Introduction
Preeclampsia (PE) is defined as the occurrence of
new onset hypertension and proteinuria or other target organ damage
occurring after 20 weeks of gestation (1). PE affects ~5% of pregnancies worldwide
and is one of the most commonly observed complications during
pregnancy (2). The disease not only
causes maternal and fetal morbidity and mortality, but also leads
to premature birth of the fetus and long-term cardiovascular
disease in pregnant women (3). In
1914, researchers found that pregnant women with toxemia,
albuminuria and eclampsia presented with an increased incidence
rate of PE compared with women with healthy pregnancies (4), which might be mediated by placental
hypoperfusion and ischemia. The development of PE is a complex
process, including abnormal homeostasis of placental cells,
oxidative stress response and angiogenesis (5). Abnormal oxidative stress leads to
abnormal spiral artery remodeling in the placenta, further
impairing angiogenesis process (6),
which also contributes to the pathophysiology of PE (7). Altering the angiogenesis process could
induce hypoperfusion and ischemia in the placenta, resulting in
placental trophoblastic cell apoptosis, which is critical for
maintaining placental homeostasis (8,9), which
leads to the occurrence of PE. Aspirin, a pharmaceutical drug,
affects the inflammatory cascade by irreversibly inhibiting the
activity of cyclooxygenase (COX)-1 and COX-2, further inhibiting
the production of prostaglandins and thromboxane (10). Inhibition of COX activity can result
in inhibition of thromboxane A2, a decrease in the release of
cytokines, growth factors and coagulation factors (11), accumulation of reactive oxygen
species (ROS) and inhibition of the oxidative stress response
process (12). A previous study
reported that pregnant women administered low-dose aspirin
treatment (60-150 mg/day) displayed a significant reduction in the
occurrence of preterm birth and PE frequency (13). Another study demonstrated that
100-160 mg aspirin treatment decreased the risk of PE starting from
16 weeks of gestation compared with patients receiving 300 mg
aspirin treatment (14). Therefore,
we hypothesize that aspirin may serve as a potential therapeutic
strategy for PE. The present study established a PE model in C57/BL
mice and evaluated the change in expression levels of antioxidative
enzymes and the AKT/mTOR signaling pathway to elucidate the effects
and mechanism of aspirin treatment on PE.
Materials and methods
Materials
A total protein extraction kit (cat. no. BC3711) and
BCA protein assay kit (cat. no. PC0020) were purchased from Beijing
Solarbio Science & Technology Co., Ltd. Primary antibodies
targeted against CAT (cat. no. ab16731), SOD1 (cat. no. ab13498),
TRX (cat. no. ab26320) and periaxin (PRX; cat. no. ab211292) were
purchased from Abcam. A total SOD assay kit (cat. no. A001-1-2) and
TRX peroxidase test kit were purchased from Nanjing Jiancheng
Bioengineering Institute. A human TRX ELISA kit (cat. no.
CSB-E09728h) and human CAT ELISA kit (cat. no. CSB-E13635h) were
purchased from Cusabio Technology LLC.
Ethics statements
The present study was approved by the Ethics
Committee of Chengdu Women's and Children's Central Hospital
(approval no. SCXK 2009-0017; Chengdu, China). The treatment of all
80 animals complied with the Guide for the Care and Use of
Laboratory Animals (15).
Animal treatment and grouping
A total of 80 female C57/BL mice (weight, 20-22 g;
age, 7 weeks) and 80 male mice (weight, 21-25 g; age, 8 weeks) were
purchased from the Laboratory Animal Center of the Academy of
Military Medical Sciences of Beijing People's Liberation Army
(Beijing, China). Female and male mice were housed at 24˚C with
60-70% humidity, 12-h dark/light cycles and free access to food and
water. Female mice were mated with male mice overnight during the
proestrus period and timed for pregnancy after 1 week of
adaptation. Mice were divided into four groups (n=20/group) as
follows: i) Negative control group (NC); ii) PE group (PE); iii) PE
with low-dose aspirin (2 mg/kg) treatment group (AL); and iv) PE
with high-dose (10 mg/kg) aspirin treatment group (AH). Mice in the
PE model group were subcutaneously injected with
L-NG-Nitroarginine methyl ester (L-NAME; 50 mg/day;
Sigma-Aldrich; Merck KGaA) at gestation day (GD) 7 until GD18. The
concentration of proteinuria was measured at GD7 and GD18 by
performing a BCA assay. Blood pressure was measured at GD 7, 10,
13, 16 and 18 using an animal non-invasive sphygmomanometer (cat.
no. BP-98A; Softron Co., Ltd.). Mice in the NC group were treated
with equal volume of normal saline.
