Introduction
Dry eye (DE) is a multifactorial disease of the tear
film and ocular surface that is characterized by multiple symptoms,
including discomfort, visual disturbance and tear film instability,
which may potentially damage the ocular surface (1). DE is accompanied by increased
osmolarity of the tear film and inflammation of the ocular surface
(2,3). Long-term progression of inflammation
at the ocular surface has the potential to aggravate symptoms and
signs, resulting in severe DE (SDE). SDE is associated with an
increased risk of infection, vision loss and ocular surface
epithelial defects (4). Numerous
patients with SDE report ocular pain, which may reduce their
quality of life due to ocular surface damage (5,6).
At present, topical 0.05% cyclosporin A (CsA;
Restasis®; Allergan), an anionic oil in water emulsion
possessing anti-inflammatory properties, has demonstrated marked
efficacy for DE treatment (7).
Following treatment with 0.05% CsA emulsion, patients with DE
exhibited ameliorated symptoms and improvements in other
indicators, including improvements in the ocular surface disease
index (OSDI), Schirmer values and corneal fluorescein staining
scores (CFS) (8-10).
However, in patients with severe inflammatory forms of DE, such as
graft-versus-host disease (GVHD) or Sjögren syndrome (SS), the use
of 0.05% CsA emulsion twice daily was found to have limitations in
controlling ocular surface inflammations (11).
In previous clinical studies published over the last
decade, marked improvements have been observed for subjective
symptoms (i.e., based on the OSDI, Schirmer test, and CFS) in
patients with severe keratoconjunctivitis, including GVHD and SS,
following treatment with 0.1% CsA cationic emulsion (CsA CE;
iKervis®; Santen Pharmaceutical Co., Ltd.) compared with
0.05% CsA emulsion (12-14).
Although clinical studies have demonstrated that 0.1% CsA CE leads
to an improvement in symptoms and indicators in patients following
treatment for longer or shorter periods of time, to the best of our
knowledge, no study has investigated the effect of 0.1% CsA CE
topical application on ocular surface inflammation and damage in
experimental DE (EDE). The aim of the present study was therefore
to investigate the therapeutic effects of topical 0.1% CsA CE
treatment on tear film parameters [tear volume and tear film
break-up time (BUT)], ocular surface damage (via evaluating CFS)
and inflammatory properties (i.e., the levels of inflammatory
cytokines and T cells) in a murine model of EDE with different
severities and to compare these effects with those of topical 0.05%
CsA emulsion treatment.
Materials and methods
Design of the mouse model and in vivo
experiments
The research protocol was approved by the Chonnam
National University School Research Institutional Animal Care and
Use Committee (approval no. CNU IACUC-H-2018-73). EDE was induced
by desiccating stress (exposure to an air draft all day; 30%
ambient humidity) and subcutaneous injection of scopolamine (0.5
mg, 0.2 ml; MilliporeSigma) three times a day (at 9 a.m., 1:30 p.m.
and 6 p.m.) as previously described (15,16).
In the present study, 8-week-old female C57BL/6 mice (weight,
16.0±2 g) were used, and EDE was induced by desiccating stress
(exposure to an air draft all day and 30% ambient humidity) and
subcutaneous injection of scopolamine (0.5 mg, 0.2 ml;
MilliporeSigma) three times a day (at 9 a.m., 1:30 p.m. and 6 p.m.)
as previously described (15,16).
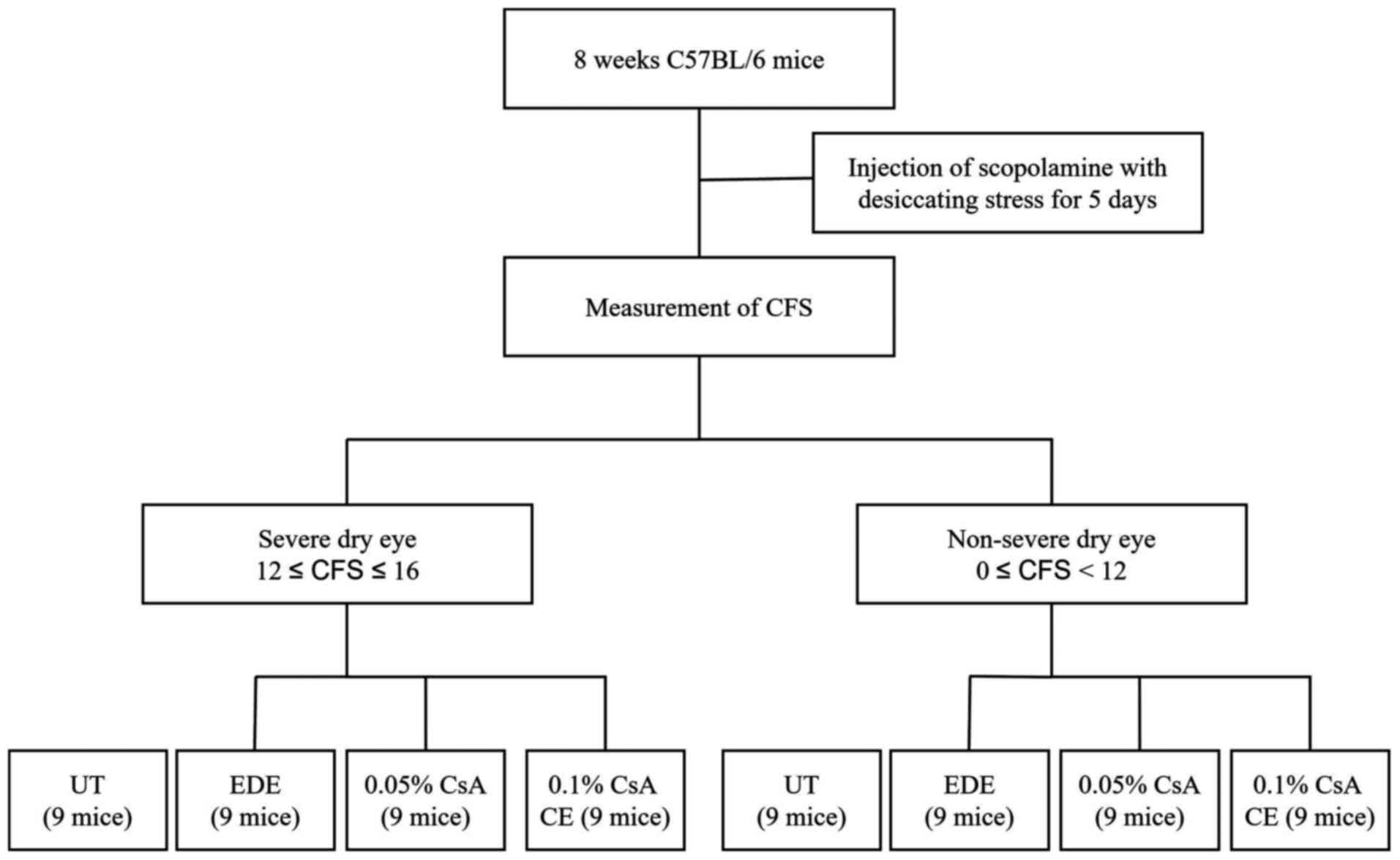
A total of 54 EDE-induced mice were divided into two groups based
on the CFSs: i) The SDE group (12≤CFS≤16); and ii) the NSDE group
(0≤CFS<12; Fig. 1). In
addition, 27 EDE-induced mice from each group were separated into
three subgroups of 9 mice according to topical treatment as
follows: i) EDE group, where mice were exposed to desiccating
stress and received no eye drops; ii) the 0.05% CsA group, where
EDE mice were treated with 2 µl 0.05% CsA emulsion twice daily
(Restasis®; Allergan); iii) the 0.1% CsA CE group, where
EDE mice were treated with 2 µl 0.1% CsA CE once daily
(iKervis®; Santen). The mice not exposed to desiccating
stress were used as untreated (UT) controls.
