Introduction
Atherosclerosis is the most common type of
arteriosclerosis, which occurs in the subendothelial layer of the
large and medium arteries, resulting in the blockage of blood flow
triggered by endothelial dysfunction and the subendothelial
retention of lipoproteins (1).
Atherosclerosis is a key cause of mortality worldwide. In Western
societies, it is the underlying cause of ~50% of all mortality
(2), and results in several medical
complications, including myocardial infarction, stroke and
peripheral arterial disease (3). A
number of inflammatory biomarkers (including IL-6, IL-1β, IL-10,
TNF-α, E-selectin, vascular cell adhesion molecule-1, adiponectin,
high-sensitivity C-reactive protein and pentraxin 3) have been
identified as independent risk factors for cardiovascular diseases,
and studies have provided evidence for low-density lipoprotein
(LDL)-induced immune activation in human atherosclerotic lesions
(4). Numerous studies have been
conducted with the aim of developing improved treatment strategies
for atherosclerosis (5,6).
Matrine is a key substance used in traditional
Chinese medicine (7). It exerts
anti-allergic, anti-inflammatory, antiviral and antifibrotic
effects and is considered to be helpful for protecting against
cardiovascular disease (8). The
anti-inflammatory mechanism of matrine in microvascular endothelial
cells has been shown to involve the increase of nitric
oxide-dependent vasodilatation and the inhibition of
lipopolysaccharide-induced inflammatory cytokines, indicating that
matrine acts as a protective agent against inflammatory tissue
damage (9). A previous study
demonstrated that matrine was effective in treating liver cancer by
inhibiting the expression of matrix metalloproteinase-9 and the
invasion of human liver cancer cells, and further showed that the
inhibitory effect was partly associated with downregulation of the
nuclear factor-κB (NF-κB) signaling pathway (10). In another study, matrine was shown
to inhibit the invasion and metastasis of melanoma cells in
vitro, and the induction of apoptosis was associated with the
downregulation of heparinase mRNA and protein expression (11).
Based on these previous findings, we hypothesized
that matrine may affect the inflammatory response and abnormal
lipid metabolism of vascular smooth muscle cells, and aimed to
investigate this and to elucidate the underlying mechanism in the
present study.
Materials and methods
Cell culture and drug treatment
Human aortic vascular smooth muscle cells (HAVSMCs;
Shenzhen Haodi Huatuo Biotechnology Co. Ltd.) were plated in 6-well
plates and routinely cultured in high-glucose Dulbecco's modified
Eagle's medium (Qingdao Jiesikang Biotechnology Co., Ltd.)
containing 10% fetal bovine serum (Shanghai Xinfan Biotechnology
Co., Ltd.) without antibiotics at 37˚C in a 5% CO2
incubator until they reached 60-70% confluency.
The proliferation of HAVSMCs treated with various
concentrations (0.0, 0.5, 1.0, 2.0, 4.0, 6.0, 8.0 and 10.0 mg/ml of
matrine (Shanghai Yuanye Biotechnology Co., Ltd.) for 24 and 48 h
was analyzed at 37˚C. In subsequent experiments, the cells were
further assigned to normal, model and matrine groups. The model
group was treated with 50 mg/ml oxidized LDL (oxLDL; Shanghai
Lianmai Biological Engineering Co., Ltd.) to establish the
atherosclerosis model. The matrine group was treated with 50 mg/ml
oxLDL and 1.0 mg/ml matrine. The normal group was treated with the
same volume of normal saline. In the western blotting experiment,
model + Bay11-7082 (NF-κB inhibitor, MedChemExpress, cat. no.
HY-13453) and matrine + Bay11-7082 groups were also established by
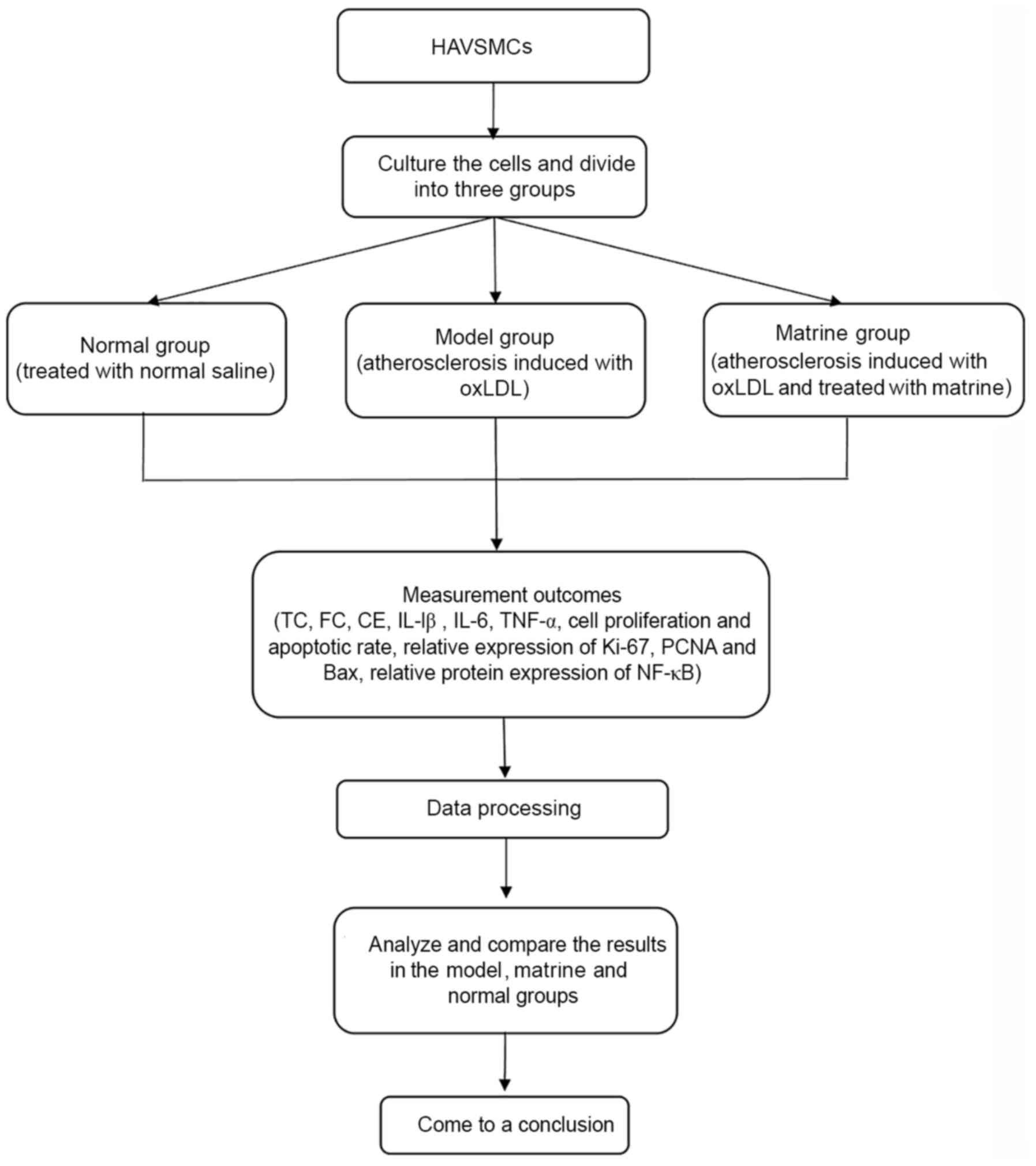
treatment with 2.5 µmol/l Bay11-7082 for 2 h at 37˚C. A flowchart
of the study protocol is shown in Fig.
