Introduction
The incidence of lung cancer in China is increasing
at ~1.63% per year (1) and the
total number of patients with lung cancer is expected to reach 1
million, which would be the largest population of lung cancer
patients in the world (2-4).
Although new therapeutic techniques for lung cancer are
continuously being developed, the 5-year survival for patients with
non-small cell lung cancer (NSCLC) remains below 15% (5). Platinum-based chemotherapy is the
first-line treatment for advanced NSCLC patients with no or unknown
driver mutations and programmed death-ligand 1 <1% or unknown,
but unfortunately, 70-80% of the patients are resistant to it
(6).
Cisplatin (DDP) enters cancer cells by cell surface
transporters, such as high affinity copper uptake protein 1 and
they form positively charged molecules that bind to DNA to form
cross-linked adducts, which inhibit transcription and replication
and induce apoptosis of the cancer cells (6). Studies have shown that resistance to
DDP in NSCLC cells was mainly represented by deletion of the DDP
transporter or reduced activity of metallothionein and
glutathione-related metabolic enzymes (such as glutathione), which
convert DDP into positively charged compounds, which loses its
ability to inhibit DNA repair (7).
Several targeted drugs aiming at this pathway have been developed
in previous years, for example picoplatin, which was expected to
increase the activity of glutathione (8). However, the efficacy of these drugs
for DDP-resistant tumors remained poor and none of them can
effectively reverse DDP resistance in NSCLCs.
Honokiol (HNK), mainly derived from the traditional
Chinese medicine Magnolia, is a representative of the
biphenyl lignans (9). It has
various pharmacological effects, including anti-infection,
anti-anxiety and anti-oxidation, and previous studies have shown
that HNK may regulate signaling pathways involved in stemness
maintenance of tumor stem cells, such as the Notch signaling
pathway (10-13).
Curcumin (CUR) is an acidic polyphenolic substance
and is a diketone compound, widely found in the root and stem of
various plants such as turmeric, medlar and calamus. CUR has a wide
range of pharmacological effects, such as anticoagulation,
hypolipidemic effects, anti-oxidation, anti-inflammatory,
anti-atherosclerosis, antitumor and antirheumatic effects, and
liver, biliary and stomach-protecting effects. CUR shows very
limited toxic side effects. Therefore, its anticancer effect has
attracted increasing attention and the National Cancer Institute of
USA has listed it as one of the third-generation cancer
chemopreventive drugs (14).
P-glycoprotein (P-gp) is a high molecular weight
glycoprotein encoded by the multidrug resistance (MDR)1 gene and
belongs to the classical ATP-binding cassette transporter family.
Overexpression of P-gp is one of the most important causes of MDR.
P-gp has been shown to regulate the AKT/ERK signaling pathway
(15,16). Caspase-3, caspase-9 and poly(ADP
ribose) polymerase (PARP) are the key proteases about cell
apoptosis. P21 is a cyclin-dependent kinase inhibitor to regulate
cancer progression (17). Matrix
metalloproteinases (MMPs) are a big family of proteinases that are
involved in numerous pathophysiological processes (18). Thus far, the role and possible
mechanisms of HNK combined with CUR on resistance of lung cancer
cells to DDP has not been elucidated. The present study
investigated the effects of HNK combined with CUR to reverse drug
resistance of A549/DDP cell lines and explored the underlying
mechanisms in vitro experiments.
Materials and methods
Reagents and instruments
Human lung cancer A549 and A549/DDP cell lines were
provided by ATCC. A549 cell culture medium RPMI-1640 and A549/DDP
cell culture specific medium (RPMI-1640 + 10% FBS + 1-2 µg/ml DDP +
1% penicillin/streptomycin) were purchased from Gibco (Thermo
Fisher Scientific, Inc.). HNK, CUR and DDP were purchased from
Sigma-Aldrich (Merck KGaA). Transwell chambers were purchased from
BD Biosciences; Becton, Dickinson and Company. Non-radioactive cell
proliferation assay (MTS) reagent and reverse
transcription-quantitative (RT-q) PCR kit were purchased from
Promega Corporation. The Apoptosis assay kit was purchased from BD
Biosciences; Becton, Dickinson and Company. The specific primary
antibodies against p-gp (cat. no. ab262880), p21 (cat. no.
ab80633;), MMP-2 (cat. no. ab2462), MMP-9 (cat. no. ab119906),
phosphorylated (p)-AKT (cat. no. ab38449), p-Erk1/2 (cat. no.
ab214362), AKT (cat. no. ab18785), ERK (cat. no. ab109282),
Cleaved-caspase-3 (cat. no. ab2302), Caspase-3 (cat. no. ab4051),
Cleaved-caspase-9 (cat. no. ab2324), Caspase-9 (cat. no. ab52298),
Cleaved-PARP (cat. no. ab32561), PARP (cat. no. ab74290) and GAPDH
(cat. no. ab181602). All of these antibodies were purchased from
Abcam. ECL immunoblot substrate was purchased from EMD Millipore.
Flow cytometry was performed with a BD FACSCalibur™ machine (BD
Biosciences; Becton, Dickinson and Company). Microplate reader and
PCR instrument were from Thermo Fisher Scientific, Inc.
Cell culture and grouping
A549 and A549/DDP cells (1x103) were
cultured in a 10 cm culture dish in a 37˚C, 5% CO2 and
humidified incubator with 90% Eagle's minimum essential medium
(EMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.).
Cells were digested with 0.25% trypsin-EDTA for passage. The cells
were divided into 8 groups: Control group of normally cultured
A549/DDP cells, HNK group treated with 5 µg/ml of HNK, CUR group
treated with 10 µg/ml of CUR, DDP group treated with 5 µg/ml DDP
and HNK + CUR, HNK + DDP, CUR + DDP and HNK + CUR + DDP groups that
received combinations of drugs implied by the group names at the
same doses as the single drug groups.
MTS assay
Each group of A549/DDP cells was cultured and
treated as described above for 24, 48 and 72 h, then the medium was
aspirated, MTS reagent was added according to the manufacture's
protocol, then optical density value was measured at 490 nm by a
microplate reader and the proliferation rate of each group of
A549/DDP cells was calculated.
Colony formation assay
The proliferation of cells was detected by colony
formation assay. Briefly, cells (200 cells/well) were cultured in
6-well plates for 12 h and then treated with the drugs as described
above and incubated for two weeks. Cells were immobilized for 15
min with 95% methanol at room temperature and then stained with
hematoxylin at room temperature for 1 min. The clones were stained
by 0.1% crystal violet at room temperature and were counted by
light microscopy (magnification, x40). The clone formation rate was
calculated by using >50 cell colonies as one clone (Clone
formation rate = number of clones/number of inoculated cells
x100%).
Flow cytometry for apoptosis
Each group of A549/DDP cells (3x104) was
treated as described above for 48 h before 20 µl of PI and 20 µl of
Annexin V-FITC (BD Biosciences) were added to each group and
incubated for 15 min, the cells were then collected and adjusted to
1x104/ml for flow cytometry to determine apoptosis of
each group. Cell apoptosis was assessed using flow cytometry
(FACScan™; BD Biosciences) and analyzed using FlowJo 10.06 software
(FlowJo LLC).
Wound healing assay for migration
After counting, the cells were seeded to 6-well
plate at 5x106 cells/well and cultured with 10% serum at
37˚C. When the cells reached confluence, a straight line was
scratched using a 200 µl tip on the cell monolayer and the width of
the scratch was recorded under a microscope. The cells were washed
3 times with PBS, serum-free medium added and then treated as
described above for 0, 24 or 48 h. Cells were observed and images
were captured under a light microscope (magnification, x200;
Olympus Corporation). A total of three parallel wells were used for
each group and the experiment was carried out in triplicate.
