Introduction
Spinal cord injury (SCI) is a serious affliction
mainly caused by trauma. Because nerve tissue is difficult to
repair, SCI can lead to a great disorder of sensory and motor
functions below the injury plane, and even cause life-long pain in
patients (1). In recent years, with
the increase in traffic and industrial accidents, the incidence of
acute SCI has been increasing in China yearly (2-4).
At present, except for the surgical relief of compression, there is
no ideal treatment method for acute SCI (5). Therefore, it is necessary to further
explore the mechanism of the pathological process of acute SCI,
discover and develop appropriate drugs to block its pathological
process and promote the recovery of nerve function.
The pathophysiological mechanism of SCI is
complicated and includes primary and secondary injury stages.
Primary injury involves cell death or degeneration induced by the
sequential activation of local and systemic inflammatory responses
and post-injury adaptation of primary injury (6). Secondary injury commences a short time
after the trauma and lasts for an extended period of time, which
can cause additional degeneration or death of neurons, and is no
less harmful to the nervous system than primary injury (7). After acute SCI, vascular injury leads
to a series of changes in spinal cord blood supply, including
abnormal spinal cord blood supply and perfusion, eventually leading
to hemorrhage, ischemia and reperfusion injury, and the death of
neurons and glial cells (8,9). The death of nerve cells is mainly
caused by apoptosis, which is considered to be a main inducer of
nervous system diseases (10). Due
to the importance and non-reproducibility of neurons, it is
particularly important to prevent neuronal apoptosis resulting from
spinal cord injury (11). A
previous study revealed that energy metabolism disorders caused by
SCI ultimately induce neuronal apoptosis (12). However, the exact mechanism remains
unclear.
Ecto-5'-nucleotidase (CD73), a nucleotide enzyme
that is attached to the plasma membrane, mediates the production of
adenosine (Ado) to control the production of purine nucleotides and
thus the signaling of nucleotides (13,14).
CD73 can mediate the binding of Ado and its receptor to form cAMP
which can reduce the permeability of the blood-brain barrier
(14). In addition, Ado can protect
the ischemic brain and spinal cord as an endogenous neuroprotective
factor (15,16). Our previous study demonstrated that
CD73 can promote the M2 polarization of microglia, weaken the
inflammatory response and inhibit caspase-3 gene expression after
SCI, indicating that CD73 has a protective effect on SCI (17). However, whether there is a
protective effect of CD73 on neurons remains to be elucidated.
Therefore, the relationship between CD73 and the apoptosis of
injured spinal cord neurons was studied through a series of
experiments to clarify the protective mechanism of CD73 in SCI.
Materials and methods
Animals
C57BL/6 CD73 knock out (CD73-/-) male
mice were kindly gifted by Professor Thompson, Oklahoma Medical
Research Foundation (Oklahoma City, USA). The Shanghai SLAC
Laboratory Animal Co., Ltd., supplied the wild-type (WT) male
C57BL/6 mice. A total of 10 CD73 knockout mice and 10 wild-type
mice were used for subsequent experiments. Each mouse was ~3 months
old and weighed ~30 g. All surgical procedures and experimental
protocols in the present study were performed in accordance with
standard guidelines approved by the Ethics Committee of
Experimental Research, Shanghai Medical College, Fudan University
(Shanghai, China). The approval number is 2019-Huashan
Hospital-JS-104.
Construction of spinal cord injury
model mice
Each of the 10 male WT C57BL/6 mice and 10
CD73-/- C57BL/6 mice were fed under temperature 28˚C,
relative humidity 40%, ventilation reach 20 times/h, light/dark
cycle durations is 12h/12h before the experiments. In the process
of modeling, 0.35% sodium pentobarbital (35 mg/kg) was injected
into the abdominal cavity to anesthetize the animals. After the
pinprick reflex and pupil reflex disappeared, the 10th lamina was
surgically exposed. The spine was removed with special vascular
forceps, the interspinous ligament was cut off with a micro
instrument, the lamina was opened and the spinal cord was exposed.
The mice were then placed on the MASCIS collimator workbench, and
the 9 and 11th dorsal vertebrae were fixed. Equipped with a 10-g
impact bar, the free fall from 2.5 cm hit the center of the spinal
cord at the 10th vertebral level. It was regarded as a sign of
successful modeling when the mice demonstrated compulsory
convulsions or paralysis of both legs. After modeling, 20,000 units
of penicillin were intramuscularly injected twice a day.
To evaluate the quality of the model, the Basso,
Beattie and Bresnahan (BBB) locomotor scale (18,19)
was used to evaluate motor function in the mice. Motor function was
divided into 22 grades, a score of 0 indicated no observable hind
limb movement, a score of 21 indicated completely normal motor
function, and scores between 1 and 20 were based on the level of
motor function. The basic contents of reference were: The number
and range of joint activity, the degree of weight bearing, the
coordination of front and rear limbs and the activity of the front
and rear claws and tail. Mice in the control group were fed under
the same conditions as the SCI group but no treatment was included.
The control and SCI groups were scored before and at 6 h, 1, 3 and
7 days after surgery.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) and immunofluorescence assay
The control and SCI mice were deeply anesthetized
with 10% chloral hydrate (3.5 ml/kg, intraperitoneally) 3 days
after surgery. NaCl (0.9%) was then used to perfuse the mice,
followed by 4% paraformaldehyde (PFA) in 0.01 M phosphate-buffered
saline (PBS, pH=7.4). Spinal cord tissue at the region of the
injury was dissected with a 0.5 cm margin on each side of the
lesion and embedded in optimum cutting temperature (OCT) compound,
after which the sections were frozen at -80°C, were
produced. Frozen sections were rewarmed, fixed with 4% PFA for 30
min at room temperature, and washed 3 times with PBS. PBS
containing 0.5% Triton X-100 was added to the sections and
incubated at room temperature for 5 min. Then, the sections were
washed 3 times with PBS. An appropriate amount of TUNEL detection
solution (FITC-conjugated) was prepared. Sections were placed in
the TUNEL detection solution, incubated at 37˚C in the dark for 60
min and washed 3 times with PBS. Next, the neuron antibody NeuN
(1:1,000; product code ab1777487; Abcam) was added to the sections
and they were incubated at 4˚C overnight. Cy3-conjugated secondary
antibody (1:1,000; product code ab6939, Abcam) was added to the
sections and they were incubated at room temperature for 2 h.
Finally, the sections were stained with Fluoroshield™
histology mounting medium (cat. no. F6057; Sigma-Aldrich; Merck
KGaA) with 10 µg/ml 4',6-diamidino-2-phenylindole (DAPI), and
sealed for microscopic observation. Imaging was performed with an
Olympus FV 1000 confocal microscope (Olympus coroporation;
magnification, x200). The observation area was randomly selected
and no less than 5 fields were subsequently imaged.
