Introduction
Tenofovir alafenamide fumarate (TAF) is a targeted
prodrug of tenofovir [phosphonomethyl pentanedioic acid (PMPA)], a
nucleotide reverse-transcriptase inhibitor. The active component,
tenofovir diphosphate, is integrated into the viral DNA by
hepatitis B virus (HBV) reverse transcriptase, resulting in the
interruption of DNA strand synthesis, which inhibits hepatitis B
virus replication (1). In November
2018, the China Food and Drug Administration approved TAF for the
treatment of chronic hepatitis B (CHB) virus infection in adults
and adolescents (2). The main goal
of treatment is to maximize the long-term inhibition of HBV
replication, prevent disease progression and the occurrence of
hepatocellular carcinoma, so as to improve the quality of life and
prolong the survival time of patients (2). As tenofovir (TFV) cannot be absorbed
by the intestine through the intestinal wall due to its own
chemical structure, it must have prodrugs. Tenofovir dipivoxil
fumarate (TDF) and TAF are both prodrugs of TFV. Compared with TDF,
another novel tenofovir prodrug, TAF exhibits superior plasma
stability and can exert more prominent liver-targeting ability. In
addition, TAF can achieve similar antiviral effects at lower doses
whilst also being associated with lower incidence of side-effects,
including renal dysfunction and reduction of bone mineral density
(3). Therefore, TAF has been
listed as the first-line drug for the antiviral treatment of CHB in
The Guidelines for the Prevention and Treatment of Chronic
Hepatitis B (2019 edition) in China (4). It has also been listed in the
Guidelines for the Prevention and Treatment of Chronic Hepatitis B
formulated by the American Association for the Study Liver Diseases
(AASLD) and the European Association for the Study of the Liver
(EASL) (5). In China, compared
with the old guidelines, the 2019 guidelines include more updates
and highlights, expanding the indications of antiviral therapy
(4). Antiviral therapy should be
recommended for patients with positive HBV DNA, elevated
transaminase (excluding other reasons), normal transaminase but
high-risk factors or liver tissue biopsy with inflammation and
fibrosis. For the initial treatment of CHB, selection of potent and
low resistance drugs is emphasized, where entecavir, TDF and TAF
are recommended (6). However, the
market time of TAF in China remains short and real-world data (RWD)
are insufficient. Therefore, the present study was conducted to
examine the efficacy and safety of TAF in the context of the new
guidelines.
Patients and methods
Patients
All cases in the present study were derived from the
follow-up study cohort of Tianjin Second People's Hospital
(Tianjin, China) between December 2019 and September 2020. The
present study retrospectively analyzed 212 patients with CHB who
were initially treated with TAF. However, 151 patients were
excluded, mainly due to incomplete follow up data.. Overall, a
total of 61 patients entered the study. Among them, 45 patients
were hepatitis B e antigen (HBeAg)-positive. A total of 61 patients
(age, 42.13±12.30) were followed up for 24 weeks, of whom 42 were
followed up for 48 weeks (age, 43.39±13.41), including 35 patients
who were tested HBeAg-positive. According to the baseline ALT
levels, 61 patients were divided into group A [ALT≤50 U/l; upper
limit of normal (ULN)=50 U/l; n=21], group B (50 U/l < ALT
<100 U/l; n=22) and group C (ALT ≥100 U/l; n=18). All patients
met the diagnostic and therapeutic criteria of the Guidelines for
the Prevention and Treatment of Chronic Hepatitis B in China
(4), which were jointly formulated
by the Chinese Society of Hepatology and Infectious Diseases in
2019. The present study was approved by the Medical Ethics
Committee of Tianjin Second People's Hospital, was performed
according to the principles of the Declaration of Helsinki and all
patients provided written informed consent prior to participation
in the study.
Criteria for patient selection
The inclusion criteria were as follows: i) Patients
diagnosed with CHB who had never been treated with NAs previously;
ii) patients with a liver stiffness measurement (LSM) indicating
evidence of fibrosis but with normal alanine aminotransferase (ALT)
levels. The ALT normal range was 9-50 U/l. The exclusion criteria
were as follows: i) Patients with other hepatitis virus
superinfections (for example, hepatitis A, C, D, E and G),
alcoholic liver disease, drug-induced liver injury or autoimmune
liver disease; ii) pregnant or lactating women; iii) patients with
decompensated cirrhosis, hepatocellular carcinoma and liver
failure; and iv) patients with CHB who had previously been treated
with NAs.
Treatment
All patients purchased and used TAF (developed and
produced by Gilead Sciences, Inc.) in the outpatient department,
who participated in the outpatient follow-up study after providing
informed consent. The patients were treated with 25 mg TAF orally,
once daily.
Detection methods and observation
indices
All blood was collected on an empty stomach in the
morning 1 week before TAF treatment (0 W), 24 weeks and 48 weeks.
Blood biochemistry was detected using a Hitachi 7180 automatic
biochemical instrument (Hitachi, Ltd.). The volume of blood
collected from each patient was 3 ml. ALT was tested through liver
function. The ALT test kit (cat. no. AUZ8705) was provided by
FujiFilm Wako Pure Chemical Corporation. The ALT normal value range
was 9-50 U/l. Renal series tests included serum creatinine (SCr),
calcium and inorganic phosphorus. SCr test kit (cat. no. KH213) was
provided by FujiFilm Wako Pure Chemical Corporation. The normal
range of SCr was 57-97 µmol/l. The Modification of Diet in Renal
Disease (MDRD) equation was used to calculate the estimated
glomerular filtration rate (eGFR) as follows: For females, eGFR =
170 x (SCr) - 1.234 x (age) - 0.179 x 0.79; and for males, eGFR =
170 x (SCr) - 1.234 x (age) - 0.179. The normal range of eGFR was
90-120 ml/min/1.73 m2. The calcium (Ca; Arsenazo III
method; cat. no. CA7290) and inorganic phosphorus (IP;
phosphomolybdate method; cat. no. IP7340) assay kits were provided
by Maccura Biotechnology Co., Ltd. The normal ranges of Ca and IP
were 2.11-2.52 and 0.85-1.51 mmol/l, respectively. The triglyceride
(TG; glycerol-3-phosphate-peroxidase method; cat. no. AUZ8611) and
total cholesterol (TC; enzyme method; cat. no. AUZ8842) test kits
were provided by Beckman Coulter Experimental System (Suzhou) Co.,
Ltd. The normal range values for TG and TC were 0.38-2.30 and
2.4-5.2 mmol/l, respectively. The low-density
lipoprotein-cholesterol (LDL-C) test kit (direct method surfactant
clearance method; cat. no. LD7152) was provided by Beijing Leadman
Biochemistry Co., Ltd. The normal range values for LDL-C were
2.07-3.37 mmol/l. Blood lipid tests included TG, TC and LDL-C. The
levels of hepatitis B surface antigen (HBsAg; cat. no. 6C36-78),
HBeAg (cat. no. 6C32-77) and hepatitis B e antibody (HBeAb; cat.