Tissue collection and processing
After L-NAME treatment at GD 18, mice were
anesthetized with isoflurane (3% for induction, 1.5% for
maintenance; Sigma-Aldrich; Merck KGaA) in an induction chamber.
Urine samples were collected from the anesthetized mice, and then
blood samples (0.5 ml) were collected by removing the eyeballs. The
blood samples were stored at 4˚C until further processing. The mice
were then sacrificed by cervical dislocation and the placental
tissues were collected by full-term caesarian section. The obtained
placental tissues were transferred in a petri dish, washed with
sterile normal saline to remove the blood and stored in liquid
nitrogen until further analysis. The obtained blood samples were
processed within 4 h of collection. Briefly, the blood samples were
centrifuged at 1,006 x g for 15 min at 4˚C to obtain serum, which
was stored at -80˚C until further analysis.
Protein extraction and western
blotting
Total protein was extracted from placental tissue
using the total protein extraction kit (cat. no. 2140; Millipore
Sigma) according to the manufacturer's protocol. Briefly, placental
tissues were homogenized with lysis buffer (Thermo Fisher
Scientific, Inc.) using a homogenizer, and the supernatant was
collected after centrifugation (16,099 x g) at 4˚C for 10 min.
Protein concentrations were determined using the BCA assay.
Subsequently, proteins (60 µg) were separated via 10% SDS-PAGE and
transferred onto 0.22 µm nitrocellulose membranes (Millipore Sigma)
using a semi-dry blotter. Following blocking with 5% non-fat milk
for 1 h at room temperature, the membranes were incubated with
primary antibodies (all Cell Signaling Technology, Inc.) against
GAPDH (1:5,000; cat. no. 5174), p-AKT (1:1,000; cat. no. 9271), AKT
(1:1,000; cat. no. 9272), p-mTOR (1:1,000; cat. no. 2971), mTOR
(1:1,000; cat. no. 2972) and PTEN (1:1,000; cat. no. 9188) at 4˚C
overnight. Subsequently, the membranes were incubated with
