Introduction
Adipose tissue which is widespread in humans is
comprised of 50% of completely developed adipocytes (1) while the remaining 50% of adipose
tissue consists of a complex population of immature adipocytes,
proinflammatory cells (macrophages and lymphocytes) and fully
differentiated cells such as endothelial and nerves cells. Two
major adipose tissue varieties are described, with different cell
phenotype and functions: white adipose tissue (WAT) and brown
adipose tissue (BAT), the latter being well developed in newborns
and small mammals, with its major function being thermoregulation
as well as playing an anti-obese role (2-5).
As a result of the heterogeneity of their structure
and function, adipose tissues are now considered to form a complex
organ provided with metabolic, endocrine and immunologic functions
(6-8).
The normal metabolic functions of WAT and their disruption involve
a mediated crosstalk between adipocytes and cells from the
stromo-vascular fraction (SVF). This crosstalk is made possible by
the active secretion of adipokines or adipocytokines from the
adipose tissue with metabolic and pro/anti-inflammatory effects
(9). As a consequence, there is a
direct link between obesity, cardiovascular diseases and in the
last 10 years cancer development (1,10,11).
The development of metabolic syndrome and cancer are
not dependent on the total amount of WAT in an organism, but rather
on the distribution of the fat depots (7,12).
Visceral adipose tissue accumulation leading to central obesity is
accepted as a source of inflammatory molecules as well as
‘oncogenic fat’ (11), whereas
subcutaneous adipose tissue is usually considered to be protective
(13). The list of central
obesity-related cancers is in active extension, most of which are
hosted in the visceral area (gastric, endometrial, ovarian,
colorectal, prostatic, renal, liver, gallbladder or esophageal)
(11,14,15).
Two main mechanisms connect visceral fat to visceral cancer: i) the
dysregulation of metabolism (through the increase in insulin
secretion and insulin growth factor synthesis in WAT) (16) and ii) mild chronic inflammation
(17,18).
At present, two important findings connect
undoubtedly visceral fat to visceral cancer: the most common site
for ovarian cancer metastasis is the epiploon, and primary human
omental adipocytes induce in vitro cell proliferation and
invasion for the majority of visceral tumors (19,20).
Even if cancer-associated adipocytes are demonstrated to supply
energy for tumor development, the role of visceral adipose tissue
from obese or lean subjects in priming tumor development is still
under debate.
The adipose tissue is one of the most plastic organs
in adults gifted with the ability of continuous remodeling,
expanding or retracting according to the energetic balance. Being a
compulsory condition for the expansion of any tissue in order to
provide the substances requested for its survival, angiogenesis may
be considered in a mechanistic approach, a common feature of
adipose and tumor tissue. WAT secretome includes various
pro-angiogenic hormones, cytokines, and growth factors secreted by
the stromo-vascular cells, namely endothelial cells, and also by
adipocytes and preadipocytes (21-23).
Visceral WAT is more vascularized (24). It expresses more adipokines with
angiogenic activity, endothelial cells exhibit more potent
angiogenic molecules and newly emerging adipocytes in obese
conditions are highly angiogenic (25). These are added to the substances
elaborated by the proinflammatory and mesenchymal cells resident in
the stromovascular fraction of the adipose depots, mainly growth
factors (26-28).
Adipokines lead to the establishment of a proangiogenic milieu.
However, it is not clear whether all the substances
secreted by the adipose tissue may have a paracrine or an endocrine
effect on visceral tumor initiation and progression, but the
proangiogenic factors may be truly incriminated, as angiogenesis is
a major common event required both for tumor and adipose
development.
Progranulin (PRG), also known as granulin
(GRN)-epithelin precursor (29),
proepithelin, (30) or acrogranin
(31), is a novel pleiotropic
growth factor acting on the proliferation and development of
fast-growing epithelial cells, cancer cells (32). It is also a proangiogenic factor
whose expression is induced in activated endothelial cells
(33) or in growing placenta
(34). PRG, recently identified as
an adipokine (7), is a 68- to
86-kDa secreted glycoprotein which can be fragmented by some matrix
metalloproteinases (MMPs) into small homologous subunits,
granulins/epithelins (35,36), with proinflammatory activity.
Previous findings emphasize the association between PRG and type 2
diabetes as well as non-fatty liver disease, and also, its function
in promoting vasodilatation (37).
Some studies demonstrated the connection between PRG, vascular
endothelial growth factor (VEGF) expression and the augmentation of
vascular density in breast cancer (38,39).
VEGF is the most potent angiogenic factor for tumor development and
invasion (40,41) and it is secreted by adipocytes as a
proangiogenic factor stimulating the migration and proliferation of
endothelial cells (23,42,43).
The aim of the present study was to examine the
possible relationship between PRG and VEGF in adipose visceral
tissue under tumorigenic or inflammatory conditions. An
immunohistochemical study was conducted to quantify the expression
of the aforementioned markers. In order to assess the degree of
maturation and functional differentiation of the vascular network
in visceral (omental) fat related to PRG and VEGF proangiogenic
activity, an immunohistochemical identification of vessels with
CD34, and collagen IV (Col IV) was performed.
Materials and methods
Tissue samples
Human adipose tissues were obtained after abdominal
surgery for visceral pathology. The subjects were included into two
groups: Group I included subjects with non-tumoral pathology (NTP)
(n=30): 15 patients with inflammatory pathology (gastric ulcer,
angiocolitis, peritonitis, pancreatitis) and 15 patients with
obstructive mechanical pathology (hernia, eventration) and all 15
subjects of the NTP group were obese; Group II included subjects
with tumoral pathology (TP): 15 patients suffering from visceral
cancer (stomach, liver, ovary, gut) from which 3 were obese.
Obese patients were those with abdominal obesity
(Ø>94 cm in men and >80 cm in women) and a body mass index
(BMI) >30, according to the criteria of the International
Federation of Diabetes (44). BMI
was calculated as weight divided by the height squared
(kg/m2).
Informed consent was obtained from all the
participants included in the study, which was approved by the
Ethics Committee of the University of Medicine and Pharmacy of
Craiova (no. 85/2019).
For each subject a fragment of omental adipose
tissue was obtained and rinsed in saline solution.
Histological staining
Immediately after sampling, the tissue fragments
were fixed in 10% buffered formalin for 24-48 h at room temperature
and then processed for paraffin embedding. Sections of 3-4 µm were
mounted on a glass slide and routinely stained with hematoxylin and
eosin (H&E), and trichrome Masson according to the producer's
data sheet (Bio-Optica).