no. 6C34-77) were measured using the Architect I2000SR
electrochemiluminescence immunoassay analyzer (Abbott
Pharmaceutical Co., Ltd.). The volume of blood collected from each
patient was 2 ml. HBsAg, HBeAg and HBeAb were obtained through
Hepatitis B five items tests. The immunoassay kits were also
provided by Abbott Pharmaceutical Co. Ltd. The HBsAg level was
expressed in IU/ml and the detection range was 0-124,950 IU/ml.
HBsAg <0.05 IU/ml was defined as negative. HBeAg and HBeAb
levels were determined semi-quantitatively, and expressed as the
ratio of absorbance to critical value (S/CO). HBeAg >1.0 S/CO
and HBeAb <1.0 S/CO were defined as positive. HBeAg
seroconversion rate was the ratio of HBeAg-negative and
HBeAb-positive patients. HBV DNA level was tested using highly
sensitive HBV DNA detection. The volume of blood collected from
each patient was 5 ml. The highly sensitive HBV DNA (cat. no.
04894570190) was extracted and amplified using the Roche COBAS
AmpliPrep automatic nucleic acid separation and purification
instrument (Roche Molecular Diagnostics). The detection reagent was
provided by Roche Diagnostics. The detection limit was 20 IU/ml, as
the early virological negative rate of HBV DNA was defined as the
proportion of HBV DNA <20 IU/ml at 12 weeks, but this was not
included in the present article.
The complete virological response rate was defined
as the rate of patients with HBV DNA <20 IU/ml. All tests were
performed at the Institute of Liver Disease, Tianjin Second
People's Hospital (Tianjin, China). The LSM and controlled
attenuation parameter (CAP) were measured through transient
elastography and used FibroScan® 502 touch (Echosens).
The normal range values for LSM and CAP were 7.3 kPa and 238 dB/m,
respectively. LSM and CAP ranges were 2.4-75 kPa and 100-400 dB/m,
respectively.
Statistical analysis
Statistical evaluation was conducted using the SPSS
21.0 statistical software (IBM Corp.). Continuous data were
expressed as the mean ± standard deviation. Comparisons between two
groups of data were assessed using Student's paired t-tests.
Comparisons at three time points were assessed using one-way
repeated measures ANOVA followed by paired t-tests with
Bonferroni's correction. Kruskal-Wallis test followed by Dunn's
post hoc test was used to compare the levels of HBV DNA and HBsAg
among the three groups A-C. Categorical count data were analyzed
using the χ2 test. P<0.05 was considered to be the
point of minimal statistical significance for all differences.
Before data analysis, the test for homogeneity of variance was
required, and a normality test was performed by the Shapiro-Wilk
test.
Results
Basic information of the patients
Among the 61 patients, 45 were male (73.8%) and 45
patients tested positive for HBeAg (73.8%). In addition, there were
seven cases of liver cirrhosis (11.5%). The baseline level of ALT
was 188.17±97.71 U/l, the proportion of patients with abnormal ALT
levels was 65.6% (40/61), the quantitative HBV DNA value was
5.49±1.95 log10 IU/ml and the quantitative HBsAg value
was 3.59±0.81 log10 IU/ml (Table I).
 | Table IBaseline characteristics of patients
with chronic hepatitis B initially treated with TAF (n=61). |
Table I
Baseline characteristics of patients
with chronic hepatitis B initially treated with TAF (n=61).
