Introduction
Osteoporosis is a common metabolic disease involving
reduced bone density, destruction of the bone microstructure and
increased bone fragility (1).
Osteoporosis is mainly caused by the inhibition of osteogenesis,
which increases the risk of fracture and predominantly affects
postmenopausal women (2). However,
little is known about the pathogenesis of postmenopausal
osteoporosis. A recent review of the literature indicated that
oxidative stress is crucial to the development of osteoporosis;
oxidative stress is a pathological state of the body in which
reactive oxygen species (ROS) cause excessive oxidation, thereby
leading to aging and disease (3).
In clinical studies, the analysis of oxidation-associated serum
biomarkers, including antioxidant enzymes and advanced oxidative
products, has indicated that the bodies of postmenopausal women
with osteoporosis are in a highly oxidative state (4). This phenomenon can be explained by the
fact that estrogen is an antioxidant and, therefore, estrogen
deficiency after menopause reduces the ability of the body to
ameliorate high oxidative stress (5). Additionally, oxidative damage in the
bone tissue has been shown to cause osteoblast apoptosis and
mediate the deterioration associated with osteoporosis (6). Therefore, improvement of the oxidative
state is an important means for reversing bone loss in
postmenopausal women.
Metformin is a traditional hypoglycemic drug that
has been used to treat tumors and delay aging and has exhibited
promising effects (7-9).
Metformin has also exhibited a therapeutic effect in
diabetes-associated osteoporosis, mediated by the amelioration of
the hyperglycemic microenvironment (10). However, the therapeutic effect was
only attributed to mitigation of the effects of high glucose. The
use of metformin in postmenopausal osteoporosis has not yet been
investigated. Since metformin is able to improve the oxidative
state in numerous diseases, including diabetes and fatty liver
(11,12), the aim of the present study was to
determine the role of metformin in the treatment of postmenopausal
osteoporosis.
Mitochondria are the main organelles involved in the
maintenance of redox balance in a cell (13). Most antioxidant enzymes are able to
eliminate peroxide and prevent the release of ROS in mitochondria.
During the development of osteoporosis, the dysfunction of
mitochondria in osteoblasts leads to the excessive generation of
ROS, which cause damage to other organelles and induce apoptosis
(14). Sirtuin3 (SIRT3) is an
important deacetylating enzyme and an upstream regulator of the
activity of numerous metabolic enzymes and mitochondrial metabolism
(15-17).
Increasing the expression of SIRT3 is expected to contribute to the
repair of mitochondrial damage in osteoblasts and attenuate the
loss of bone mass.
The present study investigated the hypothesis that
metformin can improve the activity of antioxidant enzymes and
attenuate the osteoblast apoptosis induced by
H2O2 by upregulating SIRT3 expression via the
PI3K/AKT pathway. The role of oxidative damage in the development
of postmenopausal osteoporosis was evaluated, to provide a
potential target for the treatment of osteoporosis. The direct
effect of metformin on osteoblasts and the therapeutic effect on an
animal model of postmenopausal osteoporosis were also revealed. The
findings may contribute to our understanding of the pathogenesis of
postmenopausal osteoporosis and suggest novel therapeutic
targets.
Materials and methods
Reagents and cell culture
MC3T3-E1 cells were purchased from the Cell Bank of
Type Culture Collection of the Chinese Academy of Sciences.
Metformin was purchased from Dalian Meilun Biotechnology Co., Ltd.
Antibodies against SIRT3 (1:1,000; cat. no. ab246522), catalase
(CAT) (1:2,000; cat. no. ab209211), superoxide dismutase 1 (SOD 1)
(1:2,000; cat. no. ab51254), COX IV (1:1,000; cat. no. ab16056)
cleaved caspase-3 (1:1,000; cat. no. ab214430), caspase-3 (1:1,000;
cat. no. ab184787) and Bcl-2 (1:1,000; cat. no. ab182858) were
obtained from Abcam. Bax (1:1,000; cat. no. 60267-1-Ig), cytochrome
c (1:1,000; cat no: 66264-1-Ig) and β-actin (1:2,000; cat.
no. 66009-1-Ig) antibodies and horseradish peroxidase-conjugated
anti-mouse and anti-rat secondary antibodies (both 1:2,000; cat.
nos. SA00001-1 and SA00001-15, respectively) were obtained from
ProteinTech Group, Inc. In addition, antibodies against PI3K
(1:1,000; cat. no. 4257), phosphorylated (p-)PI3K (1:1,000; cat.
no. 4228), AKT (1:1,000; cat. no. 4691) and p-AKT (1:1,000; cat.
no. 4060) were purchased from Cell Signaling Technology, Inc.
MC3T3-E1 cells were cultured in α-Minimal Essential
Medium (HyClone; Cytiva) supplemented with 10% fetal bovine serum
(HyClone; Cytiva), 100 U/ml streptomycin sulfate and 100 mg/ml
penicillin. Cells were grown in a humidified incubator with 5%
CO2 at 37˚C (18).
H2O2 was added to the medium to induce an
oxidative damage model at a concentration gradient of 0.1, 0.2 and
0.3 mM. Then, various concentrations of metformin (0.05, 0.1, 0.2,
0.3 and 0.4 mM) were added with 0.2 mM H2O2
to determine the optimal concentration based on cell apoptosis
measured by flow cytometry.
Cell viability assay
Cell Counting Kit-8 (CCK-8) colorimetric assay
(Dojindo Molecular Technologies, Inc.) was used to measure the
viability of the cells after metformin treatment to assess drug
cytotoxicity. MC3T3-E1 cells were plated into 96-well plates at
5x103 cells/well in 100 µl medium/well. Cells were
treated with the aforementioned concentrations of
H2O2 for 6 h and metformin for 24, 48 and 72
h separately in a 37˚C humidified incubator. CCK-8 solution was
added at 10 µl/well, and the cells were incubated in a humidified
incubator for another 1 h. Cell viability was then determined based
on the absorbance at 450 nm.
Apoptosis assay by flow cytometry
Various concentrations of metformin (0.05, 0.1, 0.2,
and 0.3 mM) were added with 0.2 mM H2O2 to
6-well plates seeded with 1.2x106 MC3T3-E1 cells and
incubated at 37˚C for 6 h. The cells were then harvested,
resuspended in binding buffer and stained with FITC-Annexin
V/propidium iodide for 15 min using Annexin V-FITC kit (Beyotime
Institute of Biotechnology), in the dark at room temperature
(19). Apoptosis was detected using
a FACScan flow cytometer (BD Biosciences) and analyzed with
CytExpert 2.3 (Beckman Coulter, Inc.).
Western blotting
To isolate the proteins, the culture solution was
decanted from the treated cells and phosphate-buffered saline (PBS)
was used to wash the cells three times. The Petri dishes were
placed on ice after decanting the PBS. Radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology) and
phenylmethylsulfonyl fluoride were mixed at a ratio of 1:1,000 and
100 µl mixture was incubated with the cells on ice for 30 min. The
extract was then transferred to 1.5-ml centrifuge tubes and
centrifuged at 12,000 x g and 4˚C for 5 min. The supernatant
containing total protein was collected in a new 1.5-ml centrifuge
tube and stored at -20˚C. Bone tissue protein extraction kit
(Beijing Biolab Technology Co., Ltd.) was used to extract protein
from mouse bone tissue. Bone tissue was fully soaked in saline and
washed with distilled water to remove blood and red blood cells.