Immunohistochemistry
Serial sections of 3-4 µm were dewaxed and
rehydrated. Antigen retrieval was performed after microwave
incubation of sections in citrate buffer at pH 6.0, for 20 min.
Endogenous peroxidase was blocked after incubation with 3% hydrogen
peroxide in methanol solution. After blocking, non-specific binding
with 3% skimmed milk in PBS at pH 7.4-7.6 for 30 min, the sections
were incubated overnight, at 4ºC, with one of the
primary antibodies mentioned in Table
I. Sections were then rinsed in PBS and processed for
amplification of the immune signal using a detection labeling
polymer [Histofine® Simple Stain™ MAX PO
(MULTI) 414151 Nichirei] as mentioned in Table I. The immunostaining was detected
with 3,3'-diaminobenzidine (Vector Laboratories), and finally the
nuclear counterstaining was performed with Mayer's hematoxylin
(Bio-Optica). Skimmed milk, the chemicals used for buffers,
solvents and hydrogen peroxide were purchased from Merck.
 | Table IAntibodies and immunohistochemical
techniques used for the morphological study. |
Table I
Antibodies and immunohistochemical
techniques used for the morphological study.
| Antibody | Dilution | Source | Antigen
retrieval | Amplification
method |
|---|
| Monoclonal
anti-human VEGF | 1:20 | Thermo Fisher
Scientific, Inc. JH121 | Citrate buffer pH
6.0 | HISTOFINE Simple
Stain MAX PO (MULTI) 414151 Nichirei |
| Monoclonal
anti-human collagen IV | 1:30 | Dako M0785 | Citrate buffer pH
6.0 | HISTOFINE Simple
Stain MAX PO (MULTI) 414151 Nichirei |
| Monoclonal
anti-human progranulin | 1:20 | Thermo Fisher
Scientific, Inc. 2D4-2F1 | Citrate buffer pH
6.0 | HISTOFINE Simple
Stain MAX PO (MULTI) 414151 Nichirei |
| Monoclonal
anti-human CD34 | 1:50 | Dako M7080 | Citrate buffer pH
6.0 | HISTOFINE Simple
Stain MAX PO (MULTI) 414151 Nichirei |
For each antibody tested, a negative control was
performed in which the primary antibody was replaced by 10 mM PBS,
pH 7.4-7.6.
The sections were examined and images were captured
with a Nikon Eclipse 55i microscope equipped with a 5 Mp
color-cooled CCD (charge-coupled device) camera, under the Image
ProPlus 7 AMS image analysis software (Media Cybernetics Inc.).
Each immunostaining section for the antibodies
mentioned above was examined by two different researchers according
to the following: immunohistochemical (IHC) reactions (brown
deposits in labeled structures) were graded as absent (negative
signal) or present (moderate or strong intensity of the signal).
Images were finally processed using the Microsoft Office Picture
Manager.
Morphometry
After general examination of the sections, for each
subject and each histological or IHC-stained slide with the
antibodies mentioned above, three different microscopic fields on
the same section were chosen randomly in the area of interest
captured with a x20 objective lens and examined by two different
researchers. A count of the vessels marked by tagging manually on
the field was performed by two researchers and the averages were
used as primary values in the statistical analysis.
Statistical analysis
Statistical analysis was performed using Microsoft
Excel (Microsoft Corp.), together with the XLSTAT add-on for MS
Excel (Addinsoft SARL) and IBM SPSS Statistics 20.0 (IBM Corp.) for
processing the data. Data were recorded using Microsoft Excel
files, then the data were statistically analyzed to determine the
relationship between histological parameters of the patients. A
descriptive analysis of the study group (percentages of cases for
categorical date, mean and standard deviation for numerical data)
and complex statistical tests (Chi square and Fisher's exact test,
Student's t-test) were performed using the abovementioned
statistical software.
To test the normality of the data for the parameters
involved in this study (number of blood vessels counted using
different histological markers) the Anderson-Darling test was used.
As the numerical variables investigated had a normal distribution
of data, globally or inside each studied group, parametric
statistical tests such as the Student's t-test were employed and
the results are summarized as the mean value ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Using the Pearson's ‘r’ correlation coefficient for
two normally distributed data sets (or linear correlation
coefficient), which measures the degree of relationship between two
variables, the relationship between PRG and VEGF for the two groups
of patients was analyzed. The small number of obese subjects in the
TP group prevented us from performing a statistically significant
correlation between lean and obese subjects for all the
markers.
Results
Hematoxylin and eosin or trichrome
staining
Histological staining with hematoxylin and eosin or
trichrome showed the characteristic distribution of adipose cells
in the adipose lobules. In the samples from the obese subjects a
pronounced heterogeneity of adipose cell dimension was observed
(Fig. 1A).