| Parameter | Data |
|---|
| Age, years | 43.64±14.98 |
| Male
proportion | 45 (73.8) |
| Hepatitis B e
antigen-positive proportion | 45 (73.8) |
| Cirrhosis
proportion | 7 (11.5) |
| Proportion with
abnormal ALT levels | 40 (65.6) |
| ALT level, U/l | 188.17±97.71 |
| Hepatitis B virus
DNA quantification, log10 IU/ml | 5.49±1.95 |
| Hepatitis B surface
antigen quantification, log10 IU/ml | 3.59±0.81 |
| Liver stiffness
measurement, kPa | 13.00±8.15 |
| Controlled
attenuation parameter, dB/m | 234.62±47.38 |
| Estimated
glomerular filtration rate, ml/min/1.73m2 | 105.28±12.45 |
| Calcium,
mmol/l | 2.34±0.12 |
| Inorganic
phosphorus, mmol/l | 1.11±0.19 |
Efficacy evaluation after 24 and 48
weeks of TAF treatment
After 24 and 48 weeks of TAF treatment, the
proportion patients with ALT returning to normal levels was 52.46
(32/61) and 95.24% (40/42), respectively (P<0.05). The complete
virological response rate (patients with HBV DNA levels <20
IU/ml) was 39.34 (24/61) and 69.05% (29/42) on weeks 24 and 48,
respectively (P<0.05). The HBeAg seroconversion rate was 8.57%
(3/35) and the HBsAg-negative rate was 0 at 48 weeks. The mean HBV
DNA levels among all patients at baseline was 5.49±1.95
log10 IU/ml (Table I),
which reached 1.61±0.96 (P<0.001 vs. baseline) and 1.26±0.66
log10 IU/m (P<0.001 vs. baseline) at 24 and 48 weeks,
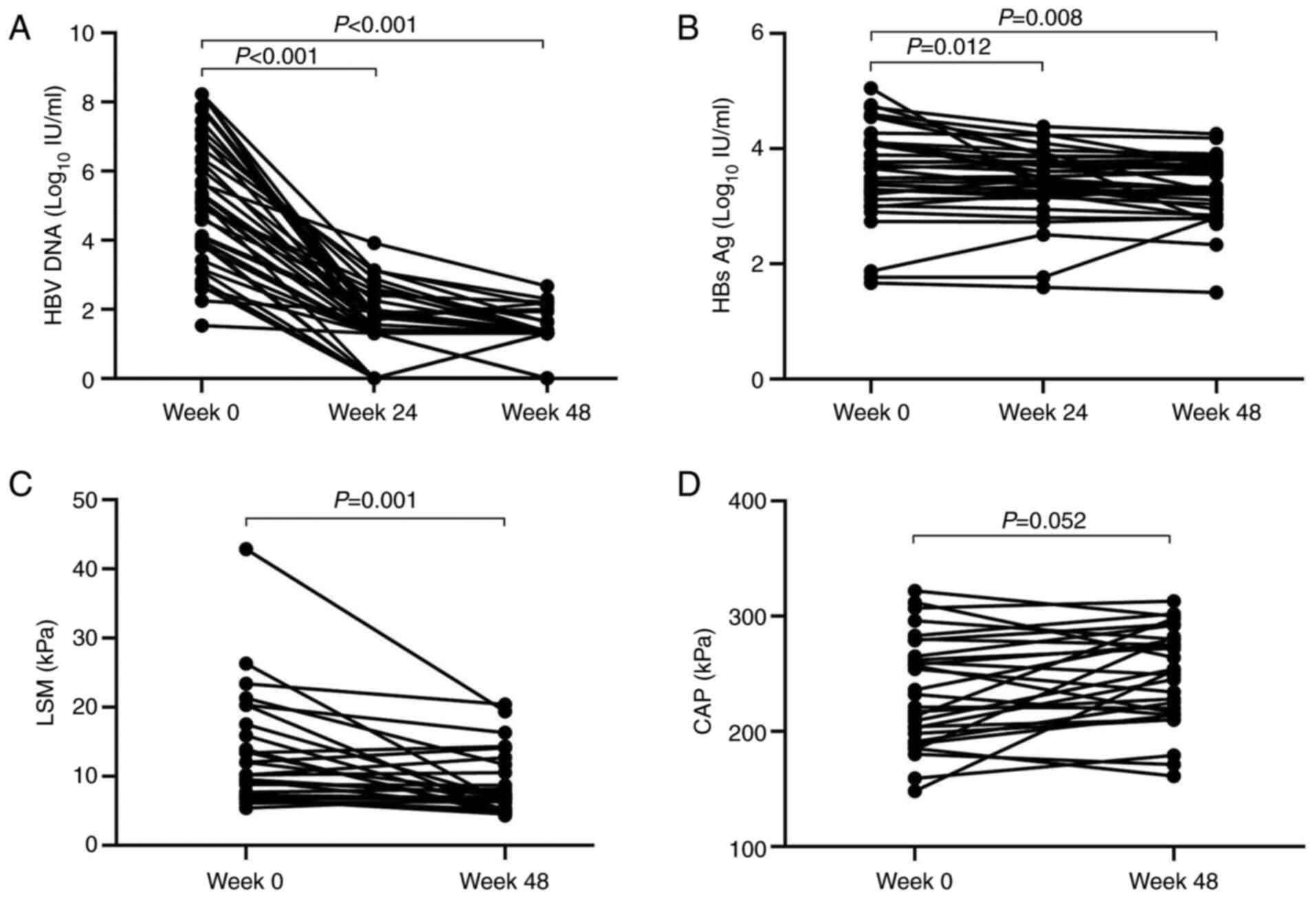
respectively (Fig. 1A). The HBsAg
level was 3.59±0.81 log10 IU/m at baseline (Table I), which reached 3.42±0.61
(P<0.05 vs. baseline) and 3.32±0.55 log10 IU/ml
(P<0.01 vs. baseline) at 24 and 48 weeks, respectively (Fig. 1B). The LSM after 48 weeks of TAF
treatment was 8.66±4.45 kPa. Compared with the baseline value of
13.00±8.15 kPa (Table I), the
difference was statistically significant (P<0.01; Fig. 1C). The CAP values before and 48
weeks after treatment were 234.62±47.38 (Table I) and 248.59±41.51 dB/m,
respectively (Fig. 1D).
