Introduction
Atrophic gastritis (AG) is the highest known
independent risk factor (risk condition) for distal, noncardial
gastric cancer (1-3).
Gastric carcinogenesis is a long and multistep
process, known as the ‘Correa's Cascade’. In this model of gastric
carcinogenesis, gastric cancer is preceded by gastric precancerous
lesions: Atrophic gastritis (AG), intestinal metaplasia (IM),
low-grade dysplasia, and high-grade dysplasia, developed
successively following chronic infection with Helicobacter
pylori (H. pylori) (4,5). Each
of these lesions is associated with an increased risk of gastric
cancer which correlates with the severity of the lesions, but AG
and IM are the most common and the most widely studied (6-9).
For the early detection of gastric cancer and to
reduce mortality, international guidelines recommend endoscopic
follow-up and gastric biopsies for subjects with atrophic
gastritis, even after H. pylori eradication (10,11).
A non-invasive tool able to easily identify
individuals with atrophic gastritis, is essential for improving the
early diagnosis of gastric cancer. To avoid numerous gastroscopies
and increase patient adhesion to surveillance several strategies
have been developed. Among them, serological markers are of growing
interest to assess the presence of gastric atrophy (12).
Numerous and potential serological biomarkers such
as serum pepsinogen 1 and 2 (PG1 and PG2, respectively), gastrin-17
(G17), antiparietal cell antibodies, IgG anti-H. pylori have
been used, separately or combined, to predict gastric mucosa status
(12).
PG1 is secreted only by oxintic glands of the
corpus, PG2 is secreted by pyloric glands and proximal duodenal
mucosa and G17 is only secreted by the G cells of the antral mucosa
(13). Serum PG1 levels and/or the
PG1/PG2 ratio appear to be lower in patients with corpus atrophic
gastritis, and low G17 serum level, in combination with positive
anti-H. pylori antibodies (H.p Ab), would indicate the
presence of antrum atrophic gastritis (13).
Some studies have tested this serologic panel
(GastroPanel) for the noninvasive diagnosis of atrophic gastritis
and have obtained encouraging results (14-19);
however, other studies do not support its usefulness (20-22).
Finally, experience with GastroPanel is limited; no
study has been carried out in a Romanian population.
Materials and methods
Patients
This was a prospective study, carried out at a
single tertiary center, namely the Second Medical Department and
the Endoscopy Laboratory, Emergency Clinical County Hospital
(Cluj-Napoca, Romania). Patient recruitment was from July 2017 to
August 2018. A total of 60 patients were included in our study: 35
(58.3%) females and 25 (41.66%) males. The mean age of the patients
was 67.63±9.36 years (range, 50-87 years). Inclusion criteria were
as follows: Patients older than 50 years, with dyspepsia. After
fulfilling this inclusion criteria, upper gastrointestinal
endoscopy was performed.
Exclusion criteria were as follows: Hepatic, lung,
renal, endocrine, metabolic, hematological or malignant diseases;
history of chemotherapy or gastric surgery, history of H.
pylori eradication; history of alcohol or drug abuse;
pregnancy. A demographic questionnaire was completed including
socio-demographic data and medical history. The Ethics Committee of
Emergency Clinical County Hospital approved the study following
European and local regulations. All admitted patients signed an
informed consent.
Investigations
Upper gastrointestinal endoscopy was performed by
gastroenterologists to all patients and biopsies were obtained (two
from the gastric corpus and two from the antrum). Pathological
examinations of biopsy samples were conducted by one single expert
pathologist and the results were reported according to the updated
Sydney system (23). Blood samples
were obtained from all patients after 10 h of fasting. Two weeks
before blood extraction, patients had ceased receiving proton pump
inhibitors (PPIs). EDTA tubes were centrifuged at 2,000 x g, for
10-15 min, at 20-25˚C. Blood was stored at -20˚C until the assay
was performed.
The determination of sPGI, sPGII, sG17 and IgG
antibody to H. pylori (H.p IgG) was performed using an
enzyme-linked immunosorbent assay (ELISA) (cat. no. 601 020.02 for
PGII; cat. no. 601 035 for G17; cat. no. 601 010.01 for PGI; cat.
no. 601 040.02 for H.p IgG; GastroPanel ELISA; Biohit Oyj).
Recommended cut-off points for GastroPanel were (as reported by the
manufacturer): sPGI: 30-120 mg/l, sPGII: 2-10 mg/l, sG17: 2-10
pmol/l and H.p IgG titre: -30 EIU. Accordingly, a value of 30 mg/l
for sPGI was assumed as a biomarker of atrophic corpus gastritis,
and a value of 2 pmol/l for sG17 was assumed to be a biomarker of
antral atrophic gastritis, in the absence of hyperchlorhydria
(22). All tests were performed at
the centralized laboratory Bioclinica, Cluj Napoca, Romania.
According to the pathological examination, subjects were classified
into four groups: non-atrophic gastritis, corpus atrophy, antral
atrophy, multifocal atrophy. Histopathology results were compared
with GastroPanel values.
