1. Introduction
Osteoporosis is a bone disorder in which the balance
between bone resorption and bone formation is disrupted, resulting
in an increase in bone resorption that decreases bone mineral
density (BMD) (1). The World
Health Organization (WHO) defines osteoporosis as a ‘progressive
systemic skeletal disease characterized by low bone mass and
microarchitectural deterioration of bone tissue, with a consequent
increase in bone fragility and susceptibility to fracture’
(2,3). Osteoporosis is a common disease,
affecting ~200 million patients worldwide, and by 2020, an
estimated 10-14 million individuals would be affected by
osteoporosis in the USA (1,4-6).
Osteoporotic fractures can be alleviated by pharmacological
treatment. Current osteoporosis treatments are either
anti-resorptive, bone-forming or dual-acting (including both types
of treatment) (5,7). Anti-resorptive drugs include
bisphosphonates, anti-receptor activator of NF-κB (RANK) ligand
(RANKL) antibodies (Denosumab), selective estrogen receptor
modulators (SERMs) and calcitonin (3,5,7).
Bone-forming drugs include parathyroid hormone (PTH) and
PTH-related protein (PTHrP) (3,5,8). An
example of a dual-acting drug is Romosozumab (3,5). The
present review highlighted the causes of osteoporosis and the
mechanisms of action underlying the drugs used to treat this
disease. In addition, the present study details an approach to drug
development that has the ability to treat osteoporosis in a more
effective manner.
2. Consideration of targets in
osteoporosis
The WHO criteria for determining osteoporosis is a
reduction in BMD of ≥2.5 standard deviations (SD) below the average
value for young healthy adults, as assessed via dual-energy X-ray
absorptiometry (DXA) (9-14).
This result is expressed as a T-score, and a low T-score (<-2.5)
is an indicator of osteoporosis (12,13).
Osteoporosis is affected by several factors,
including age and sex, and is divided accordingly into primary and
secondary osteoporosis. Primary osteoporosis includes
postmenopausal osteoporosis (type 1), which is characterized by a
decreased production of estrogen that induces bone loss. On the
other hand, secondary osteoporosis is caused by disease or drug
exposure (type 2), and is characterized by reductions in bone
function often due to malabsorption, glucocorticoid use,
hyperparathyroidism, hypogonadism or excessive alcohol consumption
(3,9,10,15,16).
Risk factors
Osteoporosis is influenced by multiple factors.
Representative causes and risk factors for osteoporosis include,
but are not limited to, increasing age, post-menopause, hormones,
genetics, ethnicity, calcium levels, body weight, exercise, poor
nutrition, early menopause, lifestyle habits, chronic disease,
rheumatoid arthritis, vitamin D deficiency, smoking and alcohol
abuse (9,17-20).
Diagnosis
Osteoporosis is a disease that has no major symptoms
unless a fracture occurs (9,17,21).
However, it has previously been demonstrated that individuals aged
≥65 years and postmenopausal women begin to lose bone density due
to several risk factors, including low calcium uptake, smoking,
alcohol abuse, certain medications and ethnic background. These
risk factors are indicative for BMD measurements (9,12,17,22).
BMD T-scores are used for diagnosis, where T-scores >-1.0 SD
denote normal bone mass; T-scores between -1.0 and -2.5 SD are
defined as osteopenia; and T-scores <-2.5 SD are indicative of
osteoporosis (9,12,13,17,22).
Bone remodeling
Bone mass is maintained by continuous bone
remodeling through bone formation by osteoblasts and bone
resorption by osteoclasts (23-25).
Bone remodeling is affected by growth factors, hormones and
cytokines, which regulate osteoclast and osteoblast activity
(23,24). The regulation of osteoclast
activity, differentiation and survival is critical in bone
remodeling, whereby RANK, the corresponding ligand RANKL and decoy
receptor osteoprotegerin (OPG) are key factors (23,26,27).
Osteoclasts
Osteoclasts differentiate from hematopoietic stem
cells, and are activated by macrophage colony-stimulating factor
and RANKL to attach to bone and begin resorption (3,28-30).
Activated osteoclasts induce bone resorption through bone mineral
dissolution and bone degradation via proteolytic enzymes and
hydrochloric acid secretion (3,23,29,31-33).
The main proteolytic enzymes released from osteoclasts are
cathepsin K and MMP9 (3,34,35).
These enzymes are released in response to PTH, and osteoclasts
activated by PTH release bone minerals into the bloodstream
(3,24). Furthermore, the RANK-RANKL
interaction activates additional signaling pathways, such as
TNF-receptor associated factor 6 (TRAF6), MAPK, NF-κB, AKT, JNK and
ERK, and increases the expression of genes associated with
osteoclastogenesis (3,23,24,28-30).
Osteoblasts
Osteoblasts differentiate from mesenchymal stem
cells (MSCs), produce hydroxyapatite and enable bone formation
through mineralized tissue formation (36-38).
The mechanism of bone formation is classified into two types:
Endochondral ossification and intramembranous ossification
(3,39). Endochondral ossification is an
essential process for the formation and growth of long bones,
healing of bone fractures and formation of cartilage by
chondrocytes (40,41), while intramembranous ossification
is essential for rudimentary bone formation and bone fracture
healing (42). Osteoblasts
interact with signaling molecules, including Runt-related
transcription factor 2 (Runx2), osterix, activating transcription
factor 4 and the activator protein 1 family (3,43-45).
In particular, Runx2 levels are increased by bone morphogenetic
proteins (BMPs), Wnt levels, receptors for lipoprotein
receptor-related protein 5 and 6 (LRP5/6) and frizzled
(FZD)-related protein. Consequently, osteoblasts form and promote
bone formation by synthesizing an extracellular matrix to maintain
bone mass while inhibiting or increasing bone resorption (3,46,47).
Osteoblasts also produce RANKL, which promotes osteoclast
differentiation, as previously described (48,49).
Osteocytes
Osteocytes are the most common cells in mature bone.
Unlike osteoclasts that survive for ~12 days and osteoblasts that
survive for about 100 days, osteocytes live in the bone matrix
>10 years (50-52).
Osteocytes are derived from MSCs through osteoblast lineage
differentiation and are the final differentiated form of
osteoblasts that do not divide. Only 10-20% of all osteoblasts
differentiate into osteocytes. In mature bones, osteocytes are
located in specific spaces called lacunae and canaliculi, and
produce several proteins that affect bone remodeling (52-56).
Osteocytes promote bone formation by releasing nitric oxide,
prostaglandin E2 and ATP, and suppress bone formation by releasing
sclerostin, Dickkopf-related protein 1 and FZD-related protein 1.
In addition, osteocytes activate osteoclastogenesis by releasing
RANKL. Sclerostin is expressed only in osteocytes, which acts as a
ligand in the Wnt signaling pathway, activates canonical Wnt
signaling, binds to LRP5/6 receptors and inhibits bone formation
(52,54,55,57,58).
OPG
OPG is a decoy receptor for RANKL and competes with
RANK for binding to RANKL; therefore, OPG inhibits bone resorption
by blocking the binding between RANK and RANKL. Furthermore, OPG
serves as a decoy receptor for TNF-related apoptosis-inducing
ligand (TRAIL), which induces osteoclastogenesis by increasing
TRAF6 and NF-κB signaling, and the expression of nuclear factor of
activated T-cells cytoplasmic 1 (NFATc1) (59,60).
Vitamin D
Vitamin D is a group of fat-soluble secosteroids
that increase the intestinal absorption of calcium, magnesium and
phosphate (61,62). Vitamin D has two main forms: D2
(ergocalciferol) and D3 (cholecalciferol). D2 is extracted from
plant sources and cannot be produced by humans, while D3 is mainly
synthesized in the human skin and is ingested through animal foods,
such as fish oil (63-66).
Vitamin D changes its structure several times during
the digestion process, and finally becomes the activated form
calcitriol, which enhances serum calcium levels by inhibiting
parathyroid gene expression and parathyroid cell proliferation
through binding to vitamin D receptor (VDR) (61,63,66).
Calcitriol acts directly on three organ targets to maintain serum
calcium levels (61,62). The first target organ is the
intestine, where calcitriol stimulates intestinal calcium
absorption; the second is the kidneys, where calcitriol along with
PTH encourages renal distal tubular reabsorption of calcium; and
the third target is bone (61-66).
Calcitriol mobilizes calcium from the bone in a
process that requires PTH. When calcium levels in serum decrease,
PTH-dependent calcitriol activation occurs, promoting the formation
and differentiation of osteoclasts (67-70).
Activation of PTH-dependent calcitriol also induces the secretion
of RANKL, which in turn induces the mobilization of calcium from
the bone. Vitamin D activates this signaling pathway through VDR,
and VDR signaling acts primarily on osteoblasts rather than
osteoclasts, directly acting on the expression of RANKL, which is
important for osteoclast production and increased bone resorption
(71-73).
Vitamin D also inhibits mineralization by increasing the levels of
pyrophosphate and osteopontin.
3. Pharmacological therapy for
osteoporosis
A number of drugs and therapeutic methods have been
evaluated for the treatment of osteoporosis (Fig. 1). Since osteoporosis is caused by
an imbalance between bone formation and bone resorption, drugs for
treating osteoporosis have been developed accordingly (Table I). To initiate pharmacological
treatment, the main purpose of which is to lower the risk of
osteoporotic fractures, it is necessary to determine the patient's
current condition and T-score and DXA are used as diagnostic
techniques (9,74-77).