Isolation and culture of dorsal root
ganglion (DRG) neurons
WT and CD73-/- mouse DRG neurons were
isolated and cultured in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Thermo Fisher Scientific, Inc.) in
a humidified atmosphere of 5% CO2 at 37°C.
The dorsal skin was removed under aseptic conditions with
ophthalmic scissors, and then a section of the spinal cord was cut.
The dorsal side was placed on a sterilized ground glass piece, the
ventral half of vertebrae was cut horizontally along the two sides
of the spinal canal under an anatomical microscope, the spinal cord
and ganglion were exposed and the ganglion was separated with
anatomical tweezers. The ganglion membrane was removed, digested
with 0.125% trypsin (37˚C for 30 min), and then diluted into a
0.2x105 cells/ml cell suspension with planting
medium(DMEM and 10% FBS). The suspension was inoculated in a 35-mm
plastic culture dish coated with collagen (type I, from rat tail)
which was used as a membrane coating for cell attachment, and 2 ml
of cell suspension was placed in each dish. The specimens were
cultured in DMEM with 10% FBS in a 5% CO2 incubator at
37˚C. After 24 h, the culture medium was removed from the culture
dish and replaced with fresh medium. On the third day of
inoculation, the cell division inhibitors 5-fluoro-2'-deoxyuridine
(15 µg/ml; Sigma-Aldrich; Merck KGaA) and uridine (35 µg/ml;
Sigma-Aldrich; Merck KGaA) were added to the culture dish to
inhibit the proliferation of non-neural cells. After 48 h, the
fresh culture medium was changed. The medium was changed twice a
week and half of the fresh culture medium was changed each
time.
Hypoxia and hypoglycemia of DRG
neurons
During anoxic and glucose-deficient culture, the DRG
cell culture medium was aspirated, washed twice with D-Hank's
solution (Sigma-Aldrich; Merck KGaA) and replaced with sugar-free
medium. The cells were then placed into a three-gas incubator
containing 1% O2, 5% CO2 and 94%
N2 for 0, 0.5, 1 and 2 h. After 0, 0.5, 1 and 2 h of
anoxia and glucose deficiency, the cells were removed from the
three-gas incubator, the sugar-free medium was replaced with the
same as that used before and the cells were placed into an ordinary
cell incubator. Before anoxic and glucose deficiency treatment,
cultured DRG neurons were treated with Ado and α,β-methylene ADP
(APCP) which is a CD73 hydrolase specific inhibitor (Sigma-Aldrich;
Merck KGaA). The Ado group was cultured in a medium containing an
Ado final concentration of 100 µmol/l for 24 h at 37˚C. The APCP
group was cultured in a medium containing an APCP final
concentration of 10 µmol/l for 24 h at 37˚C. The cells of each
group were collected and the related experiments were carried
out.
Construction of a CD73 overexpression
lentivirus
The CD73 gene CDS fragment was synthesized by Sangon
Biotech Co., Ltd. and inserted into the lentivirus vector
(pCDH1-MSCV-MCS1-EF1-GreenPuro Vector; System Biosciences LLC). The
2nd generation system of the lentivirus was used. Lentivirus
packaging plasmids included PCDH-CD73 (10 µg), psPAX2 (10 µg) and
pMD2G (10 µg), which were co-transfected into a 10 cm dish
containing 293T cells (American Type Culture Collection). Samples
were transfect using the Lipofectamine®2000 transfection
reagent (Thermo Fisher Scientific, Inc.). The supernatant was
collected after 48 h of continuous culture. Appropriate amounts of
PEG8000 were added to the supernatant and incubated overnight at
4˚C. The precipitate was resuspended in medium and stored at -80˚C.
Lentivirus titers were measured and used with an MOI of 10
according to the number of cells infected as needed. The isolated
and cultured DRG neurons were seeded into six-well-plates
(5x104 per well), after which 100 µl lentivirus was
added when cell confluence reached 70%. Infected cells were then
cultured at 37˚C for 12 h and replaced with fresh medium. Cells
were cultured until they were used for subsequent
experimentation.
Western blotting
Tissues and cells were lysed with RIPA buffer (25 mM
Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate,
0.1% SDS), and centrifuged at 4˚C for 5 min (13,800 x g), and the
supernatant was collected. Total protein concentration was measured
with a bicinchoninic acid (BCA) assay (Sangon Biotech Co., Ltd.).
Approximately 20 µg of sample was added to the loading buffer,
heated at 95˚C for 5 min, and then SDS-PAGE (12.5 and 10% of
acrylamide) electrophoresis separation was carried out. After
electrophoresis, the protein samples were transferred to PVDF
membranes. The CD73 antibody (1:1,000; product code ab175396;
Abcam), caspase-3 antibody (1:1,000; product code ab184787; Abcam),
Bcl-2 antibody (1:1,000; product code ab182858; Abcam), Bax
antibody (1:1,000; product code ab182733; Abcam), Fas antibody
(1:1,000; product code ab133619; Abcam), protein kinase A (PKA)
antibody (1:1,000; product no. 4782S; Cell Signaling Technology,
Inc.), cAMP response element-binding protein (CREB) antibody
(1:1,000; product no. 9197; Cell Signaling Technology, Inc.),
phosphorylated (p)-CREB antibody (1:1,000; product no. 9198; Cell
Signaling Technology, Inc.) and GAPDH antibody (1:2,000; product
code ab8245; Abcam) were incubated overnight at 4˚C. The membranes
were subsequently blocked with 5% skimmed milk for 1 h at room
temperature. The diluted secondary antibodies [1:2,000; cat. nos.
SA00001-1 Goat anti-mouse IgG (H + L) and SA00001-2 Goat
anti-rabbit IgG (H + L), ProteinTech Group, Inc.] labeled with HRP
was added and incubated for 2 h at room temperature. ECL reagent
(cat. no. 34580; Thermo Fisher Scientific, Inc.) was prepared
according to the manufacturer's instructions. A chemiluminescence
imaging system (Tanon Science and Technology Co., Ltd.) was used to
visualize the protein bands. The densitometric analysis of bands
was completed using ImageJ 1.52 software (National Institutes of
Health).
Detection of LDH, SOD and MDA in the
cell culture supernatant and cell viability
LDH and MDA levels as well as SOD activity were
assessed by commercialized kits (serial nos. A020-2-2, A003-4-1 and
A001-3-2, respectively; Nanjing Jiancheng Bioengineering
Institute). After treatment with 2% Triton X-100, 100 µl cell
culture supernatant was extracted from each well and used to
measure absorbance (A) values at 450 nm, 550 nm and 532 nm on a
microplate spectrophotometer according to the instructions of the
detection kit for LDH, MDA content and SOD activity. Cell viability
was assessed by Cell Counting Kit-8 (CCK-8) commercialized kits
(Beyotime Institute of Biotechnology). After treatment, 10 µl WST-8
solution from the CCK-8 was added to each well. The absorbance
value at 450 nm of each well was measured after 2 h. Cell viability
was calculated according to absorbance values at different
time-points (0.5, 1 and 2 h).
