Introduction
Following surgery, 8-34% of patients worldwide
experience chronic postsurgical pain (CPSP), which leads to a
decline in the quality of postoperative daily life (1,2). In
the clinic, the therapeutic management for CPSP is limited. In
persistent postsurgical pain, there is a series of complicated
alterations ranging from nociceptive stimulation to the occurrence
and development of postsurgical pain (3,4). It is
generally considered that peripheral sensitization is the starting
point of CPSP (5,6). Therefore, there is a requirement to
discover targets against peripheral sensitization leading to CPSP.
Caveolae, which constitute specific forms of lipid rafts, are small
invaginations of the plasma membrane that exist in numerous
mammalian cells (7,8). Previous studies have reported that
caveolae function as organizing centers for signaling molecules
(9,10). Caveolins have cytoplasmic N and C
termini, palmitoylation sites and a scaffolding domain that
facilitates interaction and organization of signaling molecules so
as to help provide coordinated and efficient signal transduction.
Such signaling components include upstream entities (such as G
protein-coupled receptors, receptor tyrosine kinases and steroid
hormone receptors) and downstream components (such as
heterotrimeric and low-molecular weight G proteins, effector
enzymes and ion channels) (11).
Caveolin-1 (Cav-1) is the principal structural and signaling
component of caveolae, which is expressed mainly in inflammatory
cells (12,13). Recent studies have revealed that
Cav-1 plays an important role in pain modulation. For example, in
mouse anterior cingulate cortex neurons, Cav-1 contributes to pain
modulation by directly binding with N-methyl D-aspartate receptor
subtype 2B, the promotion of which leads to central sensitization
(14,15). In diabetic neuropathic pain (DNP)
rats, Cav-1 in the spinal cord dorsal horn contributes to the
development of DNP through the upregulation of toll-like receptor 4
expression in the spinal cord (16). However, the role of Cav-1 in
peripheral sensitization is not clear. It is well known that tissue
injury results in endothelial hyperpermeability and increased
exudation, which can lead to increased levels of local inflammatory
agents that cause sensitization and increased excitation of the
nociceptors, thereby causing pain (17,18).
Previous studies have demonstrated that there is a positive
correlation between Cav-1 overexpression and extravascular albumin,
which suggests that upregulation of Cav-1 may be associated with
endothelial hyperpermeability (19-21).
In the present study, the skin/muscle incision and
retraction (SMIR) model (22) was
established to observe the expression and localization of Cav-1 in
the tissue around the incision and the dorsal root ganglion (DRG)
and examine the effect of Cav-1-induced acute postsurgical pain on
chronic pain.
Materials and methods
Animals
A total of 84 male Sprague-Dawley rats (weight,
200-250 g; age, 8-10 weeks) were provided with food and water ad
libitum. The ratio of light to darkness was l2:12 h, with the
temperature at 23±1˚C and a humidity of 55-60%. The rats were
provided by The Experimental Animal Center of Nantong University,
and the study procedures were approved by the Experimental Animal
Protection and Care Committee of Nantong University (approval no.
20171015S1051122; Nantong, China).
SMIR model and injection of drugs
The rats were randomly divided into five groups. In
the naive group (12 rats in total), no treatment was performed, six
rats were sacrificed and sampled 7 days later. In the sham group
(12 rats in total), the rats received an incision through the skin
and muscle. In the SMIR group (36 rats in total), after
anesthetization under isoflurane induction with 3-4% induction and
1-2% maintenance and fixed in the supine position, the rats
underwent retraction for 1 h following the skin/muscle incision. A
total of 30 rats in the SMIR group were sacrificed and sampled 1,
3, 7, 14 and 28 days after SMIR modeling with six rats sacrificed
at each time point. In the SMIR + Cav-1 small interfering (si)RNA
group (12 rats in total), Cav-1 siRNA (Guangzhou RiboBio Co., Ltd.)
intrathecal injections were performed at 1, 3 and 7 days after SMIR
modeling. For the intrathecal injections, the animals were
anesthetized via inhalation of 2% isoflurane. A spinal cord
puncture was made using a 30-gauge needle between the L4 and L5
level, and 10 nmol Cav-1 siRNA was diluted into sterile water
containing 5% dextrose (40 µl) to be delivered to the cerebral
spinal fluid. Immediately after the needle entry into the
subarachnoid space, a brisk tail flick could be observed. In the
SMIR + Negative control group (12 rats in total), the control siRNA
intrathecal injections were performed at 1, 3 and 7 days after SMIR
modeling. Intrathecal injections were performed as aforementioned.
Cav-1 siRNA and control siRNA were purchased from Santa Cruz
Biotechnology, Inc. The sequence of Cav-1 siRNA was sense,
5'-AACCAGAAGGGACACACAG-3', and antisense,
5'-CUGUGUGUCCCUUCUGGUU-3'. The control siRNA was a non-targeting
siRNA, the sequence was sense, 5'-GAGAAGCAGUGAUUACGACG-3', and
antisense, 5'-CGUCGUAUCACUGCUUCUC-3'. In the Sham, SMIR + Cav-1
siRNA and SMIR + negative control groups, six rats in each group
were sacrificed and sampled 7 days after surgery. SMIR surgery did
not cause death or any signs of illness, such as hair loss,
diarrhea or weight loss. The rats were sacrificed in a
CO2 chamber and the injured tissue around postoperative
incision and DRG were collected for subsequent experiments and
stored in a -80˚C freezer for further use.
Behavioral testing
The absence of heat hyperalgesia has been previously
reported with animal models of incisional pain (23). Therefore, the mechanical withdrawal
threshold (MWT) was detected prior to and at 1, 3, 7, 14 and 28
days following SMIR surgery in the present study. The rats were
habituated to the testing environment for at least 30 min before
testing. Mechanical allodynia was assessed using the up-down
paradigm with von Frey filaments (IITC Life Science, Inc.) ranging
between 1.4-26 g. Shrinking, swinging or paw licking were regarded
as positive reactions. Each filament was presented five times
within 30 sec to determine the response threshold. If the response
was not elicited at least twice, the next ascending von Frey
filament was applied until at least two responses were
observed.
Immunofluorescence staining
On day 7 after SMIR, the rats were anesthetized with
isoflurane (induction with 3-4%; maintenance with 1-2%) and were
transcardially perfused with PBS followed by 4% paraformaldehyde in
PBS (250 ml; pH 7.0). After perfusion, the injured tissues around
the postoperative incision and DRG tissues from the rats in the
SMIR group were extracted and post-fixed in the 4% paraformaldehyde
at 4˚C overnight, and then placed in 20% and subsequently in 30%
sucrose solution at 4˚C overnight. After embedding with OCT, the
tissues were consecutively sectioned at a 6-µm thickness and stored
at -20˚C. These sections were selected randomly and blocked with 5%
serum antibody blocking solution (Beyotime Institute of
Biotechnology) for 2 h at room temperature. The tissue slices were
then incubated with antibodies against Cav-1 (1:50; cat. no.
sc-53564; Santa Cruz Biotechnology, Inc.), CD34 (1:100; cat. no.
ab81289; Abcam), CD68 (1:50; cat. no. ab125212; Abcam), calcitonin
gene-related peptide (CGRP; 1:800; cat. no. 14959; Cell Signaling
Technology, Inc.) and neurofilament 200 (NF200; 1:2,000; cat. no.