Transwell assay for invasion
Matrigel was diluted with serum-free EMEM at 1:3 and
35-45 µl of the solution was added to the Transwell chambers and
incubated at 37˚C for 4-5 h. Cell density was adjusted to
1x105/ml with medium containing 5% FBS. Then, 0.2 ml of
the cell suspension was added the upper Transwell chambers of each
group and treated accordingly. Afterwards, 350 µl EMEM containing
20% FBS was added to the lower chambers and cultured at 37˚C, 5%
CO2 for 48 h. Cells on the upper surface of the chamber
was wiped off and the culture solution in the lower chamber was
replaced with 350 µl 0.5% Wright-Giemsa dye solution so that the
dye solution was just in contact with the lower surface of the
chamber. The lower surface of the chamber was brought into contact
with the surface of the dye solution and stained at room
temperature for 5 min. Then 175 µl of the dye was removed and 175
µl of ddH2O was added. After 10 min of incubation, 4
fields of view were imaged under a light microscope (magnification,
x400) and the number of invading cells counted using ImageJ
software V1.8.0.112 (National Institutes of Health).
RT-qPCR assay
A549/DDP cells (1x105) in logarithmic
growth phase were treated for 48 h and total RNA was extracted by
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The cDNA was
reverse transcribed by SuperScript™ IV CellsDirect™ cDNA Synthesis
Kit (Thermo Fisher Scientific, Inc.) according the following
temperature protocol: 42˚C for 60 min, 70˚C for 15 min and chilling
at 4˚C. qPCR was performed using a SYBR® Premix Ex
Taq™ (cat. no. DRR041A, Takara Bio, Inc.) on an Applied
Biosystems thermal cycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol: The
parameters for qPCR assays were denaturation at 94˚C for 3 min; 40
cycles of 95˚C for 5 sec, 65˚C for 35 sec and 72˚C for 60 sec; and
finally, 72˚C for 5 min. Fold changes were calculated using the
2-ΔΔCq method (19).
Primer sequences used were as follows: AKT forward,
5'-GGACAACCGCCATCCAGACT-3' and reverse, 5'-GCCAGGGACACCTCCATCTC-3',
Erk1/2 forward, 5'-CTAAACCACATCGGGAACCT-3' and reverse,
5'-TACTTCCGGGCTTTGATGGA-3'; P21 forward, 5'-CAGAGCCACAGGCACCAT-3'
and reverse, 5'-GCGAAGTCAAAGTTCCACC-3'; Caspase 3 forward,
5'-ATGGAGAACAATAAAACCT-3' and reverse, 5'-CTAGTGATAAAAGTAGAGTTC-3';
PARP forward, 5'-GGCATCGGAACTGGACGAGG-3' and reverse,
5'-CCCCACGAACGGAACAACCA-3'. P-gp forward,
5'-CGCAGGCAGGTGATAAGGGG-3' and reverse, 5'-GCAATGCGGTCTGCGTTCTG-3'.
MMP-2 forward, 5'-CAGGACATTGTCTTTGATGGCATCGC-3' and reverse,
5'-CAGGACATTGTCTTTGATGGCATCGC-3'; MMP-9 forward,
5'-CAGGACATTGTCTTTGATGGCATCGC-3' and reverse,
5'-CAGGACATTGTCTTTGATGGCATCGC-3' and GAPDH forward,
5'-GGACCTGACCTGCCGTCTAG-3' and reverse, 5'-GTAGCCCAGGATGCCCTTGA-3'.
GAPDH was used as an internal standard.
Immunofluorescence
The cells were permeabilized for 30 min in 0.5%
Triton X-100 and incubated for 1 h at room temperature with a
primary antibody diluted in PBS supplemented with 3% bovine serum
albumin (Beyotime Institute of Biotechnology). The cells were
rinsed with PBS and incubated with 2 µg/ml Alexa Fluor®
488-conjugated goat anti-rabbit IgG antibody (secondary antibody;
ab150085; 1:5,000) for 2 h at room temperature. Nuclei were
counterstained with DAPI with 5 min incubation at room temperature.
After washing with PBS, the slides were mounted in the Vectashield
mounting medium and examined under a FluoView FV1000 confocal
microscope (Olympus Corporation) and analyzed with the Olympus
FluoView ver. 1.7b viewer (Olympus Corporation).
Western blotting for protein levels of
P-gp, p-AKT, p-Erk1/2, P21, MMP-2, MMP-9 and apoptosis related
proteins
Each group of A549/DDP cells in the logarithmic
growth phase was treated with the corresponding treatment for 48 h
and lysed using cell lysis buffer (Beyotime Institute of
Biotechnology) to extract total protein. Protein concentrations of
the lysate were measured by the Bicinchoninic acid Protein Assay
kit (Beyotime Institute of Biotechnology) and equal amounts (5 µg)
of the protein loaded on a 12% SDS-PAGE for electrophoresis
separation. The protein bands were then transferred to PVDF
membrane. The membranes were then blocked with 5% non-fat milk in
TBS-0.1% Tween buffer at room temperature, following which they
were incubated with specific primary antibodies at 4˚C overnight,
washed, incubated with horseradish peroxidase-conjugated secondary
antibody (cat. no. ab97040; 1:1,000; Abcam) at room temperature for
1 h, washed again and developed with enhanced chemiluminescence
(EMD Millipore). GAPDH was used as the internal reference. ImageJ
software (ver. 1.48, National Institutes of Health) was used for
quantification of the band intensity. The dilutions used for each
of the specific primary antibodies were as follows: P-gp (1:1,000),
p21 (1:1,000), MMP-2 (1:1,000), MMP-9 (1:1,000), p-AKT (1:1,000),
p-Erk1/2 (1:1,000), AKT (1:1,000), ERK (1:1,000), Cleaved-caspase-3
(1:1,000), Caspase-3 (cat. no. ab4051; 1:1,000), Cleaved-caspase-9
(1:1,000), Caspase-9 (1:1,000), Cleaved-PARP (1:1,000), PARP
(1:1,000) and GAPDH (1:1,000).
Statistical analysis
All assays were repeated at least three times and
statistical analysis was performed using SPSS ver. 19.0 (IBM Corp.)
statistical software. Measurement data was expressed as mean ±
standard deviation and the difference between the groups was
compared by one-way analysis of variance followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of HNK, CUR and DDP on
A549/DDP cell viability
The MTS assay was performed to assess the effect of
HNK, CUR and DDP on the viability of A549 and A549/DDP cells, and
the optimal concentrations of HNK, CUR and DDP were selected for
further studies. A549 and A549/DDP cells were treated with
increasing concentrations of HNK (0, 5, 10, 20, 40 and 80 µg/ml),
CUR (0, 5, 10, 20, 40 and 80 µg/ml) and DDP (0, 5, 10, 20, 40 and
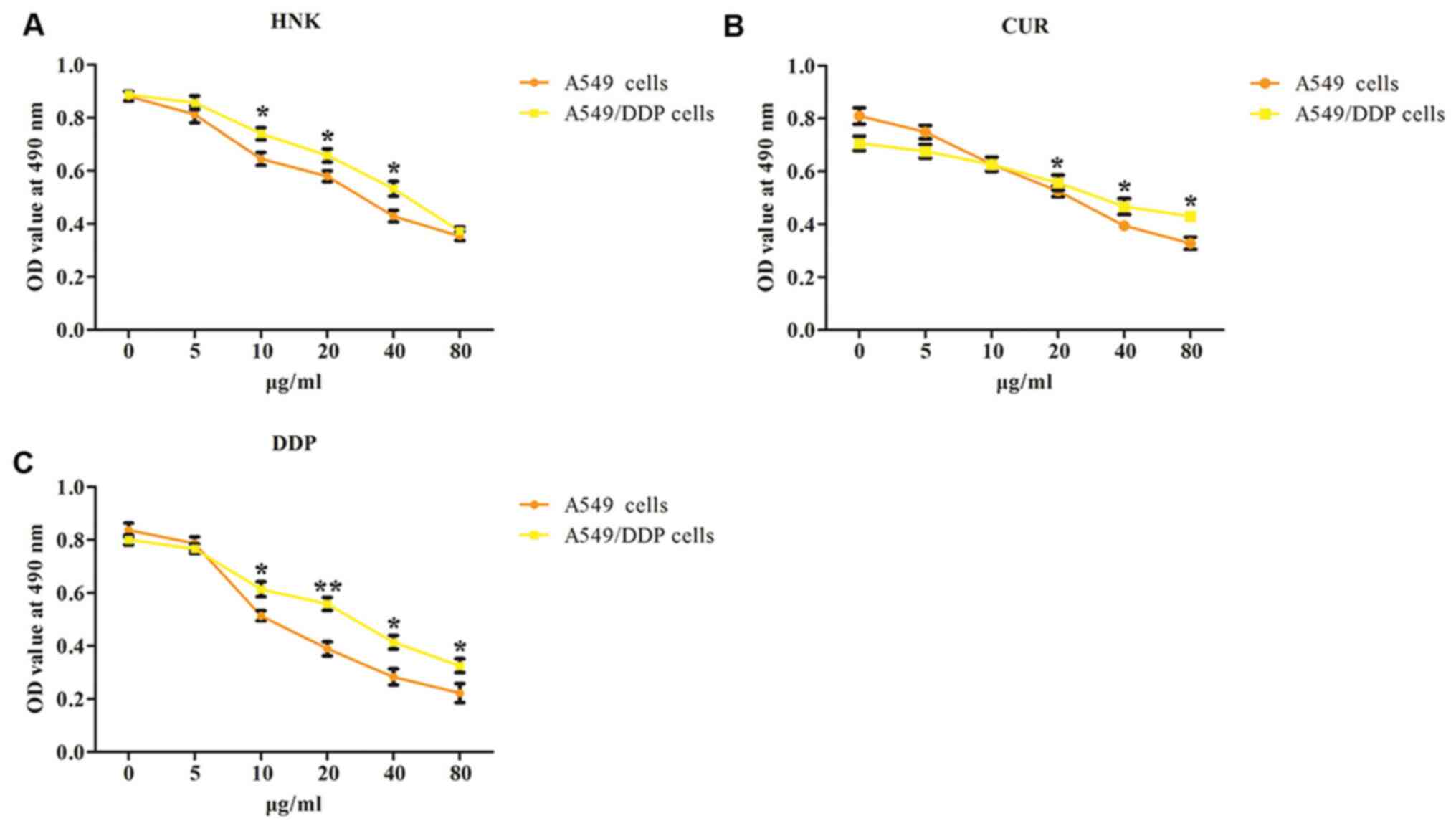
80 µg/ml) for 48 h. From the results of Fig. 1A-C, A549 cells were more sensitive
to HNK, CUR and DDP with increasing concentrations than A549/DDP
cells, in accordance with previous studies (15,20).