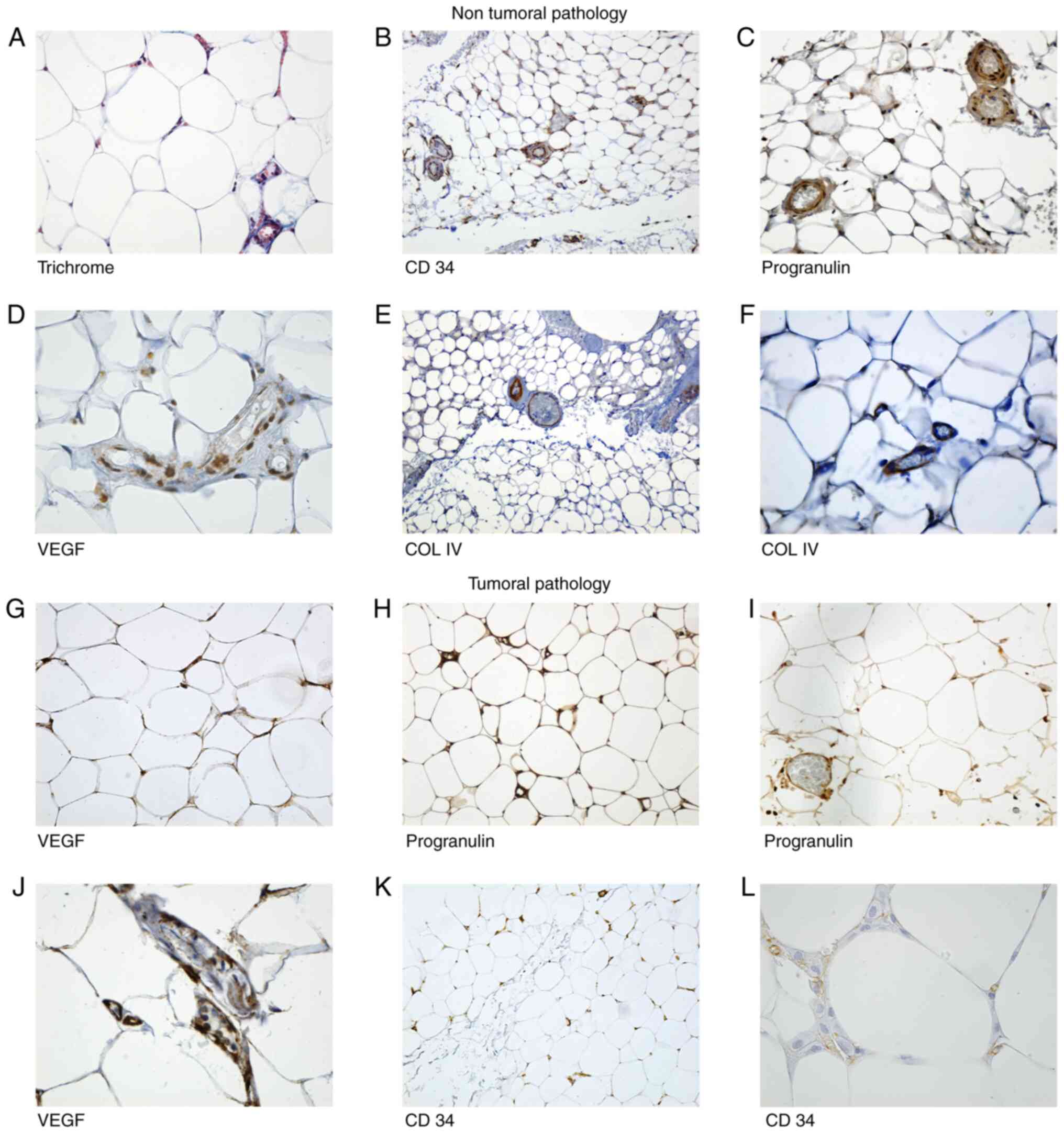 | Figure 1Microscopic aspects of the adipose
tissue sections from patients with non-tumoral pathology (NTP)
group and tumoral pathology (TP) group. Microscopic aspects of the
adipose tissue sections from patients with non-tumoral pathology
(NTP) group (A-F): (A) Trichrome. Adipose tissue from epiploon,
inflammatory cells were present in vessels and in stromo-vascular
fraction (SVF), intermingled with the adipocytes. Magnification,
x400. (B) CD34. General view of an adipose lobule: double positive
concentric rings are noted around the vessels. Magnification, x100.
(C) PRG. Positive cells for PRG in vessels and in SVF.
Magnification, x20. (D) VEGF. Endothelial positivity for VEGF in
SVF cells and in endothelial cells. Magnification, x40. (E) Col IV.
General aspect of an adipose pad, vessels on the periphery are
positive for Col IV. Magnification, x45. (F) Col IV. Few
capillaries inside of the adipose lobule immunolabeled with Col IV.
Magnification, x400. (G-L) Microscopic aspects of the adipose
tissue sections from patients with tumoral pathology (TP group):
(G) VEGF was positive in endothelial cells and in mature
adipocytes. Magnification, x400. (H) Adipose cells and capillaries
with intense reaction for PRG. Magnification, x200. (I) PRG intense
positive reaction in vessels. Magnification, x400. (J) VEGF.
Positive reaction in vessels walls. Magnification, x400. (K) CD34.
Numerous vessels positive for CD34. Magnification, x100. (L) CD34.
New forming vessels and stromal cells positive for CD34.
Magnification, x400. SVF, stromo-vascular fraction; PRG,
progranulin; VEGF, vascular endothelial growth factor; Col IV,
collagen IV. |
The Student's t-test did not reveal any significant
difference between patients with mechanical or inflammatory
pathology and those with tumor pathology, in terms of the number of
capillaries identified by trichrome or hematoxylin and eosin
staining (P=0.0787) (Table
II).
 | Table IINumber of capillaries identified with
usual histological staining. |
Table II
Number of capillaries identified with
usual histological staining.
| Pathology vs.
Trichrome/H&E | Non-tumoral
pathology | Tumoral
pathology |
|---|
| No. of cases | 30 | 15 |
| Mean | 28.23 | 32.47 |
| SD | 8.16 | 5.64 |
| C.V. (%) | 28.89% | 17.38% |
| P-value (Student's
t-test) | 0.0787 | NS |
Each lobule contained some middle vessels
(arterioles or venules) and many very small capillaries (new
capillaries) around the adipose cells (Fig. 1A). All these vessels were
consistently marked with CD34 in inflammatory and in tumoral
pathology (Fig. 1B and K) and inconsistently with PRG (Fig. 1C) and Col IV (Fig. 1E and F). In both groups, proinflammatory cells
were noted inside the vessel lumen, perivascular and in the SVF
between the adipose cells. In visceral adipose tissue from the TP
group, these stromal cells were largely positive for PRG and CD34
(Fig. 1H, I and L).
The result of the Student's t-test revealed a
significant difference (P=0.0106) between patients with mechanical
and inflammatory pathology (NTP) and those with tumor pathology
(TP) in terms of the number of capillaries identified by labeling
them for PRG (Table III).
 | Table IIINumber of capillaries labeled with
progranulin (PRG). |
Table III
Number of capillaries labeled with
progranulin (PRG).
| Pathology vs.
PRG | Non-tumoral
pathology | Tumoral
pathology |
|---|
| No. of cases | 30 | 15 |
| Mean | 16.17 | 22.53 |
| SD | 7.00 | 8.53 |
| C.V. (%) | 43.29% | 37.88% |
| P-value (Student's
t-test) | 0.0106 | NS |
There was no significant difference between the
number of blood vessels identified in normal-weight and obese
patients, not for inflammatory and mechanical pathologies, nor for
tumoral pathologies.
