Introduction
Alzheimer's disease (AD) is a chronic
neurodegenerative disease characterized by progressive cognitive
impairment and impaired learning and memory. AD is the most common
form of dementia. As the world's population continues to age and
the number of elderly people increases, the number of patients with
AD is expected to reach 140 million worldwide by 2050(1). AD will become one of the most
important diseases that affect the health of the elderly
population. However, due to the complexity of the pathogenesis of
AD and the side effects and tolerability of drugs for its
treatment, no effective method or reasonable drug treatment has
been developed yet to prevent and treat this disease. Therefore,
developing methods to prevent or delay the onset of AD is
particularly important.
Mild cognitive impairment (MCI) refers to a
condition in which an individual has obvious memory and/or other
cognitive defects but is still able to care for him/herself and has
not yet reached the diagnostic criteria for dementia, adjusted for
age and education level. MCI is currently described as a
transitional state between normal aging and AD (2-5).
The data showed that the probability of progression to AD in normal
elderly individuals was 1-2%, whereas in patients with MCI was as
high as 10-15% (6). Therefore,
early diagnosis of MCI can aid the identification of individuals at
high risk of dementia, and active, effective interventions in
patients with MCI can prevent or delay the development of AD.
The senescence-accelerated mouse prone 8 (SAMP8)
mouse model is considered a suitable, naturally derived animal
model of late-onset AD (7-9).
A previous study on age-related changes in SAMP8 mice using a
grading score, the Morris water maze (MWM) test, Golgi staining,
Nissl staining and immunohistochemical staining was conducted
(10). The results demonstrated
that SAMP8 mice exhibited obvious signs of aging, MCI, and slight
neuropathological changes starting at 5 months of age and showed
signs similar to those associated with AD at 7 months old compared
with senescence-accelerated mouse resistant 1 (SAMR1) mice. The
present study confirmed that SAMP8 mice began to enter the MCI
phase at 5 months of age, which suggested that middle-aged SAMP8
mice could be used as a reasonable animal model to examine the
transition from MCI to AD (10).
Aging is the result of interactions between genetic
and environmental factors. Enriched environment (EE) has been
widely used to study brain structural remodeling and functional
recovery after an acute brain injury, such as cerebral ischemia,
since it was proposed by Hebb in 1947(11). With advances in research, the
effects of EE on neurodegenerative disease rehabilitation have
attracted widespread attention. Animal experiments have
demonstrated that EE can improve the neurological functions and
cognitive performances associated with various neurodegenerative
diseases, including AD and Parkinson's disease (12). In addition, it has been reported
that lifestyle modifications, including diet, exercise, and
cognitive stimulation, may be effective in slowing the progression
of MCI to dementia (13). In the
present study, the effects of EE exposure on the cognitive
functions and neuropathological changes in the hippocampus of SAMP8
mice were evaluated during the MCI phase (5-months old) to
determine whether EE exposure can delay the progression from MCI to
AD.
Materials and methods
Animals and groups
A total of 30 male SAMP8 mice in the MCI phase
(5-months old) were randomly categorized into either the P8 + EE
group (n=15) or the P8 + standard environment (SE) group (n=15).
Mice were housed under EE or SE conditions for 60 days. As a
homologous control, 15 male senescence-accelerated mouse resistant
1 (SAMR1) mice aged 5 months (R1 + SE group) were housed under SE
conditions for 60 days. All mice were provided by the experimental
animal center of The First Hospital Affiliated to Tianjin Medical
University. They received a standard rodent diet and water ad
libitum and were housed at room temperature (18-22˚C) at a
relative humidity of 65-70%. No significant differences in animal
body mass were observed among the groups (P>0.05). All
experimental procedures followed the guiding principles of
mammalian neuroscience research and were approved by the Ethics
Committee for Animal Experiments at the Changzhi Medical
College.
Environments
SE: Mice were housed in standard polyethylene
squirrel cages (30x20x15 cm; five mice per cage), with the top
covered by stainless-steel wire. The cages only included nesting
material.
EE: Mice were housed in large polyethylene squirrel
cages (40x25x30 cm; five mice per cage) with the top covered by
stainless-steel wire. The cages contained a running wheel, a swing,
a slide, a tunnel, and several toys with different colors and
shapes, in addition to nesting material. Cages were cleaned and the
contents were rearranged once each day at a fixed time, and a 12-h
light/dark cycle was implemented (lights on at 7:00 AM).
MWM test
The MWM comprises three components: A circular pool,
a platform, and a video capture system (SMART 3.0 Behavioral Video
Analysis Software, Panlab). The pool was 120 cm in diameter and 60
cm in height and was filled with water (20±1˚C) made opaque by the
addition of carbon ink. The pool was divided into four quadrants.
The platform (10 cm in diameter) was placed in the center of
quadrant IV (the target quadrant) and was hidden 1 cm below the
water surface. After the mice were housed in the maze laboratory
for 2 days, each mouse was placed in the pool near the wall of one
quadrant and was allowed to swim freely for 120 sec to adapt to the
water temperature and gain familiarity with the task; using this
test, mice with existing non-cognitive impairments were identified.
The MWM test was conducted daily from 8:00 am to 12:00 pm, and the
objects around the pool, including the experimenter, remained in
the same location throughout the test.
Positioning navigation test
The mice were gently placed in the water at the
midpoint of each quadrant, by turns, facing the pool wall. The
video capture system tracked mouse movements and recorded the time
required for each mouse to find the platform (escape latency). Each
mouse was allowed to swim until it found the platform, for a
maximum duration of 120 sec, and was allowed to rest on the
platform for 20 sec. If the mice did not find the platform within
120 sec, they were guided to the platform and were maintained on
the platform for 20 sec. The test was conducted once a day for 4
consecutive days.
Space exploration test
On the fifth day, the platform was removed from the
pool to evaluate the memories of the mice regarding the platform
location. Keeping all other factors unchanged, the mice were gently
placed in the water at the midpoint of quadrant III, facing the
pool wall. The video capture system recorded the swimming paths,
time spent in the target quadrant, and number of times the platform
location was crossed during each 120-sec test session.
Transmission electron microscopy
From each group, five mice were randomly selected
and anesthetized with pentobarbital sodium (80 mg/kg) by
intraperitoneal injection, before opening the thorax to expose the
heart. An infusion needle was inserted into the left ventricle
while the right atrial appendage was cut. At room temperature, the
mice were perfused with 0.9% normal saline until clear liquid
flowed out, and fixed with a 4% glutaraldehyde solution at the same
temperature until the liver became white and the body became stiff.
The brain was removed and postfixed for 24 h in the same fixative.
The hippocampal CA1 region was examined and removed under a
stereoscopic microscope (magnification, x240), referring to the
Paxinos mouse brain stereotactic atlas. The CA1 region was fixed
with 1% osmium acid at 4˚C for 1 h, dehydrated in a graded acetone
(50%, 15 min; 60%, 15 min; 70%, 15 min; 80%, 15 min; 90%, 15 min;
and 100%, 15 min; x2) at room temperature, embedded in araldite,
and sliced into semi-thin sections (100-nm thick). Superfluous
tissues surrounding the hippocampal CA1 region were removed under
the microscope based on toluidine blue staining. Consecutive
ultrathin (70-nm thick) sections were prepared and stained with
uranium acetate and lead citrate, for 30 min each at 37˚C.
Ultrastructures were observed under an H-7500 transmission electron
microscope (Hitachi, Ltd.), and three photographs were randomly
taken from clear sections (x25,000 magnification). A square-shaped
area, defined as a counting frame, was randomly drawn onto each
photograph. Synapses were identified according to the synaptic
confirmation standard (14) and
counted according to the stereological ‘forbidden line’ method
(15). The synaptic numerical
density (Nv, number/µm3) and surface density (Sv,
µm2/µm3) were calculated using a
stereological method and an image analysis system (16). Nv was calculated according to the
formula Nv = Na/(L/Ko + T); where, Na is the total number of
synapses per square micrometer, L is the average length of synaptic
junction, T is the thickness of ultrathin section, and Ko is the
correction coefficient to correct the overestimation of synaptic
junction band length caused by Holmes effect (calculated as
follows: Ko = 1+3T/2L). Sv was calculated according to the formula
Sv = 2Ni/LtKo; where, Ni is the number of crossing points between
synaptic junction and test line, Lt is the total length of test
line, and Ko is the correction coefficient to correct the
overestimation of synaptic junction band length caused by Holmes
effect (calculated as aforementioned).
