Introduction
Activated synovial cells are involved in the
pathogenesis and progression of arthritic diseases, such as
rheumatoid arthritis and osteoarthritis (1,2). The
pro-inflammatory cytokines, tumor necrosis factor (TNF)-α and
interleukin (IL)-1β, are typical activators of synovial cells
(3). They stimulate their own
production and also induce the production of other inflammatory
cytokines and mediators, including IL-6, IL-8, nitric oxide (NO),
and prostaglandin E2, by synovial cells (4,5).
Increased levels of these inflammatory cytokines and mediators
induce persistent inflammation and cartilage degradation,
eventually leading to joint destruction (1). Therefore, the inhibition of
inflammatory cytokine and mediator production by activated synovial
cells is regarded as a potential therapeutic target for suppressing
the progression of inflammation and joint destruction in arthritic
diseases (6).
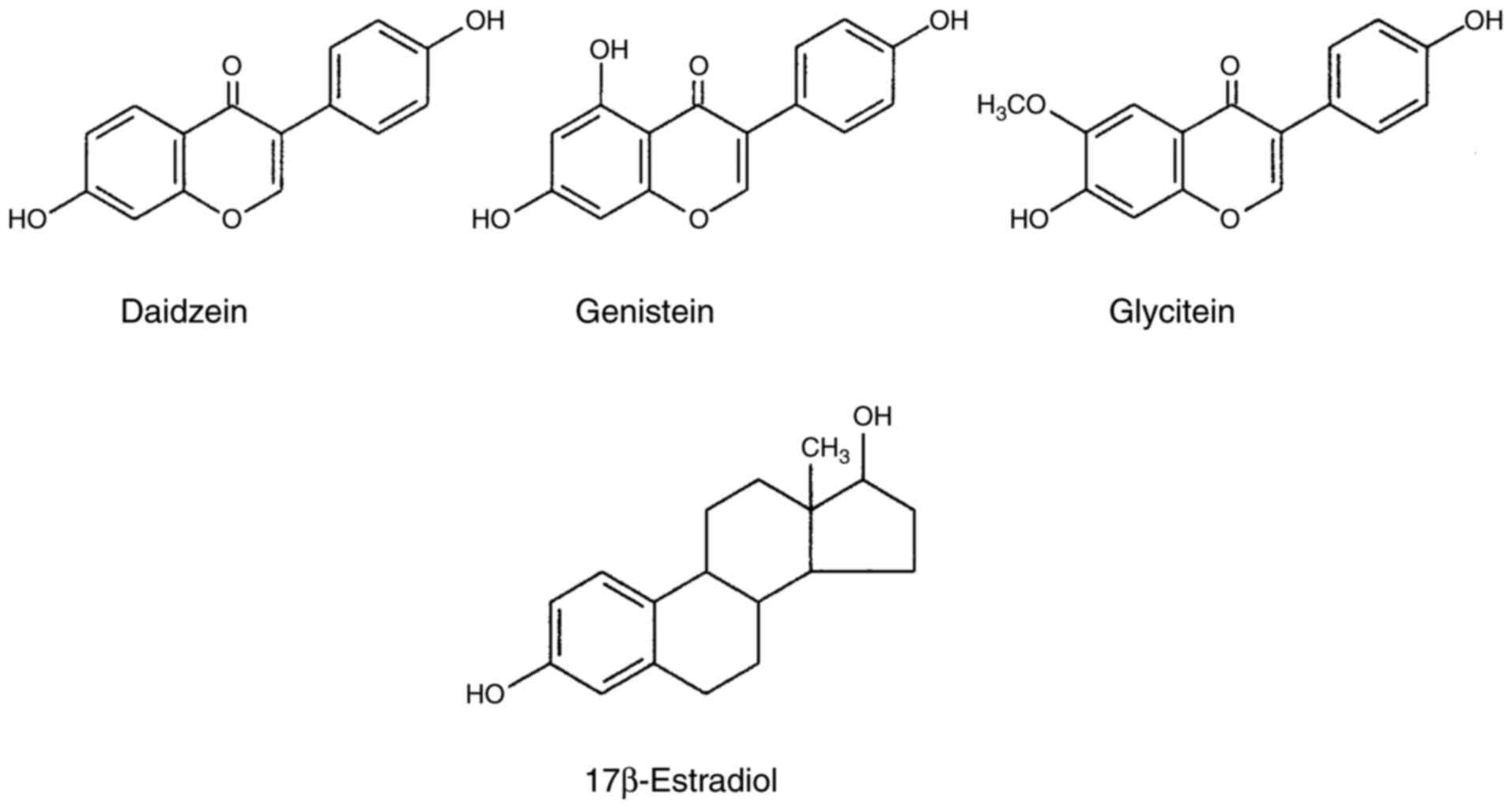
Isoflavone derivatives (isoflavones) are a class of
flavonoids that belong to a large family of polyphenolic compounds
from plants (such as soybeans, red clover, and kudzu roots)
(7-9).
Isoflavones are also called phytoestrogens because they contain an
estrogen structure (particularly 17β-estradiol) (Fig. 1), and mimic the effects of estrogens
by binding to estrogen receptors (10-12).
Isoflavones are divided into a glycosidic form with sugar chains
and an aglycone form without sugar chains in their molecular
structures (8). Daidzein,
genistein, and glycitein are the aglycone forms of isoflavones
(Fig. 1), and exhibit more potent
bioactivities than their glycoside forms (10). In addition to estrogenic activity,
isoflavones possess various biological properties (such as
antioxidant, antimicrobial, and anti-inflammatory activities)
(13-15).
The anti-inflammatory activities of isoflavones have been examined
in detail, and their therapeutic effects have been demonstrated in
various animal models with inflammatory disorders, such as lung
injury and arthritis (16,17). A previous study confirmed the
anti-inflammatory effects of genistein in a collagen-induced
rheumatoid arthritis model of rats (17). Genistein, daidzein, and glycitein
also exert anti-inflammatory effects through the inhibition of
inflammatory cytokine and mediator production by various types of
cells, including macrophages, chondrocytes, and synovial cells
(18-21).
For example, genistein reduced the LPS-stimulated up-regulation of
cyclooxygenase (COX)-2 and NO production in primary cultures of
human chondrocytes (18). Genistein
also decreased the production of IL-1β, IL-6, and IL-8 by the
TNF-α-simulated fibroblast-like synovial cell line MH7A (19). Moreover, daidzein inhibited
LPS-stimulated NO and IL-6 production by RAW264.7 mouse macrophage
cells (20), and glycitein
suppressed NO production by LPS-activated RAW264.7 cells (21).