It was found that HNK (5 µg/ml), CUR (10 µg/ml) and DDP (5 µg/ml)
displayed no significant cytotoxicity in A549/DDP cells compared
with that in A549 cells. Therefore, HNK (5 µg/ml), CUR (10 µg/ml)
and DDP (5 µg/ml) were chosen as the optimal concentrations for
further experimentation.
Effects of HNK or/and CUR on activity
and proliferation of A549 cells and A549/DDP cells
The activity and proliferation of A549 cells and
A549/DDP cells was respectively detected by MTS assay and
colony-forming assay. Compared with the control group, all groups
could inhibit cell activity in A549 (Fig. 2A). However, as shown in Fig. 2B, compared with the control group,
the cell activity of HNK, CUR, DDP and HNK + CUR groups was not
significantly changed in A549/DDP cells. In contrast, the cell
activity in HNK + DDP and CUR + DDP groups was significantly
suppressed. Meanwhile, the cell activity rate of HNK + CUR + DDP
group was significantly depressed compared with that of HNK + DDP
or CUR + DDP groups. The results of cell proliferation were
consistent with MTS assay (Fig.
2C). The data revealed that HNK or/and CUR could reverse
resistance to DDP in A549/DDP cells.
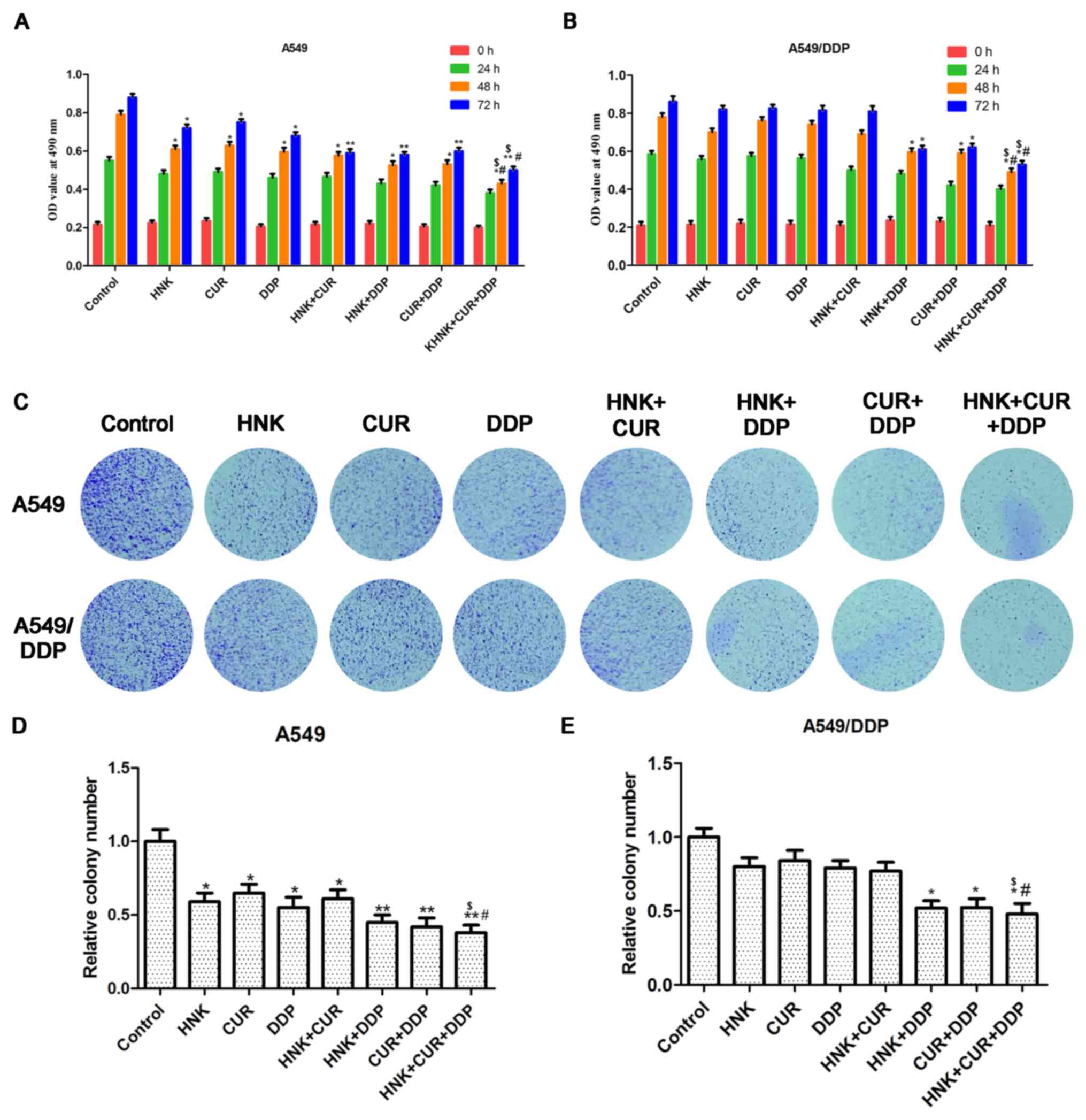 | Figure 2Effects of HNK or/and CUR on activity
and proliferation of A549/DDP cells. The activity of (A) A549 and
(B) A549/DDP cells administered HNK, CUR, DDP, HNK + CUR, HNK +
DDP, CUR + DDP and HNK + CUR + DDP were examined by MTS assay.
*P<0.05 and **P<0.01 vs. Control group,
#P<0.05 vs. HNK + DDP group, $P<0.05
vs. CUR + DDP group. (C) The proliferation of A549 and A549/DDP
cells administered with HNK, CUR, DDP, HNK + CUR, HNK + DDP, CUR +
DDP and HNK + CUR + DDP were examined by colony formation assay.
Quantification of (D) A549 and (E) A549/DDP cell colony formation.
