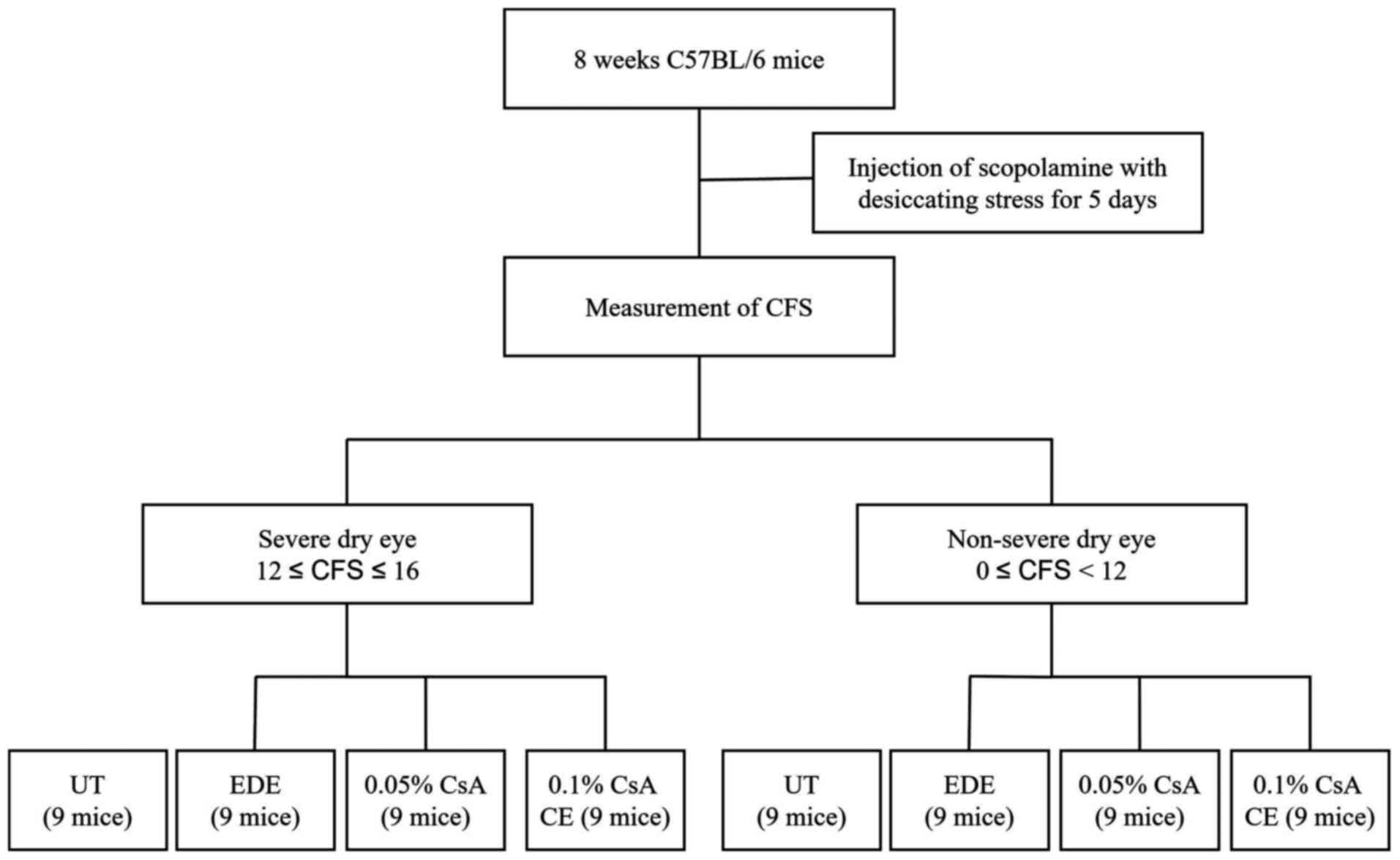
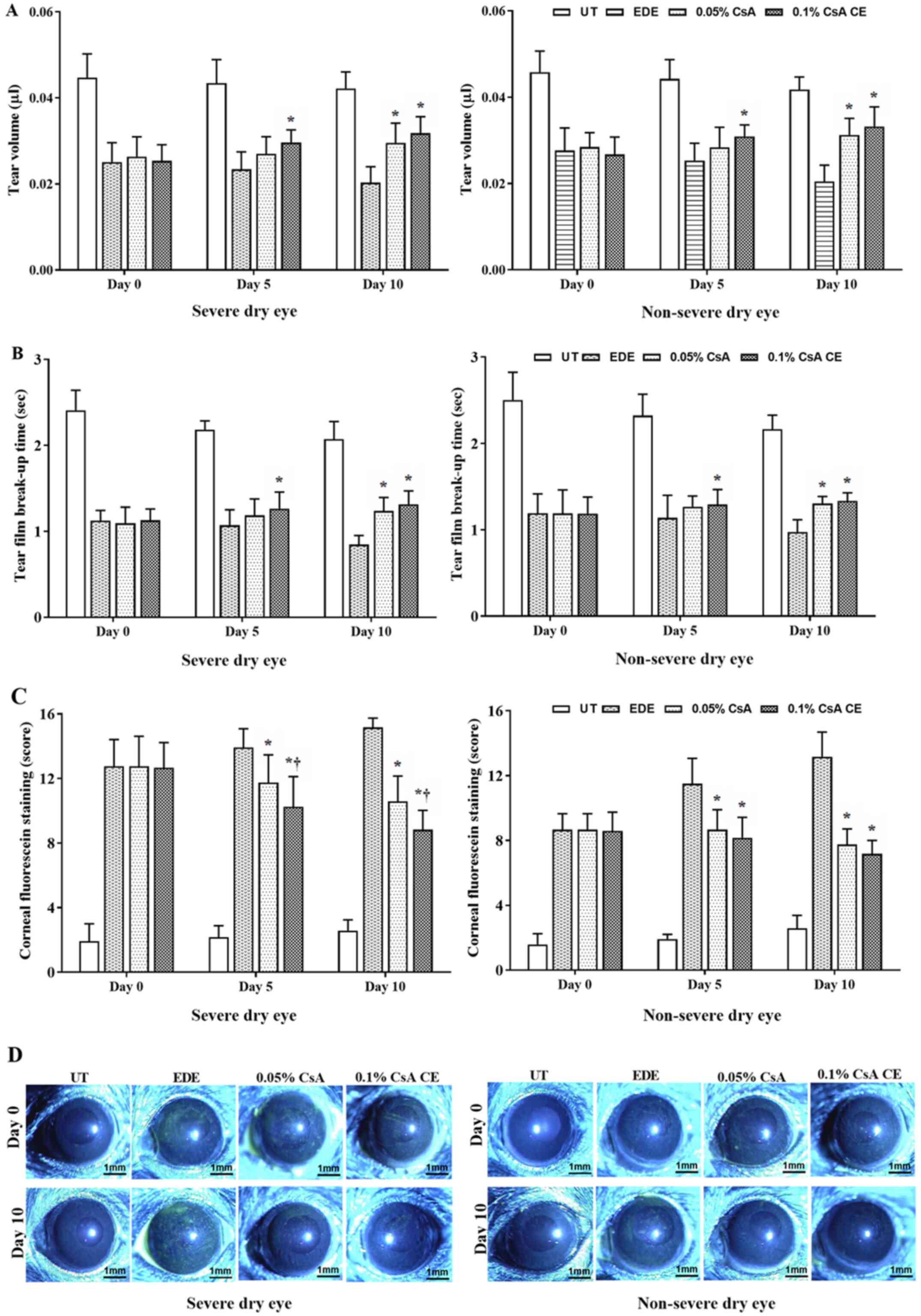
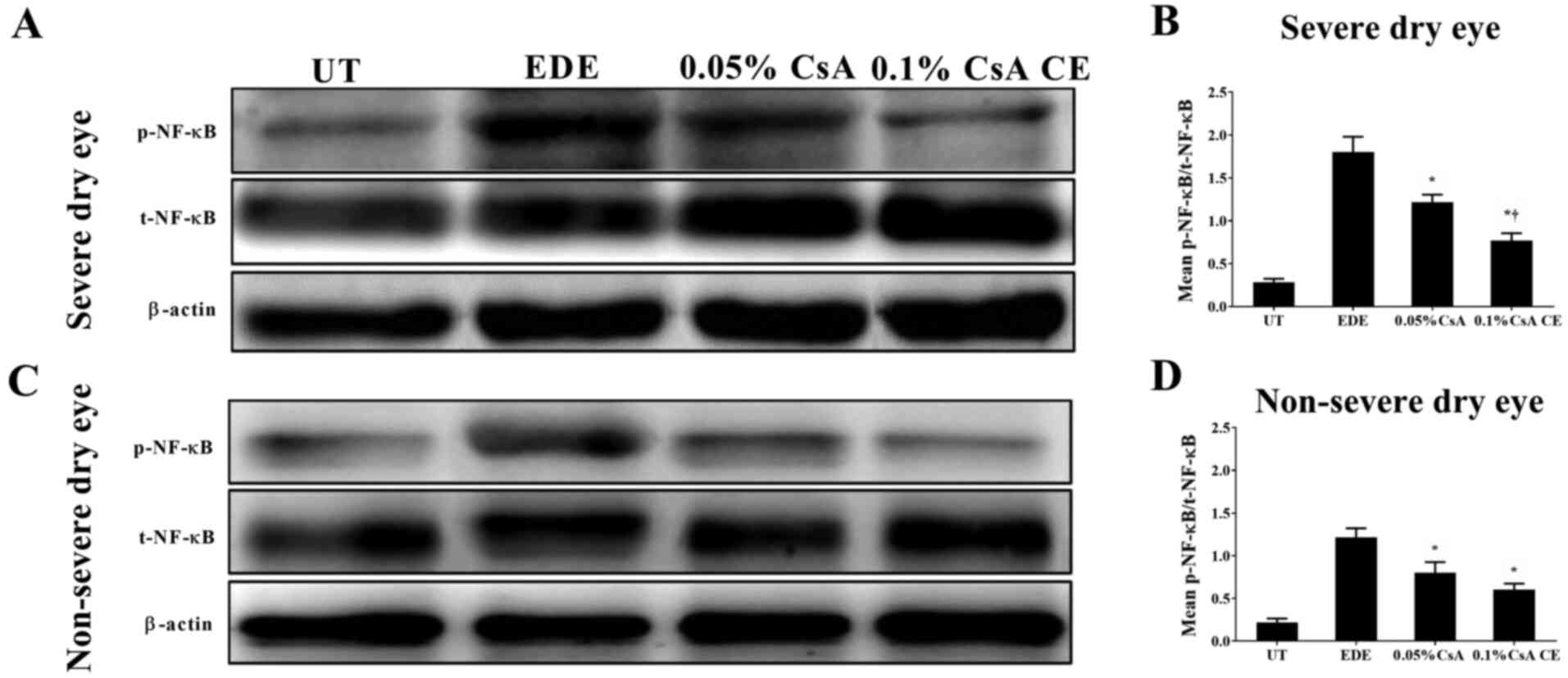
