Introduction
Rheumatoid arthritis (RA) is a chronic
immune-mediated inflammatory disease characterized by deformity of
the joints and functional disability (1). Limb swelling, pain, stiffness and bone
erosion at the joint have been reported to primarily result from
inflammatory cytokine release and propagation in the synovial joint
(2). Furthermore, it is now
accepted that osteoporosis in RA is associated with an imbalance
between bone formation and absorption, leading to a decrease in
bone mineral density (BMD) and subsequent deterioration of the
trabecular microstructure (3).
RA-associated chronic synovitis is largely due to the expression of
proinflammatory cytokines, such as IL-17, TNF-α, IL-1β and
macrophage colony-stimulating factor (M-CSF), which independently
stimulate osteoclast differentiation, thereby significantly
increasing bone resorption and formation (4).
Panax notoginseng saponins (PNS) is the
primary active compound obtained from the Traditional Chinese
Medicinal plant Panax notoginseng, which has been used to
treat musculoskeletal injuries in China for hundreds of years
(5). PNS was previously shown to
possess various pharmacological properties, including its
beneficial effects on cardiomyocytes and brain cells (6). Furthermore, PNS may attenuate
atherogenesis by promoting blood circulation and it may also exert
immunoregulatory effects according to a previous study (7). PNS has also been shown to exert a
considerable clinical effect in the relief of RA symptoms (8). Furthermore, Cai et al (9) reported that PNS had noticeably
improved the clinical manifestations of joint pain, tenderness and
swelling associated with RA at the 2-year follow-up. PNS prevents
the progression of RA, which may be associated with a decrease in
the numbers of Th17 and Th1 cells, and inhibits the production of
INF-γ and IL-17(10). PNS has also
been reported to stimulate bone formation in excess of resorption,
and to inhibit osteoclast activity in vitro (11). Jang et al (12) reported that the PNS-induced
regulation of MMPs and osteoclastogenesis is responsible, in part,
for its bone-protective effects. Therefore, it was hypothesized
that PNS may be used to treat RA-associated joint destruction and
osteoporosis. The present study was undertaken to investigate the
mechanism through which PNS relieves joint destruction, in the hope
of providing further experimental evidence to support the clinical
use of PNS in the treatment of patients with RA.
Materials and methods
Animals and treatments
PNS powder was purchased from Beijing Qihuang
Pharmaceutical Co., Ltd. Indomethacin was used as the positive
control treatment; each tablet contained 25 mg indomethacin, and
was purchased from Mundipharma International Ltd. A total of 37
adult female New Zealand rabbits, aged 6 months and weighing 2.5-3
kg, were purchased from the Beijing Experimental Animal Center.
According to previous research (13), based on the total success rate of
model establishment, estimation of sample size through power
calculations using a two-sided t-test indicated that ~6 rabbits
would be required for each group. Therefore, 37 rabbits in total
were included in this trial. All rabbits were allowed access to
water and food ad libitum and were housed at a temperature
of 23˚C and humidity of 45%, with a 12-h light/dark cycle. Body
weight, food intake and animal health were examined daily.
The antigen-induced arthritis (AIA) model was
generated as previously described (13). Briefly, all rabbits were allowed to
acclimate for 1 week, and 30 of the 37 rabbits were then
subcutaneously immunized with 500 µg ovalbumin in complete Freund's
adjuvant emulsion (Sigma-Aldrich; Merck KGaA). Injections were
administered once a week for 2 weeks, and the AIA rabbits were then
boosted with 100 µg ovalbumin via and intra-articular injection
into the knee joints, once a week for the following 4 weeks.
After 6 weeks of treatment, all 30 AIA rabbits were
scored according to the symptoms presented in Table I (14); 21 rabbits with clinical symptom
scores >5 were randomized into three groups. The first arthritic
rabbit group did not receive any treatment (AIA, n=7). The second
arthritic rabbit group was treated with PNS by means of
intragastric administration (gavage) at a dose of 75 mg/kg body
weight per day (PNS, n=7); PNS (75 mg/kg body weight per day) was
diluted in distilled water and each rabbit was administered 20 ml
by gavage. The third arthritic rabbit group received indomethacin
by gavage at a dose of 10 mg/kg body weight per day (Control, n=7);
indomethacin powder (10 mg/kg body weight) was diluted with normal
saline and shaken to generate a 20-ml suspension for gavage. The
rationale for selecting a dose of 10 mg/kg was that it equates to 9
times the clinical dose for a 70-kg adult. Rabbits in the normal
group (n=7) did not receive treatment of any type, and had free
access to distilled water and food. At 3 months post-treatment, the
rabbits were sacrificed with a lethal dose of sodium pentobarbital
(100 mg/kg, intravenous), and death was confirmed by the absence of
a heartbeat and visible breathing. The knee joint and first lumbar
vertebra were then collected for further experimentation. All
procedures were authorized by the Animal Care Committee of Shanghai
University of Traditional Chinese Medicine (Shanghai, China). All
animal experimental protocols were conducted in accordance with the
Institutional Animal Care and Use Committee of Shin Nippon
Biomedical Laboratories, Ltd. and the National Institutes of Health
Guide for Care and Use of Laboratory Animals (15).
