Introduction
IL-4 has been recognized as an important
inflammatory regulatory cytokine that activates the differentiation
of T cells into CD4+ T helper type 2 (Th2) subsets
(1,2) and exerts an anti-inflammatory effect
in macrophages (3). IL-4 functions
in combination with two types of receptors: IL-4RI (type I IL-4
receptor), a heterodimer of IL-4Rα and a common gamma-chain, and
IL-4RII (type II IL-4 receptor), which consists of an IL-4Rα and an
IL-13Rα1 chain (4,5). A previous study indicated that IL-4
mediated immune responses in different types of cells by combining
with different receptor sets and that IL-4 mutein exerted a
Th2-deviation characteristic (6).
In addition, this previous study demonstrated that the
receptor-selective IL-4 mutein Q116E modulated the inflammatory
phenotype of vascular cells and macrophages, and successfully
attenuated atherogenesis in a mouse model.
Choroidal neovascularization (CNV) is a major cause
of reduced vision in patients with such diseases as age-related
macular degeneration (AMD) and pathologic myopia (7). CNV may invade the subretinal space and
cause pathological consequences, including retinal edema,
detachment and hemorrhage (8). The
causative factors that trigger CNV formation and the cascade of
events during the pathogenesis of CNV have not been fully
elucidated. However, progressive inflammatory event cascades and
macrophage infiltration are widely considered to contribute to CNV
(8). Zandi et al (9) reported that M1 macrophages were
dominant in dry AMD, and that M2 macrophages enhanced CNV.
Moreover, two types of macrophages can be found in CNV lesion sites
of the patient at the same time (10). M2 macrophages upregulate the
expression of vascular endothelial growth factor (VEGF) and promote
neovascularization (11,12). Further studies have shown that the
polarization of M2 macrophages was induced by exogenous stimulators
inside the eyes, and intravitreal injection of M2 macrophages
induced CNV (9,13). These studies have confirmed that the
activation, aggregation and inflammatory factor release of
macrophages are closely related to the occurrence and development
of CNV. Different types of polarized macrophages play opposite
roles: M1 macrophages are related to dry AMD, and M2 macrophages
induce the occurrence of CNV (9).
To date, there is no strategy put in place to regulate the
polarization of macrophages to affect the formation of CNV.
To explore the therapeutic potential of
IL-4-mediated immune responses in CNV, the present study
investigated the effects of two receptor-selective IL-4 muteins on
laser-induced CNV in mice. Single mutant IL-4/Q116E acted as an
IL-4RI-specific agonist without activation of IL-4RII, and double
mutant IL-4/Q116E/Y120D acted as an antagonist of IL-4RI and
IL-4RII, as proven in a previous study (6). The present study hypothesized that
IL-4 and its muteins can modulate the inflammatory phenotypes of
macrophages through different receptors, and will affect CNV in a
mouse model.
Materials and methods
Experimental animals
A total of 84 male 8-week-old C57BL/6 mice (18-20 g)
(Cyagen Biosciences, Inc.) were used in the present study. Mice
were bred under standard conditions (humidity, 55%; room
temperature, 23±1˚C; and 12-h dark-light cycle) with free access to
chow and water. The study protocol was reviewed and approved by the
Animal Care and Use Committees of the Third Xiangya Hospital of
Central South University (Changsha, China).
Pre-experimental protocol for the
establishment of a laser-induced CNV mouse model
Mice were anesthetized with intraperitoneal
injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Pupils
were then dilated with a drop of 1% tropicamide (Wuhan Wujing
Medicine, Co., Ltd.). One eye of each mouse was left untreated as a
control, and the other eye was irradiated with a 532-nm laser
(integrated radiotherapy imaging system; 75 µm spot size; 0.1 sec
interval; 0.1 sec duration; 100 mw power), which created 4-6 injury
spots evenly distributed around the optic disc. The eyes were
observed in vivo with a MICRON IV microscope (Phoenix
Technology Group, LLC). A bubble formed at a laser-induced injury
spot indicated the rupture of Bruch's membrane, which serves an
important role in inducing neovascularization (7). Therefore, a total of 24 mice with eyes
presenting bubbles formed at laser-induced injury spots were
included in the study. Histological examination and
immunofluorescence microscopy were performed on day 7 after laser
coagulation. One mouse failed to wake up after laser
photocoagulation, potentially because of an intolerance to ketamine
and xylazine.
Histological examination and
immunofluorescence microscopy
The mice were sacrificed by an overdose of
pentobarbital (150 mg/kg), and death was confirmed by a lack of
pulse. The eyeballs were fixed with 4% neutral formaldehyde
solution (room temperature, 2 h). The corneas and lenses were
removed from the eyes, and the remaining eyecups were then
snap-frozen in Tissue-Tek® O.C.T.™ Compound (Sakura
Finetek Japan Co., Ltd.). Serial cryostat sections (6-µm thickness)
of the eyecups were prepared and stained with H&E by standard
methods.
For immunofluorescence microscopy, slides were
blocked with PBS containing 1% BSA and 0.3% Triton X-100 for 30 min
at room temperature, incubated with primary (4˚C, overnight) and
secondary (room temperature, 2 h) antibodies and counterstained
with DAPI (cat. no. D8417; Sigma-Aldrich; Merck KGaA) for 10 min at
room temperature. Slides were covered with coverslips, assessed and
photographed by confocal laser scanning microscopy (Nikon
Corporation). Primary antibodies included Alexa Fluor 647
anti-mouse CD206 (1:200; cat. no. 141712; BioLegend, Inc.),
fluorescein isothiocyanate anti-mouse CD80 (1:200; cat. no.
11-0801; eBioscience; Thermo Fisher Scientific, Inc.), rat
anti-mouse EGF-like module-containing mucin-like hormone
receptor-like 1 (F4/80; 1:400; cat. no. 14-4801; eBioscience,
Thermo Fisher Scientific, Inc.), goat anti-mouse delta-like 4
(Dll4; 1:400; cat. no. PA5-46974; Invitrogen; Thermo Fisher
Scientific, Inc.) and goat anti-mouse CD31 (1:200; cat. no. AF3628;
R&D Systems, Inc.). Secondary antibodies included donkey
anti-goat IgG (1:500; Alexa Fluor 488; cat. no. CA11055S;
Invitrogen; Thermo Fisher Scientific, Inc.), goat anti-rat
IgG-biotin (1:500; cat. no. BA-9400; Maravai LifeSciences) and
donkey anti-mouse IgG (1:500; Alexa Fluor 488; cat. no. CA21202S,
Invitrogen; Thermo Fisher Scientific, Inc.). Cells with
CD68-positive signals were identified as M1 macrophages, while
cells with CD206-positive signals were identified as M2 macrophages
(11).