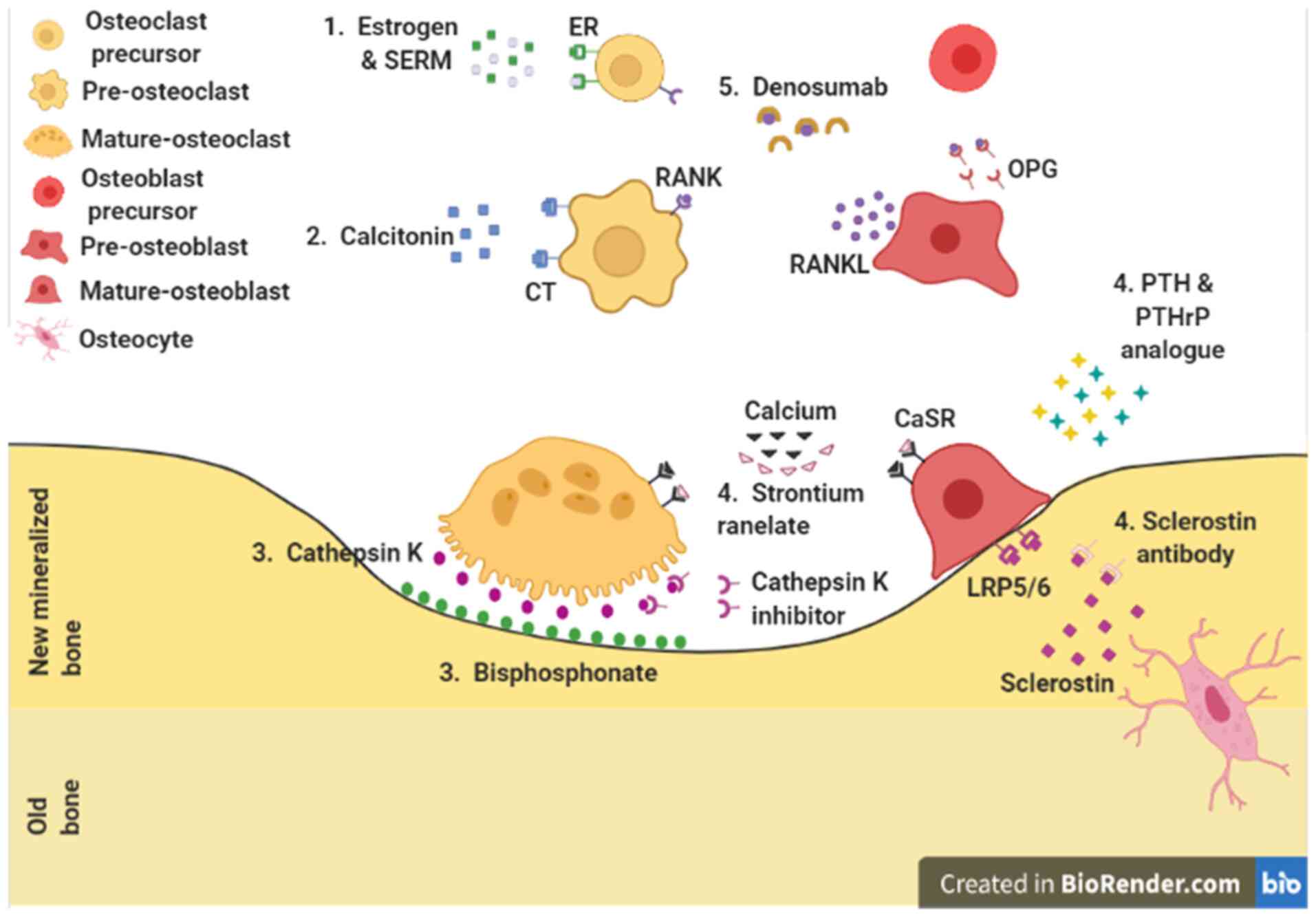 | Figure 1Pharmacological action sites for
osteoporosis. 1. Pre-osteoclast: Estrogen and SERMs. 2. Osteoclast:
Calcitonin. 3. Bone resorption site: Cathepsin K inhibitor and
bisphosphonates. 4. Osteoblast: Strontium ranelate, PTH and PTHrP
analogues, and anti-sclerostin antibody. 5. Osteoclast and
osteoblast: Denosumab. ER, estrogen receptor; SERM, selective
estrogen receptor modulator; CT, calcitonin receptor; RANK,
receptor activator of nuclear factor-κB; RANKL, receptor activator
of nuclear factor-κB ligand; CaSR, calcium sensing receptor;
LRP5/6, lipoprotein receptor-related protein 5 and 6; PTH,
parathyroid hormone; PTHrP, PTH-related protein; OPG,
osteoprotegerin. |
 | Table ISummary of pharmacological drugs used
for osteoporosis. |
Table I
Summary of pharmacological drugs used
for osteoporosis.
| A, Anti-resorptive
drugs |
|---|
| Drug class | Mechanism of
action | Name | Method of
administration | Side effects | (Refs.) |
|---|
| NBP | Inhibition of FPPS
enzyme, inhibition of the mevalonate pathway | Alendronate,
risedronate, ibandronate, zoledronate | Oral | Dysphagia, nausea,
constipation/diarrhea, gastritis, flatulence | (9,17,79,81,93) |
|
NNBP | ATP derivative
formation, induction of osteoclast apoptosis | Etidronate,
clodronate, tiludronate | Oral, IV | AFF, ONJ,
acidregurgitation, hypocalcemia, esophageal ulcers | (9,15,74,76,87) |
| RANKL
inhibitor | Competitive binding
to RANKL, osteoclast inactivation and apoptosis | Denosumab | SC | ONJ, AFF,
musculoskeletal pain, gastrointestinal symptoms | (17,29,50,93,102,103) |
| SERMs | Binding to ER by
acting in a similar manner to estrogen, induction of apoptosis of
osteoclasts | Raloxifene,
bazedoxifene, lasofoxifene, tamoxifen | Oral | Stroke, venous
thromboembolic disorder, muscle cramps | (17,102,109,110) |
| ERT | Estrogen binding to
ER promotes FASL transcription, induction of apoptosis of
osteoclasts | Estrogen | Tablet, insert
pill, patch | Breast cancer,
heart disease, stroke, venous thromboembolic disorders | (79,91,96,102,105) |
| Calcitonin | Calcitonin binding
to CT on osteoclasts, regulation of the CREB pathway | Calcitonin | Oral, intranasal
spray | Hypocalcemia, nasal
adverse reaction, anti-calcitonin antibody formation, prostate
cancer | (111-114) |
| Cathepsin K
inhibitor | Binding to
cathepsin K and inhibition of function, reduction of collagen
degradation by cathepsin K | Balicatib,
odanacatib, ONO-5334 | Oral | AFF, stroke,
pycnodysostosis | (115-119,121,122) |
| Strontium
ranelate | Binding to calcium
sensing receptors instead of calcium, osteoclast inhibition and
induction of apoptosis |
Protelos®,
Osseor® | Oral | Cardiovascular
disorder, venous thromboembolic disorder, myocardial
infarction | (45,126,128,129) |
| B, Bone-formation
drugs |
| Drug class | Mechanism of
action | Name | Method of
administration | Side effects | (Refs.) |
| PTH | PTH binding to
PTH1R on osteoblasts, increased bone formation by anabolic
effect | Teriparatide | SC | Dizziness,
headache, nausea, leg cramps, osteosarcoma | (145-149,154-156) |
| PTHrP | PTHrP binding to
PTH1R on osteoblasts, increased bone formation by anabolic
effect | Abaloparatide | SC | Gastrointestinal
symptoms, dizziness, myalgia, injection site reaction,
osteosarcoma | (148,157-162) |
| Dual-action
drugs |
| Drug class | Mechanism of
action | Name | Method of
administration | Side effects | (Refs.) |
| Anti-sclerostin
antibody | Degradation of
sclerostin, increased Wnt signaling | Romosozumab,
blosozumab | SC, IV | Stroke,
cardiovascular disorder, myocardial infarction | (163,164,166,167) |
Anti-resorptive agents
Bisphosphonates
Bisphosphonates are considered as the first-line
pharmacological treatment for osteoporosis. There are several types
of bisphosphonates, and their basic action is to attach to the bone
and induce osteoclast apoptosis, thereby inhibiting bone resorption
and increasing BMD (Table II)
(9,17,78,79).
Bisphosphonates are stable analogs of pyrophosphate and have a
P-C-P bond that provides binding affinity to hydroxyapatite. As
osteoclasts begin to resorb bones covered with bisphosphonates, the
released bisphosphonates reduce the ability of osteoclasts to form
wrinkled boundaries and produce protons necessary for bone
resorption (3,17,80-85).
 | Table IIClassification of
bisphosphonates. |
Table II
Classification of
bisphosphonates.
| A, NBPs |
|---|
| Drug name | Molecular
formula | Indication | Potency relative to
etidronate (Unit) | (Refs.) |
|---|
| Alendronate |
C4H13NO7P2 | Paget's disease,
osteoporosis in men and postmenopausal women, glucocorticoid
induced osteoporosis | 100-1,000 | (90,175) |
| Risedronate |
C7H10NNaO7P2 | Paget's disease,
osteoporosis in men and postmenopausal women, glucocorticoid
induced osteoporosis | 1,000-10,000 | (90,175) |
| Ibandronate |
C9H22NNaO7P2 | Osteoporosis in
postmenopausal women | 1,000-10,000 | (90,175) |
| Zoledronate |
C5H10N2O7P2 | Paget's disease,
osteoporosis in men and postmenopausal women, glucocorticoid
induced osteoporosis | >10,000 | (90,175) |
| Neridronate |
C6H16NNaO7P2 | Paget's disease,
osteogenesis imperfecta, osteoporosis | 100 | (90,175) |
| Pamidronate |
C3H9NNa2O7P2 | Hypercalcemia of
malignancy, Paget's disease, osteolytic lesions of multiple
myeloma | 100 | (90,175) |
| B, NNBPs |
| Drug name | Molecular
formula | Indication | Relative potency
(Unit) | (Refs.) |
| Etidronate |
C2H8O7P2 | Paget's disease,
heterotopic ossification following total hip replacement | 1 | (81,175) |
| Clodronate |
CH4Cl2O6P2 | Osteolytic bone
metastases, hypercalcemia of malignancy, transient osteoporosis of
the hip | 10 | (81,175) |
| Tiludronate |
C7H9ClO6P2S | Paget's disease,
osteoporosis | 10 | (81,175) |
Bisphosphonates are classified into two types:
Nitrogen-containing bisphosphonates (NBPs) and
non-nitrogen-containing bisphosphonate (NNBPs; Table II) (78,80,86).
NBPs inhibit the farnesyl pyrophosphate synthase enzyme in the
mevalonate pathway, which disrupts protein prenylation and causes
cytoskeletal abnormalities in osteoclasts, resulting in the release
of osteoclasts from the bone (78,80,87-89).
Alendronate, risedronate, ibandronate, zoledronate, neridronate and
pamidronate are typical NBPs (78,80).
As NNBPs do not contain nitrogen, they have a different mechanism
of action compared with NBPs. NNBPs are exchanged for one half the
ATP in terminal pyrophosphates and are metabolized after being
incorporated intracellularly by osteoclasts. The metabolites, which
act as ATP analogs, are then used instead of ATP and interfere with
cell metabolism, consequently inducing osteoclast apoptosis
(78,80,86,87).
Etidronate, clodronate and tiludronate are typical NNBPs (80). Regardless of the type,
bisphosphonates have a central carbon atom, but the length and
structure of the side chains vary. These differences determine
their affinity for specific skeletal sites (17,20,90,91).
For example, alendronate has a high binding affinity to bone but is
slow acting (91), while
risedronate has a low binding affinity to bone and its effect
appears rapidly due to its high diffusion ability (20,90).
Bisphosphonates are administered as oral tablets or
intravenous injections; oral tablets are preferred, but in case of
adverse effects, intravenous injections are used instead. Common
adverse effects of the oral administration of bisphosphonates are
dysphagia, abdominal pain, nausea, flatulence, constipation or
diarrhea, acid regurgitation, taste distortion, esophageal ulcers
and gastritis (9,17,92,93).
Relatively rare adverse effects include atypical femoral fractures
(AFFs), osteonecrosis of the jaw (ONJ), influenza-like symptoms,
hypocalcemia, uveitis and episcleritis (9,17,92,94-96).
The most common adverse effects associated with the stomach and
digestion are alleviated by reducing the number of doses, or
changing to intravenous administration or pre-prandial
administration. Rare adverse effects are alleviated by reducing the
dose or changing the time between doses, since bisphosphonates
continue to be effective even if they are stopped after the first
administration (9,48,69-71).
Despite common adverse effects, bisphosphonate is an ideal
treatment option for patients with early osteoporosis if the
administration method is followed up carefully (1,9,74,97).