Preparation and staining of tissue
sections
The remaining 10 mice in each group were
anesthetized with pentobarbital sodium (80 mg/kg) by
intraperitoneal injection, before opening the thorax to expose the
heart. An infusion needle was inserted into the left ventricle, and
the right atrial appendage was simultaneously cut. At room
temperature, the mice were perfused with 0.9% normal saline until
the clear liquid flowed out, followed by a fixative containing 4%
paraformaldehyde in 0.1 M phosphate buffer at a pH of 7.4 until the
liver became white and the body became stiff. The brain was
carefully removed and fixed with the same fixative as
aforementioned for 24 h. The tissue block between the optic chiasm
and the superior colliculus was cut and divided into two halves
along the median sagittal plane. The left tissue block was used for
Nissl staining, terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) staining, and immunohistochemical staining,
whereas the right tissue block was used for Golgi staining. The
left tissue block was dehydrated in a graded ethanol series (70%, 2
h; 80%, 2 h; 90%, overnight; 95%, 90 min x2; and 100%, 40 min x2),
cleared in xylene (20 min x2), and embedded in paraffin; it was
then cut serially from the cranial to the caudal side into 5-µm
sections. Five successive sections were taken at intervals of 50 µm
and mounted on glass slides coated with polylysine to prepare five
sets of sections. One set of sections was used for Nissl staining,
another set was used for TUNEL staining, and the remaining three
sets were used for immunohistochemical staining of
anti-synaptophysin (anti-SYN), anti-Aβ, and anti-APP.
Golgi staining
After immersion in mordant solution for 3 days,
followed by immersion in 1.5% AgNO3 solution at room
temperature for 5 days in a dark environment, the right tissue
block was dehydrated in a graded ethanol series (70%, 2 h; 80%, 2
h; 90%, overnight; 95%, 90 min x2; and 100%, 40 min x2), cleared in
xylene (20 min x2), embedded in paraffin, and cut serially into
60-µm sections. For each mouse, 12 randomly chosen neurons fully
impregnated, unobscured by neighboring neurons, from 4 sections
were analyzed, and the average value was used as the result for
this mouse. The lengths (in µm) of the secondary and tertiary
apical dendrites in the hippocampal CA1 region were measured using
digitized images under x1,000 magnification, the number of
dendritic spines was independently counted by two different
investigators, and the results were cross-checked to preclude
systematic analytical errors. In addition, the dendritic spine
density was calculated (number/µm) (17).
Nissl staining
A set of sections was conventionally deparaffinized
in xylene (10 min x2), dehydrated in a graded ethanol series (100%,
3 min x2; 95%, 3 min; 90%, 3 min; 80%, 3 min; and 70%, 3 min),
soaked in 1% cresyl violet at 60˚C for 30 min, and then washed with
distilled water. The sections were differentiated in 95% ethanol
until the Nissl body appeared to be clear, dehydrated rapidly twice
in 100% ethanol, cleared twice in xylene, and coverslipped using a
neutral balsam. The numbers of pyramidal cells per field under x400
magnification using light microscope (Olympus Corporation) in the
hippocampal CA1 region were counted.
TUNEL staining
Another set of sections was conventionally
deparaffinized in xylene (10 min x2), dehydrated in a graded
ethanol series (100%, 3 min x2; 95%, 3 min; 90%, 3 min; 80%, 3 min
and 70%, 3 min), and incubated with protease K for 30 min at room
temperature. After washing with PBS, the sections were incubated
with 50 µl TUNEL reaction mixture in a wet box at 37˚C for 60 min.
After further washing with PBS, the sections were incubated with 50
µl of transformant-peroxidase in a wet box at 37˚C for 30 min.
Finally, the sections were stained using 3,3'-diaminobenzidine,
counterstained using hematoxylin, and coverslipped using a neutral
balsam [in situ cell apoptosis test kit; Roche Diagnostics
(Shanghai) Co., Ltd.]. The number of TUNEL-positive cells and the
number of pyramidal cells were counted separately in each field
under x400 magnification using a light microscope (Olympus
Corporation) in the hippocampal CA1 region. The apoptotic index
(AI) was calculated by dividing the number of TUNEL-positive cells
by the number of pyramidal cells (18).
Immunohistochemical staining
The remaining three sets of sections were
conventionally deparaffinized in xylene (10 min x2), dehydrated in
a graded ethanol series (100%, 3 min x2; 95%, 3 min; 90%, 3 min;
80%, 3 min; 70%, 3 min), and immersed in 3%
H2O2 at room temperature for 20 min to block
the endogenous peroxidase activity. After being subjected to
antigen retrieval with citrate buffer solution (OriGene
Technologies, Inc.) for 15 min in a microwave, the sections were
incubated with 10% goat serum (OriGene Technologies, Inc.) in a wet
box at room temperature for 10 min to block nonspecific binding.
This was followed by overnight incubation with rabbit anti-SYN
polyclonal antibody (1:200; cat. no. ab14692; Abcam), rabbit
anti-Aβ polyclonal antibody (1:200; cat. no. bs-0107R; BIOSS), and
rabbit anti-APP monoclonal antibody (1:200; cat. no. ab32136;
Abcam) at 4˚C. After washing with PBS, the sections were incubated
with biotinylated goat anti-rabbit immunoglobulin G (OriGene
Technologies, Inc.) at room temperature for 1 h. The hippocampal
CA1 region was imaged under a light microscope (Olympus Coporation;
magnification, x400). Image-Pro Plus 6.0 (Media Cybernetics, Inc.)
was used to determine the average optical density (OD) for SYN, Aβ
and APP in the hippocampal CA1 region.
Data analysis
Repeated measures analysis of variance (ANOVA) was
used to analyze the main effects of environment and day (1, 2, 3
and 4 days) on escape latency in the positioning navigation test.
One-way ANOVA was used to analyze the effects of environment on the
time spent in the target quadrant and the number of platform
crossings in the space exploration test. Dendritic spine density,
Nv, Sv, number of neurons, and the OD of SYN, Aβ, and APP were
tested for normality (Kolmogorov-Smirnov test) and equal variance
(Levene's test). If both normal distribution and homogeneity of
variance were found, comparisons were analyzed using one-way ANOVA,
and Cohen's d test was used for pair-wise comparisons. All data are
presented as the mean ± standard deviation. The AI was generated
using a Cartesian inspection test. All statistical analyses were
performed using SPSS version 25.0 (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
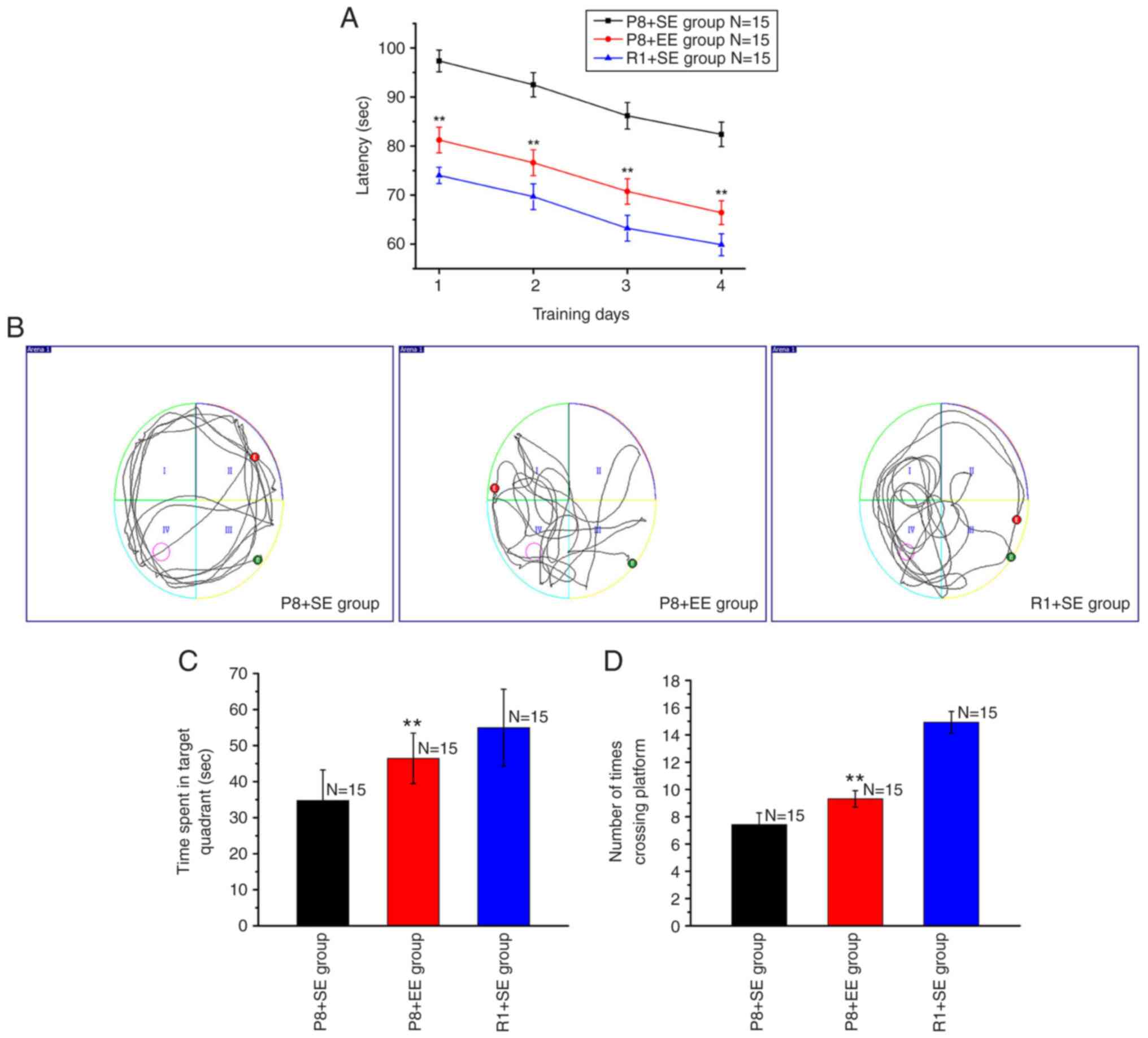
Effects of EE exposure on the
cognitive functions of SAMP8 mice during the MCI phase, assessed
using the MWM test
During the positioning navigation test, the average
escape latencies on training days 1, 2, 3 and 4 in the P8 + EE
group were significantly shorter compared with those in the P8 + SE
group (all P<0.01) but significantly longer compared with
the R1 + SE group (all P<0.01) (Fig. 1A).