Number of capillaries
CD34 and PRG immune reaction showed a stronger
positivity deep in the lobule than in the peripheral area and PRG
was constantly present in endothelial cells of the very small
neoformation capillaries (Fig. 1H
and I). In adipose tissue samples
collected from patients of the TP group, the adipose cells also
showed a positive reaction for PRG (Fig. 1H and I). In the medium vessels, CD34-positive
cells formed a double circular layer in the endothelium and in the
adventitial lamina (Fig. 1B).
Following the Student's t-test it was found that
there was a high significant difference of the number of
capillaries labeled for CD34 (P=0.0006) between patients with
mechanical or inflammatory pathology and those with tumor pathology
(Table IV).
 | Table IVNumber of capillaries labeled with
CD34. |
Table IV
Number of capillaries labeled with
CD34.
| Pathology vs.
CD34 | Non-tumoral
pathology | Tumoral
pathology |
|---|
| No. of cases | 30 | 15 |
| Mean | 21.63 | 31.93 |
| SD | 9.39 | 7.60 |
| C.V. (%) | 43.42% | 23.80% |
| P-value (Student's
t-test) | 0.0006 | HS |
VEGF displayed an inconstant positivity in the
capillaries from the NTP group, being also present in some
proinflammatory cells (Fig. 1D).
Additionally, in the TP group all the vessels, medium and new
capillaries and proinflammatory cells were positive for VEGF
(Fig. 1J). By contrast, adipocytes
from the NTP group (Fig. 1D) and
more frequently small adipocytes from the TP group (Fig. 1G) showed positivity for VEGF.
The Student's t-test did not reveal a significant
difference between the number of blood vessels identified in
normal-weight and obese patients for the NTP group (P=0.242) but we
found significant differences for the number of blood vessels in
normal-weight and obese patients in TP group (P=0.017) (Table V).
 | Table VComparison between the number of
vessels labeled for VEGF between lean and obese patients. |
Table V
Comparison between the number of
vessels labeled for VEGF between lean and obese patients.
| | Non-tumoral
pathology | Tumoral
pathology |
|---|
| Pathology
Weight | Normal weight | Obese | Normal weight | Obese |
|---|
| No of cases | 15 | 15 | 12 | 3 |
| Mean | 20.27 | 16.07 | 25.00 | 17.67 |
| SD | 11.07 | 7.91 | 4.22 | 3.79 |
| C.V. (%) | 54.64% | 49.26% | 16.88% | 21.43% |
| P-value (Student's
t-test) | 0.242 (NS) | 0.017 (S) |
After performing the Student's t-test, a significant
difference of the number of Col IV-positive capillaries between
patients with mechanical or inflammatory pathology, i.e., NTP group
and those with tumor pathology was observed (P=0.0217) (Table VI). Any significant difference was
noted between the number of blood vessels identified in
normal-weight and obese patients neither for inflammatory and
mechanical pathologies nor for tumoral pathologies.
 | Table VINumber of capillaries labeled for
collagen IV. |
Table VI
Number of capillaries labeled for
collagen IV.
| Pathology vs.
Collagen IV | Non-tumoral
pathology | Tumoral
pathology |
|---|
| No. of cases | 30 | 15 |
| Mean | 22.83 | 17.80 |
| SD | 7.55 | 4.36 |
| C.V. (%) | 33.08% | 24.51% |
| P-value (Student's
t-test) | 0.0217 | S |
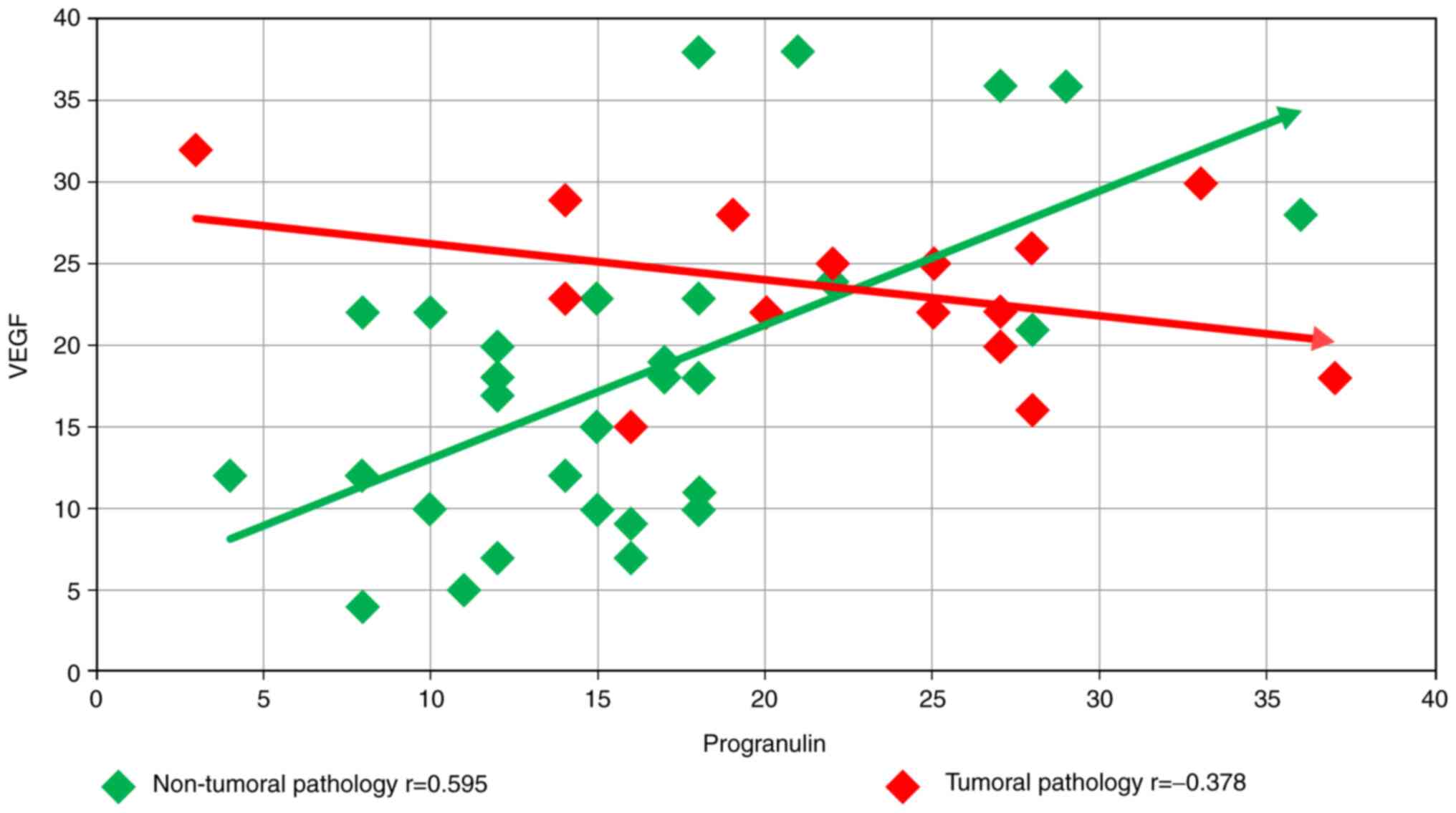
Finally, the relationship between PRG and VEGF for
the two groups of patients, NTP with inflammatory and mechanical
pathology, and TP with tumoral pathology, was examined. For the
first group, the Pearson correlation coefficient was r=0.595,
showing a statistically significant direct correlation, with a
significance level of 95% for 30 samples (P<0.05). For the
second group, the Pearson correlation coefficient was r=-0.378,
showing an inverse correlation, without having a statistical
significance for a group of 15 samples (Fig. 2).