1.
Cell growth analysis
Cell proliferation in the normal, model and matrine
groups was evaluated using a Cell Counting Kit-8 (CCK-8) assay kit
(cat. no. ab228554; Abcam). A suspension of HAVSMCs was prepared
and the 1x106 cell suspension (100 µl) was added to each
well of a 96-well plate. Three replicates were prepared for each
group and time point. Each well was treated with 20 µl CCK-8
reagent after incubation for 24, 48, 72 and 96 h at 37˚C in a 5%
CO2 incubator. After incubation for 2 h with the CCK-8
reagent, the absorbance at 490 nm was measured using an automated
microplate reader. The experiment was repeated three times.
Analysis of lipid metabolism
markers
Cell supernatants were collected from the normal,
model and matrine groups and the levels of total cholesterol (TC;
cat. no. JL19339), free cholesterol (FC; cat. no. JL20022) and
cholesterol ester (CE; cat. no. JL19339) in the cell supernatants
were determined using ELISA kits (Shanghai Jianglai Biological
Technology Co., Ltd.) in accordance with the manufacturer's
instructions. Experiments were repeated three times.
Detection of apoptotic rates
An apoptosis assay was performed on cells from the
normal, model and matrine using an apoptosis kit according to the
manufacturer's instructions (Apoptotic DNA-Ladder kit; Hangzhou
Xinjing Biological Reagent Development Co., Ltd.). Flow cytometry
(BD FACSCalibur; BD Pharmingen) was used to analyze the cells. The
experiment was repeated three times.
Western blot analysis
Cells from the various treatment groups were lysed
using radio-immunoprecipitation assay buffer (Beyotime Institute of
Biotechnology). Protein determination by was performed via the BCA
method and total protein was isolated. Equal amounts (25 µg) total
protein were separated on a 10% gel via sodium dodecyl sulfate
polyacrylamide gel electrophoresis and transferred to a
polyvinylidene difluoride (PVDF) membrane. The PVDF membrane was
blocked with 5% milk for 1 h at room temperature. The PVDF membrane
was then washed with TBST (0.1% Tween-20) three times The PVDF
membrane was incubated with primary antibodies against Ki-67
(1:1,000; cat. no. ab15580; Abcam), proliferating cell nuclear
antigen (PCNA; 1:1,000; cat. no. ab280088; Abcam), Bcl-2 (1:1,000;
cat. no. ab32124; Abcam) and Bax (1:1,000; cat. no. ab182734;
Abcam) at 4˚C overnight. Secondary horseradish peroxidase
(HRP)-rabbit antibody (1:5,000; cat. no. ab6858; Abcam) was then
added to the membrane, after which it was incubated at room
temperature for 2 h. The PVDF membrane was then washed with TBST
three times and developed using 5 ml enhanced chemiluminescence
substrate (Roche Diagnostics; cat. no. 11684817910) for 3 times and
ImageJ software (version k 1.45; National Institutes of Health) was
used for analysis.
Analysis of the mRNA expression of
inflammatory factors
Total RNA was obtained from the cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.),
following the manufacturer's instructions. The concentration and
purity of RNA were quantified using a UV spectrophotometer
(Shanghai Qinxiang Scientific Instrument Co., Ltd.) by measuring
the ratio of optical densities at 260 and 280 nm, which were 1.8
and 2.0, respectively. cDNA was synthesized from the RNA by reverse
transcription (RT) using reverse transcriptase (Shenzhen Zike
Biotechnology Co., Ltd.) and oligonucleotides. The reaction mix
contained (in 20 µl volume): 1 dNTPs, 1 primers, 4 buffer, 2
reverse transcriptase, 2 total RNA and 12 µl RNase-free water. The
reaction conditions comprised incubation at 42˚C in a water bath
for 1 h, followed by incubation at 95˚C in a water bath for 5 min.
This was followed by quantitative PCR (qPCR) amplification using a
Real-Time qPCR kit (Guangzhou Huafeng Biological Technology Co.,
Ltd.). Specific primers were used to detect the expression of
interleukin (IL)-1β, IL-6, tumor necrosis factor-α (TNF-α) and
β-actin, which served as an internal control. The qPCR reaction
mixture (20 µl) consisted of 0.4 µl each of the upstream and
downstream primers and 0.5 µl Taq DNA polymerase diluted in
ddH2O. The conditions of the qPCR were as follows: 94˚C
for 10 sec, followed by 40 cycles of 94˚C for 5 sec, 52˚C for 30
sec and 72˚C for 15 sec. All experiments were performed in
triplicate and repeated three times. The results were analyzed
using a relative quantitation method to internal controls;
specifically, the expression levels of IL-1β, IL-6 and TNF-α were
calculated using the 2-∆∆Cq method (12). The sequences of the primers used for
the qPCR analysis of inflammatory factors and the internal controls
(Suzhou Hongxun Biological Technology Co., Ltd.) are listed in
Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Name | Forward (5'-3') | Reverse (5'-3') |
|---|
| IL-1β |
CTTCAAATCTCACAGCAGCAGCATC |
GCTGTCTAATGGGAACATCACA |
| IL-6 |
GTTTGACCAGAGGACCCAGA |
TCCTTTGTTACGGCTTCCAG |
| TNF-α |
GTGCCTCAGCCTCTTCTCATT |
CTCTGCTTGGTGGTTTGCTAC |
| β-actin |
CACCCGCGAGTACAACCTTC |
CCCATACCCACCATCACAAA |
Statistical analysis
Statistical analysis was performed using SPSS 20.0
(IBM Corp.) statistical software. Data are expressed as the mean ±
standard deviation. One-way ANOVA with Tukey's post hoc test was
used for the comparison of multiple groups. P<0.05 was
considered to indicate a statistically significant result.