During the space exploration test, the mice in the
R1 + SE and P8 + EE groups primarily swam in or near the target
quadrant, whereas the mice in the P8 + SE group primarily swam
along the pool wall and rarely swam near the target quadrant
(Fig. 1B). The time spent in the
target quadrant was longer in the P8 + EE group compared with the
P8 + SE group (P<0.01) but shorter compared with the R1 +
SE group (P<0.01) (Fig.
1C). The number of platform crossings significantly increased
in the P8 + EE group compared with that in the P8 + SE group
(P<0.01) but was fewer than that in the R1 + SE group
(P<0.01) (Fig. 1D).
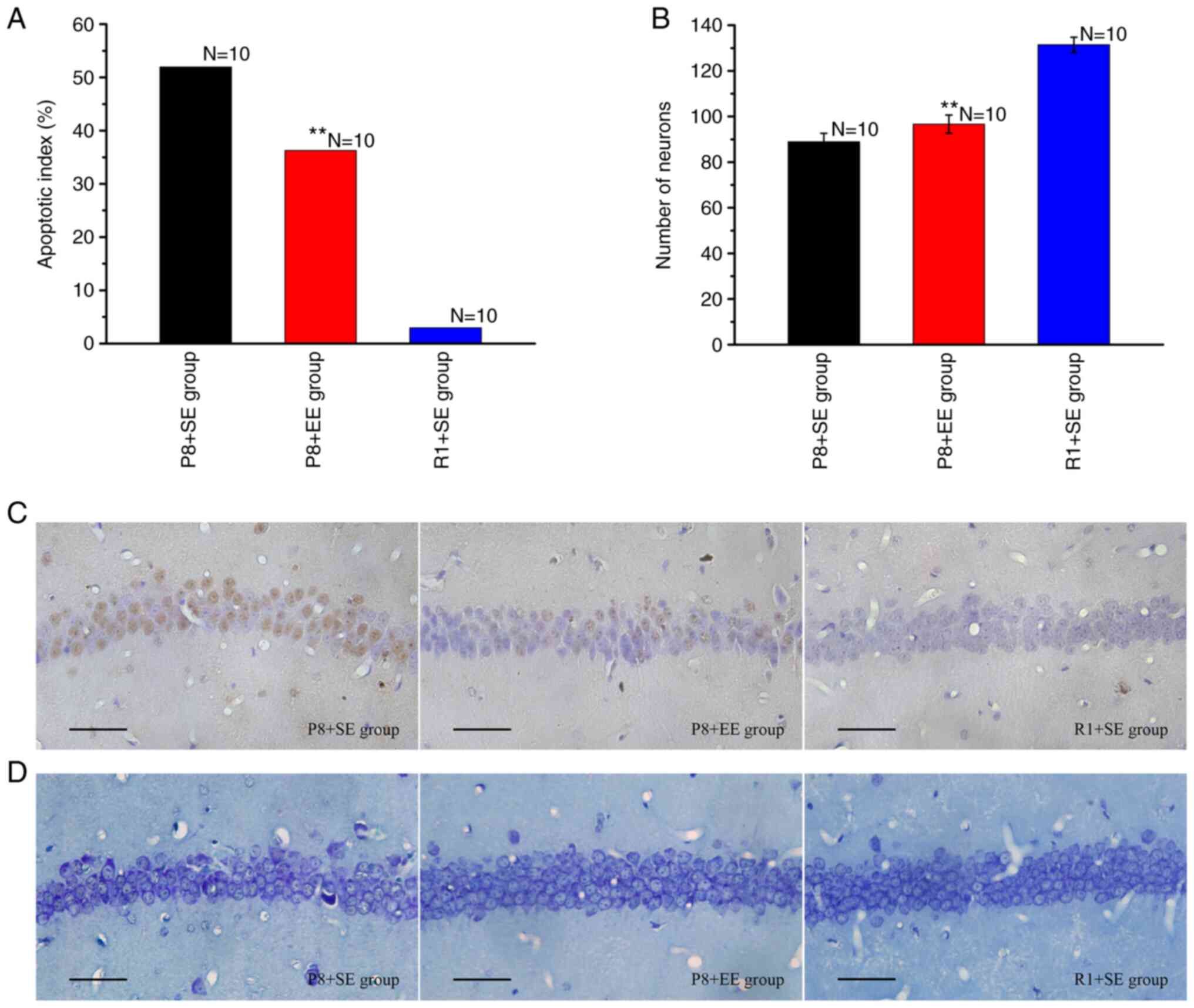
Effects of EE exposure on neuronal
loss in the hippocampal CA1 region in SAMP8 mice during the MCI
phase
TUNEL staining resulted in the nuclei of apoptotic
cells being stained brown (Fig.
2C). As shown in Fig. 2A, the
AI of the P8 + EE group was significantly decreased compared with
that of the P8 + SE group (P<0.01), but was significantly higher
than that of the R1 + SE group (P<0.01).
The Nissl staining results (Fig. 2B and D) showed that the number of neurons in the
hippocampal CA1 region was significantly higher in the P8 + EE
group (P<0.01) compared with that in the P8 + SE group but
remained significantly lower than that in the R1 + SE group
(P<0.01).