We have been investigating the anti-inflammatory
effects of various functional food materials, such as glucosamine,
citrulline, methionine, and chondroitin sulfate, on IL-6 and IL-8
production by IL-1β-stimulated synovial cells (22,23),
and revealed that these materials inhibit cytokine production by
suppressing signaling molecules, including nuclear factor (NF)-κB,
extracellular signal regulated kinases (ERK)1/2, and p38
mitogen-activated protein kinase (MAPK). However, the
anti-inflammatory effects of isoflavones (daidzein, genistein, and
glycitein) on inflammatory cytokine production by IL-1β-stimulated
synovial cells have not yet been examined. Therefore, the present
study investigated the effects of isoflavones (daidzein, genistein,
and glycitein) on the production of inflammatory cytokines (IL-6
and IL-8) by IL-1β-stimulated synovial cells. The results obtained
indicated that among these isoflavones, only daidzein inhibited
IL-6 production by IL-1β-stimulated synovial cells, and this effect
was mediated by the suppression of NF-κB and ERK1/2 activation
(phosphorylation).
Materials and methods
Reagents and antibodies
Human IL-1β (cat. no. 200-01B) was purchased from
PeproTech; daidzein (cat. no. 09388-64), genistein (cat. no.
NH010302), glycitein (cat. no. 09387-74), dimethyl sulfoxide (DMSO;
cat. no. 09659-14), RIPA (radioimmunoprecipitation assay) buffer
(50 mmol/l Tris-HCl buffer pH 7.6, 150 mmol/l NaCl, 1% Nonidet p40,
0.5% sodium deoxycholate, 0.1% SDS (sodium dodecyl sulfate),
Protease Inhibitor Cocktail; cat. no. 08714-04), 2X SDS-PAGE
(polyacrylamide gel electrophoresis) sample buffer (cat. no.
30657-12), 6X SDS-PAGE sample buffer (cat. no. 09500-64), and WB
(western blot) Stripping Solution Strong (cat. no. 05677-65) were
from Nacalai Tesque, Inc. A rabbit anti-phosphorylated NF-κB p65
polyclonal antibody (93H1; cat. no. 3033), rabbit
anti-phosphorylated ERK1/2 polyclonal antibody (Thr202/Tyr204; cat.
no. 9101), rabbit anti-NF-κB p65 (D14E12; cat. no. 8242), and
rabbit anti-ERK1/2 (cat. no. 9102) were purchased from Cell
Signaling Technology, Inc.; a mouse anti-phosphorylated p38 MAPK
monoclonal antibody (clone 30; cat. no. 612281) and mouse anti-p38
MAPK (clone 27; cat. no. 612168) from BD Bioscience Pharmingen; a
mouse anti-GAPDH monoclonal antibody (cat. no. MAB374) from
Chemicon International; and horseradish peroxidase (HRP)-conjugated
goat anti-rabbit immunoglobulin IgG (cat. no. 115-035-144) and
HRP-conjugated goat anti-mouse IgG/IgM (cat. no. 115-035-044) from
Jackson ImmunoResearch Laboratories, Inc.
Daidzein, genistein, and glycitein were dissolved in
DMSO immediately before use, sterilized by filtration, and then
used in experiments.
Cell culture
The human fibroblast-like synovial cell line MH7A
(RCB1512) obtained from the Riken Cell Bank was cultured in
RPMI-1640 medium (Nacalai Tesque, Inc.) supplemented with 10% fetal
bovine serum (FBS; Nichirei Biosciences Inc.), 100 U/ml penicillin,
and 100 µg/ml streptomycin (Nacalai Tesque, Inc.) at 37˚C in a
humidified incubator containing 5% CO2, as previously
described (24).
Measurement of IL-6 and IL-8
MH7A cells were seeded on 24-well culture plates at
a density of 3.5x104 cells per well and incubated
overnight in RPMI-1640 medium containing 10% FBS. Cells were then
pretreated without or with daidzein (1 or 5 µg/ml), genistein (1 or
5 µg/ml), and glycitein (1 or 5 µg/ml) for 3 h, and stimulated at
37˚C for 18 h with or without 50 pg/ml IL-1β in a total volume of
0.5 ml RPMI-1640 medium. Thereafter, culture supernatants were
collected, centrifuged at 3,600 x g at 4˚C for 5 min, and the
concentrations of IL-6 and IL-8 in the culture supernatants were
assessed by an enzyme-linked immunosorbent assay (ELISA) (R&D
Systems, Inc.) according to the manufacturer's instructions.
Briefly, 96-well half area plates were coated with 0.6 µg/ml of the
mouse anti-human IL-6 antibody or 0.7 µg/ml of the mouse anti-human
IL-8 antibody diluted in phosphate-buffered saline (PBS; 137 mM
NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, and 1.5 mM
KH2PO4; pH 7.4) and incubated at 4˚C
overnight. The plates were washed three times with PBS containing
0.05% Tween-20 (PBS-T) and blocked with PBS containing 1% bovine
serum albumin at room temperature for 1 h. Thereafter, plates were
washed three times with PBS-T and incubated with culture
supernatants or standards (5-1,000 pg/ml) at room temperature for 2
h. After washing three times with PBS-T, plates were incubated with
15 ng/ml of the biotinylated goat anti-human IL-6 antibody or 7
ng/ml of the biotinylated goat anti-human IL-8 antibody at room
temperature for 2 h, washed three times with PBS-T, and then
incubated with streptavidin-HRP at room temperature for 30 min.
Plates were then washed five times with PBS-T and incubated with
tetramethyl benzidine (TMB) liquid substrate at room temperature in
the dark until color developed. The reaction was terminated using 1
M H2SO4 and the absorbance of the solution
was measured with a xMARK™ Microplate Spectrophotometer (Model 680;
Bio-Rad Laboratories, Inc.) at 450 nm.
Phosphorylation of NF-κB, ERK1/2, and
p38 MAPK
MH7A cells (1.5x105/well) were seeded and
cultured in 6-well plates overnight. Cells were then pretreated
without or with 10 µg/ml daidzein for 16 h and stimulated with 200
pg/ml IL-1β at 37˚C for 15 or 30 min. Cells were washed twice with
ice-cold PBS, and then lysed in 150 µl/well RIPA buffer containing
Phosphatase Inhibitor Cocktail (Roche Diagnostics; cat. no. 04 906
837 001). Following sonication, lysates were centrifuged at 15,000
x g at 4˚C for 15 min and the supernatants were collected. The
protein concentrations of the supernatants were assessed using the
Bicinchoninic Acid (BCA) protein assay kit (Pierce Biotechnology),
and 12 µg protein/lane was used in the western blot analysis.