Results
Selection of the optimum concentration
of matrine
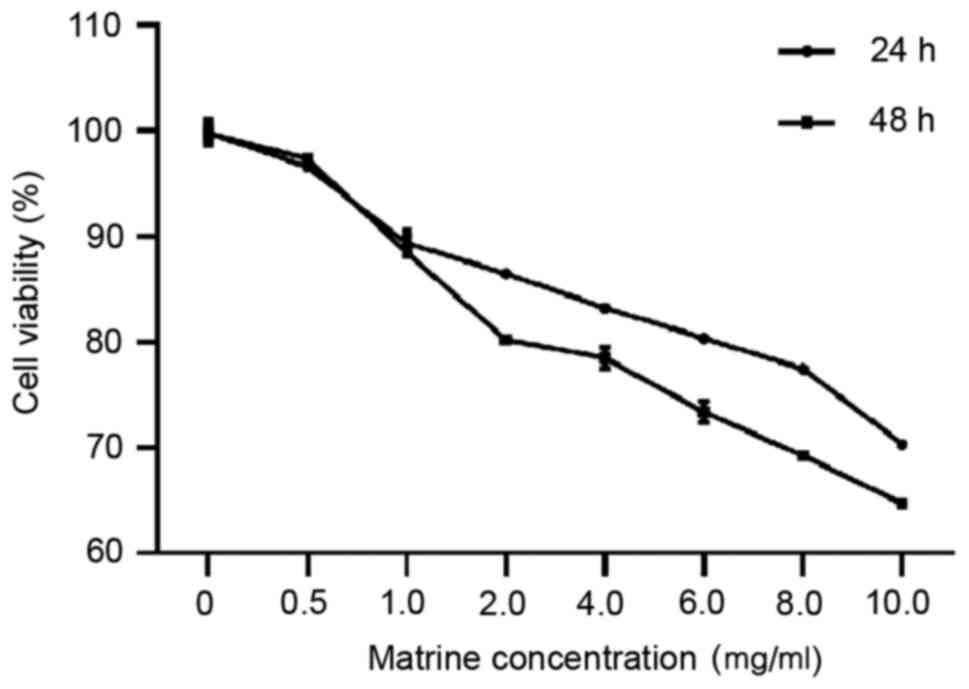
The optimal matrine concentration was determined by
treating HAVSMCs with 0.5-10 mg/ml matrine for 24 and 48 h, and
then analyzing the cell viability. The results of the CCK-8 assay
show that cell viability was >90% for cells treated with <1
mg/ml matrine, and for cells treated with matrine at concentrations
>1 mg/ml, the cell viability was inversely proportional to the
concentration. Thus, 1 mg/ml matrine was selected for subsequent
experiments (Fig. 2).
Effect of matrine on lipid
metabolism
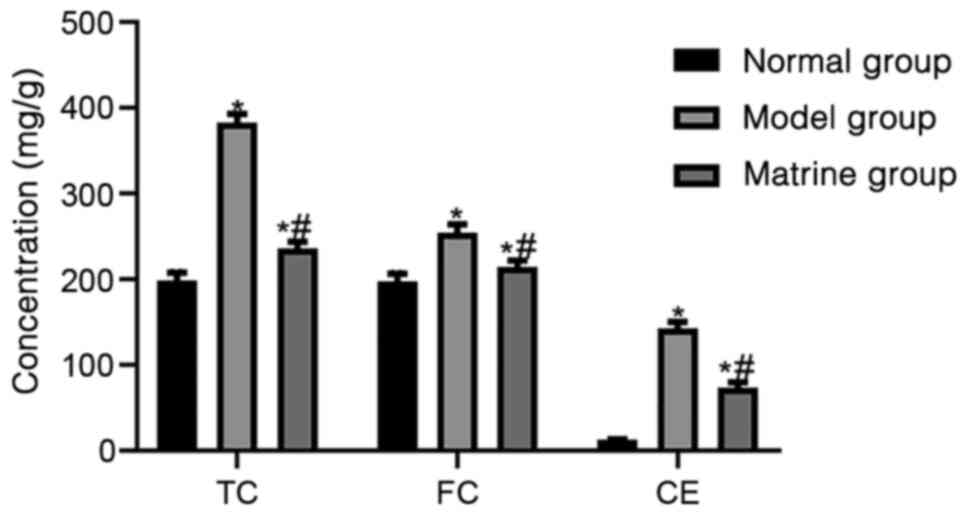
The lipid metabolism markers TC, FC and CE were
detected in the HAVSMCs in the normal, model and matrine groups.
The levels of TC, FC and CE were 198.23±9.47, 197.37±9.23 and
12.48±0.58 mg/g, respectively, in the normal group; 382.58±10.4,
254.23±10.32 and 142.67±7.35 mg/g, respectively, in the model
group; and 235.54±8.4, 214.24±7.6 and 73.24±6.54 mg/g,
respectively, in the matrine group. The results showed that the
levels TC, FC and CE were significantly higher in the oxLDL-treated
model group compared with the normal group (P<0.05).
Furthermore, the levels of TC, FC and CE in the matrine group were
lower than those in the model group, although they remained higher
than those in the normal group (P<0.05; Fig. 3)
Effect of matrine on cell
proliferation, apoptosis and associated proteins
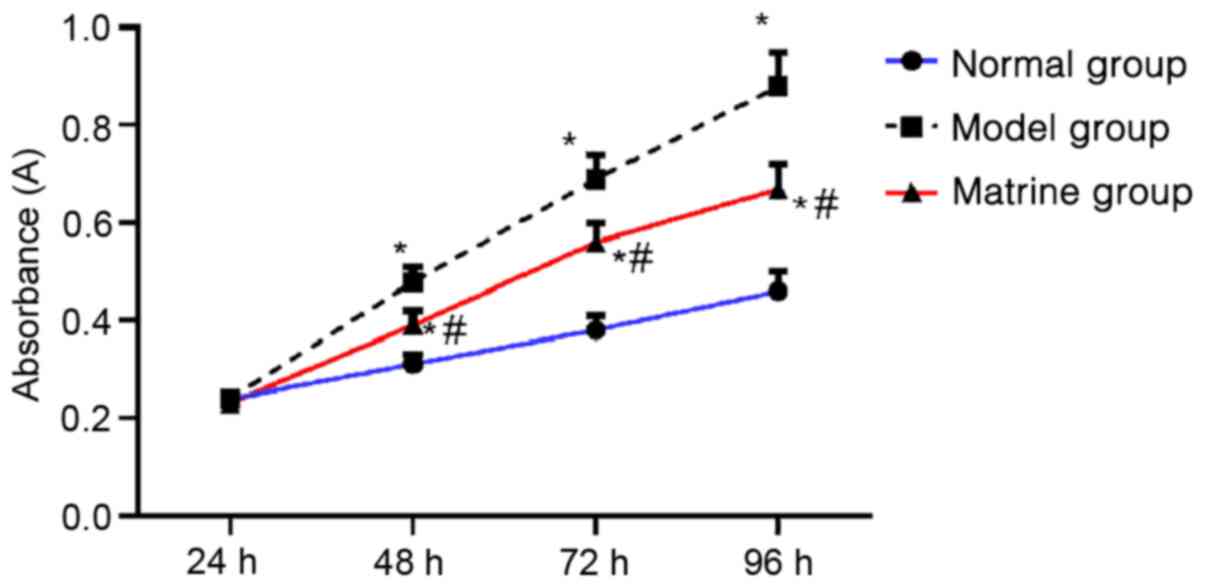
From 48-96 h, cell growth was higher in the model
and matrine groups compared with the normal group, and the cell
growth in the matrine group was reduced compared with that in the
model group (P<0.05). The cell growth in each group presented an
upward trend over time (Fig.