 | Table ICriteria for arthritis activity
score. |
Table I
Criteria for arthritis activity
score.
| Arthritis
symptoms | Score |
|---|
| No joint
swelling | 0 |
| Redness or swelling
of one joint | 1 |
| Redness and swelling
of more than one joints | 2 |
| Ankle and
tarsal-metatarsal joint involvement | 3 |
| Entire leg and paw
redness or swelling | 4 |
Morphological analysis of skeletal
muscle
Histopathological analysis of selected muscles was
conducted. The quadriceps femoris muscle of the knee joint was
dissected from the left side, washed in PBS, fixed at 4˚C overnight
in 4% polyformaldehyde, and then dehydrated in a graded ethanol
series. Tissue sections were embedded in paraffin and cut into 4-µm
sections, and then stained at 25˚C with 1% hematoxylin and eosin
(H&E) for histopathological evaluation using an BX54 optical
microscope (Olympus Corporation; magnification, x300).
Processing for transmission electron
microscopy (TEM)
Articular cartilage pieces from the left distal
femur were prepared for TEM according to the procedure described by
Chen et al (16). In order
to remove organic material, the samples were treated with 10% EDTA
for 3 weeks at a temperature of 30˚C followed by increasing
concentrations of alcohol (75-100%), slowly embedded at 37˚C in
100% Epon 812 resin for 48 h, and then cut into 50-nm sections.
Tissue samples were double-stained at 37˚C with 2% uranyl and 1%
phosphotungstic acid for TEM analysis.
Skeletal microstructure analysis
As previously described (17), the right distal femur and the first
lumbar vertebra were prepared for micro-computed tomography
(micro-CT) examination using a Locus SP scanner (GE
Healthcare). The microstructural parameters of the
trabeculae, such as trabecular thickness, trabecular bone volume
fraction (BV/TV), trabecular spacing (Tb.Sp), trabecular number
(Tb.N) and BMD, were evaluated.
Statistical analysis
Continuous variables are presented as the mean ± SD.
Mean differences between the control and experimental groups were
compared by one-way ANOVA followed by Tukey's post hoc test. All
statistical analyses were performed using SPSS 25.0 (IBM Corp.),
and two-sided P<0.05 was considered to indicate a statistically
significant difference.
Results
