improves vascular progenitor cell
functions. PLoS One. 14(e0216602)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Jin YJ, Lee JH, Kim YM, Oh GT and Lee H:
Macrophage inhibitory cytokine-1 stimulates proliferation of human
umbilical vein endothelial cells by up-regulating cyclins D1 and E
through the PI3K/Akt-, ERK-, and JNK-dependent AP-1 and E2F
activation signaling pathways. Cell Signal. 24:1485–1495.
2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Song H, Yin D and Liu Z: GDF-15 promotes
angiogenesis through modulating p53/HIF-1α signaling pathway in
hypoxic human umbilical vein endothelial cells. Mol Biol Rep.
39:4017–4022. 2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang S, Li M, Zhang W, Hua H, Wang N, Zhao
J, Ge J, Jiang X, Zhang Z, Ye D and Yang C: Growth differentiation
factor 15 promotes blood vessel growth by stimulating cell cycle
progression in repair of critical-sized calvarial defect. Sci Rep.
7(9027)2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Dong G, Zheng QD, Ma M, Wu SF, Zhang R,
Yao RR, Dong YY, Ma H, Gao DM, Ye SL, et al: Angiogenesis enhanced
by treatment damage to hepatocellular carcinoma through the release
of GDF15. Cancer Med. 7:820–830. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Whitson RJ, Lucia MS and Lambert JR:
Growth differentiation factor-15 (GDF-15) suppresses in vitro
angiogenesis through a novel interaction with connective tissue
growth factor (CCN2). J Cell Biochem. 114:1424–1433.
2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Kist M and Vucic D: Cell death pathways:
Intricate connections and disease implications. EMBO J.
40(e106700)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zhu S, Yang N, Guan Y, Wang X, Zang G, Lv
X, Deng S, Wang W, Li T and Chen J: GDF15 promotes glioma stem
cell-like phenotype via regulation of ERK1/2-c-Fos-LIF signaling.
Cell Death Discov. 7(3)2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhang W, Hu C, Wang X, Bai S, Cao S,
Kobelski M, Lambert JR, Gu J and Zhan Y: Role of GDF15 in
methylseleninic acid-mediated inhibition of cell proliferation and
induction of apoptosis in prostate cancer cells. PLoS One.
14(e0222812)2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Liu T, Bauskin AR, Zaunders J, Brown DA,
Pankhurst S, Russell PJ and Breit SN: Macrophage inhibitory
cytokine 1 reduces cell adhesion and induces apoptosis in prostate
cancer cells. Cancer Res. 63:5034–5040. 2003.PubMed/NCBI
|
|
68
|
Schlittenhardt D, Schober A, Strelau J,
Bonaterra GA, Schmiedt W, Unsicker K, Metz J and Kinscherf R:
Involvement of growth differentiation factor-15/macrophage
inhibitory cytokine-1 (GDF-15/MIC-1) in oxLDL-induced apoptosis of
human macrophages in vitro and in arteriosclerotic lesions. Cell
Tissue Res. 318:325–333. 2004.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Tarfiei GA, Shadboorestan A, Montazeri H,
Rahmanian N, Tavosi G and Ghahremani MH: GDF15 induced apoptosis
and cytotoxicity in A549 cells depends on TGFBR2 expression. Cell
Biochem Funct. 37:320–330. 2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Li J, Yang L, Qin W, Zhang G, Yuan J and
Wang F: Adaptive induction of growth differentiation factor 15
attenuates endothelial cell apoptosis in response to high glucose
stimulus. PLoS One. 8(e65549)2013.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Nickel N, Jonigk D, Kempf T, Bockmeyer CL,
Maegel L, Rische J, Laenger F, Lehmann U, Sauer C, Greer M, et al:
GDF-15 is abundantly expressed in plexiform lesions in patients
with pulmonary arterial hypertension and affects proliferation and
apoptosis of pulmonary endothelial cells. Respir Res.
12(62)2011.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Suriben R, Chen M, Higbee J, Oeffinger J,
Ventura R, Li B, Mondal K, Gao Z, Ayupova D, Taskar P, et al:
Antibody-mediated inhibition of GDF15-GFRAL activity reverses
cancer cachexia in mice. Nat Med. 26:1264–1270. 2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Chrysovergis K, Wang X, Kosak J, Lee SH,
Kim JS, Foley JF, Travlos G, Singh S, Baek SJ and Eling TE:
NAG-1/GDF-15 prevents obesity by increasing thermogenesis,
lipolysis and oxidative metabolism. Int J Obes (Lond).