Bone tissue was cut into small pieces, weighed, placed in a mortar
filled with liquid nitrogen and ground into powder. The powder was
transferred into a centrifuge tube. A total of 200-300 µl protein
extraction reagent per 100 mg bone tissue was added and mixed for
30 min on ice. Then, the mixture was centrifuged at 12,000 x g and
4˚C for 15 min. Supernatant was collected in a new 1.5-ml
centrifuge tube and stored at -20˚C. BCA assay kit (Beyotime
Institute of Biotechnology) was used to measure protein
concentration. Then, 50 µg protein/lane was resolved by 10 and 12%
SDS-PAGE and transferred to polyvinylidene difluoride membranes. The
membranes were blocked with 5% skimmed milk for 1.5 h at room
temperature. After washing with 1% TBST, the membranes were
incubated with the aforementioned primary antibodies at 4˚C
overnight and secondary antibody at 4˚C for 1.5 h on the next day.
After washing thoroughly, the protein bands were coated with an
enhanced chemiluminescence system (Analytik Jena AG) and visualized
using a chemiluminescence imaging system (Analytik Jena AG)
(20). Protein levels were
normalized to β-actin (molecular weight, 43 kDa). Finally, ImageJ
v1.8.0 software (National Institutes of Health) was used to
calculate the optical density and relative protein expression
levels. For the detection of cytochrome c, mitochondria were
separated using a Cell Mitochondria Isolation kit (Beyotime
Institute of Biotechnology) and the expression levels of cytochrome
c in the mitochondria and cytosol were analyzed.
Detection of mitochondrial membrane
potential using JC-1
A Mitochondrial Membrane Potential Assay kit with
JC-1 (Beyotime Institute of Biotechnology) was used to evaluate the
mitochondrial membrane potential of the MC3T3-E1 cells after the
aforementioned treatments. The cells were suspended in JC-1
staining working solution for 30 min in a humidified incubator at
37˚C and then washed with JC-1 buffer solution three times.
Relative changes were detected by flow cytometry (CytoFLEX System
B3-R3-V3) and analyzed with CytExpert 2.3 (both Beckman Coulter,
Inc.). Absolute changes were detected using a multifunctional
microplate reader. JC-1 fluorescence was measured at
excitation/emission wavelengths at 585/590 (red) and 510/527 nm
(green). The red/green ratios were calculated to indicate the
mitochondrial membrane potential.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
A miRNeasy RNA mini kit (Qiagen, Inc.) was used to
extract total RNA from cells. Then, GoScript™ Reverse Transcription
mix, Oligo(dT) (Promega Corporation) were used to synthesize cDNA
from the RNA according to the manufacturer's protocol. qPCR was
performed using GoTaq® qPCR Master Mix (Promega
Corporation). The thermocycling conditions were as follows:
Denaturation at 95 ˚C for 15 sec, annealing at 60˚C for 20 sec and
extension at 72˚C for 1 min, for 40 cycles. The data were collected
using a Roche Light Cycler® 480 instrument II (Roche
Diagnostics). mRNA expression was calculated using the
2-ΔΔCq method (21).
Primers for the qPCR analysis of SIRT3 and the reference gene
b-actin are listed in Table I.
 | Table IPrimers for quantitative polymerase
chain reaction. |
Table I
Primers for quantitative polymerase
chain reaction.
| Name | Forward
(5'-3') | Reverse
(5'-3') |
|---|
| SIRT3 |
ATCCCGGACTTCAGATCCCC |
CAACATGAAAAAGGGCTTGGG |
| β-actin |
GGCTGTATTCCCCTCCATCG |
CCAGTTGGTAACAATGCCATGT |
Cell transfection
SIRT3-small interfering RNAs (siRNAs) were designed
and synthesized by SynBio-Tech (Suzhou) Co., Ltd. The inhibition
efficiencies of three different siRNA sequences were determined,
and SIRT3-si-2 with the highest inhibition efficiency (73.4%) was
used in the experiments. The siRNA sequences are presented in
Table II. A total of 50 nM siRNA
duplexes were transfected into MC3T3-E1 cells for 48 h using
Lipofectamine™ 3000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Untreated cells
were used as a control to verify the effect of cell transfection.
The cells were used in the experiments 2 days after transfection at
37˚C.
 | Table IIRNA interference sequences used for
transfection. |
Table II
RNA interference sequences used for
transfection.
| Name | Forward
(5'-3') | Reverse
(5'-3') |
|---|
| SIRT3-si-1 |
CCCUGAACCCAUCUUUGAAdTdT |
UUCAAAGAUGGCUUCAGGGdTdT |
| SIRT3-si-2 |
GCAAGGUUCCUACUCCAUAdTdT |
UAUGGAGUAGGAACCUUGCdTdT |
| SIRT3-si-3 |
GGAUCUUACGCAGCGGGAAdTdT |
UUCCCGCUGCAUAAGAUCCdTdT |
Animal experiments
A total of 15 C57BL/6J female mice (8 weeks, 20-25
g) were obtained and housed in the Department of Laboratory Animal
Science of China Medical University. The laboratory environment was
maintained at a temperature of 20-26˚C, 40-70% relative humidity,
≤14 mg/m3 ammonia concentration, ≤60 dB noise and a 12-h
alternating light/dark cycle with ad libitum access to food
and drinking water. All animals were adapted to the environment for
2 weeks before the experiments. The mice were randomly divided into
three groups (n=5/group); two groups (OVX) were selected for
ovariectomy treatment, and the remaining group underwent a sham
surgery (the epidermis and peritoneum were cut, then sutured). The
mice in the OVX groups were subjected to bilateral ovariectomy
under 1.4-1.5% isoflurane inhalation anesthesia with oxygen.
Metformin was directly dissolved in 0.9% normal saline, and mice in
one of the OVX groups (OVX + Met) were injected with metformin (100
mg/kg/day) intragastrically, starting 3 days after surgery. The
mice in the OVX and sham groups were treated with normal saline in
a similar manner. After treatment for 8 weeks, all mice were
sacrificed via exsanguination under isoflurane anesthesia.
Bilateral femurs and tibias were harvested for imaging and protein
extraction. All animal experiments were performed according to
laboratory and animal welfare guidelines and were approved by the
Animal Ethics Committee of the First Affiliated Hospital of China
Medical University (approval no. 2019014).
Microcomputed tomography
(micro-CT)
The femurs were evaluated by microcomputed
tomography (Skyscan 1276 Micro-CT; Bruker Corporation). X-ray
images at each angle were reconstructed into a 3-dimensional image
analyzed by the CT-analyser software CTAn 1.19.11.1 (Bruker
Corporation). The following variables were determined: Bone
volume/tissue volume ratio (BV/TV), bone surface/bone volume ratio
(BS/BV), trabecular separation (Tb.Sp) and trabecular thickness
(Tb.Th).
