Introduction
Peripheral nerve injury is often associated with the development of a post-traumatic neuroma, posing a significant challenge to surgeons and physicians. Symptomatic neuromas occur in ~5% of patients who sustain a peripheral nerve injury (1). Furthermore, persistent pain occurs in ~60% of patients following limb amputation, and in 10% of such cases, the pain can be directly attributed to the formation of neuromas (2). A traumatic neuroma is a cluster of regenerated nerve fascicles, neurogliocytes and proliferated connective tissues (3). A total of ~3-5% of patients can develop symptomatic neuromas following peripheral nerve injuries (4). These are most common in patients who have had limb amputations (~60%) (5-7). Typical symptoms include pain hypersensitivity and neuralgic pain with a trigger point within the region affected by a neuroma (8). There are different methods to prevent and treat traumatic neuromas; conservative methods include medication (9-11); physiotherapy techniques include therapeutic massage (8); surgical approaches include nerve stump shortening, lipofilling (12), electrical stimulation (13), nerve stump transposition into bones, muscles or veins (14-21), and nerve capping techniques (22-27). However, treatment of painful neuromas remains a challenge and the mechanism of neuroma-associated pain is not yet fully understood (28). Neuropathic pain caused by traumatic neuromas imposes physical and social burdens on patients, affecting their quality of life and contributing toward high medical expenses (6). Our previous study discussed certain promising treatment strategies (29). Although a variety of treatment modalities have been introduced, only a few are widely accepted and used. The nerve capping technique is one of the few methods that are considered effective, but its application has been limited by the availability of nerve conduits in clinical practice. Transforming growth factor-beta (TGF-β) superfamily signaling pathways are key regulators of numerous cellular activities, including proliferation, differentiation and migration (30). Transmembrane type I serine/threonine kinase receptors and the downstream small-mothers against decapentaplegic (SMAD) proteins (31) are the main transducers of TGF-β signals. TGF-β1/SMAD signaling is one of the most powerful activators of connective tissue synthesis and fibroblast proliferation. It modulates myofibroblast and collagen production and the turnover of the extracellular matrix physiologically and pathologically (32-34). It is hypothesized that TGF-β1/SMAD signaling may also be responsible for the upregulation of α-smooth muscle cell actin (α-SMA).
Myofibroblasts are interstitial reactive cells that can expand the extracellular matrix and react to acute or chronic injuries (35). The presence of α-SMA is a marker for myofibroblastic phenotypes (36). Our previous study identified a positive association between α-SMA expression and neuropathic pain in traumatic neuromas (28). The upregulation of α-SMA expression may contribute toward the increased contractility of myofibroblasts; additionally, it may increase contractility in non-muscle cells, which could cause pain in a region affected by a neuroma (36-39).
Nerve capping techniques aim to cover the resected nerve stump, protecting it against the surrounding inflammation and isolating neurotrophic factors, which may lead to the recurrence of traumatic neuromas. Either biological anatomical structures, including veins, or synthetic materials, including silicone tubes, may be used for capping (19,20,35). Electrospun fiber materials are now gaining growing attention in the field of peripheral nerve injuries. Prabhakaran et al (40) evaluated the potential of electrospun aligned nanofibers of Poly (3-hydroxybutyrateco-3-hydroxyvalerate) and composite scaffolds as a substrate for nerve regeneration; they cultured nerve cells (PC12) and studied the biocompatibility effect along with neurite extension, and it was found that the cells exhibited bipolar extensions oriented along the direction of fiber alignment. In a study by Wang et al (41) aligned silk fibroin (SF) blended poly (P) L-lactic acid-co-ε-caprolactone (LLA-CL) nanofibrous scaffolds were used to promote nerve regeneration following peripheral nerve injury, and the results demonstrated that the aligned SF/P (LLA-CL) nerve guidance conduits promoted peripheral nerve regeneration significantly. SF and P LLACL (PLCL) with a weight ratio of 25:75 is a biomaterial that can be used to mimic the characteristics of the extracellular matrix, which is very important for regular tissue regeneration (41). Aligned nanofiber conduits may provide a directional framework for cell culture and tissues, promoting the correct longitudinal alignment for cell and tissue regeneration along the axes of fibers (40,42,43). Our previous study (27) demonstrated that, compared with nonaligned nanofibers, aligned nanofibers may improve linear nerve fiber regeneration and inhibit neuroma growth following a neurectomy. The present study investigated the mechanism underlying treatment with aligned nanofiber nerve conduits. This nanomaterial may provide novel insight that benefits the future management of traumatic neuromas.
Materials and methods
Drugs and antibodies
TGF-β1, TβRΙ, Smad2, p-Smad2 and GAPDH antibodies were purchased from Santa Cruz Biotechnology, Inc. Collagen I, Collagen III and NF-200 antibodies were purchased from Abcam. The α-SMA antibody was purchased from Boster Biological Technology. The TGF-β1/SMAD signaling agonist, SRI-011381 hydrochloride, and the inhibitor, SB-431542, were purchased from Selleck Chemicals unless otherwise specified.
Cell culture
Schwann cells were isolated from the sciatic nerves of one-day-old Sprague-Dawley rats and treated to remove the fibroblasts using anti-Thy1.1 antibody (cat. no. ANT-140; Prospec-Tany TechnoGene, Ltd.) and rabbit complement (cat. no. PA1-29718; 1:100; Invitrogen; Thermo Fisher Scientific, Inc.) diluted in PBS and incubated at 4˚C overnight. The final cell preparation consisted of 98% Schwann cells, as determined by immunostaining with the specific Schwann cell marker, S100β (cat. no. EP1576Y; Abcam) incubated at 4˚C overnight. Primary culture of Schwann cells was maintained in DMEM containing 10% FBS (complete medium) at 37˚C under humidified 5% CO2. Schwann cells were cultured for 24 h on the surface of the nanofiber.