Statistical analysis
The distribution of parameters was evaluated using
Kurtosis and Skewness. The normal distributed data were expressed
as the mean ± standard deviation, and abnormal distributed data
were expressed as median and 25 and 75th percentiles. Comparison
between groups was performed using the Mann-Whitney U-test and
Wilcoxon W-test for continuous and discrete variables,
respectively. The comparisons between histologic features and sPGI,
sPGII and sG17 were performed using Kruskal-Wallis test. P<0.05
was considered to indicate a statistically significant
difference.
Receiver operating characteristic (ROC) curves were
used to calculate the overall diagnostic performance of G17, PG1,
PG2, and the PG1/PG2 ratio for the diagnosis of gastric atrophy. If
the area under the ROC curve (AUC) was acceptable (0.70), the
optimal cut-off points were assessed, and then sensitivity analysis
was calculated. The accuracy of the algorithm of GastroPanel was
assessed against histology (gold standard); sensitivity,
specificity, and positive and negative predictive values were also
calculated.
Results
A total of 60 patients were included in our study;
35 (58.3%) females and 25 (41.66%) males. (Table I). There were no significant
differences between biomarker values depending on sex: G17
(P=0.969), PG1 (P=0.708), PG2 (P=0.263) or PG1/PG2 (P=0.472)
(Table II).
 | Table ILevels of biomarkers depending on
sex. |
Table I
Levels of biomarkers depending on
sex.
| Biomarkers | Sex | Median (IQ:
25-75%) |
|---|
| PG1 | M | 84.8 (36.15-136) |
| | F | 64.3
(44.6-111.4) |
| PG2 | M | 9.4 (3.6-18.25) |
| | F | 8.4 (1.6-10.7) |
| PG1/PG2 | M | 8.3 (5.49-14.1) |
| | F | 9.7 (6.1-28.1) |
| G17 | M | 5.5 (1-26.45) |
| | F | 5.3 (1-28.9) |
| Ac. H.p IgG | M | 61.8
(18.1-96.65) |
| | F | 42.7 (15-85.5) |
 | Table IIStatistical analysis of biomarkers
depending on sex. |
Table II
Statistical analysis of biomarkers
depending on sex.
| Statistical
variables | G17 | PG1 | PG2 | PG1/PG2 |
|---|
| Mann-Whitney U | 435.000 | 412.500 | 363.000 | 389.500 |
| Wilcoxon W | 1065.000 | 1042.500 | 993.000 | 714.500 |
| Z | -0.039 | -0.375 | -1.120 | -0.720 |
| Asymp. Sig.
(two-tailed) | 0.969 | 0.708 | 0.263 | 0.472 |
The mean age of patients was 67.63±9.36 years
(range, 50-87 years), with 32 (51.61%) patients between 50 and 59
years, 13 (20.96%) patients between 60 and 69 years, and 15
(24.19%) patients older than 70 years (Table III).
 | Table IIILevels of biomarkers depending on age
groups. |
Table III
Levels of biomarkers depending on age
groups.
| | GastroPanel |
|---|
| Biomarkers | Age | Median (IQ:
25-75%) |
|---|
| PG1 | 50-59 | 64.25
(42.95-107.17) |
| | 60-69 | 110.9
(43.2-150.9) |
| | >70 | 54.4
(31.5-137.2) |
| PG2 | 50-59 | 8.05
(1.27-12.1) |
| | 60-69 | 9.4
(7.25-18.65) |
| | >70 | 8 (0.9-28.1) |
| PG1/PG2 | 50-59 | 9.85
(6.17-32.82) |
| | 60-69 | 8.8 (4.4-12.5) |
| | >70 | 8.3 (4.5-35) |
| G17 | 50-59 | 1 (1-14.75) |
| | 60-69 | 6.7
(1.5-66.25) |
| | >70 | 10.3 (1-32.8) |
| Ac. H.p IgG | 50-59 | 41.05
(16.08-87.68) |
| | 60-69 | 81.3
(12.05-99) |
| | >70 | 57.1
(29.7-87.2) |
There were no significant differences between
biomarker values and age groups: G17 (P=0.121), PG1 (P=0.533), PG2
(P=0.259), PG1/PG2 (P=0.578) and ac H.p IgG (P=0.635) (Table IV).
 | Table IVStatistical analysis of biomarkers
depending on age groups. |
Table IV
Statistical analysis of biomarkers
depending on age groups.
| Statistical
variables | G17 | PGI | PG2 | PG1/PG2 | Ac. H.p IgG |
|---|
| Chi-square | 4.225 | 1.260 | 2.701 | 1.098 | 0.907 |
| Df | 2 | 2 | 2 | 2 | 2 |
| Asymp. Sig. | 0.121 | 0.533 | 0.259 | 0.578 | 0.635 |
There were no significant differences between levels
of biomarkers and localization of atrophy: G17 (P=0.599), PG1
(P=0.270), PG2 (P=0.813), PG1/PG2 (P=0.175) and ac H.p IgG
(P=0.782) (Tables V and VI).
