Introduction
Vasculitis is the inflammation of the blood vessel
wall secondary to an abnormal immune response (1,2).
Systemic vasculitis determines sufferance and fibrinoid necrosis of
the endothelial cells, events followed by vascular leakage and
blood clot formation with secondary occlusion (1,3).
Eventually, all these vascular events determine retinal ischemia
and dysfunction of the organ (3).
Retinal vasculitis can be a complication of a local
condition or it can be a retinal expression of a systemic
inflammatory disorder, which may initially be unnoticed (3-5).
The causes of retinal vasculitis are multiple and often overlap,
making both the diagnosis and the treatment options challenging
(5). Retinal vasculitis is
frequently associated with inflammation of the adjacent tissues,
such as the choroid or the vitreous, but sometimes, remote ocular
structures also appear to be caught in the inflammatory process
(6-8).
Retinal vasculitis can be associated with infectious
and non-infectious conditions (3-5).
The non-infectious causes of vasculitis include ocular disorders
and can be drug-induced, i.e., vasculitis associated with a
systemic inflammatory disease and vasculitis associated with
malignancies (9-11).
Drug-associated vasculitis is frequently difficult to identify,
because many patients follow treatments with more than one drug and
the route of administration varies (12-15).
In addition, patients may forget, neglect or hide drug intake. For
instance, an intrauterine device (IUD) is considered a medical
device. In terms of prevention of an undesired pregnancy, IUDs are
usually considered safe with rare side effects, some of which can
be severe (16). Currently, there
are two types of IUDs available: The copper IUD, which releases
copper ions, and the hormonal IUD, which releases a synthetic form
of the progesterone hormone, named levonorgestrel (17). The most frequently cited side
effects of hormonal IUDs comprise gynecological disorders,
headaches, blood-clotting issues, developing acne and breast
tenderness, which lead to a higher rate of treatment
discontinuation, more than 24% after 1 year and 33% after 2 years
(5,17-19).
Currently, no previous reports of ocular vasculitis associated with
the use of an intrauterine device are available in the
literature.
Case report
A 35-year-old female patient sought emergency care,
complaining of sudden hearing loss, headaches and blurred vision
that had started two weeks previously and had gotten progressively
worse. The headaches were continuous and located in the occipital
area, with episodes of increased pain, accompanied by a decrease in
hearing and sometimes dizziness. During the previous two weeks
before presentation, the neurological exams had not identified any
neurological signs. However, non-steroidal anti-inflammatory drugs
were prescribed, but without improvement. Furthermore, the patient
was examined by an ear, nose and throat (E.N.T.) doctor, but there
were no clinical signs to explain the hearing loss.
Based on the patient's medical history, it was
identified that she had a multinodular non-toxic thyroid goiter, no
drug allergies, she was a non-smoker and had two natural child
births. From the Emergency Room of the University Emergency
Hospital Bucharest, the patient was admitted into the neurology
department, but still without any detectable clinical neurological
signs. The dilated fundus examination revealed bilateral optic disc
swelling. An orbito-cerebral magnetic resonance imaging (MRI) and a
computed tomography (CT) scan were performed, but with no
significant findings. In addition, the blood pressure was normal.
Therefore, the patient was discharged from the neurological
department and referred to the ophthalmologist.
The ophthalmological assessment revealed a slight
decrease in the best corrected visual acuity of 20/30 in the right
eye and of 20/25 in the left eye. Slit-lamp examination showed a 1+
faint grade of flare in the anterior chamber, accompanied by a few
small corneal endothelium precipitates. Color vision, ocular
motility and pupillary light reflex were within normal limits.