Scanning electron microscopy
Harvested samples were rapidly frozen, lyophilized, fixed on an aluminum stub, and sputtered with gold. Liquid nitrogen was used to freeze the samples rapidly because this minimizes the phase separation effect on sample morphology. Subsequently, a 10 kV scanning electron microscope (magnification, x500; Hitachi Ltd.) was used to view the surfaces of the samples.
Pre-preparation of the conduits
Aligned nanofiber nerve conduits were used in the present study. As in our previous study, the conduits were made by electrospinning using natural-synthetic polymeric nanofibers (SF and PLCL; weight ratio, 25:75) (27). The conduits used were 12 mm in length. Our group has reported the fabrication of these nanofibers and addressed their biocompatibility and application in peripheral nerve regeneration following injury in previous studies on rats (27,44).
Animals and experimental groups
In total, 80 male Sprague-Dawley rats (age, 7-8 weeks; weight, 250-300 g) were purchased from the Wenzhou Medical University Centre for Laboratory Animals and transported to our animal facility 1 week prior to surgery. The animals were kept at 22-24˚C in a 12 h day-night cycle under pathogen-free conditions. All rats received food and water ad libitum. For Part I of the study, animals were randomly separated into four experimental groups (n=10 in each group) as follows: i) SRI-011381 group (SRI-011381 hydrochloride); ii) control group (traumatic neuroma); iii) SB431542 group (SB-431542); and iv) conduit group (aligned nanofiber nerve conduits), to discuss the possible connections between traumatic neuroma and proliferation of α-SMA and collagen, and to investigate the therapeutic effect of aligned nanofiber nerve conduits. In Part II, it was hypothesized that the therapeutic effect of aligned nanofiber nerve conduits functioned by suppressing TGF-β1/SMAD signaling. The animals were randomly separated into four experimental groups (n=10 in each group) as follows: i) control group (traumatic neuroma); ii) SB431542 group (SB-431542); iii) conduit group (aligned nanofiber nerve conduits); and iv) conduit + SRI group (aligned nanofiber nerve conduits + SRI-011381 hydrochloride). The rats were sacrificed, and specimens were harvested 1 month after the surgery.
Surgical procedure and animal model preparation
Intraperitoneal injections of sodium pentobarbital (50 mg/kg) were used to anesthetize the rats. The right side of the sciatic nerve was exposed between the gluteal muscles and the biceps femoris under aseptic conditions. The branch site of the posterior gluteal nerve was identified and marked with a 9-0 suture. Using a 1-cm-long silicon tube as a length marker, the sciatic nerve was then cut 1 cm distal to the marked site. In every rat, at least 15 mm of sciatic nerve distal to the transection site was removed to prevent the nerve from healing spontaneously (Fig. 1A). The proximal nerve stump was then capped 4 mm into the conduit. The epineurium and the conduit were joined using a single 11-0 monofilament nylon suture. The proximal nerve end was retained in situ in the control group. SRI-011381 hydrochloride (30 mg/kg (45), dissolved in dimethyl sulfoxide) was intraperitoneally injected into rats from the agonist group on the first day of surgery and every 2 days thereafter. The same dosage was used for rats from the conduit + agonist group. SB-431542 (10 mg/kg) (46,47) dissolved in DMSO was injected intraperitoneally into rats from the inhibitor group at the same time points. To close muscle wounds and skin incisions, 4-0 sutures were used. Daily intragastric administration of ibuprofen (50 mg/kg) was administered for analgesia until 1 week after the operations.
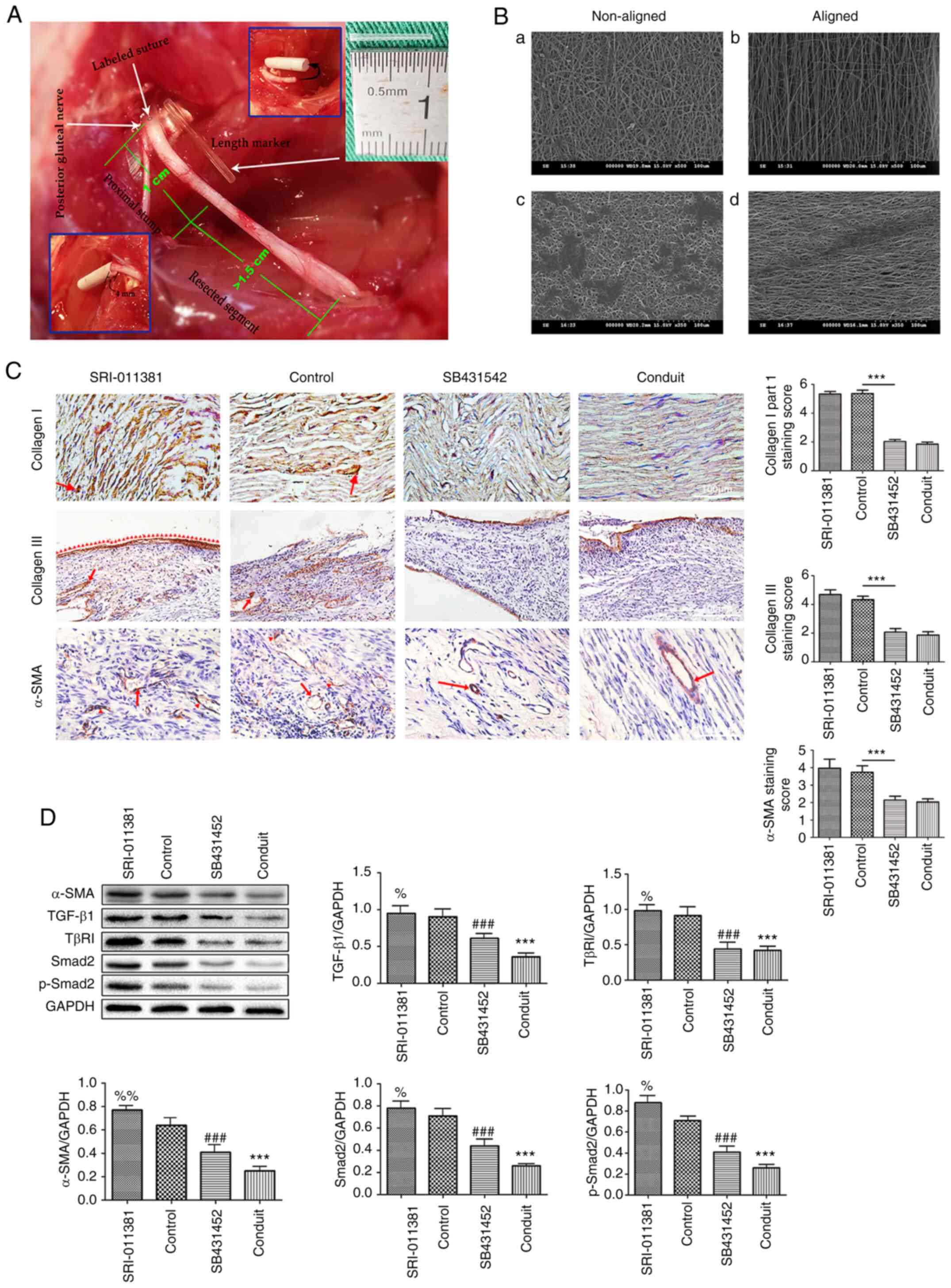 |
Figure 1
