Introduction
Lung cancer has the highest mortality rate among all
types of cancer worldwide (1); ~85%
of lung cancer cases are non-small cell lung cancer (NSCLC), which
has a 5-year survival rate of <20% (2). The majority of patients with NSCLC are
diagnosed at advanced stages owing to a lack of effective screening
and delayed symptom presentation. Surgery is also not an effective
therapeutic strategy (3). With an
increased understanding of molecular genetics, directed molecular
targeted therapy has proven to be helpful for improving clinical
outcomes (4). Despite the
identification of several biomarkers for patient diagnosis and
prognosis, elucidating additional biomarkers to further improve
therapeutic effects is important.
Gigantol is a bibenzyl compound extracted from the
Thai orchid, Dendrobium draconis (5). In China, gigantol is an active
ingredient commonly extracted from Dendrobium officinale,
which is a valuable Chinese herbal medicine (6,7).
Plants of the Dendrobium genus have been reported to display
immunomodulatory, neuroprotective, antioxidant and antitumor
effects (8). Similarly, it has been
reported that gigantol has anti-inflammation (9), antioxidant (10), antiplatelet aggregation (11) and anticancer effects (5). Furthermore, gigantol has been
indicated to inhibit aldose reductase, which may result in diabetic
cataract prevention (12,13). The anticancer activity of gigantol
has been previously reported in several types of human cancer,
including breast (14), liver
(15) and lung (16) cancer. For example, in patients with
NSCLC, a previous study revealed that gigantol inhibits metastasis
(17). However, the effects of
gigantol on cell proliferation and apoptosis, as well as its
underlying molecular mechanism of action are not completely
understood.
DEK proto-oncogene (DEK) was initially discovered in
a subtype of acute myelogenous leukemia, in which the DEK
nucleoporin 214 genes were fused (18). The DEK architectural protein is
present in the chromatin of multicellular organisms (19). DEK has two functional DNA binding
domains: One is equivalent to the SAP/scaffold attachment factor
box and the other overlaps with a di- or multimerization domain
(20). To date, it has been
revealed that DEK is involved in the tumorigenesis of human cancer
by regulating DNA repair, cell differentiation, senescence and
apoptosis (21). A previous study
suggested that DEK serves a crucial role in tumor progression and
also functions as a prognostic biomarker of NSCLC (22). However, the relationship between
gigantol and DEK is not completely understood.
The biological function of gigantol in NSCLC and its
underlying molecular mechanism were assessed in the present study.
The results indicated that gigantol suppressed proliferation and
enhanced apoptosis compared with the control group. Additionally,
DEK gene expression was significantly decreased following gigantol
treatment compared with the control group, and DEK knockdown
enhanced gigantol-induced effects on proliferation and apoptosis.
Therefore, gigantol may serve as a useful therapeutic agent and DEK
may serve as a target of gigantol in patients with NSCLC.
Materials and methods
Patients and tissues
A total of 30 paired fresh tumor tissues and
adjacent non-tumor tissues 5 mm away from tumor tissues were
collected from patients with NSCLC who underwent surgery at Baoji
Traditional Chinese Medicine Hospital (Baoji, China) between
January and December 2018. The patients included 18 males and 12
females, with a mean age of 56.77 years (range, 44-70 years).
Tissue samples were frozen in liquid nitrogen after resection and
subsequently stored at -80˚C until further use. Written informed
consent was obtained from each participant. The present study was
approved by the Ethics Committee of Baoji Traditional Chinese
Medicine Hospital (approval reference no. 201801001).
Cell culture
NSCLC cell lines (H1299, HCC827, H1650 and A549) and
the human normal bronchial epithelial cell line (BEAS-2B) were
purchased from the American Type Culture Collection. Cells were
maintained in RPMI-1640 medium (Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (HyClone; Cytiva), 100 U/ml penicillin
and 100 µg/ml streptomycin (Beyotime Institute of Biotechnology) at
37˚C with 5% CO2.
Cell viability
Gigantol (4-[2-(4-Hydroxy-3-methoxyphenyl)
ethyl]-2,6-dimethoxy-phenol; purity ≥98%) was purchased from Chroma
Biotechnology Co., Ltd. An MTT assay (Beyotime Institute of
Biotechnology) was subsequently performed to detect cell viability.
Briefly, cells (2x103 cell/well) were seeded into a
96-well plate and exposed to various concentrations of gigantol (0,
25, 50 and 100 µM) for 24 h at 37˚C. Subsequently, 10 µl MTT was
added to each well and incubated at 37˚C for 4 h. Following
incubation, 100 µl formazan solving liquid was added to each well
until samples were completely dissolved. The optical density of
each well was measured at a wavelength of 570 nM using a microplate
reader (Bio-Rad Laboratories, Inc.).
Cell transfection
A549 cells were seeded (5x104 cells/well)
into 96-well plates and cultured in complete medium containing a
series of gigantol concentrations (0, 25, 50 and 100 µM) at 37˚C
for 24 h. Following treatment, cells were transfected with 50 nM
DEK small interfering (si)RNA-1 (5'-UGUCCUCAUUAAAGAAGAA-3'), 50 nM
DEK siRNA-2 (5'-CCUUCUGGCAAACCAUUGCCGAAAU-3'), 50 nM non-targeting
siRNA negative control (siRNA-NC; 5'-UUCUCCGAACGUGUCACGUTT-3'), 10
µg pcDNA3.1 or 10 µg pcDNA3.1-DEK (all from Invitrogen; Thermo
Fisher Scientific, Inc.) using Lipofectamine® 2000
(Invitrogen, Thermo Fisher Scientific, Inc.). At 48 h
post-transfection, cells were used for subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissue specimens and
A549 cells using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). After assessing RNA integrity, total RNA
was reverse transcribed into cDNA using the FastKing RT kit
(Tiangen Biotech Co., Ltd.). The RT conditions were as follows:
42˚C for 15 min and 95˚C for 3 min before chilling on ice.