4).
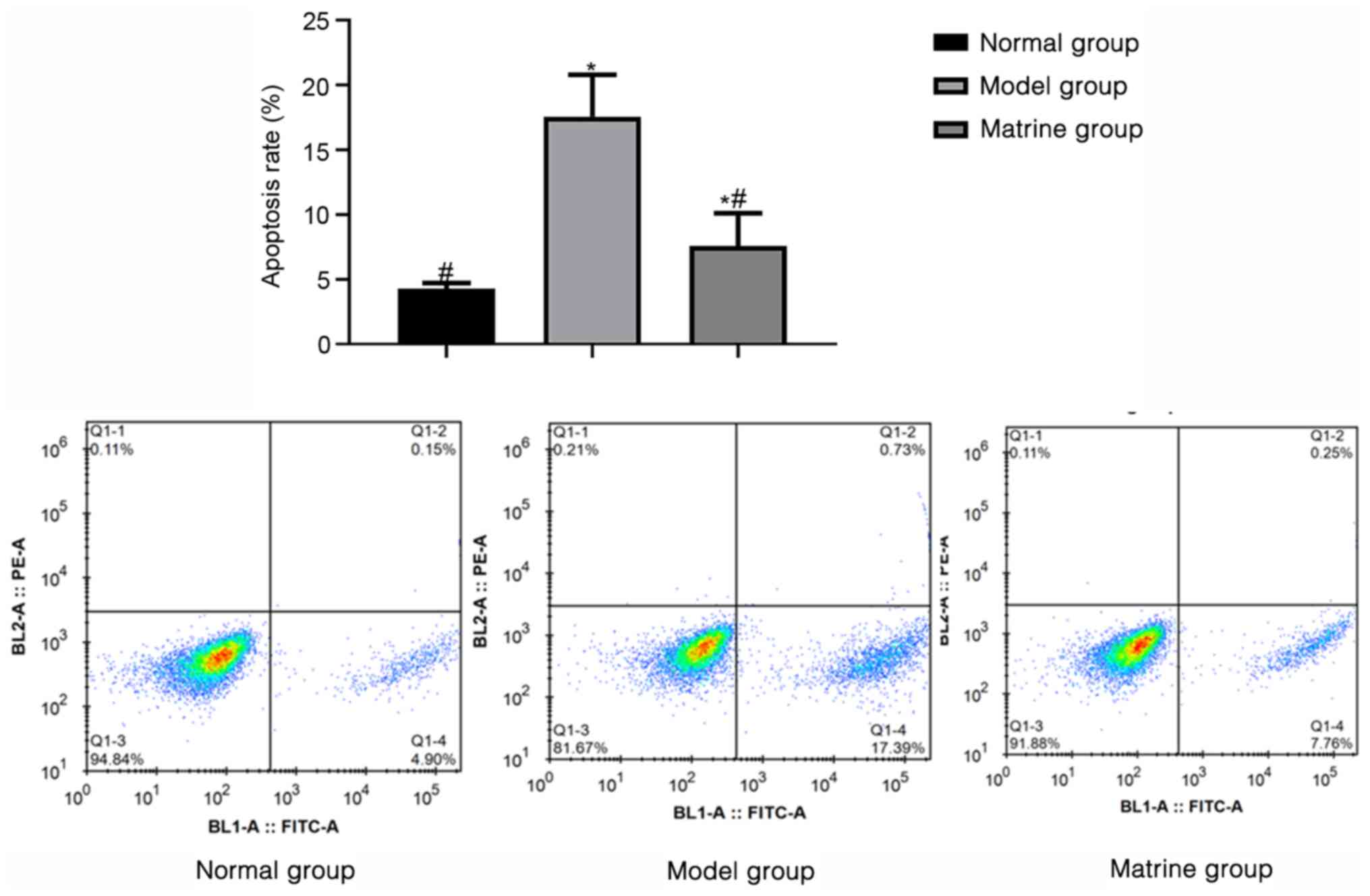
The apoptotic rates in the normal, model and matrine
groups as determined using flow cytometry were 4.28±0.43,
17.57±3.24 and 7.59±2.52%, respectively. These results indicate
that the model group had an increased apoptosis rate compared with
that of the normal group (P<0.05). Furthermore, the apoptosis
rate of the matrine group was lower compared with that of the
model, but higher than that of the normal group (P<0.05;
Fig. 5).