Detection of intracellular ROS, ATP
and cAMP content
ROS and ATP levels were measured with commercialized
kits (serial nos. E004-1-1 and A095-1-1, respectively; Nanjing
Jiancheng Bioengineering Institute). According to the
manufacturer's instructions, the DCFH-DA probe was added to the
cell medium at a final concentration of 10 µM. After culture for 1
h, the culture medium was removed, cells were collected and the
fluorescence intensity value of 525 nm was detected after 500 nm
excitation. The value of fluorescence intensity represented the ROS
content in cells. The ATP content in cells was detected by
colorimetry. After the preparation of the detection reagent, the
reaction was carried out according to the manufacturer's
instructions. Finally, the absorbance value at 636 nm was detected
and the ATP content was calculated according to the manufacturer's
instructions. The cAMP detection kit (cat. no. 581001) was
purchased from Cayman Chemical Company. The content of cAMP in the
cells was detected by ELISA according to the manufacturer's
instructions.
Detection of Ado in the cell culture
supernatant and tissues
An Ado Assay Kit (fluorometric; product code
ab211094; Abcam) was used to detect Ado in cell culture supernatant
and tissues. In this assay, Ado was measured using Ado deaminase
followed by a multi-step enzymatic approach resulting in the
generation of an intermediate that reacts with the Ado probe,
leading to the formation of a fluorescent product. The fluorescent
product can be detected at ex/em=535/587 nm, and its intensity is
proportional to the amount of Ado in the sample.
Flow cytometry assay of apoptosis
An Annexin V-FITC Apoptosis Detection Kit (cat. no.
BMS500FI-300; Thermo Fisher Scientific, Inc.) was used to evaluate
apoptosis. According to manufacturer's instructions,
1x105 cultured DRG neurons were collected and washed
once with PBS buffer. A total of 500 µl of binding buffer were
added to resuspend the cells. Then, 5 µl Annexin V-FITC and 5 µl
propidium iodide were added to mix, and the mixture was incubated
at room temperature, in the dark, for 15 min. A flow cytometric
instrument (BD FACSCalibur; BD Biosciences) was used for detection.
ModFit LT 5.0 software (Verity Software House) was used for the
analysis of flow cytometry data.
Statistical analysis
All results are expressed as the mean ± standard
deviation. Student's unpaired t-tests and two-way analysis of
variance (ANOVA) were used to analyze data. The t-test uses the
t-statistic, which is the ratio of the difference in the means to
the weighted mean standard deviation, for the two sets of
identical/heteroscedasticity data. ANOVA uses the F-statistic,
which is the ratio of intergroup variance to intragroup variance,
and is mostly used when the number of groups exceeds two. One-way
ANOVA was used to examine the difference between two, three, or
more groups of a dependent variable within a categorical
independent variable. Two-way ANOVA was used to analyze the
difference between groups of two independent variables, one of
which can be regarded as a processing variable. ANOVA with
Bonferroni or Dunnett's post hoc analysis was used where
appropriate. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
with GraphPad Prism 8.0 software (GraphPad Software, Inc.).
Results
Apoptosis of neurons from
CD73-/- mice is increased after spinal cord injury
To verify this hypothesis, a spinal cord injury
model of CD73-/- and WT mice was constructed, the degree
of injury was identified, the diseased spinal cord was stripped and
transverse sections were produced. Western blot assays were
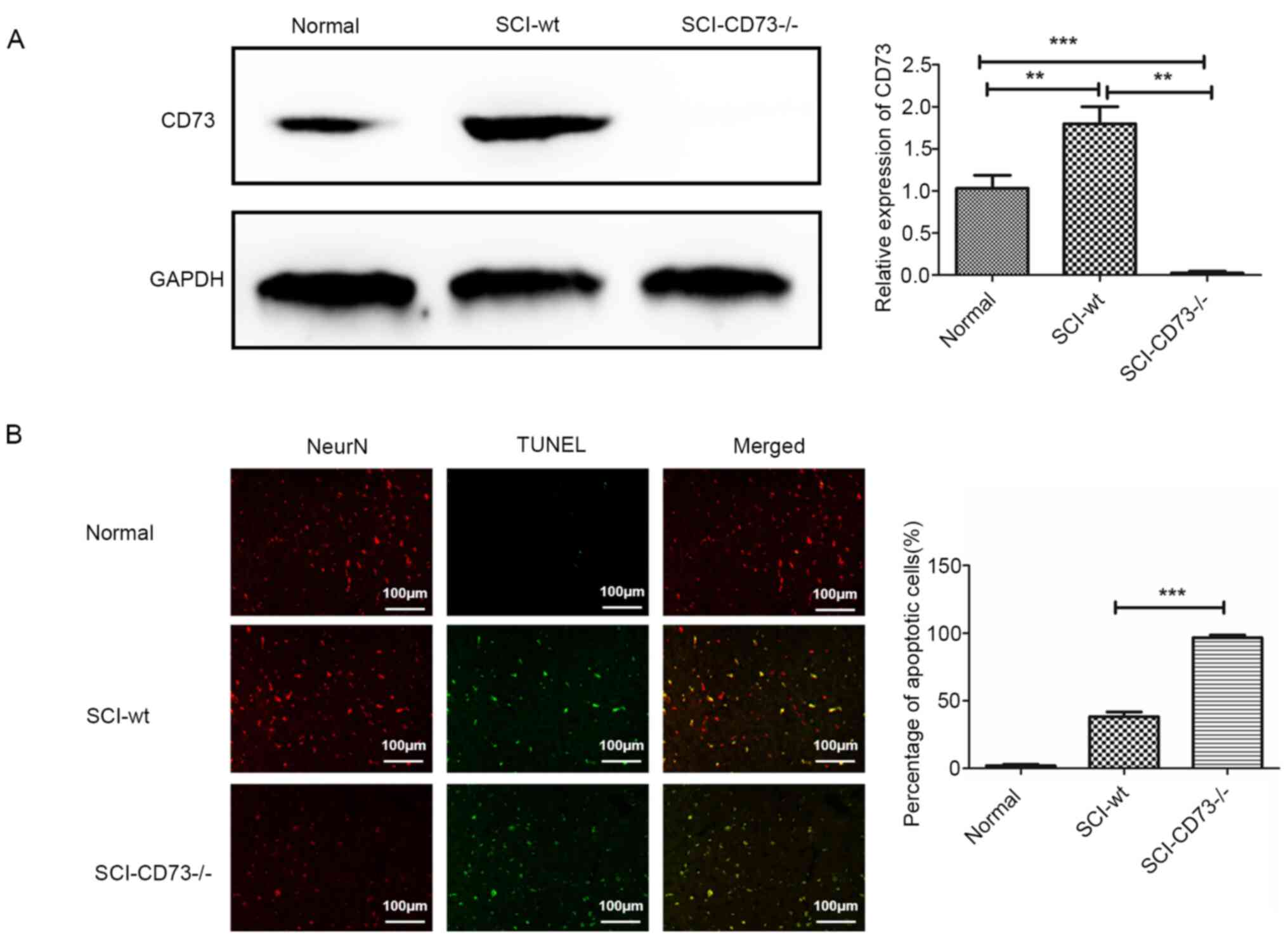
performed to detect the CD73 expression level (Fig. 1A), and double fluorescence staining
of TUNEL and a neuron marker (NeuN) was carried out in normal,
SCI-WT and SCI-CD73-/- tissues (Fig. 1B). Results revealed that the
apoptosis level of neurons caused by SCI in CD73-/- mice
was significantly higher than that in WT mice. Therefore, CD73
deficiency may aggravate neuronal apoptosis caused by SCI.