Subsequently, qPCR was performed using FastFire qPCR PreMix (SYBR
Green; Tiangen Biotech Co., Ltd.) and the ABI PRISM 7500 Real-Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The following thermocycling conditions were used for qPCR: Initial
denaturation at 95˚C for 1 min; followed by 40 cycles of
denaturation at 95˚C for 5 sec and annealing/extension at 60˚C for
10 sec. The following primers were used for qPCR: DEK forward (F),
5'-CCGAGAAAGAACCCGAAATGC-3' and reverse (R),
5'-GTGCCTGGCCTGTTGTAAAGC-3'; Ki-67 F, 5'-ACTGCAGCAGATGGAACTAGG-3'
and R, 5'-AGAACAGTAGCGTGATGTTTGG-3'; Bcl-2 F,
5'-TCATGAAATATGCATCTCACTGG-3' and R, 5'-AAATGCAATCCACTGTCACTCTT-3';
Bax F, 5'-AGGGTTTCATCCAGGATCGAG-3' and R,
5'-CACTCGCTCAGCTTCTTGGT-3'; and GAPDH F, 5'-ATGTCGTGGAGTCTACTGGC-3'
and R, 5'-TGACCTTGCCCACAGCCTTG-3'. mRNA expression levels were
quantified using the 2-ΔΔCq method (23) and normalized to the internal
reference gene GAPDH.
Western blotting
Total protein was extracted from tissue samples and
transfected cells using pre-cooled RIPA lysis buffer (Beyotime
Institute of Biotechnology). The BCA Protein Assay kit (Beyotime
Institute of Biotechnology) was used to determine the concentration
of total protein. Proteins (30 µg) were separated via 10% SDS-PAGE
and transferred onto PVDF membranes (Thermo Fisher Scientific,
Inc.). After blocking with 5% skim milk at room temperature for 1
h, the membranes were incubated at 4˚C overnight with the following
primary antibodies (all purchased from Abcam): Anti-DEK (cat. no.
ab166624; 1:1,000), anti-Ki-67 (cat. no. ab92742; 1:5,000),
anti-Bcl-2 (cat. no. ab182858; 1:2,000), anti-Bax (cat. no.
ab53154; 1:1,000) anti-Wnt10b (cat. no. ab70816; 1:500),
anti-β-catenin (cat. no. ab6302; 1:4,000) and anti-GAPDH (cat. no.
ab9485; 1:2,500). Following primary incubation, the membranes were
incubated with a horseradish peroxidase-conjugated goat anti-rabbit
IgG secondary antibody (cat. no. ab205718; 1:2,000; Abcam) for 1 h
at room temperature. Protein bands were visualized using the
EasyBlot ECL kit (Sangon Biotech, Co., Ltd.). Protein expression
levels were semi-quantified using Image J software (version 1.48U;
National Institutes of Health) with GAPDH as the loading
control.
Cell proliferation assay
Cell proliferation was assessed using the Cell
Counting Kit-8 (CCK-8) assay (MedChemExpress) following the
manufacturer's protocol. Cells were seeded (1x104
cells/well) into 96-well plates and incubated at 37˚C with 5%