Safety assessment after 48 weeks of
TAF treatment
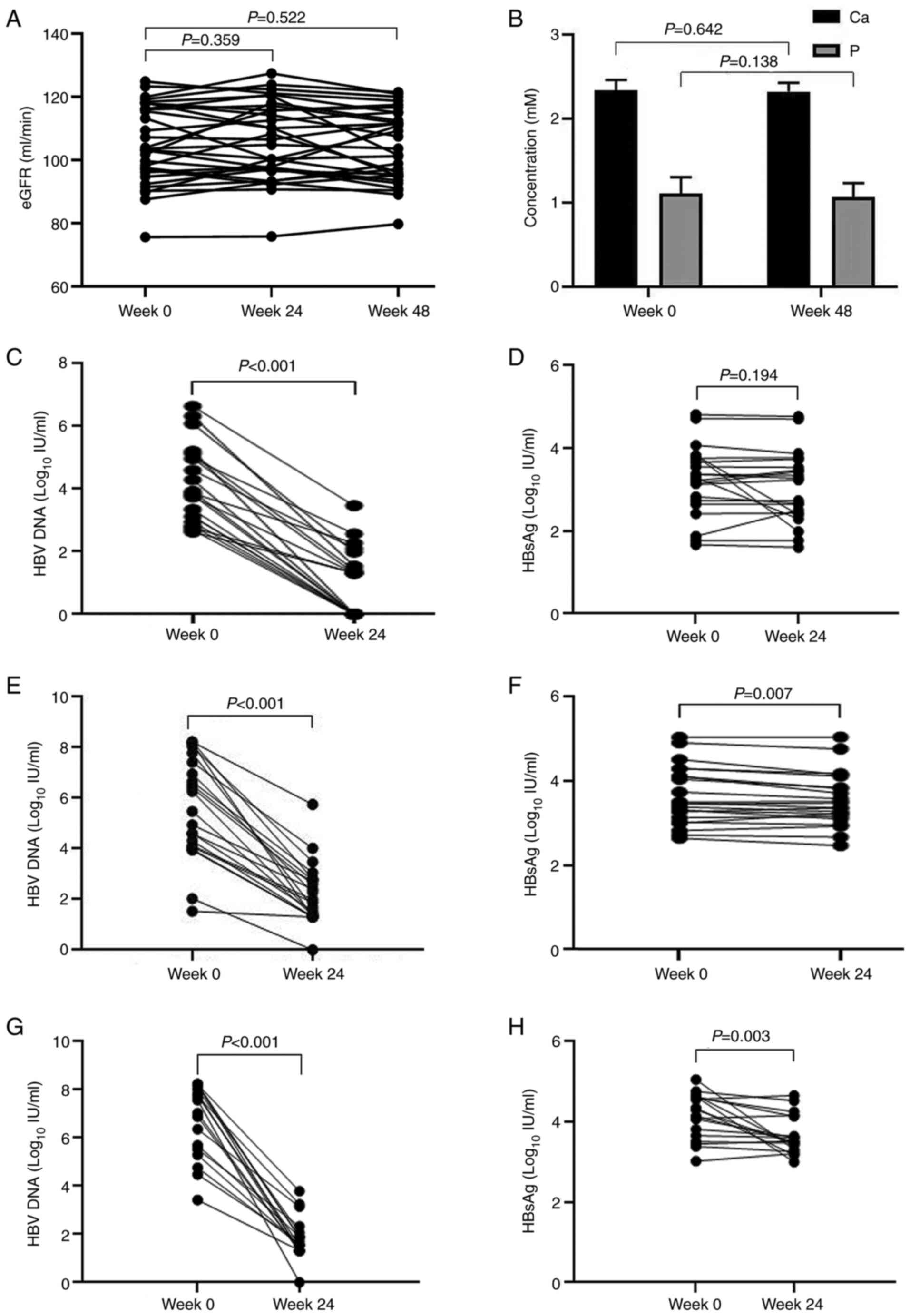
The eGFR was 105.28±12.45 ml/min/1.73 m2
at baseline (Table I), which then
reached 106.31±12.40 and 104.39±11.33 ml/min/1.73 m2 at
24 and 48 weeks, respectively (Fig.
2A). The Ca value was 2.34±0.12 (Table I) and 2.32±0.10 mmol/l at baseline
and 48 weeks, respectively (Fig.
2B). The IP value was 1.11±0.19 (Table I) and 1.07±0.16 mmol/l at baseline
and at 48 weeks, respectively (Fig.
2B).
Comparison of treatment efficacy in
the different groups according to the ALT level
In group A, which was normal at baseline for all ALT
levels, the early negative rate of HBV DNA was 52.38% (11/21) and
the HBV DNA quantification was 4.10±1.23 log10 IU/ml
before treatment and 0.97±1.07 log10 IU/ml after 24
weeks of treatment (P<0.001; Fig.
2C). After 24 weeks of treatment, the HBsAg level changed from
3.22±0.85 to 3.06±0.87 log10 IU/ml (Fig. 2D). In group B, the early
normalization rate of ALT was 84% (18/22) and the negative rate of
HBV DNA was 24% (5/22). HBV DNA quantification was 5.69±2.01
log10 IU/ml before treatment and 2.24±1.13
log10 IU/ml after 24 weeks of treatment (P<0.001;
Fig. 2E). The level of HBsAg
decreased from 3.63±0.68 to 3.53±0.63 log10 IU/ml
(P<0.01; Fig. 2F). In group C,
the normalization rate of ALT was 73.68% (13/18) and the negative
rate of HBV DNA was 26.31% (5/18) after 24 weeks of treatment. HBV
DNA quantification was 6.79±1.56 log10 IU/ml before
treatment and 1.89±0.77 log10 IU/ml after 24 weeks of
treatment (P<0.001; Fig. 2G).
The levels of HBsAg decreased from 4.15±0.57 to 3.66±0.48
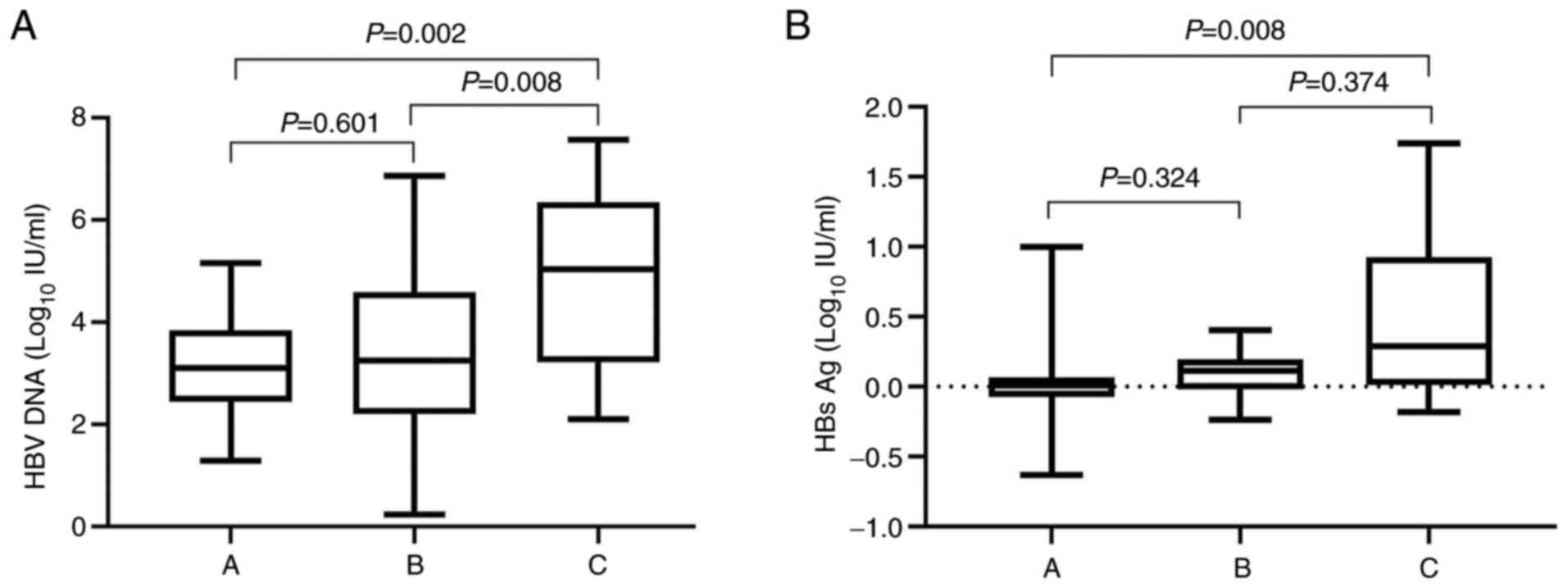
log10 IU/ml (P<0.01; Fig. 2H). The decreased levels of HBV DNA
among three groups were as follows: Group A vs. group B (P=0.601),
group A vs. group C (P=0.002) and group B vs. group C (P=0.008).