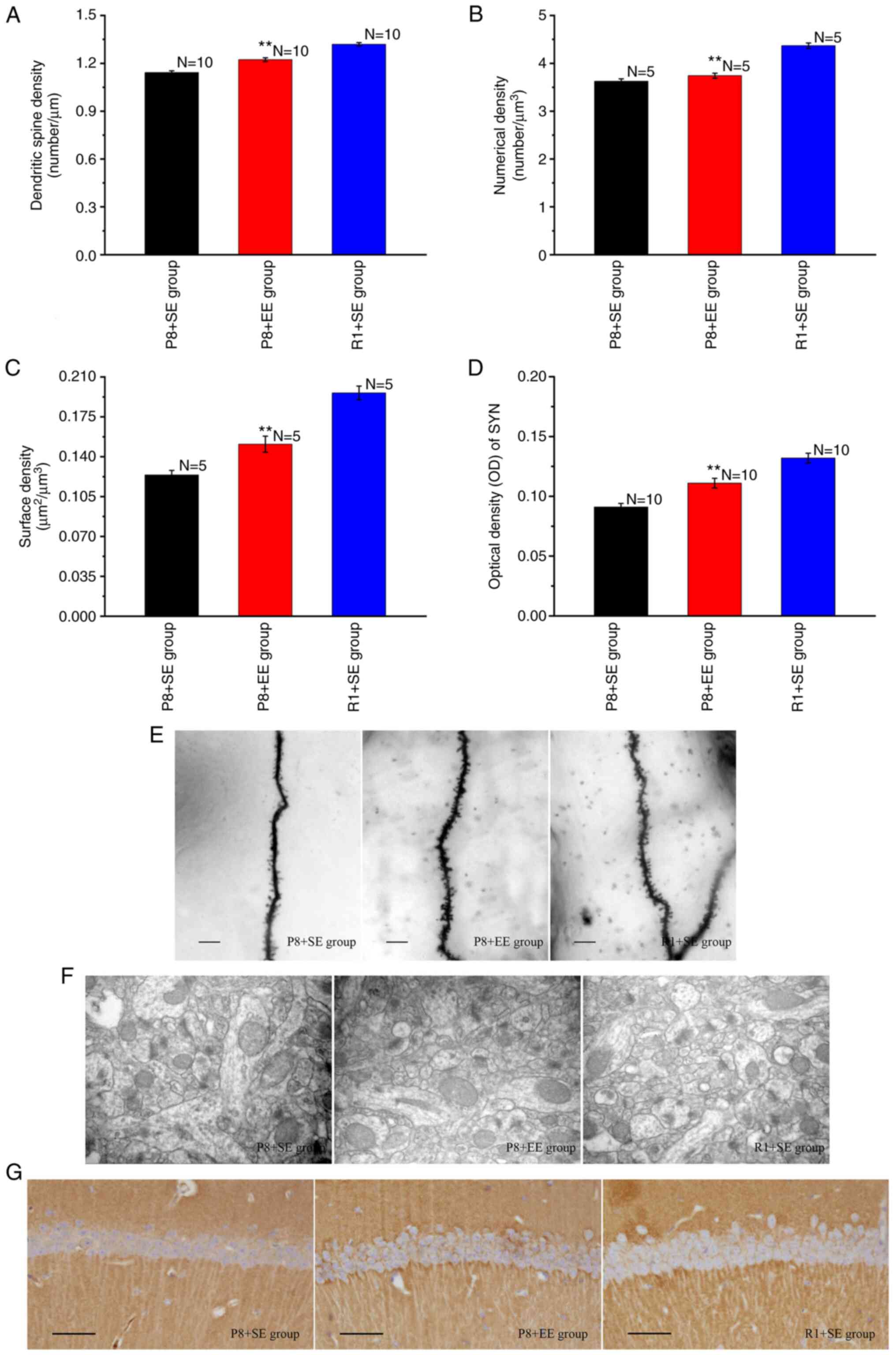
Effects of EE exposure on synaptic
plasticity in the hippocampal CA1 region of SAMP8 mice during the
MCI phase
Golgi staining was used to observe secondary and
tertiary apical dendrites in the hippocampal CA1 region (Fig. 3E). The lengths of secondary and
tertiary apical dendrites were measured, the numbers of dendritic
spines were counted, and dendritic spine densities were determined.
As shown in Fig. 3A, the dendritic
spine density of the P8 + EE group was significantly increased
compared with that of the P8 + SE group (P<0.01) but was
significantly lower than that of the R1 + SE group (P<0.01).
Transmission electron microscopy was used to observe
the ultrastructure of excitatory synapses in the hippocampal CA1
region (Fig. 3F). The Nv and Sv
were calculated using a stereological method and an image analysis
system. The results showed significant differences in the Nv and Sv
of synapses in the hippocampal CA1 region between the P8 + EE and
P8 + SE groups (all P<0.01), and the Nv and Sv of synapses in
the hippocampal CA1 region in the P8 + EE group were significantly
lower than those in the R1 + SE group (all P<0.01) (Fig. 3B and C).
The immunohistochemical staining of SYN showed that
immune reaction products, observed as a brownish-yellow color, were
distributed throughout the neuropil of pyramidal cells but not in
the nucleus and cytoplasm (Fig.
3G). The OD of the immune reaction products in each group was
measured and found that the expression of immune reaction products
in the P8 + EE group was significantly increased compared with that
in the P8 + SE group (P<0.01), but was significantly lower than
that in the R1 + SE group (P<0.01) (Fig. 3D).
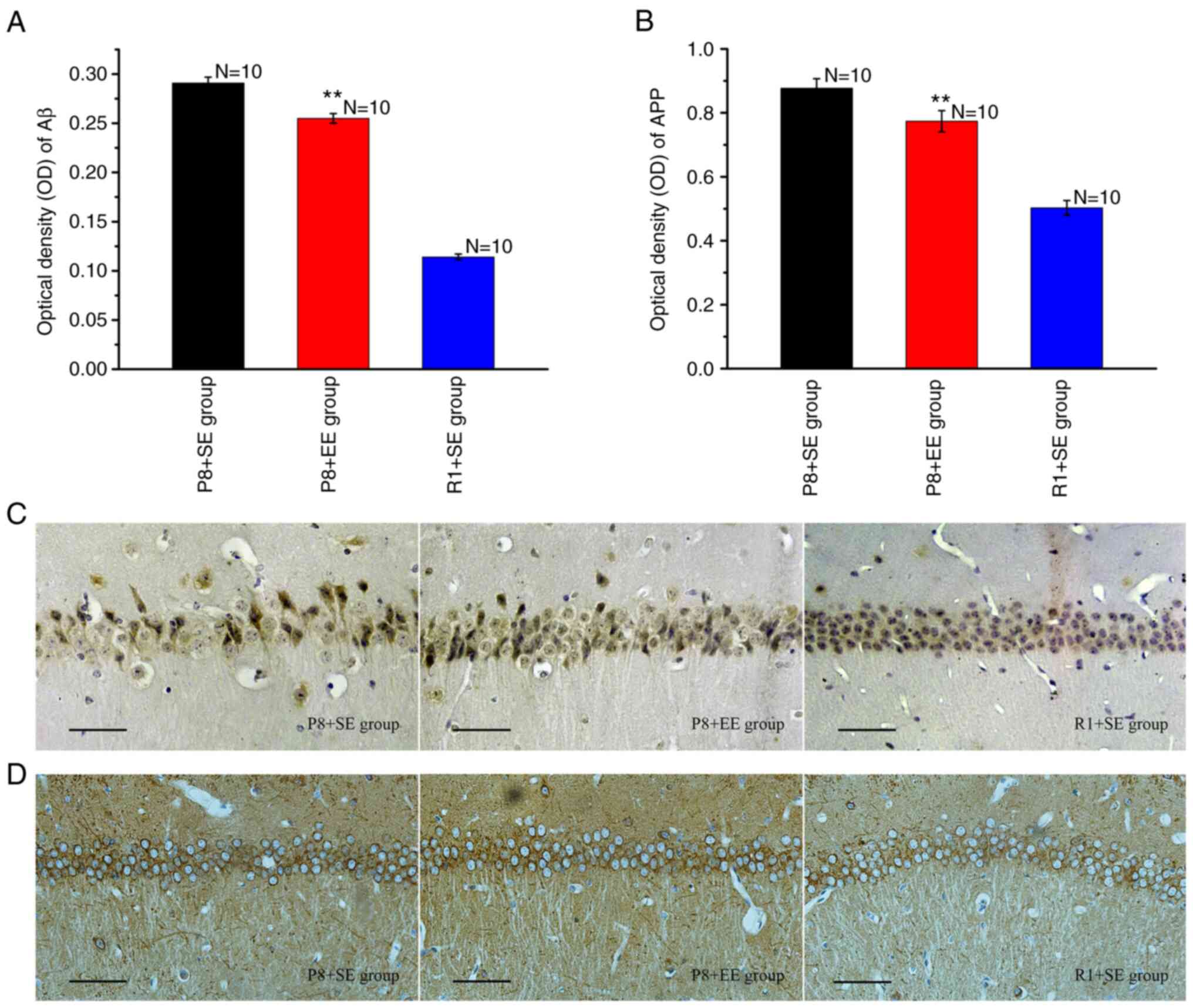
Effects of EE exposure on the
deposition of Aβ and the expression of APP in the hippocampal CA1
region of SAMP8 mice during the MCI phase
Immunohistochemical staining for Aβ showed that
Aβ-immunoreactive granules, which appeared as a dark yellow color,
were primarily distributed in the cytoplasm and protuberances
(Fig. 4C). Aβ was deposited in
large amounts in the P8 + SE group, resembling senile plaques
(Fig. 4C). The Aβ-immunoreactive
granules in the hippocampal CA1 region in the P8 + EE group were
observed at significantly reduced numbers and densities compared
with those in the P8 + SE group (Fig.