HRP-conjugated goat anti-mouse IgG secondary antibodies (1:1,000;
cat. no. PV-6001; OriGene Technologies, Inc.) for 1 h at room
temperature. Protein bands were visualized using chemiluminescence
(Amersham; Cytiva). Protein expression levels were semi-quantified
using Image-Pro Plus software (version 6.0; Media Cybernetics,
Inc.).
ELISA
ELISAs were performed using ELISA kits according to
the manufacturer's protocols. Briefly, Serum samples were added
into each well of a 96-plate. After incubation with the reaction
buffer at 37˚C, the optical density value of each well was measured
at a wavelength of 450 nm using a microplate reader.
Statistical analysis
Data are presented as the mean ± SD. Each experiment
was repeated three times independently. Dunnett's post hoc test was
used for comparisons among multiple groups following one-way ANOVA
using SPSS software version 19.0 (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
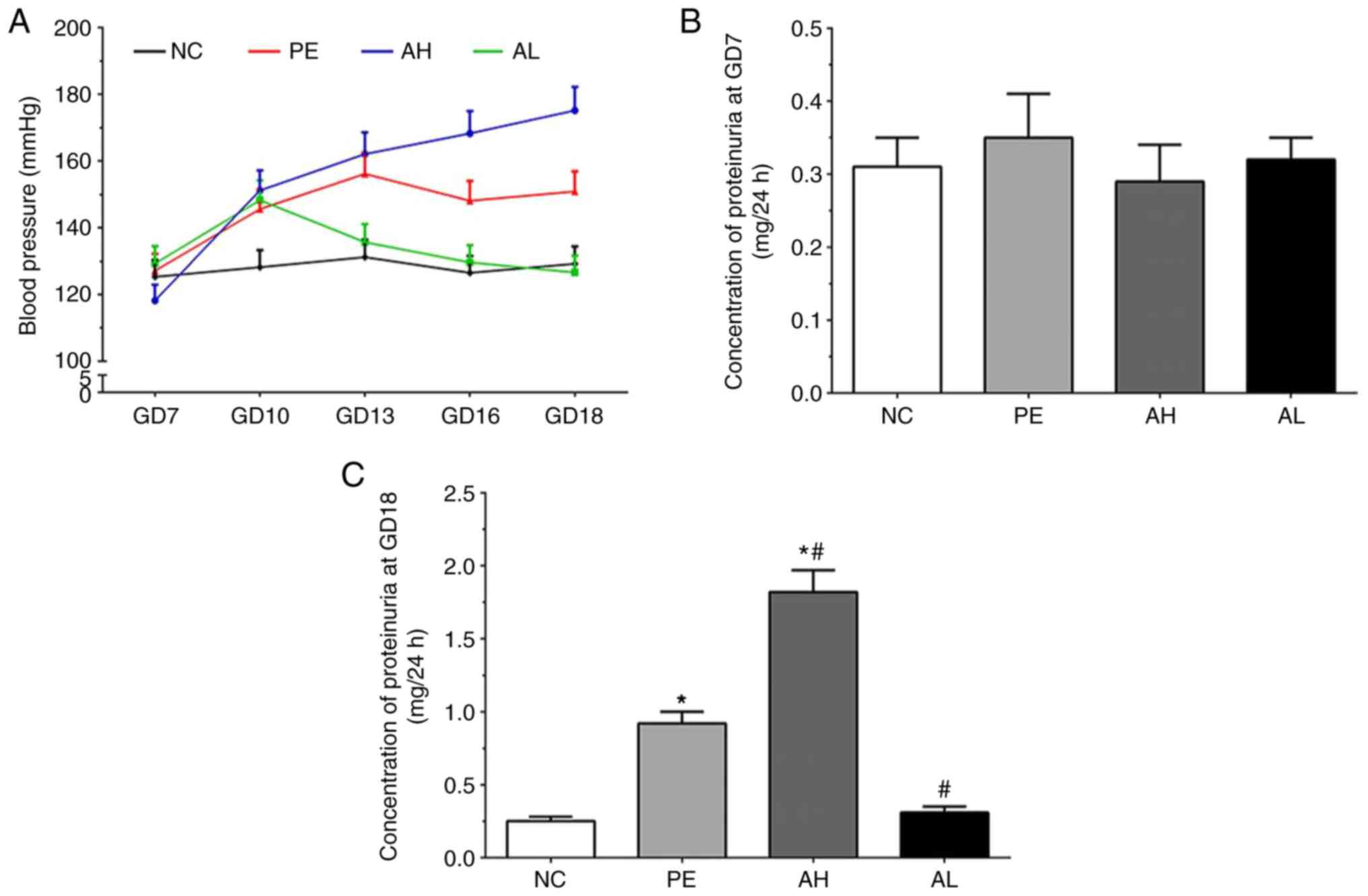
Measurement of blood pressure of mice
in each group
The blood pressure of mice in each group are
presented in Fig. 1A. Compared with
the NC group, the blood pressure was not altered at GD7 but notably
increased in all treatment groups at GD10. The blood pressure in
the PE group remained stable at GD13, 16 and 18; however, blood
pressure displayed a different trend in the AH and AL groups. Blood
pressure in the AH group gradually increased to ~175 mmHg by GD18,
whereas blood pressure in the AL group gradually decreased to ~120
mmHg by GD18.
Concentration of proteinuria in each
group
As shown in Fig. 1B,
the concentration of proteinuria at GD7 was 0.31±0.04, 0.35±0.06,
0.29±0.05 and 0.32±0.03 in the NC, PE, AH and AL groups,
respectively. At GD18, the concentration of proteinuria was
0.25±0.03, 0.92±0.08, 1.82±0.15 and 0.31±0.04 in the NC, PE, AH and
AL groups, respectively (Fig. 1C).
The concentration of proteinuria was significantly increased in PE
and AH groups compared with the NC group at GD18 (P<0.05).