CD73 can inhibit neuronal apoptosis
caused by oxygen-glucose deprivation
It was demonstrated that CD73 deficiency can
increase apoptosis of neurons induced by SCI in vivo, but
whether CD73 can affect apoptosis of normal spinal cord neurons
in vitro has not been confirmed. Therefore, DRG neurons from
the spinal cord of CD73-/- and WT mice were isolated and
cultured. The apoptosis of both CD73-/- and WT DRG
neurons was detected by flow cytometry after anoxia and glucose
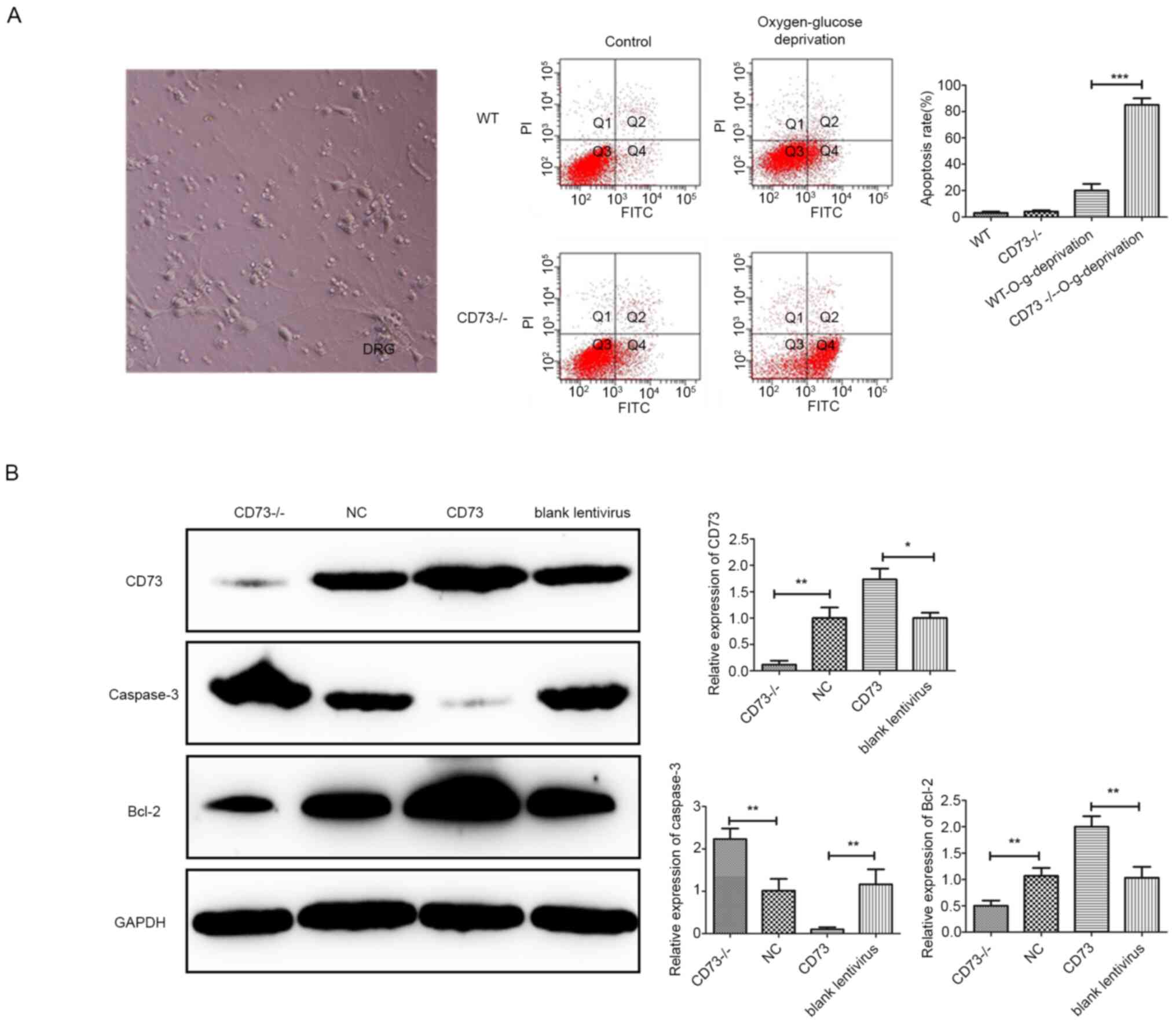
deficiency treatment (Fig. 2A).
Results revealed that the apoptosis of CD73-/- mouse DRG
neurons increased under anoxic and glucose-deficient conditions. To
further study the relationship between CD73 and neuronal apoptosis,
a CD73 overexpression lentivirus was used to enhance the CD73
expression level in DRG neurons before treatment with
oxygen-glucose deprivation. Then the expression levels of CD73, an
apoptosis gene (caspase-3) and an antiapoptotic gene (Bcl-2) in the
CD73 group (CD73 overexpression group), CD73-/- group,
blank lentivirus group and NC group were detected by western
blotting (Fig. 2B). The results
indicated that the expression level of CD73 was negatively
associated with that of caspase-3 and positively associated with
the expression level of Bcl-2.
To study the effects of CD73 on the normal
physiological function of DRG neurons, LDH, MDA, SOD contents in
cell culture medium and ATP, ROS and cell viability in cells were
measured in each group (Fig. 3).
Compared with the NC group, the release of LDH, and MDA and the
production of ROS in CD73-/- DRG neurons was increased,
while the synthesis of ATP, SOD and cell viability was decreased
after hypoxia-hypoglycemia treatment. However, the
CD73-overexpression group revealed the opposite effects. These
results demonstrated that CD73 could inhibit the apoptosis of
neurons caused by glucose deficiency and hypoxia and improve the
adaptability of neurons to harsh environments.