The mice in all treatment groups, except for the UT
group, received scopolamine injections. Nine animals in each
subgroup were used for clinical and experimental analysis and no
animal was found dead before euthanasia. Tear film parameters (tear
volume and tear film BUT) and CFSs were evaluated after 5 and 10
days of CsA application. After assessment of the clinical
parameters, mice were deeply anesthetized with 3% sevoflurane and
intraperitoneal injection of sodium pentobarbital (50 mg/kg).
Transcardial perfusion was subsequently performed with 4%
paraformaldehyde in phosphate buffer (pH 7.4) for euthanasia.
Animals were euthanized by experienced experts and when needed,
medications and supplies were available, minimizing pain and stress
for the animals. Euthanasia was performed in accordance with the
AVMA Animal Euthanasia Guidelines: 2020 Edition (https://www.avma.org). Following euthanasia, animal
death was confirmation by the absence of cardiovascular and
respiratory movements. Western blotting, multiplex immunobead assay
and flow cytometric analysis, histological analysis and TUNEL
staining were performed at day 15 after initiation of the
treatment. During these experiments, animals were treated in
accordance with ARVO Statement for the Use of Animals in Ophthalmic
and Vision Research, and animal movement, food and water intake
were not restricted (https://www.arvo.org/About/policies/statement-for-the-use-of-animals-in-ophthalmic-and-vision-research/#three).
Experiments involving EDE induction and CsA application lasted 15
consecutive days and the experiment was repeated three times. In
addition, all clinical and laboratory analyzes were performed after
each experiment. As all experiments were repeated 3 times, a total
of 216 mice were used within the present study.
Evaluation of tear film parameters and
corneal epithelial damage
Tear volume was measured using phenol red
impregnated cotton threads (Zone-Quick™; Oasis Medical, Inc.), and
the threads were placed in the lateral canthus for 20 sec as
previously described (17,18). The length of the wet red thread was
measured in mm under a photomicroscope (light microscope;
magnification, x1; SMZ 1500; Nikon Corporation).
After allowing 1 µl of 1% sodium fluorescein to fall
into the inferior conjunctival sac for 20 sec, the ocular surface
was washed with PBS and the tear film BUT (in sec) was recorded
using slit lamp biomicroscopy (BQ-900; Haag-Streit Diagnostics)
under cobalt blue light. The cornea was distributed into four
parts, which were scored separately. The CFSs were calculated
according to a 4 point scale and added together to obtain a final
score (range, 0-16) as previously described (19).
Western blotting
The expression of the nuclear factor (NF)-κB p65
protein was determined using western blotting. Proteins were
extracted from the conjunctival tissues (4 eyes per group) using
lysis buffer (RIPA buffer; GeneAll Biotechnology Co., Ltd.)
supplemented with a protease inhibitor cocktail (cat. no.
11836153001; Roche Diagnostics GmbH) on ice, and lysates were
centrifuged at 25,200 x g for 10 min at 4˚C, as previously
described (20). Proteins (20 µg)
were separated by 12% SDS-PAGE and were transferred onto PVDF
membranes. Membranes were washed with TBST-Tween-20 [TBST; 10 mM
Tris-HCl (pH 7.6), 150 mM NaCl and 0.05% Tween-20] and blocked with
5% skimmed milk in TBST for 1 h at room temperature. Membranes were
incubated for 2 h at room temperature with the following primary
antibodies: Rabbit polyclonal antibody against NF-κB p65 (cat. no.
ab16502; Abcam; diluted by 1:1,000), rabbit anti-phosphorylated
NF-κB p65 (cat. no. ab76302; Abcam; diluted by 1:500) and
anti-β-actin (cat. no. ab8227; Abcam; diluted by 1:1,000). The
membranes were washed three times with 1X TBST buffer for 5 min and
incubated with secondary antibodies goat anti-rabbit IgG H&L
(cat. no. ab205718; Abcam; diluted by 1:5,000, 1:5,000, and
1:10,000 for visualization of NF-κB p65, phosphorylated NF-κB p65
and β-actin antibodies, respectively) diluted in 1X TBST for 60 min
at room temperature. After incubation, membranes were washed five
times with TBST for 5 min. Enhanced chemiluminescence system (ECL
Blotting Analysis System; Cytiva) was used to detect the signal on
the membrane. The data were analyzed via densitometry (Alliance
MINI HD9; UVItec Ltd.) and normalized to the expression of the
internal control β-actin.
Multiplex immunobead assay
The levels of tumor necrosis factor-α (TNF-α),
interferon-γ (IFN-γ), interleukin (IL)-6, IL-17 and IL-21 in mice
conjunctiva (six eyes per group) were evaluated using MILLIPLEX MAP
Mouse Cytokine/Chemokine Magnetic Bead Panel - Immunology Multiplex
Assay kit (all from Milliplex®; MilliporeSigma; cat. no.
MCYTOMAG-70K) and the Luminex 200 detection method (Luminex
Corporation) as previously described (16). The conjunctival tissues (10 mg)
were collected, pooled and lysed in TissueLyser lysis buffer
(Qiagen, Inc.) containing protease inhibitors (cat. no.
11836153001; Roche Diagnostics GmbH) for 30 min on ice. The
extracts were subsequently centrifuged at 14,000 x g for 15 min at
4˚C. After centrifugation, the samples were added to a 96-well
plate (25 µl/well) and incubated overnight at 4˚C in the dark with
25 µl 1X beads coupled to mouse cytokine/chemokine-specific
antibodies. Serial dilutions of each cytokine/chemokine were also
performed on the same plate to generate a standard curve. The
following day, the beads were washed and mixed with 25 µl 1X
biotinylated secondary cytokine/chemokine antibody mixture for 1 h
at room temperature, followed by a wash and subsequent incubation
with 25 µl streptavidin-phycoerythrin for 30 min at room
temperature (both steps performed in the dark). After a final wash,
the beads were resuspended in 150 µl sheath fluid assay buffer. The
reactions were detected after addition of
streptavidin-phycoerythrin using an analysis system (xPONENT;
Luminex Corporation). The concentrations of cytokines in the
tissues were calculated using standard curves of known
concentrations of recombinant mouse cytokines.
Flow cytometric analysis
The percentages of CD4+ IFN-γ+
T cells and CD4+ IL-17+ T cells in mice
cornea and conjunctiva (6 eyes per group) were evaluated using flow
cytometric analysis as previously described (21). Tissues from each group were
surgically removed and immersed in PBS. Subsequently, samples were
torn apart with scissors and incubated with 0.5 mg/ml collagenase
type D (Roche Applied Science) under agitation at 37˚C for 45 min.
The samples were disrupted by grinding using a syringe plunger and
subsequently passed through a cell strainer with a pore size of 100
µm. Cells were then centrifuged for 7 min at 450 x g at 4˚C.
Subsequently, samples were resuspended in PBS containing 1% BSA,
then 2 µl of fluorescein-conjugated anti-CD4 antibody (0.5 mg/ml;
cat. no. 553651; BD Biosciences), phycoerythrin-conjugated
anti-IFN-γ-antibody (0.5 mg/ml; cat. no. 554412; BD Biosciences)
and phycoerythrin-conjugated anti-IL-17 antibody (0.5 mg/ml; cat.
no. 561020; BD Biosciences) were added for an incubation at 4˚C for
30 min. Phycoerythrin-conjugated rat IgG isotype (BD Biosciences)
was used as the control. The percentage of CD4+
IFN-γ+ and CD4+ IL-17+ T cells
were evaluated using a FACSCalibur cytometer with CellQuest
software (version 5.2.1; BD Biosciences).
Histological analysis
Mice eye and adnexa were surgically excised, fixed
in 4% paraformaldehyde overnight at 4˚C and embedded in paraffin.
Sections (thickness, 6 µm) were stained with Periodic Acid-Schiff
reagent (cat. no. 395B-1 KT; MilliporeSigma; Merck KGaA) for 15 min
at room temperature, and those obtained from four animals in each
group were subsequently examined and imaged using a light
microscope (magnification, x10; Olympus Corporation) equipped with
a digital camera. Goblet cell density in the superior and inferior
conjunctiva was measured in three sections from each eye using
Image-Pro version 10.0.5 (Medial Cybernetics, Inc.) and was
expressed as the number of goblet cells per 100 µm.