N5389; Sigma-Aldrich; Merck KGaA) at 4˚C overnight, then
co-incubated with Cy3-conjugated goat anti-rabbit (1:1,000; cat.
no. 111-165-003; Jackson ImmunoResearch Laboratories, Inc.),
FITC-conjugated goat anti-mouse secondary antibodies (1:1,000; cat.
no. 115-095-205; Jackson ImmunoResearch Laboratories, Inc.) or
FITC-conjugated isolectin B4 (IB4; 1:1,000; cat. no. PR-02;
Advanced Targeting Systems, Inc.) in the dark for 2 h at room
temperature. Five sections were randomly selected from the injured
tissue around the incision and the DRG of each rat. The
localizations of Cav-1 in the injured tissue around the incision
and the DRG were examined under a fluorescence microscope (Olympus
Corporation; magnification, x200) in the dark to capture images,
and ImageJ (National Institutes of Health) was used to quantitate
fluorescence intensity.
Detection of local tissue vascular
permeability
Rats were anesthetized as previously described.
Subsequently, 1 ml/kg of 2% Evans blue solution was slowly injected
into the left femoral vein. If the skin of the toes and ears turned
blue, the injection was successful. After 60 min, the rats' chests
were opened, and 100-200 ml of normal saline was perfused from the
ascending aorta of the left ventricle, until a clear fluid flowed
from auricula dextra. The same portion of the injured tissue around
postoperative incision was collected and placed in an Eppendorf
tube for use. The 2% Evans blue solution was diluted 100-fold, and
a concentration of 20 ng/µl of the liquid was serially diluted into
10, 5, 2.5, 1.25, 0.625, 0.313 and 0.156 ng/µl, using formamide as
blank, in order to make an Evans blue standard curve. The tissue
was placed in a 2% carboxamide bath at a ratio of 100 mg:1 ml,
homogenized, incubated at 37˚C for 48 h and then centrifuged at
high speed (13,527.8 x g) at 4˚C for 15 min, the supernatant of
which was carefully aspirated and divided into three sample tubes
(50 µl/tube). The absorbance was measured at 620 nm using a
microplate reader (BioTek Instruments, Inc.). According to the
standard curve, the Evans blue content of the sample was
calculated, and the mean value was recorded to detect the leakage
outside the blood vessel. The unit was ng Evans blue/mg tissue.
Western blotting
The rats were anesthetized and sacrificed as
previously described, and the injured tissue around the incision
and the DRG was homogenized in sodium dodecyl sulfate (SDS; cat.
no. 71736; Sigma-Aldrich; Merck KGaA) sample buffer containing a
mixture of protease and phosphatase inhibitors (Sigma-Aldrich;
Merck KGaA), and measured with a BCA protein assay kit (Beyotime
Institute of Biotechnology). For separation, 30 µg total protein
per gel lane was loaded onto 10% gels (Beyotime Institute of
Biotechnology). The separated proteins were then transferred onto
nitrocellulose membranes. The membranes were incubated for 2 h at
room temperature in TBS + 0.1% Tween-20 (TBST) blocking solution
containing 5% skimmed milk, followed by overnight incubation at 4˚C
in blocking solution containing primary antibodies against Cav-1
(1:50; cat. no. sc-53564; Santa Cruz Biotechnology, Inc.) and GAPDH
(1:5,000; cat. no. SAB2108668; Sigma-Aldrich; Merck KGaA).
Membranes were washed three times with TBST (10 min/wash) and
incubated with goat anti-mouse (cat. no. 115-035-003) and
anti-rabbit (cat. no. 111-005-003) HRP-conjugated secondary
antibodies (both 1:2,000; both from Jackson ImmunoResearch
Laboratories, Inc.) at room temperature for 2 h. Following washing
with TBST, immunolabeling was detected using the Tanon 2500 gel
imaging system (Tanon Science and Technology Co., Ltd.) and
hypersensitive ECL chemiluminescence detection kit (Absin
Bioscience, Inc.). ImageJ software (v1.8.0; National Institutes of
Health) was used to capture images and analyze the intensity of the
bands.
Reverse transcription-quantitative
(RT-q)PCR
Cav-1 mRNA expression levels in the injured tissue
around postoperative incision and the DRG were determined via
RT-qPCR. Total RNA was extracted from tissue using
TRIzol® reagent (cat. no. 15596-026; Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. cDNA was synthesized using a RevertAid RT Reverse
Transcription kit (cat. no. K1691; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. RT-PCR was performed
on cDNA using LightCycler 96 Real-Time PCR System with
UltraSYBR® Mixture (with ROX) (CoWin Biosciences). Each
sample was configured to a 25 µl reaction volume, and GAPDH was
used as reference gene. Primer sequences were as follows: Cav-1
Forward, 5'-ACCTCAACGATGACGTGGTCAAGA-3', and reverse,
5'-TGGAATAGACACGGCTGATGCACT-3'; GAPDH forward,
5'-GAAGATGGTGATGGGATTTC-3', and reverse, 5'-GAAGGTGAAGGTCGGAGTC-3'.
Reaction conditions were as follows: Pre-denaturation at 95˚C for
10 min, followed by 45 cycles of denaturation at 95˚C for 30 sec,
annealing at 52˚C for 40 sec and extension at 72˚C for 40 sec. To
ensure primer specificity, a melting curve was set after the 45
amplification cycles (95˚C for 5 sec, 65˚C for 60 sec and 97˚C for
1 sec). Results were presented as the level of mRNA relative to
endogenous and calculated using the 2-ΔΔCq method
(24).
ELISA
Concentrations of IL-6 (PicoKine ELISA kit; cat. no.
EK0411) and TNF-α (PicoKine ELISA kit; cat. no. EK0527) were
measured using respective specific ELISA kits in accordance with
the manufacturer's instructions (all supplied from Boster
Biological Technology).
Data and statistical analyses
Experimental data were analyzed using SPSS 20.0
software (IBM Corp.). Measured data were expressed as the mean ±
SEM, and behavioral data were analyzed by a mixed two-way ANOVA
followed by the Bonferroni test for multiple comparison analysis.
One-way ANOVA followed by the Bonferroni test was used for
comparison between the groups except for the behavioral data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
SMIR induces persistent mechanical
allodynia and continuous increase of vascular endothelial
permeability around the incision
To study the mechanism of postoperative chronic
pain, a SMIR model was established. Significant levels of
mechanical allodynia appeared on day 1 in SMIR rats and persisted
until day 14 after SMIR. MWT in SMIR rats returned to similar
levels as in the sham and naive rats by postsurgical day 28
(Fig. 1A).