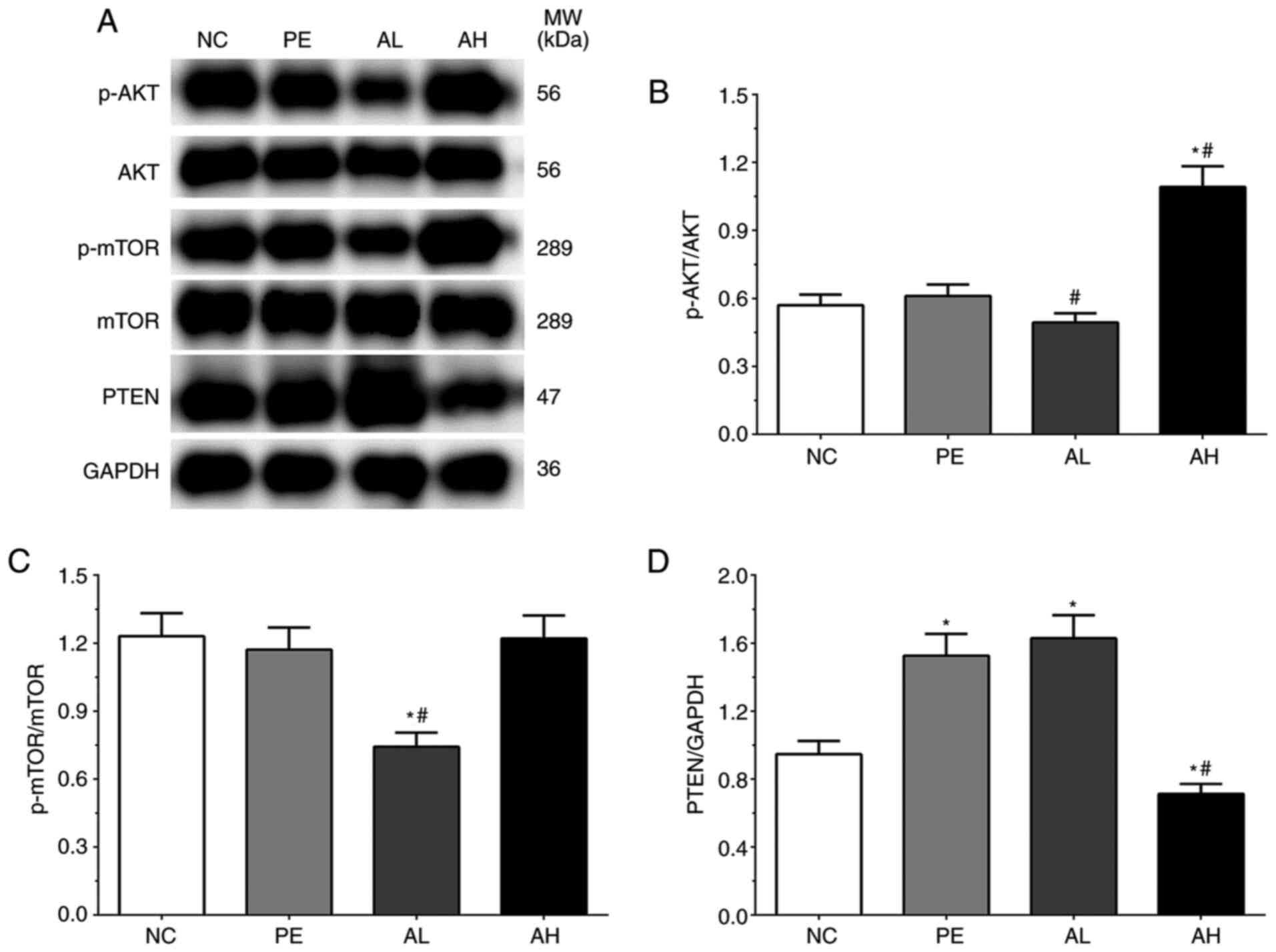
Expression levels of AKT/mTOR
signaling pathway-related proteins in placental tissues
As shown in Fig. 2A
and B, the ratios of p-AKT/AKT was
0.57±0.05, 0.61±0.05, 0.49±0.04 and 1.09±0.09 in the NC, PE, AH and
AL groups, respectively. The ratio of p-AKT/AKT was significantly
increased in the AH group (P<0.05) compared with the NC group,
but significantly decreased in the AL group (P<0.05) and
significantly increased in the AH group (P<0.05) compared with
the PE group. The ratios of p-mTOR/mTOR were 1.23±0.10, 1.17±0.10,
0.74±0.06 and 1.22±0.10 in the NC, PE, AH and AL groups,
respectively (Fig. 2A and C). The ratio of p-mTOR/mTOR was
significantly decreased in the AL group (P<0.05) compared with
the NC and PE groups. The expression levels of PTEN were 0.95±0.08,
1.53±0.13, 1.63±0.14 and 0.71±0.06 in the NC, PE, AH and AL groups,
respectively (Fig. 2A and D). Compared with the NC group, the
expression of PTEN was significantly increased in the PE and AL
groups (P<0.05), but significantly decreased in the AH group
(P<0.05). The expression of PTEN was also significantly
decreased in the AH group (P<0.05) compared with the PE
group.