Denosumab
Denosumab is the first fully human monoclonal
antibody that competitively binds to human RANKL, thus preventing
the interaction between RANK and RANKL, and inhibiting the
RANK/RANKL signaling pathway. Therefore, it inhibits osteoclast
activity and differentiation, and consequently inhibits bone
resorption, thus inhibiting osteoclast function (3,17,28,49,92,98,99).
Denosumab is injected subcutaneously into the thigh
or abdomen. Unlike bisphosphonates, it is not considered as a
first-line treatment for osteoporosis. Denosumab is usually
prescribed instead of bisphosphonates for patients with renal
failure (78,96). To confirm the efficacy of
denosumab, a study was conducted called ‘Fracture Reduction
Evaluation of Denosumab in Osteoporosis every 6 Months’ (FREEDOM),
which revealed an increase in BMD and a decrease in bone turnover
rate in the denosumab group compared with those in the placebo
group (5,17,100).
The FREEDOM study also highlighted the adverse
effects of long-term denosumab treatment, which were similar to
those associated with bisphosphonates, including ONJ, AFFs,
hypocalcemia, musculoskeletal pain and gastrointestinal symptoms
(9,78,92,101). Unlike bisphosphonates, denosumab
also weakens the immune system. As denosumab targets RANK-RANKL
interactions, lymphocytes requiring RANK-RANKL interactions are
affected, resulting in decreased lymphocyte activity and increased
risk of infection (92,101,102). Furthermore, unlike
bisphosphonates, denosumab loses its efficacy rapidly after
cessation of administration. If adverse effects occur, denosumab
administration can be stopped immediately; however, the associated
osteoporosis-suppressing effects also disappear rapidly (78,98,102,103).
Estrogen replacement therapy
(ERT)
Estrogen is the primary sex hormone secreted by
women, and estrogen secretion decreases as menopause begins.
However, in osteoporosis, estrogen regulates osteoclast apoptosis.
Estrogen binds to estrogen receptor α, which translocates to the
nucleus and binds the Fas ligand (FasL) transcription site to
promote FasL transcription. FasL subsequently binds to Fas, a
receptor present on the surface of pre-osteoclasts, inducing
cleavage of caspase 8 and promoting osteoclast apoptosis (78,101,104).
Hormone replacement therapy (HRT) is similar to ERT,
but instead uses progestin in combination with estrogen. ERT or HRT
is administered as tablets, patches on the skin or insertion of
estrogen pills under the skin (78,101,104). Long-term use of HRT and ERT
exhibit adverse effects; HRT increases the risk of breast cancer,
heart disease, stroke and venous thromboembolic disorders, while
ERT increases the risk of endometrial cancer, stroke and venous
thromboembolic disorders (1,90,95,104-107).
ERT is selectively used in menopausal women and is not considered a
first-line therapy for osteoporosis. ERT or HRT should be
discontinued upon adverse health effects; however, discontinuation
increases the risk of osteoporosis (78,101).
SERMs
SERMs are used to reduce adverse effects caused by
the long-term use of estrogen. SERMs are nonsteroidal drugs that
bind to estrogen receptors and exert selective estrogenic activity
depending on the type of cell or tissue. SERMs function in a
similar manner to estrogen, without any adverse effects on the
breast or endometrium. Commonly used SERMs include raloxifene,
bazedoxifene, lasofoxifene and tamoxifene (78,101).
SERMs increase the risk of stroke, thromboembolic
disorders and muscle cramps (52,65,76).
Thus, they are contraindicated for use in premenopausal women with
osteoporosis, while they are considered a first-line therapy for
postmenopausal women with osteoporosis (78,101,108,109).
Calcitonin
Calcitonin is a 32-amino acid hormone secreted by
thyroid C cells. The three main functions of calcitonin are: i) To
absorb calcium into the bones; ii) to inhibit calcium reuptake in
the kidneys; and iii) to inhibit calcium reuptake in the small
intestine. Thus, for osteoporosis treatment, calcitonin functions
by storing calcium in bones. The majority of cells require calcium,
which is necessary for multiple functions within cells. Among the
different tissues that store calcium, bone stores the largest
quantity of calcium by combining it with phosphoric acid to form
hydroxyapatite (110-112).
The actions of calcitonin are opposite to those of
PTH; PTH increases the concentration of blood calcium, whereas
calcitonin decreases it (111,112). Calcitonin binds to the calcitonin
receptor on osteoclasts and regulates transcription through the
cyclic adenosine monophosphate (cAMP)/protein kinase
A(PKA)-cAMP-response element binding protein pathway (110-112).
Methods for calcitonin administration include
injection, oral formulation and intranasal spray. Two types of
calcitonin are used in therapy: Human and salmon calcitonin. Salmon
calcitonin is used more often due to its high affinity for human
calcitonin receptors. Calcitonin treatment is usually considered a
second-line therapy for osteoporosis, and it is used when
first-line therapy is ineffective or intolerable (78,101,113). Calcitonin also exhibits adverse
effects, such as hypocalcemia, nasal adverse reactions, formation
of calcitonin antibodies and prostate cancer (9,101).
If adverse effects appear following the use of calcitonin
treatment, it must be replaced with alternate osteoporosis
therapies.
Cathepsin K inhibitors
Cathepsin K is a member of the papain-like cysteine
protease family and is highly expressed in osteoclasts. When
osteoclasts are activated, cathepsin K, residing in the lysosomes
of osteoclasts, is released into the resorption lacuna, initiating
bone resorption (114,115). During bone resorption,
osteoclasts form a structure called the sealing zone, which is a
dynamic actin-rich structure that defines the resorption region.
Subsequently, osteoclasts secrete several molecules, including
proteases, to break down bone material for resorption (114-116).
The bones are composed of a mineralized organic matrix consisting
of 30% organic components and 70% inorganic components. The
majority of organic components are composed of type 1 collagen,
while the inorganic components are mainly composed of
hydroxyapatite (114,115,117). There are two types of collagen,
types 1 and 2, which form a triple helix structure with two α1
chains and one α2 chain. Due to this structure, collagen resists
proteolysis by proteases, such as MMP9 and serine protease. By
contrast, cathepsin K efficiently cleaves collagen and any
telopeptides to form collagen monomers (114,115,117-119),
thus being an important marker of bone resorption and an ideal
therapeutic target. When cathepsin K is inhibited, bone resorption
activity of osteoclasts is suppressed, resulting in increased BMD
(114-117).
Cathepsin K inhibitors include balicatib, odanacatib
and 2H-Pyran-4-propanoic acid (114,119-121),
which are administered orally. Unlike other bone resorption
inhibitors, they inhibit the activity of osteoclasts, rather than
reducing the number. Cathepsin K inhibitors are good
anti-resorptive agents, but the associated adverse health effects
are yet to be fully established. Reported adverse effects include
increased risk of stroke, AFF and pycnodysostosis (114,118,121,122). The stability of cathepsin K
inhibitors is also yet to be elucidated; therefore, further studies
on the adverse effects and stability of cathepsin K inhibitors are
required (101,118,120,123,124).
Strontium ranelate
Osteoblasts and osteoclasts have calcium sensing
receptors (CaSRs), and their activity changes based on
extracellular calcium concentration. Calcium activates various
cellular pathways (40,44,125). In osteoblastic cells, elevated
extracellular calcium levels activate signaling pathways, including
phospholipase C (PLC), protein kinase C (PKC), ERK, JNK, cAMP and
PKA (3,29). The ERK pathway increases the
proliferation of osteoblastic cells, while the AKT pathway inhibits
the apoptosis of osteoblastic cells by increasing survival signals
(3,29). Additionally, calcium increases the
expression of insulin-like growth factor (IGF)-1 and IGF-2, thereby
increasing the proliferation of osteoblastic cells and inducing
differentiation by increasing the expression of cyclooxygenase 2
and prostaglandin E2 (125,126). In osteoclasts, when CaSR is
activated by high levels of extracellular calcium, PLC and NF-κB
are activated, thus inducing the apoptosis of osteoclasts (125,126).
Strontium, with an atomic number of 38, is located
just below calcium on the periodic table (127,128). The nucleus of strontium is
approximately the same size as that of calcium; thus, cells absorb
strontium instead of calcium and transport it to bone or tooth
enamel (127,128). Although the mechanism of action
of strontium ranelate has not been clearly identified, the
characteristics of strontium and the reported research (127,128) suggest that it enters the cell
through CaSRs of osteoclasts and osteoblasts, and acts in a similar
manner to calcium (125,127,128). Therefore, strontium ranelate
increases the differentiation and proliferation of osteoblasts, and
the activated osteoblasts produce OPG, which reduces the activity
of osteoclasts. Furthermore, it acts directly on the CaSRs of
osteoclasts and increase their apoptosis (125,127,129).
Strontium ranelate is administered orally. Due to
its adverse effects, strontium ranelate is considered a second-line
therapy, and is administered when other osteoporosis therapies
cannot be used or are ineffective (78,101). Common adverse effects include
cardiovascular disorders, venous thromboembolism, myocardial
infarction and symptoms of the nervous system. Rarely reported
adverse effects include allergic reactions, such as drug rash with
eosinophilia and systemic symptoms syndrome. Adverse effects
associated with the heart are particularly severe. Therefore, it is
recommended that strontium ranelate is administered only to
patients with severe osteoporosis. It is not recommended for
patients with severe renal impairment, thrombophlebitis, ischemic
heart disease, a history of peripheral artery disease,
cerebrovascular disease or hypertension (101,128,130-132).