Animal modeling and scanning electron micrograph of the nanofiber material and aligned nanofiber conduits decreases soft tissue proliferation and prevent traumatic neuroma. (A) Schematic diagram of animal modeling. The white arrows on the upper left of the panel mark the labeled suture site and the origin of the posterior gluteal nerve; the white arrow in the middle of the panel shows a length marker (silicone tube in the center), which indicates scale. The green numbers 1 and >1.5 cm show the lengths of the proximal nerve stump and the resected segment, respectively. The inset photographs show steps in the capping procedure. (B) Scanning electron micrograph of the nanofiber material. Superficial structures of (a) non-aligned and (b) aligned nanofiber materials at high magnification (x500). Superficial morphology of Schwann cell clusters after 24 h of cell culture on (c) non-aligned and (d) aligned nanofiber materials shown at high magnification (x500). Scale bar, 500 µm. (C) Immunohistochemical staining of Collagen I, III and α-SMA of Part I of the study. It can be seen that the staining score of Collagen I, III and α-SMA in the SRI and control groups are higher than in the SB431542 and conduit groups (all P<0.05), but there is no statistical difference between the SRI and control groups. Collagen I, III (magnification, x100); α-SMA (magnification, x200). (D) The protein expressions and quantitative analysis of α-SMA, TGF-β1, TβR1, Smad2 and p-Smad2 in the four groups. Values are expressed as the mean ± standard deviation, n=5 per group. ***P<0.001, %%P<0.01, %P<0.05 vs. control group; ###P<0.001 vs. control group.
|
Animal sacrifice and specimen harvest
An overdose of intraperitoneal sodium pentobarbital (200 mg/kg) was used to sacrifice all the rats 1 month postoperatively. Specimens were harvested thereafter. The proximal nerve end was removed at the labeled site together with 10 mm of contralateral normal sciatic nerve. Half the specimens (n=5) were randomly selected for histological analysis. The other five specimens were stored at -80˚C for western blot analysis.
Histological analysis
The tissue samples for pathological analysis were soaked in 4% paraformaldehyde solution, using an amount ~20 times the volume of the tissue mass. Tissues were fixed overnight at 4˚C and preserved. The fixed specimens were embedded in paraffin and cut into 4 µm sections. Paraffin sections 400-600 µm from the distal end of the specimens were randomly selected to standardize a position for histological evaluation. After excluding those that were poorly cut, 10 sections from each sample were randomly selected for immunohistochemical staining. Using a standard procedure, the paraffin sections were first deparaffinized using xylene at room temperature three times for 15 min each, then rehydrated in ethanol and incubated in 3% H2O2 for 15 min at room temperature. Subsequently, the sections were treated for 30 min with pancreatin at 37˚C to retrieve the antigens, and 5% bovine serum albumin (cat. no. AB-0986/B; Beijing Solarbio Science & Technology Co., Ltd.) was used to block the samples for 30 min at 37˚C. Next, the sections were incubated with primary antibodies: anti-Collagen I (1:400; cat. no. ab270993; Abcam), anti-Collagen III (1:400; cat. no. ab7528; Abcam) and anti-α-SMA (1:500; cat. no. ab7818; Abcam) at 4˚C overnight. Finally, tagged secondary antibodies diluted in PBS were as follows: goat anti-mouse (Alexa Fluor® 488; 1:100; cat. no. ab150117; Abcam) and goat anti-rabbit: (Alexa Fluor 488; 1:100; cat. no. ab150081; Abcam), and were incubated with the sections for 2 h at 37˚C. The incubation reactions were terminated using 3, 3-diaminobenzidine. A Nikon ECLIPSE 80i research microscope (magnification, x100 and x200; Nikon Corporation) was used to view the samples. The (A) positive cells and the (B) staining degree of positive cells in each section were scored respectively. (A) represented the percentage score of the number of positive cells under high magnification (no positive cells=0; 1-30% positive cells=1; 31-60% positive cells=2; 61-100% positive cells=3). (B) represented the intensity score of positive cell staining (negative=0; weakly positive=1; moderately positive=2; strongly positive=3). The product of the two (A x B) is the comprehensive score.