Statistical analysis
Student's t-tests and one-way ANOVA followed by
Tukey's post hoc tests were used for the statistical analysis.
Experiments were performed with three replicates and were analyzed
using SPSS 19.0 software (IBM Corp.). P<0.05 was considered
statistically significant.
Results
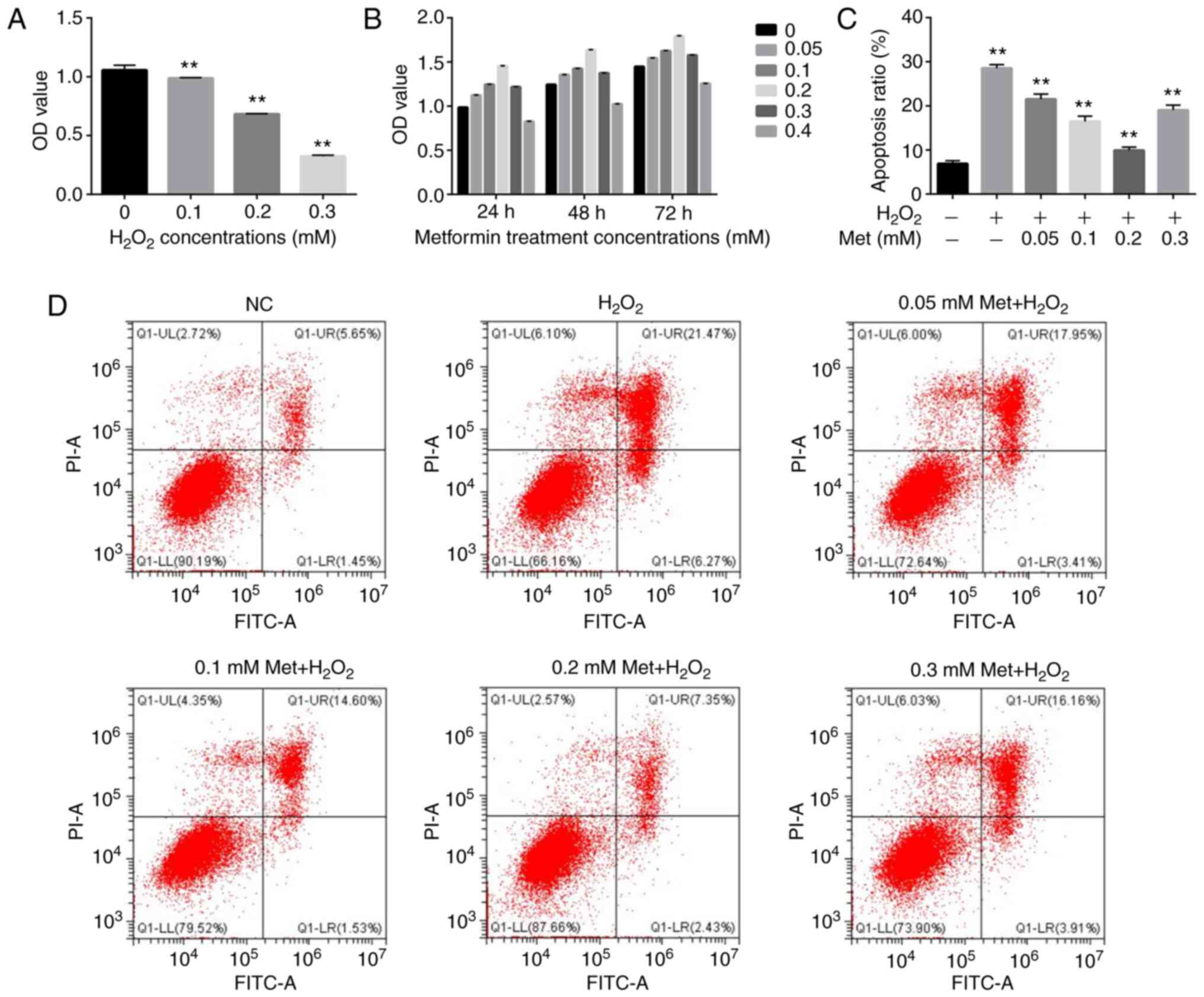
Metformin inhibits apoptosis induced
by H2O2 in MC3T3-E1 cells
The effect of metformin on osteoblasts under
H2O2-induced highly oxidative conditions was
evaluated. A CCK-8 cell viability assay was performed to
investigate the effects of different concentrations of
H2O2 on cell viability and thereby determine
the appropriate concentration for use in subsequent experiments.
H2O2 concentrations of 0.1, 0.2 and 0.3 mM
were used to treat cultured osteoblasts for 6 h. The results
revealed a weak effect of 0.1 mM, intermediate effect of 0.2 mM and
strong effect of 0.3 mM (Fig. 1A).
As the loss of viability at 0.3 mM was considered too high and
different from in vivo conditions, the 0.2-mM concentration
was deemed appropriate and selected for further experiments.
Additionally, co-treatment of the cells with 0.2 mM
H2O2 and various concentrations of metformin
(0.05, 0.1, 0.2, 0.3 and 0.4 mM) indicated that only the 0.4-mM
concentration had a negative effect on cell proliferation (Fig. 1B). Flow cytometry was used to
evaluate the alleviating effect of metformin at concentrations of
0.05-0.3 on 0.2 mM H2O2-induced osteoblast
apoptosis. All metformin concentrations inhibited apoptosis, and
treatment with 0.2 mM metformin exhibited the strongest ability to
attenuate apoptosis (Fig. 1C and
D). Therefore, this concentration
of metformin was used in subsequent experiments.