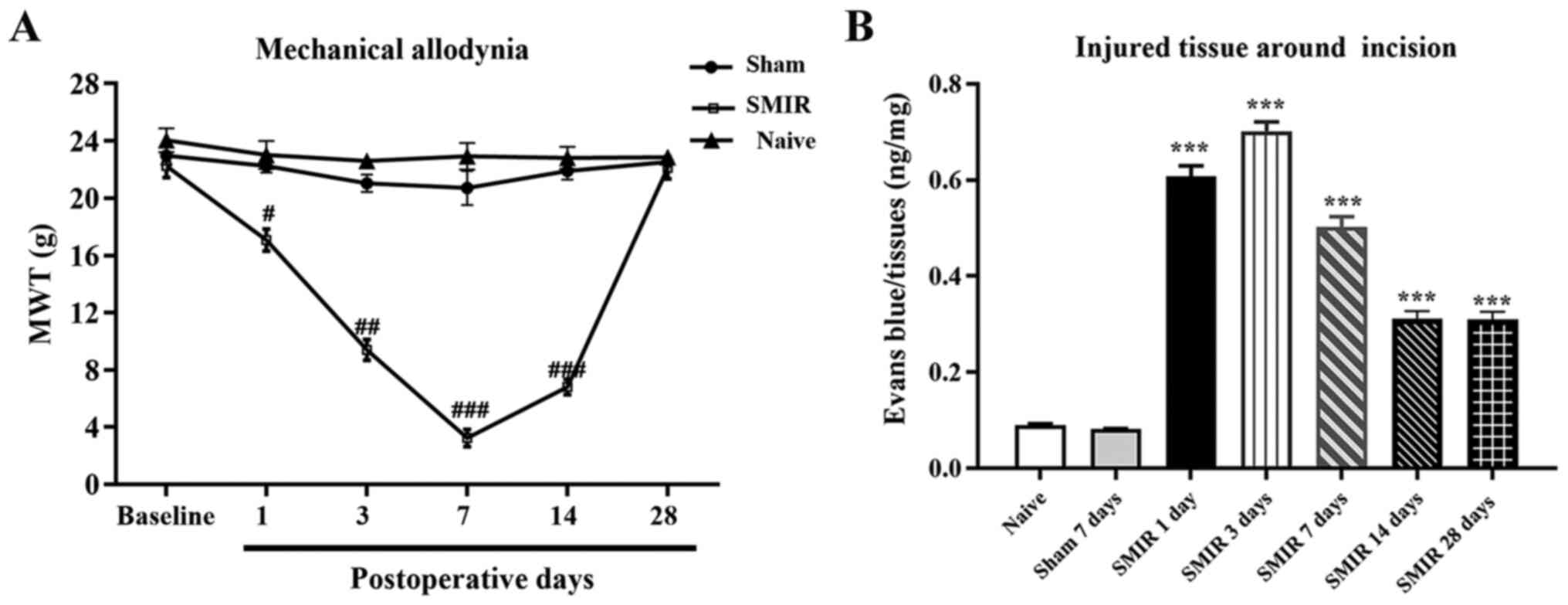 | Figure 1Detection of MWT after SMIR and the
vascular endothelial permeability around the incision. (A) MWT of
the SMIR group was significantly reduced in a time-dependent manner
on days 1, 3, 7 and 14 after surgery increased after postsurgical
day 14, whereas no statistical difference was observed in the sham
group compared with the naive group. (B) Intravascular Evans blue
extravasation of the SMIR group was significantly increased on days
1, 3, 7, 14 and 28 after surgery. n=6. #P<0.05,
##P<0.01 and ###P<0.001 vs. sham group;
***P<0.001 vs. naive and sham groups. SMIR,
skin/muscle incision and retraction; MWT, mechanical withdrawal
threshold; d, day. |
The tissues around the incision were collected on
the 1st, 3rd, 7th, 14th and 28th day after SMIR to detect vascular
endothelial permeability using Evans blue staining. It was revealed
that surgical wounds significantly induced maintenance of
endothelial hyperpermeability in the SMIR group from day 1 after
surgery, while compared with the naïve group there was no
significant change in the vascular endothelial permeability of the
sham group (Figs. 1B and S1; Table
SI).
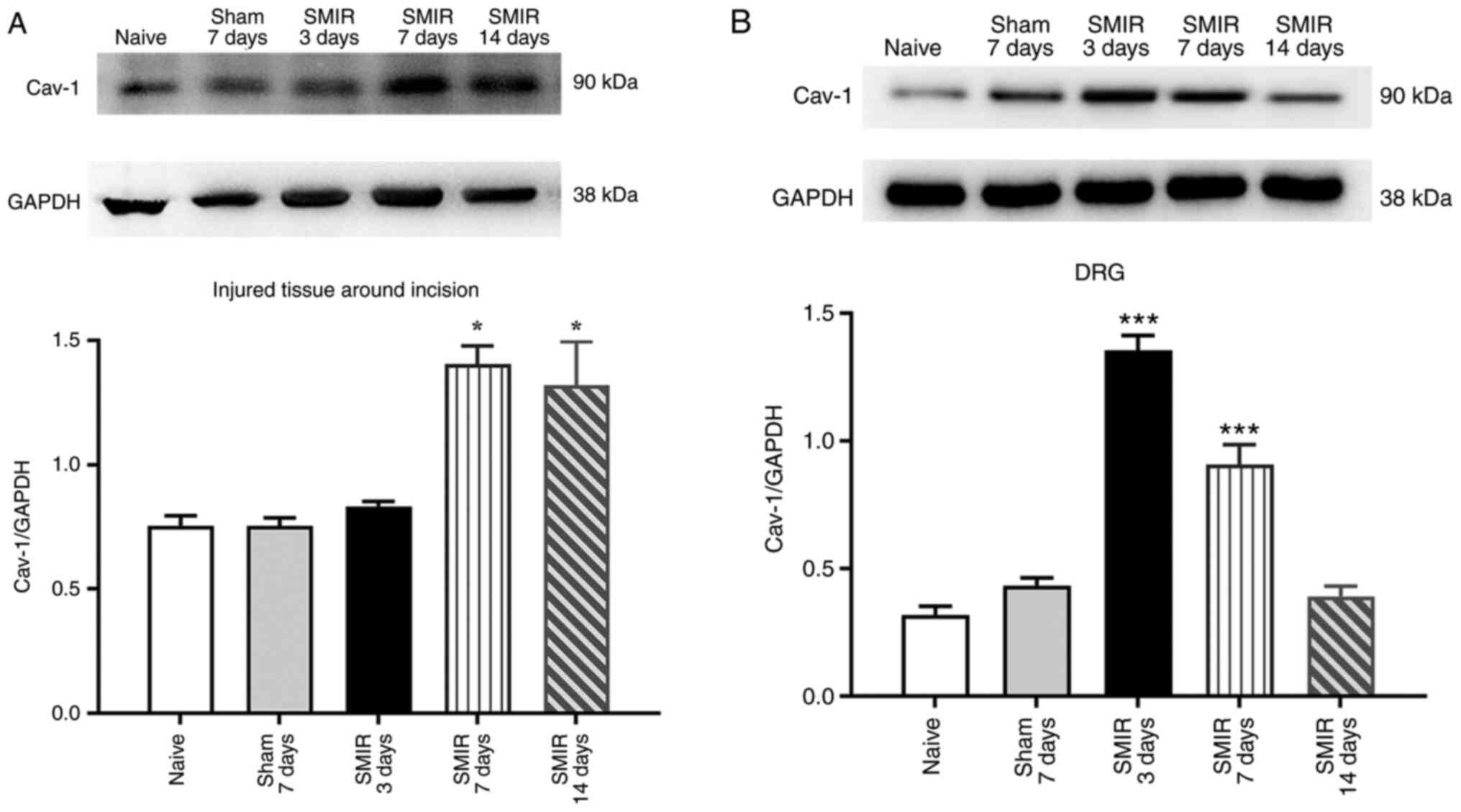
Effects of CPSP on the expression of
Cav-1 in the injured tissue around postoperative incision and the
DRG
To explore the role of Cav-1 in CPSP and its
possible mechanism, changes in the expression of Cav-1 in the
injured tissue around postoperative incision and the DRG were
examined on postsurgical days 3, 7 and 14 in the SMIR and sham
groups. Western blotting demonstrated that compared with the naive
and sham groups, the expression of Cav-1 was significantly
increased on day 7 and 14 following SMIR in the injured tissue
around postoperative incision, while there was no significant
change on postsurgical day 3 (Fig.