Western blot analysis
Lysis buffer (100 mmol/l dithiothreitol, 50 mmol/l Tris-HCl pH 6.8, 2% sodium dodecyl sulfate, and 10% glycerol) containing protease inhibitors was used to lyse the specimens. The BCA assay was used to determine total protein concentrations. The proteins (~25 µg/lane) were separated via SDS-PAGE on a 10% gel, and subsequently transferred to a PVDF membrane. A solution containing 0.05% Tween-20, TBS and 3% skimmed dried milk (Bio-Rad Laboratories, Inc.) was then prepared to block the membrane for 2 h at room temperature with agitation. The membrane was then incubated with blocking solution containing diluted primary antibodies: mouse anti-TGF-β1 (1:200; cat. no. ab215715; Santa Cruz Biotechnology, Inc.), mouse anti-TβRΙ (1:300; cat. no. ab31013; Santa Cruz Biotechnology, Inc.), mouse anti-Smad2 (1:200; cat. no. ab40855; Santa Cruz Biotechnology, Inc.), mouse anti-p-Smad2 (1:200, cat. no. ab280888; Santa Cruz Biotechnology, Inc.), and mouse anti-α-SMA (1:400; cat. no. ab5694; Boster Biological Technology) overnight at 4˚C, followed by incubation with a secondary antibody (HRP-Goat polyclonal to Papain diluted in 5% BSA; cat. no. ab181737; Abcam) for 2 h at room temperature. The membrane was washed with buffer and visualized using a ChemiDoc™ XRS+ imaging system (Bio-Rad Laboratories, Inc.). Multi Gauge Science Lab software (version 2006; Fujifilm Corporation) was used to quantify band densities.
Statistical analysis
For each group, data are expressed as the mean ± standard deviation. Student's t-tests were performed to determine significant differences between two groups. One-way ANOVA was used to determine significant differences among multiple groups, followed by a Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Aligned nanofiber nerve conduits inhibit alpha smooth muscle actin expression and collagen proliferation by suppressing TGF-β1/SMAD signaling
In the present study, TGF-β1/SMAD signaling agonist (SRI-011381 hydrochloride) and inhibitor (SB-431542) were used to upregulate and suppress the TGF-β1/SMAD pathway. Part I of the study discussed the possible connections between traumatic neuroma and proliferation of α-SMA and collagen (agonist, control and inhibitor group). It also investigated the therapeutic effect of aligned nanofiber nerve conduits (conduit group). Part II hypothesized that the therapeutic effect of aligned nanofiber nerve conduits functioned by suppressing TGF-β1/SMAD signaling (contained control, inhibitor, conduit, and conduit + agonist group). Fig. 1A is a demonstrative intraoperative image of animal modeling (Fig. 1A).
Alignment of electrospun fibers and orientation of Schwann cells
At the cellular level, the scanning electron micrographs of cell cultures revealed that the Schwann cells exhibited extensions oriented along the direction of fiber alignment, which indicated that aligned nanofiber materials are better than non-aligned materials at promoting linear growth of Schwann cells (Fig. 1B). Part I of the study revealed that aligned nanofiber nerve conduits prevented traumatic neuromas from occurring.
Histological analysis
Neuromas were observed in 9 rats from the control group and 10 rats from the SRI-011381 group. Nerve ends from rats in the SB431542 group were thin and truncated, while those from rats in the conduit group were thinner and more linear. Autotomy was observed at different levels in all groups which are not discussed in the present study. Immunohistochemical staining of Collagen I (Fig. 1C) revealed high proliferation of Collagen I associated with a disorderly arrangement of nerve fibers in the control and SRI-011381 groups (P<0.05, compared with the SB431542 and conduit groups). Less stained Collagen I in a disorderly arrangement was also present in the SB431542 group, and less stained but orderly arranged Collagen I was present in the conduit group (Fig. 1C).
Immunohistochemical staining demonstrated that the presence of Collagen III (Fig. 1C) was mainly in the epineurium and the connective tissues surrounding it (red arrowheads). Results from the control and SRI-011381 groups demonstrated the highly proliferated (P<0.05, compared with SB431542 and conduit groups) and intraneural connective tissue (red arrows), erosion of the epineurium, and haphazardly distributed nerve fascicles, indicating the presence of neuromas. In the SB431542 and conduit groups, Collagen III was present in the epineurium and connective tissues surrounding it, but there was no erosion of the epineurium or the intraneural connective tissue. Additionally, the funicular architecture of the nerve fascicles was more orderly in the conduit group (Fig. 1C). The aforementioned findings suggested that the aligned nanofiber conduit has a similar function in suppressing Collagen I & III as the TGF-β1/SMAD signaling inhibitor (SB-431542).
As mentioned earlier, α-SMA is a marker for myofibroblastic phenotypes. Fig. 1C shows the results of the immunohistochemical staining of α-SMA. High proliferations of α-SMA (red arrows and red arrowheads) were visible in the control and SRI-011381 groups (Fig. 1C; P<0.05, compared with SB431542 and conduit groups). However, only slightly stained α-SMA was observed, mainly within the vascular walls (red arrow), in the SB431542 and conduit groups (Fig. 1C). These results demonstrated that aligned nanofiber conduit serves an important role in preventing the proliferation of α-SMA; cutting down the possibility of self-contractility surrounding the injured nerve ends.
Western blot analysis
Quantitative analysis using the Western blot method revealed that α-SMA expression was highest in the agonist group and lowest in the conduit group; the differences in α-SMA protein content among the four groups were all significant (Fig. 1D). Western blot analysis of TGF-β1/SMAD-signaling-related proteins revealed that TGF-β1, TβRI, Smad2 and p-Smad2 protein contents were all highest in the agonist group and lowest in the conduit group (Fig. 1D). In accordance with histological results, this finding suggested that aligned nanofiber conduit suppressed TGF-β1/SMAD signaling and downregulated α-SMA expression.
These findings suggested that aligned nanofiber conduit may prevent traumatic neuromas from occurring. Based on our previous studies (28) and literature review (30,31,33), we hypothesized that this effect could have certain connections with the status of TGF-β1/SMAD signaling. Part II of the study revealed that aligned nanofiber nerve conduits inhibited soft tissue proliferation through regulation of TGF-β1/SMAD signaling.