Proteins were separated by 10% SDS-PAGE and transferred onto an
PVDF (polyvinylidene difluoride) membrane (EMD Millipore), followed
by blocking with Block Ace solution (Megmilk Snow Brand Co., Ltd.)
for 1 h. Membranes were then washed three times with Tris-buffered
saline (TBS; 25 mM Tris, 137 mM NaCl, 2.7 mM KCl, pH 7.4)
containing 0.05% Tween-20 (TBS-T), and incubated with the rabbit
anti-phosphorylated NF-κB p65 polyclonal antibody (1,000-fold
dilution), rabbit anti-phosphorylated ERK1/2 polyclonal antibody
(1,000-fold dilution), or mouse anti-phosphorylated p38 MAPK
monoclonal antibody (1,000-fold dilution) in TBS containing 0.05%
NaN3 at 4˚C overnight. After washing three times with
TBS-T, the membranes were incubated with HRP-conjugated goat
anti-rabbit IgG (5,000-fold dilution) or HRP-conjugated goat
anti-mouse IgG/IgM (5,000-fold dilution) in TBS containing 10%
Block Ace solution for 1 h. Following washing three times with
TBS-T, reactive proteins were detected with SuperSignal™ West Dura
Extended Duration Substrate (Pierce Biotechnology), and signals
were quantified using the LAS-3000 luminescent image analyzer
(Fujifilm Corporation) and Multi Gauge version 3.0 (Fujifilm
Corporation) with the Exposure type of Increment and the View menu
of Paint saturated data Red, in which images were analyzed before
the saturation of the images.
Membranes were then stripped with WB Stripping
Solution Strong for 30 min and reprobed with rabbit anti-NF-κB p65
(5,000-fold dilution), rabbit anti-ERK1/2 antibodies (1,000-fold
dilution), or mouse anti-p38 MAPK (2,000-fold dilution) followed by
an incubation with HRP-conjugated goat anti-rabbit IgG or
HRP-conjugated goat anti-mouse IgG/IgM secondary antibodies,
respectively.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was
detected with a mouse anti-GAPDH monoclonal antibody (50,000-fold
dilution; cat. no. MAB374; Chemicon International) and
HRP-conjugated goat anti-mouse IgG/IgM (5,000-fold dilution).
Reactive proteins were detected and their signals
were quantified, as described above.
Statistical analysis
Data were expressed as the mean ± standard
deviation, and analyzed for significant differences by a one-way
ANOVA with a post-hoc Tukey's test using GraphPad Prism software
version 6.0 (GraphPad Software, Inc.). P<0.05 was considered to
indicate a significant difference.
Results
Effects of daidzein, genistein, and
glycitein on IL-6 and IL-8 production by IL-1β-stimulated MH7A
cells
Fig. 2 shows the
effects of daidzein, genistein, and glycitein on IL-6 and IL-8
production by MH7A cells. Cells were stimulated with IL-1β in the
absence or presence of daidzein (1 or 5 µg/ml), genistein (1 or 5
µg/ml), or glycitein (1 or 5 µg/ml) for 18 h, and the levels of
IL-6 and IL-8 were measured by ELISA. The production of IL-6
(Fig. 2A-C) was approximately
28-fold higher after the IL-1β stimulation (1,090±479 pg/ml) than
that by unstimulated cells (38±35 pg/ml). Daidzein (5 µg/ml)
markedly inhibited the production of IL-6 by approximately 20% from
that by the IL-1β stimulation (P=0.3 compared with IL-1β
stimulation + DMSO, and P=0.1 compared with IL-1β stimulation
only), whereas 1 µg/ml daidzein did not significantly affect IL-6
production (Fig. 2A). On the other
hand, genistein and glycitein did not exert any significant effects
on IL-6 production in IL-1β-stimulated MH7A cells (Fig. 2B and C). Furthermore, the production of IL-8 was
approximately 127-fold higher with the IL-1β stimulation
(2,359±1,206 pg/ml) than that by unstimulated cells (19±33 pg/ml).
However, neither daidzein, genistein, nor glycitein exerted any
significant effects on IL-8 production (Fig. 2D-F).
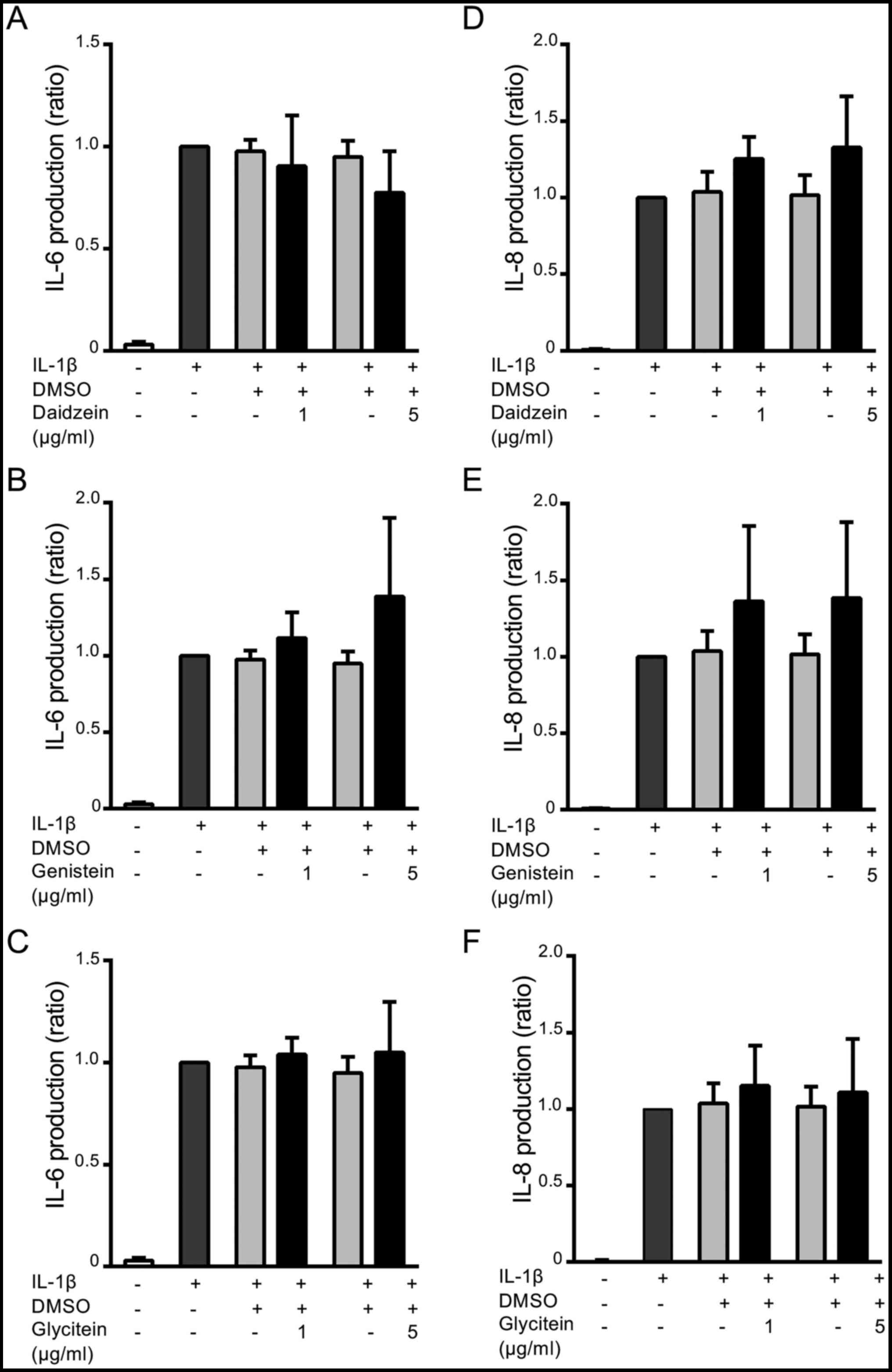 | Figure 2Effects of daidzein, genistein, and
glycitein on IL-6 and IL-8 production by IL-1β-stimulated MH7A
cells. Cells were treated without (-) or with (+) 1 or 5 µg/ml of
(A and D) daidzein, (B and E) genistein or (C and F) glycitein for
3 h, and then stimulated without (-) or with (+) 50 pg/ml IL-1β for
18 h. Alternatively, cells were treated without (-) or with (+)
0.5% DMSO (a solvent of isoflavones) in the absence of isoflavones,
and then stimulated with IL-1β. Thereafter, culture supernatants
were recovered, and (A-C) IL-6 and (D-F) IL-8 levels were measured
by ELISA. Data are expressed as a ratio to that in IL-1β-stimulated
MH7A cells without isoflavone derivatives, and compared between
without and with isoflavones. Results are shown as the means ± SD
of five independent experiments. DMSO, dimethyl sulfoxide. |
A morphological analysis of the cytotoxic effects of
daidzein on IL-1β-stimulated human synovial MH7A cells was
performed under light microscopy. Daidzein did not induce cell
death (such as apoptosis and necrosis) in IL-1β-stimulated MH7A
cells during an incubation at 5 and 10 µg/ml (data not shown).