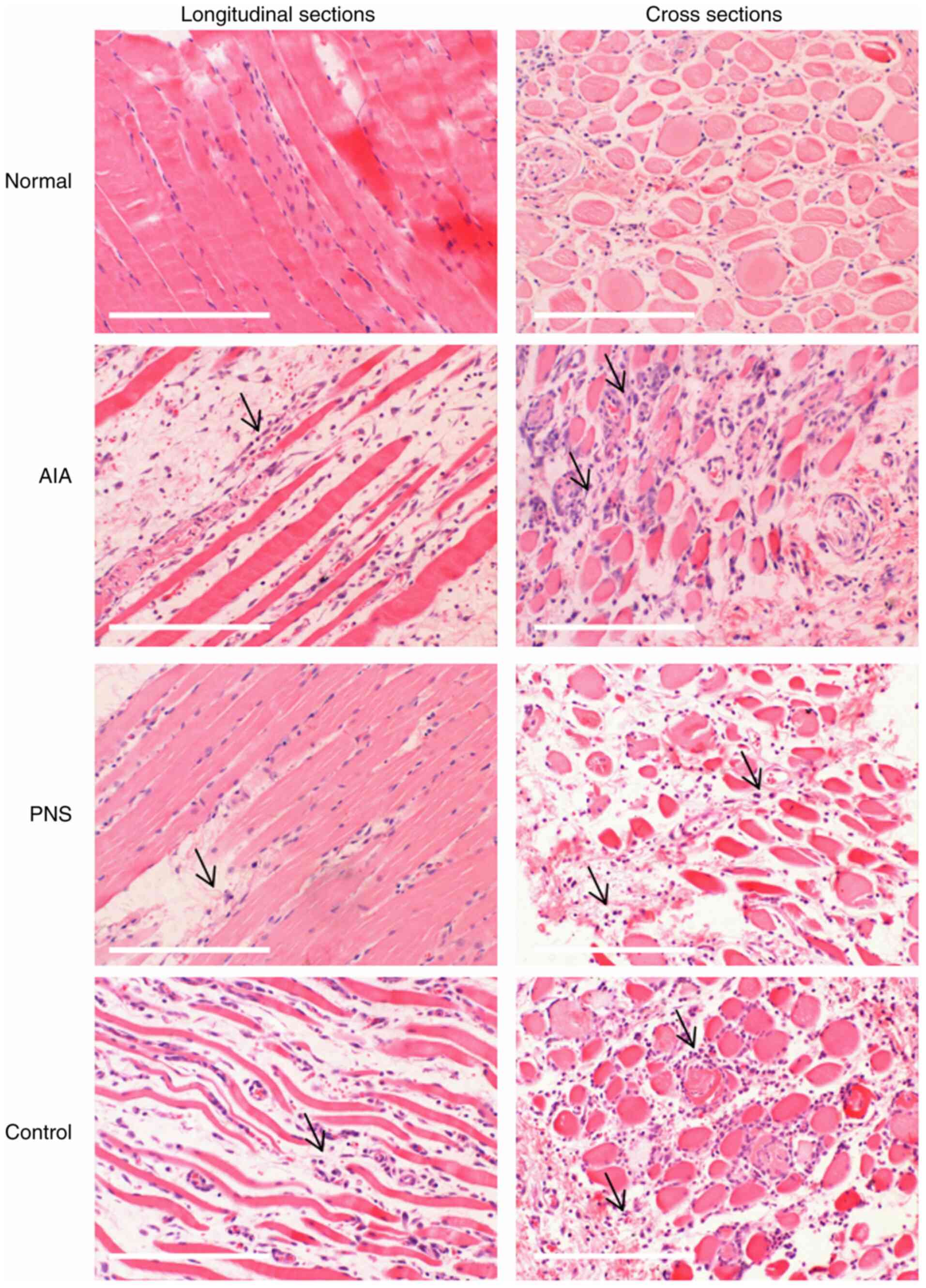
PNS reduces arthritic muscular atrophy
and inflammation
The morphological characteristics of rabbit skeletal
muscle fibers were observed following H&E staining. Skeletal
muscle from the normal group (in longitudinal sections) exhibited a
compact structure with no gaps between the fibers, deeply stained
nuclei in a linear arrangement around intact muscle fiber cells,
and few inflammatory cells. The AIA group exhibited various degrees
of inflammatory cell infiltration into the empty spaces between
fibers, and the skeletal muscle fibers displayed increased atrophy
compared with the other groups. However, narrower intercellular
spaces and less prominent fiber atrophy were observed in the
PNS-treated muscle fibers. In addition, cross sections of the
skeletal muscle fibers in the PNS-treated groups revealed a
considerable decrease in inflammatory cell numbers. Moreover, the
number of inflammatory cells in the indomethacin-treated control
group was lower compared with that in the AIA group (Fig. 1).
PNS inhibits articular chondrocyte
apoptosis
TEM images of articular cartilage and the
extracellular matrix (ECM) are displayed in Fig. 2. Examination of the TEM images
revealed that the typical chondrocyte exhibited heterochromatic
regions within the cell nucleus. The endoplasmic reticulum was
expansive and prominent, the Golgi bodies were highly developed,
and numerous mitochondria were visible. Altered chondrocyte
ultrastructure was apparent in the AIA model; the cells had
irregular hyperchromatic nuclei, and the cell membrane possessed
short filopodia. Moreover, the intracellular organelles, such as
mitochondria and Golgi bodies, were markedly reduced, whereas short
fibril forms and numerous abnormal collagen heterofibrils were
observed in the ECM. However, treatment with PNS decreased
chondrocyte apoptosis. In addition, the cytoplasm was shown to
contain an abundance of active organelles, and the cell membrane
possessed longer filopodia in the rabbits treated with PNS compared
with the control group. Furthermore, a highly complex ECM with a
visible underlying collagen fibril meshwork, as well as fibers, was
observed in the PNS-treated group compared with the control
group.