Histological analysis
Neuromas were observed in eight rats from the control group. Nerve endings from rats in the SB431542 group were thin, while the nerve endings from rats in the conduit and conduit + SRI (applied with both conduit and SRI-011381) groups were truncated and linear. Immunohistochemical staining of Collagen I showed the highest proliferation of haphazardly distributed Collagen I fibers in control group (Fig. 2A; P<0.05, vs. per groups). In the SB431542 group, less stained Collagen I had regenerated in a disorderly arrangement, less stained Collagen I were observed in the conduit and conduit + SRI groups, with an orderly arrangement of nerve fibers (Fig. 2A).
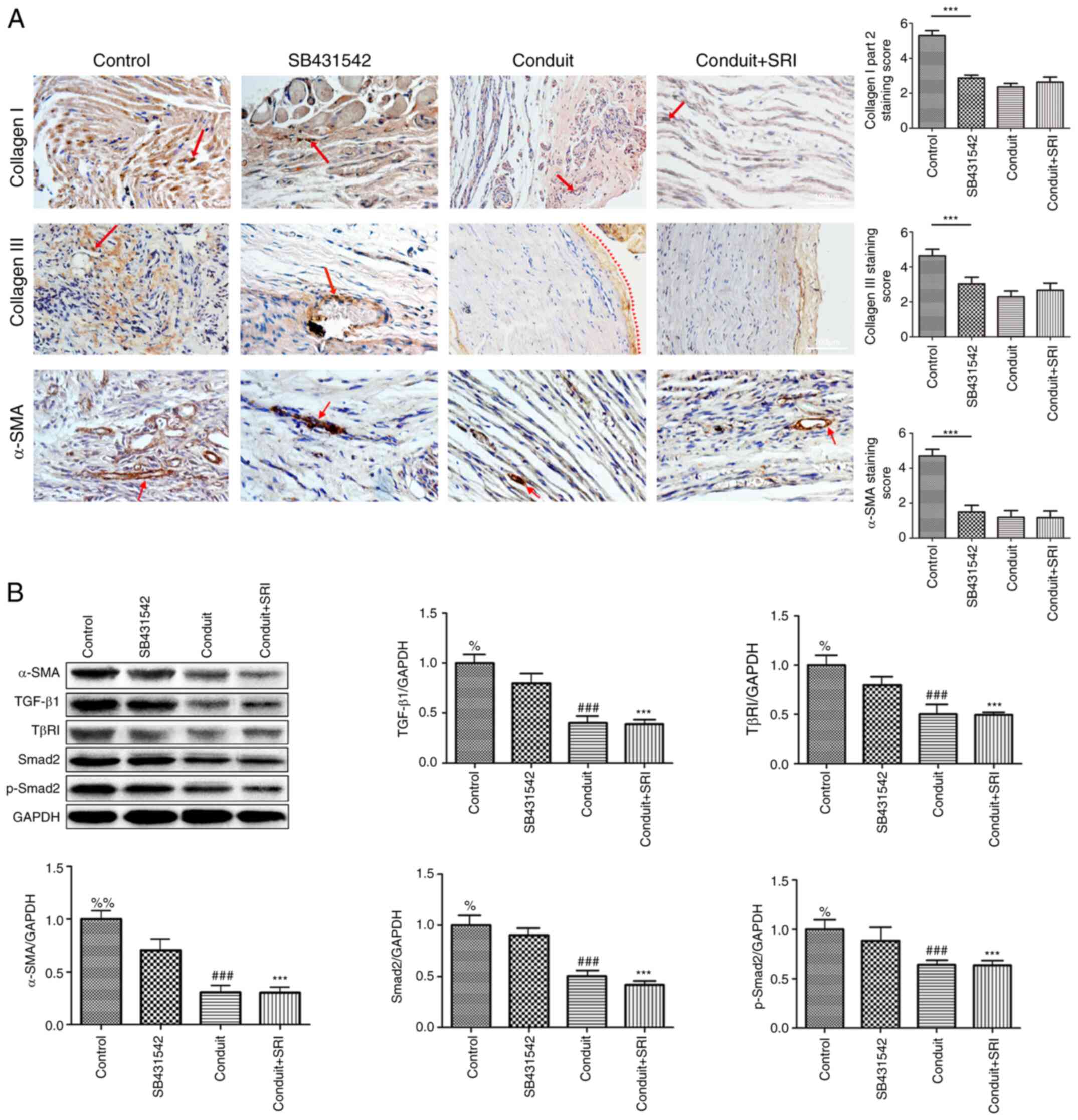 |
Figure 2
Aligned nanofiber conduits suppress TGF-β1/SMAD-signaling and prevent traumatic neuroma. (A) Immunohistochemical staining of Collagen I, III and α-SMA in Part II of the study. The staining score of Collagen I, III and α-SMA are highest in the control group (vs. per groups, all P<0.05). Collagen I, III (magnification, x100) and α-SMA (magnification, x200). (B) Protein expression and quantitative analysis of α-SMA, TGF-β1, TβR1, Smad2 and p-Smad2 in the four groups. Values are expressed as the mean ± standard deviation, n=5 per group. ***P<0.001, %%P<0.01, %P<0.05 vs. control group; ###P<0.001 vs. control group.
|
Immunohistochemical staining of Collagen III showed the highest proliferation of collagen: severe erosion of the epineurium, and intraneural connective tissue interspersed with a disorderly arrangement of nerve fascicles in control group (Fig. 2A; P<0.05 vs. per groups). Moderate regeneration of collagen was present in the SB431542 group. In the conduit and conduit + SRI groups, collagen was observed surrounding the nerve tract (red arrowheads) with an orderly arrangement of nerve fibers (Fig. 2A). These findings suggested that our aligned nanofiber conduit may prevent the proliferation of Collagen I & III, and this function would not be blocked by TGF-β1/SMAD signaling agonist (SRI-011381 hydrochloride).