Construction and in vivo transfection
of recombinant IL-4-expressing adenoviruses
Adenovirus vectors expressing murine IL-4
were constructed as previously described (6). Briefly, the sequence of murine
IL-4 in pcDNA3.1 (Thermo Fisher Scientific, Inc.)was
subcloned into the shuttle vector pCMVAdvLink1 (Thermo Fisher
Scientific, Inc.), and then co-transfected with the human
adenovirus mutant dl7001 into AD293 cells (Agilent
Technologies, Inc.) to generate an adenovirus-expressed IL-4
wild-type (WT) vector, AdIL-4/Q116E (adenovirus-expressed IL-4,
with Q116E mutation) and AdIL-4/Q116D/Y119D (adenovirus-expressed
IL-4, with Q116D and Y119D mutations) vectors by homologous
recombination. AdLacZ (adenovirus-expressed β-galactosidase) vector
was used as a control in each experiment. For each mouse,
1x108 plaque forming units of adenovirus vectors were
diluted in 0.1 ml PBS and injected into the tail vein. For the CNV
study, mice were injected with recombinant adenovirus vectors (n=3
for each condition) and then received laser treatment 1 day later.
At day 7 after laser coagulation, the tissue samples were collected
for use. Secreted IL-4 protein in the serum was measured by ELISA
(cat. no. S4050; R&D Systems, Inc.) according to the
manufacturer's instructions. IL-4 protein (~2-3 ng/ml) was detected
7 days after adenovirus infection (Fig. S1), indicating comparable IL-4
concentrations in the serum through adenovirus injection.
Fluorescein fundus angiography (FFA) and optical coherence
tomography (OCT). Mice were anesthetized with an intraperitoneal
injection of ketamine (100 mg/kg) and xylazine (10 mg/kg), and the
pupils were dilated with a drop of 1% tropicamide. Then, FFA and
OCT were performed on both eyes of each mouse using a MICRON IV
retinal imaging microscope (Phoenix Technology Group, LLC) 3 min
after intraperitoneal injection of 2.5% fluorescein sodium
(Guangzhou Baiyunshan Mingxing Pharmaceutical Co., Ltd.). All
images were recorded within 5-8 min of injection. For images of
FFA, the fluorescent leakage intensity was graded as follows: 1,
normal; 2, nonperfusion; 3, slight leakage; 4, moderate leakage;
and 5, obvious leakage. For the OCT data, the images were graded as
follows: 1, normal; 2, subretinal hyperreflective material with
nonfibrotic scar; 3, subretinal hyperreflective material with
fibrotic scar; and 4, intraretinal fibrotic scar.
Western blotting
Whole protein extracts were isolated from isolated
retinal pigment epithelium (RPE)-choroid tissues with RIPA buffer
(Santa Cruz Biotechnology, Inc.) and were then quantified with
Quick Start™ Bradford Protein Assay kit (Bio-Rad Laboratories,
Inc.). Subsequently, 20 mg of protein extracts were resolved by 10%
SDS-PAGE gel and transferred onto PVDF membranes (Immobilon™;
Sigma-Aldrich; Merck KGaA). The membranes were blocked with 5%
fat-free milk for 30 min at room temperature, probed for overnight
at 4˚C with rabbit polyclonal anti-Dll4 (1:200; Abcam), goat
polyclonal anti-delta-like 1 (Dll1; 1:200; cat. no. ab85346,
Abcam), goat polyclonal anti-CD80 (1:500; cat. no. AF740, R&D
Systems, Inc.), rabbit polyclonal anti-CD206 (1:500, cat. no.
ab64693, Abcam), rabbit monoclonal anti-monocyte to macrophage
differentiation associated (MMD) (1:200; cat. no. ab173967, Abcam),
anti-β-actin antibody (1:2,000; cat. no. AC-15; Sigma-Aldrich;
Merck KGaA), and then incubated with peroxidase-conjugated
anti-rabbit-IgG (cat. no. A21020) or anti-goat-IgG (cat. no.
A21030) (both 1:1,000; Abbkine Scientific Co., Ltd.) for 2 h at
room temperature. The proteins were detected using ImmunoStar LD
reagents (Wako Pure Chemical Industries, Ltd.) and visualized with
a luminescent imager (Ez-Capture; ATTO Corporation).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from isolated RPE-choroid
tissues with TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). A total of 1 µg of extracted RNA per sample was
reverse transcribed using a PrimeScript™ II Reverse Transcriptase
kit (Takara Bio, Inc.) in a total volume of 20 µl, according to the
manufacturer's instructions. Subsequently, 1 mg cDNA was used for
qPCR with Fast™ SYBR-Green fluorescence dye (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Taq DNA polymerase was activated
at 94˚C for 10 min, followed by 40 cycles of 94˚C for 20 sec and
65˚C for 40 sec. Amplification reactions were performed in
duplicate, fluorescence curves were analyzed with the included
software of the StepOne real-time PCR system, and quantified using
the 2-ΔΔCq method (14).
All results were normalized to the expression of β-actin. The
primer sequences used in the present study are listed in Table I.
 | Table ISequences of primers for reverse
transcription-quantitative PCR. |
Table I
Sequences of primers for reverse
transcription-quantitative PCR.
| Mouse gene | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| CD68 |
GCTACATGGCGGTGGAGTACAA |
ATGATGAGAGGCCAGCAAGATGG |
| CD80 |
TATTGCTGCCTTGCCGTTACA |
AACAGATTCTGGTCCCGTTGA |
| Arg-1 |
AGACAGCAGAGGAGGTGAAGAG |
CGAAGCAAGCCAAGGTTAAAG |
| YM-1 |
TCACAGGTCTGGCAATTCTTCTG |
TGCATTCCAGCAAAGGCATAC |
| MMD |
TGGATCAATGCGGTTCAGGA |
GCAATTGGCAGCATGTTCGTAG |
| Dll1 |
GACGCTGAGGGGTATGTGATG |
CTTGAGGCATACGCGAAAGAAGGTC |
| Dll4 |
GGGCACCTACTGTGAACTCC |
GCTGCCCACAAAGCCATAAG |
| β-actin |
CAGCCTTCCTTCTTGGGTAT |
TGGCATAGAGGTCTTTACGG |
Statistical analysis
The statistical analysis was performed using the
SPSS 18.0 program (SPSS, Inc.). Image analysis was performed using
ImageJ software (version 1.46; National Institutes of Health). All
data are expressed as the mean ± SD. Mean values were compared with
one-way ANOVA followed by Dunnett's test, categorical data were
scaled according to the definition and compared using
Kruskal-Wallis test. P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
3 times.