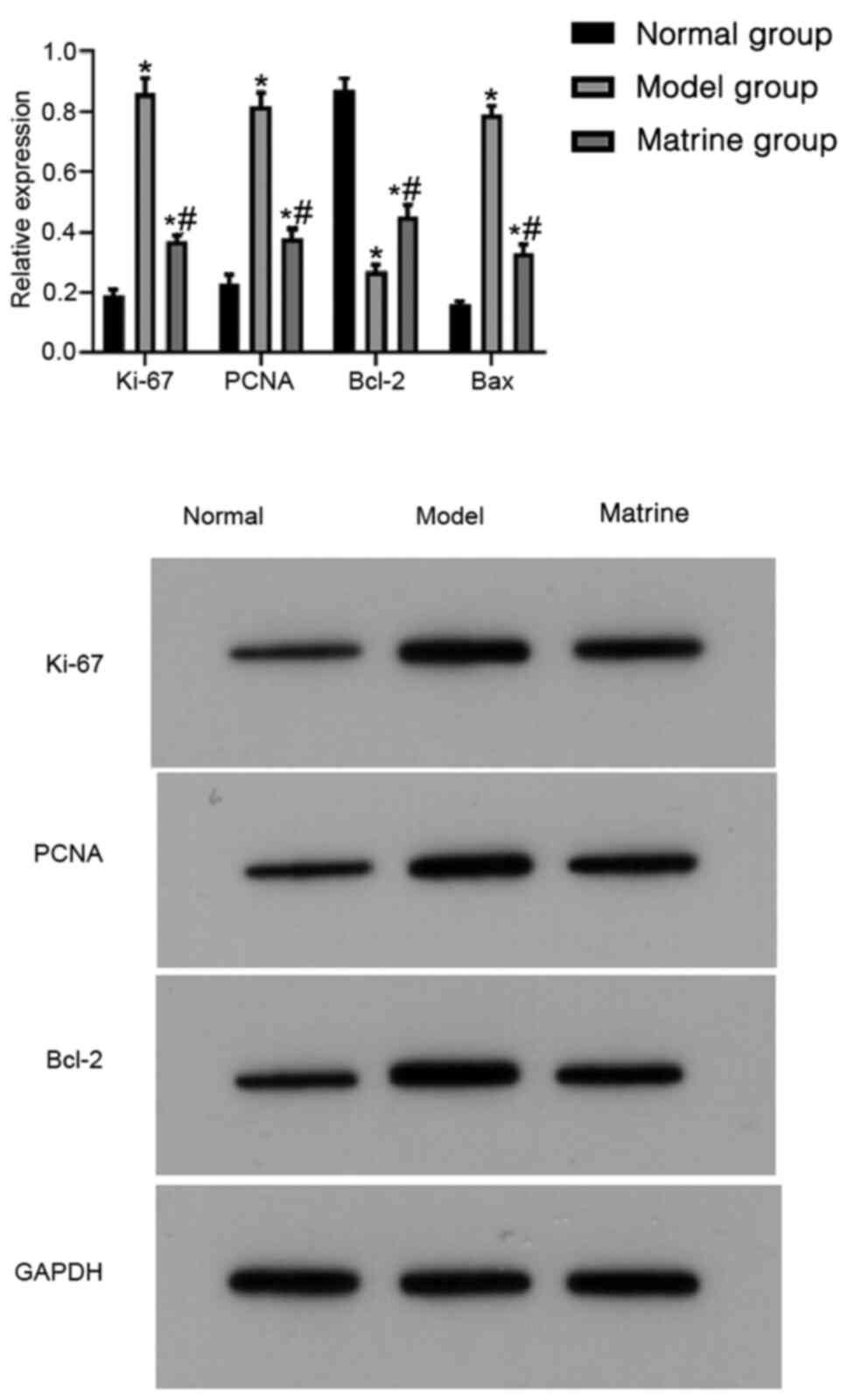
The expression of proliferation- and
apoptosis-associated proteins was evaluated using western blotting.
The relative expression levels of Ki-67, PCNA, Bcl-2 and Bax were
0.19±0.02, 0.23±0.03, 0.87±0.04 and 0.16±0.01, respectively, in the
normal group; 0.86±0.05, 0.82±0.04, 0.27±0.02 and 0.79±0.03,
respectively, in the model group; and 0.37±0.02, 0.38±0.03,
0.45±0.04 and 0.33±0.03, respectively, in the matrine group. In the
model group, the expression levels of Ki-67, PCNA and Bax were
increased while those of Bcl-2 were decreased compared with those
in the normal group (P<0.05). In addition, the matrine group
showed decreased expression levels of Ki-67, PCNA and Bax and
increased expression levels of Bcl-2 compared with those in the
model group (P<0.05; Fig.
6).
Matrine regulates cell proliferation
and apoptosis through the NF-κB pathway
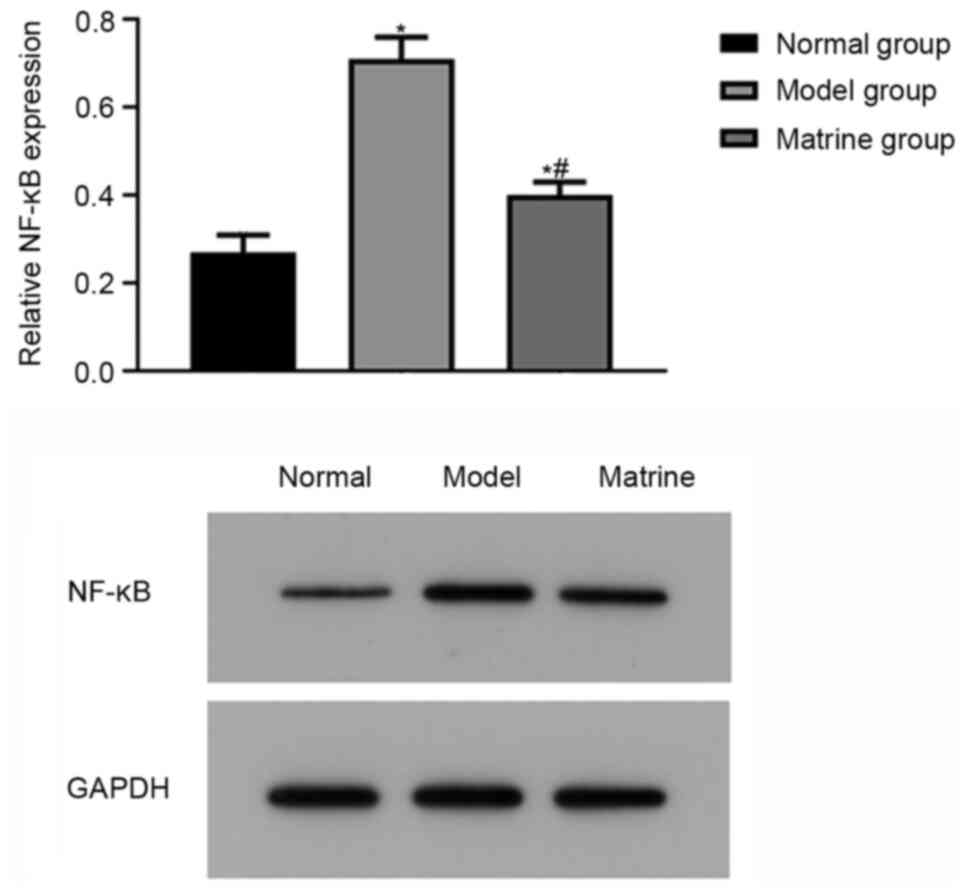
To further investigate the underlying mechanism of
matrine, the expression of NF-κB was evaluated using western
blotting. The relative protein expression levels of NF-κB in the
normal, model and matrine groups were 0.27±0.04, 0.71±0.05 and
0.40±0.03, respectively, indicating that the relative protein
expression level of NF-κB in the model group was higher compared
with that in the normal group (P<0.05). In the matrine group,
the relative expression of NF-κB protein was lower than that in the
model group but higher than that in the normal group (P<0.05;
Fig. 7).