Consistent with this result, similar amounts of GAPDH, an internal
control, were recovered from the cell lysates of unstimulated MH7A
cells, IL-1β-stimulated MH7A cells, and IL-1β-stimulated MH7A cells
incubated with daidzein (Figs. 3
and 4).
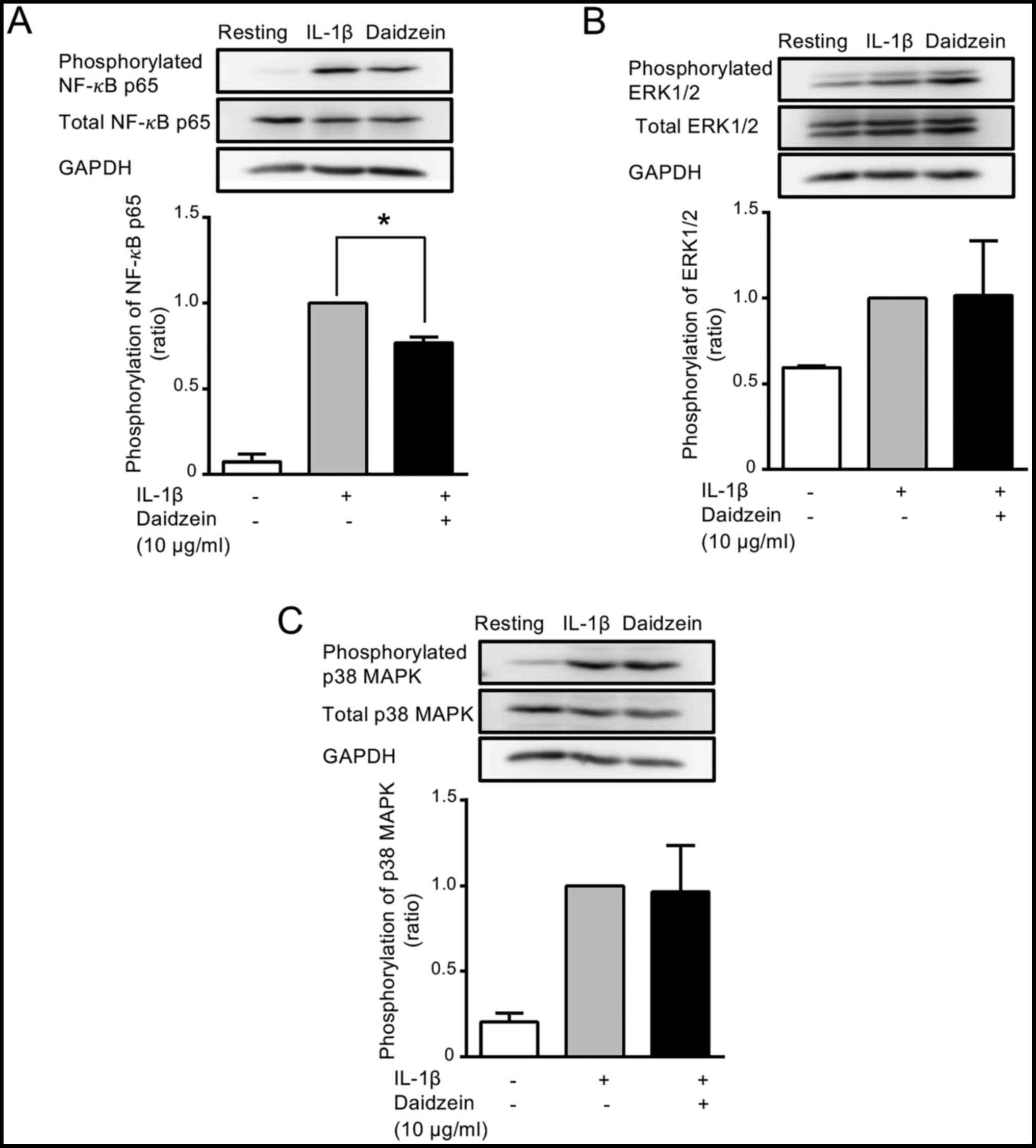 | Figure 3Effects of daidzein on the
phosphorylation of NF-κB, ERK1/2, and p38 MAPK. Cells were treated
without (-) or with (+) 10 µg/ml daidzein for 16 h, and then
stimulated without (-) or with (+) 200 pg/ml IL-1β for 15 min.
Cells were harvested, the supernatants (containing 12 µg protein)
obtained from cell lysates were resolved on 10% SDS-PAGE, and
phosphorylated and total (A) NF-κB p65, (B) ERK1/2 and (C) p38 MAPK
were evaluated by western blotting. GAPDH (an internal control) was
also detected by western blotting. Images are representative of
three separate experiments. The phosphorylation of NF-κB p65,
ERK1/2, and p38 MAPK was normalized with total NF-κB p65, ERK1/2,
and p38 MAPK, respectively, and expressed as a ratio to that in
IL-1β-stimulated MH7A cells without daidzein. Data were compared
between without and with daidzein. Results are expressed as the
means ± SD of three independent experiments. *P<0.05.