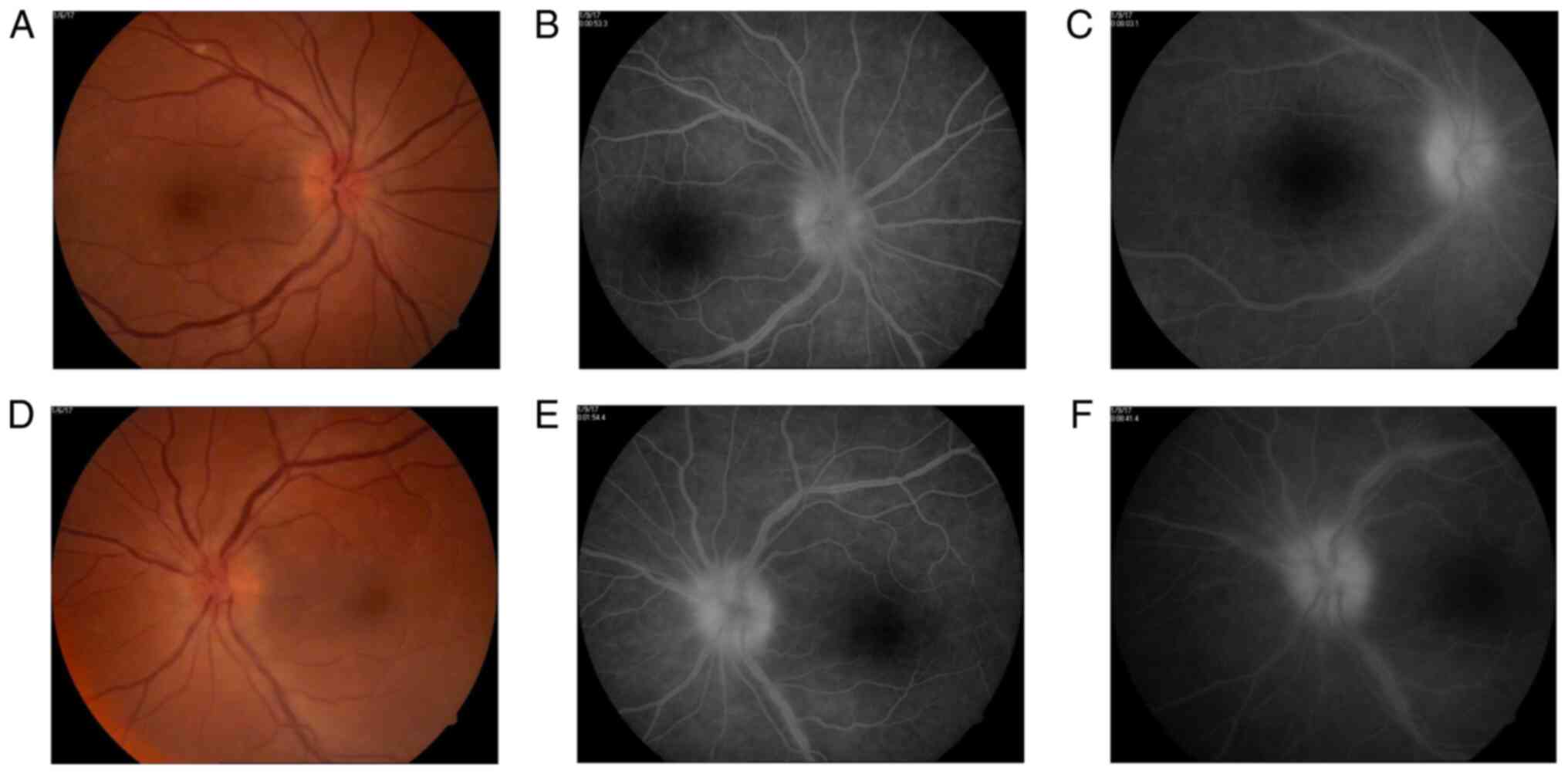
Fundus examination reveals bilateral optic disc
swelling, hyperemia, loss of the optic cup, optic disc vessels
drowned in edema, retinal edema around the optic disc, normal
aspect of arteries, but sinuous and dilated veins. The veins also
presented a larger diameter than expected and some venous branches
were more dilated than others. Their diameter did not constantly
decrease towards the periphery, with some medium-sized venous
branches greater than the large-sized venous branches (Fig. 1).
Therefore, a fundus fluorescein angiography was
carried out, with a delay of 2-3 sec in the appearance of the
laminar venous flow and an unequal filling with dye of some
temporal medium-sized venous branches. The late phase shows optic
disc hyperfluorescence due to venous and capillary leakage.