4C). In contrast, the deposition of Aβ was rarely observed in
the R1 + SE group (Fig. 4C). The OD
of Aβ in the P8 + EE group was significantly decreased compared
with that in the P8 + SE group (P<0.01) but remained
significantly higher than that in the R1 + SE group (P<0.01)
(Fig. 4A).
The immunohistochemical staining of APP revealed
that immune reaction products, observed in brown color, were
distributed in the cytoplasm and the axons of neurons (Fig. 4D). The measurement of the OD of the
immune reaction products in each group showed that the expression
of immune reaction products in the P8 + EE group was significantly
decreased compared with the P8 + SE group (P<0.01) but was
significantly higher than that in the R1 + SE group (P<0.01)
(Fig. 4B).
Discussion
In humans, the aging process is accompanied by a
decline in cognitive function, which is often associated with
progressive declines in the functions of multiple systems,
including the visual, auditory, olfactory, masticatory and motor
systems. An active lifestyle that includes mental and social
aspects could improve the functions of these systems; this has been
shown to have positive effects on the brain regions that mediate
cognitive function, thereby mitigating declines in cognitive
ability (19,20). The concept of EE was first
introduced by the American scholar Hebb in 1947(11). For more than 70 years, EE has been
demonstrated to be beneficial for the brain, particularly the
hippocampus, at the molecular, cellular and behavioral levels. At
the behavioral level, EE not only improves spatial, non-spatial and
long-term memory in normal animals (21-23)
but also ameliorate human-like neurological symptoms in animal
models of human neurological pathology, including the cognitive
decline associated with AD (24-28).
Currently, accumulated evidence has demonstrated that EE exposure
can mitigate cognitive decline in young SAMP8 mice (29-31).
Griñán-Ferré et al (29,30)
raised SAMP8 mice under EE conditions from 21 days after birth
until 3 months of age and found a better generalized performance in
SAMP8 mice raised under EE conditions compared with those raised
under SE conditions, assessed by behavioral and learning tests.
Yuan et al (31) housed
3-month-old female SAMP8 mice under EE conditions for 3 months, and
the results demonstrated that EE exposure improved cognitive
performance, assessed by the MWM test and a step-down avoidance
experiment. In the present study, compared with mice under SE
conditions, mice under EE conditions were housed in large,
polyethylene squirrel cages, that provided opportunities to perform
more activities, including a running wheel, a swing and a slide,
increasing the opportunities for voluntary exercise and likely
increased aerobic exercise. In addition, mice housed under the EE
conditions had access to a tunnel and several toys with different
colors and shapes. Additionally, the cages were cleaned and
positions of the objects rearranged once a day at a fixed time to
enhance visual stimulation. After 2 months, the learning and memory
abilities of middle-aged SAMP8 and SAMR1 mice housed under
different environments were evaluated using the MWM test. The
results showed that mice in the P8 + EE group exhibited shorter
escape latencies compared with mice in the P8 + SE group during the
positioning navigation test. P8 + EE mice also spent more time in
the target quadrant compared with mice in the P8 + SE group during
the space exploration test. Moreover, the number of platform
crossings increased significantly for the P8 + EE group compared
with that for the P8 + SE group. However, a significant difference
was observed between mice in the P8 + EE and R1 + SE groups for
escape latencies, time spent in the target quadrant, and the number
of platform crossings. These behavioral results indicated that EE
exposure could be used as a rehabilitation strategy to mitigate the
cognitive impairments of SAMP8 mice during the MCI phase and to
delay the progression from MCI to AD.
The hippocampus is an important structure in limbic
system, which is associated with learning, memory and cognition.
The hippocampal CA1 region is an important part of learning and
memory, information processing and signal transmission. It is
responsible for spatial learning and memory (32,33).
The selective vulnerability of distinct regions of the brain is a
critical factor in neurodegenerative disorders. In Alzheimer's
disease (AD), neurons in hippocampus situated in medial temporal
lobe are immensely damaged (34).
Neurons in the hippocampal CA1 region are the most sensitive of the
entire forebrain, and are considered as the most vulnerable neurons
of the hippocampus (35,36). It is the most sensitive area to
hypoxia and other damage, also known as vulnerable area (35). In the present study,
neuropathological changes in the hippocampal CA1 region were
examined after the mice were exposed to different environments for
60 days.
Compared with the number of neurons in the
hippocampus of age-matched normal elderly people, the hippocampus
of patients with AD is significantly reduced (37). Autopsies of patients with AD and
experiments performed in animal models of AD have revealed higher
numbers of apoptotic cells in the hippocampus (38), which suggests that abnormal
apoptosis is responsible for neuronal losses in the hippocampus of
patients with AD. Studies have shown that EE exposure may reduce
abnormal cell apoptosis (39,40)
and cause changes in the morphology and structure of the brain at
both macro and micro levels, such as increasing the thickness of
the cerebral cortex, area of the cerebral sulcus, and number of
neurons (41,42). Griñan-Ferré et al
demonstrated that EE exposure decreased the levels of Bax and
antiapoptotic Bcl-2 proteins and reduced caspase-3 activity,
thereby preventing neuronal apoptosis and loss in SAMP8 mice
(29). In agreement with these
results, we found that in the hippocampal CA1 region, apoptotic
neurons were reduced significantly, as assessed by TUNEL staining,
and existing neurons were significantly increased, as assessed by
Nissl staining, in the P8 + EE group compared with the P8 + SE
group. However, these indicators, including AI and the number of
existing neurons, also differed significantly between the P8 + EE
and R1 + SE groups. Mitochondria play a pivotal role in the process
of integrating all types of death signal stimulation and
determining cell fate. By releasing cytochrome c and other related
apoptotic factors, mitochondria can initiate caspase protein
cascade reaction and induce cell apoptosis; it can also produce a
large number of free radicals and destroy the electron transmission
of respiratory chain to cause ATP synthesis disorder and induce
cell necrotic death (43). Combined
with these findings, it is speculated that EE condition may
decrease apoptosis by affecting mitochondrial function, including
caspase activity and ATP production; this hypothesis was be
verified through relevant experiments in the present study.
The synaptic plasticity is the neurobiological basis
for learning and memory activities (44,45).
Synapses, which are the basic unit of transmission of information
in the central nervous system, form connections and transmit
electrical and chemical signals between neurons (46). Structural and functional damage to
synapses has been identified as one of the pathological bases of
learning and memory impairment in patients with AD (47,48).
Dendritic spines, which are protrusions that extend outward from
the neuronal dendrite, form the structural bases of neuronal
connections in the brain and are the primary sites of synapse
formation (49). Dendritic spines
accept and transfer information, and their upregulation can
increase communication and transmission (50,51).
During learning and memory processes, changes in synaptic
structural plasticity are frequently accompanied by changes in
dendritic spines, including formation, expansion, atrophy and
abscission. Accumulating studies have identified changes in the
morphology and quantity of dendritic spines associated with
neurodegenerative diseases such as AD (52,53).
Several studies have demonstrated that the dendritic spine density
of the secondary and tertiary dendrites in hippocampal neurons is
significantly lower in SAMP8 mice compared with SAMR1 mice
(10,17). EE exposure can modulate the shape,
number and function of dendritic spines (54,55)
and considerably increase dendritic spine densities of neocortical,
hippocampal and cerebellar neurons (56-58).
In the present study, it was found that the dendritic spine density
in SAMP8 mice raised under EE conditions was significantly
increased compared with that in SAMP8 mice raised under SE
conditions, but was significantly lower compared with that in SAMR1
mice raised under SE conditions. To further elucidate the effects
of EE exposure on the synapses in SAMP8 mice, the ultrastructure of
the hippocampal CA1 region was examined using transmission electron
microscopy and calculated synaptic count indices, including Nv and
Sv, using a stereological method and an image analysis system. The
results showed that EE exposure increased the density of synaptic
contacts on the dendritic spines of hippocampal CA1 neurons.