2A). The expression of Cav-1 was significantly increased on
postsurgical day 3 and 7 in the DRG, while there was no significant
change on postsurgical day 14 (Fig.
2B). In addition, compared with the naïve group, there was no
significant change in the expression of Cav-1 in the injured tissue
around postoperative incision and the DRG in the sham group
(Fig. 2A and B).
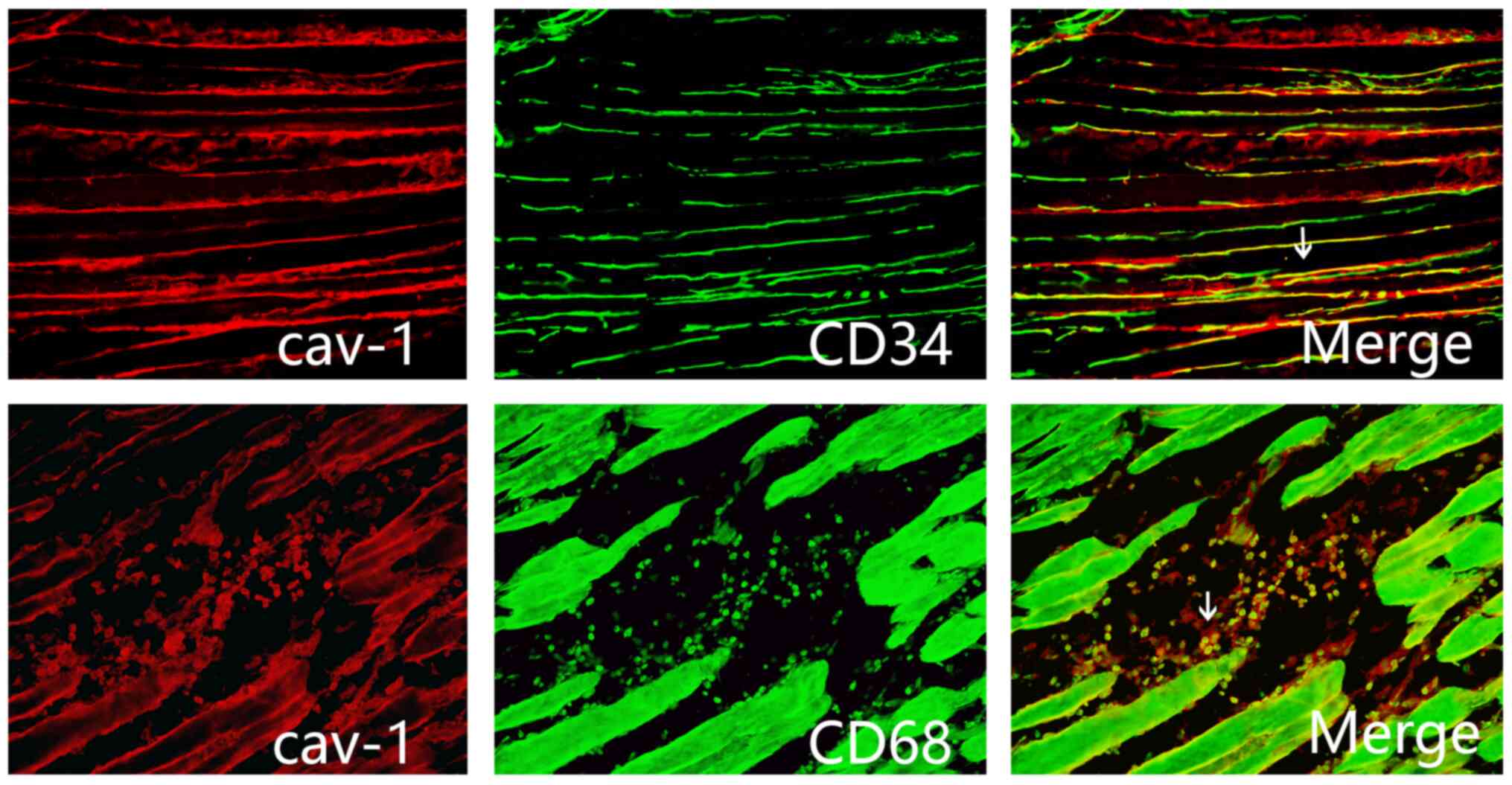
Immunofluorescence staining
demonstrates localization of Cav-1 in the injured tissue around
postoperative incision
To study the localization of Cav-1, the tissue
sections around the postoperative incision of rats in the SMIR
group at postsurgical day 7 were stained with the macrophage marker
CD68 and the endothelial cell marker CD34. Immunofluorescence
staining indicated that Cav-1 was expressed by macrophages and
endothelial cells in the injured tissue around postoperative
incision (Fig. 3).
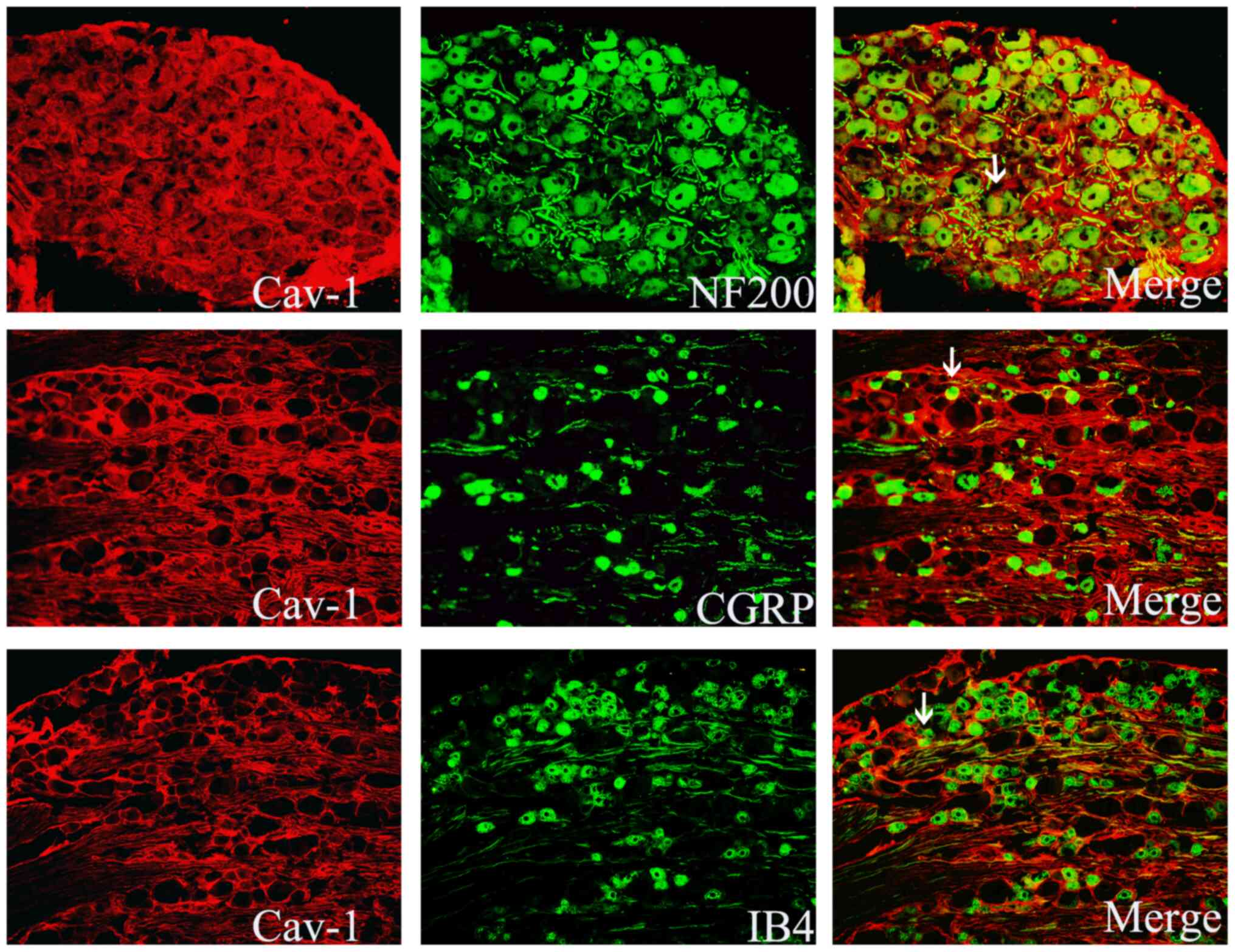
Immunofluorescence staining
demonstrates localization of Cav-1 in the DRG
At postsurgical day 7, DRG tissue sections were
stained with Cav-1, the medium and large neuronal marker NF200 or
the medium and small neuronal markers CGRP and IB4. The results
revealed that Cav-1 was mainly distributed in the NF200-positive
medium and large neurons, and a small part of it was distributed in