Analyzing the venous branches, segmental diffuse hyperfluorescence
in the early phases and staining in the later phases of the
angiogram were evident (Fig. 1). No
additional retinal or choroidal fluorescein lesions were
identified.
In this context, the patient's diagnosis was
bilateral retinal vasculitis, papilledema and anterior uveitis,
headache and hypoacusis. In order to identify the cause of the
retinal vasculitis, various blood tests were performed based on the
medical history, symptomatology and clinical assessment, trying to
avoid unnecessary and exhaustive investigations. The purpose was to
identify an immune or infectious cause of the retinal vasculitis.
All serological tests for syphilis, tuberculosis, HSV-1, HSV-2,
HZV, CMV, HIV, and ELISA for toxoplasmosis were negative. In
addition, IgM and IgG anti-β2- glycoprotein antibodies, IgM and IgG
anti-cardiolipin antibodies, lupus anticoagulant, homocysteine
level, antibody anti-DNA double catenary, C-ANCA, P-ANCA,
anti-Ro/SSA and anti-La/SSB antibodies, ANA, and rheumatoid factor
were negative (Table I). Full blood
count, biochemical blood profile and cerebrospinal fluid analysis
were non-contributory.
 | Table ISpecific blood tests performed. |
Table I
Specific blood tests performed.
| Immunology | Value | Normal range | Observation |
|---|
| Anti-β2 glycoprotein
antibodies (IgM) (U/ml) | 3.3 | <5 | Negative |
| Anti-β2 glycoprotein
antibodies (IgG) (U/ml) | 1.3 | <5 | Negative |
| Anti-cardiolipin Ab
(IgG) (U GPL/ml) | <2 | <20 | Negative |
| Anti-cardiolipin Ab
(IgM) (U MPL/ml) | <2 | <20 | Negative |
| Lupus anticoagulant
(ratio) | 0.98 | <1.2 | Negative |
| Homocysteine level
µmol/l | 10.1 | <10 | Risk for
cardio-vascular disease, mild |
| Anti-DNA double
catenary antibodies (U/ml) | 6.6 | <25 | Normal |
| Anti C-ANCA
antibodies (U/ml) | 1.1 | <5 | Negative |
| Anti P-ANCA
antibodies (U/ml) | <2 | <13 | Negative |
| Anti-Ro/SSA
antibodies (U/ml) | 2.6 | <15 | Negative |
| Anti-La/SSB
antibodies (U/ml) | 3.9 | <15 | Negative |
| ANA | <1:100 | Titer 0<1:100 | Negative |
| Anti-TPO antibodies
(U/ml) | 1.44 | <5.61 | Normal |
| Anti-thyroglobulin
antibodies (UI/ml) | 1 | <4.11 | Normal |
| Calcitonin
(pg/l) | 2 | <5 | Normal |
| Triiodothyronine (T3)
(ng/dl) | 0.98 | 0.58-1.59 | Normal |
| Free thyroxine (T4)
(ng/dl) | 0.89 | 0.70-1.48 | Normal |
| Parathormone
(pg/l) | 37.8 | 11-67 | Normal |
| Erythrocyte
sedimentation rate (ESR) mm/h | 13 | <29 | Normal |
| Rheumatoid
factor | | | Negative |
| Hematology | | | |
|
White blood
cells (WBCs) (103/µl) | 7.4 | 3.8-11.8 | Normal |
|
Monocytes
(%) | 12.4 | 4.3-10 | High |
|
Red blood
cells (RBCs) (106/µl) | 4.37 | 3.63-4.92 | Normal |
|
Platelets
(103/µl) | 290 | 179-408 | Normal |
|
Factor V
Leiden | 3.18 | 2.45-3.32 | Normal |
|
Protein C
(%) | 104.42 | 70-140 | Normal |