Magnification, x40. The results were expressed as the mean ±
standard deviation of three independent experiments and each was
performed in triplicate. *P<0.05 and
**P<0.01 vs. control group, #P<0.05 vs.
HNK + DDP group, $P<0.05 vs. CUR + DDP group. HNK,
honokiol; CUR, curcumin; DDP, cisplatin; OD, optical density. |
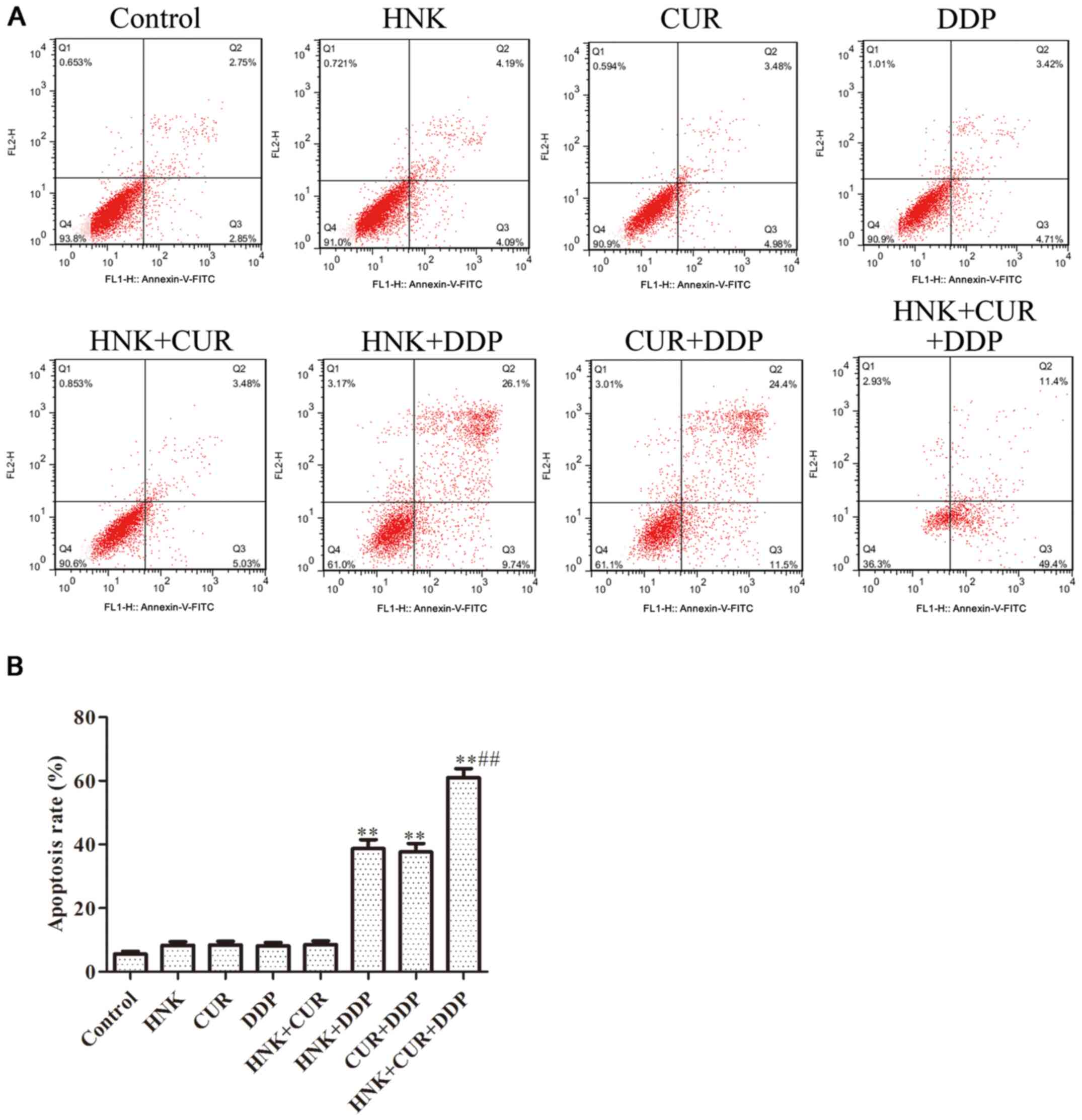
Effects of HNK or/and CUR on apoptosis
of A549/DDP cells by flow cytometry assay
The results of Fig.
3 indicated that after treatment with HNK, CUR, DDP and HNK +
CUR, the cell apoptosis rate was as same as that in the control
group. While A549/DDP cells were treated with HNK + DDP and CUR +
DDP, the apoptosis rate of A549/DDP cells was obviously enhanced.
Further, compared with HNK + DDP or CUR + DDP groups, the cell
apoptosis rate of HNK + Cur + DDP group was significantly
upregulated.
Effects of HNK or/and CUR on migration
of A549/DDP cells by wound healing assay
Following wound healing assay, the results of
Fig. 4 suggested that there was no
difference between the control group and HNK, CUR, DDP and HNK +
CUR groups. In addition, the wound healing rates were significantly
depressed with HNK + DDP and CUR + DDP treatment in 24 and 48 h
compared with wound healing rate of the control group. The results
identified that the wound healing rate of HNK + CUR + DDP group was
significantly downregulated compared with that of HNK + DDP or CUR
+ DDP groups in 24 and 48 h, respectively.
 | Figure 4Effects of HNK or/and CUR on
migration of A549/DDP cells by wound healing assay. (A) The
migration abilities of A549/DDP cells treated with HNK, CUR, DDP,
HNK + CUR, HNK + DDP, CUR + DDP and HNK + CUR + DDP for 24 or 48 h
were examined by wound healing assay. Magnification, x200. (B)
Wound healing percentage are shown in chart. *P<0.05
and **P<0.01 vs. control group, #P<0.05
vs. HNK + DDP group or CUR + DDP group. HNK, honokiol; CUR,
curcumin; DDP, cisplatin. |
Effects of HNK or/and CUR on invasion
of A549/DDP cells by Transwell assay
The data from Fig. 5
demonstrated that the invading cell numbers of HNK, CUR, DDP and
HNK + CUR groups were the same as the control group. After
treatment with HNK + DDP and CUR + DDP, the invading cell numbers
were significantly suppressed compared with the control group.
Compared with HNK + DDP or Cur + DDP groups, the invading cell
numbers of the HNK + CUR + DDP group was significantly
decreased.