 | Table VLevels of biomarkers depending on
localization of atrophy. |
Table V
Levels of biomarkers depending on
localization of atrophy.
| | Corpus atrophy | Antral atrophy | Multifocal
atrophy |
|---|
| Biomarkers | Presence of
atrophy | Cut-off value | Median (IQ:
25-75%) | Median (IQ:
25-75%) | Median (IQ:
25-75%) |
|---|
| PG1 | No | 30-120 | 65.25
(44.05-113.45) | 77.2
(42.2-126.75) | 64.25
(44.75-126.75) |
| | Yes | | 58.6
(33.15-222.75) | 58.7
(43.05-110.65) | 69.6
(34.55-106.8) |
| PG2 | No | 2-10 | 8.3
(1.75-15.45) | 8.55
(5.97-16.4) | 7.85
(1.75-11.85) |
| | Yes | | 9.2
(4.05-14.85) | 7.3
(0.95-12.4) | 11.5
(1.8-16.6) |
| PG1/PG2 | No | N/A | 9.25
(6.02-17.02) | 9.25
(5.64-15.05) | 10.25
(6.8-29.67) |
| | Yes | | 8.3
(5.85-34.23) | 8.8
(6.65-35.55) | 6.28
(3.11-10.4) |
| G17 | No | 2-10 | 3.25 (1-29.55) | 5.3 (1-21.9) | 3.85 (1-29.55) |
| | Yes | | 15.8
(3.2-37.55) | 1 (1-52.75) | 5.5 (1-20.1) |
| Ac. H.p IgG | No | 30 | 48
(15.52-93.18) | 61
(18.18-93.18) | 37.75
(15.52-86.23) |
| | Yes | | 61.2
(22.85-86.95) | 34.9
(16.1-84.65) | 82.4
(42.45-97.25) |
 | Table VIStatistical analysis of biomarkers
depending on atrophy. |
Table VI
Statistical analysis of biomarkers
depending on atrophy.
| Statistical
variables | G17 | PGI | PG2 | PG1/PG2 | Ac. H.p IgG |
|---|
| Chi-Square | 1.024 | 2.615 | 0.413 | 3.483 | 0.491 |
| Df | 2 | 2 | 2 | 2 | 2 |
| Asymp. Sig. | 0.599 | 0.270 | 0.813 | 0.175 | 0.782 |
GastroPanel values were not significantly altered in
patients with antral atrophy or corpus atrophy compared to those
without atrophy (Tables VII and
VIII).
 | Table VIIStatistical analysis of biomarkers
depending on antral atrophy. |
Table VII
Statistical analysis of biomarkers
depending on antral atrophy.
| Statistical
variables | G17 | PGI | PG2 | PG1/PG2 | Ac. H.p IgG |
|---|
| Mann-Whitney U | 392.500 | 346.000 | 324.500 | 363.000 | 351.000 |
| Wilcoxon W | 1133.500 | 577.000 | 555.500 | 1104.000 | 582.000 |
| Z | -0.108 | -0.839 | -1.182 | -0.570 | -0.760 |
| Asymp. Sig.
(two-tailed) | 0.914 | 0.401 | 0.237 | 0.569 | 0.447 |
 | Table VIIIStatistical analysis of biomarkers
depending on corpus atrophy. |
Table VIII
Statistical analysis of biomarkers
depending on corpus atrophy.
| Statistical
variables | G17 | PGI | PG2 | PG1/PG2 | Ac. H.p IgG |
|---|
| Mann-Whitney U | 87.000 | 130.000 | 126.500 | 125.500 | 124.500 |
| Wilcoxon W | 1572.000 | 1615.000 | 1611.500 | 1610.500 | 1609.500 |
| Z | -1.366 | -0.136 | -0.232 | -0.259 | -0.286 |
| Asymp. Sig.
(two-tailed) | 0.172 | 0.892 | 0.817 | 0.796 | 0.775 |
| Exact Sig.
[2*(1-tailed Sig.)] | 0.203 | 0.906 | 0.823 | 0.802 | 0.782 |
In addition, in cases of multifocal atrophy the
values of G17, PG1, PG2, H.p IgG were not statistically altered
compared to those without atrophy: G17 (P=0.894), PG1 (P=0.370),
PG2 (P=0.415), PG1/PG2 (P=0.060) and ac H.p IgG (P=0.139). However,
the ratio PG1/PG2 was lower in patients with multifocal atrophy;
the difference being close to the threshold of statistical
significance 6,2 (3,1; 10,4) vs. 10,2 (6,8; 29,6) P=0.060 (Table IX).
 | Table IXStatistical analysis of biomarkers
depending on multifocal atrophy. |
Table IX
Statistical analysis of biomarkers
depending on multifocal atrophy.
| Statistical
variables | G17 | PG1 | PG2 | PG1/PG2 | Ac. H.p IgG |
|---|
| Mann-Whitney U | 292.000 | 250.000 | 254.500 | 196.000 | 218.000 |
| Wilcoxon W | 383.000 | 341.000 | 1335.500 | 287.000 | 1299.000 |
| Z | -0.134 | -0.896 | -0.816 | -1.884 | -1.481 |
| Asymp. Sig.