Discussion
The development of a newly formed network of blood
vessels through the secretion of angiogenetic factors is a common
attribute of tumor growth and adipose tissue expanding in the case
of a high caloric regime. In both circumstances, the rapid growth
of blood vessels is stimulated by various tumorigenic-secreted
molecules or adipokines synthetized in excess of caloric
intake.
The results provided in the present study sustain
the direct suggestion of progranulin (PRG) as an angiogenetic
factor in omental adipose tissue in subjects developing abdominal
tumors and we compared the proangiogenic activity of PRG and VEGF
in visceral pads under inflammatory and tumoral conditions.
The results of the present study demonstrated that
the number of vessels positively marked with PRG was significantly
higher in the TP group than that noted in the inflammatory
pathology group (NTP). However, no significant difference was
observed between the lean and obese patients, for the inflammatory
and mechanical pathologies, nor for the tumoral pathologies. The
results may be due to the small number of obese patients included
in the tumoral pathology (TP) group; on the other hand, two
patients from this group declared during the medical history dialog
that they had lost weight up until the time of the surgery; thus,
it may be possible that they were obese at the onset of the
pathology. Youn et al obtained similar results for the
visceral adipose tissue from obese subjects, demonstrating that the
adipocytes and the cells from the SVF contribute to PRG expression
in adipose tissue (45).
Correlating these results with the serum level of PRG authors of
that study suggested that PRG contributes to the crosstalk between
macrophages and adipocytes in the adipose tissue and could be one
of the molecules involved in the increase of the macrophage number
in the visceral adipose tissue of obese subjects.
PRG is less expressed in quiescent vessels and more
intensely in tissue with active angiogenesis: in normal tissues
(developing placenta or wound healing) (33,34) or
tumoral diseases (46-48).
The results of the present study demonstrated that the vessels
marked with PRG were more numerous in the adipose pads of the
tumoral milieu than in the inflammatory ones, and the positivity
observed from the IHC reaction was not restricted to vessels, but
was expressed also in adipocytes and migrated cells. Thus, PRG
overexpression in the vessel walls and equally in adipocytes and
stromal cells may be induced by proangiogenic molecules synthetized
by the neighboring tumor.
The angiogenic action of PRG seems to be performed
at least under certain circumstances (such as in breast cancer and
breast cancer cell lines) (39)
under the influence of the main proangiogenic factor, VEGF. In
order to assess the collaboration between the two molecules, we
performed an IHC study for the expression of VEGF in blood vessels
of the same lots. A significant difference between patients with
mechanical and inflammatory pathology (NTP) and those with tumor
pathology (TP) in terms of the number of capillaries identified,
similar to PRG, was not evident. However, VEGF expression showed a
significant difference between lean and obese subjects in the TP
group. This aspect may be explained by the canonical model of
vascular development whereby the oncogenic vascularization and the
vascular network in adipose pads are both induced by the rapid
growth of the tissue and the onset of hypoxic conditions, with an
increase in VEGF expression (49).
As observed in the IHC reactions for PRG and VEGF,
not only the capillaries demonstrate an immune positivity for the
two molecules, but also the adipocytes and cells from the stromal
tissue. Similar results were reported by others showing that both
SVF and mature adipocytes express VEGF (24,50).
In obese subjects, when the blood supply becomes
deficient, as in all fast-growing tissues, the whole human adult
adipose tissue can induce angiogenesis from its own cells,
adipocytes or stromal cells, or can recruit distant endothelial
cells (24). In human and animal
models, it was demonstrated that VEGF is produced by adult
adipocytes and stromovascular cells (2,51).
Corroborating our results with findings by others (24,52),
an interesting hypothesis to understand the adaptation of the
vascular network density to the adipose pads volume was that the
synthesis of adipogenic factors is related to the variation of
weight rather than to the absolute weight. Our results demonstrated
that weight may influence the expression of VEGF in tumoral
pathology but not in inflammatory or mechanical diseases, and that
weight does not seem to have the same influence for PRG
expression.
The present study was carried out on a small number
of cases, and must therefore be considered a pilot study. Research
on a larger group of patients should be conducted to perform a
comparison with significant results between VEGF and PRG in lean
and obese subjects.
When and how locally secreted PRG and VEGF could
influence individually or by molecular crosstalk the initiation and
the development of visceral tumors is a controversial subject. It
is often emphasized that the tumor microenvironment provides
angiogenetic factors released by the tumor cells or by the stromal
cells surrounding the tumors (20,53).
VEGF, leptin and recently described PRG are similarly expressed in
adipose tissue and the tumoral vascular network development
(25,50,54-56).
Overexpression of PRG was reported in renal, breast or ovarian
cancers (57-59),
organs embedded in an active metabolic adipose tissue.
Tangkeangsirisin et al demonstrated that PRG could promote
tumor angiogenesis and metastasis in human breast cancer by
stimulating the synthesis of VEGF and MMP9(39).
In a mouse developmental model, Toh et al
demonstrated that the expression of VEGF and its receptors is
compulsory for early vasculogenesis in embryo, but PRG was
expressed later, its synthesis being instead necessary for vessel
growth and stability and may represent a novel angiogenic pathway
(48). The results reported by
Tangkeangsirisin and Serrero for breast tumors support the
hypothesis that the increased VEGF expression was determined rather
than an indirect effect of stromal cells found in the tumor
environment by the PRG direct stimulation of VEGF expression
(39).