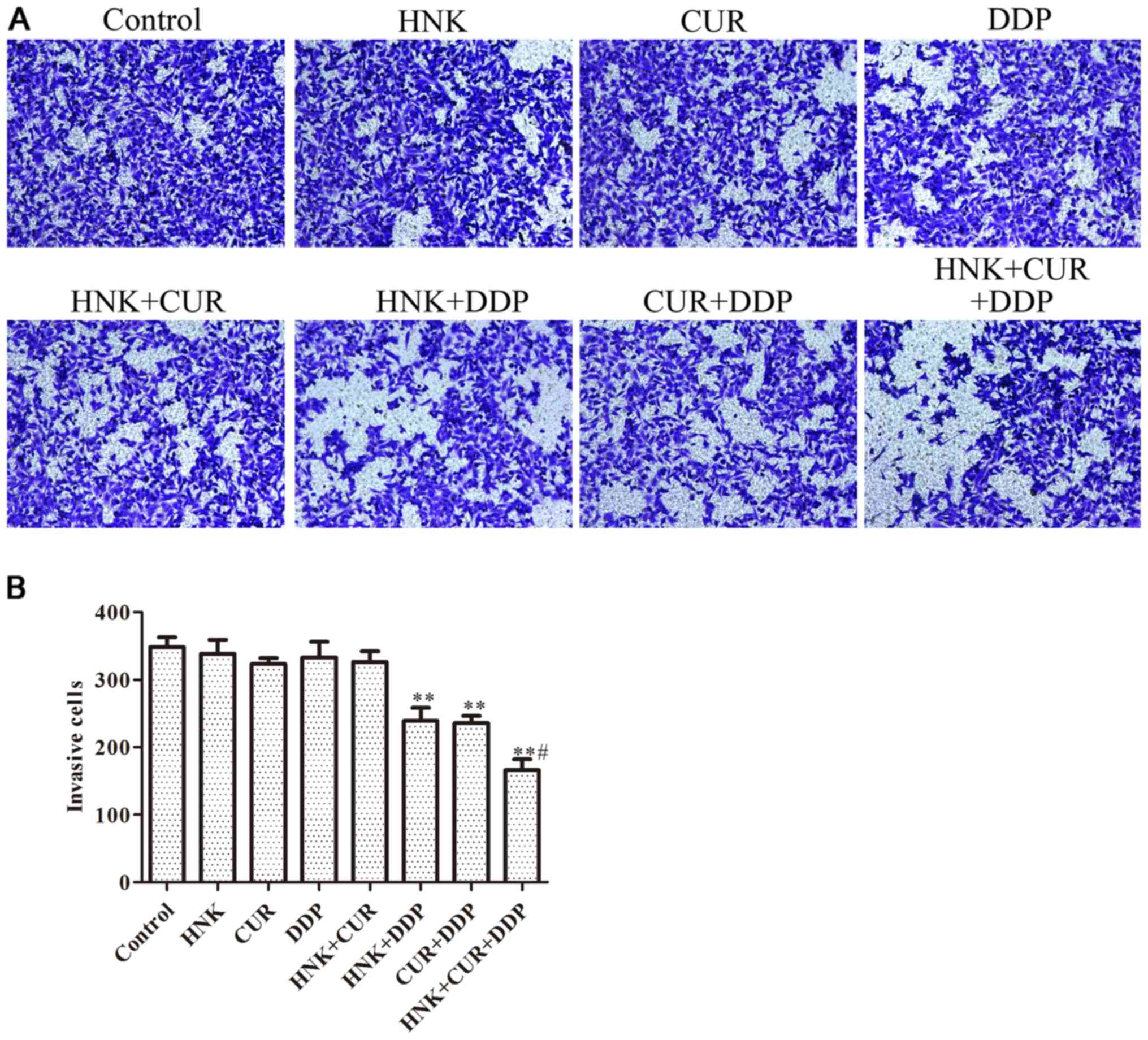 | Figure 5Effects of HNK or/and CUR on invasion
of A549/DDP cells by Transwell assay. (A) The invasion abilities of
A549/DDP cells treated with HNK, CUR, DDP, HNK + CUR, HNK + DDP,
CUR + DDP and HNK + CUR + DDP for 48 h were evaluated Transwell
invasion assay and (B) analyzed. Magnification, x200. The results
were expressed as the mean ± standard deviation of three
independent experiments and each was performed in triplicate.
**P<0.01 vs. control group, #P<0.05 vs.
HNK + DDP group or CUR + DDP group. HNK, honokiol; CUR, curcumin;
DDP, cisplatin. |
Effects of HNK or/and CUR on P-gp
protein nuclear volume of A549/DDP cells by immunofluorescence
assay
Following immunofluorescence, the results of
Fig. 6 demonstrated that the P-gp
nuclear volume of HNK, CUR, DDP and HNK + CUR groups was similar to
that of the control group. In addition, the P-gp nuclear volume of
HNK + DDP and CUR + DDP groups were significantly inhibited
compared with the control group, and the P-gp nuclear volume of HNK
+ CUR + DDP group was significantly downregulated compared with
that of HNK + DDP or CUR + DDP groups.
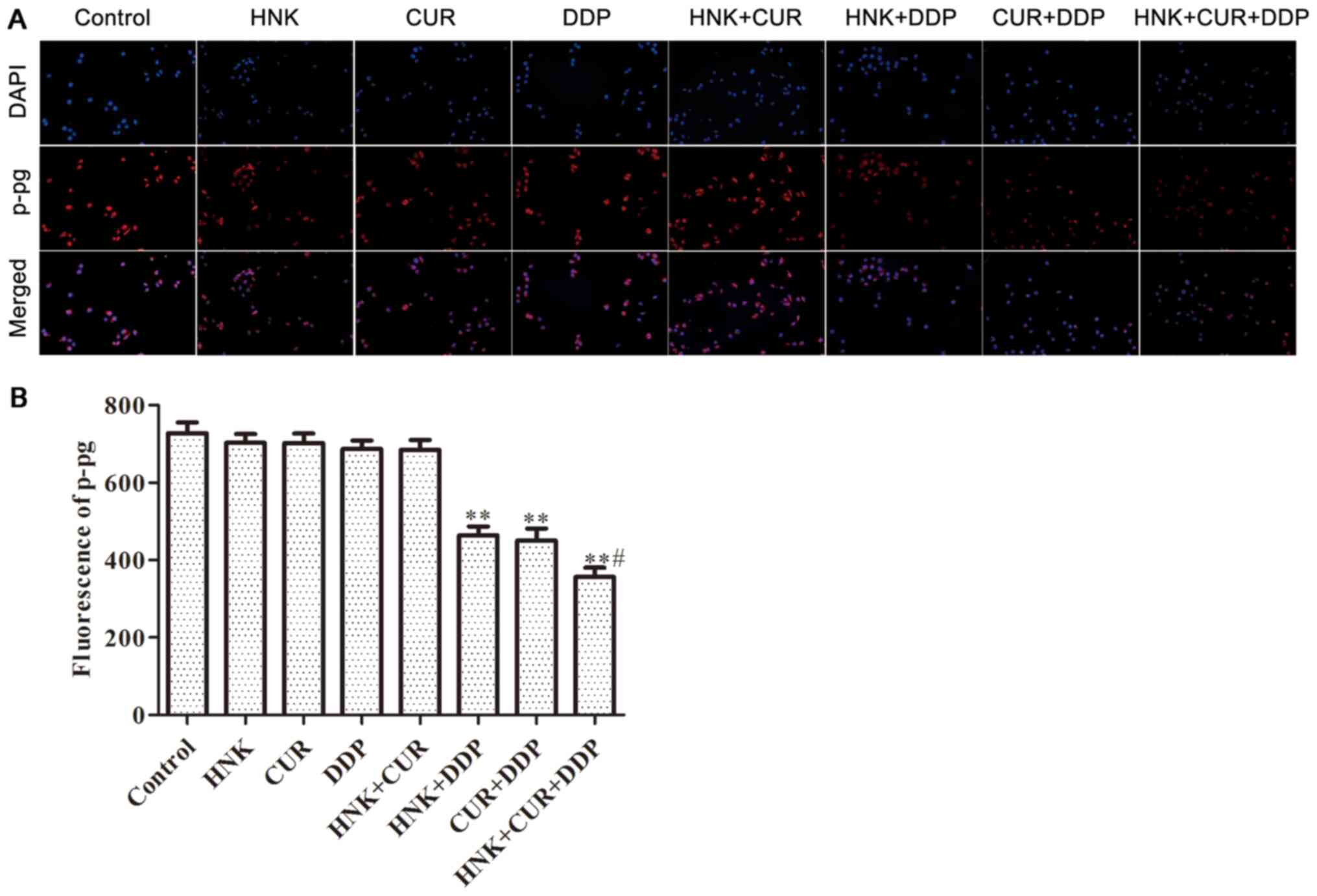 | Figure 6Effects of HNK or/and CUR on P-gp
protein nuclear volume of A549/DDP cells by immunofluorescence
assay. (A) The P-gp protein nuclear volume of A549/DDP cells
treated with HNK, CUR, DDP, HNK + CUR, HNK + DDP, CUR + DDP and HNK
+ CUR + DDP for 48 h were determined by immunofluorescence assay
and (B) analyzed. Magnification, x200. **P<0.01 vs.
control group, #P<0.05 vs. HNK + DDP group or CUR +
DDP group. HNK, honokiol; CUR, curcumin; P-gp, P-glycoprotein; DDP,
cisplatin. |
Effects of HNK or/and CUR on the mRNA
expression of the AKT/Erk signaling pathway by RT-qPCR assay
As demonstrated in Fig.