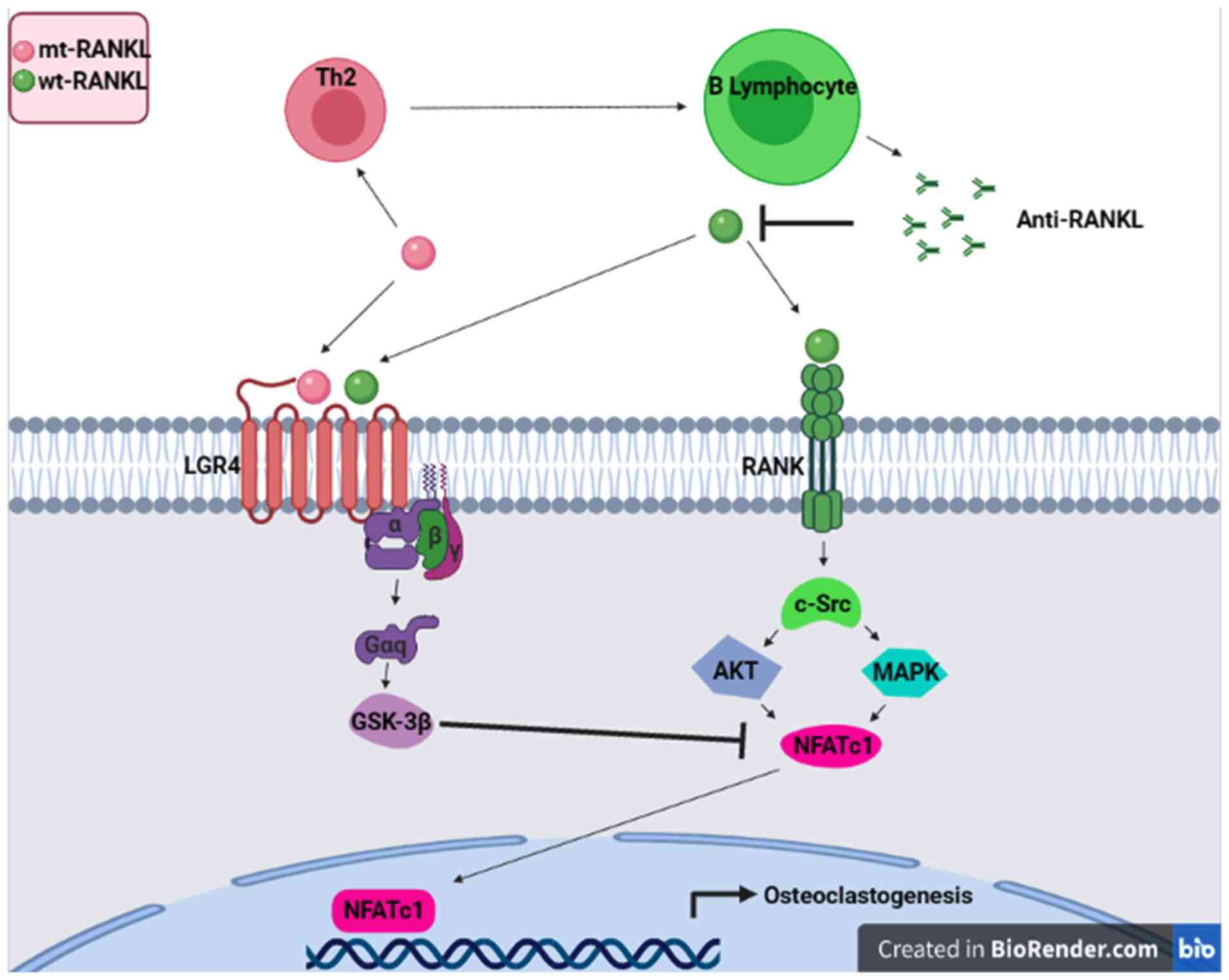
Mutant RANKL
Mutations within the TNF-like core domain of RANKL
have been reported for creating a novel therapy for osteoporosis.
The identification of RANKL as the final effector in the
pathogenesis of osteoporosis has led to an improved understanding
of bone remodeling. When RANKL binds to its receptor (RANK),
osteoclastic differentiation and activation are initiated. RANKL is
a member of TNF superfamily, which is a group of cytokines involved
in cell proliferation and cell death consisting of 19 multimeric
ligands interacting with cognate receptor molecules, the majority
of which require trimerization to initiate their signaling cascade
(133). Ligands belonging to the
TNF superfamily are mostly type II transmembrane glycoproteins,
containing a C-terminal, receptor-interacting ectodomain, a
transmembrane domain and an N-terminal intracellular tail. The
extracellular domain is either cleaved by the proteolytic activity
of metalloproteases or produced by alternative splicing (133). Three-dimensional structures of
TNF-α, TRAIL and RANKL (alone and in complex with their respective
receptors) have revealed remarkably similar overall structures that
comprise unique conserved elements involved in receptor binding
(134). Ko et al (135) and Jang et al (136) proposed a strategy using a RANKL
variant as a competitive inhibitor for RANKL/RANK signaling. They
suggest that this RANKL variant activates leucine rich repeat
containing G protein-coupled receptor 4 (LGR4) signaling, which
competitively regulates RANK and acts as an immunogen that induces
anti-RANKL antibody production, demonstrating a strategy in the
development of general immunotherapy. The RANKL variant did not
bind RANK in osteoclast progenitor cells, but activated LGR4
through the GSK3-β signaling pathway, thereby suppressing the
expression and activity of activated T cell cytoplasmic NFATc1
during osteoclastogenesis (Fig.
2). The aforementioned RANKL variant generated high levels of
RANKL-specific antibodies, blocked osteoclastogenesis and inhibited
osteoporosis in ovariectomized mouse models. Generated anti-RANKL
antibodies demonstrated a high inhibitory effect on
osteoclastogenesis in vivo and in vitro. In addition,
Liu et al (137)
demonstrated that immunization with a RANKL mutant generates an
inter-species anti-RANKL antibody, which blocks the interaction
between RANKL and its receptor, and further prevents the formation
of osteoclasts and improves the bone density in rats with
ovariectomy (OVX). Furthermore, the development of an unnatural
amino acid into a RANKL vaccine has been proposed as a therapeutic
approach to inhibit RANKL activity (138). An anti-RANKL vaccine,
Y234pNO2Phe, was constructed by substituting a single
tyrosine residue (Tyr234) in murine RANKL with p-nitrophenylalanine
(pNO2Phe), and it was demonstrated that
Y234pNO2Phe induced a high titer antibody response in
C57BL/6 mice and prevented OVX-induced bone loss in mice (138). The potential advantage of a
vaccine-type approach to RANKL inhibition compared with that of
antibody-based approaches is that patients who discontinue
denosumab experience rapid increases in bone remodeling and an
increased risk of multiple vertebral fractures. It is possible,
although not clearly demonstrated, that this vaccine approach may
not induce rapid high-turnover bone loss, either by causing more
durable RANKL inhibition or by allowing a more gradual resumption
of remodeling when vaccinations are discontinued. It would be
reasonable to evaluate this vaccine approach for its ability to
minimize the risk of high-turnover bone loss in situations where
vaccinations and boosters are discontinued.
Non-coding RNA
Long non-coding RNAs (lncRNAs) represent a group of
non-protein-coding RNA transcripts that have been reported to play
pivotal roles in various biological functions such as gene
expression, cell proliferation and differentiation (139). As a ‘bridge’ between DNA and
protein, RNA serves a complex regulatory role. In eukaryotic cells,
protein-coding RNA (mRNA) only accounts for ~2% of the genome, and
the remaining transcripts are categorized as non-protein-coding
RNAs (133). Unlike ribosomal RNA
and transfer RNA, which are well known, other non-coding RNAs were
previously considered to be transcriptional ‘noise’ (140). However, a recent study by Zhu
et al (141) revealed that
lncRNAs serve a vital role in regulating bone and cartilage
development and remodeling processes. Research on microRNAs
(miRNAs/miRs) and lncRNAs in the context of osteoporosis is
limited. Often, there are conflicting results in the literature,
such as miR-223 having the ability to both promote and inhibit
osteoclastogenesis. Wijnen et al (142) suggested that miRNAs provided both
positive and negative cross-talk between different regulatory
pathways, thereby leading to the aforementioned phenomenon. Another
potential explanation is that miRNA is present in different
clinical specimens. For example, Mandourah et al (143) found that while both miR-122-5p
and miR-4516 were suitable biomarkers for osteoporosis, miR-122-5p
was detectable in serum, while miR-4516 was found in plasma
(143). A large cohort study of
682 women found that there was no association between bone
parameters and circulating levels of miRNAs, although these results
changed after age adjustment. The authors suggested that this
observation could be due to the fact that age was also strongly
associated with the serum levels of the 32 miRNAs they selected.
These factors may be associated with fragility fracture and low BMD
in patients with osteoporosis, and may provide new insights into
the modulation and potential treatment of osteoporosis.
Bone formation therapies PTH
analogue
PTH is an 84-amino acid peptide hormone that is
synthesized in the parathyroid glands and regulates serum calcium
concentration. PTH function is mediated by PTH-1 receptor (PTH1R),
which is a G protein-coupled receptor (GPCR) expressed in
osteoblasts and oocytes (144-146).
When PTH1R is stimulated, it activates several GPCR-related
signaling pathways, such as the cAMP/PKA, PLC/PKC and ERK signaling
pathways. Furthermore, PTH regulates the Wnt signaling pathway by
downregulating sclerostin, a Wnt antagonist (145-149).
The effects of PTH are divided into anabolic and catabolic effects.
The anabolic effect of PTH increases the differentiation and growth
of osteoblasts, thereby increasing bone formation, while its
catabolic effect increases bone resorption indirectly, since
osteoclasts are activated by RANKL secreted by osteoblasts
(146-148).
PTH analogues are administered in a continuous or
intermittent manner. When a PTH analogue is administered, markers
of bone formation are initially increased, and those of bone
resorption are activated at a later point; the period in which bone
formation is higher than bone resorption is called the anabolic
window, during which maximum bone formation occurs. After the
anabolic window period, bone resorption gradually increases
(150-152).
PTH analogues are not recommended as first-line therapy for
osteoporosis due to their high cost and difficulty of
administration by subcutaneous injections. Adverse effects of PTH
analogues include dizziness, headache, nausea and leg cramps. In
addition, in animal experiments, osteosarcoma was reported to be
induced based on the duration of the treatment; therefore, the
administration of PTH analogues should be limited to 2 years
(153-155).
PTHrP analogue
PTHrP, a member of the PTH family, is secreted by
MSCs. PTH and PTHrP share 8 of the first 13 amino acids, have
similar secondary structures, an overall homologous sequence and
bind to the same receptor (147,156,157). PTH1R has two conformations: i) G
protein-dependent RG conformation; and ii) a G protein-independent
R0 conformation. The two conformations activate the same signaling
pathway, yet demonstrate different response patterns depending on
the activated conformation (158-160).
Long-lasting signaling was observed when the R0 conformation was
activated, whereas short-lasting signaling was observed when the RG
conformation was activated. Since anabolic and catabolic effects
are activated as a long-acting signal in the R0 conformation, the
anabolic effect is low; whereas in the case of the RG conformation,
the anabolic effect is high with a strong signal that lasts for a
short time. The binding affinities of the ligands for these two
conformations are different. PTHrP analogues have a higher affinity
for the RG conformation than that of PTH analogues. Thus, PTHrP
analogues are expected to exert an improved anabolic effect than
that of PTH analogues (144,147,159-161).
The adverse effects of PTHrP analogues are similar
to those of PTH analogues, including gastrointestinal complaints,
injection-site reactions, dizziness and myalgia. Furthermore, a
mouse model demonstrated that PTHrP analogs were associated with
osteosarcoma and osteoblastoma, similarly to the observations with
PTH analogues. Thus, PTHrP analogue administration is limited to 2
years (153-155,158).
Dual-action therapy Anti-sclerostin
antibody
Sclerostin is a glycoprotein secreted by
osteocytes; it functions as an antagonist of BMP and suppresses the
canonical Wnt signaling pathway. Sclerostin interacts with pro-BMP7
and mature BMP7 to increase the intracellular accumulation of BMP7,
leading to its degradation. Thus, sclerostin inhibits the BMP7
signaling pathway (162,163). The canonical Wnt signaling
pathway is activated by the binding of a Wnt ligand to a receptor
complex composed of LRP5/6 and FZD receptors. FZD mediates the
recruitment of axin to form a complex that inhibits β-catenin
phosphorylation by GSK-3β. Non-phosphorylated β-catenin then
accumulates in the cytoplasm, resulting in its nuclear
translocation, and thereby triggers the transcription of genes
involved in bone formation. It also induces the internalization of
LRP5/6, which forms a complex with axin and adenomatous polyposis
coli protein, which then degrades phosphorylated β-catenin
(144,162-164).