TUNEL staining
A TUNEL assay was used to detect the 3'hydroxyl ends
of the fragmented DNA as an early event in the apoptotic cascade
and to identify apoptotic cells. Mice eye and adnexa were
surgically excised, fixed in 4% paraformaldehyde overnight at 4˚C
and embedded in paraffin. After deparaffinization and washing, the
samples were rehydrated by sequential immersion. Graded ethanol
washes (100, 95, 85, 70 and 50%) were performed at room temperature
for 3 min each. After rehydration, the tissues were immersed in a
4% methanol-free formaldehyde solution in PBS for 15 min at room
temperature to fix the tissue. The slides were then incubated in 20
µg/ml proteinase K for 10 min at room temperature, before being
rinsed with PBS for 5 min. The samples were subsequently incubated
in terminal deoxynucleotidyl transferase, recombinant, enzyme
containing equilibration buffer and nucleotide mix for 60 min at
37˚C in the dark. The reaction was terminated by adding 2X
saline-sodium citrate buffer for 15 min. The samples were washed
three times with PBS for 5 min and stained with
VECTASHIELD® and DAPI. Staining was evaluated using the
DeadEnd™ Fluorometric TUNEL System (Promega Corporation) according
to the manufacturer's instructions. The images were observed on a
Leica TCS SP5 AOBS laser scanning confocal microscope (Zeiss GmbH)
under an LSM 800 10x (N.A. 0.4) oil objective. Cell images were
obtained separately with the following fluorescence excitation and
emission settings: Excitation wavelengths at 405 and 488 nm and
emission wavelengths between 424-472 and 502-550 nm for TUNEL assay
and DAPI staining, respectively. TUNEL positive cells and nuclear
staining of cells with DAPI in the cornea were viewed under a
fluorescent microscope (magnification, x20).
Statistical analyses
SPSS software (version 18.0; SPSS, Inc.) was used
for all statistical analyses. Data were presented as the means ±
standard deviation. The normal distribution of the data was
verified using Kolmogorov-Smirnov test. Statistical differences for
tear volume, tear film BUT and CSS among the groups were determined
using one-way ANOVA tests followed by Dunnett's post hoc tests
(sphericity assumptions were evaluated with a Mauchly's test, and
in the case of violation, the data were adjusted with an Epsilon
Greenhouse-Geisser statistic). A Kruskal-Wallis test followed by a
Dunn's multiple comparisons post-hoc test was used to compare the
expression levels of NF-κB, the cytokine levels and the goblet cell
density and apoptotic cell density data derived from the flow
cytometric analysis experiments between the groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Tear film parameters on the ocular
surface
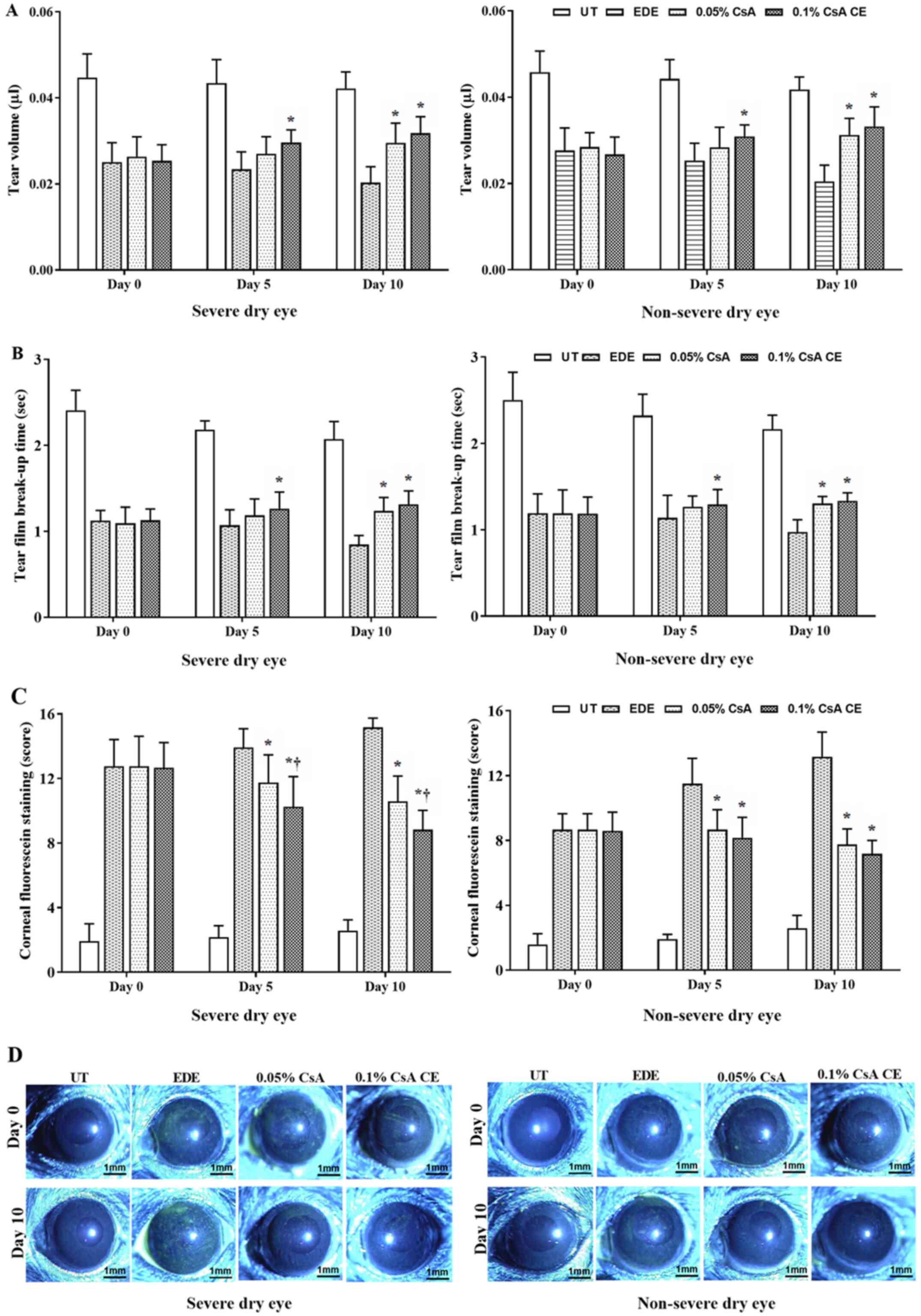
Mean tear volumes of the SDE group at 5 and 10 days
were 0.043±0.005 µl and 0.042±0.004 µl in UT mice, 0.023±0.004 µl
and 0.02±0.004 µl in EDE mice, 0.027±0.004 µl and 0.03±0.005 µl in
0.05% CsA-treated mice, and 0.03±0.003 µl and 0.032±0.004 µl in
0.1% CsA CE-treated mice, respectively. In addition, mean tear
volumes of the NSDE group at 5 and 10 days were 0.044±0.004 µl and
0.042±0.003 µl in UT mice, 0.025±0.004 µl and 0.020±0.004 µl in EDE
mice, 0.028±0.005 µl and 0.031±0.004 µl in 0.05% CsA-treated mice,
and 0.031±0.003 µl and 0.033±0.005 µl in 0.1% CsA CE-treated mice,
respectively (Fig. 2A). Mice
treated with 0.1% CsA CE in the SDE and NSDE groups exhibited a
significant increase in tear volume compared with EDE mice at 5 and
10 days, whereas 0.05% CsA-treated mice in both groups had an
improvement in tear volume at 10 days (all P<0.05). No
significant differences were observed between 0.05% CsA and 0.1%
CsA CE-treated mice from the two groups.