(two-tailed) | 0.894 | 0.370 | 0.415 | 0.060 | 0.139 |
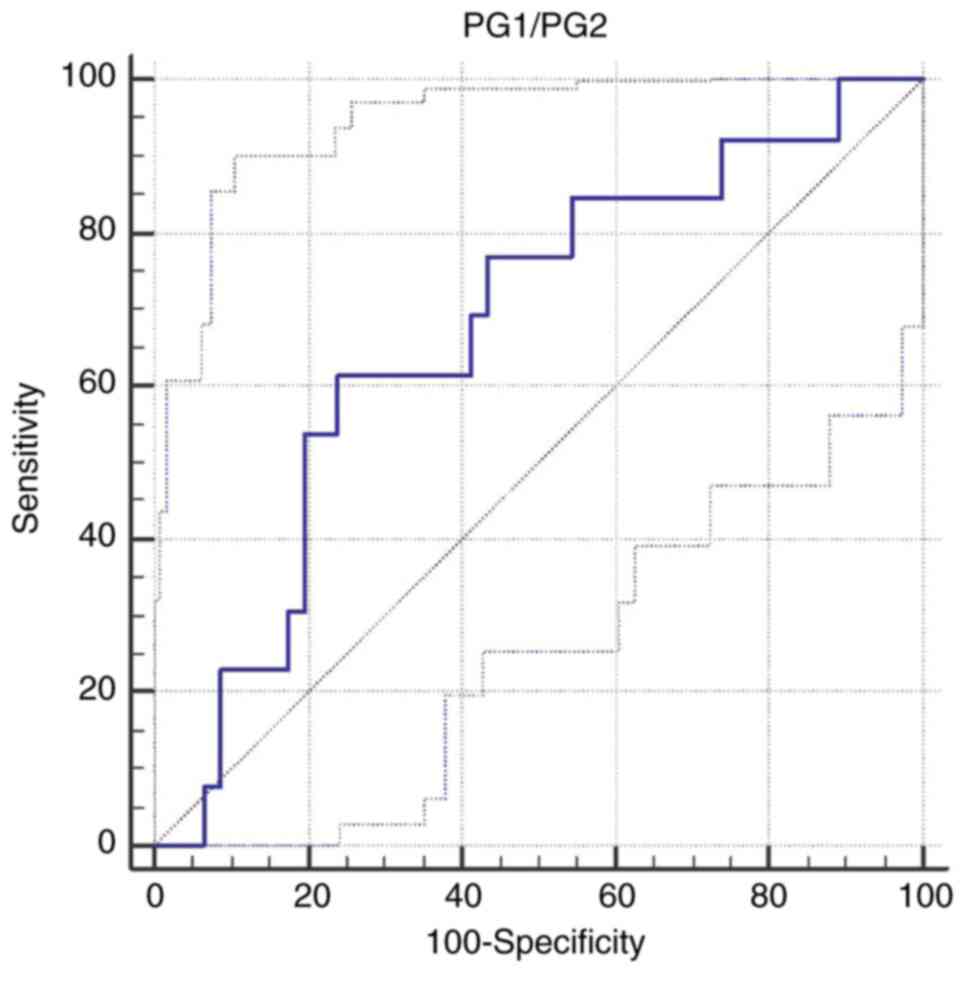
A cut-off value for PG1/PG2 of <6.59 was
calculated to differentiate multifocal atrophy patients from the
other patients [AUC 0.672; Se 61.5% (95% CI 31.6-86.1), Sp 76% (95%
CI 61.2-87.4); P=0.04] (Table IX
and Fig. 1).
Furthermore in cases of intestinal metaplasia the
values of G17, PG1, PG2, H.p IgG were not statistically altered
compared to those without intestinal metaplasia: G17 (P=0.791), PG1
(P=0.532), PG2 (P=0.962), PG1/PG2 (P=0.083) an ac H.p IgG
(P=0.806); only the ratio PG1/PG2 was lower in intestinal
metaplasia; the difference being almost statistically significant
[7,4 (4,4; 12,4) vs. 11 (6,4; 29,6); P=0.083] (Tables X and XI).
 | Table XLevels of biomarkers depending on
intestinal metaplasia. |
Table X
Levels of biomarkers depending on
intestinal metaplasia.