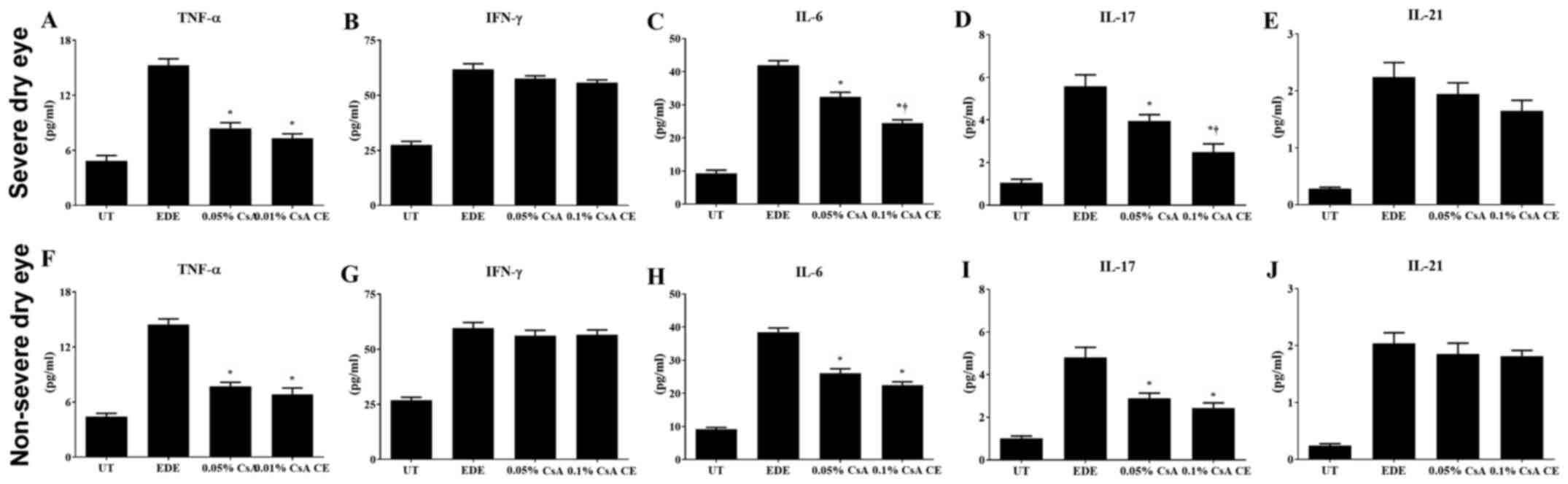
|
1
|
Wolffsohn JS, Arita R, Chalmers R,
Djalilian A, Dogru M, Dumbleton K, Gupta PK, Karpecki P, Lazreg S,
Pult H, et al: TFOS DEWS II Diagnostic methodology report (2017).