However, significant differences in Nv and Sv were observed between
the P8 + EE and R1 + SE groups.
SYN is a glycoprotein that is located on the
membranes of presynaptic vesicles, and SYN expression can
accurately and objectively reflect the distribution and density of
synapses (59), since SYN is
closely associated with synapse formation and reconstruction
(60). The degree of cognitive
decline in animal models of AD has been correlated with changes in
SYN expression in the hippocampus (17,61-64).
The present study demonstrated that EE exposure increased the
expression of SYN in the hippocampal CA1 region, which further
indicates that EE exposure was able to increase neuronal synapses
and improve synaptic plasticity in SAMP8 mice. Collectively, these
results suggest that the observed declines in learning and memory
abilities in SAMP8 mice are closely associated with synaptic
plasticity in the hippocampal CA1 region and that EE exposure
effectively regulates synaptic plasticity and reduces synaptic
loss.
The deposition of Aβ is a common pathological change
identified in brain regions associated with neurodegenerative
diseases such as AD and MCI (65),
and the present results showed that the average Aβ absorbance in
the P8 + SE group significantly increased compared with that in the
R1 + SE group. Accumulating research has demonstrated that EE
exposure may decrease the Aβ content in the hippocampus. Male
APPswe X PS1E9 mice were exposed to an EE for 5 months, starting at
the age of 1 month, and the results of biochemical and histological
analyses showed decreases in both the steady-state levels of Aβ
peptides and Aβ deposition in the brains of mice exposed to EE
compared with mice exposed to SE (66). Four months of continuous EE
stimulation in 30-day-old female TgCRND8 mice resulted in
significant reductions in Aβ plaques in the neocortex and
hippocampus (67). Berardi et
al (68) found that AD11 mice
exposed to EE from 2 to 7 months of age exhibited marked reductions
in the presence of Aβ clusters in the hippocampus.
Ziegler-Waldkirch et al (69) exposed pregnant 5xFAD mice to EE for
18 days and found that hippocampal and cortical Aβ plaque pathology
was significantly decreased compared with that in pregnant mice
housed under SE conditions, reaching an Aβ-plaque load similar to
that in the non-pregnant control group, which was housed under SE
conditions. A review examining the neuroprotective effects of
physical activity on the brain in patients with AD concluded that
physical activity may improve cognitive dysfunction by reducing Aβ
deposition in the brain (70). In
the present study, it was also found that EE exposure significantly
decreased the deposition of Aβ in the hippocampal CA1 region in
SAMP8 mice. Aβ is a product of APP that is formed under the action
of β-secretase or γ-secretase. Previous studies have shown that EE
exposure significantly decreases APP levels in the hippocampus
(29,71). In addition, the present study
demonstrated that EE exposure significantly decreased the
expression of APP in the hippocampal CA1 region in SAMP8 mice,
indicating that EE exposure can decrease the production and
deposition of Aβ, which is likely mediated by decreasing APP
expression. Aβ is a neurotoxic peptide that can promote neuronal
loss and mediate synaptic loss and dysfunction in specific regions
by disrupting the mitochondrial membrane potential, decreasing ATP
generation, enhancing intracellular reactive oxygen species
production and activating apoptosis factors and mitophagy (46,72-79).
Based on the aforementioned findings, it is
speculated that EE exposure may effectively decrease neuronal loss
and regulate neuronal synaptic plasticity by reducing the
expression of APP and the deposition of Aβ in the hippocampal CA1
region, so as to mitigate cognitive decline in SAMP8 mice during
the MCI phase and delay the progression from MCI to AD. EE alone
can play a role in ameliorating human-like neurological symptoms
observed in animal models of human neurological pathology.
Remarkably, the combination of drugs and EE effectively reduces
drug-associated side effects and can promote the therapeutic
effects of the drugs (71,76,80).
Therefore, as a low-cost, low-risk and very effective method, EE
exposure should attract widespread attention for the rehabilitation
of neurodegenerative diseases.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Scientific Research
Program of Health Commission in Shanxi of China (grant no. 2015158;
to JZL) and the Scientific and Technological Innovation Programs of
Higher Education Institutions in Shanxi of China (grant no.
2019L0676 to JZL). Funders had no involvement in the study design;
data collection, analysis and interpretation, manuscript writing or
decision to submit the paper for publication.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
Study design: JZL and XHH. Experiment
implementation: JZL, HPW and ML. Data analysis: XML and ZBW.
Manuscript writing: JZL and XHH. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All experimental procedures followed the guiding
principles of mammalian neuroscience research and were approved by
the Ethics Committee for Animal Experiments at Changzhi Medical
College (approval no. DW2015018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ribarič S: The rationale for insulin
therapy in Alzheimer's disease. Molecules. 21(689)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Petersen RC, Smith GE, Waring SC, Ivnik
RJ, Tangalos EG and Kokmen E: Mildcognitive impairment: Clinical
characterization and outcome. Arch Neurol. 56:303–308.
1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Winblad B, Palmer K, Kivipelto M, Jelic V,
Fratiglioni L, Wahlund LO, Nordberg A, Bäckman L, Albert M,
Almkvist O, et al: Mild cognitive impairment-beyond controversies,
towards a consensus: Report ofthe international working group on
mild cognitive impairment. J Intern Med. 256:240–246.
2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Albert MS, DeKosky ST, Dickson D, Dubois
B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen
RC, et al: The diagnosis of mild cognitive impairment due to
Alzheimer's disease: Recommendations from the national institute on
aging-Alzheimer's association workgroups on diagnostic guidelines
for Alzheimer's disease. Alzheimers Dement. 7:270–279.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yi D, Choe YM, Byun MS, Sohn BK, Seo EH,
Han J, Park J, Woo JI and Lee DY: Differences in functional brain
connectivity alterations associated with cerebral amyloid
deposition in amnestic mild cognitive impairment. Front Aging
Neurosci. 7(15)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Min BQ, Zhang LN, Lu Y, Zhou AH, Wei CB
and Jian JP: Degeneration of optic nerve and retina in patients of
mild cognitive impairment and Alzheimer's disease. J Neurosci Ment
Heal. 12:491–494. 2012.
|
|
7
|
Cheng XR, Zhou WX and Zhang YX: The
behavioral, pathological and therapeutic features of the
senescence-accelerated mouse prone 8 strain as an Alzheimer's
disease animal model. Ageing Res Rev. 13:13–37. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang JH, Ye FQ, Cheng XR, Zhang XR, Liu F,
Liu G, Ni M, Qiao SY, Zhou WX and Zhang YX: The effects of LW-AFC
on intestinal microbiome in senescence-accelerated mouse prone 8
strain, a mouse model of Alzheimer's disease. J Alzheimers Dis.
53:907–919. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Griñán-Ferré C, Corpas R,
Puigoriol-Illamola D, Palomera-Ávalos V, Sanfeliu C and Pallàs M:
Understanding epigenetics in the neurodegeneration of Alzheimer's
disease: SAMP8 mouse model. J Alzheimers Dis. 62:943–963.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kang L, Li S, Xing ZG, Li JZ, Su YH, Fan
P, Wang L and Cui HX: Dihydrotestosterone treatment delays the
conversion from mild cognitive impairment to Alzheimer's disease in
SAMP8 mice. Horm Behav. 65:505–515. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hebb DO: The effects of early experience
on problem-solving at maturity. Am Psychol. 2:306–307. 1947.
|
|
12
|
Meng FT, Zhao J, Ni RJ, Fang H, Zhang LF,
Zhang Z and Liu YJ: Beneficial effects of enriched environment on
behaviors were correlated with decreased estrogen and increased
BDNF in the hippocampus of male mice. Neuro Endocrinol Lett.
36:490–497. 2015.PubMed/NCBI
|
|
13
|
Sanford AM: Mild cognitive impairment.
Clin Geriatr Med. 33:325–337. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
He YS, Yao ZB, Gu YM, Kuang GB and Chen
YC: Nerve growth factor promotes collateral sprouting of
cholinergic filbers in the septohippocampal cholinergic system of
aged rats with fimbria transaction. Brain Res. 586:27–35.
1992.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jones DG and Calverley RK: Frequency of
occurrence of perforated synapses in developing rat neocortex.