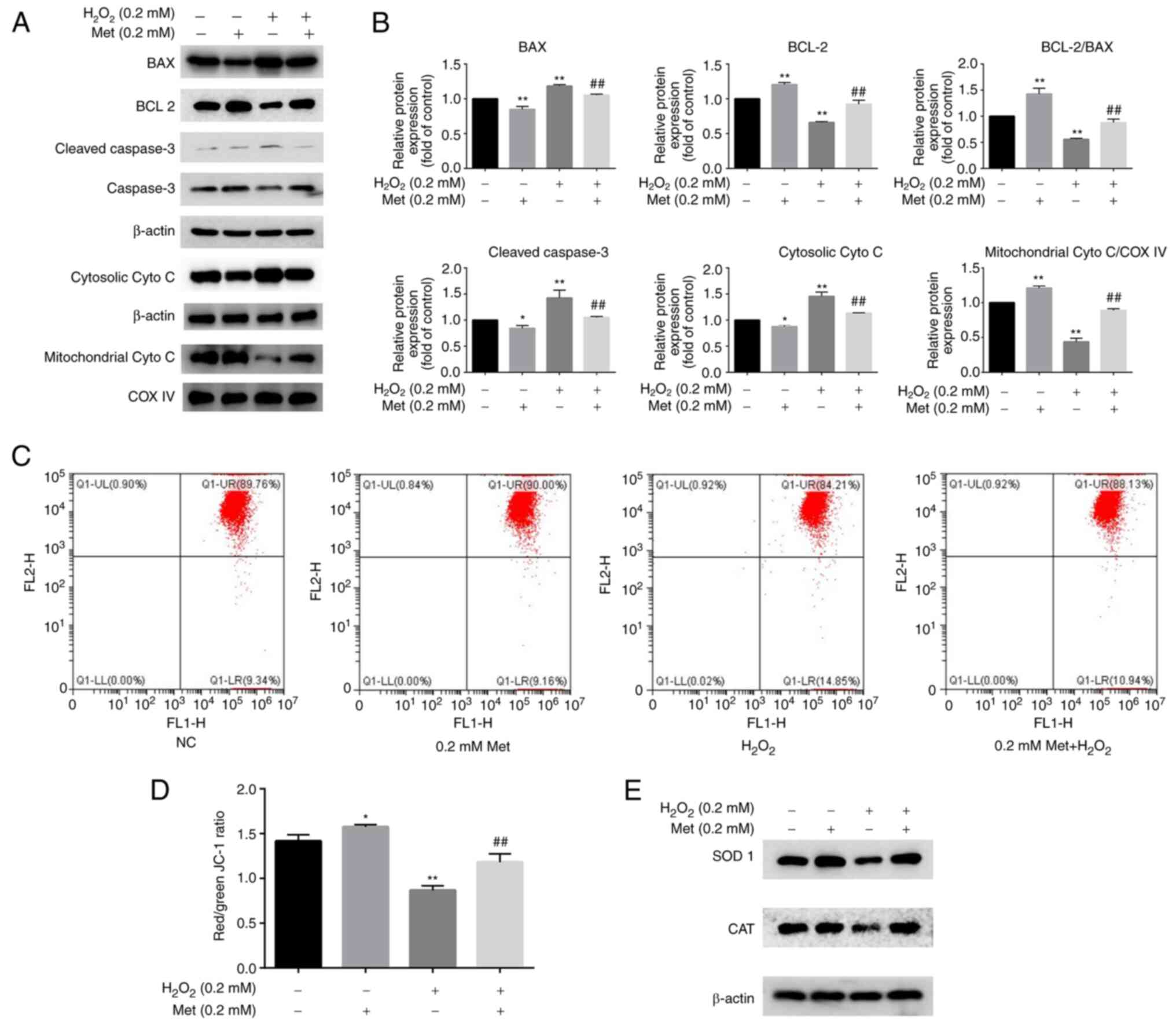
Metformin reverses osteoblast
apoptosis induced by H2O2 via the
mitochondrial pathway
The western blot analysis of proteins involved in
the mitochondrial apoptotic pathway indicated that the levels of
Bax, cleaved caspase-3 and cytosolic cytochrome c were
increased, and the level of Bcl-2 and mitochondrial cytochrome
c were decreased following induction with 0.2 mM
H2O2; these changes were attenuated by
treatment with 0.2 mM metformin (Fig.
2A and B). JC-1 is a
fluorescent probe used to detect the mitochondrial membrane
potential. A reduction in mitochondrial membrane potential is an
important event in the early stage of apoptosis (22). Changes in the mitochondrial membrane
potential were detected by a mitochondrial membrane potential assay
using JC-1. The results indicated that metformin inhibited
H2O2-induced mitochondrial injury (Fig. 2C and D). Additionally, the expression levels of
SOD 1 and CAT, which are important regulators of ROS production in
mitochondria, were determined. The expression levels of these
proteins were decreased after treatment with
H2O2 and increased by metformin co-treatment
(Fig. 2E). These results suggest
that H2O2 induces apoptosis via the
mitochondrial pathway by changing the mitochondrial membrane
potential and causing an imbalance in ROS, and these effects are
reversed by metformin.
Metformin ameliorates osteoblast
apoptosis induced by H2O2 by upregulating
SIRT3 expression via the PI3K/AKT pathway
To determine the role of SIRT3 in the reversal of
H2O2-induced apoptosis by metformin, the
SIRT3 gene was knocked down by transfection of the cells with
SIRT3-siRNA (Fig. 3A). The data in
Fig. 3B and C indicate that in cells treated with
H2O2 and metformin, the targeted knockdown of
SIRT3 attenuated the metformin-induced reduction in apoptosis.
Marker proteins of the mitochondrial apoptosis pathway were
detected by western blotting. The levels of Bax, cleaved caspase-3
and cytosolic cytochrome c increased and the level of Bcl-2
was decreased in the H2O2 and
metformin-treated cells with SIRT3 knockdown compared with those
without SIRT3 knockdown (Fig. 3D).
Additionally, the direct effect of metformin on the expression of
SIRT3 was detected, and the results indicated that metformin
attenuated the H2O2-induced reduction in
SIRT3 expression in osteoblasts (Fig.
3E). The mitochondrial membrane potential was also measured
using a microplate reader, and the results show that the ability of
metformin to inhibit the H2O2-induced change
in mitochondrial membrane potential was attenuated by SIRT3
knockdown, indicating that SIRT3 is involved in the mechanism by
which metformin protects the mitochondria (Fig. 3F). In the H2O2
and metformin-treated cells, the protein levels of SOD 1 and CAT
were decreased following SIRT3 knockdown (Fig. 3G), indicating that the oxidative
state and ROS levels were increased. The PI3K/AKT signaling pathway
is closely associated with cell proliferation, differentiation and
apoptosis (23). In order to
investigate whether this pathway is involved in the protective
mechanism of metformin against H2O2-induced
osteoblast apoptosis, the levels of proteins in the PI3K/AKT
pathway were measured. The results in Fig. 3H show that the activation of
PI3K/AKT was inhibited in osteoblasts under apoptosis-inducing
conditions and preserved with metformin co-treatment. When SIRT3
was silenced, the preventive effect of metformin was suppressed.
These results suggest that metformin protects the mitochondria and
prevents the H2O2-induced
apoptosis of MC3T3-E1 cells by enhancing SIRT3 expression via the
PI3K/AKT signaling pathway.