CO2. for 0, 12, 24 and 48 h. Subsequently, CCK-8 reagent
(10 µl) was added to each well at 37˚C with 5% CO2 for 4
h. Absorbance was measured at a wavelength of 450 nM using a
microplate reader (Bio-Rad Laboratories, Inc.).
Apoptosis assay
Apoptosis was assessed using the Annexin V Apoptosis
Detection Kit FITC (Sangon Biotech Co., Ltd.). Transfected cells
were resuspended in binding buffer at a density of 2x105
cells/ml. Following incubation with 5 µl Annexin V-FITC and 10 µl
PI for 15 min at room temperature in the dark, flow cytometry was
performed. Early and late apoptotic cells were analyzed using the
CytoFLEX flow cytometer (Beckman Coulter, Inc.) carrying CellQuest
Pro software (version 5.1; BD Biosciences).
Statistical analysis
Each experiment was repeated at least three times.
Statistical analyses were performed using GraphPad Prism software
(version 6.0; GraphPad Software, Inc.). Data are presented as the
mean ± SEM. Comparisons between two unrelated groups were analyzed
using unpaired Student's t-test. However, comparisons between tumor
tissues and adjacent normal tissues used paired Student's t-test.
Comparisons among multiple groups were analyzed using one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
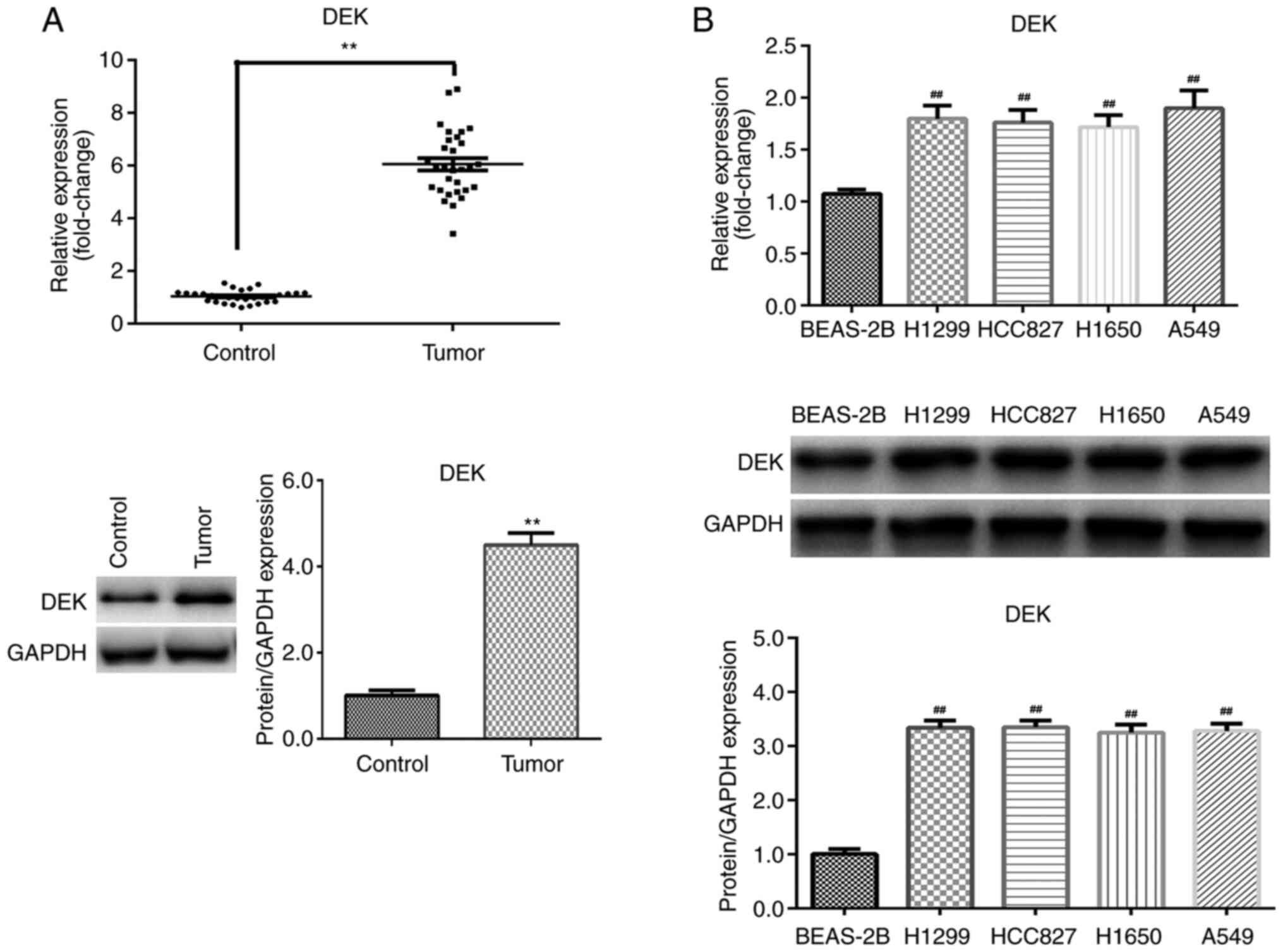
DEK expression levels are increased in
NSCLC
To determine the function of DEK in NSCLC, mRNA and
protein expression levels in tissues and cells were first assessed
by RT-qPCR and western blotting, respectively. The results
indicated that DEK expression was significantly upregulated in
tumor tissues compared with the paired adjacent non-tumor tissue
control group (P<0.01; Fig. 1A).
Additionally, compared with BEAS-2B cells, DEK expression levels
were significantly increased in NSCLC cell lines (P<0.01;
Fig. 1B), most notably in A549
cells. Therefore, A549 cells were used in subsequent
experiments.
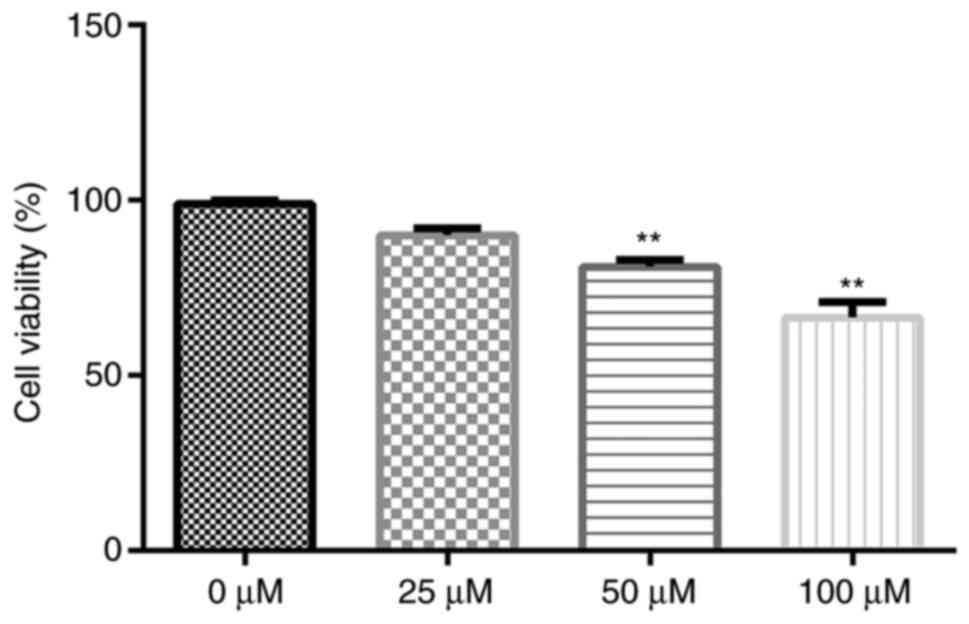
Cytotoxicity of gigantol in A549
cells
To determine the cytotoxicity of gigantol, A549
cells were exposed to a series of gigantol concentrations (0, 25,
50 and 100 µM). Subsequently, cell viability was analyzed by
performing an MTT assay. The results indicated that gigantol
decreased cell viability in a dose-dependent manner (Fig. 2). Compared with the 0 µM group, only
50 and 100 µM gigantol significantly decreased cell viability
(P<0.01). However, there was no significant difference between
the 0 and 25 µM groups. Therefore, the non-toxic concentration of
25 µM was used for further experiments.