Anti-sclerostin antibody treatment removes sclerostin from the Wnt
signaling pathway, thus activating the canonical Wnt signaling
pathway, and consequently promoting bone formation and inhibiting
bone resorption. β-catenin inhibits osteoclastogenesis by
increasing the production of OPG in osteoblasts and by regulating
the RANK/RANKL/OPG signaling pathway. Therefore, activation of the
canonical Wnt signaling pathway not only increases bone formation
but also decreases bone resorption (144,162,164).
Anti-sclerostin antibody treatment is administered
via subcutaneous injection, and the most common adverse effects are
stroke, cardiovascular events and myocardial infarction.
Furthermore, Wnt signaling is associated with cancer, which has
been reported as an adverse effect in the Fracture Study in
Postmenopausal Women with Osteoporosis (FRAME) study (144). Long-term anti-sclerostin antibody
treatment, therefore, is not recommended, since it poses a high
risk to the heart and may cause cancer (144,165,166).
Combination therapy
Since pharmacological therapies for osteoporosis
have limitations, several studies have been conducted to determine
more effective therapies. An example of which is combination
therapy, which is expected to exert a synergistic effect by using
either two anti-resorptive drugs or an anti-resorptive drug with an
anabolic drug.
In several studies, combination therapy was
evaluated using existing drugs. The PTH and alendronate study used
a combination of PTH and alendronate, and demonstrated no increase
in BMD compared with that of the individual use of PTH or
alendronate. Another study used a combination of PTH and SERMs, and
also demonstrated no increase in BMD. By contrast, a combination of
denosumab and teriparatide slightly increased BMD (76,101,167-169).
Another study reported that teriparatide can be used alone or in
combination with denosumab and abaloparatide (170). Previous studies have indicated
that PTH and PTHrP-related protein analogues, whether as
monotherapy, in combination or in sequence with anti-resorptive
agents, serve an important role in the management of osteoporosis
(170). Since the benefit of
combination therapy is only a slight increase in BMD, it is
generally not recommended for osteoporosis due to its combined
adverse effects and increased cost. Therefore, combination therapy
is limited to patients with high risk of fractures or when other
therapies are ineffective.
Sequential therapy
Since combination therapies are associated with
more adverse effects than clear advantages, studies to identify
other effective therapies, such as sequential therapy, have been
conducted. Sequential therapy has been used to overcome the
limitation of prolonged treatment time, which is a common problem
with osteoporosis drugs.
In the denosumab and teriparatide transitions in
postmenopausal osteoporosis (DATA-Switch) study, BMD increased in
the spine and hips upon switching to denosumab after teriparatide
treatment (100,171). In the Abaloparatide Comparator
Trial in Vertebral Endpoints study, switching from abaloparatide to
alendronate increased BMD and maintained lowered fracture risk
(165,171,172). In the FRAME study, switching from
romosozumab to denosumab also maintained a lower fracture risk. In
an active-controlled fracture study in postmenopausal women with
high risk of osteoporosis, switching from romosozumab to
alendronate increased BMD and decreased non-vertebral fracture risk
(171-175).
As demonstrated in a number of studies, sequential therapy is
effective against osteoporosis as it increases BMD compared with
the effects of single sustained therapy. However, it has adverse
effects, most of which appear to be similar to that of single
therapies.
4. Conclusions
Osteoporosis, a chronic and difficult-to-cure
disease, occurs naturally with age. As the lifespan of a person
increases, so does the incidence of osteoporosis and the length of
disease (1,9). Therefore, effective long-term
treatment options for osteoporosis are required. Among the various
treatments for osteoporosis that are currently in use,
pharmacological therapy is the most efficient and accessible, and
has been rigorously studied (9,10).
Currently used therapies include those that inhibit bone
resorption, promote bone formation and dual-action therapies
(9,17). Pharmacological therapies are used
in patients with osteoporosis to reduce the risk of fracture and
increase BMD, but their use is limited by adverse effects, which
are determined by multiple factors, including the patient's
nutritional status, genetic factors and past medical history
(7-9,13,17).
To reduce these adverse effects, studies on individual variability,
such as treatment time, concentration and timing of the
administration of drugs, as well as drugs' mechanisms of action,
such as osteoclast inhibition and osteoblast growth promotion, have
been conducted (13,17,20).
In addition to single therapies with drugs, combination therapy and
sequential therapy are under investigation to treat osteoporosis
more effectively. However, it is not yet possible to completely
cure osteoporosis, and there are serious adverse effects develop
due to the long-term use of the current drugs. Therefore, there is
a need to develop novel drugs that have the ability to effectively
treat osteoporosis while minimizing adverse effects, regardless of
variable patient-related factors.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science Research
Program through the National Research Foundation of Korea (NRF),
funded by the Ministry of Education in Korea (grant nos.
NRF-2021R1I1A3046499, NRF-2021R1I1A1A01049248 and
NRF-2019R1G1A1100099).
Availability of data and materials
Not applicable.
Authors' contributions
BK, YJC and WL were responsible for project
conceptualization. BK and YJC were responsible for writing and
original draft preparation:. WL was responsible for writing,
reviewing and editing the manuscript, and creating the figures. BK
and YJC were responsible for the assessment of all the raw data. WL
was responsible for project supervision and administration. BK, YJC
and WL were responsible for funding acquisition. Data
authentication is not applicable. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tu KN, Lie JD, Wan CKV, Cameron M, Austel
AG, Nguyen JK, Van K and Hyun D: Osteoporosis: A review of
treatment options. P T. 43:92–104. 2018.PubMed/NCBI
|
|
2
|
Genant HK, Cooper C, Poor G, Reid I,
Ehrlich G, Kanis J, Nordin BE, Barrett-Connor E, Black D, Bonjour
JP, et al: Interim report and recommendations of the World Health
Organization task-force for osteoporosis. Osteoporosis Int.
10:259–264. 1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Noh JY, Yang Y and Jung H: Molecular
mechanisms and emerging therapeutics for osteoporosis. Int J Mol
Sci. 21(7623)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wright NC, Looker AC, Saag KG, Curtis JR,
Delzell ES, Randall S and Dawson-Hughes B: The recent prevalence of
osteoporosis and low bone mass in the United States based on bone
mineral density at the femoral neck or lumbar spine. J Bone Miner
Res. 29:2520–2526. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Langdahl BL: Overview of treatment
approaches to osteoporosis. Br J Pharmacol. 178:1891–1906.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Burge R, Dawson-Hughes B, Solomon DH, Wong
JB, King A and Tosteson A: Incidence and economic burden of
osteoporosis-related fractures in the United States, 2005-2025. J
Bone Miner Res. 22:465–475. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Langdahl BL and Harsløf T: Medical
treatment of osteoporotic vertebral fractures. Ther Adv
Musculoskelet Dis. 3:17–29. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Camacho PM, Petak SM, Binkley N, Clarke
BL, Harris ST, Hurley DL, Kleerekoper M, Lewiecki EM, Miller PD,
Narula HS, et al: American association of clinical endocrinologists
and American college of endocrinology clinical practice guidelines
for the diagnosis and treatment of postmenopausal
osteoporosis-2016. Endocr Pract. 22 (Suppl 4):S1–S42.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Akkawi I and Zmerly H: Osteoporosis:
Current concepts. Joints. 6:122–127. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
De Martinis M, Sirufo MM and Ginaldi L:
Osteoporosis: Current and emerging therapies targeted to
immunological checkpoints. Curr Med Chem. 27:6356–6372.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Milat F and Ebeling PR: Osteoporosis
treatment: A missed opportunity. Med J Aust. 205:185–190.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cosman F, de Beur SJ, LeBoff MS, Lewiecki
EM, Tanner B, Randall S and Lindsay R: National Osteoporosis
Foundation. Clinician's guide to prevention and treatment of
osteoporosis. Osteoporos Int. 25:2359–2381. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nuti R, Brandi ML, Checchia G, Di Munno O,
Dominguez L, Falaschi P, Fiore CE, Iolascon G, Maggi S, Michieli R,
et al: Guidelines for the management of osteoporosis and fragility
fractures. Intern Emerg Med. 14:85–102. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Marshall D, Johnell O and Wedel H:
Meta-analysis of how well measures of bone mineral density predict
occurrence of osteoporotic fractures. BMJ. 312:1254–1259.
1996.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Szulc P and Delmas PD: Biochemical markers
of bone turnover: Potential use in the investigation and management
of postmenopausal osteoporosis. Osteoporos Int. 19:1683–1704.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dempster D, Cauley J, Bouxsein M and
Cosman F (eds.): Lessons from bone histomorphometry on the
mechanisms of action of osteoporosis drugs. In: Marcus and
Feldman's Osteoporosis. 5th edition. Academic Press, Cambridge, MA,
USA, pp1835-1863, 2020.
|
|
17
|
Schuiling KD, Robinia K and Nye R:
Osteoporosis update. J Midwifery Womens Health. 56:615–627.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zmuda JM, Sheu YT and Moffett SP: The
search for human osteoporosis genes. J Musculoskelet Neuronal
Interact. 6:3–15. 2006.PubMed/NCBI
|
|
19
|
Sadler C and Huff M: African-American
women: Health beliefs, lifestyle, and osteoporosis. Orthop Nurs.
26:96–101. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kanis JA, Burlet N, Cooper C, Delmas PD,
Reginster JY, Borgstrom F and Rizzoli R: European Society for
Clinical and Economic Aspects of Osteoporosis and Osteoarthritis
(ESCEO). European guidance for the diagnosis and management of
osteoporosis in postmenopausal women. Osteoporos Int. 19:399–428.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kuo TR and Chen CH: Bone biomarker for the
clinical assessment of osteoporosis: Recent developments and future
perspectives. Biomark Res. 5(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Qaseem A, Snow V, Shekelle P, Hopkins R
Jr, Forciea MA and Owens DK: Clinical Efficacy Assessment
Subcommittee of the American College of Physicians. Pharmacologic
treatment of low bone density or osteoporosis to prevent fractures:
A clinical practice guideline from the American college of
physicians. Ann Intern Med. 149:404–415. 2008.PubMed/NCBI
|
|
23
|
Wada T, Nakashima T, Hiroshi N and
Penninger JM: RANKL-RANK signaling in osteoclastogenesis and bone
disease. Trends Mol Med. 12:17–25. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li H, Xiao Z, Quarles LD and Li W:
Osteoporosis: Mechanism, molecular target and current status on
drug development. Curr Med Chem. 28:1489–1507. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yasuda H, Shima N, Nakagawa N, Yamaguchi
K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A,
et al: Osteoclast differentiation factor is a ligand for
osteoprotegerin/osteoclastogenesis-inhibitory factor and is
identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 95:3597–3602.
1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wong BR, Rho J, Arron J, Robinson E,
Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS III,
Frankel WN, et al: TRANCE is a novel ligand of the tumor necrosis
factor receptor family that activates c-Jun N-terminal kinase in T
cells. J Biol Chem. 272:25190–25194. 1997.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Park JH, Lee NK and Lee SY: Current
understanding of RANK signaling in osteoclast differentiation and
maturation. Mol Cells. 40:706–713. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu F and Teitelbaum SL: Osteoclasts: New
insights. Bone Res. 1:11–26. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yamashita T, Takahashi N and Udagawa N:
New roles of osteoblasts involved in osteoclast differentiation.