The mean tear film BUTs in the SDE group at 5 and 10
days were 2.18±0.10 and 2.07±0.21 sec in UT mice, 1.07±0.18 and
0.85±0.10 sec in EDE mice, 1.18±0.19 and 1.24±0.16 sec in 0.05%
CsA-treated mice, and 1.26±0.19 and 1.32±0.15 sec in 0.1% CsA
CE-treated mice, respectively. Tear film BUTs in the NSDE group at
5 and 10 days were 2.32±0.25 and 2.16±0.16 sec in the UT mice,
1.14±0.26 and 0.97±0.14 sec in the EDE mice, 1.27±0.12 and
1.30±0.08 sec in the 0.05% CsA mice, and 1.29±0.18 and 1.34±0.09
sec in 0.1% CsA CE-treated mice, respectively (Fig. 2B). Mice treated with 0.1% CsA CE in
the NSDE and SDE groups had a significantly higher tear film BUT
compared with EDE treated mice at 5 and 10 days (all P<0.05);
however, no significant differences were identified with 0.05%
CsA-treated mice. The 0.05% CsA-treated mice in the two groups did
present with an increased tear film BUT compared with EDE mice at
10 days (all P<0.05).
Ocular surface damages
The mean CFSs in the SDE group for UT, EDE, 0.05%
CsA and 0.1% CsA CE-treated mice at days 5 and 10 were 2.17±0.72
and 2.58±0.67, 13.92±1.17 and 15.17±0.58, 11.75±1.71 and
10.58±1.56, and 10.25±1.87 and 8.83±1.19, respectively. Mean CFSs
in the NSDE group at 5 and 10 days were 1.92±0.29 and 2.58±0.79 (UT
mice), 11.50±1.57 and 13.17±1.53 (EDE mice), 8.67±1.23 and
7.75±0.97 (0.05% CsA-treated mice), and 8.17±1.27 and 7.17±0.84
(0.1% CsA CE-treated mice), respectively (Fig. 2C and D). Mice treated with 0.1% CsA CE and
0.05% CsA in both groups exhibited a significantly decreased CFS
compared with EDE mice at 5 and 10 days (all P<0.05). In
addition, in the SDE group, 0.1% CsA CE-treated mice had a
significantly lower CFS compared with 0.05% CsA-treated mice at 5
and 10 days (both P<0.05).
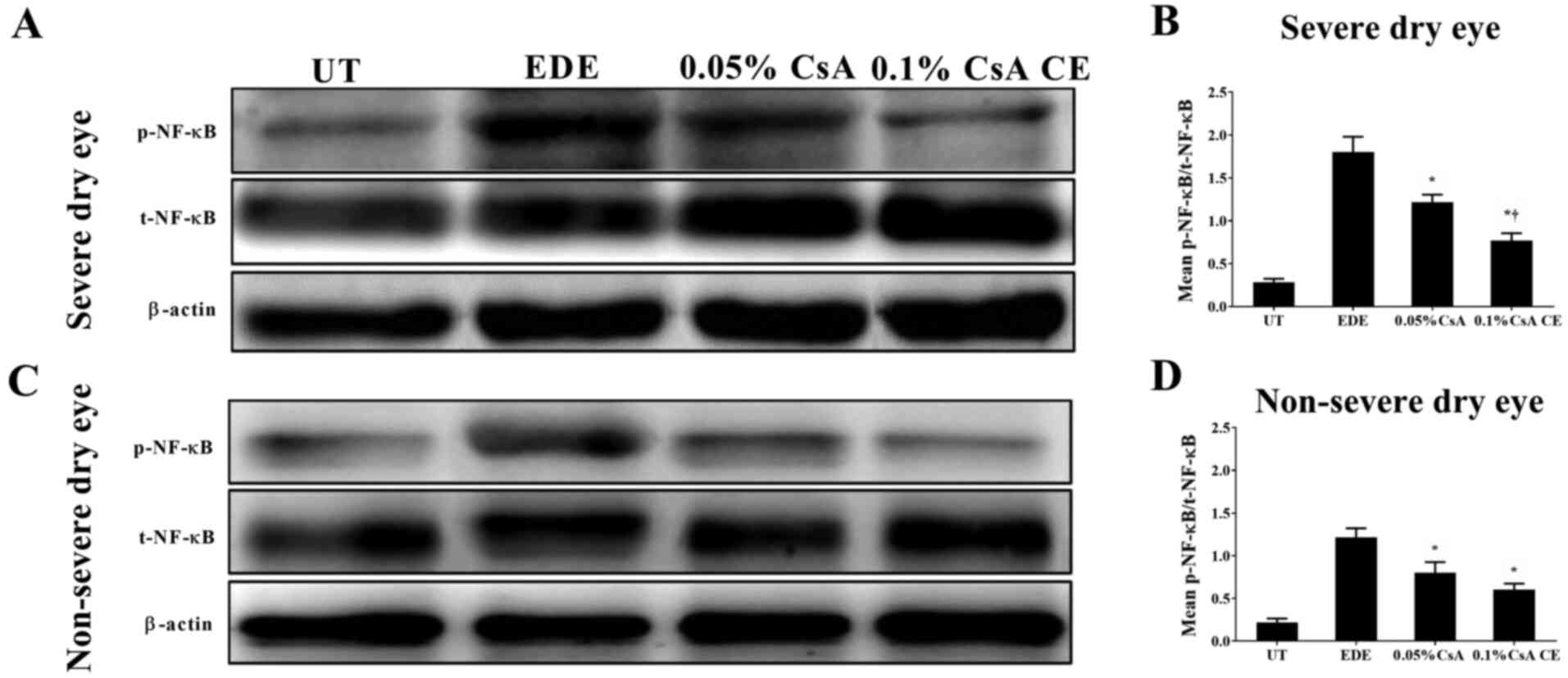
Expression of NF-κB in mice
conjunctiva
To investigate the involvement of NF-κB activation
in the conjunctiva, the expression of total NF-κB p65 and
phosphorylated-NF-κB p65 was evaluated in conjunctival tissues
(Fig. 3). CsA-treated mice in the
SDE and NSED groups showed a decreased expression of NF-κB in the
conjunctiva. Furthermore, in the SDE group, mice treated with 0.1%
CsA CE had a lower NF-κB expression compared with those treated
with 0.05% CsA (all P<0.05).
Inflammatory cytokine levels in
conjunctival tissues
Significantly decreased levels of TNF-α, IL-6 and
IL-17 were observed in the conjunctiva of 0.1% CsA CE and 0.05%
CsA-treated mice compared with EDE mice (all P<0.05). In the SDE
group, mice treated with 0.1% CsA CE exhibited significantly lower
IL-6 and IL-17 levels compared with 0.05% CsA-treated mice (both
P<0.05; Fig. 4A-J).