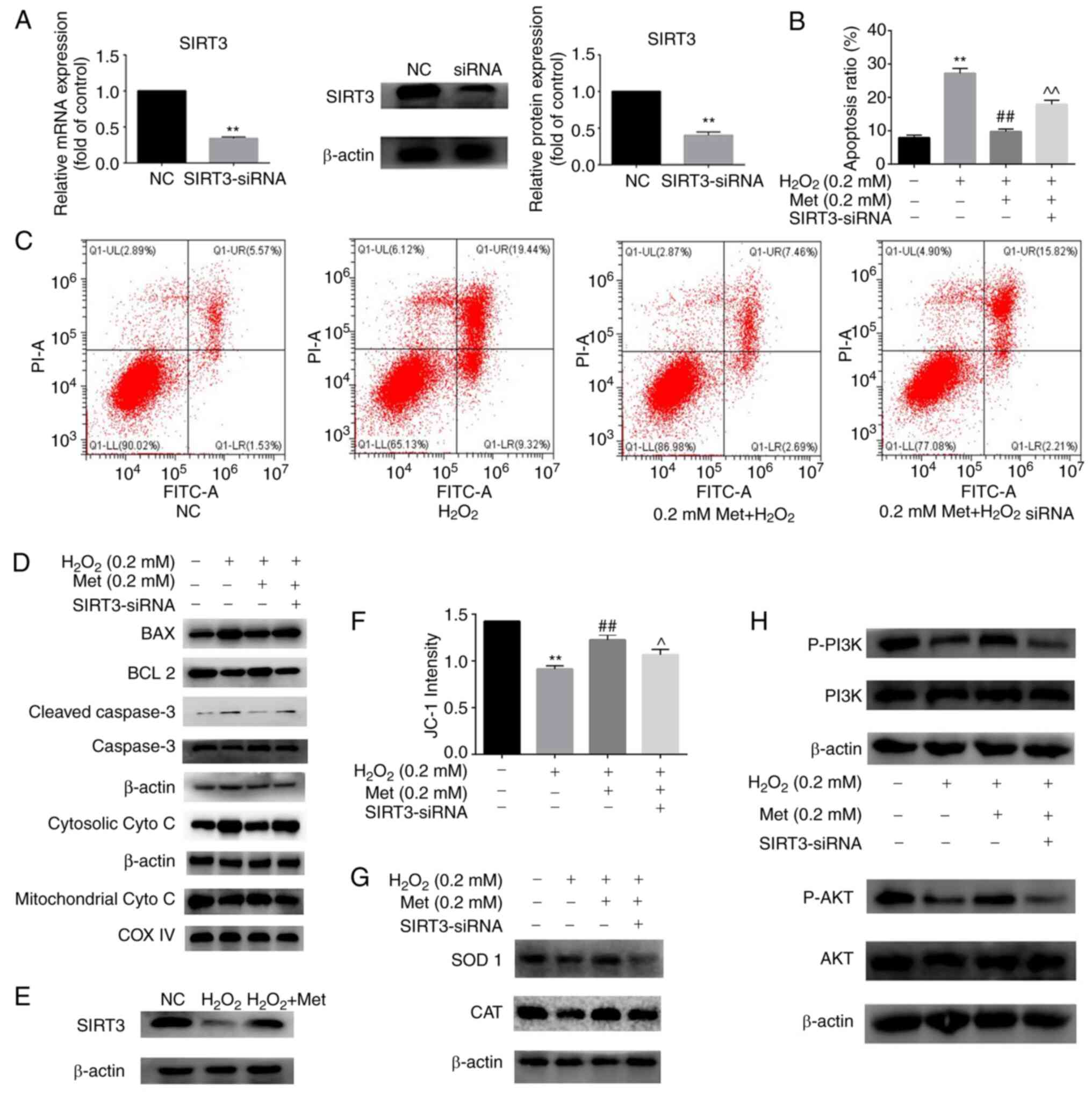 | Figure 3Met ameliorates osteoblast apoptosis
induced by H2O2 by upregulating SIRT3
expression via the PI3K/AKT pathway. (A) Transfection efficiency of
SIRT3 knockdown was detected at the mRNA and protein levels. (B)
Apoptosis rate of MC3T3-E1 cells after incubation for 6 h in the
control, H2O2, H2O2 +
0.2 mM Met and H2O2 + 0.2 mM Met +
SIRT3-siRNA group. (C) Detection of cell apoptosis in the four
groups by flow cytometry. (D) Marker proteins in the mitochondrial
apoptosis pathway were detected by western blotting in the four
groups. (E) Effect of Met on the protein expression of SIRT3. (F)
Quantitative changes in mitochondrial membrane potential were
detected using a full-wavelength multifunctional microplate reader.
(G) Protein expression of the antioxidant enzymes SOD 1 and CAT
tested by western blotting shows that SIRT3-siRNA interferes with
the anti-oxidative effect of Met. (H) Western blotting of proteins
in the PI3K-AKT pathway shows the involvement of this pathway in
the SIRT3-mediated protective effect of Met against osteoblast
apoptosis. Experiments were performed in triplicate. Data are
presented as the mean ± SD. **P<0.01 vs. control
cells, ##P<0.01 vs. H2O2 alone
and ^P<0.05 and ^^P<0.01 vs.
H2O2 + Met analyzed using ANOVA followed by
Tukey's post hoc tests. Met, metformin; SIRT3, sirtuin3; siRNA,
small interfering RNA; NC, negative control; cyto c,
cytochrome c; COX IV, cyto c oxidase subunit 4; SOD
1, superoxide dismutase 1; CAT, catalase; p-, phosphorylated. |
Metformin ameliorates bone loss in OVX
mice
To verify whether metformin can prevent the
development of osteoporosis in postmenopausal mice, a menopausal
mouse model was generated by bilateral ovariectomy. After
observation for 3 days, the ovariectomized mice were divided into
two groups with or without intragastric metformin. A sham group was
also established to verify the reliability of the model. The bone
mass was assessed and microstructure was observed by micro-CT
analysis. As shown in Fig. 4A, the
trabecular bone mass was lower in the OVX group compared with the
sham group, and the phenomenon was attenuated by metformin feeding.
The data obtained by micro-CT scanning showed that Tb.Th and the
BV/TV ratio were decreased in OVX mice compared with those in the
sham group but were increased in the OVX + Met group compared with
the OVX group. In addition, Tb.Sp and the BS/BV ratio were higher
in the OVX group than those in the sham group and were decreased
after metformin feeding (Fig. 4B).
All the data indicate that bilateral ovariectomy led to a reduction
in the bone mass of female mice and that intragastric metformin can
prevent this deterioration.