The statistical correlations between the two
angiogenetic factors analyzed in our experiment showed interesting
differences. As shown in Fig. 2,
for the first group (NTP), the Pearson correlation coefficient
showed a statistically significant direct correlation (r=0.595),
while for the second smaller group (TP), the Pearson coefficient
showed an inverse correlation, without having any statistical
significance (r=-0.378). The results may indicate that the
angiogenetic factors are simultaneously present stimulating vessel
formation in inflammatory conditions, or in different steps,
thereby correlating our results with the influence of VEGF on PRG
synthesis as emphasized by Toh et al in the experimental
model (48). An interesting
question is whether the duration of evolution of the disease which
induces blood vessel formation is important for the concomitance or
the individuality action of the angiogenetic factors.
The angiogenesis process includes several
chronological steps; in sprouting angiogenesis, the endothelial
cells break down the existing basal membrane, and during the last
step the formation of the basal membrane (of which the type IV
collagen is a main component) by the newly formed endothelial cells
and probably by the pericytes occurs (60,61).
Col IV is the main constituent of the lamina densa of the
basal membrane, secreted by the endothelial cells and pericytes in
the final step of the sequential angiogenic process (61) and required for blood vessel
maturation and homeostasis (62).
In order to assess the incidence of mature
capillaries in the two lots of subjects we performed an IHC study
using the Col IV antibody. The results showed a significant
difference between patients with mechanical and inflammatory
pathology (NTP group and those with TP), in terms of the number of
capillaries identified. In the TP group, few vessels were labeled
inside the adipose lobules, and those located superficially and the
small new forming vessels were inconstantly positive. Considering
Col IV as a marker for mature functional vascular wall, we may
affirm that in the adipose tissue of the TP group, PRG stimulates
the formation of newly less mature and functional vessels, lacking
a basal membrane. Considering this observation, we supposed that a
proportion of the remaining hidden capillaries may be newly formed.
We used CD34, a major endothelial cell marker present on
hematopoietic stem cells and on progenitor cells (63), in order to label these presumed
capillaries. In terms of the number of capillaries identified, a
highly significant difference (P<0.001) between patients with
mechanical or inflammatory pathology and those with tumor pathology
was found.
Stromal vascular fraction of adipose pads contains a
population of CD34-positive cells, identified as adipose
tissue-derived stem cells (ADSC), considered adult stem cells which
reside in a perivascular location (64-66).
Our results are in accordance with those of Zannettino et al
as we observed the abundance of CD34-positive cells inside the
adipose lobules, in endothelial or stromal cells (67). In that study, it was found that
mesenchymal cells including adipose stem cells are resident in
perivascular niches. The double circle of CD34-positive cells that
authors of the present study and Lin et al (65) observed around the lumen of the
arterioles and venules is described as being formed by endothelial
cells in the inner circle and adventitial
CD34+CD31- cells in the outer one. In aortic
adventitia, this population was described as vascular progenitor
cells (68).
Considering these results together with the
significant number of vessels marked by PRG in the TP group, we
were able to sustain that in the PRG omental adipose pads had a
significant contribution to induce angiogenesis in a tumoral
ambience, and that this capacity is complementary with VEGF action
in NTP.
In summary, PRG is an adipokine able to act as an
effective proangiogenic factor in visceral adipose tissue, with the
formation of new capillaries in visceral tumors. However, whether
the angiogenic response to PRG synthesis is influenced or not by
inflammation and obesity remains to be determined.
VEGF is more efficient in stimulating the formation
of new vessels in inflammatory conditions depending on weight. The
collaboration of the proangiogenic activity of PRG and VEGF in the
adipose tissue under tumor conditions may be dependent on the
visceral tumor type.
Acknowledgements
We are thankful for the technical support provided
by Cristina Stănciucă from the Histology Department, University of
Medicine and Pharmacy of Craiova, Romania.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IB, MCM, DA and SJ made equal contributions to the
conception and editing of this manuscript. IB, IMB, MCM, SJ and CGP
were involved in literature research and wrote the manuscript. Data
curation was performed by IB, IMB and CGP. DA supported the
statistical analysis and reviewed the results. IB, IMB, SJ and CGP
conceived, planned and followed the execution of the experiments.
AMA, MCM and IMB performed morphometry. All authors contributed to
manuscript revision, read and approved the final version. AMA, MCM,
IB, IMB, SJ and CGP are all responsible for the authenticity of the
data obtained.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
The University of Medicine and Pharmacy of Craiova, Romania
(approval no. 85/2019). All patients included in the study provided
informed consent for data publication.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
This study is part of the Ph.D. thesis of Ioana
Binisor, from the University of Medicine and Pharmacy of Craiova,
Romania.
References
|
1
|
Divella R, De Luca R, Abbate I, Naglieri E
and Daniele A: Obesity and cancer: The role of adipose tissue and
adipo-cytokines-induced chronic inflammation. J Cancer.
7:2346–2359. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marlatt KL and Ravussin E: Brown adipose
tissue: An update on recent findings. Curr Obes Rep. 6:389–396.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yoneshiro T, Aita S, Matsushita M,
Kayahara T, Kameya T, Kawai Y, Iwanaga T and Saito M: Recruited
brown adipose tissue as an antiobesity agent in humans. J Clin
Invest. 123:3404–3408. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Andrei AM, Berbecaru-Iovan A, Din-Anghel
FRI, Stănciulescu CE, Berbecaru-Iovan S, Baniţă IM and Pisoschi CG:
Chapter 16. Interplay between hypoxia, inflammation and adipocyte
remodeling in the metabolic syndrome. In: Hypoxia and Human
Diseases. Zheng J (ed). IntechOpen, pp303-329, 2017.
|
|
5
|
Andrei AM, Berbecaru-Iovan A, Din-Anghel
FRI, Binişor ID, Marinescu RM, Goga LD, Baniță IM and Pisoschi CG:
New insights into brown adipose tissue as a pharmacological target
in obesity. Farmacia. 68:1–7. 2020.
|
|
6
|
Fusaru AM, Stănciulescu CE, Surlin V,
Taisescu C, Bold A, Pop OT, Baniţă IM, Crăiţoiu S and Pisoschi CG:
Role of innate immune receptors TLR2 and TLR4 as mediators of the
inflammatory reaction in human visceral adipose tissue. Rom J
Morphol Embryol. 53 (3 Suppl):693–701. 2012.PubMed/NCBI
|
|
7
|
Francisco V, Pino J, Gonzalez-Gay MA, Mera
A, Lago F, Gómez R, Mobasheri A and Gualillo O: Adipokines and
inflammation: Is it a question of weight? Br J Pharmacol.