Ocul Surf. 15:539–574. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li Y, Cui L, Lee HS, Kang YS, Choi W and
Yoon KC: Comparison of 0.3% hypotonic and isotonic sodium
hyaluronate eye drops in the treatment of experimental dry eye.
Curr Eye Res. 42:1108–1114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yoon KC, De Paiva CS, Qi H, Chen Z, Farley
WJ, Li DQ and Pflugfelder SC: Expression of Th-1 chemokines and
chemokine receptors on the ocular surface of C57BL/6 mice: Effects
of desiccating stress. Invest Ophthalmol Vis Sci. 48:2561–2569.
2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pflugfelder SC: Integrating restasis into
the management of dry eye. Int Ophthalmol Clin. 46:101–103.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stapleton F, Alves M, Bunya VY, Jalbert I,
Lekhanont K, Malet F, Na KS, Schaumberg D, Uchino M, Vehof J, et
al: TFOS DEWS II Epidemiology report (2017). Ocul Surf. 15:334–365.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Baudouin C, Creuzot-Garcher C, Hoang-Xuan
T, Rigeade MC, Brouquet Y, Bassols A, Guillemin I, Benmedjahed K
and Arnould B: Severe impairment of health-related quality of life
in patients suffering from ocular surface diseases. J Fr Ophtalmol.
31:369–378. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Perry HD and Donnenfeld ED: Topical 0.05%
cyclosporin in the treatment of dry eye. Expert Opin Pharmacother.
5:2099–2107. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schultz C: Safety and efficacy of
cyclosporine in the treatment of chronic dry eye. Ophthalmol Eye
Dis. 6:37–42. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Deveney T and Asbell PA: Patient and
physician perspectives on the use of cyclosporine ophthalmic
emulsion 0.05% for the management of chronic dry eye. Clin
Ophthalmol. 12:569–576. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim HS, Kim TI, Kim JH, Yoon KC, Hyon JY,
Shin KU and Choi CY: Evaluation of clinical efficacy and safety of
a novel cyclosporin A nanoemulsion in the treatment of dry eye
syndrome. J Ocul Pharmacol Ther. 33:530–538. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wirta DL, Torkildsen GL, Moreira HR,
Lonsdale JD, Ciolino JB, Jentsch G, Beckert M, Ousler GW, Steven P
and Krösser S: A clinical phase II study to assess efficacy,
safety, and tolerability of waterfree cyclosporine formulation for
treatment of dry eye disease. Ophthalmology. 126:792–800.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Leonardi A, Van Setten G, Amrane M, Ismail
D, Garrigue JS, Figueiredo FC and Baudouin C: Efficacy and safety
of 0.1% cyclosporine A cationic emulsion in the treatment of severe
dry eye disease: A multicenter randomized trial. Eur J Ophthalmol.