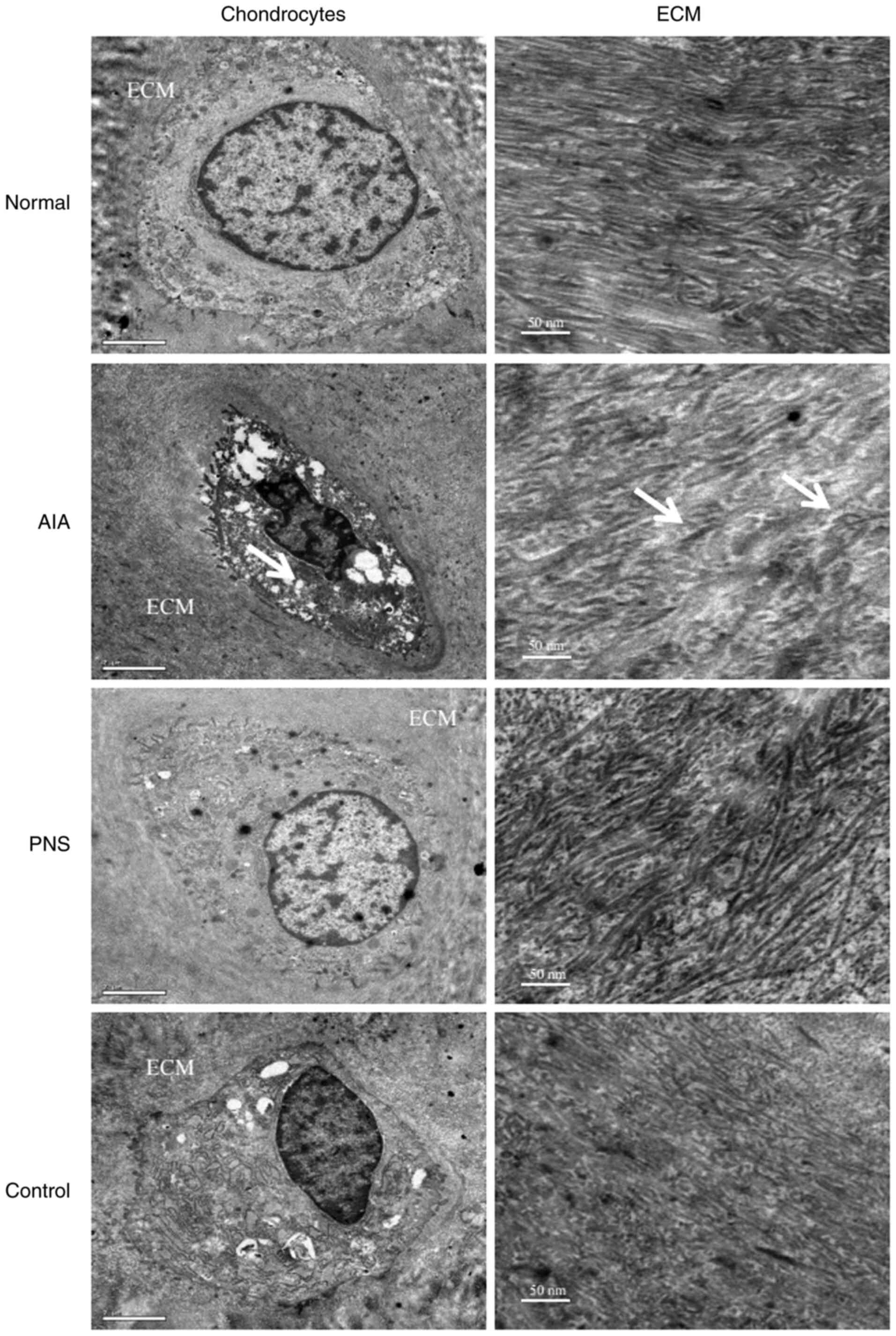 | Figure 2TEM images of chondrocytes and ECM of
the articular cartilage. The number of intracellular organelles,
such as mitochondria and Golgi bodies, was markedly reduced in the
AIA chondrocytes, and short fibril forms and numerous abnormal
collagen heterofibrils (arrows) were observed in the ECM.
Chondrocytes: Original magnification, x10,000; scale bar, 2 µm.
ECM: Original magnification, x12,000; scale bar, 50 nm. TEM,
transmission electron microscopy; ECM, extracellular matrix; AIA,
antigen-induced arthritis; PNS, Panax notoginseng
saponins. |
PNS inhibits lumbar vertebral and
articular bone destruction
Micro-CT revealed that AIA-induced osteoporosis was
evident in the femoral condyle and lumbar spine (Fig. 3A). The BMD value in both the distal
femoral and the lumbar vertebra was significantly lower in the
model rabbits compared with the normal rabbits (P<0.05). By
contrast, the BMD value of the PNS-treated group was higher
compared with that of the model rabbits (P<0.05; Fig. 3B). Furthermore, AIA significantly
decreased trabecular BV/TV, Tb.Th and Tb.N compared with the normal
group; by contrast, Tb.Sp was increased as a result of AIA-induced
osteoporosis (P<0.05). Moreover, as regards the
microarchitecture of the trabecular bone in the PNS-treated group,
the BV/TV, Tb.Th and Tb.N were markedly increased (P<0.05), and
Tb.Sp was significantly decreased (P<0.05). PNS significantly
inhibited microstructural deterioration of the trabeculae. However,
indomethacin did not significantly affect the BMD and
microarchitecture of the trabecular bone when compared with the AIA
group (P>0.05). Furthermore, the degree of apoptosis was
compared between the lumbar vertebra and knee joint in the same
group, and the data indicated that differences in the BMD value and
microarchitecture of the trabecular bone did not achieve
statistical significance (P>0.05; Fig. 3C-F).