NF-κB, nuclear factor-κB; ERK, extracellular signal regulated
kinases; p38 MAPK, p38 mitogen-activated protein kinase. |
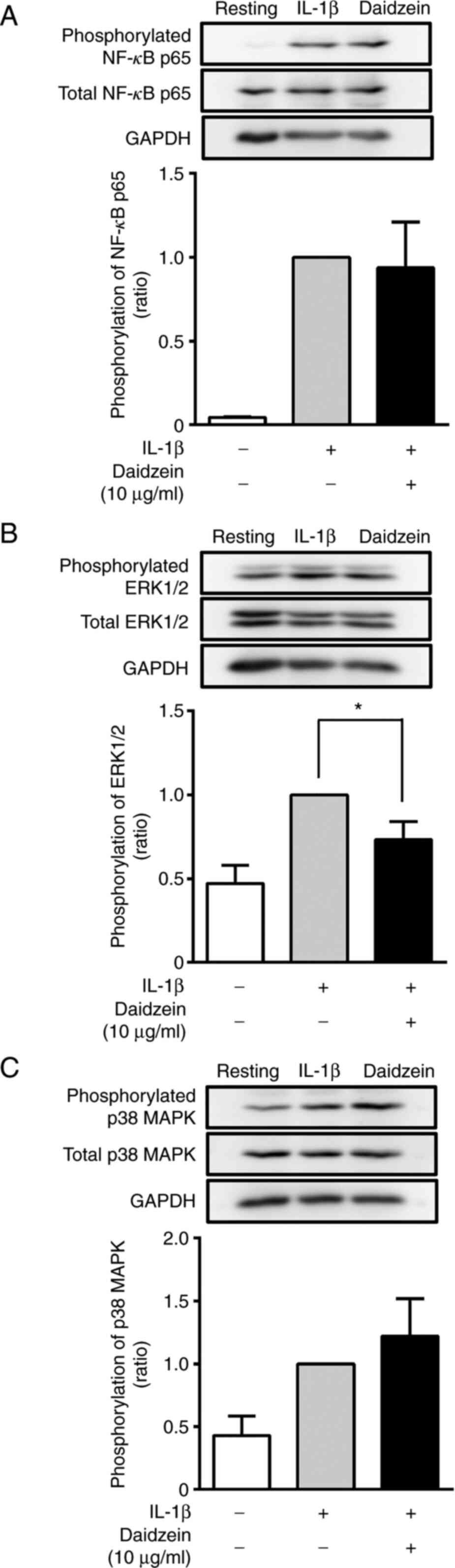 | Figure 4Effects of daidzein on the
phosphorylation of NF-κB, ERK1/2, and p38 MAPK. Cells were treated
without (-) or with (+) 10 µg/ml daidzein for 16 h and then
stimulated without (-) or with (+) 200 pg/ml IL-1β for 30 min.
Thereafter, cells were harvested, the supernatants (containing 12
µg protein) obtained from cell lysates were resolved on 10%
SDS-PAGE, and phosphorylated and total (A) NF-κB p65, (B) ERK1/2
and (C) p38 MAPK were evaluated by western blotting. GAPDH (an
internal control) was also detected by western blotting. Images are
representative of three separate experiments. The phosphorylation
of NF-κB p65, ERK1/2, and p38 MAPK was normalized to total NF-κB
p65, ERK1/2, and p38 MAPK, respectively, and expressed as a ratio
to that in IL-1β-stimulated MH7A cells without daidzein. Data are
compared between without and with daidzein. Results are expressed
as the means ± SD of three independent experiments.
*P<0.05. NF-κB, nuclear factor-κB; ERK, extracellular
signal regulated kinases; p38 MAPK, p38 mitogen-activated protein
kinase. |
Effects of daidzein on the
phosphorylation of NF-κB, ERK1/2, and p38 MAPK
The activation of the NF-κB and MAPK (ERK1/2 and p38
MAPK) signaling pathways is involved in the production of
inflammatory cytokines. Therefore, to elucidate the mechanisms
underlying the daidzein-mediated suppression of IL-6 production, we
examined the activation (phosphorylation) of NF-κB p65, ERK1/2, and
p38 MAPK. MH7A cells were stimulated with 200 pg/ml IL-1β for 15 or
30 min after the treatment with or without 10 µg/ml daidzein for 16
h. The phosphorylation of NF-κB p65, ERK1/2, and p38 MAPK was
evaluated by western blotting. When cells were stimulated with
IL-1β for 15 min, the phosphorylation of NF-κB p65 was
approximately 17-fold higher (Fig.
3A) than that in unstimulated cells. Daidzein (10 µg/ml)
significantly inhibited the phosphorylation of NF-κB p65 by 23%
from that by the IL-1β stimulation only (Fig. 3A). The phosphorylation of ERK1/2 and
p38 MAPK was also increased by approximately 1.7- and 5-fold,
respectively, by the IL-1β stimulation (Fig. 3B and C). However, daidzein (10 µg/ml) did not
show exert any significant effects on the phosphorylation of ERK1/2
or p38 MAPK (Fig. 3B and C).
We also examined the effects of daidzein (10 µg/ml)
on the phosphorylation of NF-κB p65, ERK1/2, and p38 MAPK after the
stimulation with IL-1β for 30 min. After the stimulation, the
phosphorylation of NF-κB p65, ERK1/2, and p38 MAPK was
approximately 23-, 2-, and 2.6-fold higher, respectively, that that
in unstimulated cells (Fig. 4).
Daidzein (10 µg/ml) significantly inhibited the IL-1β-induced
phosphorylation of ERK1/2 by 50% (Fig.
4B). In contrast, daidzein did not exert any significant
effects on the phosphorylation of p65 or p38 MAPK after the 30-min
stimulation (Fig. 4A and C).
Therefore, daidzein inhibited the IL-1β-induced
phosphorylation of NF-κB p65 (after 15 min) and ERK1/2 (after 30
min) in MH7A cells.
Discussion
The present study investigated the effects of
isoflavone derivatives (daidzein, genistein, and glycitein) on the
production of inflammatory cytokines (IL-6 and IL-8) by
IL-1β-stimulated synovial MH7A cells. The results obtained
indicated that daidzein markedly inhibited the production of IL-6,
but not IL-8. In contrast, neither genistein nor glycitein exerted
any effects on IL-6 or IL-8 production by IL-1β-stimulated synovial
cells.