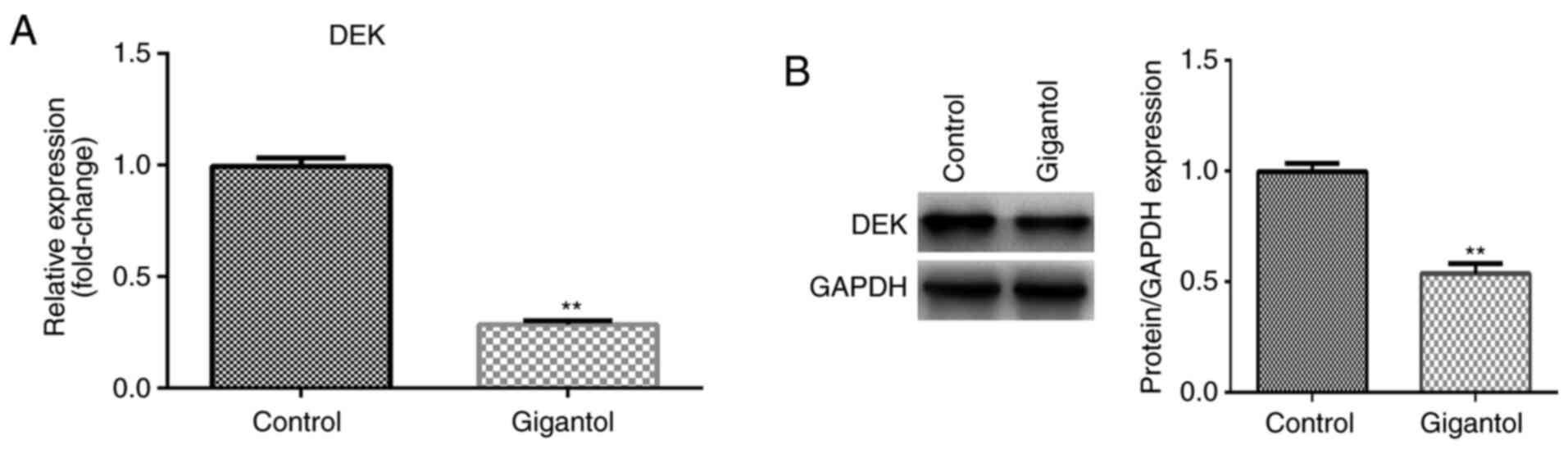
Gigantol regulates DEK expression in
A549 cells
To investigate whether gigantol influenced DEK, the
expression of DEK was assessed in gigantol-treated cells. The
results suggested that DEK mRNA and protein expression levels were
significantly decreased by gigantol treatment compared with the
untreated control group (P<0.01; Fig. 3A and B, respectively).
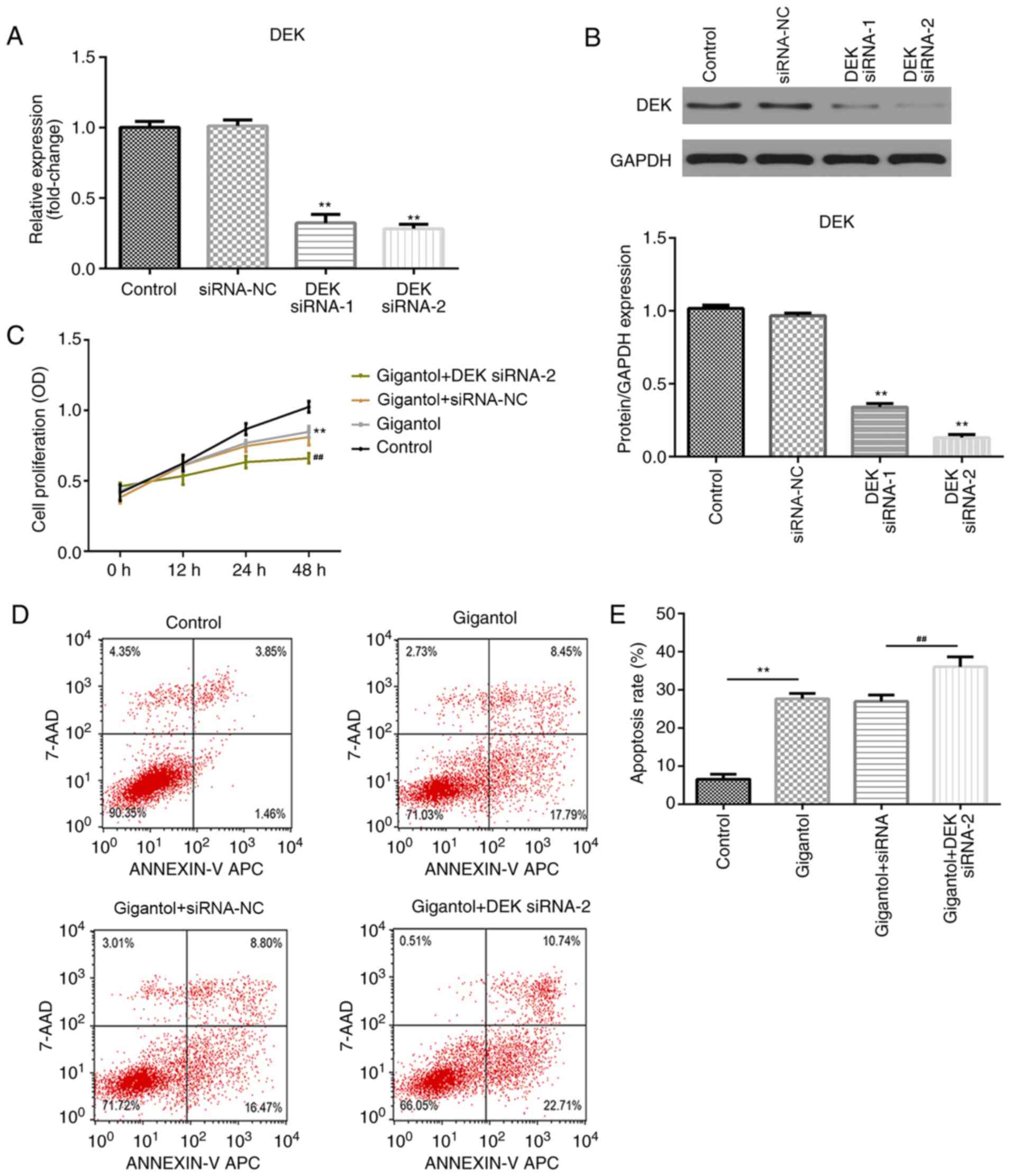
DEK knockdown enhances
gigantol-mediated inhibition of proliferation and increases
gigantol-induced apoptosis
To evaluate the role of DEK in gigantol-treated
cells, A549 cells were transfected with DEK siRNA. The transfection
efficiency of two DEK siRNAs were determined, which demonstrated
that DEK mRNA and protein expression levels were significantly
downregulated by DEK knockdown compared with the siRNA-NC group
(both P<0.01; Fig. 4A and
B), particularly in the DEK siRNA-2
group. Cell proliferation and apoptosis were subsequently analyzed
by performing CCK-8 and flow cytometry assays, respectively.