Results
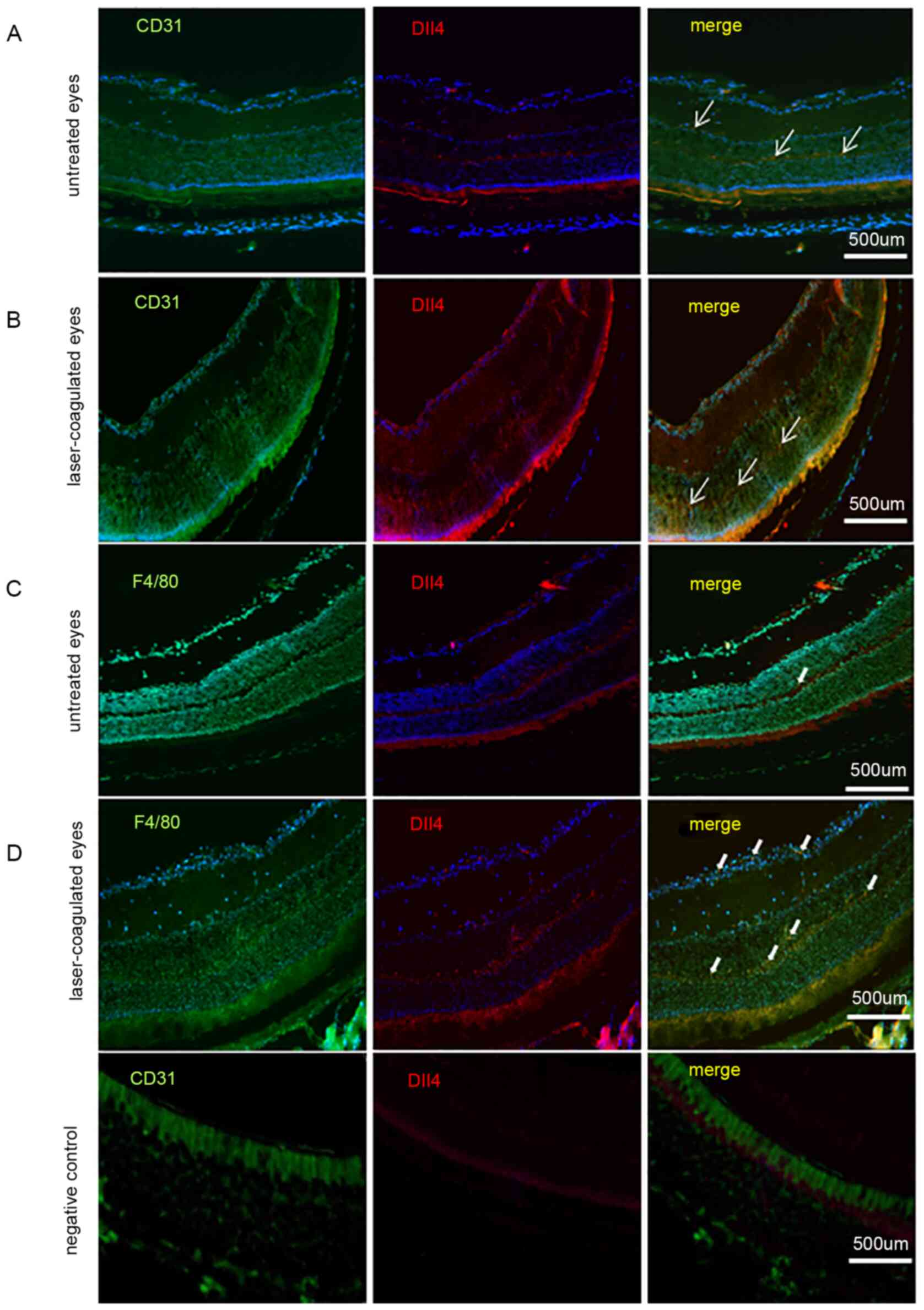
Dll4 signaling activation in
macrophages of CNV
The 12 eyes of 6 mice were included in the present
study. The expression of Dll4 was observed not only in endothelial
cells (CD31-positive cells; Fig. 1A
and B) but also in macrophages
(F4/80-positive cells; Fig. 1C and
D). Dll4 was notably observed in
endothelial cells (CD31-positive cells) of the outer and inner
plexiform layers, not only in untreated eyes but also in
laser-coagulated eyes. For macrophages (F4/80-positive cells), a
low level of Dll4 expression was detected in the neuronal retina
layer of untreated eyes, but the expression of Dll4 was notably
increased in the neuronal retina layer of laser-coagulated
eyes.