26:287–296. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Eroglu YI: A comparative review of Haute
Autorité de Santé and National Institute for Health and Care
Excellence health technology assessments of Ikervis® to
treat severe keratitis in adult patients with dry eye disease which
has not improved despite treatment with tear substitutes. J Mark
Access Health Policy. 5(1336043)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Baudouin C, Figueiredo FC, Messmer EM,
Ismail D, Amrane M, Garrigue JS, Bonini S, Leonardi A and Baudouin
C: A randomized study of the efficacy and safety of 0.1%
cyclosporine A cationic emulsion in treatment of moderate to severe
dry eye. Eur J Ophthalmol. 27:520–530. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
de Oliveira RC and Wilson SE: Practical
guidance for the use of cyclosporine ophthalmic solutions in the
management of dry eye disease. Clin Ophthalmol. 13:1115–1122.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yoon KC, De Paiva CS, Qi H, Chen Z, Farley
WJ, Li DQ, Stern ME and Pflugfelder SC: Desiccating environmental
stress exacerbates autoimmune lacrimal keratoconjunctivitis in
non-obese diabetic mice. J Autoimmun. 30:212–221. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yoon KC, Ahn KY, Choi W, Li Z, Choi JS,
Lee SH and Park SH: Tear production and ocular surface changes in
experimental dry eye after elimination of desiccating stress.
Invest Ophthalmol Vis Sci. 52:7267–7273. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Villareal AL, Farley W and Pflugfelder SC:
Effect of topical ophthalmic epinastine and olopatadine on tear
volume in mice. Eye Contact Lens. 32:272–276. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pauly A, Brignole-Baudouin F, Labbé A,
Liang H, Warnet JM and Baudouin C: New tools for the evaluation of
toxic ocular surface changes in the rat. Invest Ophthalmol Vis Sci.
48:5473–5483. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li Y, Jin R, Li L, Yoon HJ, Choi JH, Park
JH, Liu Z, Li W, Li Z and Yoon KC: Expression and role of
nucleotide-binding oligomerization domain 2 (NOD2) in the ocular
surface of murine dry eye. Invest Ophthalmol Vis Sci. 60:2641–2649.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoon KC, Park CS, You IC, Choi HJ, Lee KH,
Im SK, Park HY and Pflugfelder SC: Expression of CXCL9, -10, -11,
and CXCR3 in the tear film and ocular surface of patients with dry
eye syndrome. Invest Ophthalmol Vis Sci. 51:643–650.
2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
The definition and classification of dry
eye disease. Report of the Definition and Classification
Subcommittee of the International Dry Eye WorkShop (2007). Ocul
Surf. 5:75–92. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Baudouin C, Aragona P, Messmer EM,
Tomlinson A, Calonge M, Boboridis KG, Akova YA, Geerling G,
Labetoulle M and Rolando M: Role of hyperosmolarity in the
pathogenesis and management of dry eye disease: Proceedings of the
OCEAN group meeting. Ocul Surf. 11:246–258. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ambroziak AM, Szaflik J, Szaflik JP,
Ambroziak M, Witkiewicz J and Skopiński P: Immunomodulation on the
ocular surface: A review. Cent Eur J Immunol. 41:195–208.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lallemand F, Daull P, Benita S, Buggage R
and Garrigue JS: Successfully improving ocular drug delivery using
the cationic nanoemulsion, novasorb. J Drug Deliv.
2012(604204)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rabinovich YI, Vakarelski IU, Brown SC,
Singh PK and Moudgil BM: Mechanical and thermodynamic properties of
surfactant aggregates at the solid-liquid interface. J Colloid
Interface Sci. 270:29–36. 2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Royle L, Matthews E, Corfield A, Berry M,
Rudd PM, Dwek RA and Carrington SD: Glycan structures of ocular
surface mucins in man, rabbit and dog display species differences.
. 25:763–773. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Daull P, Guenin S, Hamon de Almeida V and
Garrigue JS: Anti-inflammatory activity of CKC-containing cationic
emulsion eye drop vehicles. Mol Vis. 24:459–470. 2018.PubMed/NCBI
|
|
29
|
Hwang SB, Park JH, Kang SS, Kang DH, Lee
JH, Oh SJ, Lee JY, Kim JY and Tchah H: Protective effects of
cyclosporine A emulsion versus cyclosporine A cationic emulsion
against desiccation stress in human corneal epithelial cells.
Cornea. 39:508–513. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jurisic V, Terzic T, Colic S and Jurisic
M: The concentration of TNF-alpha correlate with number of
inflammatory cells and degree of vascularization in radicular
cysts. Oral Dis. 14:600–605. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang S, Wang J, Brand DD and Zheng SG:
Role of TNF-TNF Receptor 2 signal in regulatory T cells and its
therapeutic implications. Front Immunol. 9(784)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pegoretti V, Baron W, Laman JD and Eisel
UL: Selective modulation of TNF-TNFRs signaling: Insights for
multiple sclerosis treatment. Front Immunol. 9(925)2018.PubMed/NCBI View Article : Google Scholar
|