Immunohistochemical staining with α-SMA revealed the highest staining level of α-SMA and a disorderly arrangement of regenerated nerve fibers in the control group (Fig. 2A; P<0.05 vs. per groups). In the SB431542 group, moderate expression of α-SMA was observed. Less staining with α-SMA, mainly within the artery walls, was observed in the conduit and conduit + SRI groups (Fig. 2A). The nerve fascicles in these two conduit groups had an orderly arrangement compared with the control and SB431542 groups. As with Part I, the results of Part II revealed that aligned nanofiber conduit may prevent heterotopic-expression of α-SMA.
Western blot analysis
Quantitative analysis using the Western blot method demonstrated that α-SMA expression was highest in the control group and significantly lower in the conduit and conduit + agonist groups. Differences in α-SMA content among all the groups were significant (Fig. 2B). Western blot analysis of TGF-β1/SMAD-signaling-related proteins revealed that TGF-β1, TβRI, Smad2 and p-Smad2 protein content were all highest in the control group (Fig. 2B). The content of these signaling-related proteins was significantly lower in the conduit and conduit + agonist groups (Fig. 2B), but no other significant differences between these two groups were observed. This finding suggested that aligned nanofiber conduit suppressed α-SMA expression by suppressing TGF-β1/SMAD signaling.
From the histological findings in Part II, it was observed that aligned nanofiber conduit may remove the stimulation of the TGF-β1/SMAD signaling agonist, suggesting that the conduit may serve its role by suppressing TGF-β1/SMAD signaling. These findings supported our earlier hypothesis; and quantitative analysis further proved these findings.
Discussion
Traumatic neuromas can be a troublesome consequence of peripheral nerve injuries (1). There have been various recommendations regarding how to prevent and treat traumatic neuromas, but no consensus has yet been reached (7,8,14,20,26). One major reason for this is probably uncertainty regarding the specific pathophysiological mechanisms of neuropathic pain (2,9,48).
Several studies have reported satisfying treatment results following resecting the nerve stump and transferring it into muscles, veins or bones (14,18,49). This treatment method is based on the theory that external mechanical stimulation from connective tissues surrounding the neuroma may lead to neuroma-associated pain (3). Inconsistencies in the success of the nerve capping technique imply that there may be several sources of neuroma-associated pain.
Border and Noble (33) reported that transforming growth factor beta served a positive role in tissue fibrosis. Furthermore, the association between nerve growth factor and TGF-β1 was studied by Coassin et al (34), revealing the potential function of TGF-β1 in the regeneration of connective tissue in the nervous system. Xie et al (31) suggested that SMAD regulated the mono-ubiquitination of TGF-β superfamily signaling. Furthermore, Arora and Mcculloch (37) reported that collagen remodeling depended on α-SMA expression by fibroblasts. The results of the present study strongly suggest that, fibroblasts in traumatic neuroma are regulated by TGF-β1/SMAD signaling. Further studies have illustrated the essential role of soft tissue proliferation, which could be a cause of neuropathic pain (3,5,29). In our previous study, formation of traumatic neuromas was significantly inhibited in the nerve conduit group with relatively ‘normal’ structural and morphological features and no occurrence of autotomy and significantly lower expression of pain marker (c-fos) compared with the no-capping group (26). Furthermore, significantly higher levels of α-SMA and the pain marker, c-fos, were observed in traumatic neuroma. Additionally, a strong correlation between autotomy scores and the expression level of α-SMA was reported (R=0.957; P<0.001) and the expression level of α-SMA was positively associated with the autotomy scores (R2=0.915; P<0.001) (28). It is widely known that the pathophysiology of neuroma formation depends on the presence of a chaotic admixture of neurite outgrowth and fibrous tissue, and since α-SMA is one of the markers of a myofibroblastic phenotype (32) and is common in the muscular layer of vascular walls, upregulated α-SMA expression may increase the contractility of myofibroblasts and non-muscle cells, and this could be a cause of pain in the region affected by a neuroma (36-39). Nanofibrous scaffolds manufactured by the electrospinning technique have been discussed previously and are becoming increasingly important in the study of nanomaterials and tissue engineering (44,50,51). Ceballos et al (52) demonstrated that, compared with random collagen gels, aligned gels could enhance neurite extension. At the cellular level, Pandey et al (53) reported that polymer nanofibers could provide an ideal three-dimensional scaffold for the attachment and growth of mesenchymal stem cells. Our previous study also discussed this characteristic of aligned nanofiber conduits and demonstrated that these nerve conduits greatly improved the propensity of regenerated nerve fibers to adopt a linear alignment (27).
The results of the present study demonstrated that regeneration of nerve fibers can be affected by the proliferation of surrounding connective tissues. In particular, the activation of TGF-β1/SMAD signaling upregulates the expression of α-SMA and collagen, leading to chaotic tissue proliferation, which is a typical pathological sign of traumatic neuromas. Scanning electron micrographs of cell cultures revealed that aligned nanofiber materials are better than non-aligned materials at promoting the linear growth of Schwann cells. Combined with the results of the present study, we hypothesized that the inhibitory effect of aligned nanofiber nerve conduits on α-SMA expression occurs through TGF-β1/SMAD signaling in traumatic neuroma.
These results suggested that the aligned nanofiber conduits prevent the development of post-traumatic neuroma by suppressing TGF-β1/SMAD signaling-related α-SMA and collagen proliferation. Furthermore, our recent study has demonstrated that aligned nanofiber nerve conduit inhibits painful traumatic neuroma formation also through regulation of the RhoA/ROCK signaling pathway (54). These findings support the theory that aligned nanofiber conduits may be used clinically in peripheral nerve injuries.