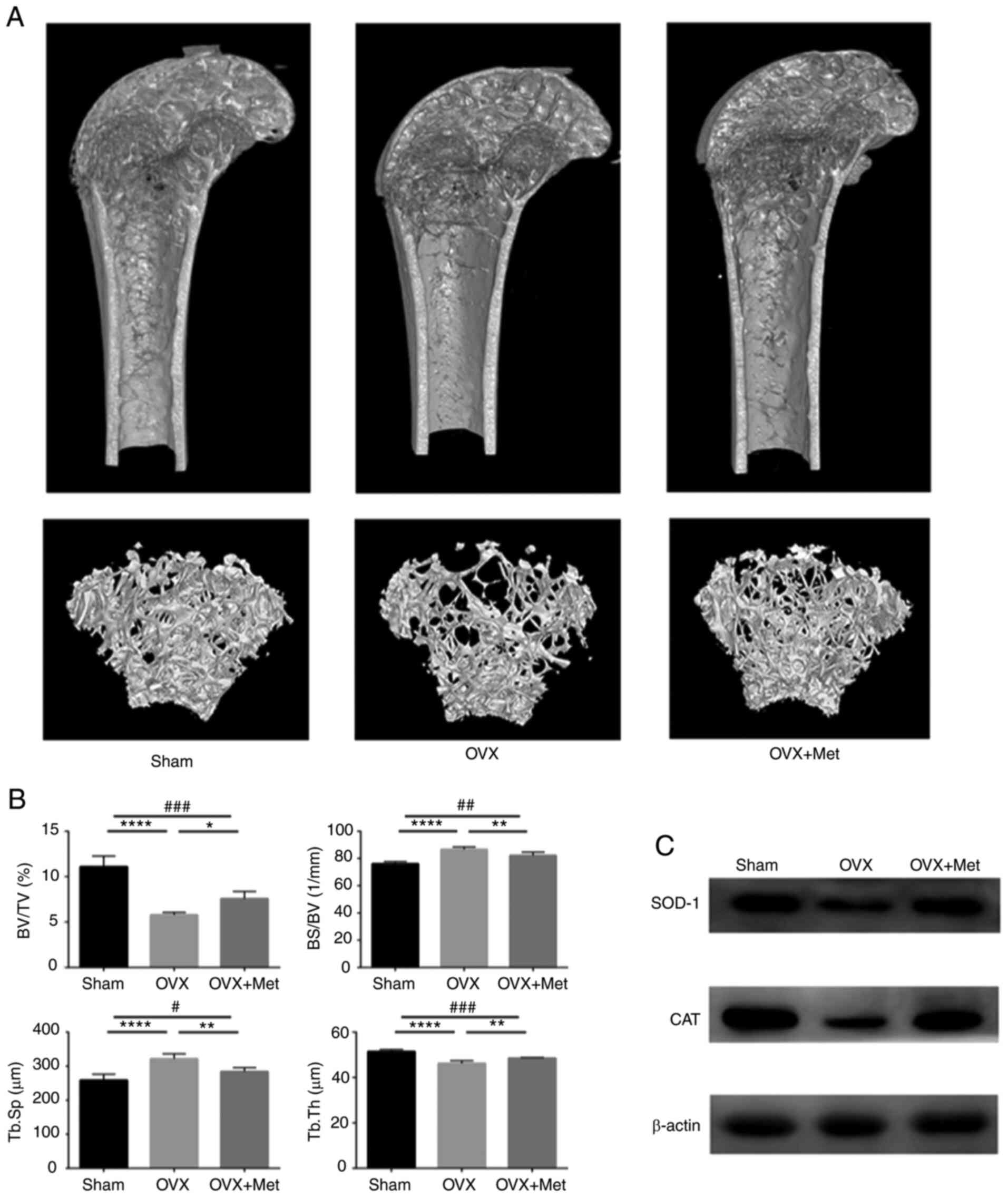 | Figure 4Met attenuates bone loss in
ovariectomized mice. (A) 3-Dimensional reconstruction of the
micro-CT images of femurs extracted from mice from the sham, OVX
and OVX + Met groups. (B) BV/TV, BS/BV, Tb.Th and Tb.Sp values
determined by micro-CT. (C) Protein level of the antioxidant
enzymes SOD 1 and CAT in bone tissue extracted from femurs. Data
are presented as the mean ± SD (n=5 specimens/group).
*P<0.05, **P<0.01 and
****P<0.0001 vs. OVX, and #P<0.05,
##P<0.01 and ###P<0.001 sham vs. OVX +
Met analyzed using ANOVA followed by Tukey's post hoc tests. Met,
metformin; BV/TV, bone volume/tissue volume (percentage bone
volume); BS/BV, bone surface/bone volume (bone surface/volume
ratio; Tb.Th, trabecular thickness; Tb.Sp trabecular separation;
CT, computed tomography; OVX, ovariectomy; SOD 1, superoxide
dismutase 1; CAT, catalase. |
To evaluate the oxidative level in the bone tissue
of the mice, the protein levels of SOD 1 and CAT were assayed by
western blotting. The results indicate that SOD 1 and CAT
expression levels were lower in OVX mice compared with those in the
sham group and were increased after treatment with metformin
(Fig. 4C). These changes indicate
that estrogen deficiency disrupts the redox balance and induces a
high-oxidation state of the bone, which may lead to the apoptosis
of osteoblasts and development of osteoporosis.
Discussion
At present, the treatment of osteoporosis is focused
on direct action on osteoblasts and osteoclasts; for example,
teriparatide promotes bone formation and bisphosphonates inhibit
bone resorption. However, the condition of the patients remains
unstable and is recurrent following drug withdrawal (24,25).
The targeted treatment of osteoblasts or osteoclasts is limited,
and the systemic regulation of osteoporosis is the focus of further
studies. Oxidative stress is regarded as the main factor leading to
body aging and organ damage (26-28).
Studies have indicated that postmenopausal women generally have
reduced antioxidant capacity and are in a highly oxidized state
(29,30). The present study is consistent with
this; OVX mice were established and shown to have reduced bone
levels of antioxidative enzymes. Following treatment with
metformin, the results indicated that the bone mass increased and
the oxidative state of bone tissue was improved after the
treatment.
The present study also investigated the effect and
mechanism of action of metformin on oxidative modulation in
osteoblasts. Oxidative damage is caused by the excessive generation
of ROS that attack cell membranes and destroy protein structures
and genetic materials (31). Under
physiological conditions, ROS are produced in the mitochondria and
are partially eliminated by antioxidants, such as SOD and CAT, to
maintain a dynamic balance. When excessive amounts of free radicals
are produced and the antioxidant function is decreased, the ability
of the body to eliminate and self-repair may be exceeded;
therefore, the release of copious quantities of free radicals
induces apoptosis (32). Thus,
H2O2 was used in the present study to mimic
the hyperoxidative state in postmenopausal osteoporosis and induce
osteoblast apoptosis. The results indicated that the
H2O2-induced changes in apoptosis and
antioxidant levels were reversed by metformin treatment. Thus,
metformin may prevent H2O2-induced osteoblast
apoptosis by improving mitochondrial function.
Subsequently, the mechanism by which metformin
regulates mitochondrial function was investigated. The SIRT protein
family is important for the regulation of metabolism and aging
(33). SIRT3 is associated with
mitochondrial biogenesis and regulates the acetylation levels of
metabolic enzymes in the mitochondria (34,35).
SIRT3 has been demonstrated to increase the expression of CAT and
SOD via the upregulation of forkhead box O3a in the nucleus
(36,37). The excessive production of ROS can
induce p53-mediated apoptosis (38). However, enhancing the expression of
SIRT3 can eliminate ROS, while a deficiency in SIRT3 leads to
oxidative damage and accelerates aging (39,40).
SIRT3 weakens the binding between p53 and Bcl-2 associated
athanogene 2 to activate the Bcl-2-mediated apoptosis-inhibiting
pathway (41). A reduction in the
Bax/Bcl-2 ratio inhibits the expression of cytochrome c,
thereby reducing the activation of caspase-3(42). Additionally, studies have shown that
SIRT3 expression is decreased in an oxidative damage model of
osteoblasts induced by H2O2 and in a rat
model of postmenopausal osteoporosis (43,44).
This evidence indicates that SIRT3 may play an important role in
the metformin-dependent reversal of the apoptosis of osteoblasts
caused by oxidative damage. In the present study, transfection
technology was used to knockdown the expression of SIRT3, and the
data indicate that the therapeutic effect of metformin was
inhibited by the knockdown of SIRT3. These results indirectly
indicate that metformin reverses the mitochondrial injury caused by
H2O2 by upregulating the expression of SIRT3.