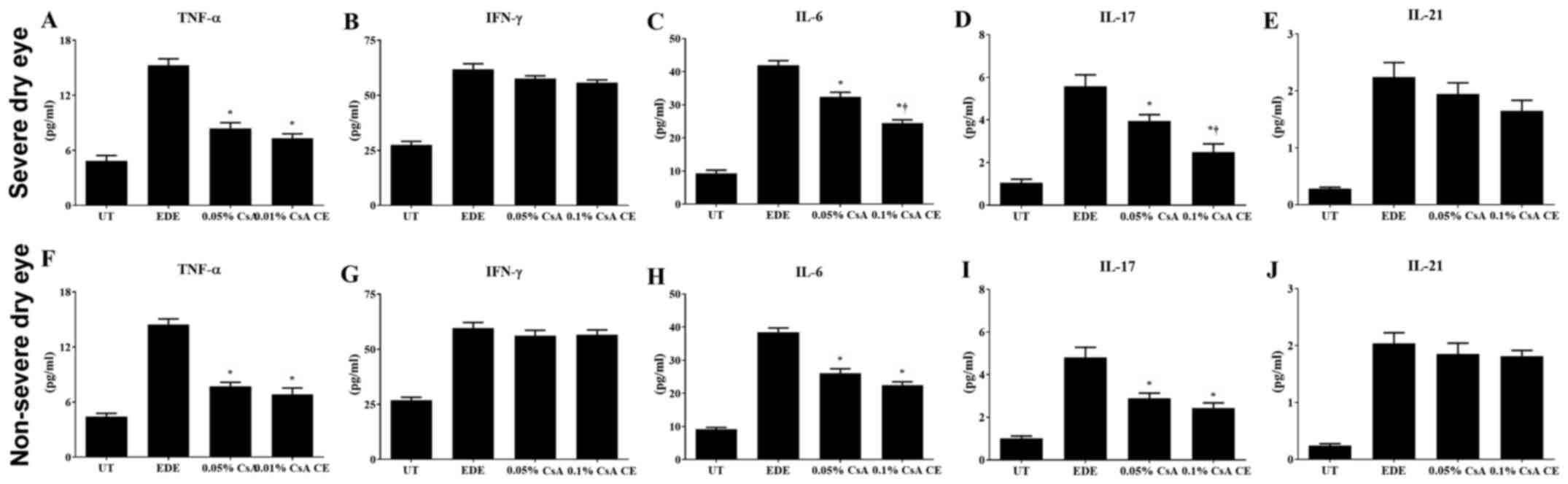 | Figure 4Multiplex immunobead assay for
inflammatory levels. Levels of TNF-α, IFN-γ, IL-6, IL-17, and IL-21
in the conjunctiva in UT, EDE, 0.05% CsA emulsion and 0.1% CsA
CE-treated mice from the (A-E) severe dry eye and (F-J) non-severe
dry eye groups at day 10. *P<0.05 vs. EDE;
†P<0.05 vs. 0.05% CsA. UT, untreated control; EDE,
experimental dry eye; CsA, cyclosporin A; CE, cationic emulsion;
TNF-α, tumor necrosis factor-α; IFN, interferon-γ; IL,
interleukin. |
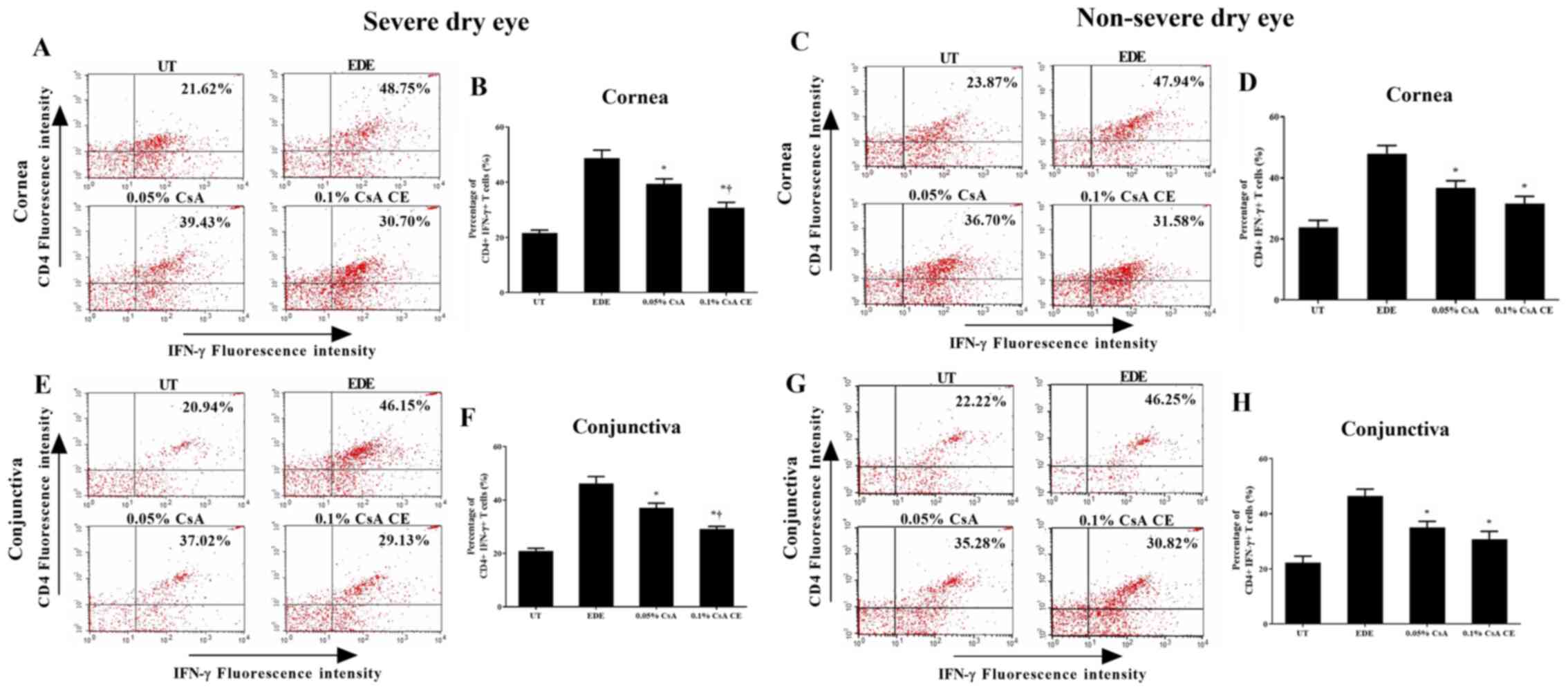
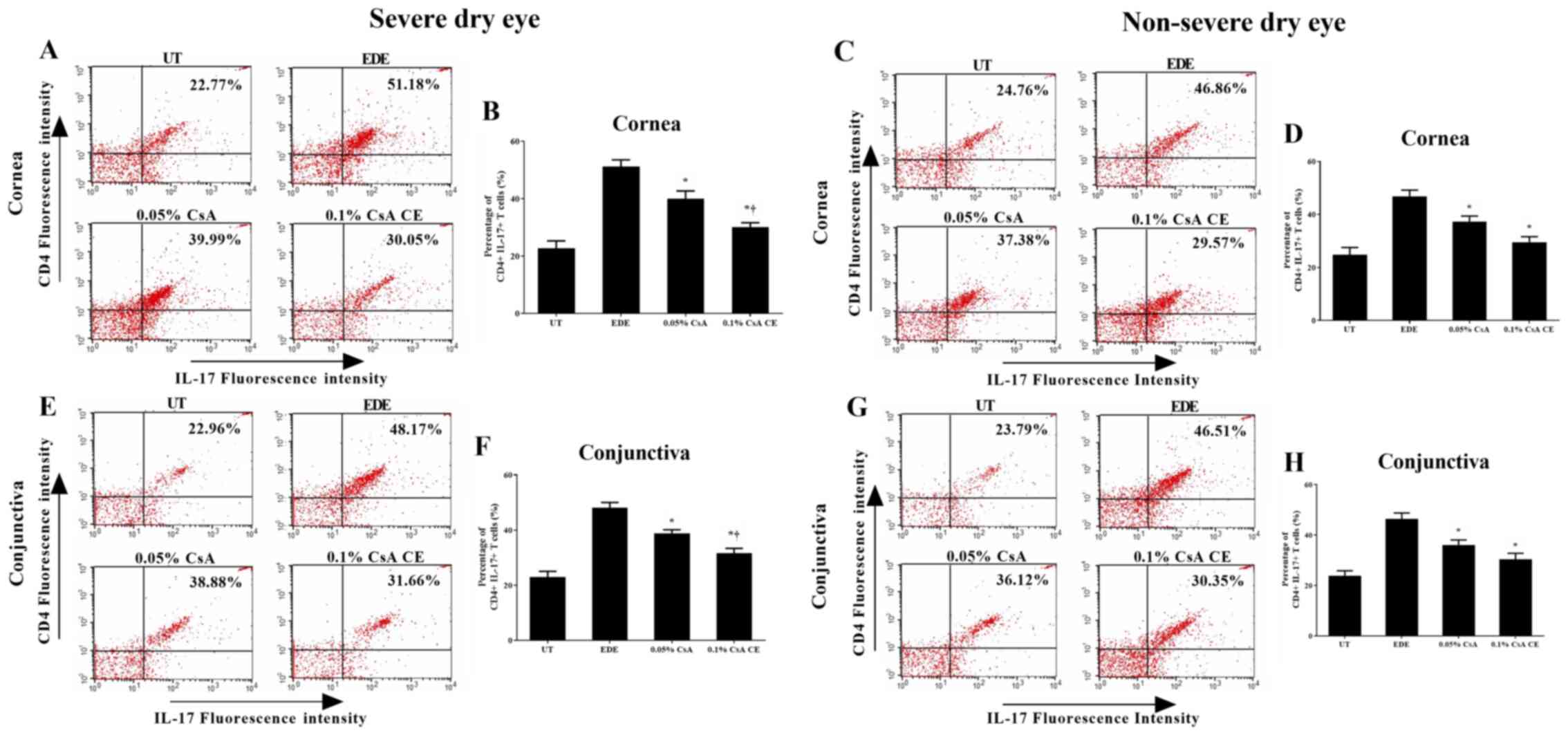
Flow cytometric analysis
The percentage of CD4+ IFN-γ+
and CD4+ IL-17+ T cells in the cornea and
conjunctiva of the SDE and NSDE groups from the UT, EDE, 0.05% CsA,
and 0.1% CsA CE mice treatment groups are presented in Figs. 5 and 6. Mice treated with 0.1% CsA CE and 0.05%
CsA in both groups showed a decreased percentage of CD4+
IFN-γ+ and CD4+ IL-17+ T cells at
10 days (all P<0.05). In addition, in the SDE group, 0.1% CsA
CE-treated mice had significantly lower percentages of
CD4+ IFN-γ+ and CD4+
IL-17+ T cells compared with 0.05% CsA-treated mice (all
P<0.05).