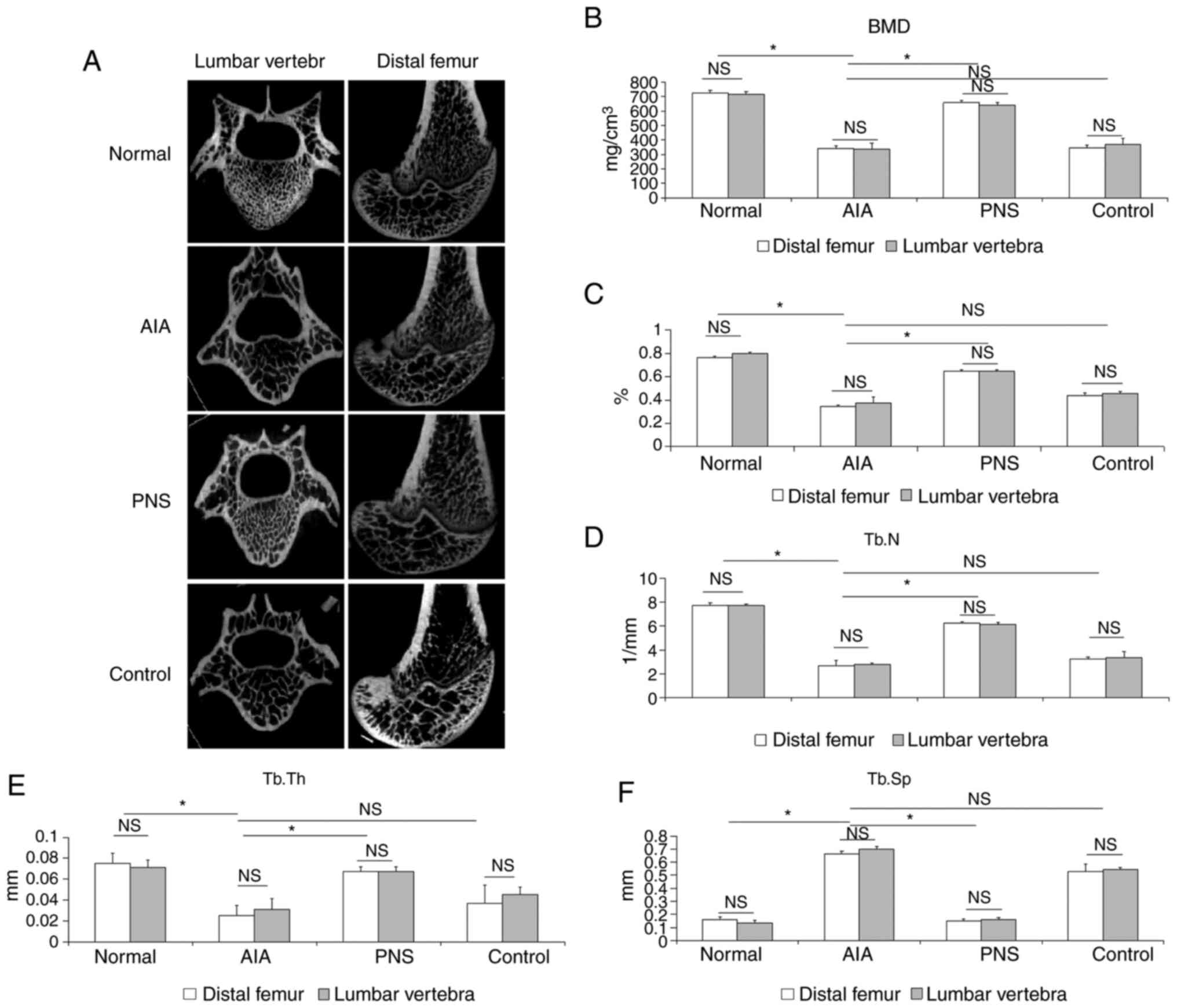 | Figure 3Micro-CT analysis of the BMD value,
bone structure and quality of the femoral condyle and the first
lumbar vertebra. (A) Micro-CT images of the femoral condyle and the
first lumbar vertebra of rats from the different groups. (B)
Comparison of BMD values among the groups. Compared with model
rabbits, (C) BV/TV, (D) Tb.N (D) and (E) Tb.Th were significantly
increased, whereas (F) Tb.Sp was significantly decreased in
PNS-treated rabbits. The results are presented as the mean ± SD.