| Biomarkers | Presence of
intestinal metaplasia | Cut-off value | Intestinal
metaplasia median (IQ: 25-75%) |
|---|
| PG1 | No | 30-120 | 65.25
(44.87-120.02) |
| | Yes | | 58.6
(32.75-128.1) |
| PG2 | No | 2-10 | 8.45
(1.7-14.62) |
| | Yes | | 7.7 (3.3-16.1) |
| PG1/PG2 | No | N/A | 11
(6.47-29.67) |
| | Yes | | 7.4
(4.44-12.4) |
| G17 | No | 2-10 | 5.3 (1-21.9) |
| | Yes | | 2 (1-56) |
| Ac. H.p IgG | No | 30 | 41.05
(14.68-93.18) |
| | Yes | | 57.1
(18.85-83.95) |
 | Table XIStatistical analysis of biomarkers
depending on intestinal metaplasia. |
Table XI
Statistical analysis of biomarkers
depending on intestinal metaplasia.
| Statistical
variables | G17 | PG1 | PG2 | PG1/PG2 | Ac. H.p IgG |
|---|
| Mann-Whitney U | 383.000 | 359.500 | 396.000 | 289.500 | 383.500 |
| Wilcoxon W | 1124.000 | 590.500 | 1137.000 | 520.500 | 1124.500 |
| Z | -0.265 | -0.626 | -0.048 | -1.734 | -0.245 |
| Asymp. Sig.
(two-tailed) | 0.791 | 0.532 | 0.962 | 0.083 | 0.806 |
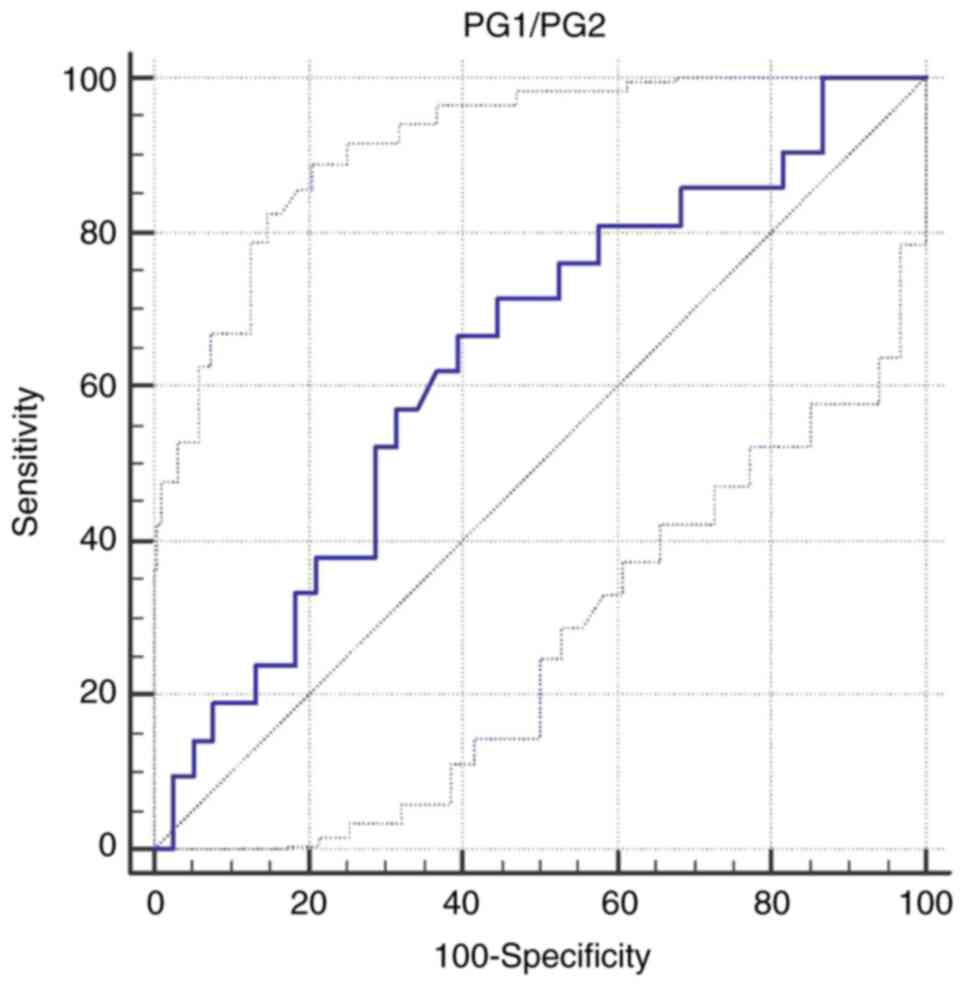
A cut-off value for PG1/PG2 of <8.8 was
calculated to differentiate intestinal metaplasia patients from the
other patients [AUC 0.637; Se 66.6% (95% CI 43.0-85.4), Sp 60.5%
(95% CI 43.4-76.0); P=0.07] (Table
XI and Fig. 2).