The decreased levels of HBsAg among three groups were as follows:
Group A vs. group B (P=0.324), group A vs. group C (P=0.008) and
group B vs. group C (P=0.374). In addition, compared with group A
and group B, levels of HBV DNA and HBsAg were increased by the most
significant extent in group C before and 24 weeks after treatment
(P<0.05; Fig. 3A and B).
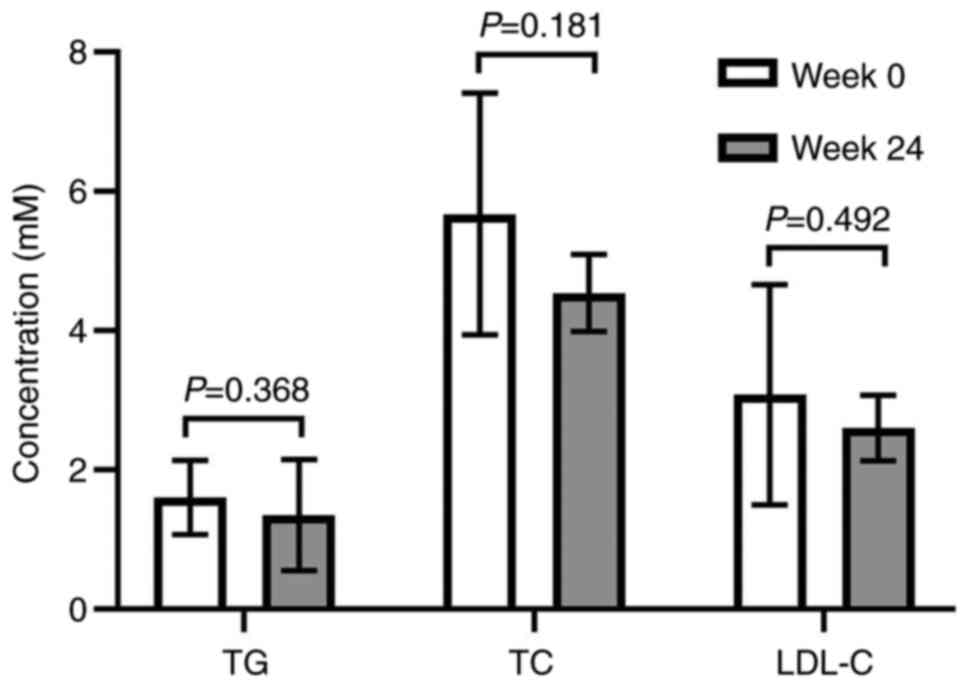
After 48 weeks of TAF treatment, the TG levels
(1.35±0.80 mmol/l) were decreased compared with the baseline value
(1.60±0.53 mmol/l), whereas the TC level (4.54±0.55 mmol/l) was
decreased compared with the baseline value (5.68±1.74 mmol/l) and
the LDL-C level (2.60±0.47 mmol/l) was decreased compared with the
baseline value (3.08±1.58 mmol/l). However, no statistical
significance was observed (Fig.
4).
Discussion
HBV infection remains a global health concern due to
its high incidence and mortality rates. According to the estimates
made by the World Health Organization (WHO) in 2016, there were
~292 million individuals infected with HBV worldwide (7). There is sufficient evidence to
indicate that CHB is a major cause of liver failure and
hepatocellular carcinoma (8,9). To
reduce the risk of HBV infection, Anti-hepatitis B vaccinations and
perinatal antiviral treatment, which are effective and safe
(10), have been applied. TAF is a
phosphonamide prodrug of tenofovir that is converted into its
corresponding active metabolite tenofovir diphosphate, which is
effective against both HBV and HIV infection (4,11).
In view of the shortest marketing time of TAF in China, the lack of
RWD derived from the medical environment and insufficient
information reflecting the actual diagnosis, treatment process and
health status of patients available, the present study aimed to
investigate the efficacy of TAF in patients with CHB. The majority
of data on the efficacy and safety of TAF in patients with chronic
HBV infection were obtained from global multicenter, randomized,
double-blind, controlled studies (12-14).
In the present study, results after 24 and 48 weeks
of TAF treatment revealed that the ALT normalization and complete
virological response rates were gradually increased as the duration
of TAF treatment increased, where the HBeAg seroconversion rate was
8.57% at 48 weeks, similar to 10% reported in a previous study on
TAF (4). In addition, the ALT
normalization rate of ALT after 24 weeks of TAF treatment was only
52.46%, which was <81.8% (13)
but increased to 95.24% at 48 weeks, which was higher compared with
72% and 83% reported by previous TAF global phase III clinical
trials (6,8). In Study 108, 425 patients with CHB
who were tested HBeAg-negative were enrolled, whilst 873 patients
tested positive for HBeAg were enrolled in Study 110(8). It was hypothesized that the reason
for the above situation was that the cases in the present study
were derived from the ‘real world’, where some patients with
abnormal liver function had unstable transaminase activity during
the early stages of antiviral treatment. As the antiviral treatment
time increases, HBV DNA levels were reduced, where some patients
may have taken hepatoprotective drugs, resulting in a significant
increase in the normalization rate of ALT at 48 weeks.