Compared with the control group, cell proliferation was
significantly inhibited by gigantol (P<0.01) and further
suppressed by DEK knockdown (P<0.01; Fig. 4C). Moreover, apoptosis was
significantly increased in the gigantol group compared with the
control group (P<0.01). Gigantol-induced apoptosis was not
affected by siRNA-NC transfection, but was significantly increased
following DEK siRNA-2 transfection (P<0.01; Fig. 4D and E).
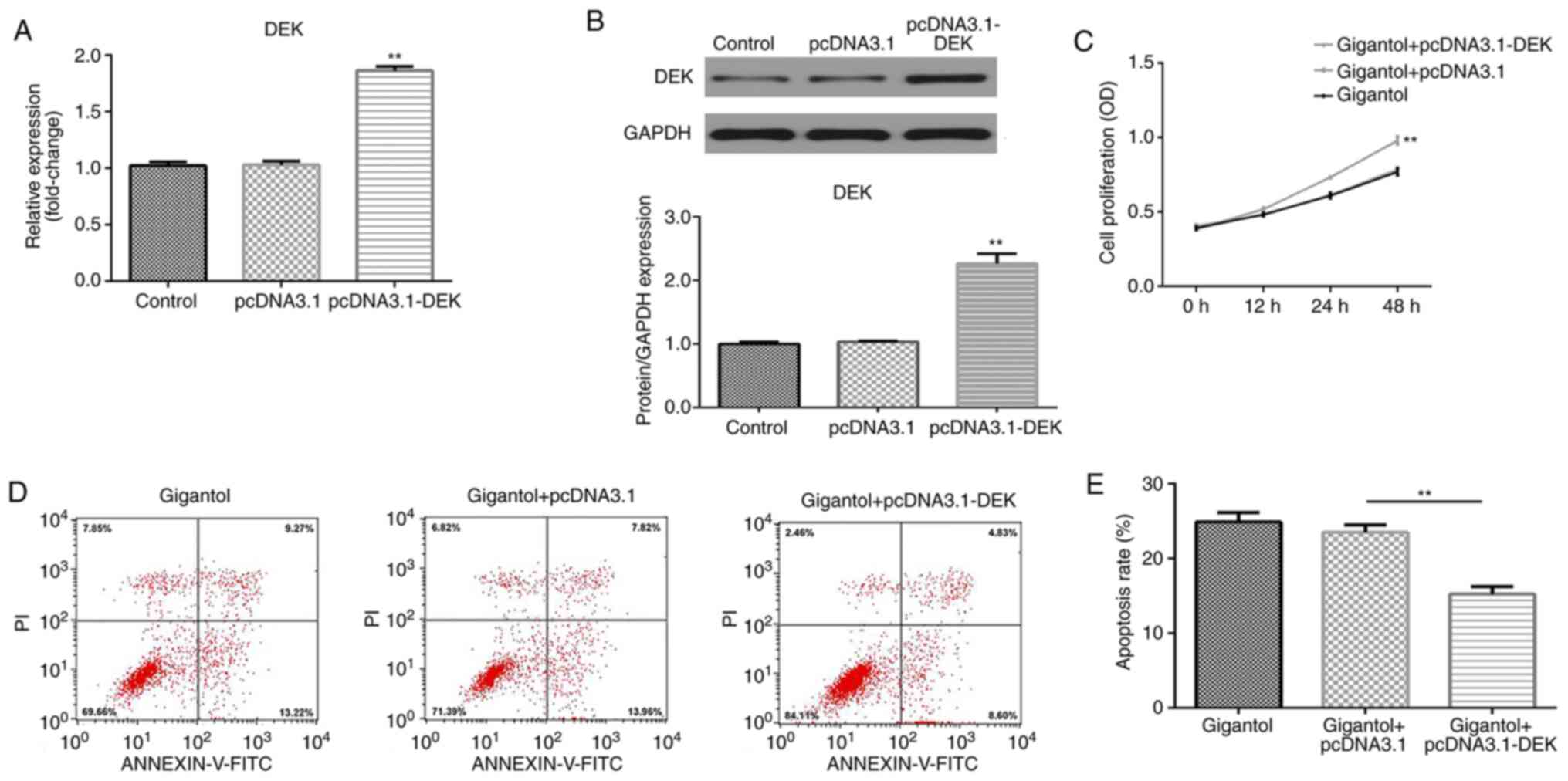
DEK overexpression promotes cell
proliferation and suppresses apoptosis in gigantol-treated
cells
A549 cells were transfected with pcDNA3.1-DEK
overexpression vector and transfection efficiency was measured by
RT-qPCR and western blotting. The results indicated that DEK mRNA
and protein expression levels were significantly upregulated by
pcDNA3.1-DEK compared with pcDNA3.1 empty vector transfection
(P<0.01; Fig. 5A and B). Moreover, compared with the gigantol +
pcDNA3.1 group, DEK overexpression significantly enhanced cell
proliferation in gigantol-treated cells (P<0.01; Fig. 5C). By contrast, gigantol-induced
apoptosis was decreased by DEK overexpression, compared with
pcDNA3.1 (P<0.01; Fig. 5D and
E).
Gigantol suppresses Ki-67 and Bcl-2
expression, increases Bax expression and inactivates the
Wnt/β-catenin signaling pathway by regulating DEK
The expression levels of Ki-67 (a proliferation
marker) and apoptosis-related factors Bcl-2 and Bax were assessed.
Compared with the control group, gigantol significantly reduced
Ki-67 and Bcl-2 mRNA expression levels, but increased Bax mRNA
expression levels (all P<0.01; Fig.