CGRP- or IB4-positive small and medium-sized neurons in the DRG
(Fig. 4)
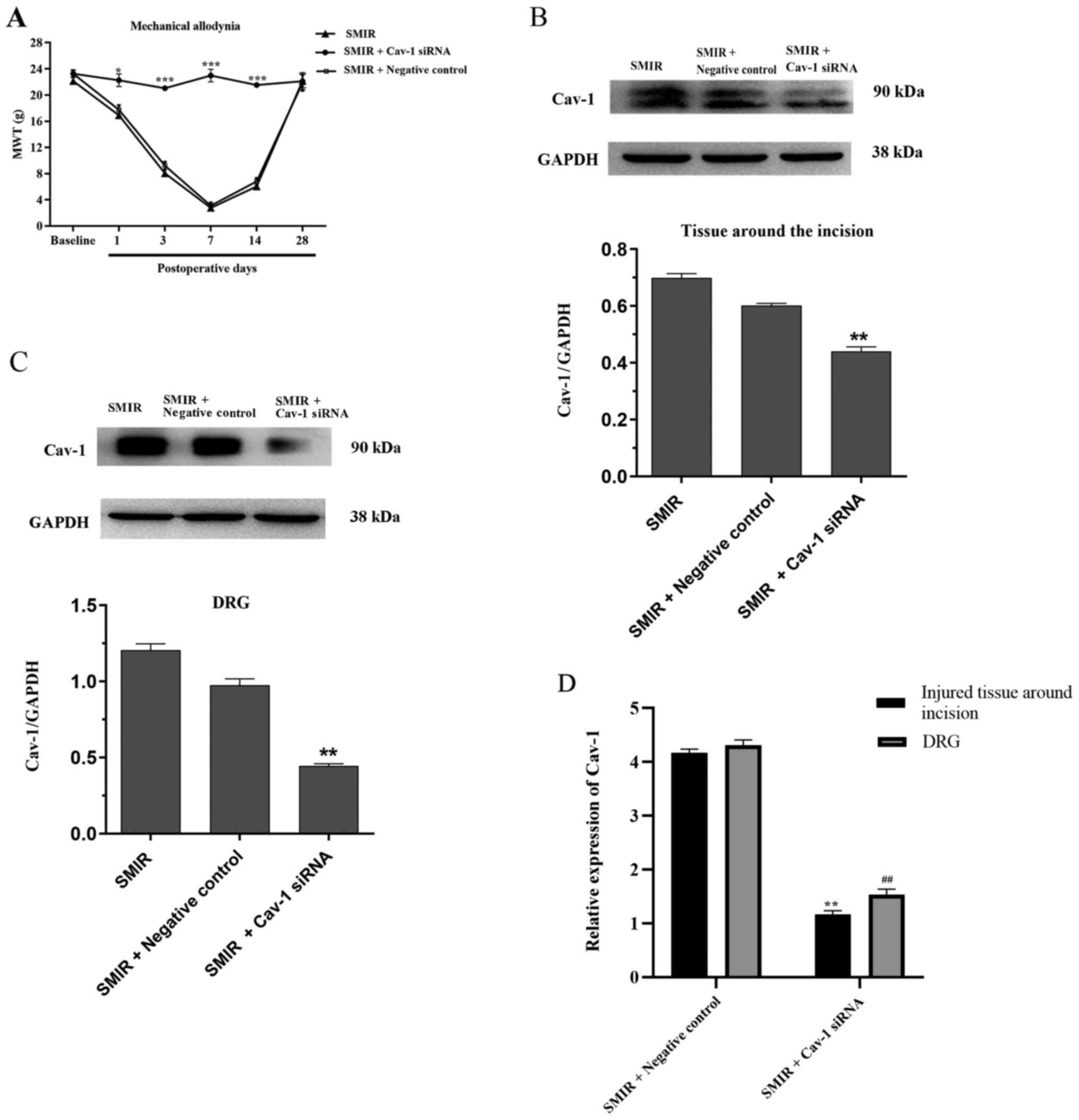
Intrathecal injection of Cav-1 siRNA
attenuates mechanical allodynia and decreases Cav-1 mRNA and
protein expression in the injured tissue around postoperative
incision and the DRG
To further investigate the role of Cav-1 in CPSP,
Cav-1 siRNA was used to silence Cav-1 gene expression at 1, 3 and 7
days following SMIR. Compared with the SMIR + Negative control
group, intrathecal injection of Cav-1 siRNA significantly increased
MWT on postsurgical days 1, 3, 7 and 14 (Fig. 5A). As presented in Fig. 5B and C, the expression of Cav-1 in the Cav-1
siRNA group significantly decreased in the injured tissue around
postoperative incision and the DRG, as compared with the SMIR +
Negative control group. RT-qPCR was performed to examine the mRNA
levels of Cav-1 in the injured tissue around the postoperative
incision and the DRG after intrathecal injection of Cav-1 siRNA. As
presented in Fig. 5D, Cav-1 siRNA
significantly decreased the expression levels of Cav-1 mRNA in the
injured tissue around the incision and the DRG compared with the
negative control on postsurgical day 7, reversing the increase in
the expression levels of Cav-1 associated with SMIR.