Neurosci Lett. 129:189–192. 1991.PubMed/NCBI View Article : Google Scholar
|
|
16
|
He JF, Qian G, Ling X, Wu MS, Guo P and
Luo SY: Compound ruikangxin can reverse the structural plasticity
of synapses in hippocampus, amygdala and nucleus accumbens in
morphine withdrawal rats. Acta Anatomica Sin. 42:300–306. 2011.(In
Chinese).
|
|
17
|
Li S, Kang L, Zhang C, Xie GS, Li N, Zhang
Y, Du J and Cui HX: Effects of dihydrotestosterone on synaptic
plasticity of hippocampus in male SAMP8 mice. Exp Gerontol.
48:778–785. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo LZ, Li LM and Zhang C: Effects of
S14G-humanin on Aβ31-35-induced dysfunction of learning and memory
and neuronal apoptosis in rat. Chin J Neuroanatomy. 27:164–168.
2011.(In Chinese).
|
|
19
|
Haesner M, O'Sullivan JL, Gövercin M and
Steinhagen-Thiessen E: Requirements of older adults for a daily use
of an internet-based cognitive training platform. Inform Health Soc
Care. 40:139–153. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Leon M and Woo C: Environmental enrichment
and successful aging. Front Behav Neurosci. 12(155)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bruel-Jungerman E, Laroche S and Rampon C:
New neurons in the dentate gyrus are involved in the expression of
enhanced long-term memory following environmental enrichment. Eur J
Neurosci. 21:513–521. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Leggio MG, Mandolesi L, Federico F,
Spirito F, Ricci B, Gelfo F and Petrosini L: Environmental
enrichment promotes improved spatial abilities and enhanced
dendritic growth in the rat. Behav Brain Res. 163:78–90.
2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rampon C, Tang YP, Goodhouse J, Shimizu E,
Kyin M and Tsien JZ: Enrichment induces structural changes and
recovery from nonspatial memory deficits in CA1 NMDAR1-knockout
mice. Nat Neurosci. 3:238–244. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Arranz L, De Castro NM, Baeza I,
Giménez-Llort L and De la Fuente M: Effect of environmental
enrichment on the immunoendocrine aging of male and female
triple-transgenic 3xTg-AD mice for Alzheimer's disease. J
Alzheimers Dis. 25:727–737. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Polito L, Chierchia A, Tunesi M,
Bouybayoune I, Kehoe PG, Albani D and Forloni G: Environmental
enrichment lessens cognitive decline in APP23 mice without
affecting brain sirtuin expression. J Alzheimers Dis. 42:851–864.
2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Stuart KE, King AE, Fernandez-Martos CM,
Dittmann J, Summers MJ and Vickers JC: Mid-life environmental
enrichment increases synaptic density in CA1 in a mouse model of
Aβ-associated pathology and positively influences synaptic and
cognitive health in healthy ageing. J Comp Neurol. 525:1797–1810.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Griñán-Ferré C, Izquierdo V, Otero E,
Puigoriol-Illamola D, Corpas R, Sanfeliu C, Ortuño-Sahagún D and
Pallàs M: Environmental enrichment improves cognitive deficits, AD
hallmarks and epigenetic alterations presented in 5xFAD mouse
model. Front Cell Neurosci. 12(224)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Stuart KE, King AE, King NE, Collins JM,
Vickers JC and Ziebell JM: Late-life environmental enrichment
preserves short-term memory and may attenuate microglia in male
APP/PS1 mice. Neuroscience. 408:282–292. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Griñan-Ferré C, Pérez-Cáceres D,
Gutiérrez-Zetina SM, Camins A, Palomera-Avalos V, Ortuño-Sahagún D,
Rodrigo MT and Pallàs M: Environmental enrichment improves
behavior, cognition, and brain functional markers in young
senescence-accelerated prone mice (SAMP8). Mol Neurobiol.
53:2435–2450. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Griñan-Ferré C, Puigoriol-Illamola D,
Palomera-Ávalos V, Pérez-Cáceres D, Companys-Alemany J, Camins A,
Ortuño-Sahagún D, Rodrigo MT and Pallàs M: Environmental enrichment
modified epigenetic mechanisms in SAMP8 mouse hippocampus by
reducing oxidative stress and inflammaging and achieving
neuroprotection. Front Aging Neurosci. 8(241)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yuan ZY, Wang MW, Yan BY, Gu P, Jiang XM,
Yang XF and Cui DS: An enriched environment improves cognitive
performance in mice from the senescence-accelerated prone mouse 8
strain: Role of upregulated neurotrophic factor expression in the
hippocampus. Neural Regen Res. 7:1797–1804. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Khan UA, Liu L, Provenzano FA, Berman DE,
Profaci CP, Sloan R, Mayeux R, Duff KE and Small SA: Molecular
drivers and cortical spread of lateral entorhinal cortex
dysfunction in preclinical Alzheimer's disease. Nat Neurosci.
17:304–311. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Witter MP: Organization of the
entorhinal-hippocampal system: A review of current anatomical data.
Hippocampus. 3:33–44. 1993.PubMed/NCBI
|
|
34
|
Dharshini SAP, Taguchi YH and Gromiha MM:
Exploring the selective vulnerability in Alzheimer disease using
tissue specific variant analysis. Genomics. 111:936–949.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Schmidt-Kastner R and Freund TF: Selective
vulnerability of the hippocampus in brain ischemia. Neuroscience.
40:599–636. 1991.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Morrison JH and Hof PR: Selective
vulnerability of corticocortical and hippocampal circuits in aging
and Alzheimer's disease. Prog Brain Res. 136:467–486.
2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zilkova M, Koson P and Zilka N: The hunt
for dying neurons: Insight into the neuronal loss in Alzheimer's
disease. Bratisl Lek Listy. 107:366–373. 2006.PubMed/NCBI
|
|
38
|
Tian XQ, Zhang L, Yang L, Huang P, Qian X,
Huang PL and Zhang LD: A possible anti-apoptosis mechanism of
hyperbaric oxygen in rats with memory impairments induced by
Aβ25-35. Chin J Phys Med Rehabil. 36:7–11. 2014.
|
|
39
|
van Praag H, Kempermann G and Gage FH:
Neural consequences of environmental enrichment. Nat Rev Neurosci.
1:191–198. 2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Brown J, Cooper-Kuhn CM, Kempermann G, Van
Praag H, Winkler J, Gage FH and Kuhn HG: Enriched environment and
physical activity stimulate hippocampal but not olfactory bulb
neurogenesis. Eur J Neurosci. 17:2042–2046. 2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kobilo T, Liu QR, Gandhi K, Mughal M,
Shaham Y and van Praag H: Running is the neurogenic and
neurotrophic stimulus in environmental enrichment. Learn Mem.
18:605–609. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Herring A, Ambree O, Tomm M, Habermann H,
Sachser N, Paulus W and Keyvani K: Environmental enrichment
enhances cellular plasticity in transgenic mice with Alzheimer-like
pathology. Exp Neurol. 216:184–192. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ji XY, Zhang LN, Liu R, Liu YZ, Song JF,
Dong H, Jia YF and Zhou ZG: Potential targets for protecting
against hippocampal cell apoptosis after transient cerebral
ischemia-reperfusion injury in aged rats. Neural Regen Res.
9:1122–1128. 2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hitti FL and Siegelbaum SA: The
hippocampal CA2 region is essential for social memory. Nature.
508:88–92. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Han WN, Yuan L, Liu XJ, Zhou LW, Wu MN and
Qi JS: The correlation study between spatial memory and hippocampal
long term potentiation in rats. Chin J Behav Med Brain Sci.
21:630–633. 2012.
|
|
46
|
Zhao CH, Su P, Lv C, Guo LM, Cao GQ, Qin
CX and Zhang WS: Berberine alleviates amyloid β-induced
mitochondrial dysfunction and synaptic loss. Oxid Med Cell Longev.
2019(7593608)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Serrano-Pozo A, Frosch MP, Masliah E and
Hyman BT: Neuropathological alterations in Alzheimer disease. Cold
Spring Harb Perspect Med. 1(a006189)2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Tu S, Okamoto S, Lipton SA and Xu H:
Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease.