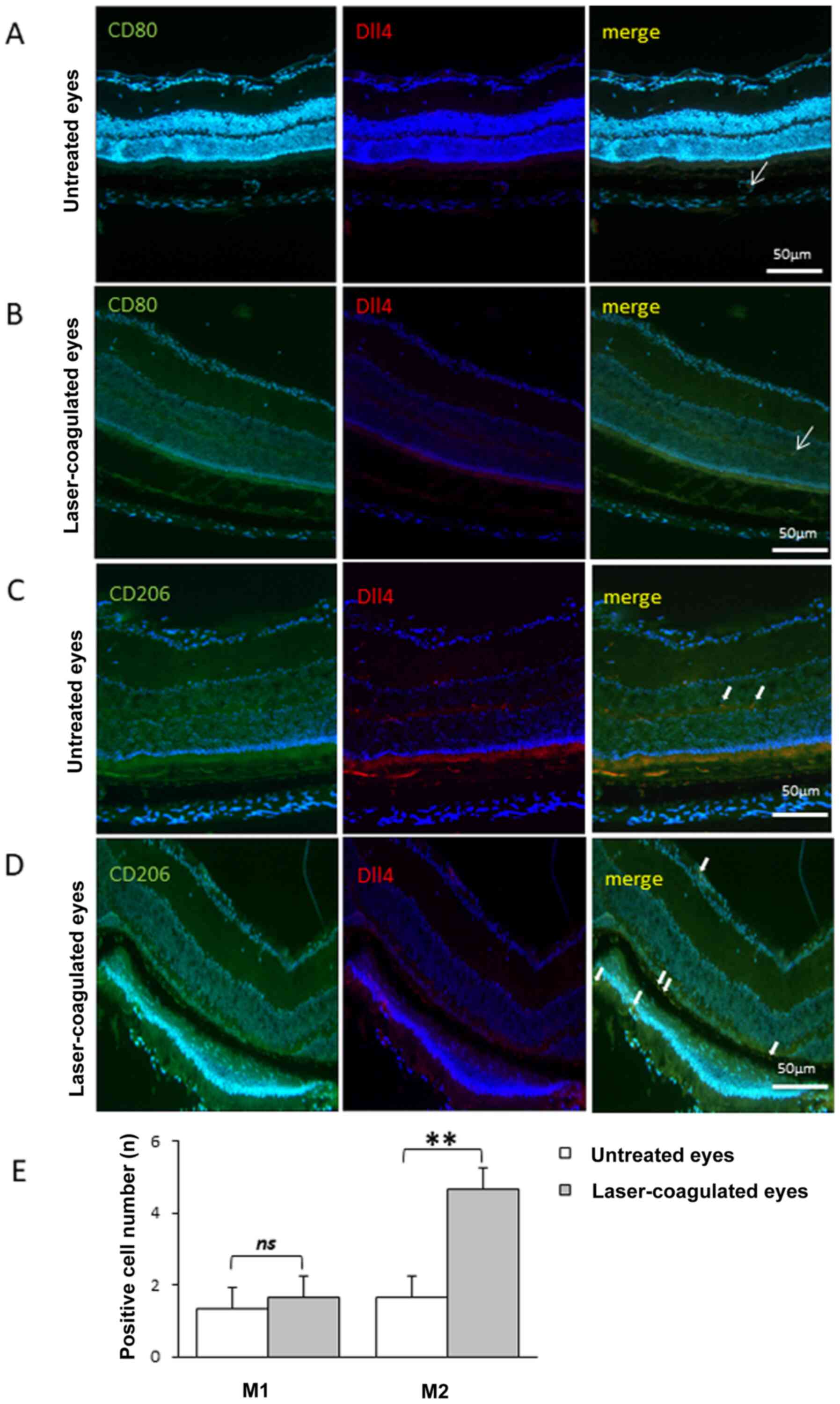
Polarization shift of M2 macrophages
in laser-coagulated eyes
No difference was identified in the number of M1
macrophages (CD80-positive cells) before and 7 days after laser
coagulation (Fig. 2A, B and E).
Meanwhile, the number of M2 macrophages (CD206-positive cells) was
significantly increased at 7 days after laser coagulation compared
with that before laser coagulation (Fig. 2C-E), indicating a shift in
macrophage polarization in eyes subjected to laser coagulation.
IL-4/Q116E attenuates laser-induced
CNV in mice
Fundus images indicated a marked laser lesion around
the optic disc in laser-coagulated eyes (Fig. 3A). Histological examination revealed
that the retina was disordered in laser-coagulated eyes compared to
untreated eyes (Fig. S2). The
structure of the retinal neuronal layer was disrupted and unclear
in laser-coagulated eyes compared with untreated eyes.
AdIL-4/LacZ-injected and AdIL-4/WT-injected mice had the most
severe laser-induced retinal injury, in which the retinal nerve
fiber layer, inner nuclear layer and outer nuclear layer could not
be distinguished (Fig. 3A).
Furthermore, FFA revealed notable fluorescence leakage in the
laser-induced retinal lesions of AdLacZ-injected mice and low
levels of fluorescence leakage in other AdIL-4 vector-infected
mice, suggesting the establishment of CNV (Fig. 3A and C). OCT images showed disruptions in a
highly reflective layer corresponding to the RPE and
choriocapillaris in the laser-induced retinal lesions of AdLacZ-,
AdIL-4/WT- and AdIL-4/Q116D/Y119D-infected mice, but not in
AdIL-4/Q116E-infected mice (Fig. 3A
and B).
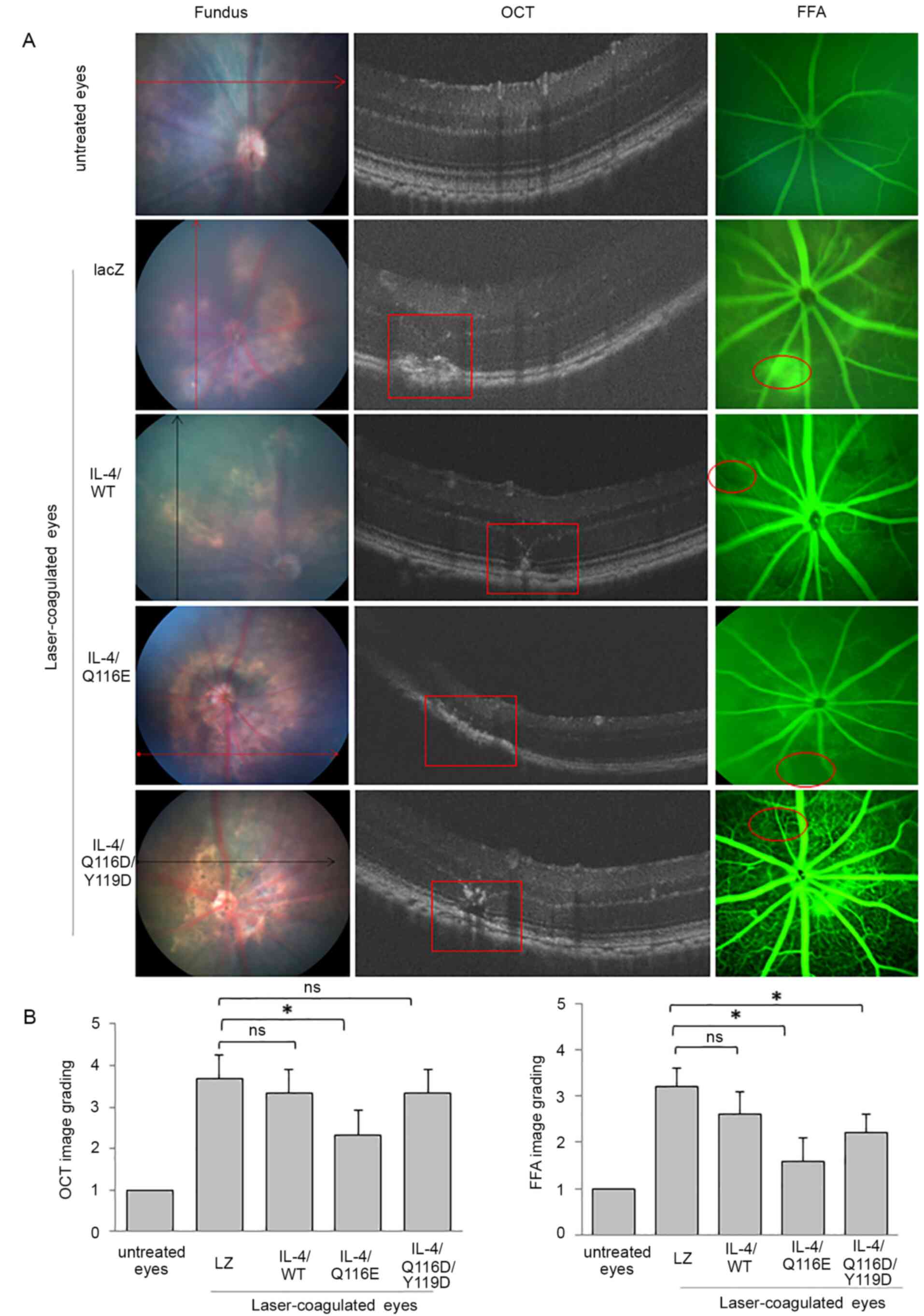 | Figure 3CNV induction through
photocoagulation, and IL-4/Q116E attenuation of CNV in
laser-induced mice. C57BL/6 mice were injected with the control
vector AdLZ or the three recombinant IL-4 vectors and subsequently
received laser coagulation 1 day later. On day 7 after laser
coagulation, one eye of each mouse was removed. Fundus images, FFA
and OCT of the laser-treated eyes of adenovirus-injected mice were
performed at 3 min after intraperitoneal injection of 2.5%
fluorescein sodium. (A) Untreated eyes revealed normal images
without laser injury spots, with clear lays and no fluorescence
leakage. Laser-treated eyes exhibited laser injury spots (as