6A-C, respectively). DEK knockdown enhanced the effects of
gigantol on the expression levels of Ki-67, Bcl-2 and Bax
(P<0.01). Similar effects were observed for Ki-67, Bcl-2 and Bax
protein expression levels (P<0.01, Fig. 6D and E). In addition, the expression levels of
Wnt10b and β-catenin were significantly decreased following
gigantol treatment compared with the control group (P<0.01), and
DEK knockdown increased gigantol-mediated downregulation
(P<0.01; Fig. 6D and E).
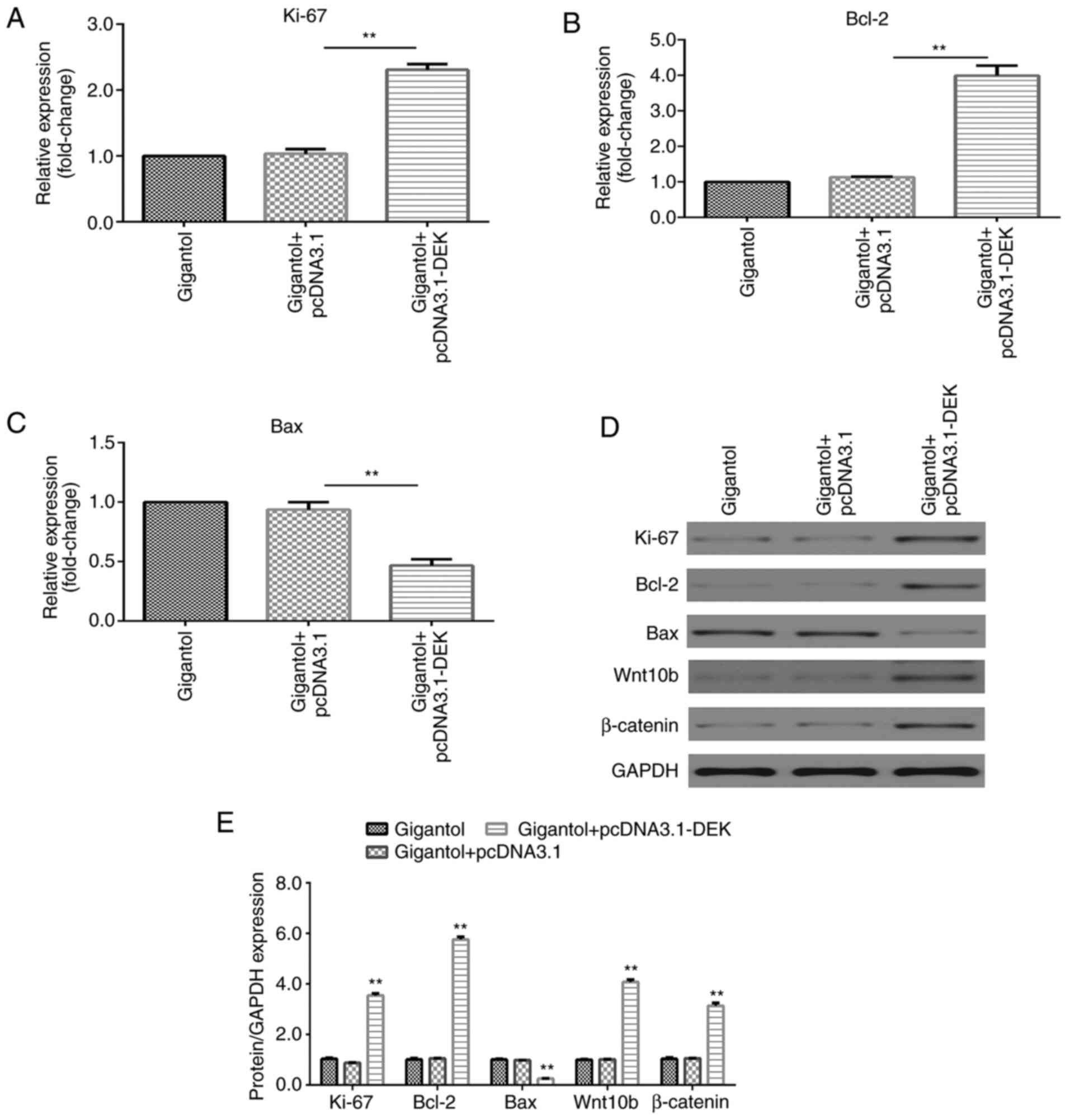
DEK overexpression promotes
gigantol-mediated effects on increasing Ki-67 and Bcl-2 expression
levels, decreasing Bax expression levels and activating the
Wnt/β-catenin signaling pathway
RT-qPCR and western blotting results indicated that
the mRNA and protein expression levels of Ki-67 and Bcl-2 were
significantly increased (P<0.01), but Bax expression levels were
significantly decreased (P<0.01) by pcDNA3.1-DEK in
gigantol-treated A549 cells (Fig.
7A-E). Additionally, gigantol-induced protein expression levels
of Wnt10b and β-catenin were significantly increased by DEK
overexpression (P<0.01; Fig. 7D
and E).
Discussion
Results from the present study demonstrated that DEK
expression levels were increased in NSCLC tissues and cells
compared with adjacent non-tumor tissues and BEAS-2B cells,
respectively. Gigantol treatment inhibited NSCLC cell proliferation
and induced apoptosis compared with the control. Furthermore, DEK
knockdown enhanced gigantol-mediated inhibition of cell
proliferation and promotion of apoptosis. Therefore, gigantol may
serve as a potential therapeutic agent through regulating DEK
expression for the trepary of NSCLC.
DEK has been identified as an oncogene that
regulates cellular processes in many types of cancer. For example,
decreased DEK expression inhibited cell proliferation, induced
apoptosis and blocked the cell cycle at the
G0/G1 phase in astrocytic tumors (24). Furthermore, DEK overexpression
induces the epithelial-mesenchymal transition (EMT) process in
osteosarcoma cells (25).
Additionally, miR-1292-5p inhibits gastric carcinoma cell
proliferation, migration and invasion through suppressing DEK,
suggesting DEK is an oncogene (26). DEK is also a prognosis factor in
gastric cancer (27), colorectal
cancer (28) and NSCLC (22). Moreover, in lung cancer, DEK was
highly expressed in tumor tissues, which promoted cell
proliferation and invasion (29).