A previous study reported that genistein
significantly inhibited the production of IL-6 and IL-8 by
TNF-α-stimulated synovial MH7A cells (19). However, in the present study,
genistein did not inhibit the production of IL-6 or IL-8 by
IL-1β-stimulated synovial MH7A cells. Therefore, genistein may
exert different effects on cytokine production depending on the
stimuli used (different stimuli of IL-1β and TNF-α). In addition,
the present study indicated that glycitein did not affect IL-6 or
IL-8 production by IL-1β-stimulated synovial cells. To the best of
our knowledge, the effects of glycitein on cytokine production have
not yet been examined.
The signal transduction pathways of NF-κB and MAPK
(ERK1/2 and p38 MAPK) play a critical role in the production of
inflammatory cytokines and mediators (25-27).
Therefore, to elucidate the molecular mechanisms underlying the
daidzein-mediated inhibition of IL-6 production, we examined the
effects of daidzein on the phosphorylation (activation) of NF-κB
p65, ERK1/2, and p38 MAPK. The results obtained indicated that
daidzein significantly inhibited the phosphorylation of NF-κB p65
(15 min) and ERK1/2 (30 min) by MH7A cells after the IL-1β
stimulation. In contrast, daidzein did not exert any effects on the
phosphorylation of p38 MAPK. A previous study reported that
daidzein inhibited the phosphorylation of NF-κB p65 in
lipopolysaccharide (LPS)-stimulated murine J774 macrophages
(28). However, the effects of
daidzein on the phosphorylation of ERK1/2 currently remain unknown.
Following the IL-1β stimulation, TAK1 (transforming growth
factor-β-activated kinase 1; an isoform of MAP kinase kinase
kinase; MAPKKK) was shown to be activated, and activated TAK1
phosphorylated the inhibitor of κB (IκB) kinase (IKK) (Fig. 5) (4,29,30).
Activated IKK phosphorylates IκB in the complex with NF-κB, and
phosphorylated IκB is degraded by the ubiquitin-proteasome system,
which allows the release and nuclear translocation of NF-κB,
leading to the transcription of inflammatory cytokine genes
(Fig. 5) (29-32).
Moreover, activated IKK activates Tpl2 (tumor progression locus 2;
an isoform of MAPKKK), which, in turn, activates MKK
(mitogen-activated protein kinase kinase; MAPKK) 1/2, leading to
the activation of ERK1/2 (Fig. 5)
(33). Alternatively, TAK1
activates the p38 MAPK pathway via the activation of MKK3 and
MKK6(33). p38 MAPK may also be
activated by TAK1 via the IKK/Tpl2 pathway (33). The present study revealed that
daidzein inhibited the phosphorylation of NF-κB p65 and ERK1/2
(Figs. 3A and 4B), but not p38MAPK in IL-1β-stimulated
MH7A cells. Therefore, it is reasonable to speculate that daidzein
primarily inhibits the TAK1/IKK-mediated activation of NF-κB p65
and ERK1/2, but not the TAK1/MKK3/MKK6-mediated activation of p38
MAPK. Moreover, daidzein significantly inhibited the
phosphorylation of NF-κB p65 (15 min) and ERK1/2 (30 min) by MH7A
cells after the IL-1β stimulation. The time difference in the
inhibitory effects of daidzein on the phosphorylation of NF-κB p65
(15 min) and ERK1/2 (30 min) may reflect differences in the
activation of NF-κB p65 (activation at an early stage) and ERK1/2
(activation at a later stage) via the different signal transduction
pathways starting from TAK1 by the IL-1β stimulation in MH7A
cells.
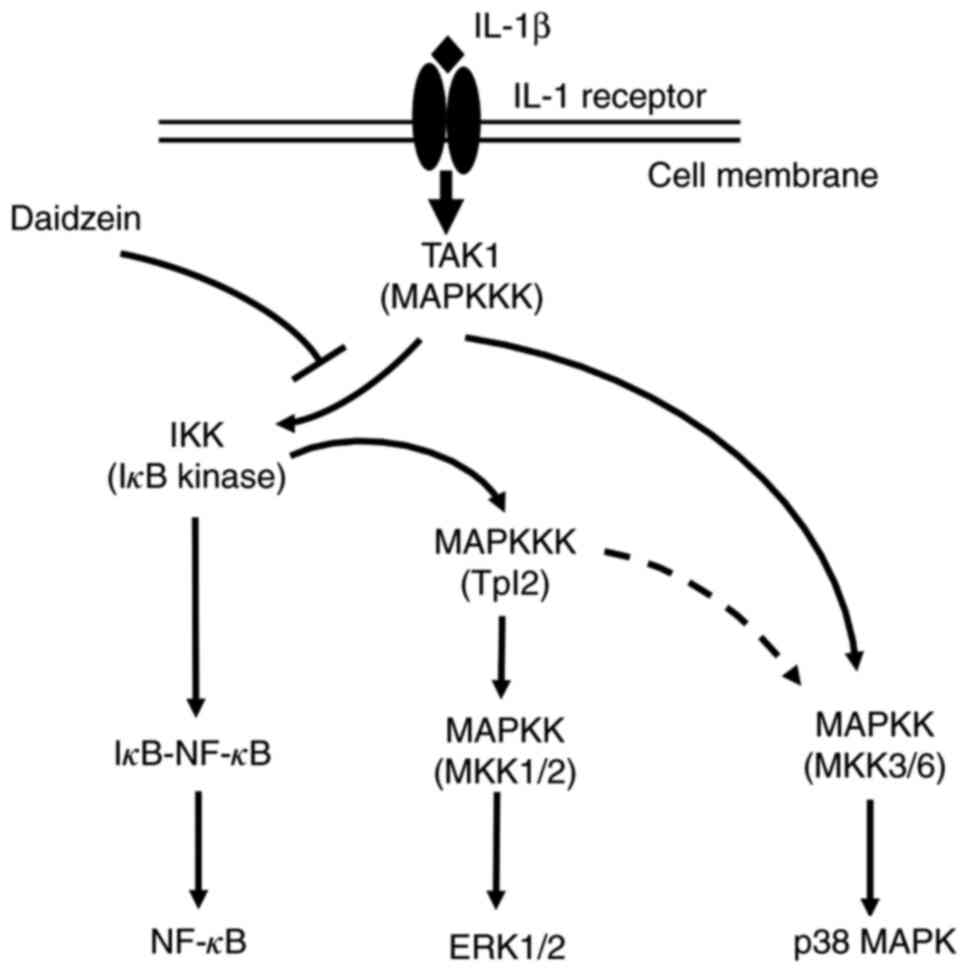 | Figure 5Schematic model of IL-1β-stimulated
signaling pathways of NF-κB p65, ERK1/2, and p38 MAPK, and
inhibitory effects of daidzein on NF-κB/ERK1/2 signaling in
synovial cells. TAK1 is activated by a stimulation with IL-1β, and
activated TAK1 phosphorylates the IKK. Activated IKK phosphorylates
IκB in the complex with NF-κB, and phosphorylated IκB is degraded
by the ubiquitin-proteasome system, which allows the release and
nuclear translocation of NF-κB, leading to the transcription of
inflammatory cytokine genes. Moreover, activated IKK activates
Tpl2, which, in turn, activates MKK1/2, leading to the activation
of ERK1/2. Alternatively, TAK1 activates the p38 MAPK pathway via
the activation of MKK3 and MKK6. p38 MAPK may also be activated by
TAK1 via the IKK/Tpl2 pathway (dotted line). Since daidzein
inhibited the phosphorylation of NF-κB p65 and ERK1/2, but not
p38MAPK in IL-1β-stimulated MH7A cells, it appears to inhibit the
TAK1/IKK-mediated activation of NF-κB p65 and ERK1/2, but not the
TAK1/MKK3/MKK6-mediated activation of p38 MAPK. IKK, IκB kinase;
IκB, inhibitor of κB. NF-κB, nuclear factor-κB; ERK, extracellular
signal regulated kinases; p38 MAPK, p38 mitogen-activated protein
kinase; TAK1, transforming growth factor-β-activated kinase 1;
MAPKKK, MAP kinase kinase kinase; IκB, inhibitor of κB; IKK, IκB
kinase; Tpl2, tumor progression locus 2; MKK and MAPKK,
mitogen-activated protein kinase kinase. |
NF-κB is a pivotal transcription factor for the
expression of inflammatory cytokines, such as IL-6 and IL-8
(25,34). After its stimulation, NF-κB
translocates to the nucleus and binds to the NF-κB binding site of
the promoters for cytokine genes, leading to cytokine gene
expression (35-37).