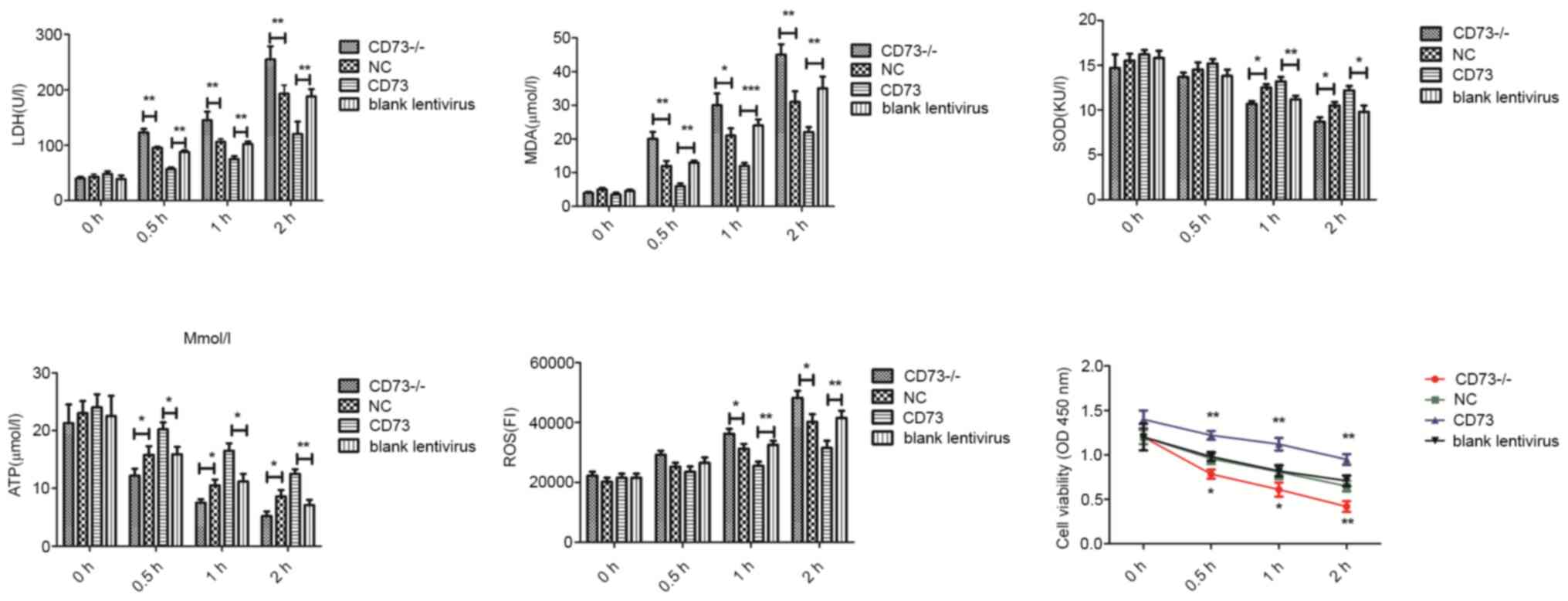 | Figure 3LDH, MDA, SOD contents in cell culture
medium and ATP, ROS and cell viability in CD73-/- and
CD73 mouse dorsal root ganglion neurons under oxygen-glucose
deprivation. The treatment time-points were 0, 0.5, 1 and 2 h.
Blank Lentivirus, empty lentivirus-transfected cells.
*P<0.05, **P<0.01 and
***P<0.001. LDH, lactate dehydrogenase; MDA,
malondialdehyde; SOD, superoxide dismutase; ATP, adenosine
triphosphate; ROS, reactive oxygen species; CD73,
ecto-5'-nucleotidase; NC, negative control. |
CD73 decreases neuronal apoptosis by
promoting Ado synthesis
Firstly, the relationship between CD73 and Ado was
examined. The extracellular Ado concentration in DRG neurons was
significantly decreased after CD73 was knocked out, while it was
significantly increased after CD73 was overexpressed (Fig. 4A). Similarly, Ado concentration
levels in the spinal cord of CD73-/- mice were
significantly lower than those of control WT mice (Fig. 4B). These results indicated that the
expression level of CD73 was positively associated with the
concentration of Ado. Whether the protective effect of CD73 on
neurons is related to its metabolic Ado function is unclear.
Therefore, DRG neurons were treated with APCP and Ado to inhibit
and enhance the CD73-mediated Ado synthesis pathway before low
glucose and hypoxia treatment. Then western blot analysis was
performed to detect the expression of apoptosis-related proteins in
neurons (Fig. 4C and E). The results demonstrated that both
blocking the Ado synthesis pathway with APCP and knocking out CD73
could increase the neuronal apoptosis induced by glucose deficiency
and hypoxia. Proapoptotic genes such as those of the death receptor
pathway (Fas) and the mitochondrial pathway (Bax and caspase-3)
were both induced, while antiapoptotic gene Bcl-2 was decreased.
However, this effect could be reversed by overexpressing the CD73
gene or adding Ado to the culture medium. Thus, it was demonstrated
that CD73 could protect spinal cord neurons from external hypoxia
and a low glucose environment by influencing Ado metabolism.