|
Protein S
(%) | 86.9 | 54.7-123.7 | Normal |
|
Coagulation | | | |
|
International
normalized ratio (INR) | 0.91 | 0.8-1.2 | Normal |
|
Activated
partial thromboplastin time (APTT) (sec) | 24.5 | 22-36 | Normal |
|
Fibrinogen
mg/dl | 302 | 239-498 | Normal |
|
Molecular
Biology | | | |
|
PAI-1
gene mutation | 675 4G homozygote
844A - AA genotype | | Positive (high risk
of thrombophilia in association with Leiden V mutation |
|
MTHFR-gene
mutation | 677 C | | Negative |
| | 1298 A | | Positive |
| Biochemistry | | | |
|
Alkaline
phosphatase (U/l) | 52 | 40-150 | Normal |
|
Total serum
calcium (mg/dl) | 8.76 | 8.4-10. | Normal |
|
Serum
phosphate (mg/dl) | 3.5 | 2.6-4.5 | Normal |
|
Serum
magnesium (mg/dl) | 2. | 1.6-2.8 | Normal |
| | | | |
| Serology | | | |
|
Anti-HIV-1/HIV-2
antibodies | | | Negative |
|
Anti-HCV
antibodies | | | Negative |
|
Tuberculosis
IgG/IgM | | | Negative |
|
Antistreptolysin
O (ASLO) | | | Negative |
|
Venereal
disease research laboratory (VDRL) | | | Non-reactive |
|
Rapid plasma
reagin (RPR) | | | Non-reactive |
|
Hepatitis B
surface antigen (HBsAg) | | | Negative |
|
Toxoplasmosis
(IgM) Index | 0.1 | <0.499 | Non-reactive |
|
Toxoplasmosis
(IgG) (U/ml) | 0.1 | <1.6 | Non-reactive |
|
Cytomegalovirus
(IgM) (S/CO) | 0.05 | <0.85 | Non-reactive |
|
Cytomegalovirus
(IgG) (AU/ml) | 250 | <6 | Reactive |
|
HSV-1 (IgM)
(Uml) | 7.8 | <20 | Negative |
|
HSV-1 (IgG)
Index | 51.1 | <0.9 | Negative |
|
Anti HVS2
(IgM) (Uml) | 8.4 | <20 | Negative |
|
Anti-HVS2
(IgG) Index | <0.5 | <0.9 | Negative |
|
Angiotensin
convertase enzyme (ACE) (U/l) | 17 | 20-70 | Negative |
Not being able to identify the cause of the retinal
vasculitis, the patient's medical history was reviewed, this time
focusing on rheumatological diseases, drug intake or other medical
procedures undergone in the recent period. As a result, it was
identified that two weeks prior to onset of the symptoms and one
month prior to her presentation at the Emergency Room, the patient
underwent a gynecological procedure, in which a 13.5 mg
levonorgestrel intrauterine contraceptive device (Bayer Inc.) was
implanted.
Systemic vasculitis secondary to the IUD was
subsequently considered the final diagnosis. After the IUD was
removed, treatment with methylprednisolone pulse therapy (MPPT) of
500 mg/daily for 5 consecutive days commenced. Throughout the
treatment, the patient was closely monitored for any possible side
effects. After the pulse therapy was completed, the patient was
discharged from the University Emergency Hospital Bucharest,
Ophthalmology Clinic and continued the oral treatment with
methylprednisolone at 0.8 mg/kg/daily with a gradual decrease of
the doses every 3 days at home. During the treatment with
corticosteroids, the patient also received a proton-pump inhibitor
once daily.