*P<0.05. NS, not significant; Micro-CT,
micro-computed tomography; AIA, antigen-induced arthritis; PNS,
Panax notoginseng saponins; BMD, bone mineral density;
BV/TV, trabecular bone volume fraction; Tb.N, trabecular number;
Tb.Sp, trabecular spacing; Tb.Th, trabecular thickness. |
Discussion
RA is a progressive inflammatory disorder that
involves not only bone and joint destruction, but also muscle
wasting. RA results in osteoporosis, impaired bone and joint
function (18); thus, preventing
osteoporosis and joint damage is crucial for RA treatment (19). AIA is a well-developed experimental
arthritis model that is widely used in the study of RA (20,21).
Unlike the ovariectomy-induced osteoporosis model (22), joint swelling and a pluricellular
infiltrate are observed in AIA, which may progress to a chronic
condition characterized by synovial inflammation and matrix
degradation. Specifically, the severity of synovitis in animal
models may be modified by altering the number or dosage of the
intra-articular injections (13).
In the present study, the AIA experimental model was generated
using injection of ovalbumin in complete Freund's adjuvant
emulsion, resulting in severe synovial lesions and osteoporosis.
Shen et al (22) reported
that PNS at a dose of 300 mg/kg/day significantly prevented the
ovariectomy-induced reduction of bone mass in rats. Therefore, in
the present study, the 75 mg/kg dose of PNS in rabbits was
calculated by converting the rat dosage, and the results
demonstrated that PNS exerted a positive effect on bone loss and
cartilage erosion in a rabbit model of inflammatory arthritis.
The pathological characteristics of RA include joint
destruction and muscle wasting. Multiple factors promote muscle
atrophy in RA, such as joint pain, swelling and local inflammation.
It is also known that RA-associated muscle atrophy may be mediated
by pro-inflammatory factors, such as IL-17, TNF-α and inducible
nitric oxide synthase (iNOS) (23).
Yamada et al (24) reported
that muscles from AIA rats exhibited increased levels of TNF-α and
high-mobility group box 1, as well as increased NO and superoxide
production, leading to the formation of actin aggregates and
decreased actomyosin ATPase activity in skeletal muscles,
ultimately resulting in intrinsic contractile dysfunction of
skeletal muscles. Previous evidence indicated that PNS treatment
improved joint tenderness and swelling in collagen-induced
arthritic rats by reducing the expression of pro-inflammatory
factors, including MMPs, iNOS and TNF-α (25). In the present study, AIA was shown
to cause inflammatory dysfunction in skeletal muscles. Degeneration
and necrosis of the muscle fibers, reduced muscle bundle width and
irregularities in muscle fiber size were observed in AIA animals.
The muscle fibers in the PNS-treated groups were less atrophic, and
the number of inflammatory cells was markedly lower. These findings
indicate the potential therapeutic value of PNS for muscle wasting
in patients with rheumatic conditions or other inflammatory
diseases. Indomethacin, which is a non-steroidal anti-inflammatory
drug commonly used in patients with RA, was used as the control
drug in the present study. It was previously demonstrated that
indomethacin significantly inhibited pro-inflammatory cytokine
expression in the synovial tissues of AIA rats (26). In the present study, the number of
inflammatory cells in the indomethacin-treated control group was
lower compared with that in the AIA group.
Articular cartilage comprises ECM and chondrocytes.
Collagen II is the main structural protein component of the ECM
(27), and chondrocyte injury plays
a crucial role in joint destruction in RA (28). As previously reported (29), chondrocytes from the cartilage of
the normal group possessed centrally located nuclei, the staining
had a finely granular appearance, and the chondrocytes were
encompassed by the ECM, while, in damaged cartilage, the
chondrocytes were encompassed by a reduced amount of cartilaginous
matrix (30). In the present study,
TEM images revealed chondrocyte apoptosis in the AIA model,
resulting in failure to form an adequate quantity of cytoplasm and
cellular organelles, such as endoplasmic reticulum, mitochondria
and Golgi bodies. However, chondrocytes were adequately preserved
in rabbits treated with PNS, and the collagen fibrils in the ECM
were denser and thicker compared with those in the model
rabbits.
Micro-CT was previously used to determine whether
PNS could maintain bone quality, and the BMD values of the lumbar
vertebrae and knee joints in the PNS group were higher compared
with those in the AIA model group (31). Systemic osteoporosis in RA has a
complex multifactorial etiology, in which an autoimmune
inflammatory response plays a significant role. Bone loss in RA, as
evidenced by the release of osteoclast-activating cytokines (for
example, IL-17 and M-CSF) by synovial cells, directly enhanced the
expression of tumor necrosis factor ligand superfamily member 11
(TNFSF11) (4). Various bioactive
compounds derived from foods or plants, such as icariin and
theaflavins, have been shown to prevent bone loss, decrease
osteoclastic bone resorption, and stimulate osteoblast activity in
AIA models (17,26). Our previous study indicated that the
levels of TNFSF11 and Osteoprotegerin expression were directly
associated with bone formation and resorption in AIA rabbits
(16). Further analysis of the
trabecular microarchitecture revealed that the BV/TV, Tb.Th and
Tb.N were also significantly increased, whereas trabecular
separation was markedly decreased following PNS treatment,
suggesting that PNS may exert a therapeutic effect on osteoporosis.