38:1555–1564. 2014.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Tsai VW, Zhang HP, Manandhar R, Schofield
P, Christ D, Lee-Ng KKM, Lebhar H, Marquis CP, Husaini Y, Brown DA
and Breit SN: GDF15 mediates adiposity resistance through actions
on GFRAL neurons in the hindbrain AP/NTS. Int J Obes (Lond).
43:2370–2380. 2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Zhang Z, Xu X, Tian W, Jiang R, Lu Y, Sun
Q, Fu R, He Q, Wang J, Liu Y, et al: ARRB1 inhibits non-alcoholic
steatohepatitis progression by promoting GDF15 maturation. J
Hepatol. 72:976–989. 2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Zhang M, Sun W, Qian J and Tang Y: Fasting
exacerbates hepatic growth differentiation factor 15 to promote
fatty acid β-oxidation and ketogenesis via activating XBP1
signaling in liver. Redox Biol. 16:87–96. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Luan HH, Wang A, Hilliard BK, Carvalho F,
Rosen CE, Ahasic AM, Herzog EL, Kang I, Pisani MA, Yu S, et al:
GDF15 is an inflammation-induced central mediator of tissue
tolerance. Cell. 178:1231–1244.e11. 2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Wu Q, Jiang D, Schaefer NR, Harmacek L,
O'Connor BP, Eling TE, Eickelberg O and Chu HW: Overproduction of
growth differentiation factor 15 promotes human rhinovirus
infection and virus-induced inflammation in the lung. Am J Physiol
Lung Cell Mol Physiol. 314:L514–L527. 2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Abulizi P, Loganathan N, Zhao D, Mele T,
Zhang Y, Zwiep T, Liu K and Zheng X: Growth differentiation
factor-15 deficiency augments inflammatory response and exacerbates
septic heart and renal injury induced by lipopolysaccharide. Sci
Rep. 7(1037)2017.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Li A, Zhao F, Zhao Y, Liu H and Wang Z:
ATF4-mediated GDF15 suppresses LPS-induced inflammation and MUC5AC
in human nasal epithelial cells through the PI3K/Akt pathway. Life
Sci. 275(119356)2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Li M, Song K, Huang X, Fu S and Zeng Q:
GDF15 prevents LPS and D-galactosamine-induced inflammation and
acute liver injury in mice. Int J Mol Med. 42:1756–1764.
2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Hu X, Wang T and Jin F: Alzheimer's
disease and gut microbiota. Sci China Life Sci. 59:1006–1023.
2016.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Chen YG: Research progress in the
pathogenesis of Alzheimer's disease. Chin Med J (Engl).
131:1618–1624. 2018.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Strelau J, Sullivan A, Bottner M, Lingor
P, Falkenstein E, Suter-Crazzolara C, Galter D, Jaszai J,
Krieglstein K and Unsicker K: Growth/differentiation
factor-15/macrophage inhibitory cytokine-1 is a novel trophic
factor for midbrain dopaminergic neurons in vivo. J Neurosci.
20:8597–8603. 2000.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Schober A, Bottner M, Strelau J, Kinscherf
R, Bonaterra GA, Barth M, Schilling L, Fairlie WD, Breit SN and
Unsicker K: Expression of growth differentiation
factor-15/macrophage inhibitory cytokine-1 (GDF-15/MIC-1) in the
perinatal, adult, and injured rat brain. J Comp Neurol. 439:32–45.
2001.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Fuchs T, Trollor JN, Crawford J, Brown DA,
Baune BT, Samaras K, Campbell L, Breit SN, Brodaty H, Sachdev P and
Smith E: Macrophage inhibitory cytokine-1 is associated with
cognitive impairment and predicts cognitive decline-the Sydney
memory and aging study. Aging Cell. 12:882–889. 2013.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Chai YL, Hilal S, Chong JPC, Ng YX, Liew
OW, Xu X, Ikram MK, Venketasubramanian N, Richards AM, Lai MKP and
Chen CP: Growth differentiation factor-15 and white matter
hyperintensities in cognitive impairment and dementia. Medicine
(Baltimore). 95(e4566)2016.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Low JK, Ambikairajah A, Shang K, Brown DA,
Tsai VW, Breit SN and Karl T: First behavioural characterisation of
a knockout mouse model for the transforming growth factor (TGF)-β
superfamily cytokine, MIC-1/GDF15. PLoS One.