Expression of antioxidative proteins
in placental tissues
As shown in Fig. 3,
the expression levels of CAT, SOD1, TRX and PRX were detected by
performing western blotting. The expression levels of CAT in the
NC, PE, AH and AL groups were 1.06±0.09, 0.71±0.06, 0.55±0.05 and
0.89±0.07, respectively. The expression of CAT was significantly
decreased in the PE and AH groups (P<0.05) compared with the NC
group. Compared with the PE group, the expression of CAT was
significantly decreased in the AH group (P<0.05), but
significantly increased in the AL group (P<0.05). The expression
levels of PRX in the NC, PE, AH and AL groups were 0.83±0.07,
0.74±0.06, 0.65±0.05 and 0.90±0.07, respectively. Compared with the
NC group, the expression of PRX was significantly decreased in the
AH group (P<0.05). Compared with the PE group, the expression of
PRX was significantly increased in the AL group (P<0.05). The
expression levels of TRX were 0.90±0.08, 0.81±0.07, 0.66±0.06 and
0.95±0.08 in the NC, PE, AH and AL groups, respectively. The
expression of TRX was significantly decreased in the AH group
(P<0.05) compared with the NC and PE groups, but significantly
increased in the AL group (P<0.05) compared with the PE group.
The expression levels of SOD1 were 1.03±0.09, 0.81±0.07, 0.80±0.07
and 0.98±0.08 in the NC, PE, AH and AL groups, respectively. The
expression of SOD1 was significantly decreased in the PE and AH
groups (P<0.05) compared with the NC group. By contrast,
compared with the PE group, the expression of SOD1 was
significantly increased in the AL group (P<0.05).
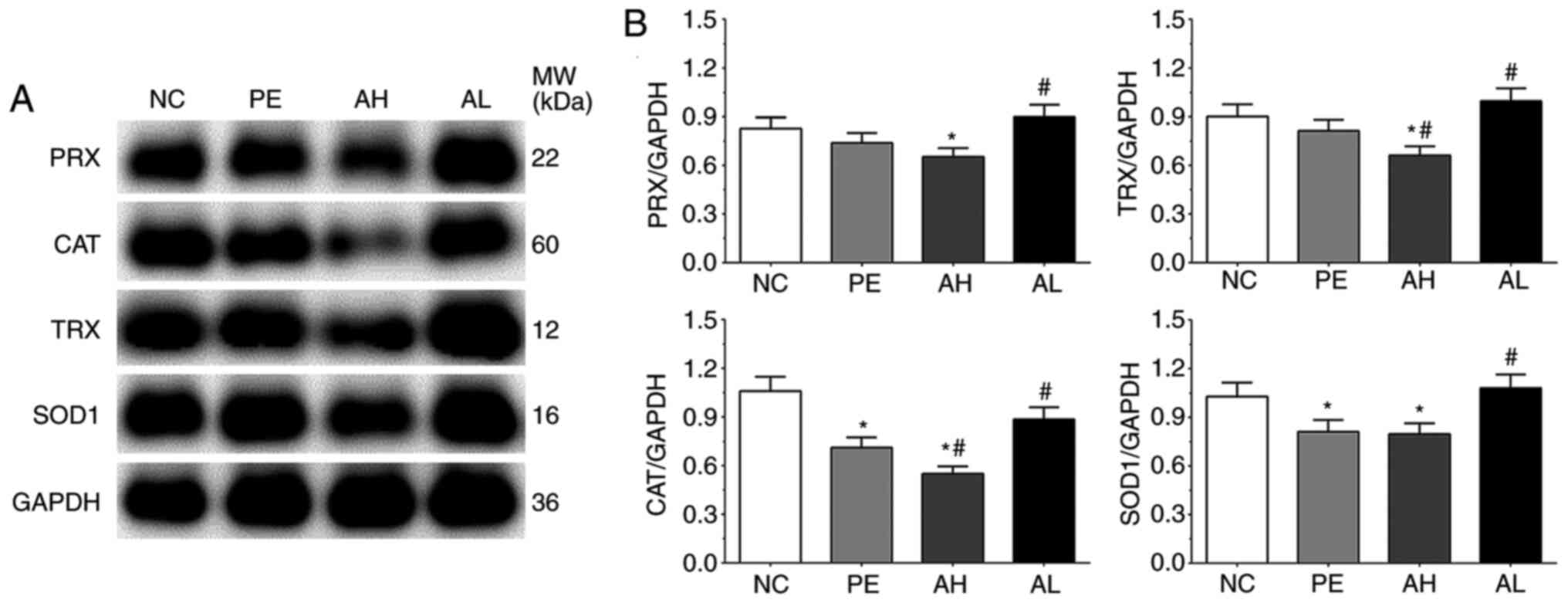 | Figure 3Antioxidative enzyme expression levels
in placental tissue of PE model mice. PRX, CAT, TRX and SOD1
protein expression levels were (A) determined by western blotting
and (B) semi-quantified. Each experiment was repeated three times
independently. Data are presented as the mean ± SD.