World J Orthop. 3:175–181. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rucci N: Molecular biology of bone
remodelling. Clin Cases Miner Bone Metab. 5:49–56. 2008.PubMed/NCBI
|
|
32
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hienz SA, Paliwal S and Ivanovski S:
Mechanisms of bone resorption in periodontitis. J Immunol Res.
2015(615486)2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Das S and Crockett JC: Osteoporosis-a
current view of pharmacological prevention and treatment. Drug Des
Devel Ther. 7:435–448. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Henriksen K, Bollerslev J, Everts V and
Karsdal MA: Osteoclast activity and subtypes as a function of
physiology and pathology-implications for future treatments of
osteoporosis. Endocr Rev. 32:31–63. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Arnett T: Regulation of bone cell function
by acid-base balance. Proc Nutr Soc. 62:511–520. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Blair HC, Zaidi M, Huang CL and Sun L: The
developmental basis of skeletal cell differentiation and the
molecular basis of major skeletal defects. Biol Rev Camb Philos
Soc. 83:401–415. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nakashima K and de Crombrugghe B:
Transcriptional mechanisms in osteoblast differentiation and bone
formation. Trends Genet. 19:458–466. 2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Brighton CT and Hunt RM: Histochemical
localization of calcium in the fracture callus with potassium
pyroantimonate. Possible role of chondrocyte mitochondrial calcium
in callus calcification. J Bone Joint Surg Am. 68:703–715.
1986.PubMed/NCBI
|
|
41
|
de Crombrugghe B, Lefebvre V and Nakashima
K: Regulatory mechanisms in the pathways of cartilage and bone
formation. Curr Opin Cell Biol. 13:721–727. 2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Brighton CT and Hunt RM: Early
histological and ultrastructural changes in medullary fracture
callus. J Bone Joint Surg Am. 73:832–847. 1991.PubMed/NCBI
|
|
43
|
Soltanoff CS, Yang S, Chen W and Li YP:
Signaling networks that control the lineage commitment and
differentiation of bone cells. Crit Rev Eukaryot Gene Expr 19,
2009.
|
|
44
|
Chau JF, Leong WF and Li B: Signaling
pathways governing osteoblast proliferation, differentiation and
function. Histol Histopathol. 24:1593–1606. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Meyer MB, Benkusky NA and Pike JW: The
RUNX2 cistrome in osteoblasts: Characterization, down-regulation
following differentiation, and relationship to gene expression. J
Biol Chem. 289:16016–16031. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lin GL and Hankenson KD: Integration of
BMP, Wnt, and notch signaling pathways in osteoblast
differentiation. J Cell Biochem. 112:3491–3501. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Burch J, Rice S, Yang H, Neilson A, Stirk
L, Francis R, Holloway P, Selby P and Craig D: Systematic review of
the use of bone turnover markers for monitoring the response to
osteoporosis treatment: The secondary prevention of fractures, and
primary prevention of fractures in high-risk groups. Health Technol
Assess. 18:1–180. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Mori G, D'Amelio P, Faccio R and Brunetti
G: The interplay between the bone and the immune system. Clin Dev
Immunol. 2013(720504)2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lacey DL, Boyle WJ, Simonet WS, Kostenuik
PJ, Dougall WC, Sullivan JK, San Martin J and Dansey R: Bench to
bedside: Elucidation of the OPG-RANK-RANKL pathway and the
development of denosumab. Nat Rev Drug Discov. 11:401–419.
2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Noble BS: The osteocyte lineage. Arch
Biochem Biophys. 473:106–111. 2008.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Klein-Nulend J, Nijweide PJ and Burger EH:
Osteocyte and bone structure. Curr Osteoporos Rep. 1:5–10.
2003.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Rochefort GY: The osteocyte as a
therapeutic target in the treatment of osteoporosis. Ther Adv
Musculoskelet Dis. 6:79–91. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Bonewald LF: The amazing osteocyte. J Bone
Miner Res. 26:229–238. 2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Nakashima T, Hayashi M, Fukunaga T, Kurata
K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, et
al: Evidence for osteocyte regulation of bone homeostasis through
RANKL expression. Nat Med. 17:1231–1234. 2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Goldring SR: The osteocyte: Key player in
regulating bone turnover. RMD Open. 1 (Suppl
1)(e000049)2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Moriishi T, Fukuyama R, Ito M, Miyazaki T,
Maeno T, Kawai Y, Komori H and Komori T: Osteocyte network; a
negative regulatory system for bone mass augmented by the induction
of Rankl in osteoblasts and Sost in osteocytes at unloading. PLoS
One. 7(e40143)2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kramer I, Halleux C, Keller H, Pegurri M,
Gooi JH, Weber PB, Feng JQ, Bonewald LF and Kneissel M: Osteocyte
Wnt/beta-catenin signaling is required for normal bone homeostasis.
Mol Cell Biol. 30:3071–3085. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bellido T: Osteocyte apoptosis induces
bone resorption and impairs the skeletal response to
weightlessness. IBMS BoneKEy. 4(252)2007.
|
|
59
|
Chamoux E, Houde N, L'eriger K and Roux S:
Osteoprotegerin decreases human osteoclast apoptosis by inhibiting
the TRAIL pathway. J Cell Physiol. 216:536–542. 2008.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yen ML, Hsu PN, Liao HJ, Lee BH and Tsai
HF: TRAF-6 dependent signaling pathway is essential for TNF-related
apoptosis-inducing ligand (TRAIL) induces osteoclast
differentiation. PLoS One. 7(e38048)2012.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Bikle DD: Vitamin D metabolism, mechanism
of action, and clinical applications. Chem Biol. 21:319–329.
2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Norman AW: From vitamin D to hormone D:
Fundamentals of the vitamin D endocrine system essential for good
health. Am J Clin Nutr. 88:491S–499S. 2008.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Valero Zanuy M and Hawkins Carranza F:
Metabolism, endogenous and exogenous sources of vitamin D. Rev Esp
Enferm Metab Oseas. 16:63–70. 2007.
|
|
64
|
Silva MC and Furlanetto TW: Intestinal
absorption of vitamin D: A systematic review. Nutr Rev. 76:60–76.
2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Compston JE, Merrett AL, Hammett FG and
Magill P: Comparison of the appearance of radiolabelled vitamin D3
and 25-hydroxy-vitamin D3 in the chylomicron fraction of plasma
after oral administration in man. Clin Sci (Lond). 60:241–243.
1981.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Gil A, Plaza-Diaz J and Mesa MD: Vitamin
D: Classic and novel actions. Ann Nutr Metab. 72:87–95.
2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Naveh-Many T, Marx R, Keshet E, Pike JW
and Silver J: Regulation of 1, 25-dihydroxyvitamin D3 receptor gene
expression by 1, 25-dihydroxyvitamin D3 in the parathyroid in vivo.
J Clin Invest. 86:1968–1975. 1990.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Glendenning P, Ratajczak T, Dick IM and
Prince RL: Calcitriol upregulates expression and activity of the 1b
isoform of the plasma membrane calcium pump in immortalized distal
kidney tubular cells. Arch Biochem Biophys. 380:126–132.
2000.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Kuchuk NO, van Schoor NM, Pluijm SM,
Chines A and Lips P: Vitamin D status, parathyroid function, bone
turnover, and BMD in postmenopausal women with osteoporosis: Global
perspective. J Bone Miner Res. 24:693–701. 2009.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Harada S, Mizoguchi T, Kobayashi Y,
Nakamichi Y, Takeda S, Sakai S, Takahashi F, Saito H, Yasuda H,
Udagawa N, et al: Daily administration of eldecalcitol (ED-71), an
active vitamin D analog, increases bone mineral density by
suppressing RANKL expression in mouse trabecular bone. J Bone Miner
Res. 27:461–473. 2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Takeda S, Yoshizawa T, Nagai Y, Yamato H,
Fukumoto S, Sekine K, Kato S, Matsumoto T and Fujita T: Stimulation
of osteoclast formation by 1, 25-dihydroxyvitamin D requires its
binding to vitamin D receptor (VDR) in osteoblastic cells: studies
using VDR knockout mice. Endocrinology. 140:1005–1008.
1999.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Christakos S, Dhawan P, Verstuyf A,
Verlinden L and Carmeliet G: Vitamin D: Metabolism, molecular
mechanism of action, and pleiotropic effects. Physiol Rev.
96:365–408. 2016.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kim S, Yamazaki M, Zella LA, Shevde NK and
Pike JW: Activation of receptor activator of NF-kappaB ligand gene
expression by 1, 25-dihydroxyvitamin D3 is mediated through
multiple long-range enhancers. Mol Cell Biol. 26:6469–6486.
2006.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Ukon Y, Makino T, Kodama J, Tsukazaki H,
Tateiwa D, Yoshikawa H and Kaito T: Molecular-based treatment
strategies for osteoporosis: A literature review. Int J Mol Sci.
20(2557)2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Watts NB, Bilezikian JP, Camacho PM,
Greenspan SL, Harris ST, Hodgson SF, Kleerekoper M, Luckey MM,
McClung MR, Pollack RP, et al: American association of clinical
endocrinologists medical guidelines for clinical practice for the
diagnosis and treatment of postmenopausal osteoporosis. Endocr
Pract. 16 (Suppl 3):S1–S37. 2010.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Kim SY, Zhang M and Bockman R: Bone
mineral density response from teriparatide in patients with
osteoporosis. HSS J. 13:171–177. 2017.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Estell EG and Rosen CJ: Emerging insights
into the comparative effectiveness of anabolic therapies for
osteoporosis. Nat Rev Endocrinol. 17:31–46. 2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Pavone V, Testa G, Giardina SMC, Vescio A,
Restivo DA and Sessa G: Pharmacological therapy of osteoporosis: A
systematic current review of literature. Front Pharmacol.
8(803)2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
North American Menopause Societ.
Management of osteoporosis in postmenopausal women: 2006 position
statement of The North American menopause society. Menopause.
13:340–367; quiz 368-9. 2006.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Drake MT, Clarke BL and Khosla S:
Bisphosphonates: mechanism of action and role in clinical practice.
Mayo Clin Proc. 83:1032–1045. 2008.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Colucci S, Minielli V, Zambonin G, Cirulli
N, Mori G, Serra M, Patella V, Zambonin Zallone A and Grano M:
Alendronate reduces adhesion of human osteoclast-like cells to bone
and bone protein-coated surfaces. Calcif Tissue Int. 63:230–235.
1998.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Sato M, Grasser W, Endo N, Akins R,
Simmons H, Thompson DD, Golub E and Rodan GA: Bisphosphonate
action. Alendronate localization in rat bone and effects on
osteoclast ultrastructure. J Clin Invest. 88:2095–2105.
1991.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Papapoulos SE: Bisphosphonates: How do
they work? Best Pract Res Clin Endocrinol Metab. 22:831–847.