Additionally, the PI3K/AKT pathway makes an important contribution
to mitochondrial apoptosis. In the present study, the results also
indicated that the SIRT3-mediated protective effect of metformin
against osteoblast apoptosis was mediated via the PI3K/AKT
signaling pathway. In previous studies, attention has focused on
how metformin inhibits apoptosis associated with oxidative damage
(45,46). However, the present study proceeded
from the effect of metformin on apoptosis, and focused on the
protection of mitochondrial function and regulation of antioxidant
enzymes by metformin.
Due to a limited understanding of the pathogenesis
of osteoporosis, the current drugs for treating this condition are
mainly aimed at the inhibition of osteoclasts (47,48).
However, a simple decline in the effect of osteoclasts can only
delay the deterioration induced by osteoporosis, and a decline in
osteogenesis will continue to cause continuous bone mass loss
concomitant to aging. Inhibition of the apoptosis of osteoblasts is
an effective means for the treatment of osteoporosis. The present
study demonstrates that oxidative damage is involved in the
pathogenesis of postmenopausal osteoporosis, and metformin is an
effective drug therapy. Additionally, the study provides a
potential target for treatment, SIRT3, thus contributing to drug
design and development. Further clarification of the role of
oxidative damage in the development of osteoporosis and the effect
of metformin on osteoclasts will be the focus of future
research.
Acknowledgements
Not applicable.
Funding
Funding: The study was supported by the National Natural Science
Foundation of China (grant no. 81472044), Construction of Clinical
Medical Research Center of Orthopaedics and Sports Rehabilitation
Diseases in Liaoning Province (grant no. 2019416030).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KY was responsible for annotating and maintaining
research data, formal analysis and writing the original draft of
the manuscript. LP contributed to methodology, and software support
for data acquisition and analysis. SZ performed the experiments. LT
was responsible for conceptualization and validation. YZ was
involved in conceptualization, funding acquisition, project
administration, providing resources, and reviewing and editing the
manuscript. All authors read and approved the final manuscript. KY
and YZ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics Committee of the First Affiliated Hospital of China Medical
University (no. 2019014).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Glaser DL and Kaplan FS: Osteoporosis.
Definition and clinical presentation. Spine (Phila Pa 1976). 22
(Suppl 24):S12–S16. 1997.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514.
2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sies H and Jones DP: Reactive oxygen
species (ROS) as pleiotropic physiological signalling agents. Nat
Rev Mol Cell Biol. 21:363–383. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhou Q, Zhu L, Zhang D, Li N, Li Q, Dai P,
Mao Y, Li X, Ma J and Huang S: Oxidative stress-related biomarkers
in postmenopausal osteoporosis: A systematic review and
meta-analyses. Dis Markers. 2016(7067984)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guetta V, Quyyumi AA, Prasad A, Panza JA,
Waclawiw M and Cannon RO III: The role of nitric oxide in coronary
vascular effects of estrogen in postmenopausal women. Circulation.
96:2795–2801. 1997.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nollet M, Santucci-Darmanin S, Breuil V,
Al-Sahlanee R, Cros C, Topi M, Momier D, Samson M, Pagnotta S,
Cailleteau L, et al: Autophagy in osteoblasts is involved in
mineralization and bone homeostasis. Autophagy. 10:1965–1977.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dahmani Z, Addou-Klouche L, Gizard F,
Dahou S, Messaoud A, Chahinez Djebri N, Benaissti MI, Mostefaoui M,
Terbeche H, Nouari W, et al: Metformin partially reverses the
inhibitory effect of co-culture with ER-/PR-/HER2+ breast cancer
cells on biomarkers of monocyte antitumor activity. PLoS One.
15(e0240982)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim Y, Vagia E, Viveiros P, Kang CY, Lee
JY, Gim G, Cho S, Choi H, Kim L, Park I, et al: Overcoming acquired
resistance to PD-1 inhibitor with the addition of metformin in
small cell lung cancer (SCLC). Cancer Immunol Immunother.
70:961–965. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pryor R, Norvaisas P, Marinos G, Best L,
Thingholm LB, Quintaneiro LM, De Haes W, Esser D, Waschina S, Lujan
C, et al: Host-Microbe-Drug-Nutrient screen identifies bacterial
effectors of metformin therapy. Cell. 178:1299–1312.e29.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shen CL, Kaur G, Wanders D, Sharma S,
Tomison MD, Ramalingam L, Chung E, Moustaid-Moussa N, Mo H and
Dufour JM: Annatto-extracted tocotrienols improve glucose
homeostasis and bone properties in high-fat diet-induced type 2
diabetic mice by decreasing the inflammatory response. Sci Rep.
8(11377)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rahimi G, Heydari S, Rahimi B, Abedpoor N,
Niktab I, Safaeinejad Z, Peymani M, Seyed Forootan F, Derakhshan Z,
Esfahani MHN and Ghaedi K: A combination of herbal compound (SPTC)
along with exercise or metformin more efficiently alleviated
diabetic complications through down-regulation of stress oxidative
pathway upon activating Nrf2-Keap1 axis in AGE rich diet-induced
type 2 diabetic mice. Nutr Metab (Lond). 18(14)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Satapati S, Kucejova B, Duarte JA,
Fletcher JA, Reynolds L, Sunny NE, He T, Nair LA, Livingston KA, Fu
X, et al: Mitochondrial metabolism mediates oxidative stress and
inflammation in fatty liver. J Clin Invest. 125:4447–4462.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Tremblay BP and Haynes CM: Mitochondrial
distress call moves to the cytosol to trigger a response to stress.
Nature. 579:348–349. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jing X, Du T, Chen K, Guo J, Xiang W, Yao
X, Sun K, Ye Y and Guo F: Icariin protects against iron
overload-induced bone loss via suppressing oxidative stress.
Journal of cellular physiology. 234:10123–10137. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu S, Su Y, Sun B, Hao R, Pan S, Gao X,
Dong X, Ismail AM and Han B: Luteolin protects against CIRI,
potentially via regulation of the SIRT3/AMPK/mTOR signaling
pathway. Neurochem Res. 45:2499–2515. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li M, Wu C, Muhammad JS, Yan D, Tsuneyama
K, Hatta H, Cui ZG and Inadera H: Melatonin sensitises
shikonin-induced cancer cell death mediated by oxidative stress via
inhibition of the SIRT3/SOD2-AKT pathway. Redox Biol.
36(101632)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim HS, Patel K, Muldoon-Jacobs K, Bisht
KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage
J, Owens KM, et al: SIRT3 is a mitochondria-localized tumor
suppressor required for maintenance of mitochondrial integrity and
metabolism during stress. Cancer Cell. 17:41–52. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li P, Mao WW, Zhang S, Zhang L, Chen ZR
and Lu ZD: Sodium hydrosulfide alleviates dexamethasone-induced
cell senescence and dysfunction through targeting the miR-22/sirt1
pathway in osteoblastic MC3T3-E1 cells. Exp Ther Med.