*P<0.05 vs. NC; #P<0.05 vs. PE. PE,
preeclampsia; PRX, periaxin; CAT, catalase; TRX, thioredoxin; SOD1,
superoxide dismutase 1; NC, negative control; AH, PE with high-dose
(10 mg/kg) aspirin treatment; AL, PE with low-dose aspirin (2
mg/kg) treatment; MW, molecular weight. |
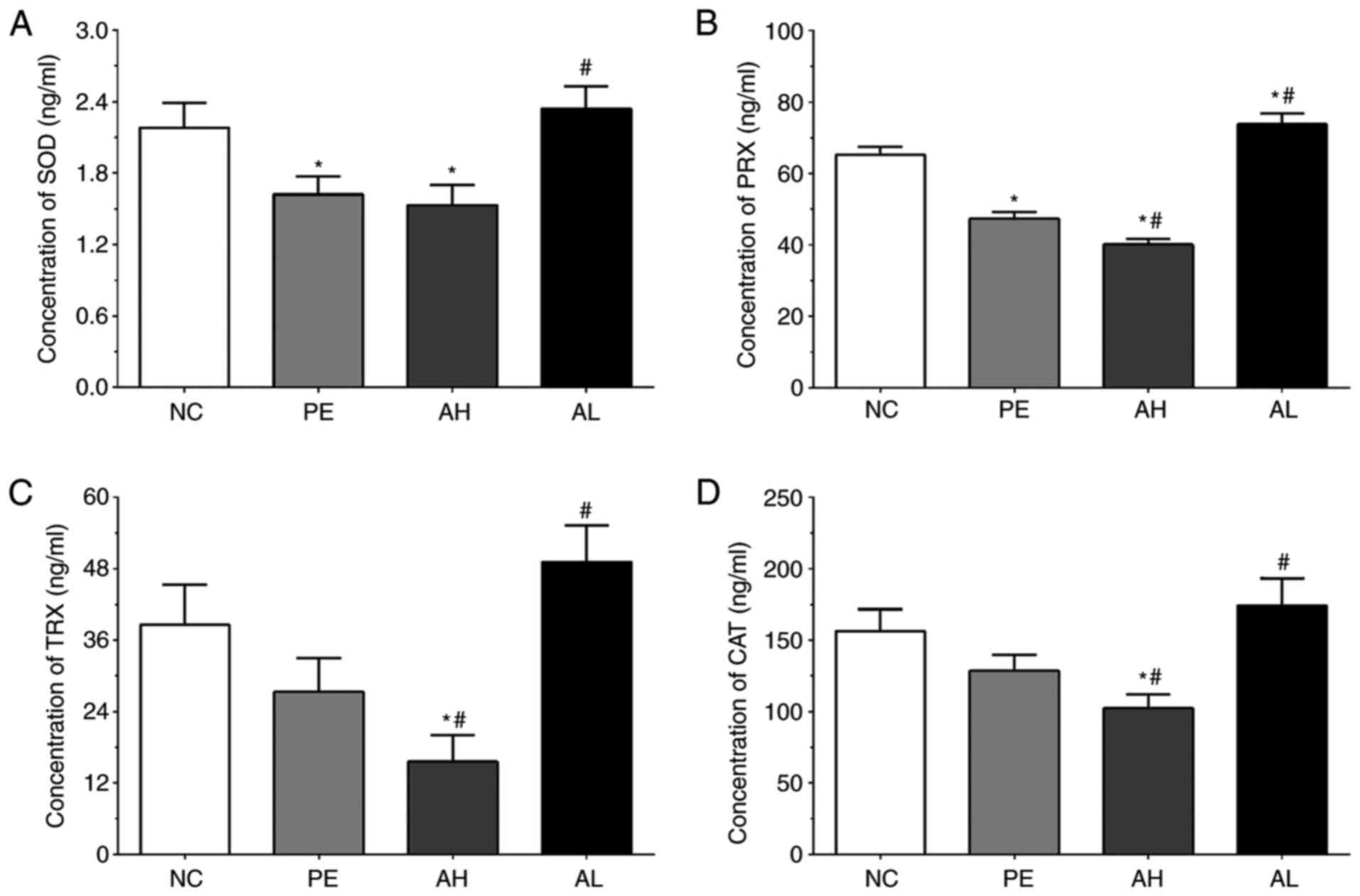
Measurement of oxidative enzymes in
serum samples of mice
As shown in Fig. 4A,
the concentrations of SOD in serum samples of the NC, PE, AH and AL
groups were 2.18±0.21, 1.62±0.15, 1.53±0.17 and 2.34±0.19 ng/ml,
respectively. The concentration of SOD was significantly decreased
in the PE and AH groups (P<0.05) compared with the NC group, but
significantly increased in the AL group (P<0.05) compared with
the PE group. The concentrations of PRX in serum samples of the NC,
PE, AH and AL groups were 65.26±2.31, 47.31±1.98, 40.51±1.62 and
73.83±3.05 ng/ml, respectively. Compared with the NC group, the
concentration of PRX was significantly decreased in PE and AH
groups (P<0.05), but significantly increased in the AL group
(P<0.05). The concentration of PRX was also significantly
decreased in the AH group (P<0.05) and significantly increased
in the AL group (P<0.05) compared with the PE group. The
concentrations of TRX in the NC, PE, AH and AL groups were
38.56±6.82, 27.31±5.65, 15.58±4.51 and 49.13±6.21 ng/ml,
respectively. The concentration of TRX was significantly decreased
in the AH group (P<0.05) compared with the NC and PE groups, but
significantly increased in the AL group (P<0.05) compared with
the PE group. The concentrations of CAT were 156.22±15.42,
128.43±11.23, 102.31±9.81 and 174.15±19.17 pg/ml in the NC, PE, AH
and AL groups, respectively. The concentration of CAT was
significantly decreased in the AH group (P<0.05) compared with
the NC and PE groups, but significantly increased in the AL group