indicated by arrows), retinal tomostructure (as indicated by red
squares) and fluorescence leakage (as indicated by red circles) in
the scanned area. All images were recorded within 5-8 min. (B)
Image grading for OCT and FFA images was established. Data are
expressed as the means ± SD (n=3 mice per group).
*P<0.05 as indicated. CNV, choroidal
neovascularization; FFA, fluorescein fundus angiography; IL-4,
adenovirus-expressed IL-4 vector; LacZ, adenovirus-expressed
β-galactosidase; ns, no significance; OCT, optical coherence
tomography; WT, wild-type. |
IL-4/Q116E exerts an immunomodulatory
effect in mice
The protein expression of CD80 was increased in both
AdIL-4/Q116E- and AdIL-4/Q116E/Y119D-injected laser-coagulated
eyes, whereas the expression of CD206 was decreased (Fig. 4A). Consistent with the results of
western blotting, compared with laser-coagulated eyes with the LacZ
negative control, CD68 and CD80 mRNA expression levels were
decreased in AdIL-4/WT-infected laser-coagulated eyes and increased
in AdIL-4/Q116E-infected laser-coagulated eyes (Fig. 4B). Compared with laser-coagulated
eyes treated with the AdLZ negative control, arginase 1 (Arg-1) was
downregulated in AdIL-4/Q116E-infected laser-coagulated eyes, CD80
was upregulated in AdIL-4/Q116D/Y119D-infected laser-coagulated
eyes, and no difference in expression was observed for the
chitinase-like protein YM-1 (Fig.
4B).
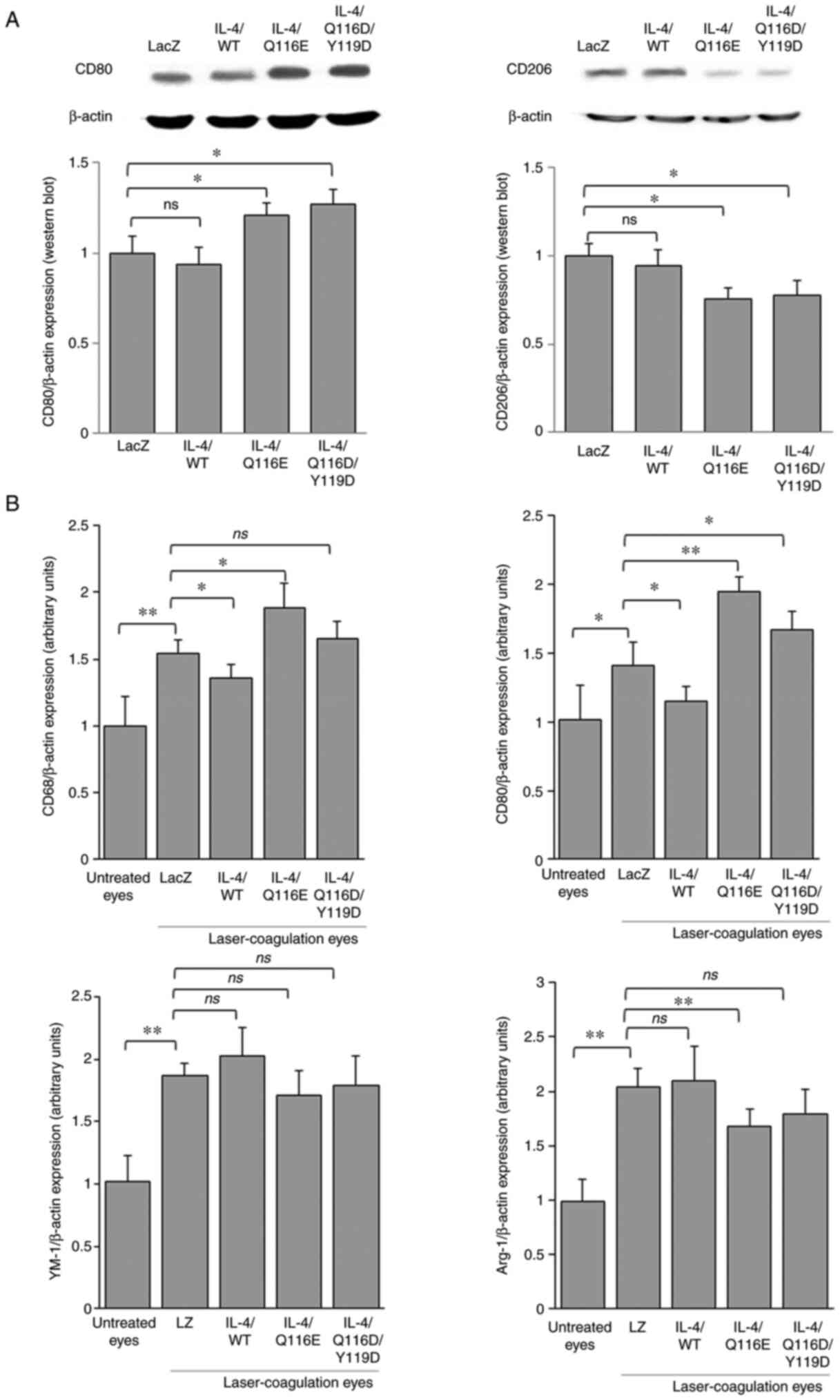 | Figure 4Distribution of M1 and M2 macrophages
in RPE-choroid tissues of laser-induced CNV. RPE-choroid tissues
were resected from mice exhibiting laser-induced CNV lesions. (A)
The expression of CD80 and CD206 was determined by western
blotting, with β-actin as a control. Each experiment was repeated
three times. (B) Reverse transcription-quantitative PCR was
performed, and the expression levels of CD68, CD80, Arg-1 and YM-1
were normalized to β-actin. Data are expressed as the mean ± SD
(n=3 mice per group). *P<0.05 and
**P<0.01 as indicated. Arg-1, arginase 1; CNV,
choroidal neovascularization; IL-4, adenovirus-expressed IL-4
vector; LacZ and LacZ, adenovirus-expressed β-galactosidase; ns, no
significance; RPE, retinal pigment epithelium; WT, wild-type; YM-1,
chitinase-like protein 3. |
Taken together, these results suggested that IL-4
led to a polarization shift of M2/M1 macrophages in different
adenovirus-transfected eyes.