One week later, we re-evaluated the patient. All the
previous neurological symptoms disappeared and the hearing loss was
completely recovered. At the anterior pole examination, there was
improvement, the corneal endothelial edema was remitted and the
keratic precipitates were reduced in number and size. The fundus
examination revealed a decrease of the optic disc edema and in the
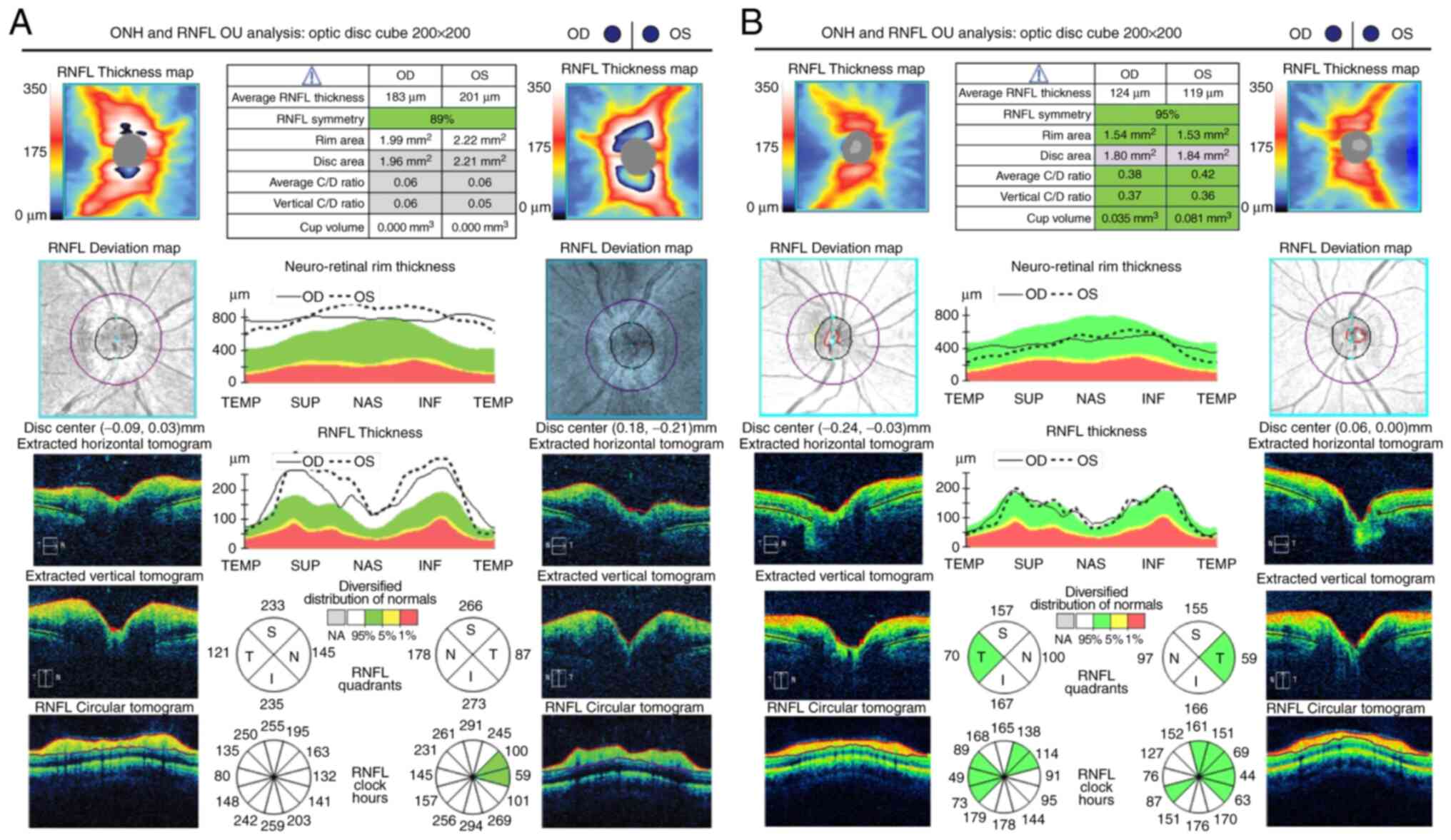
diameter of the retinal veins and their branches (Fig. 2). The optical coherence tomography
(OCT) confirmed the identified clinical aspects (Fig. 3). One month later, fundus
fluorescein angiography presented with a decrease of the optic disc
hyperfluorescence and fewer venous segments of slightly diffuse
hyperfluorescence secondary to decreased vascular leakage (Fig. 2).
Ethics approval was obtained from the University
Emergency Hospital (Bucharest, Romania). The patient provided
written informed consent.