In the present study, daidzein significantly inhibited the
phosphorylation of NF-κB p65 (Fig.
3A) and production of IL-6 (Fig.
2A), but not IL-8 (Fig. 2D).
IL-6 production was approximately 28-fold higher after the IL-1β
stimulation than that by unstimulated cells, whereas IL-8
production was increased by approximately 127-fold by the IL-1β
stimulation. Daidzein inhibited the phosphorylation of NF-κB p65 by
only 20% (Fig. 3A). Therefore, the
expression of IL-6 appears to be more sensitive to the inhibitory
effects of daidzein than that of IL-8, and the weak
daidzein-induced inhibition of NF-κB p65 phosphorylation
(approximately 20%) may suppress the moderate elevations in IL-6
expression, but not the marked increases in IL-8 expression.
However, further studies are needed to clarify the mechanisms
responsible for the different effects of daidzein on IL-6 and IL-8
production.
In the present study, the concentrations of daidzein
(5 and 10 µg/ml) and the incubation periods with daidzein (3 and 16
h) are different between ELISA (detection of cytokines IL-6 and
IL-8) and western blot analysis (detection of the phosphorylation
of NF-κB, ERK1/2 and p38 MAPK). This is based on the facts that the
detection of cytokines by ELISA is highly sensitive. Thus, cytokine
production was markedly increased by a low concentration of IL-1β
(50 pg/ml), and especially IL-6 production was significantly
suppressed by incubation with 5 µg/ml daidzein for 3 h (as shown in
Fig. 2A). In contrast, the
detection of the phosphorylation of NF-κB, ERK1/2 and p38 MAPK was
low-sensitive, and the phosphorylation was significantly enhanced
by a high concentration of IL-1β (200 pg/ml). Thus, to make the
suppressive effect of daidzein more effective, MH7A cells were
incubated with a high concentration of daidzein (10 µg/ml) for a
long period (16 h) to detect the effect of daidzein on the
phosphorylation of NF-κB, ERK1/2 and p38 MAPK (Figs. 3 and 4), compared with the effect of daidzein on
cytokine production (5 µg/ml daidzein and incubation period 3 h)
(Fig. 2).
In conclusion, the present study revealed that among
the isoflavone derivatives examined (daidzein, genistein, and
glycitein), daidzein inhibited the production of IL-6, but not IL-8
by IL-1β-stimulated synovial MH7A cells, possibly via the
suppression of NF-κB p65 and ERK1/2 activation. IL-6 is a
pleiotropic cytokine that plays a pivotal role in the
pathophysiology of arthritis, which is found in the synovial fluid,
and its level correlates with disease activity and joint
destruction (38). Furthermore,
IL-6 may promote synovitis and joint destruction by stimulating
neutrophil migration, osteoclast maturation, and vascular
endothelial growth factor (VEGF)-stimulated pannus proliferation
(38). In contrast, IL-8 is an
inflammatory chemokine that is involved in the pathological
processes of arthritis, including the release of matrix
metalloproteinase-13 (MMP-13), neutrophil accumulation, and
leukocyte homing to the synovium (39). Based on these findings, daidzein has
potential as a therapeutic agent for arthritic disorders by mainly
suppressing IL-6 production, thereby ameliorating the progression
of inflammation and joint destruction.
Acknowledgements
The authors would like to thank Dr Keiji Miyazawa
(Kissei Pharmaceutical Co., Ltd., Nagano, Japan) for the
establishment of MH7A cells, and Dr Mamoru Igarashi, Dr Kaori
Suzuki, Dr Taisuke Murakami and Dr Yumi Kumagai Department of Host
Defense and Biochemical Research, Juntendo University, Graduate
School of Medicine, for their technical assistance and helpful
discussions.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NM substantially contributed to the conception and
design of this study, and the acquisition of data. AS and IN
confirmed the authenticity of all the raw data. NM, AS and IN were
involved in the analysis and interpretation of data, and drafting
and revising the manuscript for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huber LC, Distler O, Tarner I, Gay RE, Gay
S and Pap T: Synovial fibroblasts: Key players in rheumatoid
arthritis. Rheumatol (Oxford). 45:669–675. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mathiessen A and Conaghan PG: Synovitis in
osteoarthritis: Current understanding with therapeutic
implications. Arthritis Res Ther. 19(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Storch H, Zimmermann B, Resch B,
Tykocinski LO, Moradi B, Horn P, Kaya Z, Blank N, Rehart S, Thomsen
M, et al: Activated human B cells induce inflammatory fibroblasts
with cartilage-destructive properties and become functionally
suppressed in return. Ann Rheum Dis. 75:924–32. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014(561459)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pelletier JP, Martel-Pelletier J and
Abramson SB: Osteoarthritis, an inflammatory disease: Potential
implication for the selection of new therapeutic targets. Arthritis
Rheum. 44:1237–1247. 2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Malemud CJ: Biologic basis of
osteoarthritis: State of the evidence. Curr Opin Rheumatol.
27:289–294. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Barnes S, Prasain J, D'Alessandro T,
Arabshahi A, Botting N, Lila MA, Jackson G, Janle E and Weaver CM:
The metabolism and analysis of isoflavones and other dietary
polyphenols in foods and biological systems. Food Funct. 2:235–244.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Barnes S: The biochemistry, chemistry and
physiology of the isoflavones in soybeans and their food products.