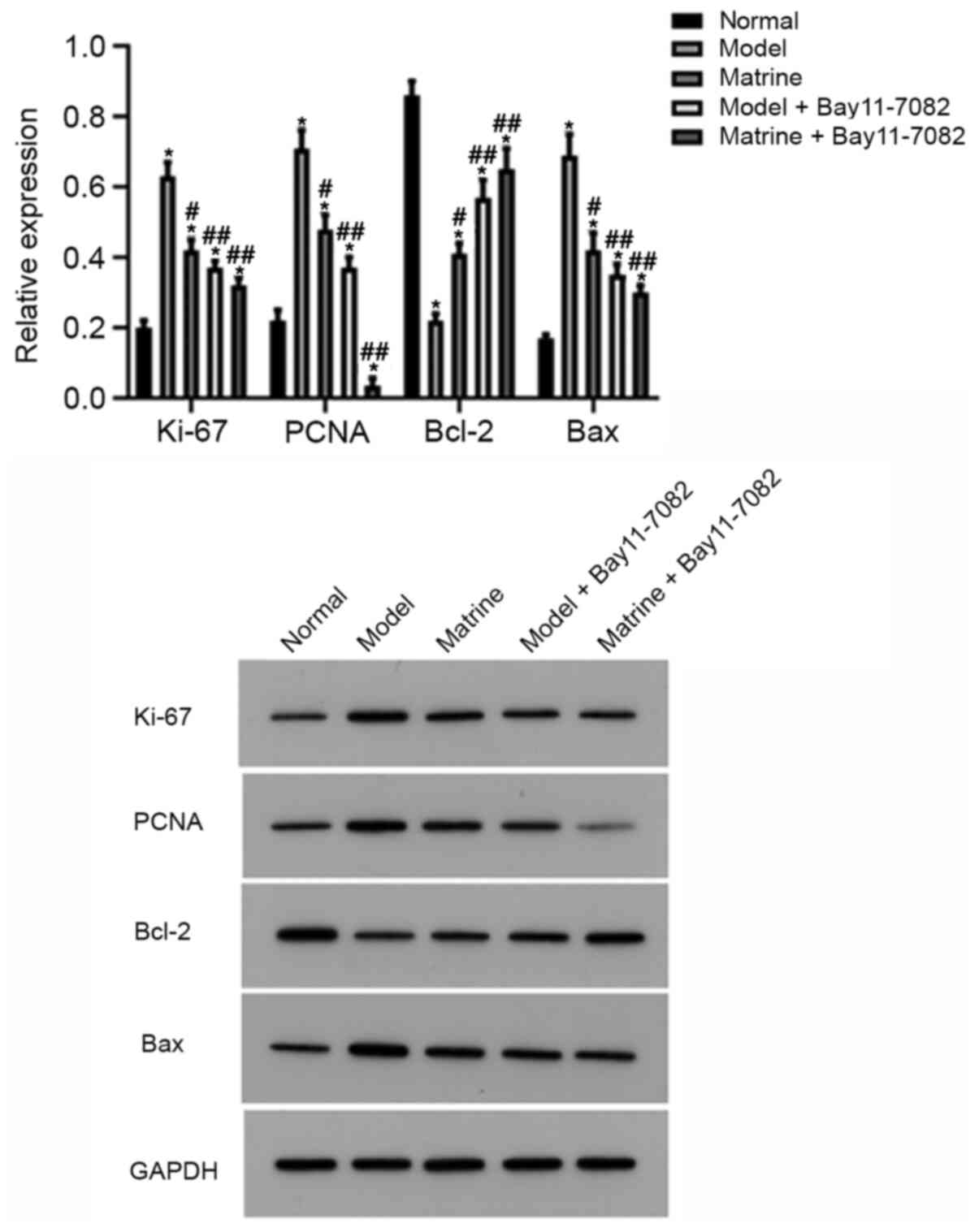
After the analysis of the effects of modeling and
matrine administration on the cells, additional groups, namely the
model + Bay11-7082 group and matrine + Bay11-7082 group were
established, and the effects of Bay11-7082 on the expression of
proliferation- and apoptosis-associated proteins were assessed
using western blotting. Consistent with the aforementioned results,
compared with the normal group, the relative expression of Ki-67,
PCNA and Bax increased in the model group, while that of Bcl-2
decreased (P<0.05). However, the matrine, model + Bay11-7082 and
matrine + Bay11-7082 groups exhibited lower relative expression
levels of Ki-67, PCNA and Bax and higher relative expression levels
of Bcl-2 compared with those in the model group (P<0.05;
Fig. 8).
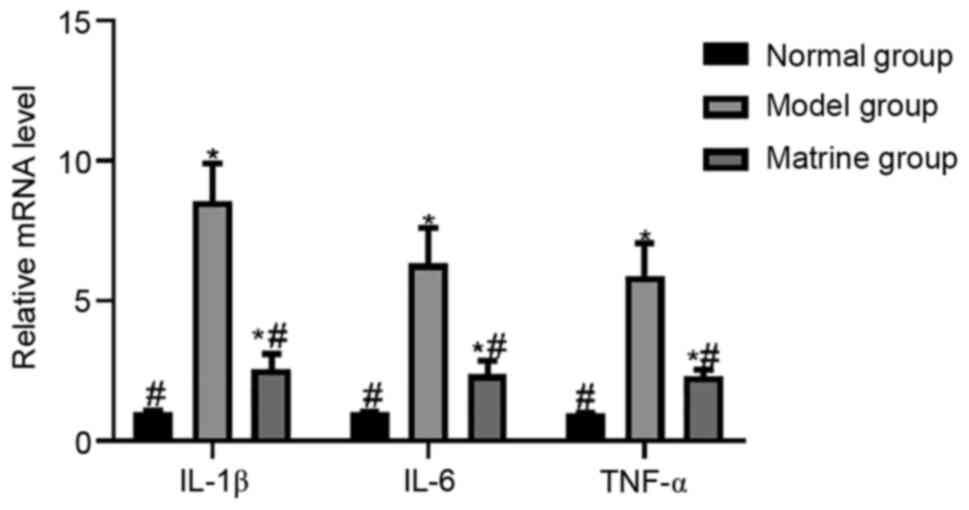
Matrine reduces inflammatory factors
through the NF-κB pathway
The effect of matrine on oxLDL-induced inflammatory
factors was assessed using RT-qPCR. The relative mRNA levels of
IL-1β, IL-6 and TNF-α were 1.03±0.02, 1.02±0.01 and 0.99±0.01,
respectively, in the normal group; 8.57±1.34, 6.32±1.29 and
5.87±1.18, respectively, in model group; and 2.57±0.54, 2.38±0.48
and 2.29±0.25, respectively, in the matrine group. The results
indicated that exposure to oxLDL in the model group significantly
increased the relative mRNA levels of IL-1β, IL-6 and TNF-α
compared with those in the normal group (P<0.05). The relative
mRNA levels of IL-1β, IL-6 and TNF-α in the matrine group were
lower than those in the model group, but higher than those in the
normal group (P<0.05; Fig.