IL-4/Q116E regulates the expression of
MMD and Dll4
AdIL-4/Q116E injection in laser-coagulated eyes
increased the expression of MMD and Dll4 but did not affect the
expression of Dll1 at the protein level (Fig. 5A). At the mRNA level, AdIL-4/Q116E
increased the expression of MMD, Dll1 and Dll4 (Fig. 5B).
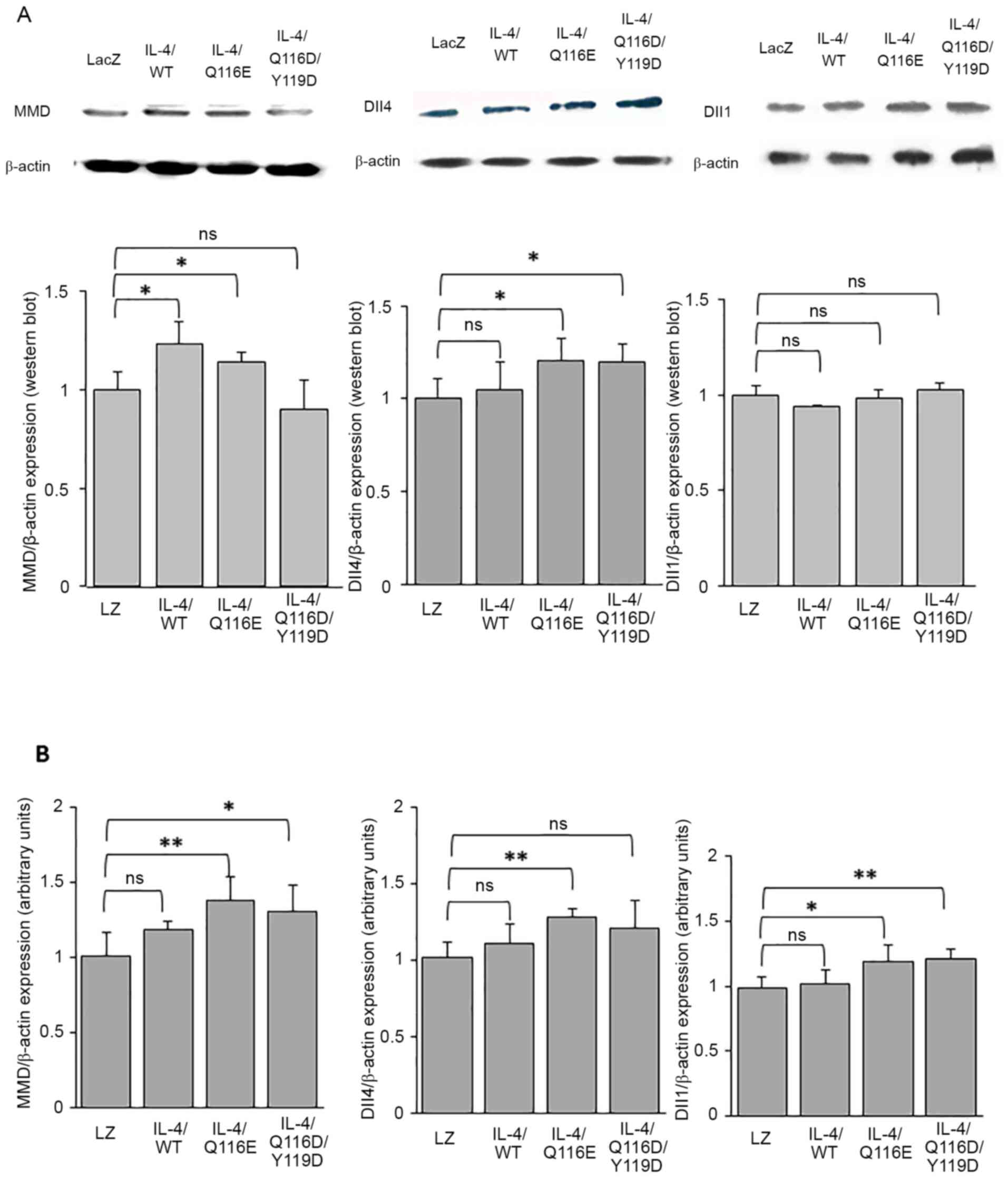 | Figure 5MMD-Dll4 pathway expression in
RPE-choroid tissues of laser-induced CNV. RPE-choroid tissues were
resected from mice exhibiting laser-induced CNV. (A) The expression
of MMD, Dll1 and Dll4 was determined by western blotting, with
β-actin as a control. Each experiment was repeated three times.
Statistical analysis was performed using ImageJ software. (B)
Reverse transcription-quantitative PCR was performed, and the
expression of MMD, Dll4 and Dll1 was normalized to β-actin. Data
are expressed as the mean ± SD (n=3 mice per group).