Results from the present study demonstrated that DEK expression
levels were increased in NSCLC tissues and cells compared with
adjacent non-tumor tissues and BEAS-2B cells, respectively, which
was consistent with previous studies (22,29).
Gigantol exhibits anticancer activities that
regulate cellular processes in various types of human cancer
(14-17).
A number of studies have investigated the function of gigantol in
lung cancer. For example, gigantol suppressed stem-like malignancy
phenotypes of lung cancer cells, reducing anchorage-independent
growth and the ability to form tumor spheroids (30). Additionally, gigantol inhibited EMT
processes in H460 NSCLC cells (16,31).
Furthermore, gigantol suppressed NSCLC cell migration and invasion
(17). In the present study, the
effects of gigantol on NSCLC cell proliferation and apoptosis, and
its underlying molecular mechanisms were assessed. The results
showed that gigantol attenuated cell viability and decreased DEK
expression levels in A549 cells compared with the control group.
Additionally, DEK knockdown by siRNA enhanced gigantol-induced
inhibition of proliferation, as well as gigantol-induced apoptosis.
The results suggested that gigantol may inhibit proliferation and
induced apoptosis by suppressing DEK expression in NSCLC.
Ki-67 is present in proliferating cells and is
therefore used to determine the proportion of proliferating cells
in tumors (32). Bax is a
proapoptotic factor of the Bcl-2 family, of which Bcl-2 is also an
apoptosis-related factor (33). A
previous study demonstrated that DEK knockdown downregulated Bcl-2
expression and upregulated Bax expression (34). High DEK expression was also
associated with increased Ki-67 expression (34). The Wnt/β-catenin signaling pathway
serves a pivotal role in NSCLC tumorigenesis, prognosis and
resistance to therapy (35). A
previous study reported that DEK promoted proliferation and
β-catenin activity by regulating Wnt10b in breast cancer (36). In the present study, compared with
the control group, gigantol reduced Ki-67, Bcl-2, Wnt10b and
β-catenin expression levels, and increased Bax expression levels.
Additionally, DEK siRNA-mediated knockdown enhanced these
gigantol-induced effects. Collectively, the results indicated that
gigantol suppressed decreased Ki-67 and Bcl-2 expression levels,
promoted increased Bax expression levels and inactivated the
Wnt/β-catenin signaling pathway possibly by regulating DEK.
The present study had a number of limitations.
Firstly, only one NSCLC cell line was selected to explore the role
of DEK. Secondly, the in vitro results were not verified
in vivo. Therefore, further investigation is required.
In conclusion, the present study indicated that DEK
was upregulated in NSCLC tissues and cells. Gigantol inhibited cell
viability and decreased DEK expression levels. Furthermore,
gigantol suppressed the proliferation and enhanced the apoptosis of
NSCLC cells by decreasing Ki-67 and Bcl-2 expression, increasing
Bax expression and inactivating the Wnt/β-catenin signaling
pathway, and this may be through DEK regulation. The results
suggested that DEK knockdown may be associated with the anticancer
activity of gigantol in NSCLC in vitro. Therefore, DEK may
serve as a novel target to enhance the therapeutic effects of
gigantol in patients with NSCLC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and BR designed this study. YC, YH, HX and KC
performed the experiments and the statistical analysis. YH and HX
confirm the authenticatity of all the raw data. YC wrote and BR
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Baoji Traditional Chinese Medicine Hospital (Boaji,
China; approval no. 201801001). Written informed consent was
obtained from each participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Carbone DP, Gandara DR, Antonia SJ,
Zielinski C and Paz-Ares L: Non-small-cell lung cancer: Role of the
immune system and potential for immunotherapy. J Thorac Oncol.
10:974–984. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gu H, Chen J, Song Y and Shao H: Gastric
adenocarcinoma predictive long intergenic non-coding RNA promotes
tumor occurrence and progression in non-small cell lung cancer via
regulation of the miR-661/eEF2K signaling pathway. Cell Physiol
Biochem. 51:2136–2147. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gridelli C, Rossi A, Carbone DP, Guarize
J, Karachaliou N, Mok T, Petrella F, Spaggiari L and Rosell R:
Non-small-cell lung cancer. Nat Rev Dis Primers.
1(15009)2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kumarakulasinghe NB, van Zanwijk N and Soo
RA: Molecular targeted therapy in the treatment of advanced stage
non-small cell lung cancer (NSCLC). Respirology. 20:370–378.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lam Y, Ng TB, Yao RM, Shi J, Xu K, Sze SC
and Zhang KY: Evaluation of chemical constituents and important
mechanism of pharmacological biology in Dendrobium plants. Evid
Based Compement Alternat Med. 2015(841752)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen X, Wang F, Wang Y, Li X, Wang A, Wang
C and Guo S: Discrimination of the rare medicinal plant Dendrobium
officinale based on naringenin, bibenzyl, and polysaccharides. Sci
China Life Sci. 55:1092–1099. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zheng S, Zhu Y, Jiao C, Shi M, Wei L, Zhou
Y, Jin Q and Cai Y: Extraction and analysis of gigantol from
Dendrobium officinale with response surface methodology. Molecules.
23(818)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ng TB, Liu J, Wong JH, Ye X, Wing Sze SC,
Tong Y and Zhang KY: Review of research on Dendrobium, a prized
folk medicine. Appl Microbiol Biotechnol. 93:1795–1803.
2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Won JH, Kim JY, Yun KJ, Lee JH, Back NI,
Chung HG, Chung SA, Jeong TS, Choi MS and Lee KT: Gigantol isolated
from the whole plants of Cymbidium goeringii inhibits the
LPS-induced iNOS and COX-2 expression via NF-kappaB inactivation in
RAW 264.7 macrophages cells. Planta Med. 72:1181–1187.
2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Simmler C, Antheaume C and Lobstein A:
Antioxidant biomarkers from Vanda coerulea stems reduce irradiated
HaCaT PGE-2 production as a result of COX-2 inhibition. PLoS One.