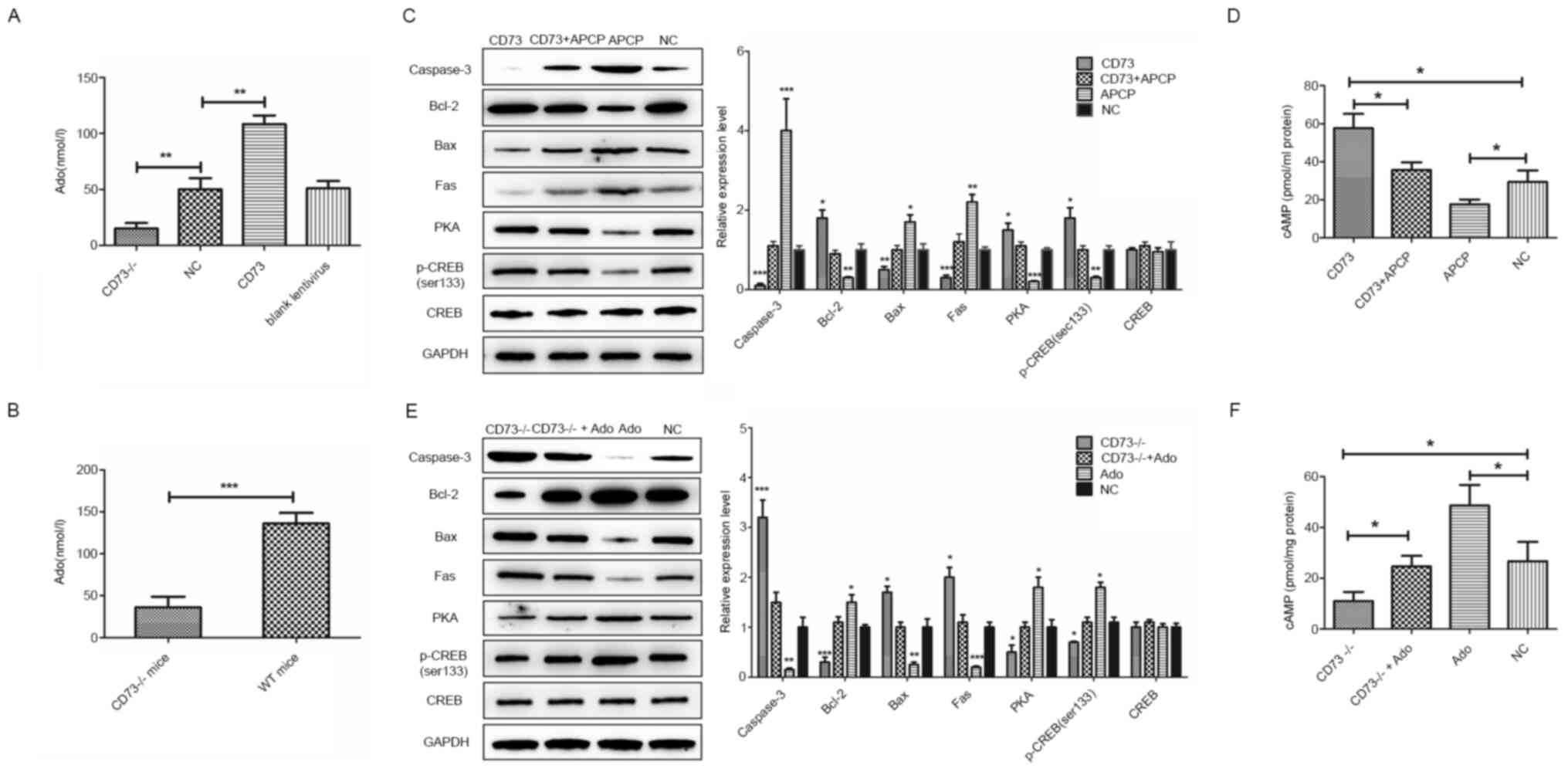 | Figure 4Effects of CD73, APCP and Ado on DRG
neurons in hypoxic and hypoglycemic environments. (A) The
extracellular Ado concentration in CD73-/- and CD73 DRG
neurons. (B) The Ado concentration in the spinal cord of
CD73-/- and WT mice. (C) The expression of
apoptosis-related genes and PKA/CREB signaling pathway genes in
CD73- and APCP-treated mouse DRG neurons under oxygen-glucose
deprivation. (D) The content of cAMP in CD73- and APCP-treated
mouse DRG neurons under oxygen-glucose deprivation. (E) The
expression of apoptosis-related genes and PKA/CREB signaling
pathway genes in CD73-/-- and Ado-treated mouse DRG
neurons under oxygen-glucose deprivation. (F) The content of cAMP
in CD73-/-- and Ado-treated mouse DRG neurons under
oxygen-glucose deprivation. *P<0.05,
**P<0.01 and ***P<0.001. CD73,
ecto-5'-nucleotidase; APCP, α,β-methylene ADP; Ado, adenosine; DRG,
dorsal root ganglion; WT, wild-type; PKA/CREB, protein kinase
A/cAMP response element-binding protein; NC, negative control. |
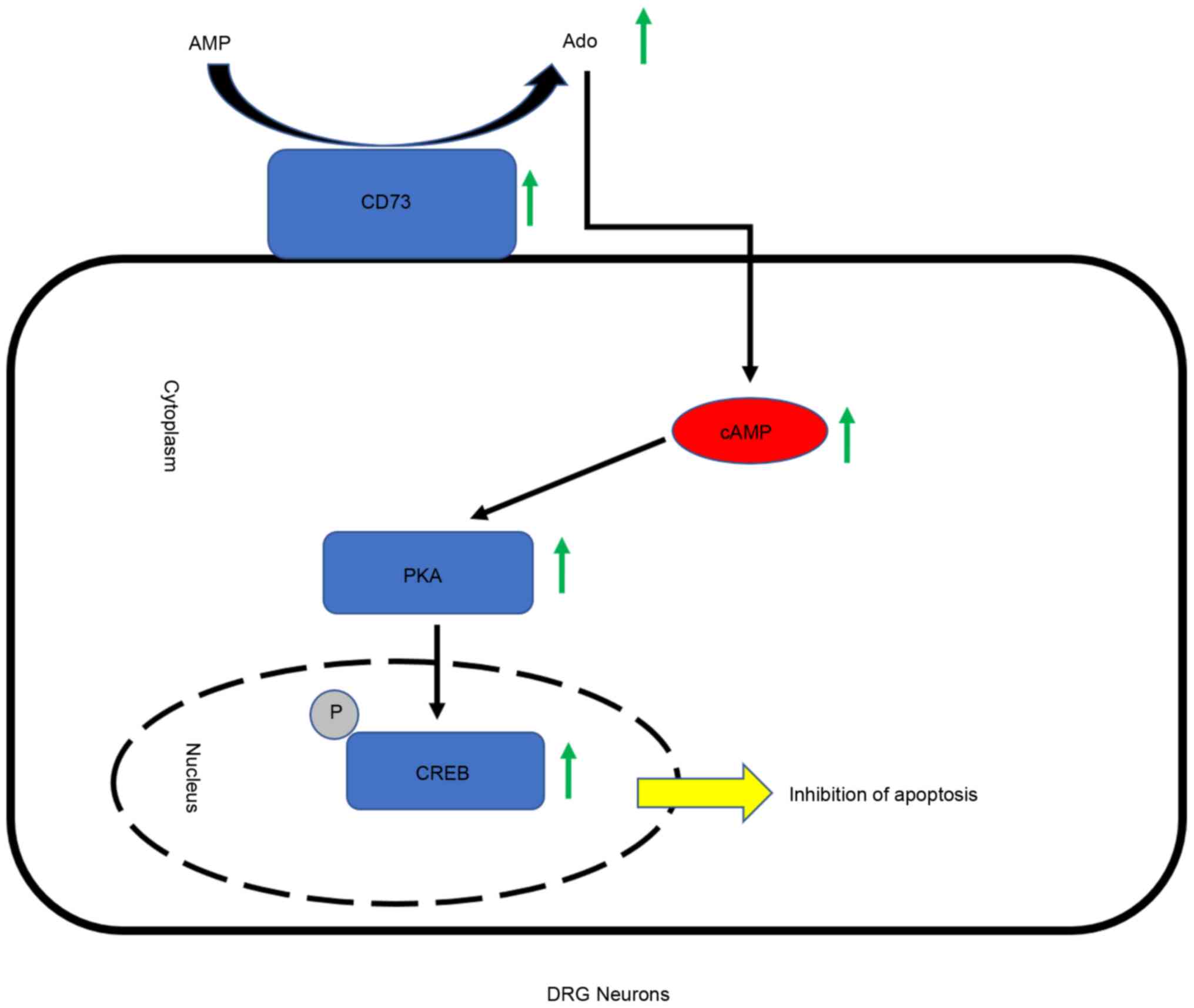
CD73 activates the cAMP/PKA/CREB
pathway by enhancing Ado synthesis
A previous study has revealed that Ado is closely
related to cAMP which has been shown to protect damaged neurons by
activating PKA and CREB (20).