Patients included in the study were divided into 2
groups: patients without gastric atrophy (n=21) 35%; and patients
with gastric atrophy (n=39) 65%. Those with atrophy (n=39) were
divided into 2 subgroups with mild atrophy (n=26) and moderate
atrophy (n=13). No patients with severe atrophy were found. In the
non-atrophic group there were patients with spotty gastritis and
erosive gastritis. H. pylori infection was found in 45% of
patients (n=27).
GastroPanel values did not differ depending on the
severity of the atrophy: G17 (P=0.599), PG1 (P=0.270), PG2
(P=0.813), PG1/PG2 (P=0.175) and ac H.p IgG (P=0.782) (Tables VI and XII).
 | Table XIILevels of biomarkers depending on
histological grading (severity of atrophy). |
Table XII
Levels of biomarkers depending on
histological grading (severity of atrophy).
| Histological
grading | n | PG1 median (IQ:
25-75%) | PG2 median (IQ:
25-75%) | PG1/PG2 median (IQ:
25-75%) | G17 median (IQ:
25-75%) | Ac. H.p IgG median
(IQ: 25-75%) |
|---|
| No activity | 21 | 100.6
(44.85-136) | 8.4 (6.4-16.5) | 11.5
(5.85-15.1) | 2 (1-23.9) | 39.4
(13.55-90.90) |
| Mild | 26 | 58.85
(37-113.45) | 7.75
(1.32-16.55) | 9.35
(5.98-34.42) | 7.1 (1-37.52) | 56.8
(18.68-87.5) |
| Moderate | 13 | 55.2
(21.5-107.05) | 8.8
(2.9-14.65) | 6.59
(3.27-8.5) | 1 (1-34.2) | 60.8
(17.95-96.65) |
Discussion
Several authors have suggested a non-invasive test
defined as a ‘serological biopsy’, aimed at providing a gastric
function serum profile, especially of gastric atrophy (17,19,24,25).
The results of a recent meta-analysis suggest that
the combination of pepsinogen, G17 and anti-H. pylori
antibody serum assays is a reliable tool for the diagnosis of the
presence and site of atrophic gastritis (26).
Thus, GastroPanel could be a useful noninvasive
method to reduce unnecessary gastroscopies. The results of our
study did not support this theory.
In our study it was demonstrated that GastroPanel
values (biomarkers G17, PG1, PG2) were not significantly altered in
patients with antral atrophy or corpus atrophy compared to those
without atrophy. The measurement of G17 and PG2 for the diagnosis
of antral atrophy had an unacceptably low accuracy.
In addition, in cases of multifocal atrophy the
values of G17, PG1, PG2, H.p IgG were not statistically altered
compared to those without atrophy: However, the ratio PG1/PG2 was
lower in patients with multifocal atrophy; the difference being
close to the threshold of statistical significance.
In the cases of multifocal atrophy, a sensitivity of
61.5% and specificity of 76% were determined (P=0.04).
In this regard, our results are supported by another
study, which found discouraging results. The study revealed that
PG1 differences between patients with or without corpus atrophy
were not significant (112 vs. 117 µg/l), and no statistically
significant differences for PG1/PG2 for the localization of the
atrophy were reported; but, compared to our results, they found
that the mean levels of G17 were significantly reduced in patients
with atrophy in the antrum (5 vs. 13 pmol/l; P<0.01) (27).
The ratio PG1/PG2 (in our study) was lower in
patients with intestinal metaplasia; the difference being almost
statistically significant (P=0.083) with a sensitivity of 66.6% and
a specificity of 60.5% (P=0.07).
According to our findings, PG and G17 were not valid
enough to differentiate between patients with or without atrophic
gastritis. Nasrollahzadeh et al similarly reported a
relatively low validity for PG and G17 to distinguish non-atrophic
gastritis (28).
The suboptimal accuracy of GastroPanel (and the
individual biomarkers) may be negatively affected by some other
variables, but these unknown altering variables (such as a possible
spotty gastritis with ‘normal function’) arise from real clinical
practice experience.
The main limitation of the present study is that we
did not find patients with severe atrophy in the study population.
This theory is supported by a group of French researchers who claim
that GastroPanel has an insufficient diagnostic performance in case
of mild gastric atrophy. However, it can be useful in selected
groups of patients at high risk for gastric cancer, in particular
to detect severe atrophy and corpus atrophy (29).
In conclusion, our study indicated that biomarkers
used by GastroPanel do not have enough accuracy for use in the
diagnosis of atrophy in the population studied.
An association was only revealed for the ratio
PG1/PG2 which was lower in patients with multifocal atrophy.
However, our present data exhibited low accuracy in detecting
intestinal metaplasia.
These results suggest that the serological approach
may not be the best method to screen for gastric mild atrophy or
gastric cancer in people from low prevalence areas, such as
Romania.