5(e13713)2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fan C, Wang W, Wang Y, Qin G and Zhao W:
Chemical constituents from Dendrobium densiflorum. Phytochemistry.
57:1255–1258. 2001.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu J, Li X, Wan W, Yang Q, Ma W, Chen D,
Hu J, Chen CO and Wei X: Gigantol from Dendrobium chrysotoxum
Lindl. binds and inhibits aldose reductase gene to exert its
anti-cataract activity: An in vitro mechanistic study. J
Ethnopharmacol. 198:255–261. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu J, Li X, Fang H, Yi Y, Chen D, Long Y,
Gao X, Wei X and Chen CY: Investigation of synergistic mechanism
and identification of interaction site of aldose reductase with the
combination of gigantol and syringic acid for prevention of
diabetic cataract. BMC Complement Altern Med.
16(286)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yu S, Wang Z, Su Z, Song J, Zhou L, Sun Q,
Liu S, Li S, Li Y, Wang M, et al: Gigantol inhibits Wnt/β-catenin
signaling and exhibits anticancer activity in breast cancer cells.
BMC Complement Altern Med. 18(59)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen H, Huang Y, Huang J, Lin L and Wei G:
Gigantol attenuates the proliferation of human liver cancer HepG2
cells through the PI3K/Akt/NF-κB signaling pathway. Oncol Rep.
37:865–870. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Unahabhokha T, Chanvorachote P and
Pongrakhananon V: The attenuation of epithelial to mesenchymal
transition and induction of anoikis by gigantol in human lung
cancer H460 cells. Tumour Biol. 37:8633–8641. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Charoenrungruang S, Chanvorachote P,
Sritularak B and Pongrakhananon V: Gigantol, a bibenzyl from
Dendrobium draconis, inhibits the migratory behavior of non-small
cell lung cancer cells. J Nat Prod. 77:1359–1366. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
von Lindern M, Breems D, van Baal S,
Adriaansen H and Grosveld G: Characterization of the translocation
breakpoint sequences of two DEK-CAN fusion genes present in t(6;9)
acute myeloid leukemia and a SET-CAN fusion gene found in a case of
acute undifferentiated leukemia. Genes Chromosomes Cancer.
5:227–234. 1992.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Waldmann T, Scholten I, Kappes F, Hu HG
and Knippers R: The DEK protein-an abundant and ubiquitous
constituent of mammalian chromatin. Gene. 343:1–9. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hu HG, Scholten I, Gruss C and Knippers R:
The distribution of the DEK protein in mammalian chromatin. Biochem
Biophys Res Commun. 358:1008–1014. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Riveiro-Falkenbach E and Soengas MS:
Control of tumorigenesis and chemoresistance by the DEK oncogene.
Clin Cancer Res. 16:2932–2938. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu X, Qi D, Qi J, Mao Z, Li X, Zhang J,
Li J and Gao W: Significance of DEK overexpression for the
prognostic evaluation of non-small cell lung carcinoma. Oncol Rep.
35:155–162. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta DeltaC(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Feng T, Liu Y, Li C, Li Z and Cai H: DEK
proto-oncogene is highly expressed in astrocytic tumors and
regulates glioblastoma cell proliferation and apoptosis. Tumour
Biol. 39(1010428317716248)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wise-Draper TM, Mintz-Cole RA, Morris TA,
Simpson DS, Wikenheiser-Brokamp KA, Currier MA, Cripe TP, Grosveld
GC and Wells SI: Overexpression of the cellular DEK protein
promotes epithelial transformation in vitro and in vivo. Cancer
Res. 69:1792–1799. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hui W, Ma X, Zan Y, Song L, Zhang S and
Dong L: MicroRNA-1292-5p inhibits cell growth, migration and
invasion of gastric carcinoma by targeting DEK. Am J Cancer Res.
8:1228–1238. 2018.PubMed/NCBI
|
|
27
|
Piao J, Shang Y, Liu S, Piao Y, Cui X, Li
Y and Lin Z: High expression of DEK predicts poor prognosis of
gastric adenocarcinoma. Diagn Pathol. 9(67)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin L, Piao J, Gao W, Piao Y, Jin G, Ma Y,
Li J and Lin Z: DEK over expression as an independent biomarker for
poor prognosis in colorectal cancer. BMC Cancer.
13(366)2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhou QC, Deng XF, Yang J, Jiang H, Qiao
MX, Liu HH, Qian Z, Hou LL and Hu HG: Oncogene DEK is highly
expressed in lung cancerous tissues and positively regulates cell
proliferation as well as invasion. Oncol Lett. 15:8573–8581.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bhummaphan N and Chanvorachote P: Gigantol
suppresses cancer stem cell-like phenotypes in lung cancer cells.
Evid Based Complement Alternat Med. 2015(836564)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Unahabhokha T, Chanvorachote P, Sritularak
B, Kitsongsermthon J and Pongrakhananon V: Gigantol inhibits
epithelial to mesenchymal process in human lung cancer cells. Evid
Based Complement Alternat Med. 2016(4561674)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gerdes J, Schwab U, Lemke H and Stein H:
Production of a mouse monoclonal antibody reactive with a human
nuclear antigen associated with cell proliferation. Int J Cancer.
31:13–20. 1983.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Harris MH and Thompson CB: The role of the
Bcl-2 family in the regulation of outer mitochondrial membrane
permeability. Cell Death Differ. 7:1182–1191. 2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lin L, Piao J, Ma Y, Jin T, Quan C, Kong
J, Li Y and Lin Z: Mechanisms underlying cancer growth and
apoptosis by DEK overexpression in colorectal cancer. PLoS One.
9(e111260)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst.
106(djt356)2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Privette Vinnedge LM, Benight NM, Wagh PK,
Pease NA, Nashu MA, Serrano-Lopez J, Adams AK, Cancelas JA, Waltz
SE and Wells SI: The DEK oncogene promotes cellular proliferation
through paracrine Wnt signaling in Ron receptor-positive breast
cancers. Oncogene. 34:2325–2336. 2015.PubMed/NCBI View Article : Google Scholar
|