These results are consistent with previous findings according to
which PNS prevented ovariectomy-related osteoporosis in rats, and
that these beneficial effects may be the result of stimulating bone
formation and decreasing resorption (31). In conclusion, the results of the
present study indicated that PNS may relieve osteoporosis and
prevent joint bone destruction in rabbits with AIA.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Shanghai Science
and Technology Commission (grant no. 19401935400), the construction
project of Li Fei Yue's National Famous TCM (grant no.
MLZJGZS-2017001), the Construction Project of Li Fei Yue's Shanghai
Famous TCM Research Studio (grant no. SHGZS-2017010) the National
Natural Science Foundation of China (grant no. 81302987) and the
National Training Program for Innovative Talents of Traditional
Chinese Medicine.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LFY and CCW designed the study. CCW drafted the
manuscript. FTY performed the experiments. CCW and CT verified and
statistically analyzed the data, and proofread the manuscript . All
the authors have read and approved the final manuscript. CCW and CT
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the Animal Care Committee of Shanghai University of
Traditional Chinese Medicine (approval no. 201601217117).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Croia C, Bursi R, Sutera D, Petrelli F,
Alunno A and Puxeddu I: One year in review 2019: Pathogenesis of
rheumatoid arthritis. Clin Exp Rheumatol. 37:347–357.
2019.PubMed/NCBI
|
|
2
|
Gao W, McGarry T, Orr C, McCormick J,
Veale DJ and Fearon U: Tofacitinib regulates synovial inflammation
in psoriatic arthritis, inhibiting STAT activation and induction of
negative feedback inhibitors. Ann Rheum Dis. 75:311–315.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gaudio A, Pennisi P, Bratengeier C,
Torrisi V, Lindner B, Mangiafico RA, Pulvirenti I, Hawa G, Tringali
G and Fiore CE: Increased sclerostin serum levels associated with
bone formation and resorption markers in patients with
immobilization-induced bone loss. J Clin Endocrinol Metab.
95:2248–2253. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kwan Tat S, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: Interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pumpa KL, Fallon KE, Bensoussan A and
Papalia S: The effects of Panax notoginseng on delayed onset
muscle soreness and muscle damage in well-trained males: A double
blind randomised controlled trial. Complement Ther Med. 21:131–140.
2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhou N, Tang Y, Keep RF, Ma X and Xiang J:
Antioxidative effects of Panax notoginseng saponins in brain
cells. Phytomedicine. 21:1189–1195. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu Y, Zhang HG, Jia Y and Li XH: Panax
notoginseng saponins attenuate atherogenesis accelerated by
zymosan in rabbits. Biol Pharm Bull. 33:1324–1330. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li XY: Immunomodulating Chinese herbal
medicines. Mem Inst Oswaldo Cruz. 86 (Suppl 2):S159–S164.
1991.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cai H, Zhang QY, Zhao ZM and Yao RB: Two
years follow-up of Panax notoginseng saponins combined with
DMARDs on treating rheumatoid arthritis. J Med Postgrad.
26:505–507. 2013.(In Chinese).
|
|
10
|
Wei JR, Wen X, Bible PW, Li Z, Nussenblatt
RB and Wei L: Panax notoginseng saponin controls IL-17
expression in helper T cells. J Ocul Pharmacol Ther. 33:285–289.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li XD, Chang B, Chen B, Liu ZY, Liu DX,
Wang JS, Hou GQ, Huang DY and Du SX: Panax notoginseng
saponins potentiate osteogenesis of bone marrow stromal cells by
modulating gap junction intercellular communication activities.