Therefore, whether CD73 affects the cAMP/PKA/CREB signaling
pathways by regulating Ado synthesis was investigated. Western blot
assays and cAMP content detection were performed to verify the
relationship of CD73, Ado and the cAMP/PKA/CREB signaling pathway
(Fig. 4C-F). Results indicated that
the intracellular content of cAMP and the expression of PKA and
p-CREB were upregulated by CD73 overexpression or Ado but
downregulated by CD73-/- and APCP. In summary, CD73
could activate the cAMP/PKA/CREB signaling pathway by enhancing Ado
synthesis.
Discussion
It was observed in a previous study that CD73
deficiency exacerbated the motor dysfunction, inflammatory
responses, and neuronal apoptosis induced by SCI (17). Therefore, focus was directed on the
neuroprotective effect of CD73 in SCI. Firstly, SCI models with
CD73-/- mice and WT mice were constructed. Subsequently,
immunofluorescence and TUNEL staining assays revealed that the
apoptosis level of spinal cord neurons in CD73-/- SCI
mice was significantly higher than that in the control group.
Results indicated that CD73 may inhibit neuronal apoptosis caused
by SCI in vivo. To further investigate the relationship
between CD73 and neuronal apoptosis, CD73-/-, CD73 and blank
lentivirus group neurons and NC spinal DRG neurons were cultured
before anoxia and glucose deficiency treatment. It was demonstrated
that CD73 could inhibit the apoptosis of DRG neurons, suppress the
release of LDH, MDA and ROS, and increase the synthesis of ATP, SOD
and cell viability caused by glucose deficiency and hypoxia. CD73
was revealed to protect neurons both in vitro and in
vivo, and its mechanism is worthy of further study.
CD73 is a multifunctional extracellular nuclease, an
enzyme that mainly catalyzes AMP to Ado. Numerous studies have
confirmed that Ado, as an important molecule in the process of
nucleotide metabolism, has a clear protective effect on ischemic
neurons (21-25).
Therefore, the initiation of the endogenous adenosine pathway has
become an important candidate for the protection of ischemic
neurons. The cAMP molecule, which is regulated by Ado, is an
important second messenger of intracellular signal transduction
(20). PKA, which is
cAMP-dependent, is the classic signal transduction pathway
(26). The activated PKA catalytic
subunit can enter the nucleus and regulate cell metabolism and gene
expression by catalyzing the phosphorylation of serine or threonine
residues of proteins in the cell, the most famous of which is CREB
(27,28). Apoptosis induced by ischemia and
hypoxia can be initiated by various endogenous and exogenous
signals, which are ultimately regulated by genes. In this process,
caspases of cysteine are an important intracellular initiating
mechanism for apoptosis (29), and
Bcl-2 can participate in the regulation of this caspase pathway,
thus indirectly affecting the occurrence of apoptosis in disease
(30). Meller et al reported
that phosphate-acidified CREB after the prestimulation of ischemia
could mediate the expression of the antiapoptotic gene Bcl-2, which
could reduce the apoptosis of cells and thus play a role in
protecting nerve cells (31). Shieh
et al reported that the phosphorylation of CREB activated
the expression of brain-derived neurotrophic factor (BDNF) which is
widely distributed in the central nervous system, playing an
important role in the survival, differentiation, growth and
development of neurons, preventing the death of damaged neurons,
improving the pathological state of neurons, and promoting the
regeneration and differentiation of injured neurons and other
biological effects (32).
Therefore, it was hypothesized that CD73 could affect Ado synthesis
and then indirectly affect the content of intracellular cAMP, and
the increased cAMP could activate the PKA/CREB signaling pathway,
thereby inhibiting cell apoptosis.
To verify this hypothesis, a series of in
vitro cell experiments was conducted and the results
demonstrated that CD73 and Ado could inhibit the expression of
proapoptotic genes (Fas, Bax and caspase-3) and induce an
antiapoptotic gene (Bcl-2) when neurons encountered a hostile
environment. In addition, CD73 and Ado also promoted the synthesis
of cAMP and activated the cAMP/PKA/CREB signaling pathway. The
molecular mechanism of CD73 inhibition of DRG neuronal apoptosis
can be described as follows: When DRG neurons encounter harsh
living conditions, highly expressed CD73 can generate more Ado,
which can promote an increase in intracellular cAMP content. PKA
can be activated and promote the phosphorylation level of CREB.
p-CREB subsequently activates a number of signaling pathways to
reduce the apoptosis caused by the harsh environment and improve
the adaptability of neurons (Fig.
5).
In conclusion, it was revealed that CD73 activated
the cAMP/PKA/CREB signaling pathway by increasing Ado synthesis to
protect neurons from apoptosis. This finding will deepen the
understanding of the neuroprotective mechanism of CD73 and Ado, and
provide a new theoretical basis for the treatment of SCI and other
neurological injuries in the future. However, the specific
molecular mechanism by which CD73 inhibits neuronal apoptosis
through signaling pathways and whether CD73 has other ways to
affect neuronal apoptosis remain to be further investigated. In the
future, more CD73-regulated genes and signaling pathways will be
studied through high-throughput sequencing technology. Through the
in-depth study of them, it is anticipated to deepen the
understanding of the function of CD73 and provide novel insights
into the treatment of diseases related to neuronal apoptosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81871552) and the National Science
Foundation for Distinguished Young Scholars of China (grant no.
81802145).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MS constructed the animal model and performed DRG
cell isolation and culture, performed lentivirus cell infection,
analyzed the data and wrote the original draft of the manuscript.