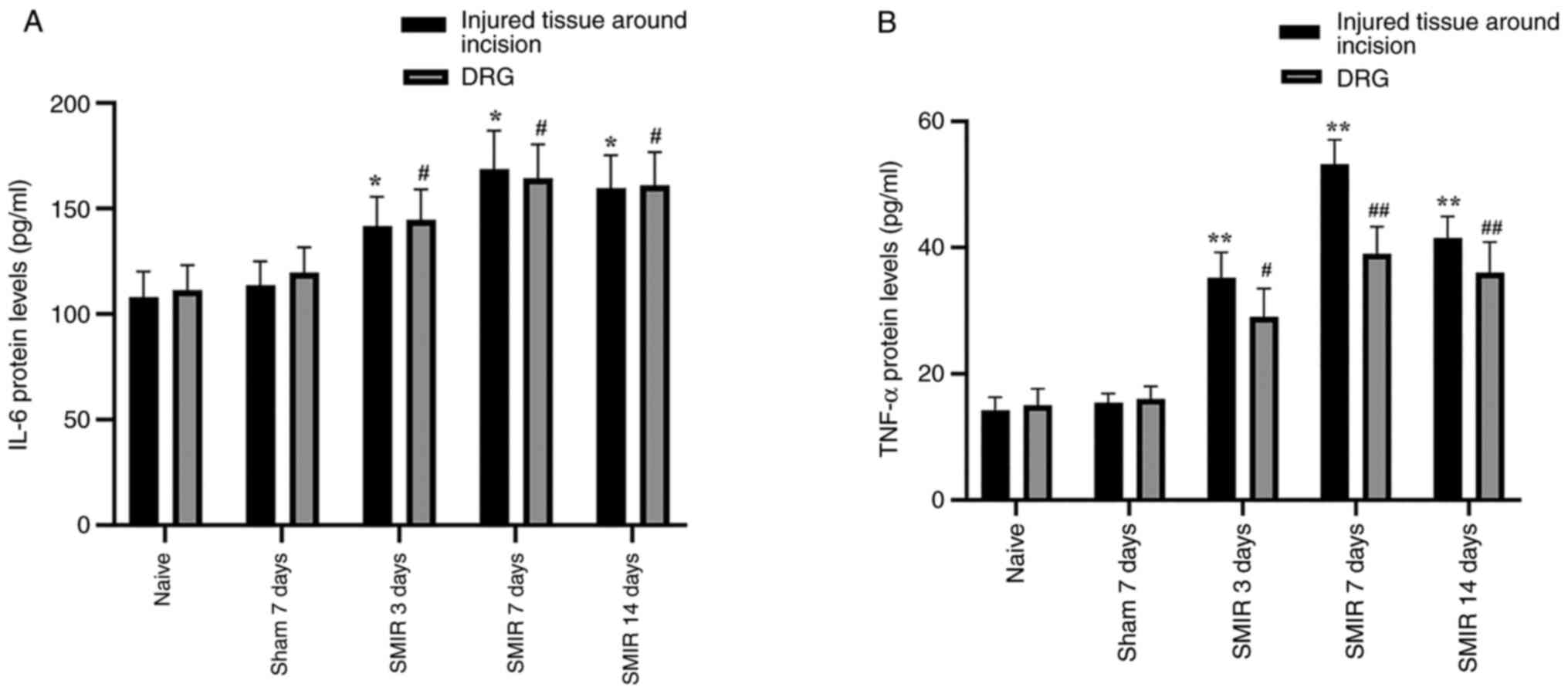
Effects of CPSP on the expression of
IL-6 and TNF-α protein levels in the injured tissue around
postoperative incision and the DRG
The IL-6 and TNF-α protein levels were detected in
the injured tissue around postoperative incision and the DRG
following SMIR surgery. ELISA assays demonstrated that IL-6 and
TNF-α expression levels were significantly increased in the injured
tissue around postoperative incision and the DRG on postsurgical
days 3, 7 and 14 compared with the naive and sham groups (Fig. 6A and B).
Discussion
Peripheral sensitization serves an important role in
the occurrence and maintenance of CPSP (25,26).
The purpose of the present study was to explore the role of Cav-1
in peripheral sensitization, starting with the alterations of the
peripheral incision tissue. The caveolae are a special form of
lipid raft, which is a small invagination of the plasma membrane
that is present in a number of mammalian cells, such as endothelial
cells, adipocytes and skeletal muscle (27,28).
Previous studies have reported that caveolae are the cellular
center of signaling molecules (9,10,29).
The formation and stability of the caveolae mainly depends on
caveolin (30). Cav-1 is the main
structural and signaling component of the fossa, which is mainly
expressed in inflammatory cells (31-33).
Recent studies have demonstrated that Cav-1 serves a notable role
in pain modulation (14,15), but its specific mechanism of action
requires further investigation. Our previous study has demonstrated
that SMIR increased the infiltration of macrophages and endothelial
cells in the injured tissue around the postoperative incision
(6), suggesting that the
inflammatory response in the tissue around the incision was
aggravated, which led to the occurrence of CPSP. In the current
study, the results indicated that SMIR increased the permeability
of vascular endothelial cells in the muscle tissue around the
incision at an early stage (on postoperative day 1). In addition,
the expression of Cav-1 increased following SMIR and was localized
in macrophages and endothelial cells, suggesting that Cav-1 may be
involved in the inflammatory response of the tissue around the
incision.
Endothelial cells regulate leukocyte activity,
inflammation and platelet aggregation by secreting various active
substances, while endothelial hyperpermeability induces
inflammatory response in local tissues (34). Due to the minor trauma in the sham
operation group, the vascular permeability of the local tissues did
not significantly change, therefore there was less local
inflammatory exudation accompanied by Cav-1 expression in the local
tissues and the DRG. The results of the present study indicated
that Cav-1 was upregulated after the initiation of the inflammatory
response in the injured tissue around the postoperative incision.
Moreover, a positive correlation between Cav-1 upregulation and the
levels of extravascular albumin has been demonstrated, which
suggested that upregulation of Cav-1 may be associated with
endothelial hyperpermeability (35,36).
It was hypothesized that SMIR increased the
expression level of Cav-1 in the tissue around the incision, which
resulted in an increase in the permeability of vascular endothelial
cells in the tissue. The increase of vascular endothelial
permeability then led to a continuous and significant increase in
exudation, which aggravated the inflammatory response of the tissue
around the incision. This promoted the transmission of pain
information to the higher center and, therefore, resulted in CPSP.
The pain signals are transmitted along the DRGs to the spinal cord
and then to the corresponding zone of the brain, including the
thalamus, forebrain, brainstem and midbrain, and finally to the
cerebral cortex (37-39).
Therefore, as the soma assembly of the first-order neurons for the
pain pathway, the excitability of DRGs is important in pain
signaling (37-39).
As reported in the literature, early nociceptive
stimuli may induce vigorous production of cytokines, such as IL-6
and TNF-α, in the DRG, and the cytokines may be transported to
central terminals of primary afferents (40,41).
Furthermore, the cytokines further activate glial cells and neurons
to release more activating substances, such as ATP,
pro-inflammatory factors and reactive oxygen species (42). These activating substances further
enhance pain and may transform acute pain into chronic pain
(43). In the present study, the
expression levels of the inflammatory factors IL-6 and TNF-α not
only increased in the DRG, but also in the tissues surrounding
incision after noxious injury, which provided evidence towards the
aforementioned hypothesis. In addition, the results of the present
study demonstrated that the increase of Cav-1 in the DRG occurred
in the SMIR group on the 3rd day after operation, while the
increase of Cav-1 in the peripheral tissue was relatively delayed
until postoperative day 7; therefore, the early nociceptive stimuli
may stimulate the alteration of Cav-1 expression levels in the DRG
first.
A previous study has revealed that Cav-1 is present
at excitatory synapses and concentrates at the postsynaptic density
during the later stage development of presynaptic components
(44), suggesting that Cav-1 may
play a notable role in synapse formation and plasticity. The
results of the present study indicated that Cav-1 was mainly
distributed in NF200-positive large and medium neurons in the DRG.