Cell Physiol Biochem. 26:1081–1092. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jang YJ, Kim ME and Ko SY: n-Butanol
extracts of Panax notoginseng suppress LPS-induced MMP-2
expression in periodontal ligament fibroblasts and inhibit
osteoclastogenesis by suppressing MAPK in LPS-activated RAW264.7
cells. Arch Oral Biol. 56:1319–1327. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Largo R, Roman-Blas JA, Moreno-Rubio J,
Sánchez-Pernaute O, Martínez-Calatrava MJ, Castañeda S and
Herrero-Beaumont G: Chondroitin sulfate improves synovitis in
rabbits with chronic antigen-induced arthritis. Osteoarthritis
Cartilage. 18 (Suppl 1):S17–S23. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ochaion A, Bar-Yehuda S, Cohn S, Del Valle
L, Perez-Liz G, Madi L, Barer F, Farbstein M, Fishman-Furman S,
Reitblat T, et al: Methotrexate enhances the anti-inflammatory
effect of CF101 via up-regulation of the A3 adenosine receptor
expression. Arthritis Res Ther. 8(R169)2006.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Derrell CJ, Gebhart GF, Gonder JC, Keeling
ME and Kohn DF: Special report: The 1996 guide for the care and use
of laboratory animals. ILAR J. 38:41–48. 1997.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen CW, Fan TY, Li YM, Sun J and Chen YQ:
Total glucosides of paeony prevents juxta-articular bone loss in
experimental arthritis. BMC Complement Altern Med.
13(186)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wei CC, Ping DQ, You FT, Qiang CY and Tao
C: Icariin prevents cartilage and bone degradation in experimental
models of arthritis. Mediators Inflamm.
2016(9529630)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jang EJ, Lee YK, Choi HJ, Ha YC, Jang S,
Shin CS and Cho NH: Osteoporotic fracture risk assessment using
bone mineral density in Korean: A community-based cohort study. J
Bone Metab. 23:34–39. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Maruotti N, Corrado A and Cantatore FP:
Osteoporosis and rheumatic diseases. Reumatismo. 66:125–135.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lei Z, Feng G, Xu N, Wei Q, Liu J, Bian T
and Zou T: Early extremity MRI findings and pathological synovial
changes in antigen-induced arthritis rabbit model. J Magn Reson
Imaging. 39:1366–1373. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lewthwaite J, Blake SM, Hardingham TE,
Warden PJ and Henderson B: The effect of recombinant human
interleukin 1 receptor antagonist on the induction phase of antigen
induced arthritis in the rabbit. J Rheumatol. 21:467–472.
1994.PubMed/NCBI
|
|
22
|
Shen Y, Li YQ, Li SP, Ma L, Ding LJ and Ji
H: Alleviation of ovariectomy-induced osteoporosis in rats by
Panax notoginseng saponins. J Nat Med. 64:336–345.
2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Horai N, Nagaoka T, Higuchi I, Kasai H,
Yoshioka T, Umekita Y, Fukuzaki K, Nagata R, Miyata A and Abeyama
K: Muscle wasting associated with pathologic change is a risk
factor for the exacerbation of joint swelling in collagen-induced
arthritis in cynomolgus monkeys. BMC Musculoskelet Disord.
14(205)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yamada T, Abe M, Lee J, Tatebayashi D,
Himori K, Kanzaki K, Wada M, Bruton JD, Westerblad H and Lanner JT:
Muscle dysfunction associated with adjuvant-induced arthritis is
prevented by antioxidant treatment. Skelet Muscle.
5(20)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chang SH, Choi Y, Park JA, Jung DS, Shin
J, Yang JH, Ko SY, Kim SW and Kim JK: Anti-inflammatory effects of
BT-201, an n-butanol extract of Panax notoginseng, observed
in vitro and in a collagen-induced arthritis model. Clin Nutr.
26:785–791. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ramadan G, El-Beih NM, Talaat RM and Abd
El-Ghffar EA: Anti-inflammatory activity of green versus black tea
aqueous extract in a rat model of human rheumatoid arthritis. Int J
Rheum Dis. 20:203–213. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Raya JG: Techniques and applications of in
vivo diffusion imaging of articular cartilage. J Magn Reson
Imaging. 41:1487–1504. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Huh YH, Lee G, Lee KB, Koh JT, Chun JS and
Ryu JH: HIF-2α-induced chemokines stimulate motility of
fibroblast-like synoviocytes and chondrocytes into the
cartilage-pannus interface in experimental rheumatoid arthritis
mouse models. Arthritis Res Ther. 17(302)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Toh ML, Bonnefoy JY, Accart N, Cochin S,
Pohle S, Haegel H, De Meyer M, Zemmour C, Preville X, Guillen C, et
al: Bone- and cartilage-protective effects of a monoclonal antibody
against colony-stimulating factor 1 receptor in experimental
arthritis. Arthritis Rheumatol. 66:2989–3000. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bustamante MF, Garcia-Carbonell R,
Whisenant KD and Guma M: Fibroblast-like synoviocyte metabolism in
the pathogenesis of rheumatoid arthritis. Arthritis Res Ther.
19(110)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fan JZ, Wang Y, Meng Y, Li GW, Chang SX,
Nian H and Liang YJ: Panax notoginseng saponins mitigate
ovariectomy-induced bone loss and inhibit marrow adiposity in rats.
Menopause. 22:1343–1350. 2015.PubMed/NCBI View Article : Google Scholar
|