The present results are contrary to expectations and
contrary to some authors who claim that GastroPanel is ‘even more
reliable than a histology biopsy’ (30).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CG, EG and AP performed the literature search for
relevant publications on the topic. CG and SG performed endoscopies
with biopsy. CG and SG collected the data. EG and AP analyzed the
data. CG and SG were responsible for original draft preparation. DD
conceived this study, surveyed its progress and contributed to the
writing. All the authors read, verified and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Emergency Clinical Hospital
Cluj County approved the study following European and local
regulations. Emergency Clinical Hospital Cluj County is a
University hospital and all admitted patients signed an informed
consent by which they agree that their data are available for
academic and scientific purposes.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sipponen P, Kekki M, Haapakoski J, Ihamäki
T and Siurala M: Gastric cancer risk in chronic atrophic gastritis:
Statistical calculations of cross-sectional data. Int J Cancer.
35:173–177. 1985.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ohata H, Kitauchi S, Yoshimura N, Mugitani
K, Iwane M, Nakamura H, Yoshikawa A, Yanaoka K, Arii K, Tamai H, et
al: Progression of chronic atrophic gastritis associated with
Helicobacter pylori infection increases risk of gastric
cancer. Int J Cancer. 109:138–143. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Varis K, Sipponen P, Laxén F, Samloff IM,
Huttunen JK, Taylor PR, Heinonen OP, Albanes D, Sande N, Virtamo J
and Härkönen M: Implications of serum pepsinogen I in early
endoscopic diagnosis of gastric cancer and dysplasia. Helsinki
Gastritis Study Group. Scand J Gastroenterol. 35:950–956.
2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Correa P: A human model of gastric
carcinogenesis. Cancer Res. 48:3554–3560. 1988.PubMed/NCBI
|
|
5
|
de Vries AC, van Grieken NC, Looman CW,
Casparie MK, de Vries E, Meijer GA and Kuipers EJ: Gastric cancer
risk in patients with premalignant gastric lesions: A nationwide
cohort study in the Netherlands. Gastroenterology. 134:945–952.
2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Song H, Ekheden IG, Zheng Z, Ericsson J,
Nyrén O and Ye W: Incidence of gastric cancer among patients with
gastric precancerous lesions: Observational cohort study in a low
risk western population. BMJ. 351(h3867)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chapelle N, Péron M, Mosnier JF,
Quénéhervé L, Coron E, Bourget A, Cauchin E, Touchefeu Y and
Matysiak-Budnik T: Prevalence, characteristics and endoscopic
management of gastric premalignant lesions in France. Dig Dis.
38:286–292. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
den Hoed CM, Holster IL, Capelle LG, de
Vries AC, den Hartog B, Ter Borg F, Biermann K and Kuipers EJ:
Follow-up of premalignant lesions in patients at risk for
progression to gastric cancer. Endoscopy. 45:249–256.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
den Hollander WJ, Holster IL, den Hoed CM,
Capelle LG, Tang TJ, Anten MP, Prytz-Berset I, Witteman EM, Ter
Borg F, Hartog GD, et al: Surveillance of premalignant gastric
lesions: A multicentre prospective cohort study from low incidence
regions. Gut. 68:585–593. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dinis-Ribeiro M, Areia M, de Vries AC,
Marcos-Pinto R, Monteiro-Soares M, O'Connor A, Pereira C,
Pimentel-Nunes P, Correia R, Ensari A, et al: Management of
precancerous conditions and lesions in the stomach (MAPS):
Guideline from the European society of gastrointestinal endoscopy
(ESGE), European helicobacter study group (EHSG), European society
of pathology (ESP), and the sociedade portuguesa de endoscopia
digestiva (SPED). Endoscopy. 44:74–94. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Malfertheiner P, Megraud F, O'Morain CA,
Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton
J, Graham DY, et al: Management of Helicobacter pylori
infection the maastricht V/florence consensus report. Gut. 66:6–30.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
di Mario F and Cavallaro LG: Non-invasive
tests in gastric diseases. Dig Liver Dis. 40:523–530.
2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Agréus L, Kuipers EJ, Kupcinskas L,
Malfertheiner P, Di Mario F, Leja M, Mahachai V, Yaron N, van Oijen
M, Perez Perez G, et al: Rationale in diagnosis and screening of
atrophic gastritis with stomach-specific plasma biomarkers. Scand J
Gastroenterol. 47:136–147. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Di Mario F, Moussa AM, Caruana P, Merli R,
Cavallaro LG, Cavestro GM, Dal Bò N, Iori V, Pilotto A, Leandro G,
et al: ‘Serological biopsy’ in first-degree relatives of patients
with gastric cancer affected by Helicobacter pylori
infection. Scand J Gastroenterol. 38:1223–1227. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Germaná B, Di Mario F, Cavallaro LG,
Moussa AM, Lecis P, Liatoupolou S, Comparato G, Carloni C, Bertiato
G, Battiestel M, et al: Clinical usefulness of serum pepsinogens I
and II, gastrin-17 and anti-Helicobacter pylori antibodies
in the management of dyspeptic patients in primary care. Dig Liver
Dis. 37:501–508. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Graham DY, Nurgalieva ZZ, El-Zimaity HM,
Opekun AR, Campos A, Guerrero L, Chavez A and Cardenas V:
Noninvasive versus histologic detection of gastric atrophy in a
Hispanic population in North America. Clin Gastroenterol Hepatol.