Discussion
First, the predominant optic disc edema accompanied
by very fine ocular signs suggested a neurological condition,
misleading the clinicians. However, a careful examination related
to ancillary tests including fundus fluorescein angiography and OCT
provides the information necessary for a proper diagnosis (4,20).
Rarely, retinal vasculitis may be isolated, and idiopathic without
any other signs. Typical, retinal vasculitis is a manifestation of
a systemic disease or of a retinal inflammatory condition. We
followed up the guides in order to determine the cause of the
disease; however, the tests did not offer the information required.
A very thorough examination of the medical history supplied the
missing piece of information that was necessary to elucidate the
cause. To the best of our knowledge, medical literature has not
previously reported any case of retinal vasculitis related to
Jaydess® 13.5 mg levonorgestrel intrauterine
contraceptive device (21).
However, some studies in literature indicate the occurrence of
vasculitis related to oral contraceptive administration, but with
mild vascular involvement (16,18,22,23).
Mosovich et al published a case of necrotizing vasculitis
caused by the Microgynon pills (levonorgestrel, 0.15 mg and
ethinyl-oestradiol, 0.03 mg) (13).
The diagnosis of generalized vasculitis with retinal
involvement is, similar to other drug-induced vasculitis, based on
the temporal relationship between the drug administration and the
appearance of the clinical signs of disease (24). In addition, the absence of other
causes that could explain the clinical picture, as systemic
autoimmune diseases or infectious diseases (the ancillary test was
negative or non-reactive), the remission of the disease after the
device has been removed and the positive response to the systemic
steroid therapy also indicate a direct relationship between the IUD
and disease.
In addition, we focused on thyroid disorders in
order to exclude a secondary cause, being aware of the possible
relationship with primary or secondary ANCA vasculitis. The test
results excluded other retinal vasculitis associated with systemic
inflammatory diseases, such as systemic lupus erythematosus,
Wegener granulomatosis, microscopic polyangiitis, antiphospholipid
syndrome and ANCA-associated vasculitis (25,26).
As the patient was complaining of hearing
dysfunction, and in the context of a mild anterior uveitis, Cogan
syndrome was suspected (27).
However, in the absence of any cardiac signs, normal blood level of
leukocytes, a slight increase of the erythrocyte sedimentation
rate, a normal C-reactive protein, we excluded this syndrome
(27,28).
Finally, the Naranjo adverse drug reaction (ADR)
probability scale was performed and suggested an outcome to our
clinical evidence (29,30) (Table
II). The last question is slightly contentious, since it was
based on the patient's response. The patient claimed that when she
was previously under treatment with similar drugs, such as oral
contraceptive (similar to levonorgestrel), she presented a low
intensity similar response, but could not provide medical evidence
to support these aspects.
 | Table IIThe Naranjo adverse drug reaction
(ADR) probability scale for the intrauterine contraceptive device
used. |
Table II
The Naranjo adverse drug reaction
(ADR) probability scale for the intrauterine contraceptive device
used.
| Questions | Yes | No | Do not know | Score |
|---|
| 1. Are there
previous conclusive reports on this reaction? | +1 | 0 | 0 | 1 |
| 2. Did the adverse
event appear after the suspected drug was readministered? | +2 | 0 | 0 | 2 |
| 3. Did the adverse
reaction improve when the drug was discontinued or was a specific
antagonist administered? | +1 | 0 | 0 | 1 |
| 4. Did the adverse
reaction reappear when the drug was readministered? | 0 | 0 | 0 | 0 |
| 5. Are there
alternative causes (other than the drug) that, on their own, could
have caused the reaction? | 0 | 2 | 0 | 2 |
| 6. Did the reaction
reappear when a placebo was given? | 0 | 0 | 0 | 0 |
| 7. Was the blood
detected in the blood (or other fluids) in concentrations known to
be toxic? | 0 | 0 | 0 | 0 |
| 8. Was the reaction
more severe when the dose was increased or less severe when the
dose was decreased? | 0 | 0 | 0 | 0 |
| 9. Did the patient
have a similar reaction to the same or similar drugs in any
previous exposure? | +1 | 0 | 0 | 1 |
| 10. Was the adverse
event confirmed by any objective evidence? | +1 | | | |
| Total | | | | 9 |
The Naranjo criteria classifies the probability that
an adverse event is related to a specific drug therapy, based on a
list of weighted questions, which examine factors such as the
temporal association of drug administration and the event,
alternative causes that can explain the event, drug levels,
dose-response relationship and the patient's previous experience
with that drug (29-31).