7, the mRNA expression of P-gp, p21, MMP-2, MMP-9, AKT, Erk1/2,
caspase-3, caspase-9 and PARP in HNK, CUR, DDP and HNK + CUR groups
was not obviously upregulated or downregulated compared with the
control group. Conversely, the P-gp, MMP-2, MMP-9, AKT and Erk1/2
mRNA expression of HNK + DDP and CUR + DDP groups was significantly
downregulated and the p21, caspase-3, caspase-9 and PARP mRNA
expression of the HNK + DDP and CUR + DDP groups was significantly
upregulated compared with those of control group. The effects of
HNK + CUR + DDP on the mRNA expression of P-gp, MMP-2, MMP-9, AKT
and Erk1/2 was increased compared than those of HNK + DDP or CUR +
DDP groups. P21, caspase-3, caspase-9 and PARP mRNA expression were
enhanced in HNK + CUR + DDP group.
 | Figure 7Effects of HNK or/and CUR on the
related mRNA expressions of AKT/Erk signal pathway by RT-qPCR. The
mRNA expression of (A) P-gp, (B) P21, (C) MMP-2, (D) MMP-9, (E)
Akt, (F) Erk1/2, (G) caspase-3, (H) caspase-9, (I) and PARP in
A549/DDP cells treated with HNK, CUR, DDP, HNK + CUR, HNK + DDP,
CUR + DDP and HNK + CUR + DDP for 48 h were evaluated by RT-qPCR
assay. The results were expressed as the mean ± standard deviation
of three independent experiments and each was performed in
triplicate. **P<0.01 vs. control group,
#P<0.05 vs. HNK + DDP group or CUR + DDP group. HNK,
honokiol; CUR, curcumin; RT-qPCR, reverse
transcription-quantitative PCR; P-gp, P-glycoprotein; MMP, matrix
metalloproteinase; PARP, poly ADP-ribose polymerase; DDP,
cisplatin; p, phosphorylated. |
Effects of HNK or/and CUR on the
protein levels of AKT/Erk signal pathway by western blot assay
The results of Fig.
8 demonstrated that there was no significant difference in
protein expression of P-gp, p21, MMP-2, MMP-9, p-AKT, AKT,
p-Erk1/2, ERK, caspase-3, cleaved-caspase-3, caspase-9, cleaved
caspase-9, PARP and cleaved PARP between the control group and HNK,
CUR, DDP and HNK + CUR groups. Following treatment with HNK + DDP,
the protein levels of P-gp, MMP-2, MMP-9, p-AKT and p-Erk1/2 were
respectively decreased by 54, 26, 38, 28 and 33%, and the protein
expression of p21, cleaved-caspase-3, cleaved caspase-9 and cleaved
PARP was respectively increased by 35, 21, 29 and 23%, compared
with the control. Following treatment with CUR + DDP, the protein
expression of P-gp, MMP-2, MMP-9, p-AKT and p-Erk1/2 was
respectively decreased by 58, 30, 36, 27 and 34%, and the protein
expression of p21, cleaved-caspase-3, cleaved caspase-9 and cleaved
PARP was respectively increased by 39, 20, 31 and 26%, compared
with the control. Following treatment with HNK + CUR + DDP, the
protein expression of P-gp, MMP-2, MMP-9, p-AKT and p-Erk1/2 was
respectively decreased by 65, 45, 51, 40 and 50%, and the protein
expression of p21, cleaved-caspase-3, cleaved caspase-9 and cleaved
PARP was respectively increased by 58, 34, 47 and 48%, compared
with the control.
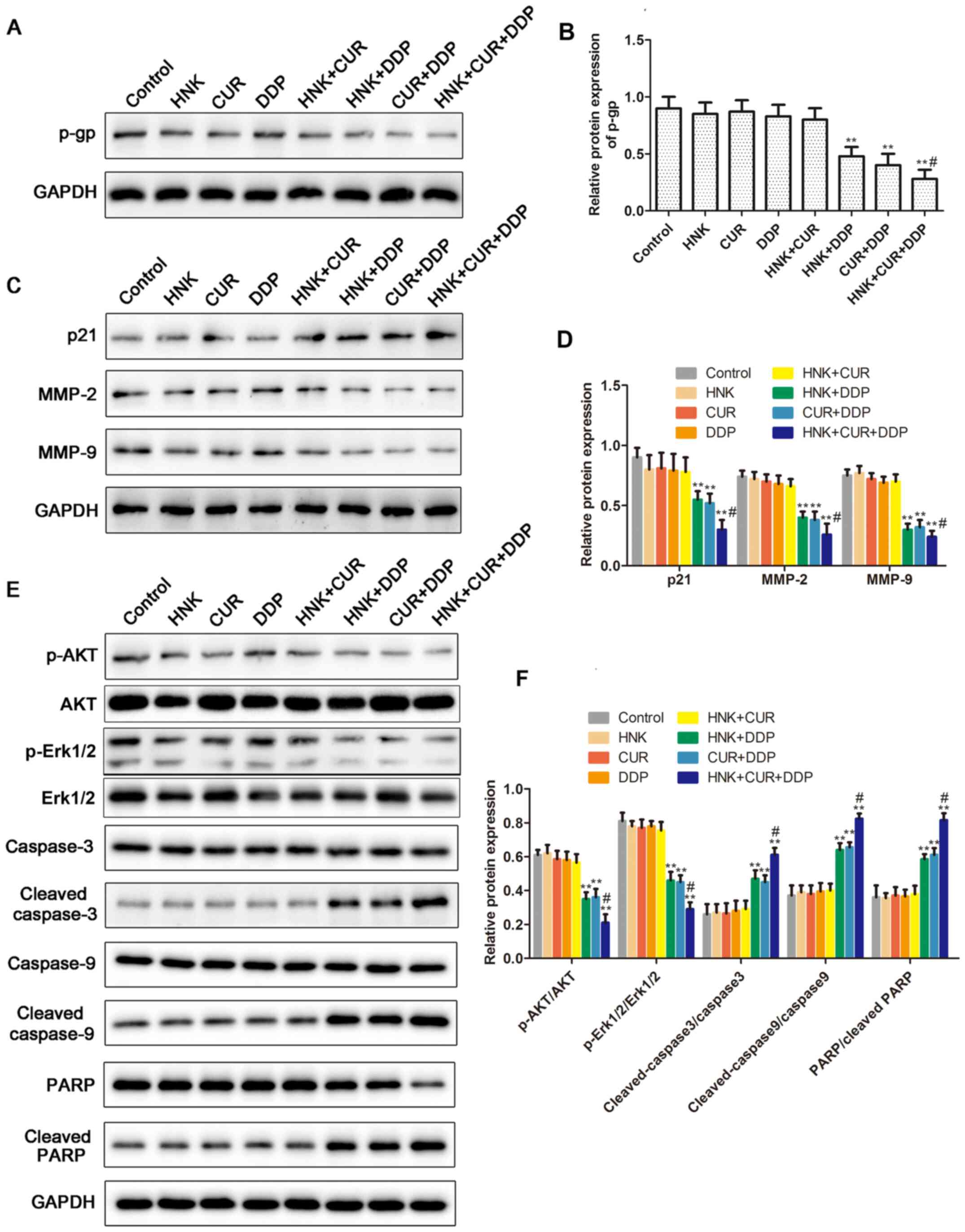 | Figure 8Effects of HNK or/and CUR on the
related protein expression of the AKT/Erk signal pathway by western
blot assay. (A) The protein expression of P-gp and (B) analysis.
(C) The protein expression of P21, MMP-2 and MMP-9 and (D) analysis
of in A549/DDP cells treated with HNK, CUR, DDP, HNK + CUR, HNK +
DDP, CUR + DDP and HNK + CUR + DDP for 48 h were evaluated by
western blot assay. (E) Protein levels of p-AKT, AKT, p-Erk1/2,
Erk1/2, caspase-3, cleaved caspase-3, caspase-9, cleaved caspase-9,
PARP and cleaved PARP and (F) densitometry analysis. The band
intensity was quantified by ImageJ software. **P<0.01
vs. control group, #P<0.05 vs. HNK + DDP group or CUR
+ DDP group. HNK, honokiol; CUR, curcumin; MMP, matrix
metalloproteinase; DDP, cisplatin. |
Discussion
HNK is a natural compound that not only shows
antibacterial, anti-inflammatory and anti-anxiety effects, but also
promotes the apoptosis of tumor cells (21). Relevant studies have confirmed that
HNK can reverse drug resistance of both solid tumors (such as
breast cancer) and hematopoietic tumors (such as myeloma) (22-24).