175:1569–1579. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schäffler A and Schölmerich J: Innate
immunity and adipose tissue biology. Trends Immunol. 31:228–235.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hotamisligil GS: Inflammation and
metabolic disorders. Nature. 444:860–867. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cozzo AJ, Fuller AM and Makowski L:
Contribution of adipose tissue to development of cancer. Compr
Physiol. 8:237–282. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Venniyoor A: The most important questions
in cancer research and clinical oncology-question 2-5.
Obesity-related cancers: More questions than answers. Chin J
Cancer. 36(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mendonça F and Soares R: Obesity and
cancer phenotype: Is angiogenesis a missed link? Life Sci.
139:16–23. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Booth AD, Magnuson AM, Fouts J, Wei Y,
Wang D, Pagliassotti MJ and Foster MT: Subcutaneous adipose tissue
accumulation protects systemic glucose tolerance and muscle
metabolism. Adipocyte. 7:261–272. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Arnold M, Pandeya N, Byrnes G, Renehan
PAG, Stevens GA, Ezzati PM, Ferlay J, Miranda JJ, Romieu I, Dikshit
R, et al: Global burden of cancer attributable to high body-mass
index in 2012: A population-based study. Lancet Oncol. 16:36–46.
2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lauby-Secretan BP, Scoccianti C, Loomis D,
Grosse Y, Bianchini F and Straif K: International Agency for
Research on Cancer Handbook Working Group. Body fatness and
cancer-viewpoint of the IARC working group. N Engl J Med.
375:794–798. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pollak M: The insulin and insulin-like
growth factor receptor family in neoplasia: An update. Nat Rev
Cancer. 12:159–169. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Colotta F, Allavena P, Sica A, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer: Links to genetic instability. Carcinogenesis.
30:1073–1081. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Khandekar MJ, Cohen P and Spiegelman BM:
Molecular mechanisms of cancer development in obesity. Nat Rev
Cancer. 11:886–895. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Nieman KM, Kenny HA, Penicka CV, Ladanyi
A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB,
Hotamisligil GS, et al: Adipocytes promote ovarian cancer
metastasis and provide energy for rapid tumor growth. Nat Med.
17:1498–1503. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Nieman KM, Romero IL, Van Houten B and
Lengyel E: Adipose tissue and adipocytes support tumorigenesis and
metastasis. Biochim Biophys Acta. 1831:1533–1541. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rehman J, Traktuev D, Li J, Merfeld-Clauss
S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV
and March KL: Secretion of angiogenic and antiapoptotic factors by
human adipose stromal cells. Circulation. 109:1292–1298.
2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Halberg N, Wernstedt-Asterholm I and
Scherer PE: The adipocyte as an endocrine cell. Endocrinol Metab
Clin North Am. 37:753–768. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Christiaens V and Lijnen HR: Angiogenesis
and development of adipose tissue. Mol Cell Endocrinol. 318:2–9.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ledoux S, Queguiner I, Msika S, Calderari
S, Rufat P, Gasc JM, Corvol P and Larger E: Angiogenesis associated
with visceral and subcutaneous adipose tissue in severe human
obesity. Diabetes. 57:3247–3257. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun K, Kusminski CM and Scherer PE:
Adipose tissue remodeling and obesity. J Clin Invest.
121:2094–2101. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Cao Y: Angiogenesis and vascular functions
in modulation of obesity, adipose metabolism, and insulin
sensitivity. Cell Metab. 18:478–489. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lijnen HR: Angiogenesis and obesity.
Cardiovasc Res. 78:286–293. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hammarstedt A, Gogg S, Hedjazifar S,
Nerstedt A and Smith U: Impaired adipogenesis and dysfunctional
adipose tissue in human hypertrophic obesity. Physiol Rev.
98:1911–1941. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zanocco-Marani T, Bateman A, Romano G,
Valentinis B, He ZH and Baserga R: Biological activities and
signaling pathways of the granulin/epithelin precursor. Cancer Res.
59:5331–5340. 1999.PubMed/NCBI
|
|
30
|
Shoyab M, McDonald VL, Byles C, Todaro GJ
and Plowman GD: Epithelins 1 and 2: Isolation and characterization
of two cysteine-rich growth-modulating proteins. Proc Natl Acad Sci
USA. 87:7912–7916. 1990.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Anakwe OO and Gerton GL: Acrosome
biogenesis begins during meiosis: Evidence from the synthesis and
distribution of an acrosomal glycoprotein, acrogranin, during
guinea pig spermatogenesis. Biol Reprod. 42:317–328.
1990.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jian J, Konopka J and Liu C: Insights into
the role of progranulin in immunity, infection, and inflammation. J
Leukoc Biol. 93:199–208. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
He Z, Ong CH, Halper J and Bateman A:
Progranulin is a mediator of the wound response. Nat Med.
9:225–229. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Desmarais J, Cao M, Bateman A and Murphy
B: Spatiotemporal expression pattern of progranulin in embryo
implantation and placenta formation suggests a role in cell
proliferation, remodeling, and angiogenesis. Reproduction.
136:247–257. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jian J, Li G, Hettinghouse A and Liu C:
Progranulin: A key player in autoimmune diseases. Cytokine.
101:48–55. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wei J, Hettinghouse A and Liu C: The role
of progranulin in arthritis. Ann NY Acad Sci. 1383:5–20.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Korolczuk A and Bełtowski J: Progranulin,
a new adipokine at the crossroads of metabolic syndrome, diabetes,
dyslipidemia and hypertension. Curr Pharm Des. 23:1533–1539.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li LQ, Min LS, Jiang Q, Ping JL, Li J and
Dai LC: Progranulin expression in breast cancer with different
intrinsic subtypes. Pathol Res Pract. 208:210–216. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tangkeangsirisin W and Serrero G: PC
cell-derived growth factor (PCDGF/GP88, progranulin) stimulates
migration, invasiveness and VEGF expression in breast cancer cells.
Carcinogenesis. 25:1587–1592. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ferrara N: Vascular endothelial growth
factor: Basic science and clinical progress. Endocr Rev.