CZ performed the western blotting, conducted the flow cytometry
assay and analyzed the data. XM performed the remaining experiments
and contributed to the writing, reviewing and editing of the
manuscript. FL designed, conceived and supervised the study. MS and
FL confirm the authenticity of all the raw data. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
All surgical procedures and experimental protocols
in the present study were performed in accordance with standard
guidelines approved by the Ethics Committee of Experimental
Research, Shanghai Medical College, Fudan University (Shanghai,
China). The approval no. is 2019-Huashan Hospital-JS-104.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eckert MJ and Martin MJ: Trauma: Spinal
cord injury. Surg Clin North Am. 97:1031–1045. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li J, Liu G, Zheng Y, Hao C, Zhang Y, Wei
B, Zhou H and Wang D: The epidemiological survey of acute traumatic
spinal cord injury (ATSCI) of 2002 in Beijing municipality. Spinal
Cord. 49:777–782. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ning GZ, Yu TQ, Feng SQ, Zhou XH, Ban DX,
Liu Y and Jiao XX: Epidemiology of traumatic spinal cord injury in
Tianjin, China. Spinal Cord. 49:386–390. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chang FS, Zhang Q, Sun M, Yu HJ, Hu LJ, Wu
JH, Chen G, Xue LD and Lu J: Epidemiological study of spinal cord
injury individuals from halfway houses in Shanghai, China. J Spinal
Cord Med. 41:450–458. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rabchevsky AG, Patel SP and Springer JE:
Pharmacological interventions for spinal cord injury: Where do we
stand? How might we step forward? Pharmacol Ther. 132:15–29.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hou JM, Sun TS, Xiang ZM, Zhang JZ, Zhang
ZC, Zhao M, Zhong JF, Liu J, Zhang H, Liu HL, et al: Alterations of
resting-state regional and network-level neural function after
acute spinal cord injury. Neuroscience. 277:446–454.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hurlbert RJ: Methylprednisolone for the
treatment of acute spinal cord injury: Point. Neurosurgery. 61
(Suppl 1):S32–S35. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oyinbo CA: Secondary injury mechanisms in
traumatic spinal cord injury: A nugget of this multiply cascade.
Acta Neurobiol Exp (Wars). 71:281–299. 2011.PubMed/NCBI
|
|
9
|
Balsam LB: Spinal cord
ischemia-reperfusion injury: MicroRNAs and mitophagy at a
crossroads. J Thorac Cardiovasc Surg. 154:1509–1510.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Knoblach SM, Huang X, VanGelderen J,
Calva-Cerqueira D and Faden AI: Selective caspase activation may
contribute to neurological dysfunction after experimental spinal
cord trauma. J Neurosci Res. 80:369–380. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nardone R, Pikija S, Mutzenbach JS, Seidl
M, Leis S, Trinka E and Sellner J: Current and emerging treatment
options for spinal cord ischemia. Drug Discov Today. 21:1632–1641.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu W, Chi L, Xu R, Ke Y, Luo C, Cai J, Qiu
M, Gozal D and Liu R: Increased production of reactive oxygen
species contributes to motor neuron death in a compression mouse
model of spinal cord injury. Spinal Cord. 43:204–213.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Antonioli L, Pacher P, Vizi ES and Haskó
G: CD39 and CD73 in immunity and inflammation. Trends Mol Med.
19:355–367. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kulesskaya N, Võikar V, Peltola M,
Yegutkin GG, Salmi M, Jalkanen S and Rauvala H: CD73 is a major
regulator of adenosinergic signalling in mouse brain. PLoS One.
8(e66896)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tsutsui S, Schnermann J, Noorbakhsh F,
Henry S, Yong V, Winston BW, Warren K and Power C: A1 adenosine
receptor upregulation and activation attenuates neuroinflammation
and demyelination in a model of multiple sclerosis. J Neurosci.
24:1521–1529. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee JY, Jhun BS, Oh YT, Lee JH, Choe W,
Baik HH, Ha J, Yoon KS, Kim SS and Kang I: Activation of adenosine
A3 receptor suppresses lipopolysaccharide-induced TNF-alpha
production through inhibition of PI 3-kinase/Akt and NF-kappaB
activation in murine BV2 microglial cells. Neurosci Lett. 396:1–6.
2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu S, Zhu W, Shao M, Zhang F, Guo J, Xu H,
Jiang J, Ma X, Xia X, Zhi X, et al: Ecto-5'-nucleotidase (CD73)
attenuates inflammation after spinal cord injury by promoting
macrophages/microglia M2 polarization in mice. J Neuroinflammation.
15(155)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Basso DM, Beattie MS and Bresnahan JC: A
sensitive and reliable locomotor rating scale for open field
testing in rats. J Neurotrauma. 12:1–21. 1995.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Basso DM, Beattie MS and Bresnahan JC:
Graded histological and locomotor outcomes after spinal cord
contusion using the NYU weight-drop device versus transection. Exp
Neurol. 139:244–256. 1996.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gerlo S, Kooijman R, Beck IM, Kolmus K,
Spooren A and Haegeman G: Cyclic AMP: A selective modulator of
NF-κB action. Cell Mol Life Sci. 68:3823–3841. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kitagawa H, Mori A, Shimada J, Mitsumoto Y
and Kikuchi T: Intracerebral adenosine infusion improves
neurological outcome after transient focal ischemia in rats. Neurol
Res. 24:317–323. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gervitz LM, Nalbant D, Williams SC and
Fowler JC: Adenosine-mediated activation of Akt/protein kinase B in
the rat hippocampus in vitro and in vivo. Neurosci Lett.
328:175–179. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zalewska-Kaszubska J: Neuroprotective
mechanisms of adenosine action on CNS neurons. Neurol Neurochir
Pol. 36:329–336. 2002.PubMed/NCBI(In Polish).
|
|
24
|
Wan Q, Zhuang WF, Yao H, Liu ZW, Huang YH
and Ding AS: Effects of adenosine on intracellular free calcium in
cultured rat hippocampal CA1 neurons during anoxia. Sheng Li Xue
Bao. 49:545–550. 1997.PubMed/NCBI(In Chinese).
|
|
25
|
Wan Q, Yao H and Wang F: Involvement of
K(+) channels in the inhibitory effects of adenosine on
anoxia-induced [Ca(2+) ](i) increase in cultured rat hippocampal
CA1 neurons. Biol Signals Recept. 8:309–315. 1999.
|
|
26
|
Walsh DA, Perkins JP and Krebs EG: An
adenosine 3',5'-monophosphate-dependant protein kinase from rabbit
skeletal muscle. J Biol Chem. 243:3763–3765. 1968.PubMed/NCBI
|
|
27
|
Mayr B and Montminy M: Transcriptional
regulation by the phosphorylation-dependent factor CREB. Nat Rev
Mol Cell Biol. 2:599–609. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Montminy MR, Gonzalez GA and Yamamoto KK:
Regulation of cAMP-inducible genes by CREB. Trends Neurosci.
13:184–188. 1990.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang H, Li Q, Li Z, Mei Y and Guo Y: The
protection of Bcl-2 overexpression on rat cortical neuronal injury
caused by analogous ischemia/reperfusion in vitro. Neurosci Res.
62:140–146. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ruffolo SC and Shore GC: BCL-2 selectively
interacts with the BID-induced open conformer of BAK, inhibiting
BAK auto-oligomerization. J Biol Chem. 278:25039–25045.
2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Meller R, Minami M, Cameron JA, Impey S,
Chen D, Lan JQ, Henshall DC and Simon RP: CREB-mediated Bcl-2
protein expression after ischemic preconditioning. J Cereb Blood
Flow Metab. 25:234–246. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shieh PB, Hu SC, Bobb K, Timmusk T and
Ghosh A: Identification of a signaling pathway involved in calcium
regulation of BDNF expression. Neuron. 20:727–740. 1998.PubMed/NCBI View Article : Google Scholar
|