In the present study, in which patients with
HBeAg-positive CHB accounted for 73.8% of the cases, the HBV DNA
response rate (HBV-DNA <20 IU/ml) at 24 weeks of TAF treatment
was 39.34%, which was higher compared with the value of 36.4%
obtained in the study by Ibrahim et al (15). Considering HBV DNA response rate
was HBV-DNA <10 IU/ml in the study by Ibrahim et al
(15), this was likely due to the
different sensitivities of the tests used for HBV DNA detection
between the two studies. In addition, the proportion of
HBeAg-positive patients in the study by Ibrahim et al
(15) was 75.4%, differed from the
present study's follow-up cases, which could have resulted in
differences in the HBV DNA level at baseline and differences in the
complete virological response rate after 24 weeks of TAF treatment.
In the present study, the seroconversion rate of HBeAg at 48 weeks
was consistent with that in Study 110 (10%) (8). Results of the present study
demonstrated that TAF effectively inhibited viral replication
whilst also alleviating liver inflammation and fibrosis, which is
consistent with findings previously reported regarding the effects
of TAF (16-19).
It was previously demonstrated that the annual incidence of
spontaneous HBsAg clearance worldwide is 1-2% (20). However, data on TDF treatment for
patients with HBeAg-positive CHB after 5 years demonstrated that
the HBsAg clearance rate is only 10% (21). In the present study, after 48 weeks
of TAF treatment, although the overall HBsAg level was
significantly lower compared with that at baseline, there was no
HBsAg clearance. Therefore, TAF could not affect the low HBsAg
clearance rate, meaning that NA would generally require long-term
treatment plans (22,23).
For patients testing positive for serum HBV DNA with
abnormally elevated levels of ALT (excluding elevated ALT levels
due to other causes), with normal ALT levels but with high-risk
factors or liver biopsy results indicating inflammation and
fibrosis, the 2019 Guidelines recommended the initiation of
antiviral therapy, which is higher than the grade of evidence and
recommendation made in the 2015 version Guidelines (24,25).
Previous studies performed a large number of histopathological
examinations on the liver tissues from patients with CHB with
normal ALT levels, which found varying degrees of liver
inflammation, necrosis and fibrosis (26-28).
Therefore, by comparing changes in the HBV DNA and HBsAg levels
among the normal ALT (group A) and abnormal ALT (group B and group
C) groups, the present study found that TAF exerted a potent
inhibitory effect on viral replication in the different subgroups.
Additionally, these aforementioned results also confirmed that ALT
levels did not affect the inhibitory effects of TAF on HBV DNA,
which was consistent with previous findings (29). Although TDF and TAF are most
effective in both HBeAg-positive and -negative populations, TAF is
more effective in general (29).
Although abnormal ALT is no longer the most important indication
for antiviral treatment in the new guidelines (4), it was found that the higher the ALT
levels during the course of TAF treatment, the larger the decrease
observed in the HBV DNA and HBsAg levels. It is considered that the
increase in ALT levels may affect the process of HBV clearance
(30). Therefore, it is suggested
that high levels of inflammation during early stages of antiviral
therapy may lead to improved virological inhibition and immune
clearance. However, whether the more potent liver-targeting effects
of TAF will lead to potential immune regulation warrants further
investigation. Tong et al (31) reported that low levels of HBV DNA
(<2,000 IU/ml) and HBsAg (<1,000 IU/ml) were the basic
criteria for defining low risk of hepatocellular carcinoma. Results
of the present study demonstrated that TAF potently reduced HBV DNA
and HBsAg levels in 24 weeks, particularly in the population with
elevated ALT levels. Therefore, long-term observation of the
effects of TAF treatment on the risk of hepatocellular carcinoma
may become the focus of further research.
Considering the good stability of TAF in plasma and
the high efficiency of hepatocyte-targeted delivery, only a
<1/10 of the TDF dose (300 mg) required can achieve similar
antiviral effects (6,8). According to data from a previous
clinical trial (32), this
difference with TDF may improve the safety of TAF, particularly in
patients with abnormal renal function and bone mineral density. A
recent study (33) also confirmed
that TAF treatment significantly reduced the incidence of renal
adverse events during long-term antiviral treatment, which is
almost consistent with the results of the present study. The eGFR,
Ca and IP exhibited no significant changes before and after TAF
treatment, suggesting that TAF exerted little to no nephrotoxicity
after short-term treatment. A previous study demonstrated that
transforming TDF into TAF significantly improved the eGFR of
patients (34), which further
supports the renal safety of TAF. Although previous studies have
indicated that the conversion from TDF to TAF can increase blood
lipid and TC levels due to the increasing LDL-C, the ratio of
TC/high-density lipoprotein remain unaltered (21,35).
The present study demonstrated that TAF treatment did not affect
blood lipid levels or the degree of hepatic steatosis within 48
weeks. Therefore, whether TAF may increase the degree of hepatic
steatosis require further investigation.
In conclusion, as demonstrated by the findings of
the present study, TAF is effective and safe for NA-naive patients
with CHB, leading to a higher biochemical and virological response
rate, decreased HBsAg levels and improved liver fibrosis with a
stable renal safety profile. Further analysis revealed that the
higher the baseline ALT levels, the more significant the reduction
in HBV DNA and HBsAg levels after 24 weeks. However, due to the
small sample size and short follow-up time, the data may be biased
and the efficacy of TAF cannot be evaluated more objectively. In
addition, renal tubular and bone indicators were not evaluated,
which renders it impossible to fully evaluate the safety of TAF. At
present, in terms of RWD, particularly in China, there remains to
be a lack of clinical randomized, double-blind, controlled studies
on TAF with large sample sizes. Therefore, difficulty remains to
fully address the efficacy and potential safety problems of TAF.