Lymphat Res Biol. 8:89–98. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Beck V, Rohr U and Jungbauer A:
Phytoestrogens derived from red clover: An alternative to estrogen
replacement therapy? J Steroid Biochem Mol Biol. 94:499–518.
2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Munro IC, Harwood M, Hlywka JJ, Stephen
AM, Doull J, Flamm WG and Adlercreutz H: Soy isoflavones: A safety
review. Nutr Rev. 61:1–33. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vitale DC, Piazza C, Melilli B, Drago F
and Salomone S: Isoflavones: Estrogenic activity, biological effect
and bioavailability. Eur J Drug Metab Pharmacokinet. 38:15–25.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Morito K, Hirose T, Kinjo J, Hirakawa T,
Okawa M, Nohara T, Ogawa S, Inoue S, Muramatsu M and Masamune Y:
Interaction of phytoestrogens with estrogen receptors alpha and
beta. Biol Pharm Bull. 24:351–356. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rodriguez-Roque MJ, Rojas-Grau MA,
Elez-Martinez P and Martin-Belloso O: Soymilk phenolic compounds,
isoflavones and antioxidant activity as affected by in vitro
gastrointestinal digestion. Food Chem. 136:206–212. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chacko BK, Chandler RT, D'Alessandro TL,
Mundhekar A, Khoo NK, Botting N, Barnes S and Patel PP:
Anti-inflammatory effects of isoflavones are dependent on flow and
human endothelial cell PPARgamma. J Nutr. 137:351–356.
2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hong H, Landauer MR, Foriska MA and Ledney
GD: Antibacterial activity of the soy isoflavone genistein. J Basic
Microbiol. 46:329–335. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kang JL, Lee HW, Lee HS, Pack IS, Chong Y,
Castranova V and Koh Y: Genistein prevents nuclear factor-kappa B
activation and acute lung injury induced by lipopolysaccharide. Am
J Respir Crit Care Med. 164:2206–2212. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Verdrengh M, Jonsson IM, Holmdahl R and
Tarkowski A: Genistein as an anti-inflammatory agent. Inflamm Res.
52:341–346. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hooshmand S, Soung do Y, Lucas EA,
Madihally SV, Levenson CW and Arjmandi BH: Genistein reduces the
production of proinflammatory molecules in human chondrocytes. J
Nutr Biochem. 18:609–614. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li J, Li J, Yue Y, Hu Y, Cheng W, Liu R,
Pan X and Zhang P: Genistein suppresses tumor necrosis factor
alpha-induced inflammation via modulating reactive oxygen
species/Akt/nuclear factor κB and adenosine monophosphate-activated
protein kinase signal pathways in human synoviocyte MH7A cells.
Drug Des Devel Ther. 8:315–323. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Choi EY, Jin JY, Lee JY, Choi JI, Choi IS
and Kim SJ: Anti-inflammatory effects and the underlying mechanisms
of action of daidzein in murine macrophages stimulated with
Prevotella intermedia lipopolysaccharide. J Periodontal Res.
47:204–211. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sheu F, Lai HH and Yen GC: Suppression
effect of soy isoflavones on nitric oxide production in RAW 264.7
macrophages. J Agric Food Chem. 49:1767–1772. 2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yamagishi Y, Someya A, Imai K, Nagao J and
Nagaoka I: Evaluation of the anti-inflammatory actions of various
functional food materials including glucosamine on synovial cells.
Mol Med Rep. 16:1353–1359. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yamagishi Y, Someya A and Nagaoka I:
Citrulline cooperatively exerts an anti-inflammatory effect on
synovial cells with glucosamine and N-acetylglucosamine. Biomed
Rep. 13:37–42. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Someya A, Ikegami T, Sakamoto K and
Nagaoka I: Glucosamine downregulates the IL-1beta-induced
expression of proinflammatory cytokine genes in human synovial MH7A
cells by O-GlcNAc modification-dependent and -independent
mechanisms. PLoS One. 11(e0165158)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: A pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kyriakis JM and Avruch J: Mammalian MAPK
signal transduction pathways activated by stress and inflammation:
A 10-year update. Physiol Rev. 92:689–737. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Hamalainen M, Nieminen R, Vuorela P,
Heinonen M and Moilanen E: Anti-inflammatory effects of flavonoids:
genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and
NF-kappaB activations, whereas flavone, isorhamnetin, naringenin,
and pelargonidin inhibit only NF-kappaB activation along with their
inhibitory effect on iNOS expression and NO production in activated
macrophages. Mediators Inflamm. 2007(45673)2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Weber A, Wasiliew P and Kracht M:
Interleukin-1 (IL-1) pathway. Sci Signal. 3(cm1)2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kawai T and Akira S: TLR signaling. Semin
Immunol. 19:24–32. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zandi E and Karin M: Bridging the gap:
Composition, regulation, and physiological function of the IkappaB
kinase complex. Mol Cell Biol. 19:4547–4551. 1999.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zandi E, Chen Y and Karin M: Direct
phosphorylation of IkappaB by IKKalpha and IKKbeta: Discrimination
between free and NF-kappaB-bound substrate. Science. 281:1360–1363.
1998.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sabio G and Davis RJ: TNF and MAP kinase
signalling pathways. Semin Immunol. 26:237–245. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Georganas C, Liu H, Perlman H, Hoffmann A,
Thimmapaya B and Pope RM: Regulation of IL-6 and IL-8 expression in
rheumatoid arthritis synovial fibroblasts: The dominant role for
NF-kappa B but not C/EBP beta or c-Jun. J Immunol. 165:7199–7206.
2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Saccani S, Pantano S and Natoli G:
p38-Dependent marking of inflammatory genes for increased NF-kappa
B recruitment. Nat Immunol. 3:69–75. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Hoffmann E, Dittrich-Breiholz O, Holtmann
H and Kracht M: Multiple control of interleukin-8 gene expression.
J Leukoc Biol. 72:847–855. 2002.PubMed/NCBI
|
|
37
|
Hoffmann E, Thiefes A, Buhrow D,
Dittrich-Breiholz O, Schneider H, Resch K and Kracht M:
MEK1-dependent delayed expression of Fos-related antigen-1
counteracts c-Fos and p65 NF-kappaB-mediated interleukin-8
transcription in response to cytokines or growth factors. J Biol
Chem. 280:9706–9718. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Srirangan S and Choy EH: The role of
interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther
Adv Musculoskelet Dis. 2:247–56. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Takahashi A, de Andres MC, Hashimoto K,
Itoi E and Oreffo RO: Epigenetic regulation of interleukin-8, an
inflammatory chemokine, in osteoarthritis. Osteoarthritis
Cartilage. 23:1946–1954. 2015.PubMed/NCBI View Article : Google Scholar
|