2008.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Russell RG: Bisphosphonates: The first 40
years. Bone. 49:2–19. 2011.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Barnsley J, Buckland G, Chan PE, Ong A,
Ramos AS, Baxter M, Laskou F, Dennison EM, Cooper C and Patel HP:
Pathophysiology and treatment of osteoporosis: Challenges for
clinical practice in older people. Aging Clin Exp Res. 33:759–773.
2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Garg MK and Kharb S: Dual energy X-ray
absorptiometry: Pitfalls in measurement and interpretation of bone
mineral density. Indian J Endocrinol Metab. 17:203–210.
2013.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Rogers MJ: From molds and macrophages to
mevalonate: A decade of progress in understanding the molecular
mode of action of bisphosphonates. Calcif Tissue Int. 75:451–461.
2004.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Russell RG: Bisphosphonates: Mode of
action and pharmacology. Pediatrics. 119 (Suppl 2):S150–S162.
2007.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Dunford JE: Molecular targets of the
nitrogen containing bisphosphonates: The molecular pharmacology of
prenyl synthase inhibition. Curr Pharm Des. 16:2961–2969.
2010.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Kanis JA, Cooper C, Rizzoli R and
Reginster JY: Scientific Advisory Board of the European Society for
Clinical and Economic Aspects of Osteoporosis (ESCEO) and the
Committees of Scientific Advisors and National Societies of the
International Osteoporosis Foundation (IOF). European guidance for
the diagnosis and management of osteoporosis in postmenopausal
women. Osteoporos Int. 30:3–44. 2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Simon JA: Are all bisphosphonates the
same? Potential reasons for clinical differences: A perspective. J
Womens Health (Larchmt). 19:719–727. 2010.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Qaseem A, Forciea MA, McLean RM and
Denberg TD: Clinical Guidelines Committee of the American College
of Physicians. Barry MJ, Cooke M, Fitterman N, Harris RP, Humphrey
LL, et al: Treatment of low bone density or osteoporosis to prevent
fractures in men and women: A clinical practice guideline update
from the American College of Physicians. Ann Intern Med.
166:818–839. 2017.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Nelson HD, Haney EM, Dana T, Bougatsos C
and Chou R: Screening for osteoporosis: An update for the US
preventive services task force. Ann Intern Med. 153:99–111.
2010.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Park-Wyllie LY, Mamdani MM, Juurlink DN,
Hawker GA, Gunraj N, Austin PC, Whelan DB, Weiler PJ and Laupacis
A: Bisphosphonate use and the risk of subtrochanteric or femoral
shaft fractures in older women. JAMA. 305:783–789. 2011.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Rossini M, Adami S, Bertoldo F, Diacinti
D, Gatti D, Giannini S, Giusti A, Malavolta N, Minisola S, Osella
G, et al: Guidelines for the diagnosis, prevention and management
of osteoporosis. Reumatismo. 68:1–39. 2016.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Pazianas M and Abrahamsen B: Osteoporosis
treatment: Bisphosphonates reign to continue for a few more years,
at least? Ann N Y Acad Sci. 1376:5–13. 2016.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Lorentzon M: Treating osteoporosis to
prevent fractures: Current concepts and future developments. J
Intern Med. 285:381–394. 2019.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Tsourdi E, Langdahl B, Cohen-Solal M,
Aubry-Rozier B, Eriksen EF, Guañabens N, Obermayer-Pietsch B,
Ralston SH, Eastell R and Zillikens MC: Discontinuation of
denosumab therapy for osteoporosis: A systematic review and
position statement by ECTS. Bone. 105:11–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Takeuchi T, Tanaka Y, Ishiguro N, Yamanaka
H, Yoneda T, Ohira T, Okubo N, Genant HK and van der Heijde D:
Effect of denosumab on Japanese patients with rheumatoid arthritis:
A dose-response study of AMG 162 (Denosumab) in patients with
RheumatoId arthritis on methotrexate to Validate inhibitory effect
on bone Erosion (DRIVE)-a 12-month, multicentre, randomised,
double-blind, placebo-controlled, phase II clinical trial. Ann
Rheum Dis. 75:983–990. 2016.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Bone HG, Wagman RB, Brandi ML, Brown JP,
Chapurlat R, Cummings SR, Czerwiński E, Fahrleitner-Pammer A,
Kendler DL, Lippuner K, et al: 10 years of denosumab treatment in
postmenopausal women with osteoporosis: Results from the phase 3
randomised FREEDOM trial and open-label extension. Lancet Diabetes
Endocrinol. 5:513–523. 2017.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Tabatabaei-Malazy O, Salari P, Khashayar P
and Larijani B: New horizons in treatment of osteoporosis. DARU.
25(2)2017.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Bonani M, Frey D, de Rougemont O, Mueller
NJ, Mueller TF, Graf N and Wüthrich RP: Infections in de novo
kidney transplant recipients treated with the RANKL inhibitor
denosumab. Transplantation. 101:2139–2145. 2017.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Cummings SR, Ferrari S, Eastell R,
Gilchrist N, Jensen JB, McClung M, Roux C, Törring O, Valter I,
Wang AT and Brown JP: Vertebral fractures after discontinuation of
denosumab: A post hoc analysis of the randomized placebo-controlled
FREEDOM trial and its extension. J Bone Miner Res. 33:190–198.
2018.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Krum SA and Brown M: Unraveling estrogen
action in osteoporosis. Cell Cycle. 7:1348–1352. 2008.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Kanis JA, McCloskey EV, Johansson H,
Cooper C, Rizzoli R and Reginster JY: Scientific Advisory Board of
the European Society for Clinical and Economic Aspects of
Osteoporosis and Osteoarthritis (ESCEO) and the Committee of
Scientific Advisors of the International Osteoporosis Foundation
(IOF). European guidance for the diagnosis and management of
osteoporosis in postmenopausal women. Osteoporos Int. 24:23–57.
2013.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Lobo RA, Pickar JH, Stevenson JC, Mack WJ
and Hodis HN: Back to the future: Hormone replacement therapy as
part of a prevention strategy for women at the onset of menopause.
Atherosclerosis. 254:282–290. 2016.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Cartwright B, Robinson J, Seed PT,
Fogelman I and Rymer J: Hormone replacement therapy versus the
combined oral contraceptive pill in premature ovarian failure: A
randomized controlled trial of the effects on bone mineral density.
J Clin Endocrinol Metab. 101:3497–3505. 2016.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Rossouw JE, Anderson GL, Prentice RL,
LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA,
Howard BV, Johnson KC, et al: Risks and benefits of estrogen plus
progestin in healthy postmenopausal women: Principal results from
the Women's Health Initiative randomized controlled trial. JAMA.
288:321–333. 2002.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Maximov PY, Lee TM and Jordan VC: The
discovery and development of selective estrogen receptor modulators
(SERMs) for clinical practice. Curr Clin Pharmacol. 8:135–155.
2013.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Siddiqui JA and Partridge NC:
Physiological bone remodeling: Systemic regulation and growth
factor involvement. Physiology (Bethesda). 31:233–245.
2016.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Gooi JH, Pompolo S, Karsdal MA, Kulkarni
NH, Kalajzic I, McAhren SH, Han B, Onyia JE, Ho PW, Gillespie MT,
et al: Calcitonin impairs the anabolic effect of PTH in young rats
and stimulates expression of sclerostin by osteocytes. Bone.
46:1486–1497. 2010.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Keller J, Catala-Lehnen P, Huebner AK,
Jeschke A, Heckt T, Lueth A, Krause M, Koehne T, Albers J, Schulze
J, et al: Calcitonin controls bone formation by inhibiting the
release of sphingosine 1-phosphate from osteoclasts. Nat Commun.
5(5215)2014.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Bandeira L, Lewiecki EM and Bilezikian JP:
Pharmacodynamics and pharmacokinetics of oral salmon calcitonin in
the treatment of osteoporosis. Expert Opin Drug Metab Toxicol.
12:681–689. 2016.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Drake MT, Clarke BL, Oursler MJ and Khosla
S: Cathepsin K inhibitors for osteoporosis: Biology, potential
clinical utility, and lessons learned. Endocr Rev. 38:325–350.
2017.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Dai R, Wu Z, Chu HY, Lu J, Lyu A, Liu J
and Zhang G: Cathepsin K: The action in and beyond bone. Front Cell
Dev Biol. 8(433)2020.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Takito J, Inoue S and Nakamura M: The
sealing zone in osteoclasts: A self-organized structure on the
bone. Int J Mol Sci. 19(984)2018.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Fonović M and Turk B: Cysteine cathepsins
and extracellular matrix degradation. Biochim Biophys Acta.
1840:2560–2570. 2014.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Mullard A: Merck & Co. drops
osteoporosis drug odanacatib. Nat Rev Drug Discov.
15(669)2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Brömme D and Lecaille F: Cathepsin K
inhibitors for osteoporosis and potential off-target effects.
Expert Opin Investig Drugs. 18:585–600. 2009.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Eastell R, Nagase S, Ohyama M, Small M,
Sawyer J, Boonen S, Spector T, Kuwayama T and Deacon S: Safety and
efficacy of the cathepsin K inhibitor ONO-5334 in postmenopausal
osteoporosis: The OCEAN study. J Bone Miner Res. 26:1303–1312.
2011.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Stoch SA, Zajic S, Stone JA, Miller DL,
van Bortel L, Lasseter KC, Pramanik B, Cilissen C, Liu Q, Liu L, et
al: Odanacatib, a selective cathepsin K inhibitor to treat
osteoporosis: safety, tolerability, pharmacokinetics and
pharmacodynamics-results from single oral dose studies in healthy
volunteers. Br J Clin Pharmacol. 75:1240–1254. 2013.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Khan B, Ahmed Z and Ahmad W: A novel
missense mutation in cathepsin K (CTSK) gene in a consanguineous
Pakistani family with pycnodysostosis. J Investig Med. 58:720–724.
2010.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Iñiguez-Ariza NM and Clarke BL: Bone
biology, signaling pathways, and therapeutic targets for
osteoporosis. Maturitas. 82:245–255. 2015.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Chapurlat RD: Odanacatib: A review of its
potential in the management of osteoporosis in postmenopausal
women. Ther Adv Musculoskelet Dis. 7:103–109. 2015.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Marie PJ: The calcium-sensing receptor in
bone cells: A potential therapeutic target in osteoporosis. Bone.
46:571–576. 2010.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Spector TD, Calomme MR, Anderson SH,
Clement G, Bevan L, Demeester N, Swaminathan R, Jugdaohsingh R,
Berghe DA and Powell JJ: Choline-stabilized orthosilicic acid
supplementation as an adjunct to calcium/vitamin D3 stimulates
markers of bone formation in osteopenic females: A randomized,
placebo-controlled trial. BMC Musculoskelet Disord.
9(85)2008.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Hamdy NA: Strontium ranelate improves bone
microarchitecture in osteoporosis. Rheumatology (Oxford). 48 (Suppl
4):iv9–iv13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Pilmane M, Salma-Ancane K, Loca D, Locs J
and Berzina-Cimdina L: Strontium and strontium ranelate: Historical
review of some of their functions. Mater Sci Eng C Mater Biol Appl.
78:1222–1230. 2017.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Delany AM, Amling M, Priemel M, Howe C,
Baron R and Canalis E: Osteopenia and decreased bone formation in
osteonectin-deficient mice. J Clin Invest. 105:915–923.