Therefore, further clinical research is required to provide
sufficient evidence on the efficacy and safety of TAF.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by Fund of Health
Commission of Tianjin, China (grant no. TQGB20210175).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YZ, WW and PC were responsible for the conception
and design of the study. WK and FL were responsible for acquisition
of data. BJ, LJ and HL were responsible for data interpretation.
All authors have read and approved the final manuscript. PC and WK
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of Tianjin Second People's Hospital (Tianjin, China), was
performed according to the principles of the Declaration of
Helsinki and all patients provided written informed consent prior
to participation in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Agarwal K, Brunetto M, Seto WK, Lim YS,
Fung S, Marcellin P, Ahn SH, Izumi N, Chuang WL, Bae H, et al:
GS-US-320-0110; GS-US-320-0108 Investigators. 96 weeks treatment of
tenofovir alafenamide vs. tenofovir disoproxil fumarate for
hepatitis B virus infection. J Hepatol. 68:672–681. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hou J, Ning Q, Duan Z, Chen Y, Xie Q, Wang
FS, Zhang L, Wu S, Tang H, Li J, et al: GS-US-320-0110 and
GS-US-320-0108 China Investigators: 3-year Treatment of Tenofovir
Alafenamide vs. Tenofovir Disoproxil Fumarate for Chronic HBV
Infection in China. J Clin Transl Hepatol. 9:324–334.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Agarwal K, Fung SK, Nguyen TT, Cheng W,
Sicard E, Ryder SD, Flaherty JF, Lawson E, Zhao S, Subramanian GM,
et al: Twenty-eight day safety, antiviral activity, and
pharmacokinetics of tenofovir alafenamide for treatment of chronic
hepatitis B infection. J Hepatol. 62:533–540. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chinese Society of Infectious Diseases,
Chinese Medical Association; Chinese Society of Hepatology, Chinese
Medical Association. The guidelines of prevention and treatment for
chronic hepatitis B (2019 version). Zhonghua Gan Zang Bing Za Zhi.
27:938–961. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
5
|
Roade L, Loglio A, Borghi M,
Riveiro-Barciela M, Soffredini R, Facchetti F, di Paolo D,
Tabernero D, Lunghi G, Esteban R, et al: Application of EASL 2017
criteria for switching hepatitis B patients from tenofovir
disoproxil to entecavir or tenofovir alafenamide. Dig Liver Dis.
52:1164–1169. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Buti M, Gane E, Seto WK, Chan HL, Chuang
WL, Stepanova T, Hui AJ, Lim YS, Mehta R, Janssen HL, et al:
GS-US-320-0108 Investigators: Tenofovir alafenamide versus
tenofovir disoproxil fumarate for the treatment of patients with
HBeAg-negative chronic hepatitis B virus infection: A randomised,
double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol
Hepatol. 1:196–206. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Buti M, Roade L, Riveiro-Barciela M and
Esteban R: Optimal management of chronic hepatitis B patients
receiving nucleos(t)ide analogues. Liver Int. 40 (Suppl 1):15–21.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chan HL, Fung S, Seto WK, Chuang WL, Chen
CY, Kim HJ, Hui AJ, Janssen HL, Chowdhury A, Tsang TY, et al:
GS-US-320-0110 Investigators: Tenofovir alafenamide versus
tenofovir disoproxil fumarate for the treatment of HBeAg-positive
chronic hepatitis B virus infection: A randomised, double-blind,
phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol.
1:185–195. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chang TT, Lai CL, Kew Yoon S, Lee SS,
Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N, et
al: Entecavir treatment for up to 5 years in patients with
hepatitis B e antigen-positive chronic hepatitis B. Hepatology.
51:422–430. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Funk AL, Lu Y, Yoshida K, Zhao T,
Boucheron P, van Holten J, Chou R, Bulterys M and Shimakawa Y:
Efficacy and safety of antiviral prophylaxis during pregnancy to
prevent mother-to-child transmission of hepatitis B virus: A
systematic review and meta-analysis. Lancet Infect Dis. 21:70–84.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chao DT, Lim JK, Ayoub WS, Nguyen LH and
Nguyen MH: Systematic review with meta-analysis: The proportion of
chronic hepatitis B patients with normal alanine transaminase ≤40
IU/L and significant hepatic fibrosis. Aliment Pharmacol Ther.
39:349–358. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Razavi-Shearer D, Gamkrelidze I, Nguyen
MH, Chen D-S, Van Damme P, Abbas Z, Abdulla M, Abou Rached A, Adda
D, Aho I, et al: Polaris Observatory Collaborators: Global
prevalence, treatment, and prevention of hepatitis B virus
infection in 2016: A modelling study. Lancet Gastroenterol Hepatol.
3:383–403. 2018.
|
|
13
|
Ogawa E, Furusyo N and Nguyen MH:
Tenofovir alafenamide in the treatment of chronic hepatitis B:
Design, development, and place in therapy. Drug Des Devel Ther.