12(e0168416)2017.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Nasrabady SE, Rizvi B, Goldman JE and
Brickman AM: White matter changes in Alzheimer's disease: A focus
on myelin and oligodendrocytes. Acta Neuropathol Commun.
6(22)2018.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Jiang J, Trollor JN, Brown DA, Crawford
JD, Thalamuthu A, Smith E, Breit SN, Liu T, Brodaty H, Baune BT, et
al: An inverse relationship between serum macrophage inhibitory
cytokine-1 levels and brain white matter integrity in
community-dwelling older individuals. Psychoneuroendocrinology.
62:80–88. 2015.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Jiang J, Wen W, Brown DA, Crawford J,
Thalamuthu A, Smith E, Breit SN, Liu T, Zhu W, Brodaty H, et al:
The relationship of serum macrophage inhibitory cytokine-1 levels
with gray matter volumes in community-dwelling older individuals.
PLoS One. 10(e0123399)2015.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Mu Y and Gage FH: Adult hippocampal
neurogenesis and its role in Alzheimer's disease. Mol Neurodegener.
6(85)2011.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Strelau J, Strzelczyk A, Rusu P, Bendner
G, Wiese S, Diella F, Altick AL, von Bartheld CS, Klein R, Sendtner
M and Unsicker K: Progressive postnatal motoneuron loss in mice
lacking GDF-15. J Neurosci. 29:13640–13648. 2009.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Carrillo-Garcia C, Prochnow S, Simeonova
IK, Strelau J, Hölzl-Wenig G, Mandl C, Unsicker K, von Bohlen Und
Halbach O and Ciccolini F: Growth/differentiation factor 15
promotes EGFR signalling, and regulates proliferation and migration
in the hippocampus of neonatal and young adult mice. Development.
141:773–783. 2014.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Kim DH, Lee D, Chang EH, Kim JH, Hwang JW,
Kim JY, Kyung JW, Kim SH, Oh JS, Shim SM, et al: GDF-15 secreted
from human umbilical cord blood mesenchymal stem cells delivered
through the cerebrospinal fluid promotes hippocampal neurogenesis
and synaptic activity in an Alzheimer's disease model. Stem Cells
Dev. 24:2378–2390. 2015.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Sudhof TC: Molecular neuroscience in the
21st Century: A personal perspective. Neuron. 96:536–541.
2017.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Liu DD, Lu JM, Zhao QR, Hu C and Mei YA:
Growth differentiation factor-15 promotes glutamate release in
medial prefrontal cortex of mice through upregulation of T-type
calcium channels. Sci Rep. 6(28653)2016.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Ballard C, Gauthier S, Corbett A, Brayne
C, Aarsland D and Jones E: Alzheimer's disease. Lancet.
377:1019–1031. 2011.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Tesseur I, Zou K, Esposito L, Bard F,
Berber E, Can JV, Lin AH, Crews L, Tremblay P, Mathews P, et al:
Deficiency in neuronal TGF-beta signaling promotes
neurodegeneration and Alzheimer's pathology. J Clin Invest.
116:3060–3069. 2006.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Das P and Golde T: Dysfunction of TGF-beta
signaling in Alzheimer's disease. J Clin Invest. 116:2855–2857.
2006.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Koh SH and Park HH: Neurogenesis in stroke
recovery. Transl Stroke Res. 8:3–13. 2017.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Barthels D and Das H: Current advances in
ischemic stroke research and therapies. Biochim Biophys Acta Mol
Basis Dis. 1866(165260)2020.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Xiang Y, Zhang T, Guo J, Peng YF and Wei
YS: The association of growth differentiation factor-15 gene
polymorphisms with growth differentiation factor-15 serum levels
and risk of ischemic stroke. J Stroke Cerebrovasc Dis.
26:2111–2119. 2017.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Brown DA, Breit SN, Buring J, Fairlie WD,
Bauskin AR, Liu T and Ridker PM: Concentration in plasma of
macrophage inhibitory cytokine-1 and risk of cardiovascular events
in women: A nested case-control study. Lancet. 359:2159–2163.