4:306–314. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hartleb M, Wandzel P, Waluga M, Matyszczyk
B, Bołdys H and Romañczyk T: Non-endoscopic diagnosis of multifocal
atrophic gastritis; efficacy of serum gastrin-17, pepsinogens and
Helicobacter pylori antibodies. Acta Gastroenterol Belg.
67:320–326. 2004.PubMed/NCBI
|
|
18
|
Nardone G, Rocco A, Staibano S, Mezza E,
Autiero G, Compare D, De Rosa G and Budillon G: Diagnostic accuracy
of the serum profile of gastric mucosa in relation to histological
and morphometric diagnosis of atrophy. Aliment Pharmacol Ther.
22:1139–1146. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Väänänen H, Vauhkonen M, Helske T,
Kääriäinen I, Rasmussen M, Tunturi-Hihnala H, Koskenpato J, Sotka
M, Turunen M, Sandström R, et al: Non-endoscopic diagnosis of
atrophic gastritis with a blood test. Correlation between gastric
histology and serum levels of gastrin-17 and pepsinogen I: A
multicentre study. Eur J Gastroenterol Hepatol. 15:885–891.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Masci E, Pellicano R, Mangiavillano B,
Luigiano C, Stelitano L, Morace C, Viale E, Freschi M, Locatelli M,
Ieri R, et al: GastroPanel® test for non-invasive
diagnosis of atrophic gastritis in patients with dyspepsia. Minerva
Gastroenterol Dietol. 60:79–83. 2014.PubMed/NCBI
|
|
21
|
Peitz U, Wex T, Vieth M, Stolte M, Willich
S, Labenz J, Jaspersen D, Lind T and Malfertheiner P: Correlation
of serum pepsinogens and gastrin-17 with atrophic gastritis in
gastroesophageal reflux patients: A matched-pairs study. J
Gastroenterol Hepatol. 26:82–89. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Koivusalo AI, Pakarinen MP and Kolho KL:
Is GastroPanel serum assay useful in the diagnosis of
Helicobacter pylori infection and associated gastritis in
children? Diagn Microbiol Infect Dis. 57:35–38. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dixon MF, Genta RM, Yardley JH and Correa
P: Classification and grading of gastritis. The updated sydney
system. International workshop on the histopathology of gastritis,
Houston 1994. Am J Surg Pathol. 20:1161–1181. 1996.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bodger KI, Wyatt JI and Heatley R:
Variation in serum pepsinogens with severity and topography of
Helicobacter pylori-associated chronic gastritis in
dyspeptic patients referred for endoscopy. Helicobacter. 6:216–224.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Urita Y, Hike K, Torii N, Kikuchi Y, Kanda
E, Sasajima M and Miki K: Serum pepsinogens as a predicator of the
topography of intestinal metaplasia in patients with atrophic
gastritis. Dig Dis Sci. 49:795–801. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zagari RM, Rabitti S, Greenwood DC, Eusebi
LH, Vestito A and Bazzoli F: Systematic review with meta-analysis:
Diagnostic performance of the combination of pepsinogen, gastrin-17
and anti-Helicobacter pylori antibodies serum assays for the
diagnosis of atrophic gastritis. Aliment Pharmacol Ther.
46:657–667. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
McNicholl AG, Forné M, Barrio J, De la
Coba C, González B, Rivera R, Esteve M, Fernandez-Bañares F,
Madrigal B, Gras-Miralles B, et al: Accuracy of GastroPanel for the
diagnosis of atrophic gastritis. Eur J Gastroenterol Hepatol.
26:941–948. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nasrollahzadeh D, Aghcheli K, Sotoudeh M,
Shakeri R, Persson EC, Islami F, Kamangar F, Abnet CC, Boffetta P,
Engstrand L, et al: Accuracy and cut-off values of pepsinogens I,
II and gastrin 17 for diagnosis of gastric fundic atrophy:
Influence of gastritis. PLoS One. 6(26957)2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chapelle N, Petryszyn P, Blin J, Leroy M,
Le Berre-Scoul C, Jirka I, Neunlist M, Moussata D, Lamarque D,
Olivier R, et al: A panel of stomach-specific biomarkers
(GastroPanel®) for the diagnosis of atrophic gastritis:
A prospective, multicenter study in a low gastric cancer incidence
area. Helicobacter. 25(e12727)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Storskrubb T, Aro P, Ronkainen J, Sipponen
P, Nyhlin H, Talley NJ, Engstrand L, Stolte M, Vieth M, Walker M
and Agréus L: Serum biomarkers provide an accurate method for
diagnosis of atrophic gastritis in a general population: The
Kalixanda study. Scand J Gastroenterol. 43:1448–1455.
2008.PubMed/NCBI View Article : Google Scholar
|