Li et al (25) found that
HNK inhibited the expression of ZEB2 by upregulating microRNA-141,
thereby reducing the stemness of renal cancer cells. Treatment of
oral cancer stem cells with magnolol induced stem cell apoptosis
and inhibited the Wnt/β-catenin signaling pathway, which is also
important for stemness maintenance (26). In colon cancer cells, HNK
significantly inhibited activity of the Notch signaling pathway
(27). HNK can also damage glioma
stem cells and reverse drug-resistance of glioma cells (28). CUR is a phenolic pigment extracted
from Curcuma longa, a plant of the ginger family. CUR shows
strong effects to induce apoptosis of tumor cells and to inhibit
tumor cell proliferation and invasion (29). Previous studies have demonstrated
that CUR can also effectively reverse MDR of multiple types of
tumors (30-32).
Almanaa et al (33) found
that CUR treatment not only kills normal esophageal cancer cells,
but also kills esophageal cancer stem cells. CUR can induce
apoptosis of colorectal cancer stem cells by binding to CD44
antigen on the surface of colorectal cancer stem cells (34). The E-cadherin/β-catenin negative
feedback signal in breast cancer cells can be amplified by CUR,
which leads to the obstruction of tumor stem cell migration
(35). The results of the present
study indicated that HNK or CUR reversed drug resistance of
A549/DDP cells to DDP alone and combination of HNK and CUR
demonstrated stronger drug resistance reversal effects.
P-gp binds to the chemotherapeutic drug molecules
after they enter the tumor cells and pumps the drugs out of the
cell using energy derived from ATP hydrolysis, thus reducing
intracellular drug concentration, hence overexpression of P-gp
causes resistance to the drug (36). The results of the present study
demonstrated that HNK or CUR combined with DDP reduced P-gp
expression and reduced nuclear import of P-gp, which are considered
an important part in reversing drug resistance of A549/DDP cells.
HNK and CUR combined with DDP had a stronger inhibitory effect on
P-gp expression.
Activation of the AKT/ERK signaling pathway is
closely related to enhanced tumor activity and reducing P-gp
expression has been shown to effectively inhibit occurrence and
progression of tumors (37-39).
In the present study, HNK or CUR combined with DDP decreased
activation of p-AKT and p-ERK1/2 compared with the control in
A549/DDP cells, and the effect of HNK and CRU was more evident.
Caspase-3 and caspase-9 are the key proteases concerned with cell
apoptosis. Activated caspase-3 and caspase-9 can be split into
cleaved caspase-3 and cleaved caspase-9, and then hydrolyze cell
structural proteins to promote the disintegration of apoptotic
cells. Furthermore, PARP is split into cleaved-PARP, which
ultimately leads to apoptosis. Cleavage levels of caspase-3,
caspase-9 and PARP can reflect the level of apoptosis (40,41).
The present study demonstrated that the ratios of cleaved
caspase-3/caspase-3, cleaved caspase-9/caspase-9 and cleaved
PARP/PARP were increased following activation of AKT/ERK signal
pathway in A549/DDP cells. The results revealed that HNK or CRU
combined with DDP inhibited cell proliferation, induced apoptosis
and increased the ratios of cleaved caspase-3/caspase-3, cleaved
caspase-9/caspase-9 and cleaved PARP/PARP in A549/DDP cells, and
the effect of HNK and CRU was clear. A previous study found that
p21 is also involved in the migration and invasion of cancer cells
(42). MMP-2/9 has been shown to be
involved in tumor invasion and migration (43). MMPs are the main rate-limiting
enzymes regulating the metabolism of the extracellular matrix and
are named for their ability to degrade extracellular matrix and
their need for metal ions such as Ca2 + and Zn2
+ as cofactors (44). MMP-2
and MMP-9 can degrade gelatin, laminin and type IV collagen, and
play an important role in invasion and metastasis of various
malignant tumors (45,46). The results of the present study
demonstrated that HNK or/and CUR inhibited invasion and metastasis
of A549/DDP cells, and decreased the expression of p21, MMP-2 and
MMP-9. These effects could reverse the resistance of A549/DDP cells
to DDP by increasing P-gp expression, by inhibiting cell
proliferation and promoting apoptosis, and by inhibiting cell
migration and invasion.
HNK and CUR are extracts in traditional Chinese
medicine, which possess low toxicity, and have been shown to have
antitumor effects. Although the reversal effect of the two drugs is
weak when they are used alone, the effect is enhanced when they are
used together. The present study demonstrated that the combination
of the two low-toxicity drugs with synergistic effect can
significantly enhance the reversal effect. This provides a strong
theoretical and experimental basis for the clinical application of
new drugs to reverse tumor MDR. In summary, the results of the
present study demonstrated that HNK and CUR could reverse drug
resistance of lung cancer cells and the two in combination
demonstrated synergistic effects and could significantly reverse
the drug resistance of lung cancer cell lines. The present study
described the inhibitory effects of HNK or/ and CUR on lung cancer
resistance in vitro, but not in vivo. Moreover, some
of the images are of poor quality.
Acknowledgements
Not applicable.
Funding
Funding: This present study was supported by the National
Natural Science Foundation of Jiangsu Higher Education Institutions
(grant no. 17KJB360008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MQ designed the experiments. XC and LB were the
major contributors to the interpretation of data for the work. HZ
and JM performed the experiments. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zheng R, Zeng H, Zhang S, Fan Y, Qiao Y,
Zhou Q and Chen W: Lung cancer incidence and mortality in China,
2010. Thorac Cancer. 5:330–336. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
She J, Yang P, Hong Q and Bai C: Lung
cancer in China: Challenges and interventions. Chest.
143:1117–1126. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schwartz AM and Rezaei MK: Diagnostic
surgical pathology in lung cancer: Diagnosis and management of lung
cancer, 3rd ed: American College of Chest Physicians evidence-based
clinical practice guidelines. Chest. 143 (Suppl 5):Se251–Se262.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Collins LG, Haines C, Perkel R and Enck
RE: Lung cancer: Diagnosis and management. Am Fam Physician.
75:56–63. 2007.PubMed/NCBI
|
|
5
|
Goldstraw P, Ball D, Jett JR, Le Chevalier
T, Lim E, Nicholson AG and Shepherd FA: Non-small-cell lung cancer.
Lancet. 378:1727–1740. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fennell DA, Summers Y, Cadranel J, Benepal
T, Christoph DC, Lal R, Das M, Maxwell F, Visseren-Grul C and Ferry
D: Cisplatin in the modern era: The backbone of first-line
chemotherapy for non-small cell lung cancer. Cancer Treat Rev.
44:42–50. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Amable L: Cisplatin resistance and
opportunities for precision medicine. Pharmacol Res. 106:27–36.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Holford J, Sharp SY, Murrer BA, Abrams M
and Kelland LR: In vitro circumvention of cisplatin resistance by
the novel sterically hindered platinum complex AMD473. Br J Cancer.
77:366–373. 1998.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Averett C, Arora S, Zubair H, Singh S,
Bhardwaj A and Singh AP: Molecular targets of Honokiol: A promising
phytochemical for effective cancer management. Enzymes. 36:175–193.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Park E, Min H, Chung H, Hong J, Kang Y,
Hung T, Youn U, Kim Y, Bae K, Kang S and Lee S: Down-regulation of
c-Src/EGFR-mediated signaling activation is involved in the
honokiol-induced cell cycle arrest and apoptosis in MDA-MB-231
human breast cancer cells. Cancer Lett. 277:133–140.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fan Y, Xue W, Schachner M and Zhao W:
Honokiol eliminates glioma/glioblastoma stem cell-like cells via
JAK-STAT3 signaling and inhibits tumor progression by targeting
epidermal growth factor receptor. Cancers (Basel).
11(22)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sengupta S, Nagalingam A, Muniraj N,
Bonner MY, Mistriotis P, Afthinos A, Kuppusamy P, Lanoue D, Cho S,
Korangath P, et al: Activation of tumor suppressor LKB1 by honokiol
abrogates cancer stem-like phenotype in breast cancer via
inhibition of oncogenic Stat3. Oncogene. 36:5709–5721.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu H, Yin Z, Wang L, Li F and Qiu Y:
Honokiol improved chondrogenesis and suppressed inflammation in
human umbilical cord derived mesenchymal stem cells via blocking
nuclear factor-κB pathway. BMC Cell Biol. 18(29)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li Y and Zhang T: Targeting cancer stem
cells by curcumin and clinical applications. Cancer Lett.