25:581–611. 2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tammela T, Enholm B, Alitalo K and
Paavonen K: The biology of vascular endothelial growth factors.
Cardiovasc Res. 65:550–563. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ikeda K: Adipose tissue angiogenesis: An
emerging therapeutic target for obesity and methabolic disease.
Austin J Clin Cardiolog. 1(1005)2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lemoine AY, Ledoux S and Larger E: Adipose
tissue angiogenesis in obesity. Thromb Haemost. 110:661–668.
2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Alberti KG, Eckel RH, Grundy SM, Zimmet
PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith
SC Jr, et al: Harmonizing the metabolic syndrome. A joint interim
statement of the international diabetes federation task force on
epidemiology and prevention; national heart, lung, and blood
institute; American heart association; world heart federation;
international atherosclerosis society; and international
association for the study of obesity. Circulation. 120:1640–1645.
2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Youn BS, Bang SI, Klöting N, Park JW, Lee
N, Oh JE, Pi KB, Lee TH, Ruschke K, Fasshauer M, et al: Serum
progranulin concentrations may be associated with macrophage
infiltration into omental adipose tissue. Diabetes. 58:627–636.
2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Davidson B, Alejandro E, Flørenes VA,
Goderstad JM, Risberg B, Kristensen GB, Trope CG and Kohn EC:
Granulin-epithelin precursor is a novel prognostic marker in
epithelial ovarian carcinoma. Cancer. 100:2139–2147.
2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gonzalez EM, Mongiat M, Slater SJ, Baffa R
and Iozzo RV: A novel interaction between perlecan protein core and
progranulin: Potential effects on tumor growth. J Biol Chem.
278:38113–38116. 2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Toh H, Cao M, Daniels E and Bateman A:
Expression of the growth factor progranulin in endothelial cells
influences growth and development of blood vessels: A novel mouse
model. PLoS One. 8(e64989)2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Corvera S and Gealekman O: Adipose tissue
angiogenesis: Impact on obesity and type-2 diabetes. Biochim
Biophys Acta. 1842:463–472. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhang QX, Magovern CJ, Mack CA,
Budenbender KT, Ko W and Rosengart TK: Vascular endothelial growth
factor is the major angiogenic factor in omentum: Mechanism of the
omentum-mediated angiogenesis. J Surg Res. 67:147–154.
1997.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Fusaru AM, Pisoschi CG, Bold A, Taisescu
C, Stănescu R, Hîncu M, Crăiţoiu S and Baniţă IM: Hypoxia induced
VEGF synthesis in visceral adipose depots of obese diabetic
patients. Rom J Morphol Embryol. 53:903–909. 2012.PubMed/NCBI
|
|
52
|
Voros G, Maquoi E, Demeulemeester D, Clerx
N, Collen D and Lijnen HR: Modulation of angiogenesis during
adipose tissue development in murine models of obesity.
Endocrinology. 146:4545–4554. 2005.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29 (Suppl 16):S15–S18.
2002.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Papetti M and Herman IM: Mechanisms of
normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol.
282:C947–C970. 2002.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Gonzalez-Perez RR, Lanier V and Newman G:
Leptin's pro-angiogenic signature in breast cancer. Cancers
(Basel). 5:1140–1162. 2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Lu R and Serrero G: Inhibition of PC
cell-derived growth factor (PCDGF, epithelin/granulin precursor)
expression by antisense PCDGF cDNA transfection inhibits
tumorigenicity of the human breast carcinoma cell line MDA-MB-468.
Proc Natl Acad Sci USA. 97:3993–3998. 2000.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Donald CD, Laddu A, Chandham P, Lim SD,
Cohen C, Amin M, Gerton GL, Marshall FF and Petros JA: Expression
of progranulin and the epithelin/granulin precursor acrogranin
correlates with neoplastic state in renal epithelium. Anticancer
Res. 21:3739–3742. 2001.PubMed/NCBI
|
|
59
|
Jones MB, Michener CM, Blanchette JO,
Kuznetsov VA, Raffeld M, Serrero G, Emmert-Buck MR, Petricoin EF,
Krizman DB, Liotta LA and Kohn EC: The granulin-epithelin
precursor/PC-cell-derived growth factor is a growth factor for
epithelial ovarian cancer. Clin Cancer Res. 9:44–51.
2003.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Sand JMB, Genovese F, Gudmann NS and
Karsdal MA: Chapter 4-type IV collagen. In: Biochemistry of
Collagens, Laminins and Elastin. Karsdal MA (ed). 2nd edition.
Academic Press, pp37-49, 2019.
|
|
61
|
Maragoudakis ME: The role of basement
membrane in angiogenesis. In: Vascular Endothelium. Catravas JD,
Gillis CN and Ryan US (eds). Springer, Boston, MA, pp111-120,
1989.
|
|
62
|
Pöschl E, Schlötzer-Schrehardt U,
Brachvogel B, Saito K, Ninomiya Y and Mayer U: Collagen IV is
essential for basement membrane stability but dispensable for
initiation of its assembly during early development. Development.
131:1619–1628. 2004.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Goncharov NV, Nadeev AD, Jenkins RO and
Avdonin PV: Markers and biomarkers of endothelium: When something
is rotten in the state. Oxid Med Cell Longev.
2017(9759735)2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Daquinag AC, Zhang Y and Kolonin MG:
Vascular targeting of adipose tissue as an anti-obesity approach.
Trends Pharmacol Sci. 32:300–307. 2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lin G, Garcia M, Ning H, Banie L, Guo YL,
Lue TF and Lin CS: Defining stem and progenitor cells within
adipose tissue. Stem Cells Dev. 17:1053–1063. 2008.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Sidney LE, Branch MJ, Dunphy SE, Dua HS
and Hopkinson A: Concise review: Evidence for CD34 as a common
marker for diverse progenitors. Stem Cells. 32:1380–1389.
2014.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Zannettino ACW, Paton S, Arthur A, Khor F,
Itescu S, Gimble JM and Gronthos S: Multipotential human
adipose-derived stromal stem cells exhibit a perivascular phenotype
in vitro and in vivo. J Cell Physiol. 214:413–421. 2008.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Hu Y, Zhang Z, Torsney E, Afzal AR,
Davison F, Metzler B and Xu Q: Abundant progenitor cells in the
adventitia contribute to atherosclerosis of vein grafts in
ApoE-deficient mice. J Clin Invest. 113:1258–1265. 2004.PubMed/NCBI View Article : Google Scholar
|