If ADR score is ≥9, the adverse drug reaction is considered as
definite, if the score is between 5 and 8 it is interpreted as
probable, possible for a score between 1 and 4, and doubtful if the
score is 0(31). The Naranjo
criteria does not take into account drug interactions. Drugs are
evaluated individually for causality, and points are deducted if
another factor may have resulted in the adverse event, thereby
weakening the causal association.
In conclusion, to the best of our knowledge no
previous case of retinal disease and optic disc edema associated
with auditory problems (possible vascular) caused by an
intrauterine device has been reported. A proper examination
correlated with a very thorough medical history could identify rare
diseases and associations, in order to provide adequate medical
care.
Acknowledgements
Professional editing, linguistic and technical
assistance were performed by Irina Radu.
Funding
Funding: No funding was received.
Availability of data and materials
Further information regarding the case is available
from the corresponding author upon reasonable request.
Authors' contributions
AMG had a substantial contribution to the conception
and design of the work, analyzed and interpreted the data being
also the first investigator and coordinator of the article. AMG
also gave the final approval of the version to be published. CA
collected all the data from the patient's medical history and
produced the figures and tables. SI performed ocular coherence
tomography, fundus fluorescein angiography, and verified that the
information and data were accurate. AE performed the Naranjo
adverse drug reaction (ADR) probability scale and revised the
paper. ACG was responsible for literature research of the current
data, drafted the study, and revised it critically for important
intellectual content. AMG and ACG assessed the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the University
Emergency Hospital, 050098 Bucharest, Romania. The patient provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guillevin L and Dorner T: Vasculitis:
Mechanisms involved and clinical manifestations. Arthritis Res
Ther. 9 (Suppl 2)(S9)2007.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Langford CA: Vasculitis. J Allergy Clin
Immunol. 125 (Suppl 2):S216–S225. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Trese MGJ, Yonekawa Y, Thomas BJ and
Randhawa S: Vasculitic central retinal vein occlusion: The
presenting sign of seronegative rheumatoid arthritis. Am J
Ophthalmol Case Rep. 2:26–29. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rosenbaum JT, Sibley CH and Lin P: Retinal
vasculitis. Curr Opin Rheumatol. 28:228–235. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jennette JC: Overview of the 2012 revised
International chapel hill consensus conference nomenclature of
vasculitides. Clin Exp Nephrol. 17:603–606. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Apinyawasisuk S, Rothova A, Kunavisarut P
and Pathanapitoon K: Clinical features and etiology of retinal
vasculitis in Northern Thailand. Indian J Ophthalmol. 61:739–742.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fukunaga H, Kaburaki T, Shirahama S,
Tanaka R, Murata H, Sato T, Takeuchi M, Tozawa H, Urade Y, Katsura
M, et al: Analysis of inflammatory mediators in the vitreous humor
of eyes with pan-uveitis according to aetiological classification.
Sci Rep. 10(2783)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kaburaki T, Fukunaga H, Tanaka R, Nakahara
H, Kawashima H, Shirahama S, Izawa H, Komae K, Takamoto M, Soga H
and Aihara M: Retinal vascular inflammatory and occlusive changes
in infectious and non-infectious uveitis. Jpn J Ophthalmol.
64:150–159. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Agarwal A, Karkhur S, Aggarwal K,
Invernizzi A, Singh R, Dogra MR, Gupta V, Gupta A, Do DV and Nguyen
QD: Epidemiology and clinical features of inflammatory retinal
vascular occlusions: Pooled data from two tertiary-referral
institutions. Clin Exp Ophthalmol. 46:62–74. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Grau RG: Drug-induced vasculitis: New
insights and a changing lineup of suspects. Curr Rheumatol Rep.