9).
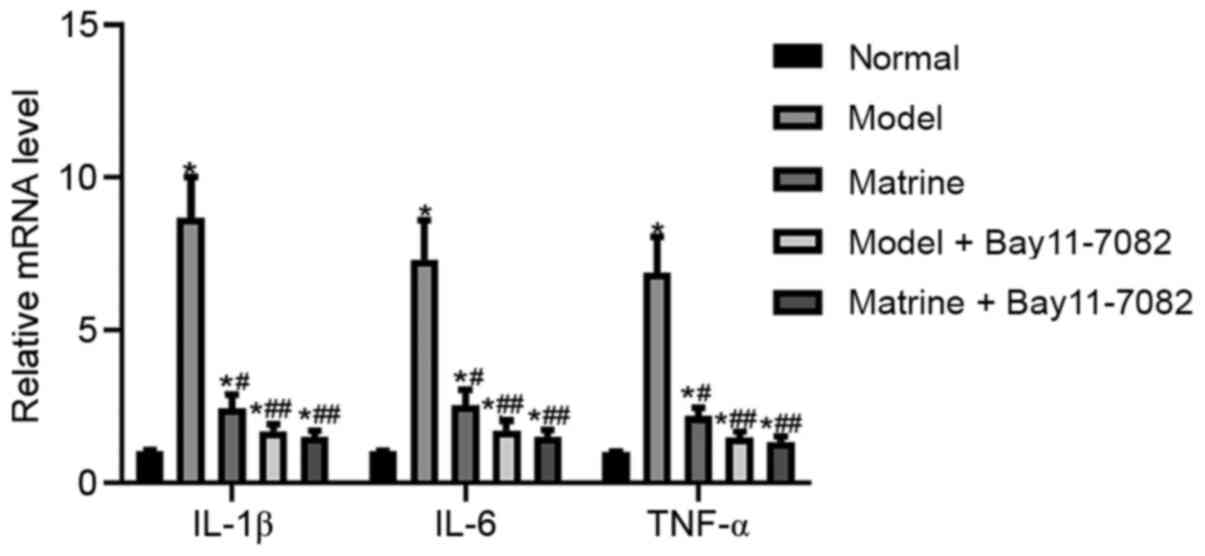
The effect of Bay11-7082 on these inflammatory
factors was also evaluated. As in the aforementioned results, the
model group showed significantly upregulated mRNA levels of IL-1β,
IL-6 and TNF-α compared with those in the normal group (P<0.05).
However, the matrine, model + Bay11-7082 and matrine + Bay11-7082
groups exhibited lower mRNA levels of 1β, IL-6 and TNF-α compared
with those in the model group (P<0.05; Fig. 10).
Discussion
Atherosclerosis is considered to be a chronic
inflammatory disease of the vascular walls, while matrine has
anti-inflammatory effects and also affects the cardiovascular
system. Therefore, we hypothesized that matrine may have potential
therapeutic use for preventing the progression of atherosclerotic
lesions. Furthermore, exploration of the potential role and
mechanism of matrine as an anti-atherosclerotic treatment may
support research into the properties of traditional Chinese
medicine and pharmacology. Inflammation is associated with the
pathogenesis of atherosclerosis (13), and acts as a key regulatory process
that links the risk factors for atherosclerosis (14). IL-1β is a key mediator of the host
response to infection and inflammation (15). Although it helps in resisting
pathogens, it also exacerbates damage during chronic diseases and
acute tissue injury (16). A study
suggested that IL-1β may play a local role in the formation and
stability of atherosclerosis by inducing macrophages, endothelial
cells and smooth muscle cells to produce cytokines and proteolytic
enzymes (17). IL-6 is an
inflammatory factor that plays a central role in the inflammatory
response. It exists in cells and in extracellular deposits of
connective tissue matrix in the human atherosclerotic wall and may
be a crucial pro-atherosclerotic cytokine (18,19).
IL-6 has also been shown to be involved in the development of human
atherosclerosis and is highly expressed in atherosclerosis
(20). As a pro-inflammatory
cytokine, TNF-α is associated with metabolic disorders and may have
a significant effect on the development of cardiovascular disease
(21). The expression of TNF-α
increases in atherosclerotic cardiovascular diseases (22). A previous study demonstrated that
the administration of matrine to oxLDL-exposed macrophages reduced
the protein and mRNA expression of inflammatory cytokines in a
concentration-dependent manner (23). In the present study, the relative
mRNA levels of IL-1β, IL-6 and TNF-α in the oxLDL-treated cell
model were significantly higher than those in the normal group, but
after treatment with matrine, the relative mRNA levels of IL-1β,
IL-6 and TNF-α were significantly reduced. These results indicate
that the expression levels of IL-1β, IL-6 and TNF-α were increased
under conditions simulating those of atherosclerosis, and matrine
inhibited the expression of inflammatory cytokines.
As an acidic nuclear protein, PCNA is considered a
histological marker of the G1/S phase in the cell cycle
(24). Ki-67 and PCNA are two
nuclear markers commonly used to signal the proliferation phase
(25). Bcl-2 family proteins are
the main regulators of cell cycle, and among them, Bax is
pro-apoptotic, whereas Bcl-2 inhibits apoptosis (26). A previous study revealed the ability
of matrine to inhibit and induce the differentiation of K-562 cells
(27). In the present study, the
results demonstrated that in the matrine group, the expression
levels of Ki-67, PCNA and Bax were significantly decreased while
those of Bcl-2 was increased compared with the respective levels in
the model group. These results indicate that matrine inhibited the
proliferation and apoptosis of vascular smooth muscle cells in this
atherosclerotic model.
The NF-κB pathway is well known as a typical
pro-inflammatory signaling pathway (28), and NF-κB has been shown to regulate
the expression of proteins that inhibit apoptosis and promote
proliferation (29). In a previous
study, matrine inhibited vascular cell adhesion molecule 1 and
intercellular adhesion molecule 1 expression in TNF-α-stimulated
human aortic smooth muscle cells by inhibiting the production of
reactive oxygen species and activating the NF-κB and MAPK pathways,
which suggests its potential for the prevention of atherosclerosis
(30). In the present study,
whether matrine affected inflammatory factors and pro-apoptotic
proteins through the NF-κB pathway was investigated. The results
suggest that matrine may exert anti-inflammatory effects and
inhibit cell proliferation by inhibiting activation of the NF-κB
pathway. However, only cell experiments were performed, which
limits the translational clinical value of the results.