*P<0.05 and **P<0.01 as indicated.
Dll1, delta-like 1; Dll4, delta-like 4; IL-4, adenovirus-expressed
IL-4 vector; LacZ and LacZ, adenovirus-expressed β-galactosidase;
MMD, monocyte to macrophage differentiation-associated; ns, no
significance; RPE, retinal pigment epithelium; WT, wild-type. |
AdIL-4/Q116D/Y119D injection of laser-coagulated
eyes increased the expression of Dll4 but did not affect the
expression of MMD and Dll1 at the protein level (Fig. 5A). At the mRNA level,
AdIL-4/Q116D/Y119D injection increased the expression levels of MMD
and Dll1 but not Dll4 (Fig.
5B).
Discussion
The present study indicated that IL-4 muteins
affected CNV development by modulating macrophage phenotypes. The
IL-4 mutein IL-4/Q116E attenuated laser-induced CNV in mice.
Furthermore, IL-4/Q116E not only remodeled the anatomical structure
of the retina and choroid but also reduced vascular leakage
according to OCT and FFA data.
Pre-experimental data indicated that not only
endothelial cells but also macrophages participated in
laser-induced retinal lesions. Additionally, M2 macrophages
(CD206-positive cells) were increased after laser coagulation and
M1 macrophages (CD80-positive cells) were not clearly increased
compared with untreated eyes and laser-coagulated eyes. The results
confirmed that M2 macrophages were dominant in laser-induced
retinal lesions. A previous study also reported that laser-induced
retinal lesions are one of the causes of CNV (11). In addition, the investigation of the
inflammatory factors in RPE-choroid tissue from mice infected with
different AdvIL-4 vectors revealed that AdIL-4/Q116E led to the
polarization shift of macrophages from the M2 to M1 phenotype; the
expression levels of CD68 and CD80 were increased, and the
expression level of Arg-1 was decreased. These results are in
contradiction with those reported by another group (15), who found that macrophage
infiltration accentuated neovascularization by a direct effect on
endothelial cell proliferation in situations of inflammatory
neovascularization. Our previous study reported that Th2 deviation
in splenocyte responses and reduced atherosclerotic area in
AdvIL-4/Q116E-infected mice. Additionally, the results demonstrated
that IL-4/Q116E served a diverse inflammatory role in different
cell types. For example, IL-4/Q116E increased the expression level
of vascular cell adhesion molecule 1 (VCAM-1) in endothelial cells
and partially inhibited the effects of IL-4 on different
macrophages (6). Therefore, it can
be hypothesized that IL-4/Q116E has a cell-specific effect on
inflammation, serving similar or opposite roles in different cell
types. However, these findings are not sufficient to explain how
IL-4/Q116E attenuates CNV. Other immune cells, such as lymphocytes,
may serve an important role, as IL-4/Q116E regulates Th1/Th2
deviation, as our previous reports (6). Therefore, further experiments on local
and systemic immune responses in CNV are required.
A previous study reported that Notch signaling
serves a pivotal role in the inflammatory response and angiogenesis
by regulating the polarization of macrophages. It also participates
in the development of certain diseases, such as CNV (16). Dll4 is a ligand of Notch signaling
that has the same function as VEGF (17). When the allele signaling molecule of
Dll4 is deleted, it can cause vascular abnormalities at the
embryonic stage, suggesting that Dll4 serves an important role in
vascular development and homeostasis in the same manner as VEGF
(17). The present study indicated
that the expression level of Dll4 in the CNV model was
significantly increased not only in the neuronal retina layer, but
also in the subretinal area compared with its expression in
untreated eyes. In addition, the present study reported that,
similar to Dll4-positive endothelial cells, Dll4-positive
macrophages also participated in CNV, which is consistent with
other studies (7,18). However, Camelo et al
(15) hypothesized that Dll4
stimulated CNV through macrophages, as VEGF was upregulated after
the overexpression of Dll4. In the same study, it was reported that
the expression levels of IL-1β, IL-6 and TNF-α were increased,
which confirmed that overexpression of Dll4 stimulated macrophage
shift to M1 polarization. These results are consistent with the
present data.
To investigate the possible signaling pathway of
IL-4 muteins, the expression levels of genes involved in Notch
signaling in CNV were quantified. The results revealed that the
expression levels of MMD and Dll4 were increased in RPE-choroid
tissue obtained from IL-4/Q116E-infected mice. MMD was identified
in 1995 by analyzing the molecular mechanism of macrophage
maturation and was speculated to participate in the differentiation
and functional process of macrophages (19). Subsequently, Liu et al
(20) reported that MMD positively
regulated the activation of ERK, protein kinase B and the
production of TNF-α and nitric oxide in macrophages, which was
consistent with the present data to some extent, where MMD
upregulated the expression of M1 cytokine. Therefore, MMD may
participate in the differentiation of macrophages and stimulate the
secretion of cytokines from M1 macrophages. The present study
reported that AdIL-4/Q116E stimulated M1-macrophage shift,
including upregulation of CD68 and CD80 and downregulation of CD206
and Arg-1. However, there is no notable reason to certify that MMD
is downstream of the Notch-Dll4 signaling pathway that affects
macrophage polarization, as experiments with an MMD blocker were
not performed in the present study. The present data reported the
possibility that IL-4 mutein attenuated CNV by regulating
macrophage polarization. It also demonstrated that Notch-Dll4-MMD
could be a possible signaling pathway involved in these events. In
future studies, it will be investigated if regulating the
expression of Dll4 or MMD, such as MMD overexpression, or using a
Dll4 blocker, could change the shift of macrophage polarization.
Furthermore, future studies should identify the downstream
signaling pathways involved, such as ERK, AKT and mitogen-activated
protein kinase.
In summary, the present results demonstrated that
IL-4/Q116E regulated the inflammatory response in laser-induced
CNV, increased the expression of CD68 and CD80, decreased the
expression of Arg-1 in RPE-choroid tissues and attenuated CNV
development. The results suggested that targeting macrophage
polarization and its inflammatory reaction may be a possible
treatment strategy for CNV. Furthermore, the present results
provide a foundation for further research investigating the
MMD-Dll4 signaling pathway as a potential target of IL-4 muteins to
treat CNV and other intraocular inflammatory disorders.
In conclusion, IL-4RI-selective mutein AdIL-4/Q116E
regulated macrophage polarization and alleviated CNV.