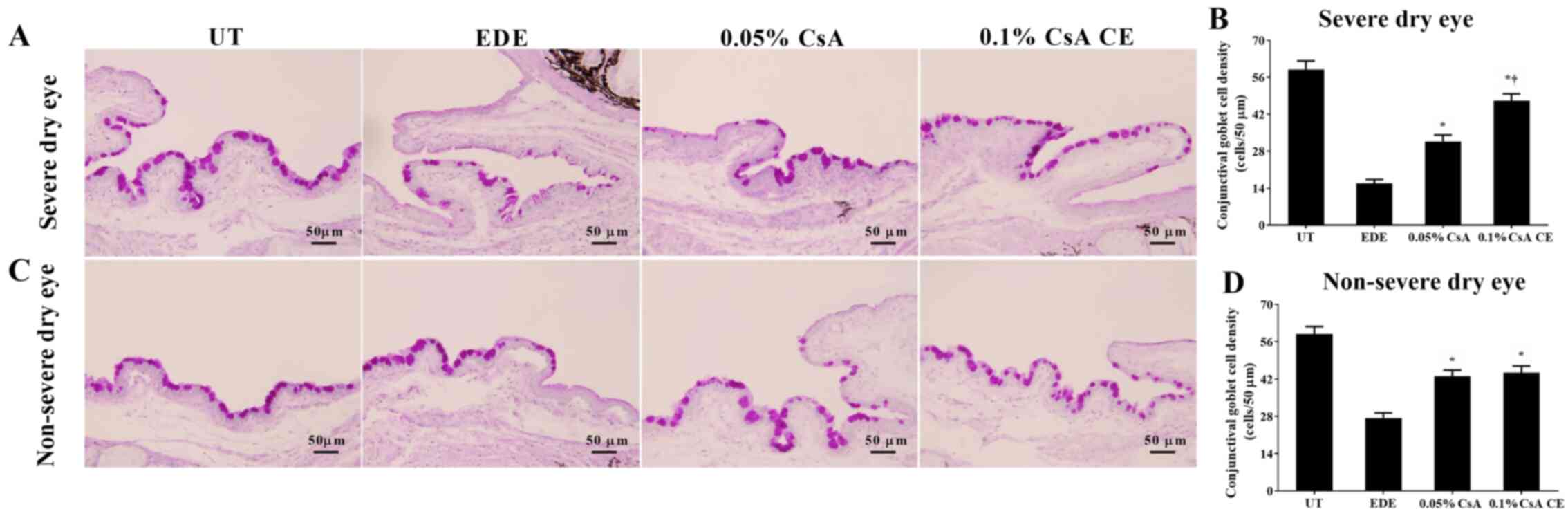
Conjunctival goblet cell density
Mean goblet cell densities in the SDE group for UT,
EDE, 0.05% CsA and 0.1% CsA CE-treated mice were 59.00±7.85
cells/50 µm, 15.83±3.60 cells/50 µm, 31.67±6.12 cells/50 µm and
47.17±6.24 cells/50 µm, respectively (Fig. 7A and B). Mean goblet cell densities in the NSDE
group were 58.83±6.94 cells/50 µm, 27.33±4.89 cells/50 µm,
43.00±5.69 cells/50 µm and 44.33±6.35 cells/50 µm for the UT, EDE,
0.05% CsA and 0.1% CsA CE mice treatment groups, respectively
(Fig. 7C and D). Mice treated with CsA CE in the SDE
and NSDE groups showed a significantly increased density of
conjunctival goblet cells compared with EDE mice (all P<0.05).
Furthermore, in the SDE group, mice treated with 0.1% CsA CE
exhibited significantly higher conjunctival goblet cell densities
compared with those treated with 0.05% CsA (P<0.05).
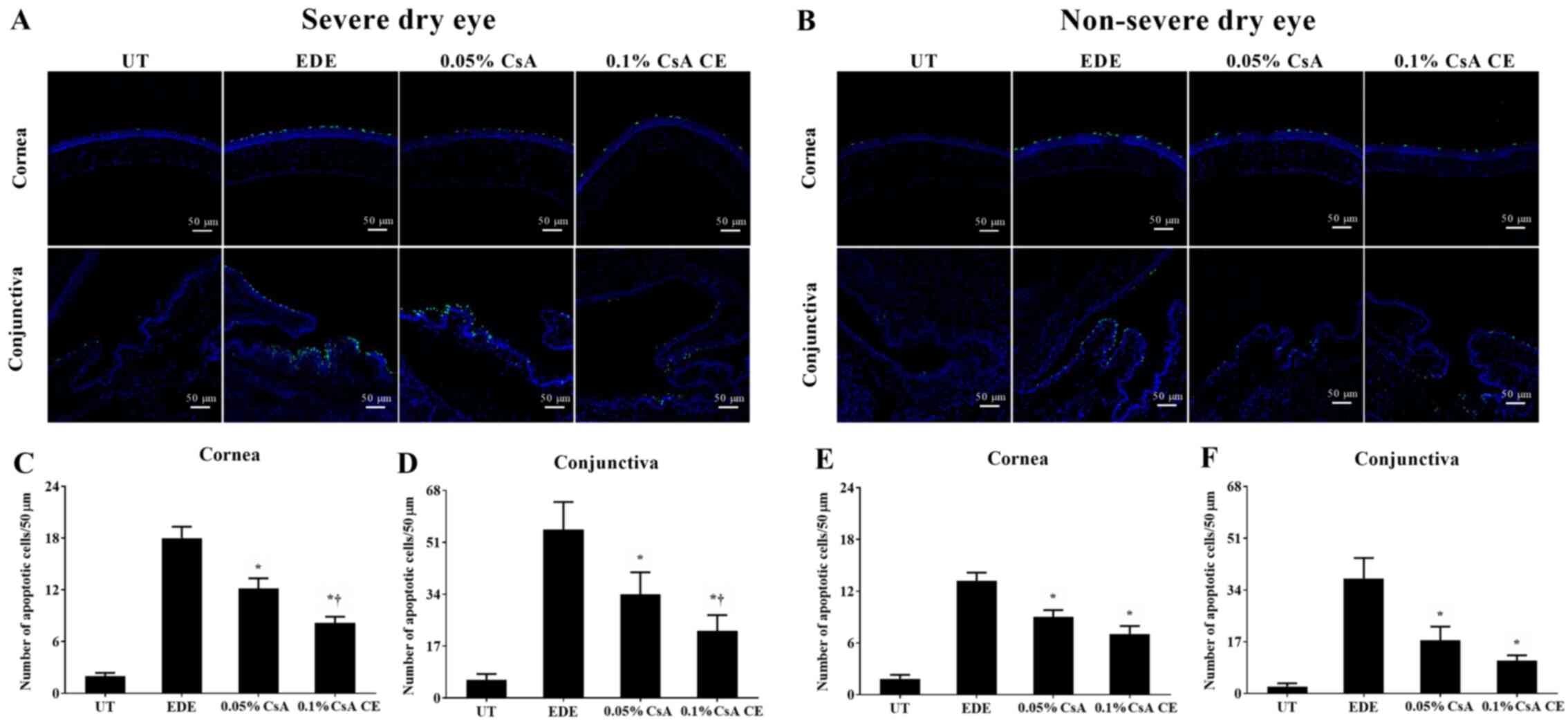
TUNEL staining
Mean apoptotic cell counts in the SDE group for UT,
EDE, 0.05% CsA, and 0.1% CsA CE-treated mice were 2.00±0.89
cells/50 µm, 18.00±3.23 cells/50 µm, 12.17±2.86 cells/50 µm and
8.17±1.72 cells/50 µm in the cornea, and 5.00±2.04 cells/50 µm,
54.83±8.59 cells/50 µm, 34.00±7.16 cells/50 µm and 22.00±5.14
cells/50 µm in the conjunctiva, respectively (Fig. 8A, C and D).