In summary, the present study demonstrates that
matrine attenuated the inflammatory response, abnormal lipid
metabolism and proliferation of vascular smooth muscle cells
exposed to oxLDL, and suggests that these effects were mediated via
the NF-κB pathway.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GW and HW designed the experiments, CJ and CW
carried out the experiments and ZL and AQ analyzed the experimental
results. GW wrote the manuscript and HW revised the manuscript. GW
and HW confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tabas I, García-Cardeña G and Owens GK:
Recent insights into the cellular biology of atherosclerosis. J
Cell Biol. 209:13–22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gisterå A and Hansson GK: The immunology
of atherosclerosis. Nat Rev Nephrol. 13:368–380. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Feinberg MW and Moore KJ: MicroRNA
regulation of atherosclerosis. Circ Res. 118:703–720.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bäck M and Hansson GK: Anti-inflammatory
therapies for atherosclerosis. Nat Rev Cardiol. 12:199–211.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Libby P and Everett BM: Novel
antiatherosclerotic therapies. Arterioscler Thromb Vasc Biol.
39:538–545. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han
X, Tang D and Chen R: Research progress on the relationship between
atherosclerosis and inflammation. Biomolecules.
8(80)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang MJ and Huang J: Recent research
progress of anti-tumor mechnism matrine. Zhongguo Zhong Yao Za Zhi.
29:115–118. 2004.PubMed/NCBI(In Chinese).
|
|
8
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Anti-tumor activities of matrine and oxymatrine: Literature
review. Tumour Biol. 35:5111–5119. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Suo Z, Liu Y, Ferreri M, Zhang T, Liu Z,
Mu X and Han B: Impact of matrine on inflammation related factors
in rat intestinal microvascular endothelial cells. J
Ethnopharmacol. 125:404–409. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yu HB, Zhang HF, Li DY, Zhang X, Xue HZ
and Zhao SH: Matrine inhibits matrix metalloproteinase-9 expression
and invasion of human hepatocellular carcinoma cells. J Asian Nat
Prod Res. 13:242–250. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu XY, Fang H, Yang ZG, Wang XY, Ruan LM,
Fang DR, Ding YG, Wang YN, Zhang Y, Jiang XL and Chen HC: Matrine
inhibits invasiveness and metastasis of human malignant melanoma
cell line A375 in vitro. Int J Dermatol. 47:448–456.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
de Boer OJ, van der Wal AC and Becker AE:
Atherosclerosis, inflammation, and infection. J Pathol.
190:237–243. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Libby P, Ridker PM and Hansson GK: Leducq
Transatlantic Network on Atherothrombosis. Inflammation in
atherosclerosis: From pathophysiology to practice. J Am Coll
Cardiol. 54:2129–2138. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Faggioni R, Fantuzzi G, Fuller J,
Dinarello CA, Feingold KR and Grunfeld C: IL-1beta mediates leptin
induction during inflammation. Am J Physiol. 274:R204–R208.
1998.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lopez-Castejon G and Brough D:
Understanding the mechanism of IL-1β secretion. Cytokine Growth
Factor Rev. 22:189–195. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bhaskar V, Yin J, Mirza AM, Phan D,
Vanegas S, Issafras H, Michelson K, Hunter JJ and Kantak SS:
Monoclonal antibodies targeting IL-1 beta reduce biomarkers of
atherosclerosis in vitro and inhibit atherosclerotic plaque
formation in Apolipoprotein E-deficient mice. Atherosclerosis.
216:313–320. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rus HG, Vlaicu R and Niculescu F:
Interleukin-6 and interleukin-8 protein and gene expression in
human arterial atherosclerotic wall. Atherosclerosis. 127:263–271.
1996.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Stenvinkel P, Barany P, Heimbürger O,
Pecoits-Filho R and Lindholm B: Mortality, malnutrition, and
atherosclerosis in ESRD: What is the role of interleukin-6? Kidney
Int. Suppl:103–108. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Seino Y, Ikeda U, Ikeda M, Yamamoto K,
Misawa Y, Hasegawa T, Kano S and Shimada K: Interleukin 6 gene
transcripts are expressed in human atherosclerotic lesions.
Cytokine. 6:87–91. 1994.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Skoog T, Dichtl W, Boquist S,
Skoglund-Andersson C, Karpe F, Tang R, Bond MG, de Faire U, Nilsson
J, Eriksson P and Hamsten A: Plasma tumour necrosis factor-alpha
and early carotid atherosclerosis in healthy middle-aged men. Eur
Heart J. 23:376–383. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Suarez EC, Lewis JG and Kuhn C: The
relation of aggression, hostility, and anger to
lipopolysaccharide-stimulated tumor necrosis factor (TNF)-alpha by
blood monocytes from normal men. Brain Behav Immun. 16:675–684.
2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhou J, Ma W, Wang X, Liu H, Miao Y, Wang
J, Du P, Chen Y, Zhang Y and Liu Z: Matrine suppresses reactive
oxygen species (ROS)-mediated MKKs/p38-induced inflammation in
oxidized low-density lipoprotein (ox-LDL)-stimulated macrophages.
Med Sci Monit. 25:4130–4136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhong W, Peng J, He H, Wu D, Han Z, Bi X
and Dai Q: Ki-67 and PCNA expression in prostate cancer and benign
prostatic hyperplasia. Clin Invest Med. 31:E8–E15. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kayaselçuk F, Zorludemir S, Gümürdühü D,
Zeren H and Erman T: PCNA and Ki-67 in central nervous system
tumors: Correlation with the histological type and grade. J
Neurooncol. 57:115–121. 2002.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Reed JC: Proapoptotic multidomain
Bcl-2/Bax-family proteins: Mechanisms, physiological roles, and
therapeutic opportunities. Cell Death Differ. 13:1378–1386.
2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang LP, Jiang JK, Tam JW, Zhang Y, Liu
XS, Xu XR, Liu BZ and He YJ: Effects of matrine on proliferation
and differentiation in K-562 cells. Leuk Res. 25:793–800.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1(a001651)2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Moynagh PN: The NF-kappaB pathway. J Cell
Sci. 118:4589–4592. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu J, Zhang L, Ren Y, Gao Y, Kang L and
Lu S: Matrine inhibits the expression of adhesion molecules in
activated vascular smooth muscle cells. Mol Med Rep. 13:2313–2319.
2016.PubMed/NCBI View Article : Google Scholar
|