Supplementary Material
sIL-4 concentration in the serum
measured by ELISA. Approximately 2-3 ng/ml of IL-4 protein was
detected in IL-4-infected mice seven days after adenovirus
injection. sIL-4, secreted IL-4; IL-4, adenovirus-expressed IL-4
vector; LacZ, adenovirus-expressed β-galactosidase; WT,
wild-type.
Histological examination of the
retinas of mice after adenovirus vector infection and laser
coagulation. Histograms show the thickness of the INL, ONL and
RNFL. IL-4, adenovirus-expressed IL-4 vector; INL, inner nuclear
layer; LacZ, adenovirus-expressed β-galactosidase; ONL, outer
nuclear layer; RNFL, retinal nerve fiver layer; WT, wild-type.
Acknowledgements
Not applicable.
Funding
This study was supported in part by Grant-in-Aids for Young
Scientists (grant nos. 2017JJ3465 and 2017JJ3473) from the Hunan
Natural Science Foundation, P.R. China.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LG was responsible for data curation, carrying out
the study and writing (original draft). WJ was responsible for
analyzing the data and ophthalmologic examination, and HL, for
literature searching and analyzing the data. ZC was responsible for
the study conception and design. YL contributed to study conception
and supervision, as well as manuscript writing editing. LG and YL
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by the
Animal Care and Use Committees of the Third Xiangya Hospital,
Central South University (Changsha, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Paul WE: Interleukin-4: A prototypic
immunoregulatory lymphokine. Blood. 77:1859–1870. 1991.PubMed/NCBI
|
|
2
|
Boothby M, Mora AL, Aronica MA, Youn J,
Sheller JR, Goenka S and Stephenson L: IL-4 signaling, gene
transcription regulation, and the control of effector T cells.
Immunol Res. 23:179–191. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hamilton TA, Ohmori Y and Tebo J:
Regulation of chemokine expression by antiinflammatory cytokines.
Immunol Res. 25:229–245. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ramalingam TR, Pesce JT, Sheikh F, Cheever
AW, Mentink-Kane MM, Wilson MS, Stevens S, Valenzuela DM, Murphy
AJ, Yancopoulos GD, et al: Unique functions of the type II
interleukin 4 receptor identified in mice lacking the interleukin
13 receptor alpha1 chain. Nat Immunol. 9:25–33. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
LaPorte SL, Juo ZS, Vaclavikova J, Colf
LA, Qi X, Heller NM, Keegan AD and Garcia KC: Molecular and
structural basis of cytokine receptor pleiotropy in the interleukin
4/13 system. Cell. 132:259–272. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lin Y, Chen Z and Kato S:
Receptor-selective IL-4 mutein modulates inflammatory vascular cell
phenotypes and attenuates atherogenesis in apolipoprotein
E-knockout mice. Exp Mol Pathol. 99:116–127. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dou GR, Li N, Chang TF, Zhang P, Gao X,
Yan XC, Liang L, Han H and Wang YS: Myeloid-specific blockade of
Notch signaling attenuates choroidal neovascularization through
compromised macrophage infiltration and polarization in mice. Sci
Rep. 6(28617)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cherepanoff S, McMenamin P, Gillies MC,
Kettle E and Sarks SH: Bruch's membrane and choroidal macrophages
in early and advanced age-related macular degeneration. Br J
Ophthalmol. 94:918–925. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zandi S, Nakao S, Chun KH, Fiorina P, Sun
D, Arita R, Zhao M, Kim E, Schueller O, Campbell S, et al:
ROCK-isoform-specific polarization of macrophages associated with
age-related macular degeneration. Cell Rep. 10:1173–1186.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang Y, Liu F, Tang M, Yuan M, Hu A, Zhan
Z, Li Z, Li J, Ding X and Lu L: Macrophage polarization in
experimental and clinical choroidal neovascularization. Sci Rep.
6(30933)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhou Y, Yoshida S, Kubo Y, Yoshimura T,
Kobayashi Y, Nakama T, Yamaguchi M, Ishikawa K, Oshima Y and
Ishibashi T: Different distributions of M1 and M2 macrophages in a
mouse model of laser-induced choroidal neovascularization. Mol Med
Rep. 15:3949–3956. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jetten N, Verbruggen S, Gijbels MJ, Post
MJ, De Winther MP and Donners MM: Anti-inflammatory M2, but not
pro-inflammatory M1 macrophages promote angiogenesis in vivo.
Angiogenesis. 17:109–118. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang P, Wang H, Luo X, Liu H, Lu B, Li T,
Yang S, Gu Q, Li B, Wang F, et al: MicroRNA-155 inhibits
polarization of macrophages to M2-type and suppresses choroidal
neovascularization. Inflammation. 41:143–153. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Camelo S, Raoul W, Lavalette S, Calippe B,
Cristofaro B, Levy O, Houssier M, Sulpice E, Jonet L, Klein C, et
al: Delta-like 4 inhibits choroidal neovascularization despite
opposing effects on vascular endothelium and macrophages.
Angiogenesis. 15:609–622. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Michelucci A, Heurtaux T, Grandbarbe L,
Morga E and Heuschling P: Characterization of the microglial
phenotype under specific pro inflammatory and anti inflammatory
conditions: Effects of oligomeric and fibrillar amyloid beta. J
Neuroimmunol. 210:3–12. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu ZQ, Rowe RG, Lim KC, Lin Y, Willis A,
Tang Y, Li XY, Nor JE, Maillard I and Weiss SJ: A Snail1/Notch1
signalling axis controls embryonic vascular development. Nat
Commun. 5(3998)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yan X, Yang Z, Chen Y, Li N, Wang L, Dou
G, Liu Y, Duan J, Feng L, Deng S, et al: Endothelial cells-targeted
soluble human Delta-like 4 suppresses both physiological and
pathological ocular angiogenesis. Sci China Life Sci. 58:425–431.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rehli M, Krause SW, Schwarzfischer L,
Kreutz M and Andreesen R: Molecular cloning of a novel macrophage
maturation-associated transcript encoding a protein with several
potential transmembrane domains. Biochem Biophys Res Commun.
217:661–667. 1995.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu Q, Zheng J, Yin DD, Xiang J, He F,
Wang YC, Liang L, Qin HY, Liu L, Liang YM, et al: Monocyte to
macrophage differentiation-associated (MMD) positively regulates
ERK and Akt activation and TNF-α and NO production in macrophages.
Mol Biol Rep. 39:5643–5650. 2012.PubMed/NCBI View Article : Google Scholar
|