In addition, the increased expression of Cav-1 in the DRG after
SMIR was associated with a decreased MWT of rats with SMIR.
Previous studies have demonstrated that large-sized neurons in the
DRG are connected with A-β fibers, which transmit the mechanical
stimuli (45,46). Central fibers of large-sized neurons
in the DRG mainly project to the deep layers of the spinal cord,
which is involved in mechanical allodynia (47,48).
Therefore, it is possible that in the models of the present study,
high expression of Cav-1 in large-sized neurons of the DRG after
SMIR sensitized the neurons and changed the transmission mode of
noxious stimuli, resulting in mechanical pain hypersensitivity.
To further confirm whether the increase of Cav-1 was
involved in pain modulation in rats with CPSP, Cav-1 siRNA was used
to silence Cav-1 gene expression. The results indicated that the
intrathecal delivery of Cav-1 siRNA decreased the expression levels
of Cav-1 mRNA and protein in the tissue around the incision and in
the DRG. Furthermore, it significantly prevented the development of
hyperalgesia in rats. These results further indicated the
association between Cav-1 and CPSP.
The present study is limited in that, except for
using Evans blue staining, the expression profiles of intercellular
adhesion molecule 1 or vascular cell adhesion protein 1 were not
detected to provide evidence of alteration in the vascular
permeability.
In summary, peripheral noxious stimuli or injury
stimulated Cav-1 expression in the tissue around the incision and
in the DRG. The high expression of Cav-1 in the tissue around the
incision was accompanied by high permeability of endothelial cells,
which modeled the local chronic, inflammatory and nutrient rich
microenvironment at the beginning of nociceptive information
transmission, thus transmitting the abnormal pain signal to neurons
of the DRG. The high expression levels of Cav-1 sensitized large
neurons in the DRG and changed the transmission mode of harmful
stimuli, which resulted in mechanical pain hypersensitivity. Cav-1
may represent the key link and initial event of peripheral
sensitization of CPSP. Targeted Cav-1 intervention may be a
potential therapeutic strategy to inhibit peripheral sensitization
and provide novel ideas for the treatment of CPSP.
Supplementary Material
Evans blue staining results.
Evan exudation in the tissue around
the incision increased in SMIR group. Evan exudation in the tissue
around the incision increased on days 1, 3 and 7 after surgery in
SMIR group. SMIR, skin/muscle incision and retraction.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The National Natural
Science Foundation of China (grant no. 81701106).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SSH and SC designed the study. SSH, SRS and CEL
acquired and interpreted the data. YBQ, SRS and CEL analyzed the
data and assisted SSH in revising the manuscript. SSH and SC
prepared the manuscript and supervised the study. SSH and SC
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All experiments in the current study were approved
by The Experimental Animal Protection and Care Committee of Nantong
University (approval no. 20171015S1051122; Nantong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim DH, Pearson-Chauhan KM, McCarthy RJ
and Buvanendran A: Predictive factors for developing chronic pain
after total knee arthroplasty. J Arthroplasty. 33:3372–3378.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Maguire J, Thibodeau ML and Oliver J: CPSP
2014 results: What have we learned? Paediatr Child Health.
20:435–436. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Uemoto Y, Uchida M, Kondo N,
Wanifuchi-Endo Y, Fujita T, Asano T, Hisada T, Nishikawa S,
Katagiri Y, Terada M, et al: Predictive factors for patients who
need treatment for chronic post-surgical pain (CPSP) after breast
cancer surgery. Breast Cancer 22: doi: 10.1007, 2021.
|
|
4
|
Blichfeldt-Eckhardt MR: From acute to
chronic postsurgical pain: The significance of the acute pain
response. Dan Med J. 65(B5326)2018.PubMed/NCBI
|
|
5
|
Fregoso G, Wang A, Tseng K and Wang J:
Transition from acute to chronic pain: Evaluating risk for chronic
postsurgical pain. Pain Physician. 22:479–488. 2019.PubMed/NCBI
|
|
6
|
Pan P, Huang SS, Shen SR, Lu CE, Qin YB,
Zhang JL and Cao S: Role of p120 catenin in Epac1-induced chronic
postsurgical pain in rats. Pain Res Manag.
2019(9017931)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Parton RG, McMahon KA and Wu Y: Caveolae:
Formation, dynamics, and function. Curr Opin Cell Biol. 65:8–16.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tiruppathi C, Regmi SC, Wang DM, Mo GCH,
Toth PT, Vogel SM, Stan RV, Henkemeyer M, Minshall RD, Rehman J and
Malik AB: EphB1 interaction with caveolin-1 in endothelial cells
modulates caveolae biogenesis. Mol Biol Cell. 31:1167–1182.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Huang Q, Zhong W, Hu Z and Tang X: A
review of the role of cav-1 in neuropathology and neural recovery
after ischemic stroke. J Neuroinflammation. 15(348)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
de Souza GM, de Albuquerque Borborema ME,
de Lucena TMC, da Silva Santos AF, de Lima BR, de Oliveira DC and
de Azevêdo Silva J: Caveolin-1 (CAV-1) up regulation in metabolic
syndrome: All roads leading to the same end. Mol Biol Rep.
47:9245–9250. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Patel HH, Murray F and Insel PA: Caveolae
as organizers of pharmacologically relevant signal transduction
molecules. Annu Rev Pharmacol Toxicol. 48:359–391. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Codrici E, Albulescu L, Popescu ID, Mihai
S, Enciu AM, Albulescu R, Tanase C and Hinescu ME:
Caveolin-1-knockout mouse as a model of inflammatory diseases. J
Immunol Res. 29(2498576)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang Y, Luo H, Lv X, Liu J, Chen X, Li Y,
Liu A and Jiang Y: Axin-1 binds to caveolin-1 to regulate the
LPS-induced inflammatory response in AT-I cells. Biochem Biophys
Res Commun. 513:261–268. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang JX, Hua L, Li YQ, Jiang YY, Han D,
Liu H, Tang QQ, Yang XN, Yin C, Hao LY, et al: Caveolin-1 in the
anterior cingulate cortex modulates chronic neuropathic pain via
regulation of NMDA receptor 2B subunit. J Neurosci. 35:36–52.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen JL, Lu JH, Xie CS, Shen YJ, Wang JW,
Ye XY, Zhang MB, Jia GL, Tao YX, Li J and Cao H: Caveolin-1 in
spinal cord modulates type-2 diabetic neuropathic pain through the
Rac1/NOX2/NR2B signaling pathway. Am J Transl Res. 12:1714–1727.