2000.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Kanis JA, Johansson H, Oden A and
McCloskey EV: A meta-analysis of the effect of strontium ranelate
on the risk of vertebral and non-vertebral fracture in
postmenopausal osteoporosis and the interaction with FRAX®.
Osteoporos Int. 22:2347–2355. 2011.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Bolland MJ and Grey A: A comparison of
adverse event and fracture efficacy data for strontium ranelate in
regulatory documents and the publication record. BMJ Open.
4(e005787)2014.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Mi B, Xiong W, Xu N, Guan H, Fang Z, Liao
H, Zhang Y, Gao B, Xiao X, Fu J and Li F: Strontium-loaded titania
nanotube arrays repress osteoclast differentiation through multiple
signalling pathways: In vitro and in vivo studies. Sci Rep.
7(2328)2017.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Gupta S, Termini JM, Kanagavelu S and
Stone GW: Design of vaccine adjuvants incorporating TNF superfamily
ligands and TNF superfamily molecular mimics. Immunol Res.
57:303–310. 2013.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Liu C, Walter TS, Huang P, Zhang S, Zhu X,
Wu Y, Wedderburn LR, Tang P, Owens RJ, Stuart DI, et al: Structural
and functional insights of RANKL-RANK interaction and signaling. J
Immunol. 184:6910–6919. 2010.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Ko YJ, Sohn HM, Jang Y, Park M, Kim B, Kim
B, Park JI, Hyun H, Jeong B, Hong C and Lim W: A novel modified
RANKL variant can prevent osteoporosis by acting as a vaccine and
an inhibitor. Clin Transl Med. 11(e368)2021.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Jang Y, Sohn HM, Ko YJ, Hyun H and Lim W:
Inhibition of RANKL-Induced Osteoclastogenesis by Novel Mutant
RANKL. Int J Mol Sci. 22(434)2021.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Liu C, Zhao Y, He W, Wang W, Chen Y, Zhang
S, Ma Y, Gohda J, Ishida T, Walter TS, et al: A RANKL mutant used
as an inter-species vaccine for efficient immunotherapy of
osteoporosis. Sci Rep. 5(14150)2015.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Wu T, Li F, Sha X, Li F, Zhang B, Ma W,
Liu M, Yang W, Li H and Tao H: A novel recombinant RANKL vaccine
prepared by incorporation of an unnatural amino acid into RANKL and
its preventive effect in a murine model of collagen-induced
arthritis. Int Immunopharmacol. 64:326–332. 2018.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Ling L, Hu HL, Liu KY, Ram YI, Gao JL and
Cao YM: Long noncoding RNA MIRG induces osteoclastogenesis and bone
resorption in osteoporosis through negative regulation of miR-1897.
Eur Rev Med Pharmacol Sci. 23:10195–10203. 2019.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Wu QY, Li X, Miao ZN, Ye JX, Wang B, Zhang
F, Xu RS, Jiang DL, Zhao MD and Yuan FL: Long Non-coding RNAs: A
new regulatory code for osteoporosis. Front Endocrinol (Lausanne).
9(587)2018.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Zhu J, Yu W, Wang Y, Xia K, Huang Y, Xu A,
Chen Q, Liu B, Tao H, Li F and Liang C: lncRNAs: function and
mechanism in cartilage development, degeneration, and regeneration.
Stem Cell Res Ther. 10(344)2019.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Wijnen WJ, Pinto YM and Creemers EE: The
therapeutic potential of miRNAs in cardiac fibrosis: Where do we
stand? J Cardiovasc Transl Res. 6:899–908. 2013.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Mandourah AY, Ranganath L, Barraclough R,
Vinjamuri S, Hof RV, Hamill S, Czanner G, Dera AA, Wang D and
Barraclough DL: Circulating microRNAs as potential diagnostic
biomarkers for osteoporosis. Sci Rep. 8(8421)2018.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Rachner TD, Hofbauer LC, Göbel A and
Tsourdi E: Novel therapies in osteoporosis: PTH-related peptide
analogs and inhibitors of sclerostin. J Mol Endocrinol.
62:R145–R154. 2019.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Borba VZ and Mañas NC: The use of PTH in
the treatment of osteoporosis. Arq Bras Endocrinol Metabol.
54:213–219. 2010.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Silva BC and Bilezikian JP: Parathyroid
hormone: Anabolic and catabolic actions on the skeleton. Curr Opin
Pharmacol. 22:41–50. 2015.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Gardella T: Interactions of PTH with
Receptors and Signaling. The Parathyroids: Basic and Clinical
Concepts. Third ed. Elsevier Inc: 65-80, 2015.
|
|
148
|
Datta NS and Abou-Samra AB: PTH and PTHrP
signaling in osteoblasts. Cell Signal. 21(8):1245–1254.
2009.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Migliaccio S, Brama M and Malavolta N:
Management of glucocorticoids-induced osteoporosis: Role of
teriparatide. Ther Clin Risk Manag. 5:305–310. 2009.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Recker RR, Marin F, Ish-Shalom S, Möricke
R, Hawkins F, Kapetanos G, de la Peña MP, Kekow J, Farrerons J,
Sanz B, et al: Comparative effects of teriparatide and strontium
ranelate on bone biopsies and biochemical markers of bone turnover
in postmenopausal women with osteoporosis. J Bone Miner Res.
24:1358–1368. 2009.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Rubin MR and Bilezikian JP: New anabolic
therapies in osteoporosis. Endocrinol Metab Clin North Am.
32:285–307. 2003.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Glover SJ, Eastell R, McCloskey EV, Rogers
A, Garnero P, Lowery J, Belleli R, Wright TM and John MR: Rapid and
robust response of biochemical markers of bone formation to
teriparatide therapy. Bone. 45:1053–1058. 2009.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Cosman F: Abaloparatide: A new anabolic
therapy on the horizon. Bonekey Rep. 4(661)2015.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Neer RM, Arnaud CD, Zanchetta JR, Prince
R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S,
Genant HK, et al: Effect of parathyroid hormone (1-34) on fractures
and bone mineral density in postmenopausal women with osteoporosis.
N Engl J Med. 344:1434–1441. 2001.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Fukumoto S and Matsumoto T: Recent
advances in the management of osteoporosis. F1000Research.
6(625)2017.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Cheloha RW, Gellman SH, Vilardaga JP and
Gardella TJ: PTH receptor-1 signalling-mechanistic insights and
therapeutic prospects. Nat Rev Endocrinol. 11:712–724.
2015.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Hattersley G, Dean T, Corbin BA, Bahar H
and Gardella TJ: Binding selectivity of abaloparatide for
PTH-type-1-receptor conformations and effects on downstream
signaling. Endocrinology. 157:141–149. 2016.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Boyce EG, Mai Y and Pham C: Abaloparatide:
Review of a next-generation parathyroid hormone agonist. Ann
Pharmacother. 52:462–472. 2018.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Gensure RC, Gardella TJ and Jüppner H:
Parathyroid hormone and parathyroid hormone-related peptide, and
their receptors. Biochem Biophys Res Commun. 328:666–678.
2005.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Wysolmerski JJ: Parathyroid
hormone-related protein: An update. J Clin Endocrinol Metab.
97:2947–2956. 2012.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Iolascon G, Moretti A, Toro G, Gimigliano
F, Liguori S and Paoletta M: Pharmacological therapy of
osteoporosis: What's new? Clin Interv Aging. 15:485–491.
2020.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Pietrzyk B, Smertka M and Chudek J:
Sclerostin: Intracellular mechanisms of action and its role in the
pathogenesis of skeletal and vascular disorders. Adv Clin Exp Med.
26:1283–1291. 2017.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Lerner UH and Ohlsson C: The WNT system:
background and its role in bone. J Intern Med. 277:630–649.
2015.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang
J, Harris SE and Wu D: Sclerostin binds to LRP5/6 and antagonizes
canonical Wnt signaling. J Biol Chem. 280:19883–19887.
2005.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Cosman F, Crittenden DB, Adachi JD,
Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki
EM, Miyauchi A, et al: Romosozumab treatment in postmenopausal
women with osteoporosis. N Engl J Med. 375:1532–1543.
2016.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Canalis E: Management of endocrine
disease: Novel anabolic treatments for osteoporosis. Eur J
Endocrinol. 178:R33–R44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Black DM, Greenspan SL, Ensrud KE, Palermo
L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP and
Rosen CJ: PaTH Study Investigators. The effects of parathyroid
hormone and alendronate alone or in combination in postmenopausal
osteoporosis. N Engl J Med. 349:1207–1215. 2003.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Lou S, Lv H, Yin P, Li Z, Tang P and Wang
Y: Combination therapy with parathyroid hormone analogs and
antiresorptive agents for osteoporosis: A systematic review and
meta-analysis of randomized controlled trials. Osteoporos Int.
30:59–70. 2019.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Leder BZ, Tsai JN, Uihlein AV,
Burnett-Bowie SA, Zhu Y, Foley K, Lee H and Neer RM: Two years of
Denosumab and teriparatide administration in postmenopausal women
with osteoporosis (The DATA Extension Study): A randomized
controlled trial. J Clin Endocrinol Metab. 99:1694–1700.
2014.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Kitaguchi K, Kashii M, Ebina K, Kaito T,
Okada R, Makino T, Noguchi T, Ishimoto T, Nakano T and Yoshikawa H:
Effects of single or combination therapy of teriparatide and
anti-RANKL monoclonal antibody on bone defect regeneration in mice.
Bone. 106:1–10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Saag KG, Petersen J, Brandi ML, Karaplis
AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD and Grauer
A: Romosozumab or alendronate for fracture prevention in women with
osteoporosis. N Engl J Med. 377:1417–1427. 2017.PubMed/NCBI View Article : Google Scholar
|
|
172
|
Langdahl BL, Libanati C, Crittenden DB,
Bolognese MA, Brown JP, Daizadeh NS, Dokoupilova E, Engelke K,
Finkelstein JS, Genant HK, et al: Romosozumab (sclerostin
monoclonal antibody) versus teriparatide in postmenopausal women
with osteoporosis transitioning from oral bisphosphonate therapy: A
randomised, open-label, phase 3 trial. Lancet. 390:1585–1594.
2017.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Bone HG, Cosman F, Miller PD, Williams GC,
Hattersley G, Hu MY, Fitzpatrick LA, Mitlak B, Papapoulos S,
Rizzoli R, et al: ACTIVExtend: 24 months of alendronate after 18
months of abaloparatide or placebo for postmenopausal osteoporosis.
J Clin Endocrinol Metab. 103:2949–2957. 2018.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Leder BZ, Tsai JN, Uihlein AV, Wallace PM,
Lee H, Neer RM and Burnett-Bowie SA: Denosumab and teriparatide
transitions in postmenopausal osteoporosis (the DATA-Switch study):
Extension of a randomised controlled trial. Lancet. 386:1147–1155.
2015.PubMed/NCBI View Article : Google Scholar
|
|
175
|
Bell NH and Johnson RH: Bisphosphonates in
the treatment of osteoporosis. Endocrine. 6:203–206.
1997.PubMed/NCBI View Article : Google Scholar
|