346:197–205. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng Q, Liao M, Hu H, Li H and Wu L:
Asiatic acid (AA) sensitizes multidrug-resistant human lung
adenocarcinoma A549/DDP cells to cisplatin (DDP) via downregulation
of P-Glycoprotein (MDR1) and its targets. Cell Physiol Biochem.
47:279–292. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen LP, Wang P, Sun YJ and Wu YJ: Direct
interaction of avermectin with epidermal growth factor receptor
mediates the penetration resistance in Drosophila larvae. Open
Biol. 6(150231)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu Z, Sun M, Lu K, Liu J, Zhang M, Wu W,
De W, Wang Z and Wang R: The long noncoding RNA HOTAIR contributes
to cisplatin resistance of human lung adenocarcinoma cells via
downregualtion of p21WAF1/CIP1 expression. PLoS One.
8:e77293–e77303. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lin Y, Duan Q and Yang YP:
Immunohistochemistry of phosphatase and tensin homolog and
metalloproteinase-9 in breast invasive micropapillary carcinoma.
Eur J Gynaecol Oncol. 40:380–383. 2019.
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dong Z, Ren L, Lin L and Li J, Huang Y and
Li J: Effect of microRNA-21 on multidrug resistance reversal in
A549/DDP human lung cancer cells. Mol Med Rep. 11:682–690.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xu D, Lu Q and Hu X: Down-regulation of
P-glycoprotein expression in MDR breast cancer cell MCF-7/ADR by
honokiol. Cancer Lett. 243:274–280. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ishitsuka K, Hideshima T, Hamasaki M, Raje
N, Kumar S, Hideshima H, Shiraishi N, Yasui H, Roccaro AM,
Richardson P, et al: Honokiol overcomes conventional drug
resistance in human multiple myeloma by induction of
caspase-dependent and -independent apoptosis. Blood. 106:1794–1800.
2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee S, Khoo C, Halstead CW, Huynh T and
Bensoussan A: Liquid chromatographic determination of honokiol and
magnolol in hou po (Magnolia officinalis) as the raw herb
and dried aqueous extract. J AOAC Int. 90:1210–1218.
2019.PubMed/NCBI
|
|
24
|
Chio CC, Chen KY, Chang CK, Chuang JY, Liu
CC, Liu SH and Chen RM: Improved effects of honokiol on
temozolomide-induced autophagy and apoptosis of drug-sensitive and
-tolerant glioma cells. BMC Cancer. 18(379)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li W, Wang Q, Su Q, Ma D, An C, Ma L and
Liang H: Honokiol suppresses renal cancer cells' metastasis via
dual-blocking epithelial-mesenchymal transition and cancer stem
cell properties through modulating miR-141/ZEB2 signaling. Mol
Cells. 37:383–388. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yao C, Lai G, Yeh C, Lai M, Shih P, Chao
W, Whang-Peng J, Chuang S and Lai T: Honokiol eliminates human oral
cancer stem-like cells accompanied with suppression of
Wnt/β-catenin signaling and apoptosis induction. Evid Based
Complement Alternat Med. 2013(146136)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wynn M, Consul N, Merajver S and Schnell
S: Inferring the effects of honokiol on the notch signaling pathway
in SW480 colon cancer cells. Cancer Inform. 13 (Suppl 5):S1–S12.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lai I, Shih P, Yao C, Yeh C, Wang-Peng J,
Lui T, Chuang S, Hu T, Lai T and Lai G: Elimination of cancer
stem-like cells and potentiation of temozolomide sensitivity by
Honokiol in glioblastoma multiforme cells. PLoS One.
10(e0114830)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ye M and Zhang J and Zhang J, Miao Q, Yao
L and Zhang J: Curcumin promotes apoptosis by activating the
p53-miR-1922-5p/215-XIAP pathway in non-small cell lung cancer.
Cancer Lett. 357:196–205. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lv L, Qiu K, Yu X, Chen C, Qin F, Shi Y,
Ou J, Zhang T, Zhu H, Wu J, et al: Amphiphilic copolymeric micelles
for doxorubicin and curcumin co-delivery to reverse multidrug
resistance in breast cancer. J Biomed Nanotechnol. 12:973–985.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yuan HB, Meng PY and Qi LJ: Curcumin
up-regulates miR-133a expression to inhibit hepatocellular
carcinoma cell migration and invasion. World Chinese J Digestol,
2019.
|
|
32
|
Hosseini A, Rasmi Y, Rahbarghazi R,
Aramwit P, Daeihassani B and Saboory E: Curcumin modulates the
angiogenic potential of human endothelial cells via FAK/P-38 MAPK
signaling pathway. Gene. 688:7–12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Almanaa T, Geusz M and Jamasbi R: Effects
of curcumin on stem-like cells in human esophageal squamous
carcinoma cell lines. BMC Complement Altern Med.
12(195)2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Huang Y, Lin Y, Chiu H and Chiang B:
Curcumin induces apoptosis of colorectal cancer stem cells by
coupling with CD44 marker. J Agric Food Chem. 64:2247–2253.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mukherjee S, Mazumdar M, Chakraborty S,
Manna A, Saha S, Khan P, Bhattacharjee P, Guha D, Adhikary A,
Mukhjerjee S and Das T: Curcumin inhibits breast cancer stem cell
migration by amplifying the E-cadherin/β-catenin negative feedback
loop. Stem Cell Res Ther. 5(116)2014.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Wilkens S: Structure and mechanism of ABC
transporters. F1000Prime Rep. 7(14)2015.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Liu CH, Huang Q, Jin ZY, Zhu CL, Liu Z and
Wang C: miR-21 and KLF4 jointly augment epithelial-mesenchymal
transition via the Akt/ERK1/2 pathway. Int J Oncol. 50:1109–1115.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhang S, Bian H, Li X, Wu H, Bi Q, Yan Y
and Wang Y: Hydrogen sulfide promotes cell proliferation of oral
cancer through activation of the COX2/AKT/ERK1/2 axis. Oncol Rep.
35:2825–2832. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Krebs K, Ruusmann A, Simonlatser G and
Velling T: Expression of FLNa in human melanoma cells regulates the
function of integrin α1β1 and phosphorylation and localisation of
PKB/AKT/ERK1/2 kinases. Eur J Cell Biol. 94:564–575.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hu Y, Hong Y, Xu Y, Liu P, Guo DH and Chen
Y: Inhibition of the JAK/STAT pathway with ruxolitinib overcomes
cisplatin resistance in non-small-cell lung cancer NSCLC.
Apoptosis. 19:1627–1636. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang W, Zhou H, Yu Y, Li J, Li H, Jiang
D, Chen Z, Yang D, Xu Z and Yu Z: Combination of gambogic acid with
cisplatin enhances the antitumor effects on cisplatin-resistant
lung cancer cells by downregulating MRP2 and LRP expression. Onco
Targets Ther. 9:3359–3368. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dai M, Alodaini AA, Filsaimé N, Villatoro
MA, Guo J, Arakelian A, Rabbani SA, Ali S and Lebrun JJ: Cyclin D1
cooperates with p21 to regulate TGFβ-mediated breast cancer cell
migration and tumor local invasion. Breast Cancer Res.
15(3246)2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Giganti Μg, Tresoldi I, Sorge R,
Melchiorri G, Triossi T, Masuelli L, Lido P, Albonici L, Foti C,
Modesti A and Bei R: Physical exercise modulates the level of serum
MMP-2 and MMP-9 in patients with breast cancer. Oncol Lett.
12:2119–2126. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Jayakumar T, Liu CH, Wu GY, Lee TY,
Manubolu M, Hsieh CY, Yang CH and Sheu JR: Hinokitiol inhibits
migration of A549 lung cancer cells via Suppression of MMPs and
induction of antioxidant enzymes and apoptosis. Int J Mol Sci.
19(939)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Laskowska M: Altered maternal serum matrix
metalloproteinases MMP-2, MMP-3, MMP-9, and MMP-13 in severe early-
and late-onset preeclampsia. Biomed Res Int.
2017(6432426)2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen J, Ren Z, Zhu M and Khalil RA:
Decreased homodimerization and increased TIMP-1 complexation of
uteroplacental and uterine arterial matrix metalloproteinase-9
during hypertension-in-pregnancy. Biochem Pharmacol. 138:81–95.
2017.PubMed/NCBI View Article : Google Scholar
|