Apoptotic cell counts in the NSDE group were 1.83±1.17 cells/50 µm
and 2.33±1.03 cells/50 µm (UT group), 13.17±2.40 cells/50 µm and
37.83±6.68 cells/50 µm (EDE group), 9.00±2.00 cells/50 µm and
17.50±4.46 cells/50 µm (0.05% CsA-treated group), and 7.00±2.37
cells/50 µm and 10.83±1.72 cells/50 µm (0.1% CsA CE-treated group)
in the cornea and conjunctiva, respectively (Fig. 8B E
and F). Mice treated with CsA in
the SDE and NSDE groups showed a significantly decreased numbers of
apoptotic cells in the corneal and conjunctival tissues compared
with those in the EDE group. Furthermore, in the SDE group, mice
treated with 0.1% CsA CE showed lower numbers of apoptotic cells in
the cornea and conjunctiva compared with those treated with 0.05%
CsA (all P<0.05).
Discussion
Inflammation is a major pathogenic mechanism
underlying DE, ultimately resulting in apoptotic cell death. The
infiltration of T cells and proinflammatory cytokines at the ocular
surface is known to initiate a cascade of events that leads to the
progression of DE indicators and symptoms (22,23).
In the absence of adequate treatment methods, the ocular surface is
gradually damaged that would eventually lead to SDE, which has a
negative impact on the patient's quality of life. The CsA emulsion,
which serves as an important agent for DE treatment, can inhibit
the activation of CD4+ T cells by blocking IL-2
production thereby decreasing apoptosis on the ocular surface
(24).
In the present study, to evaluate the change in tear
film and corneal epithelial damage following CsA emulsion
treatment, tear film BUT and CFSs were evaluated in mice with EDE
of different severities. Mice treated with 0.05% CsA emulsion and
0.1% CsA CE exhibited significantly improved tear volumes, tear
film BUTs and CFSs compared with EDE mice in the SDE and NSDE
groups. Interestingly, in SDE mice, topical application of 0.1% CsA
CE led to a significantly lower CFS on the ocular surface compared
with that of 0.05% CsA emulsion. The significant differences
between groups in terms of therapeutic effect on SDE could be
explained by the cationic property of CsA CE as well as its
concentration. This innovative formulation increases the retention
time of the nanodroplets on the ocular surface, improving therefore
the drug delivery by interacting electrostatically with the
negatively charged components of the tear film (25). A previous study reported that CsA
CE is well tolerated and effectively improves the signs and
symptoms in patients with moderate-to-severe DE to SDE over 6
months, in particular in patients with severe disease who are at
risk of irreversible corneal damage (14). Furthermore, previous clinical
studies demonstrated that 0.1% CsA CE improves the CFS, CFS OSDI
response and conjunctival expression of human leukocyte antigen DR
in SDE cases with keratitis and SS (12-14).
Consistently with these previous reports, the results from the
present study demonstrated that the application of 0.05% CsA
emulsion and 0.1% CsA CE both led to improvements in the clinical
parameters in EDE mice. In addition, the efficacy of 0.1% CsA CE
was more notable than that of the 0.05% CsA emulsion in SDE
mice.
To investigate the inflammatory changes and cell
death on the ocular surface, the expression levels of NF-κB and of
the inflammatory cytokines TNF-α, IL-6 and IL-17 were evaluated,
and the extent of apoptotic cell death on the ocular surface was
also explored. In addition, the percentage of CD4+ T
cells and conjunctival goblet cell density were also measured. The
results demonstrated that mice treated with 0.05% and 0.1% CsA CE
presented with lower expression of NF-κB and decreased levels of
inflammation on the ocular surface. In addition, CsA-treated mice
had a reduced number of apoptotic cells on the ocular surface and
an increased density of conjunctival goblet cells compared with
non-treated groups. Furthermore, in the SDE group, mice treated
with 0.1% CsA CE showed improved results in terms of the reduction
in the number of apoptosis cells and the increased density of
conjunctival goblet cells compared with mice treated with 0.05%
CsA. The CE may be able to enhance film hydration, lubrication and
stability, as the aqueous medium of the emulsion droplets may allow
rehydration, and the oily phase replenishes the lipid layer
(25-27).
In addition, the cationic vehicle of CsA CE itself has been shown
to possess anti-inflammatory effects (28,29).
Chronic inflammation in the epithelium may promote angiogenesis,
invasion and metastasis, and cytokines induce a strong inflammatory
response. Furthermore, the TNF/TNF receptor system plays an
important role in the induction of apoptotic machinery and
inflammation (30,31). Previous studies have reported that
blocking inflammatory cytokines can delay the progression of
diseases, such as rheumatoid arthritis, multiple sclerosis and
inflammatory bowel diseases (30,32,33).
In the present study, the levels of inflammatory cytokines,
including TNF-α, were significantly decreased in the SDE group
following treatment with 0.1% CsA CE. These findings suggested that
0.1% CsA CE may be effective at reducing inflammation and apoptosis
on the ocular surface by downregulating the activation of NF-κB and
cytokines in SDE compared with 0.05% CsA emulsion.
Based on the results of the present and previous
studies, it can be inferred that both higher CsA concentrations and
cationic carriers contributed to the increased therapeutic effect
of 0.1% CsA CE in SDE. However, further studies are required to
provide a more detailed comparative analysis of these two
parameters. In addition, since DE is a chronic disease, it would be
helpful to analyze the long-term therapeutic effects of 0.1% CsA
CE, and to detect CsA concentration in blood in further
experiments.
In summary, the present study demonstrated that
topical 0.1% CsA CE therapy could improve tear film parameters and
ameliorate ocular surface injury and inflammation in SDE and NSDE.
In addition, 0.1% CsA CE was more effective in improving corneal
epithelial injury and T cell-mediated inflammation in SDE than
topical 0.05% CsA emulsion.
Acknowledgements
Not applicable.
Funding
This study was supported by the Technology Innovation Program
(grant no. 20009481) through the Ministry of Trade, Industry &
Energy (MOTIE), Korea, by the Korea Health Technology R&D
project (grant no. HR20C0021050020) through the Korea Health
Industry Development Institute (KHIDI) funded by the Ministry of
Health and Welfare, Korea, and by the Chonnam National University
Hospital Biomedical Research Institute (grant no. BCRI20072).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KCY designed the experiments and revised the
manuscript. RJ, YL and LL performed the experiments. RJ, HJY and JK
analyzed and interpreted the data. RJ, YL and HJY drafted the
manuscript. KCY and RJ confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The research protocol used in the present study was
approved by the Chonnam National University Medical School Research
Institutional Animal Care and Use Committee (approval no. CNU
IACUC-H-2018-73). Maintenance of animals and in vivo
experiments were performed in accordance with the Association for
Research in Vision and Ophthalmology statement for the Use of
Animals in Ophthalmic and Vision Research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Wolffsohn JS, Arita R, Chalmers R,
Djalilian A, Dogru M, Dumbleton K, Gupta PK, Karpecki P, Lazreg S,
Pult H, et al: TFOS DEWS II Diagnostic methodology report (2017).