2002.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Worthmann H, Kempf T, Widera C, Tryc AB,
Goldbecker A, Ma YT, Deb M, Tountopoulou A, Lambrecht J, Heeren M,
et al: Growth differentiation factor 15 plasma levels and outcome
after ischemic stroke. Cerebrovasc Dis. 32:72–78. 2011.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Yin J, Zhu Z, Guo D, Wang A, Zeng N, Zheng
X, Peng Y, Zhong C, Wang G, Zhou Y, et al: Increased growth
differentiation factor 15 is associated with unfavorable clinical
outcomes of acute ischemic stroke. Clin Chem. 65:569–578.
2019.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Groschel K, Schnaudigel S, Edelmann F,
Niehaus CF, Weber-Krüger M, Haase B, Lahno R, Seegers J, Wasser K,
Wohlfahrt J, et al: Growth-differentiation factor-15 and functional
outcome after acute ischemic stroke. J Neurol. 259:1574–1579.
2012.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Dong X and Nao J: Association of serum
growth differentiation factor 15 level with acute ischemic stroke
in a Chinese population. Int J Neurosci. 129:1247–1255.
2019.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Wang X, Zhu L, Wu Y, Sun K, Su M, Yu L,
Chen J, Li W, Yang J, Yuan Z and Hui R: Plasma growth
differentiation factor 15 predicts first-ever stroke in
hypertensive patients. Medicine (Baltimore).
95(e4342)2016.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Schindowski K, von Bohlen und Halbach O,
Strelau J, Ridder DA, Herrmann O, Schober A, Schwaninger M and
Unsicker K: Regulation of GDF-15, a distant TGF-β superfamily
member, in a mouse model of cerebral ischemia. Cell Tissue Res.
343:399–409. 2011.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Dickson DW: Neuropathology of Parkinson
disease. Parkinsonism Relat Disord. 46 (Suppl 1):S30–S33.
2018.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Obeso JA, Rodriguez-Oroz MC, Goetz CG,
Marin C, Kordower JH, Rodriguez M, Hirsch EC, Farrer M, Schapira AH
and Halliday G: Missing pieces in the Parkinson's disease puzzle.
Nat Med. 16:653–661. 2010.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Maetzler W, Deleersnijder W, Hanssens V,
Bernard A, Brockmann K, Marquetand J, Wurster I, Rattay TW,
Roncoroni L, Schaeffer E, et al: GDF15/MIC1 and MMP9 cerebrospinal
fluid levels in Parkinson's disease and lewy body dementia. PLoS
One. 11(e0149349)2016.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Movement Disorder Society Task Force on
Rating Scales for Parkinson's Disease. The unified Parkinson's
disease rating scale (UPDRS): Status and recommendations. Mov
Disord. 18:738–750. 2003.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Yao X, Wang D, Zhang L, Wang L, Zhao Z,
Chen S, Wang X, Yue T and Liu Y: Serum growth differentiation
factor 15 in Parkinson disease. Neurodegener Dis. 17:251–260.
2017.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Luthman J, Fredriksson A, Sundstrom E,
Jonsson G and Archer T: Selective lesion of central dopamine or
noradrenaline neuron systems in the neonatal rat: Motor behavior
and monoamine alterations at adult stage. Behav Brain Res.
33:267–277. 1989.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Machado V, Haas SJ, von Bohlen Und Halbach
O, Wree A, Krieglstein K, Unsicker K and Spittau B:
Growth/differentiation factor-15 deficiency compromises
dopaminergic neuron survival and microglial response in the
6-hydroxydopamine mouse model of Parkinson's disease. Neurobiol
Dis. 88:1–15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Machado V, Gilsbach R, Das R, Schober A,
Bogatyreva L, Hauschke D, Krieglstein K, Unsicker K and Spittau B:
Gdf-15 deficiency does not alter vulnerability of nigrostriatal
dopaminergic system in MPTP-intoxicated mice. Cell Tissue Res.
365:209–223. 2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Hirsch E, Graybiel AM and Agid YA:
Melanized dopaminergic neurons are differentially susceptible to
degeneration in Parkinson's disease. Nature. 334:345–348.
1988.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Liu H, Liu J, Si L, Guo C, Liu W and Liu
Y: GDF-15 promotes mitochondrial function and proliferation in
neuronal HT22 cells. J Cell Biochem. 120:10530–10547.
2019.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Miyaue N, Yabe H and Nagai M: Serum growth
differentiation factor 15, but not lactate, is elevated in patients
with Parkinson's disease. J Neurol Sci. 409(116616)2020.PubMed/NCBI View Article : Google Scholar
|