The results of the present study may provide novel insights into the commonly adopted approach of treating traumatic neuromas by trans positioning the nerve stumps into biological tunnels, including bones, muscles and veins, or by covering the transected nerve ends with conduits. It is plausible that covering the nerve stumps using these methods could decrease external mechanical stimuli and prevent the formation of scar tissue. Additionally, α-SMA expression in the nerve stump may be downregulated, leading to pain relief. The main limitation of the present study was that an indirect approach was used to evaluate the mechanism of TGF-β1/SMAD signaling. Future studies using specific animal models to verify these findings will be required. In summary, it was concluded that aligned nanofiber conduits can effectively downregulate α-SMA and collagen expression, and this inhibition is mediated by suppression of TGF-β1/SMAD signaling. Aligned biomaterial conduits may be used as a basis for novel traumatic neuroma treatment strategies.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science Foundation of China (grant no. 81571185) and Wenzhou Municipal Science and Technology Bureau (grant no. Y2020040).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
HY supervised and aided in the research design. CY and XZ were major contributors in writing the manuscript. WW and CS analyzed the research data. KP performed language polishing. CY designed the research and drafted the manuscript. XZ made substantial contributions to conception and design. KP participated in the experiments design and revised the manuscript critically for important intellectual content. HY and WW confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was performed according to a protocol approved by the Institutional Animal Care Committee, Wenzhou Medical University, China. All experimental rats involved in the present study were treated humanely in accordance with the National Research Council guidelines for the care of laboratory animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Watson J, Gonzalez M, Romero A and Kerns J: Neuromas of the hand and upper extremity. J Hand Surg Am. 35:499–510. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Orza F, Boswell MV and Rosenberg SK: Neuropathic pain: Review of mechanisms and pharmacologic management. NeuroRehabilitation. 14:15–23. 2000.PubMed/NCBI
|
|
3
|
Ro LS and Chang KH: Neuropathic pain: Mechanisms and treatments. Chang Gung Med J. 28:597–605. 2005.PubMed/NCBI
|
|
4
|
Foltán R, Klíma K, Spacková J and Sedý J: Mechanism of traumatic neuroma development. Med Hypotheses. 71:572–576. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zimmermann M: Pathobiology of neuropathic pain. Eur J Pharmacol. 429:23–37. 2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Harden RN: Chronic neuropathic pain. Mechanisms, diagnosis, and treatment. Neurologist. 11:111–122. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gorkisch K, Boese-Landgraf J and Vaubel E: Treatment and prevention of amputation neuromas in hand surgery. Plast Reconstr Surg. 73:293–299. 1984.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Faith D: Therapeutic massage provides pain relief to a client with morton's neuroma: A case report. Int J Ther Massage Bodywork. 5:12–19. 2012.PubMed/NCBI
|
|
9
|
Rizzo MA: Successful treatment of painful traumatic mononeuropathy with carbamazepine: Insights into a possible molecular pain mechanism. J Neurol Sci. 152:103–106. 1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dahl E and Cohen SP: Perineural injection of etanercept as a treatment for postamputation pain. Clin J Pain. 24:172–175. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Correa-Illanes G, Calderon W, Roa R, Pineros JL, Dote J and Medina D: Treatment of localized post-traumatic neuropathic pain in scars with 5% lidocaine medicated plaster. Local Reg Anesth. 3:77–83. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ulrich D, van Doorn L and Hovius S: Fat injection for treatment of painful neuroma after episiotomy. Int J Gynaecol Obstet. 115:290–291. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Stevanato G, Devigili G, Eleopra R, Fontana P, Lettieri C, Baracco C, Guida F, Rinaldo S and Bevilacqua M: Chronic post-traumatic neuropathic pain of brachial plexus and upper limb: A new technique of peripheral nerve stimulation. Neurosurg Rev. 37:473–480. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Evans GR and Dellon AL: Implantation of the palmar cutaneous branch of the median nerve into the pronator quadratus for treatment of painful neuroma. J Hand Surg Am. 19:203–206. 1994.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yüksel F, Kişlaoğlu E, Durak N, Uçar C and Karacaoğlu E: Prevention of painful neuromas by epineural ligatures, flaps and grafts. Br J Plast Surg. 50:182–185. 1997.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sood MK and Elliot D: Treatment of painful neuromas of the hand and wrist by relocation into the pronator quadratus muscle. J Hand Surg Br. 23:214–219. 1998.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Stahl S and Rosenberg N: Surgical treatment of painful neuroma in medial antebrachial cutaneous nerve. Ann Plast Surg. 48:154–160. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Koch H, Herbert TJ, Kleinert R, Hubmer M, Scharnagl E and Pierer G: Influence of nerve stump transplantation into a vein on neuroma formation. Ann Plast Surg. 50:354–360. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Koch H, Hubmer M, Welkerling H, Sandner-Kiesling A and Scharnagl E: The treatment of painful neuroma on the lower extremity by resection and nerve stump transplantation into a vein. Foot Ankle Int. 25:476–481. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Krishnan KG, Pinzer T and Schackert G: Coverage of painful peripheral nerve neuromas with vascularized soft tissue: Method and results. Neurosurgery. 56 (Suppl 2):S369–S378. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Balcin H, Erba P, Wettstein R, Schaefer DJ, Pierer G and Kalbermatten DF: A comparative study of two methods of surgical treatment for painful neuroma. J Bone Joint Surg Br. 91:803–808. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sakai Y, Ochi M, Uchio Y, Ryoke K and Yamamoto S: Prevention and treatment of amputation neuroma by an atelocollagen tube in rat sciatic nerves. J Biomed Mater Res B Appl Biomater. 73:355–360. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Okuda T, Ishida O, Fujimoto Y, Tanaka N, Inoue A, Nakata Y and Ochi M: The autotomy relief effect of a silicone tube covering the proximal nerve stump. J Orthop Res. 24:1427–1437. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Peterson SL and Adham MN: Acellular dermal matrix as an adjunct in treatment of neuropathic pain at the wrist. J Trauma. 61:392–395. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Thomsen L, Bellemere P, Loubersac T, Gaisne E, Poirier P and Chaise F: Treatment by collagen conduit of painful post-traumatic neuromas of the sensitive digital nerve: a retrospective study of 10 cases. Chir Main. 29:255–262. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yan H, Zhang F, Kolkin J, Wang C, Xia Z and Fan C: Mechanisms of nerve capping technique in prevention of painful neuroma formation. PLoS One. 9(e93973)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yan H, Zhang F, Wang C, Xia Z, Mo X and Fan C: The role of an aligned nanofiber conduit in the management of painful neuromas in rat sciatic nerves. Ann Plast Surg. 74:454–461. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Weng W, Zhao B, Lin D, Gao W, Li Z and Yan H: Significance of alpha smooth muscle actin expression in traumatic painful neuromas: A pilot study in rats. Sci Rep. 6(23828)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yao C, Zhou X, Zhao B, Sun C, Poonit K and Yan H: Treatments of traumatic neuropathic pain: A systematic review. Oncotarget. 8:57670–57679. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gordon KJ and Blobe GC: Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta. 1782:197–228. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xie F, Zhang Z, van Dam H, Zhang L and Zhou F: Regulation of TGF-β superfamily signaling by SMAD mono-ubiquitination. Cells. 3:981–993. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gabbiani G: The biology of the myofibroblast. Kidney Int. 41:530–532. 1992.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Border WA and Noble NA: Transforming growth factor beta in tissue fibrosis. N Engl J Med. 331:1286–1292. 1994.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Coassin M, Lambiase A, Micera A, Tirassa P, Aloe L and Bonini S: Nerve growth factor modulates in vitro the expression and release of TGF-beta1 by amniotic membrane. Graefes Arch Clin Exp Ophthalmol. 244:485–491. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Otranto M, Sarrazy V, Bonté F, Hinz B, Gabbiani G and Desmoulière A: The role of the myofibroblast in tumor stroma remodeling. Cell Adh Migr. 6:203–219. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C and Brown RA: Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Arora PD and McCulloch CA: Dependence of collagen remodelling on alpha-smooth muscle actin expression by fibroblasts. J Cell Physiol. 159:161–175. 1994.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Desmoulière A, Chaponnier C and Gabbiani G: Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 13:7–12. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Serini G and Gabbiani G: Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 250:273–283. 1999.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Prabhakaran MP, Vatankhah E and Ramakrishna S: Electrospun aligned PHBV/collagen nanofibers as substrates for nerve tissue engineering. Biotechnol Bioeng. 110:2775–2784. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang CY, Zhang KH, Fan CY, Mo XM, Ruan HJ and Li FF: Aligned natural-synthetic polyblend nanofibers for peripheral nerve regeneration. Acta Biomater. 7:634–643. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Corey JM, Lin DY, Mycek KB, Chen Q, Samuel S, Feldman EL and Martin DC: Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J Biomed Mater Res A. 83:636–645. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang HB, Mullins ME, Cregg JM, Hurtado A, Oudega M, Trombley MT and Gilbert RJ: Creation of highly aligned electrospun poly-L-lactic acid fibers for nerve regeneration applications. J Neural Eng. 6(016001)2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang CY, Liu JJ, Fan CY, Mo XM, Ruan HJ and Li FF: The effect of aligned core-shell nanofibres delivering NGF on the promotion of sciatic nerve regeneration. J Biomater Sci Polym Ed. 23:167–184. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu Y, Gao L, Zhao X, Guo S, Liu Y, Li R, Liang C, Li L, Dong J, Li L and Yang H: Saikosaponin a protects from pressure overload-induced cardiac fibrosis via inhibiting fibroblast activation or endothelial cell EndMT. Int J Biol Sci. 14:1923–1934. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, Martin W, Fornwald J, Lehr R, Harling J, et al: Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 62:58–64. 2002.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ma J, Zhang L, Niu T, Ai C, Jia G, Jin X, Wen L, Zhang K, Zhang Q and Li C: Growth differentiation factor 11 improves neurobehavioral recovery and stimulates angiogenesis in rats subjected to cerebral ischemia/reperfusion. Brain Res Bull. 139:38–47. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Melzack R and Wall PD: Pain mechanisms: A new theory. Science. 150:971–979. 1965.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mass DP, Ciano MC, Tortosa R, Newmeyer WL and Kilgore ES Jr: Treatment of painful hand neuromas by their transfer into bone. Plast Reconstr Surg. 74:182–185. 1984.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Beilke MC, Zewe JW, Clark JE and Olesik SV: Aligned electrospun nanofibers for ultra-thin layer chromatography. Anal Chim Acta. 761:201–208. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Xu Y, Wu J, Wang H, Li H, Di N, Song L, Li S, Li D, Xiang Y, Liu W, et al: Fabrication of electrospun poly(L-lactide-co-ε-caprolactone)/collagen nanoyarn network as a novel, three-dimensional, macroporous, aligned scaffold for tendon tissue engineering. Tissue Eng Part C Methods. 19:925–936. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ceballos D, Navarro X, Dubey N, Wendelschafer-Crabb G, Kennedy WR and Tranquillo RT: Magnetically aligned collagen gel filling a collagen nerve guide improves peripheral nerve regeneration. Exp Neurol. 158:290–300. 1999.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Pandey S, Rathore K, Johnson J and Cekanova M: Aligned nanofiber material supports cell growth and increases osteogenesis in canine adipose-derived mesenchymal stem cells in vitro. J Biomed Mater Res A. 106:1780–1788. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhou X, Zhao B, Poonit K, Weng W, Yao C, Sun C and Yan H: An aligned nanofiber nerve conduit that inhibits painful traumatic neuroma formation through regulation of the RhoA/ROCK signaling pathway. J Neurosurg. 132:837–846. 2019.PubMed/NCBI View Article : Google Scholar
|
