11:3197–3204. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hou JL, Gao ZL, Xie Q, Zhang JM, Sheng JF,
Cheng J, Chen CW, Mao Q, Zhao W, Ren H, et al: Tenofovir disoproxil
fumarate vs adefovir dipivoxil in Chinese patients with chronic
hepatitis B after 48 weeks: A randomized controlled trial. J Viral
Hepat. 22:85–93. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ibrahim F, Campbell L, Bailey AC,
Stockwell S, Waters L, Orkin C, Johnson M, Gompels M, De
Burgh-Thomas A, Jones R, et al: Estimated glomerular filtration
rate slopes on tenofovir alafenamide. HIV Med. 21:607–612.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liang X, Gao Z, Xie Q, Zhang J, Sheng J,
Cheng J, Chen C, Mao Q, Zhao W, Ren H, et al: Long-term efficacy
and safety of tenofovir disoproxil fumarate in Chinese patients
with chronic hepatitis B: 5-year results. Hepatol Int. 13:260–269.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lampertico P, Agarwal K, Berg T, Buti M,
Janssen HLA, Papatheodoridis G, Zoulim F and Tacke F: European
Association for the Study of the Liver. Electronic address:
easloffice@easloffice.eu; European Association for the Study of the
Liver. EASL 2017 Clinical Practice Guidelines on the management of
hepatitis B virus infection. J Hepatol. 67:370–398. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Marcellin P, Buti M, Krastev Z, de Man RA,
Zeuzem S, Lou L, Gaggar A, Flaherty JF, Massetto B, Lin L, et al:
Kinetics of hepatitis B surface antigen loss in patients with
HBeAg-positive chronic hepatitis B treated with tenofovir
disoproxil fumarate. J Hepatol. 61:1228–1237. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Marcellin P, Gane E, Buti M, Afdhal N,
Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF,
Aguilar Schall R, et al: Regression of cirrhosis during treatment
with tenofovir disoproxil fumarate for chronic hepatitis B: A
5-year open-label follow-up study. Lancet. 381:468–475.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Milinkovic A, Berger F, Arenas-Pinto A and
Mauss S: Reversible effect on lipids by switching from tenofovir
disoproxil fumarate to tenofovir alafenamide and back. AIDS.
33:2387–2391. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mills A, Arribas JR, Andrade-Villanueva J,
DiPerri G, Van Lunzen J, Koenig E, Elion R, Cavassini M, Madruga
JV, Brunetta J, et al: Switching from tenofovir disoproxil fumarate
to tenofovir alafenamide in antiretroviral regimens for
virologically suppressed adults with HIV-1 infection: a randomised,
active-controlled, multicentre, open-label, phase 3,
non-inferiority study. Lancet Infect Dis. 16:43–52. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Roade L, Riveiro-Barciela M, Esteban R and
Buti M: Long-term efficacy and safety of nucleos(t)ides analogues
in patients with chronic hepatitis B. Ther Adv Infect Dis.
8(2049936120985954)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hou J, Wang G, Wang F, Cheng J, Ren H,
Zhuang H, Sun J, Li L, Li J, Meng Q, et al: Chinese Society of
Hepatology, Chinese Medical Association; Chinese Society of
Infectious Diseases, Chinese Medical Association. Guideline of
Prevention and Treatment for Chronic Hepatitis B (2015 Update). J
Clin Transl Hepatol. 5:297–318. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jia JD, Hou JL, Wei L and Zhuang H:
Highlights of the guidelines of prevention and treatment for
chronic hepatitis B (2019 version). Zhonghua Gan Zang Bing Za Zhi.
28:21–23. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
26
|
Sax PE, Wohl D, Yin MT, Post F, DeJesus E,
Saag M, Pozniak A, Thompson M, Podzamczer D, Molina JM, et al:
GS-US-292-0104/0111 Study Team: Tenofovir alafenamide versus
tenofovir disoproxil fumarate, coformulated with elvitegravir,
cobicistat, and emtricitabine, for initial treatment of HIV-1
infection: Two randomised, double-blind, phase 3, non-inferiority
trials. Lancet. 385:2606–2615. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang J, Du X, Zhou Z, Lv F and Yu Y:
Spleen thickness can predict significant liver pathology in
patients with chronic hepatitis B with persistently normal alanine
aminotransferase or minimally raised alanine aminotransferase: A
retrospective study. J Int Med Res. 47:122–132. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nguyen LH, Chao D, Lim JK, Ayoub W and
Nguyen MH: Histologic changes in liver tissue from patients with
chronic hepatitis B and minimal increases in levels of alanine
aminotransferase: A meta-analysis and systematic review. Clin
Gastroenterol Hepatol. 12:1262–1266. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Terrault NA, Lok ASF, McMahon BJ, Chang
KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH and Wong JB: Update
on prevention, diagnosis, and treatment of chronic hepatitis B:
AASLD 2018 hepatitis B guidance. Hepatology. 67:1560–1599.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang H, Luo H, Wan X, Fu X, Mao Q, Xiang
X, Zhou Y, He W, Zhang J, Guo Y, Tan W and Deng G: TNF-α/IFN-γ
profile of HBV-specific CD4 T cells is associated with liver damage
and viral clearance in chronic HBV infection. J Hepato. l72:45–56.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tong MJ, Pan CQ, Han SB, Lu DS, Raman S,
Hu KQ, Lim JK, Hann HW and Min AD: An expert consensus for the
management of chronic hepatitis B in Asian Americans. Aliment
Pharmacol Ther. 47:1181–1200. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wong WWL, Pechivanoglou P, Wong J,
Bielecki JM, Haines A, Erman A, Saeed Y, Phoon A, Tadrous M, Younis
M, et al: Antiviral treatment for treatment-naïve chronic hepatitis
B: Systematic review and network meta-analysis of randomized
controlled trials. Syst Rev. 8(207)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tao X, Lu Y, Zhou Y, Zhang L and Chen Y:
Efficacy and safety of the regimens containing tenofovir
alafenamide versus tenofovir disoproxil fumarate in fixed-dose
single-tablet regimens for initial treatment of HIV-1 infection: A
meta-analysis of randomized controlled trials. Int J Infect Dis.
93:108–117. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhou K, Contag C, Whitaker E and Terrault
N: Spontaneous loss of surface antigen among adults living with
chronic hepatitis B virus infection: A systematic review and pooled
meta-analyses. Lancet Gastroenterol Hepatol. 4:227–238.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Huhn GD, Wilkin A, Mussini C, Spinner CD,
Jezorwski J, El Ghazi M, Van Landuyt E, Lathouwers E, Brown K and
Baugh B: AMBER and EMERALD study groups. Week 96 subgroup analyses
of the phase 3, randomized AMBER and EMERALD trials evaluating the
efficacy and safety of the once daily
darunavir/cobicistat/emtricitabine/tenofovir alafenamide
(D/C/F/TAF) single-tablet regimen in antiretroviral treatment
(ART)-naïve and -experienced, virologically-suppressed adults
living with HIV-1. HIV Res Clin Pract. 21:151–167. 2021.PubMed/NCBI View Article : Google Scholar
|