17(71)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Iwahashi C, Ono H, Haruta M, Minami T,
Mashimo H, Shimojo H and Ohguro N: New onset or exacerbation of
uveitis with infliximab: Paradoxical effects? BMJ Open Ophthalmol.
4(e000250)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Agarwal A, Pilania RK, Anjani G, Choudhary
H, Gupta A and Gupta V: Retinal vasculitis with coats-like response
in a young girl with Parry-Romberg syndrome. J Clin Rheumatol: May
21, 2019 (Epub ahead of print).
|
|
13
|
Mosovich B, Biton A and Avinoach I:
Vasculitis with cutaneous necrosis induced by oral contraceptive.
Harefuah. 120:451–453. 1991.PubMed/NCBI(In Hebrew).
|
|
14
|
Trusau A and Brit ML:
Propylthiouracil-induced ANCA-negative cutaneous small vessel
vasculitis. J Community Hosp Intern Med Perspect. 8:35–37.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wiik A: Drug-induced vasculitis. Curr Opin
Rheumatol. 20:35–39. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kailasam C and Cahill D: Review of the
safety, efficacy and patient acceptability of the
levonorgestrel-releasing intrauterine system. Patient Prefer
Adherence. 2:293–302. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Andersson K, Odlind V and Rybo G:
Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during
five years of use: A randomized comparative trial. Contraception.
49:56–72. 1994.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sturridge F and Guillebaud J: A
risk-benefit assessment of the levonorgestrel-releasing
intrauterine system. Drug Saf. 15:430–440. 1996.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vrettakos C and Bajaj T: Levonorgestrel.
In: StatPearls [Internet] Treasure Island (FL): StatPearls
Publishing; 2021.
|
|
20
|
Abu El-Asrar AM, Herbort CP and Tabbara
KF: Differential diagnosis of retinal vasculitis. Middle East Afr J
Ophthalmol. 16:202–218. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wildemeersch D, Andrade A, Goldstuck ND,
Hasskamp T and Jackers G: Intrauterine levonorgestrel delivery with
frameless fibrous delivery system: Review of clinical experience.
Int J Womens Health. 9:49–58. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fekete GL and Fekete L: Cutaneous
leukocytoclastic vasculitis associated with erlotinib treatment: A
case report and review of the literature. Exp Ther Med.
17:1128–1131. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mansour D: The benefits and risks of using
a levonorgestrel-releasing intrauterine system for contraception.
Contraception. 85:224–234. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Radic M, Martinović Kaliterna D and Radic
J: Drug-induced vasculitis: A clinical and pathological review.
Neth J Med. 70:12–17. 2012.PubMed/NCBI
|
|
25
|
Papaliodis GN: Ophthalmologic
manifestations of systemic vasculitis. Curr Opin Ophthalmol.
28:613–616. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tugal-Tutkun I: Systemic vasculitis and
the eye. Curr Opin Rheumatol. 29:24–32. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Iliescu DA, Timaru CM, Batras M, De Simone
A and Stefan C: Cogan's syndrome. Rom J Ophthalmol. 59:6–13.
2015.PubMed/NCBI
|
|
28
|
Vollertsen RS: Vasculitis and Cogan's
syndrome. Rheum Dis Clin North Am. 16:433–439. 1990.PubMed/NCBI
|
|
29
|
Behera SK, Das S, Xavier AS, Velupula S
and Sandhiya S: Comparison of different methods for causality
assessment of adverse drug reactions. Int J Clin Pharm. 40:903–910.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Murayama H, Sakuma M, Takahashi Y and
Morimoto T: Improving the assessment of adverse drug reactions
using the Naranjo Algorithm in daily practice: The Japan adverse
drug events study. Pharmacol Res Perspect. 6(e00373)2018.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Naranjo CA, Busto U, Sellers EM, Sandor P,
Ruiz I, Roberts EA, Janecek E, Domecq C and Greenblatt DJ: A method
for estimating the probability of adverse drug reactions. Clin
Pharmacol Ther. 30:239–245. 1981.PubMed/NCBI View Article : Google Scholar
|