Ocul Surf. 15:539–574. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li Y, Cui L, Lee HS, Kang YS, Choi W and
Yoon KC: Comparison of 0.3% hypotonic and isotonic sodium
hyaluronate eye drops in the treatment of experimental dry eye.
Curr Eye Res. 42:1108–1114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yoon KC, De Paiva CS, Qi H, Chen Z, Farley
WJ, Li DQ and Pflugfelder SC: Expression of Th-1 chemokines and
chemokine receptors on the ocular surface of C57BL/6 mice: Effects
of desiccating stress. Invest Ophthalmol Vis Sci. 48:2561–2569.
2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pflugfelder SC: Integrating restasis into
the management of dry eye. Int Ophthalmol Clin. 46:101–103.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stapleton F, Alves M, Bunya VY, Jalbert I,
Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, et
al: TFOS DEWS II Epidemiology report (2017). Ocul Surf. 15:334–365.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Baudouin C, Creuzot-Garcher C, Hoang-Xuan
T, Rigeade MC, Brouquet Y, Bassols A, Guillemin I, Benmedjahed K
and Arnould B: Severe impairment of health-related quality of life
in patients suffering from ocular surface diseases. J Fr Ophtalmol.
31:369–378. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Perry HD and Donnenfeld ED: Topical 0.05%
cyclosporin in the treatment of dry eye. Expert Opin Pharmacother.
5:2099–2107. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schultz C: Safety and efficacy of
cyclosporine in the treatment of chronic dry eye. Ophthalmol Eye
Dis. 6:37–42. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Deveney T and Asbell PA: Patient and
physician perspectives on the use of cyclosporine ophthalmic
emulsion 0.05% for the management of chronic dry eye. Clin
Ophthalmol. 12:569–576. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim HS, Kim TI, Kim JH, Yoon KC, Hyon JY,
Shin KU and Choi CY: Evaluation of clinical efficacy and safety of
a novel cyclosporin A nanoemulsion in the treatment of dry eye
syndrome. J Ocul Pharmacol Ther. 33:530–538. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wirta DL, Torkildsen GL, Moreira HR,
Lonsdale JD, Ciolino JB, Jentsch G, Beckert M, Ousler GW, Steven P
and Krösser S: A clinical phase II study to assess efficacy,
safety, and tolerability of waterfree cyclosporine formulation for
treatment of dry eye disease. Ophthalmology. 126:792–800.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Leonardi A, Van Setten G, Amrane M, Ismail
D, Garrigue JS, Figueiredo FC and Baudouin C: Efficacy and safety
of 0.1% cyclosporine A cationic emulsion in the treatment of severe
dry eye disease: A multicenter randomized trial. Eur J Ophthalmol.
26:287–296. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Eroglu YI: A comparative review of Haute
Autorité de Santé and National Institute for Health and Care
Excellence health technology assessments of Ikervis® to
treat severe keratitis in adult patients with dry eye disease which
has not improved despite treatment with tear substitutes. J Mark
Access Health Policy. 5(1336043)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Baudouin C, Figueiredo FC, Messmer EM,
Ismail D, Amrane M, Garrigue JS, Bonini S, Leonardi A and Baudouin
C: A randomized study of the efficacy and safety of 0.1%
cyclosporine A cationic emulsion in treatment of moderate to severe
dry eye. Eur J Ophthalmol. 27:520–530. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
de Oliveira RC and Wilson SE: Practical
guidance for the use of cyclosporine ophthalmic solutions in the
management of dry eye disease. Clin Ophthalmol. 13:1115–1122.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yoon KC, De Paiva CS, Qi H, Chen Z, Farley
WJ, Li DQ, Stern ME and Pflugfelder SC: Desiccating environmental
stress exacerbates autoimmune lacrimal keratoconjunctivitis in
non-obese diabetic mice. J Autoimmun. 30:212–221. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yoon KC, Ahn KY, Choi W, Li Z, Choi JS,
Lee SH and Park SH: Tear production and ocular surface changes in
experimental dry eye after elimination of desiccating stress.
Invest Ophthalmol Vis Sci. 52:7267–7273. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Villareal AL, Farley W and Pflugfelder SC:
Effect of topical ophthalmic epinastine and olopatadine on tear
volume in mice. Eye Contact Lens. 32:272–276. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pauly A, Brignole-Baudouin F, Labbé A,
Liang H, Warnet JM and Baudouin C: New tools for the evaluation of
toxic ocular surface changes in the rat. Invest Ophthalmol Vis Sci.
48:5473–5483. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li Y, Jin R, Li L, Yoon HJ, Choi JH, Park
JH, Liu Z, Li W, Li Z and Yoon KC: Expression and role of
nucleotide-binding oligomerization domain 2 (NOD2) in the ocular
surface of murine dry eye. Invest Ophthalmol Vis Sci. 60:2641–2649.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoon KC, Park CS, You IC, Choi HJ, Lee KH,
Im SK, Park HY and Pflugfelder SC: Expression of CXCL9, -10, -11,
and CXCR3 in the tear film and ocular surface of patients with dry
eye syndrome. Invest Ophthalmol Vis Sci. 51:643–650.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
The definition and classification of dry
eye disease. Report of the Definition and Classification
Subcommittee of the International Dry Eye WorkShop (2007). Ocul
Surf. 5:75–92. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Baudouin C, Aragona P, Messmer EM,
Tomlinson A, Calonge M, Boboridis KG, Akova YA, Geerling G,
Labetoulle M and Rolando M: Role of hyperosmolarity in the
pathogenesis and management of dry eye disease: Proceedings of the
OCEAN group meeting. Ocul Surf. 11:246–258. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ambroziak AM, Szaflik J, Szaflik JP,
Ambroziak M, Witkiewicz J and Skopiński P: Immunomodulation on the
ocular surface: A review. Cent Eur J Immunol. 41:195–208.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lallemand F, Daull P, Benita S, Buggage R
and Garrigue JS: Successfully improving ocular drug delivery using
the cationic nanoemulsion, novasorb. J Drug Deliv.
2012(604204)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rabinovich YI, Vakarelski IU, Brown SC,
Singh PK and Moudgil BM: Mechanical and thermodynamic properties of
surfactant aggregates at the solid-liquid interface. J Colloid
Interface Sci. 270:29–36. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Royle L, Matthews E, Corfield A, Berry M,
Rudd PM, Dwek RA and Carrington SD: Glycan structures of ocular
surface mucins in man, rabbit and dog display species differences.
. 25:763–773. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Daull P, Guenin S, Hamon de Almeida V and
Garrigue JS: Anti-inflammatory activity of CKC-containing cationic
emulsion eye drop vehicles. Mol Vis. 24:459–470. 2018.PubMed/NCBI
|
|
29
|
Hwang SB, Park JH, Kang SS, Kang DH, Lee
JH, Oh SJ, Lee JY, Kim JY and Tchah H: Protective effects of
cyclosporine A emulsion versus cyclosporine A cationic emulsion
against desiccation stress in human corneal epithelial cells.
Cornea. 39:508–513. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jurisic V, Terzic T, Colic S and Jurisic
M: The concentration of TNF-alpha correlate with number of
inflammatory cells and degree of vascularization in radicular
cysts. Oral Dis. 14:600–605. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang S, Wang J, Brand DD and Zheng SG:
Role of TNF-TNF Receptor 2 signal in regulatory T cells and its
therapeutic implications. Front Immunol. 9(784)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pegoretti V, Baron W, Laman JD and Eisel
UL: Selective modulation of TNF-TNFRs signaling: Insights for
multiple sclerosis treatment. Front Immunol. 9(925)2018.PubMed/NCBI View Article : Google Scholar
|