2020.PubMed/NCBI
|
|
16
|
Jia GL, Huang Q, Cao YN, Xie CS, Shen YJ,
Chen JL, Lu JH, Zhang MB, Li J, Tao YX and Cao H: Cav-1
participates in the development of diabetic neuropathy pain through
the TLR4 signaling pathway. J Cell Physiol. 235:2060–2070.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang Y, Gao Y, Tian Q, Deng Q, Wang Y,
Zhou T, Liu Q, Mei K, Wang Y, Liu H, et al: TRPV1 SUMOylation
regulates nociceptive signaling in models of inflammatory pain. Nat
Commun. 9(1529)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bang S, Xie YK, Zhang ZJ, Wang Z, Xu ZZ
and Ji RR: GPR37 regulates macrophage phagocytosis and resolution
of inflammatory pain. J Clin Invest. 128:3568–3582. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Xu Q, Du F, Zhang Y, Teng Y, Tao M, Chen
AF and Jiang R: Preeclampsia serum induces human glomerular
vascular endothelial cell hyperpermeability via the
HMGB1-Caveolin-1 pathway. J Reprod Immunol. 129:1–8.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang X, Ramírez CM, Aryal B,
Madrigal-Matute J, Liu X, Diaz A, Torrecilla-Parra M, Suárez Y,
Cuervo AM, Sessa WC and Fernández-Hernando C: Cav-1 (Caveolin-1)
deficiency increases autophagy in the endothelium and attenuates
vascular inflammation and atherosclerosis. Arterioscler Thromb Vasc
Biol. 40:1510–1522. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chung JW, Kim DH, Oh MJ, Cho YH, Kim EH,
Moon GJ, Ki CS, Cha J, Kim KH, Jeon P, et al: Cav-1 (Caveolin-1)
and arterial remodeling in adult moyamoya disease. Stroke.
49:2597–2604. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Flatters SJ: Characterization of a model
of persistent postoperative pain evoked by skin/muscle incision and
retraction (SMIR). Pain. 135:119–130. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zahn PK, Pogatzki EM and Brennan TJ:
Mechanisms for pain caused by incisions. Reg Anesth Pain Med.
27:514–516. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mirra A, Spadavecchia C, Bruckmaier R,
Gutzwiller A and Casoni D: Acute pain and peripheral sensitization
following cautery disbudding in 1- and 4-week-old calves. Physiol
Behav. 84:248–260. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang Y and Wang Y: TRPV1: An important
molecule involved in the peripheral sensitization during chronic
pain and central pain modulation. Sheng Li Xue Bao. 25:677–684.
2017.PubMed/NCBI(In Chinese).
|
|
27
|
Filippini A and D'Alessio A: Caveolae and
lipid rafts in endothelium: Valuable organelles for multiple
functions. Biomolecules. 10(1218)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chow BW, Nuñez V, Kaplan L, Granger AJ,
Bistrong K, Zucker HL, Kumar P, Sabatini BL and Gu C: Caveolae in
CNS arterioles mediate neurovascular coupling. Nature. 579:106–110.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jin H, Xu Y, Shi F and Hu S: Vaccination
at different anatomic sites induces different levels of the immune
responses. Res Vet Sci. 122:50–55. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Parton RG, Tillu VA and Collins BM:
Caveolae. Curr Biol. 23:R402–R405. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Oliveira SDS, Chen J, Castellon M, Mao M,
Raj JU, Comhair S, Erzurum S, Silva CLM, Machado RF, Bonini MG and
Minshall RD: Injury-induced shedding of extracellular vesicles
depletes endothelial cells of Cav-1 (Caveolin-1) and enables TGF-β
(Transforming Growth Factor-β)-dependent pulmonary arterial
hypertension. Arterioscler Thromb Vasc Biol. 39:1191–1202.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Luo M, Xu C, Luo Y, Wang G, Wu J and Wan
Q: Circulating miR-103 family as potential biomarkers for type 2
diabetes through targeting CAV-1 and SFRP4. Acta Diabetol.
57:309–322. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang DX, Pan YQ, Liu B and Dai L: Cav-1
promotes atherosclerosis by activating JNK-associated signaling.
Biochem Biophys Res Commun. 503:513–520. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Konrad FM, Meichssner N, Bury A, Ngamsri
KC and Reutershan J: Inhibition of SDF-1 receptors CXCR4 and CXCR7
attenuates acute pulmonary inflammation via the adenosine
A2B-receptor on blood cells. Cell Death Dis.
8(e2832)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang N, Zhang D, Sun G, Zhang H, You Q,
Shao M and Yue Y: Lipopolysaccharide-induced caveolin-1
phosphorylation-dependent increase in transcellular permeability
precedes the increase in paracellular permeability. Drug Des Devel
Ther. 9:4965–4977. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Andreone BJ, Chow BW, Tata A, Lacoste B,
Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB and Gu C:
Blood-brain barrier permeability is regulated by lipid
transport-dependent suppression of caveolae-mediated transcytosis.
Neuron. 94:581–594. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bravo-Caparrós I, Ruiz-Cantero MC,
Perazzoli G, Cronin SJF, Vela JM, Hamed MF, Penninger JM, Baeyens
JM, Cobos EJ and Nieto FR: Sigma-1 receptors control neuropathic
pain and macrophage infiltration into the dorsal root ganglion
after peripheral nerve injury. FASEB J. 34:5951–5966.
2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mekhail N, Deer TR, Poree L, Staats PS,
Burton AW, Connolly AT, Karst E, Mehanny DS, Saweris Y and Levy RM:
Cost-effectiveness of dorsal root ganglion stimulation or spinal
cord stimulation for complex regional pain syndrome.
Neuromodulation. 9:708–714. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
He DD, Gao Y, Wang S, Xie Z and Song XJ:
Systematic administration of B vitamins alleviates diabetic pain
and inhibits associated expression of P2X3 and TRPV1 in dorsal root
ganglion neurons and proinflammatory cytokines in spinal cord in
rats. Pain Res Manag. 10(3740162)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li QY, Xu HY and Yang HJ: Effect of
proinflammatory factors TNF-α,IL-1β, IL-6 on neuropathic pain.
Zhongguo Zhong Yao Za Zhi. 42:3709–3712. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
41
|
Ding HH, Zhang SB, Lv YY, Ma C, Liu M,
Zhang KB, Ruan XC, Wei JY, Xin WJ and Wu SL: TNF-α/STAT3 pathway
epigenetically upregulates Nav1.6 expression in DRG and contributes
to neuropathic pain induced by L5-VRT. J Neuroinflammation.
16(29)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang ZJ, Jiang BC and Gao YJ: Chemokines
in neuron-glial cell interaction and pathogenesis of neuropathic
pain. Cell Mol Life Sci. 74:3275–3291. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li CD, Zhao JY, Chen JL, Lu JH, Zhang MB,
Huang Q, Cao YN, Jia GL, Tao YX, Li J and Cao H: Mechanism of the
JAK2/STAT3-CAV-1-NR2B signaling pathway in painful diabetic
neuropathy. Endocrine. 64:55–66. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kassan A, Egawa J, Zhang Z,
Almenar-Queralt A, Nguyen QM, Lajevardi Y, Kim K, Posadas E, Jeste
DV, Roth DM, et al: Caveolin-1 regulation of
disrupted-in-schizophrenia-1 as a potential therapeutic target for
schizophrenia. J Neurophysiol. 117:436–444. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sheikh NK and Dua A: Neuroanatomy,
substantia gelatinosa. In: StatPearls. StatPearls Publishing,
Treasure Island, FL, 2020.
|
|
46
|
Roh J, Hwang SM, Lee SH, Lee K, Kim YH and
Park CK: Functional expression of piezo1 in dorsal root ganglion
(DRG) neurons. Int J Mol Sci. 21(3834)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhang J, Harada Y and Hayashi Y: A
TLR-CXCL1 pathway in DRG neurons induces neutrophil accumulation in
the DRG and mechanical allodynia in EAE mice. Sci Rep.
9(12003)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Huang H, Wang M and Hong Y: Intrathecal
administration of adrenomedullin induces mechanical allodynia and
neurochemical changes in spinal cord and DRG. Neurosci Lett.
690:196–201. 2019.PubMed/NCBI View Article : Google Scholar
|