21(238)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hu J, Su B, Li X, Li Y and Zhao J: Klotho
overexpression suppresses apoptosis by regulating the Hsp70/Akt/Bad
pathway in H9c2(2-1) cells. Exp Ther Med. 21(486)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fathi E, Valipour B, Sanaat Z, Nozad
Charoudeh H and Farahzadi R: Interleukin-6, -8, and TGF-β secreted
from mesenchymal stem cells show functional Role in reduction of
telomerase activity of leukemia cell via Wnt5a/β-catenin and P53
pathways. Adv Pharm Bull. 10:307–314. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fathi E, Farahzadi R, Javanmardi S and
Vietor I: L-carnitine extends the telomere length of the cardiac
differentiated CD117(+)- expressing stem cells. Tissue Cell.
67(101429)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Saha PC, Bera T, Chatterjee T, Samanta J,
Sengupta A, Bhattacharyya M and Guha S: Supramolecular
dipeptide-based near-infrared fluorescent nanotubes for cellular
mitochondria targeted imaging and early apoptosis. Bioconjug Chem.
32:833–841. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang Y, Zeng G and Jiang Y: The emerging
roles of miR-125b in cancers. Cancer Manag Res. 12:1079–1088.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sim IW, Borromeo GL, Tsao C, Hardiman R,
Hofman MS, Papatziamos Hjelle C, Siddique M, Cook GJR, Seymour JF
and Ebeling PR: Teriparatide promotes bone healing in
medication-related osteonecrosis of the jaw: A placebo-controlled,
randomized trial. J Clin Oncol. 38:2971–2980. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Oryan A and Sahvieh S: Effects of
bisphosphonates on osteoporosis: Focus on zoledronate. Life Sci.
264(118681)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vatner SF, Zhang J, Oydanich M, Berkman T,
Naftalovich R and Vatner DE: Healthful aging mediated by inhibition
of oxidative stress. Ageing Res Rev. 64(101194)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Park JS, Piao J, Park G and Hong HS:
Substance-P restores cellular activity of ADSC impaired by
oxidative stress. Antioxidants (Basel). 9(978)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Krishnaswamy VKD, Alugoju P and Periyasamy
L: Effect of short-term oral supplementation of crocin on
age-related oxidative stress, cholinergic, and mitochondrial
dysfunction in rat cerebral cortex. Life sciences.
263(118545)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sack MN, Rader DJ and Cannon RO III:
Oestrogen and inhibition of oxidation of low-density lipoproteins
in postmenopausal women. Lancet. 343:269–270. 1994.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kushi LH, Folsom AR, Prineas RJ, Mink PJ,
Wu Y and Bostick RM: Dietary antioxidant vitamins and death from
coronary heart disease in postmenopausal women. N Engl J Med.
334:1156–1162. 1996.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Khaled S, Makled MN and Nader MA: Tiron
protects against nicotine-induced lung and liver injury through
antioxidant and anti-inflammatory actions in rats in vivo. Life
Sci. 260(118426)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Luo X, Gong X, Su L, Lin H, Yang Z, Yan X
and Gao J: Activatable mitochondria-targeting organoarsenic
prodrugs for bioenergetic cancer therapy. Angew Chem Int Ed Engl.
60:1403–1410. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Houtkooper RH, Pirinen E and Auwerx J:
Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol
Cell Biol. 13:225–238. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang W, Nagasawa K, Münch C, Xu Y,
Satterstrom K, Jeong S, Hayes SD, Jedrychowski MP, Vyas FS,
Zaganjor E, et al: Mitochondrial sirtuin network reveals dynamic
SIRT3-dependent deacetylation in response to membrane
depolarization. Cell. 167:985–1000.e21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hirschey MD, Shimazu T, Goetzman E, Jing
E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S,
Ilkayeva OR, et al: SIRT3 regulates mitochondrial fatty-acid
oxidation by reversible enzyme deacetylation. Nature. 464:121–125.
2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang DY, Gao T, Xu RJ, Sun L, Zhang CF,
Bai L, Chen W, Liu KY, Zhou Y, Jiao X, et al: SIRT3 transfection of
aged human bone marrow-derived mesenchymal stem cells improves cell
therapy-mediated myocardial repair. Rejuvenation Res. 23:453–464.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Perović A, Sobočanec S, Dabelić S, Balog T
and Dumić J: Effect of scuba diving on the oxidant/antioxidant
status, SIRT1 and SIRT3 expression in recreational divers after a
winter nondive period. Free Radic Res. 52:188–197. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jehan S, Zhong C, Li G, Zulqarnain
Bakhtiar S, Li D and Sui G: Thymoquinone selectively induces
hepatocellular carcinoma cell apoptosis in synergism with clinical
therapeutics and dependence of p53 status. Front Pharmacol.
11(555283)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Schumacker PT: A tumor suppressor
SIRTainty. Cancer Cell. 17:5–6. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Govindarajulu M, Ramesh S, Neel L,
Fabbrini M, Buabeid M, Fujihashi A, Dwyer D, Lynd T, Shah K,
Mohanakumar KP, et al: Nutraceutical based SIRT3 activators as
therapeutic targets in Alzheimer's disease. Neurochem Int.
144(104958)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shukla S, Sharma A, Pandey VK, Raisuddin S
and Kakkar P: Concurrent acetylation of FoxO1/3a and p53 due to
sirtuins inhibition elicit Bim/PUMA mediated mitochondrial
dysfunction and apoptosis in berberine-treated HepG2 cells. Toxicol
Appl Pharmacol. 291:70–83. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ghribi O, Herman MM, Spaulding NK and
Savory J: Lithium inhibits aluminum-induced apoptosis in rabbit
hippocampus, by preventing cytochrome c translocation, Bcl-2
decrease, Bax elevation and caspase-3 activation. J Neurochem.
82:137–145. 2002.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhou W, Liu Y, Shen J, Yu B, Bai J, Lin J,
Guo X, Sun H, Chen Z, Yang H, et al: Melatonin increases bone mass
around the prostheses of OVX rats by ameliorating mitochondrial
oxidative stress via the SIRT3/SOD2 signaling pathway. Oxid Med
Cell Longev. 2019(4019619)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chiu HC, Chiu CY, Yang RS, Chan DC, Liu SH
and Chiang CK: Preventing muscle wasting by osteoporosis drug
alendronate in vitro and in myopathy models via sirtuin-3
down-regulation. J Cachexia Sarcopenia Muscle. 9:585–602.
2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Schurman L, McCarthy AD, Sedlinsky C,
Gangoiti MV, Arnol V, Bruzzone L and Cortizo AM: Metformin reverts
deleterious effects of advanced glycation end-products (AGEs) on
osteoblastic cells. Exp Clin Endocrinol Diabetes. 116:333–340.
2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang L, Shi W, Gao X, SreeHarsha N and
Zhang D: Cardioprotective role of metformin against sodium
arsenite-induced oxidative stress, inflammation, and apoptosis.
IUBMB Life. 72:749–757. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287.
2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Styrkarsdottir U, Cazier JB, Kong A,
Rolfsson O, Larsen H, Bjarnadottir E, Johannsdottir VD,
Sigurdardottir MS, Bagger Y, Christiansen C, et al: Linkage of
osteoporosis to chromosome 20p12 and association to BMP2. PLoS
Biol. 1(E69)2003.PubMed/NCBI View Article : Google Scholar
|