Mol Neurodegener. 9(48)2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Raven F, Van der Zee EA, Meerlo P and
Havekes R: The role of sleep in regulating structural plasticity
and synaptic strength: Implications for memory and cognitive
function. Sleep Med Rev. 39:3–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Holtmaat A and Svoboda K:
Experience-dependent structural synaptic plasticity in the
mammalian brain. Nat Rev Neurosci. 10:647–658. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Rochefort NL and Konnerth A: Dendritic
spines: From structure to in vivo function. EMBO Rep. 13:699–708.
2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mavroudis IA, Fotiou DF, Manani MG, Njaou
SN, Frangou D, Costa VG and Baloyannis SJ: Dendritic pathology and
spinal loss in the visual cortex in Alzheimer's disease: A Golgi
study in pathology. Int J Neurosci. 121:347–354. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Song JM, DiBattista AM, Sung YM, Ahn JM,
Turner RS, Yang J, Pak DT, Lee HK and Hoe HS: A tetra(ethylene
glycol) derivative of benzothiazole aniline ameliorates dendritic
spine density and cognitive function in a mouse model of
Alzheimer's disease. Exp Neurol. 252:105–113. 2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Bae J, Sung BH, Cho IH, Kim SM and Song
WK: NESH regulates dendritic spine morphology and synapse
formation. PLoS One. 7(e34677)2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Petrinovic MM, Hourez R, Aloy EM, Dewarrat
G, Gall D, Weinmann O, Gaudias J, Bachmann LC, Schiffmann SN, Vogt
KE and Schwab ME: Neuronal Nogo-A negatively regulates dendritic
morphology and synaptic transmission in the cerebellum. Proc Natl
Acad Sci USA. 110:1083–1088. 2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Hu XL, Bergström SA, Brink M, Rönnbäck A
and Dahlqvist P: Enriched environment increases spinophilin mRNA
expression and spinophilin immunoreactive dendritic spines in
hippocampus and cortex. Neurosci Lett. 476:79–83. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Liu N, He S and Yu X: Early natural
stimulation throughenvironmental enrichment accelerates neuronal
development inthe mouse dentate gyrus. PLoS One.
7(e30803)2012.PubMed/NCBI View Article : Google Scholar
|
|
58
|
De Bartolo P, Florenzano F, Burello L,
Gelfo F and Petrosini L: Activity-dependent structural plasticity
of purkinje cell spines in cerebellar vermis and hemisphere. Brain
Struct Funct. 220:2895–2904. 2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Liu ZT, Yu TH, Qu TB, Li L and Chu LS:
Effect of buyanghuanwu decoction on the expression of
growth-associated protein 43 and synaptophysin after focal cerebral
ischemia in mice. Chin J Behav Med Brain Sci. 21:1070–1072.
2012.
|
|
60
|
Kwon SE and Chapman ER: Synaptophysin
regulates the kinetics of synaptic vesicle endocytosis in central
neurons. Neuron. 70:847–854. 2011.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Huo DS, Sun JF, Zhang BF, Yan XS, Wang H,
Jia JX and Yang ZJ: Protective effects of testosterone on cognitive
dysfunction in Alzheimer's disease model rats induced by oligomeric
beta amyloid peptide 1-42. J Toxicol Environ Health A. 79:856–863.
2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Jia JX, Yan XS, Cai ZP, Song W, Huo DS,
Zhang BF, Wang H and Yang ZJ: The effects of phenylethanoid
glycosides, derived from herba cistanche, on cognitive deficits and
antioxidant activities in male SAMP8 mice. J Toxicol Environ Health
A. 80:1180–1186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhou CL, Zhao L, Shi HY, Liu JW, Shi JW,
Kan BH, Li Z, Yu JC and Han JX: Combined acupuncture and HuangDiSan
treatment affects behavior and synaptophysin levels in the
hippocampus of senescence-accelerated mouse prone 8 after neural
stem cell transplantation. Neural Regen Res. 13:541–548.
2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Dong WG, Yang WD, Li FF, Guo WQ, Qian CH,
Wang F, Li CZ, Lin L and Lin RH: Electroacupuncture improves
synaptic function in SAMP8 mice probably via inhibition of the
AMPK/eEF2K/eEF2 signaling pathway. Evid Based Complement Alternat
Med. 2019(8260815)2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Hussain M, Berger M, Eckenhoff RG and
Seitz DP: General anesthetic and the risk of dementia in elderly
patients: Current insights. Clin Interv Aging. 9:1619–1628.
2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Lazarov O, Robinson J, Tang YP, Hairston
IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K and
Sisodia SS: Environmental enrichment reduces Aβ levels and amyloid
deposition in transgenic mice. Cell. 120:701–713. 2005.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ambree O, Leimer U, Herring A, Görtz N,
Sachser N, Heneka MT, Paulus W and Keyvani K: Reduction of amyloid
angiopathy and Aβ plaque burden after enriched housing in TgCRND8
mice. Am J Pathol. 169:544–552. 2006.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Berardi N, Braschi C, Capsoni S, Cattaneo
A and Maffei L: Environmental enrichment delays the onset of memory
deficits and reduces neuropathological hallmarks in a mouse model
of Alzheimer-like neurodegeneration. J Alzheimers Dis. 11:359–70.
2007.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Ziegler-Waldkirch S, Marksteiner K, Stoll
J, d'Errico P, Friesen M, Eiler D, Neudel L, Sturn V, Opper I,
Datta M, et al: Environmental enrichment reverses Aβ pathology
during pregnancy in a mouse model of Alzheimer's disease. Acta
Neuropathol Commun. 6(44)2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Phillips C, Baktir MA, Das D, Lin B and
Salehi A: The link between physical activity and cognitive
dysfunction in Alzheimer disease. Phys Ther. 95:1046–1060.
2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Dong JD, Zhou M, Wu XQ, Du MY and Wang XS:
Memantine combined with environmental enrichment improves spatial
memory and alleviates Alzheimer's disease-like pathology in
senescence-accelerated prone-8 (SAMP8) mice. J Biomed Res.
26:439–447. 2012.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Tian Q, Jia J, Qu XH, Chen Y, Guo YL and
Zhang MZ: Effect of neuregulin on expressions of apoptosis and
nuclear factor kappa B in hippocampus of Alzheimer's disease model
rats. Chin J Behav Med Brain Sci. 21:26–29. 2012.
|
|
73
|
Izzo NJ, Xu JB, Zeng CB, Kirk MJ, Mozzoni
K, Silky C, Rehak C, Yurko R, Look G, Rishton G, et al: Alzheimer's
therapeutics targeting amyloid beta1-42 oligomers II:
Sigma-2/PGRMC1 receptors mediate Abeta 42 oligomer binding and
synaptotoxicity. PLoS One. 9(e111899)2014.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Squitti R: Copper subtype of Alzheimer's
disease (AD): Meta-analyses, genetic studies and predictive value
of non-ceruloplasmin copper in mild cognitive impairment conversion
to full AD. J Trace Elem Med Biol. 28:482–485. 2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Zhang HJ, Zhao CH, Lv C, Liu XL, Du SJ, Li
Z, Wang YY and Zhang WS: Geniposide alleviates amyloid-induced
synaptic injury by protecting axonal mitochondrial trafficking.
Front Cell Neurosci. 10(309)2017.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Asih PR, Tegg ML, Sohrabi H, Carruthers M,
Gandy SE, Saad F, Verdile G, Ittner LM and Martins RN: Martins RN
multiple mechanisms linking type 2 diabetes and Alzheimer's
disease: Testosterone as a modifer. J Alzheimers Dis. 59:445–466.
2017.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Song L, Li XP, Bai XX, Gao J and Wang CY:
Calycosin improves cognitive function in a transgenic mouse model
of Alzheimer's disease by activating the protein kinase C pathway.
Neural Regen Res. 12:1870–1876. 2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Wang XN, Hu XJ, Yang Y, Takata T and
Sakurai T: Nicotinamide mononucleotide protects against β-amyloid
oligomer-inducedc ognitive impairment and neuronal death. Brain
Res. 1643:1–9. 2016.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Yan XS, Yang ZJ, Jia JX, Song W, Fang X,
Cai ZP, Huo DS and Wang H: Protective mechanism of testosterone on
cognitive impairment in a rat model of Alzheimer's disease. Neural
Regen Res. 14:649–657. 2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Stairs DJ and Bardo MT: Neurobehavioral
effects of environmental enrichment and drug abuse vulnerability.
Pharmacol Biochem Behav. 92:377–382. 2009.PubMed/NCBI View Article : Google Scholar
|