(P<0.05) compared with the PE group.
Discussion
PE is defined as new onset hypertension and
proteinuria or other damage in organs that occurs after 20 weeks of
gestation (16). PE affects ~5% of
pregnancies worldwide and remains a leading complication of
pregnancy (17), causing maternal
and fetal morbidity and mortality. Aspirin, an antiplatelet
reagent, is widely used for the treatment of cardiovascular disease
(10). A previous study found that
the vascular production of prostacyclin was deficient in PE, and
the increased production of thromboxane could lead to the
aggregation of platelets, which is commonly observed in patients
with PE (18). A previous study
indicated that low-dose aspirin might be useful for protecting
pregnant women against the development of PE (19). The present study established a PE
model in pregnant mice and explored the protective role of low-dose
aspirin for PE. Compared with the NC group, the expression levels
and concentrations of antioxidative enzymes, including CAT, TRX,
PRX and SOD, in serum samples and placental tissues were decreased
in the PE model, respectively. The expression levels and
concentrations of these antioxidative enzymes were further
decreased after high-dose aspirin treatment, whereas PE-mediated
effects were significantly reversed after low-dose aspirin
treatment. The results indicated that aspirin treatment-induced
alterations were mediated via the AKT/mTOR signaling pathway.
Pregnancy has been reported to increase the
oxidative stress status of pregnant women, resulting in an increase
in circulating ROS. The major organ that regulates ROS during
pregnancy is the placenta (20).
The function of antioxidative stress defenses is also impaired by
the downregulation of antioxidant enzymes (21). Multiple enzymes participate in the
maintenance of oxidative status balance, including CAT, glutathione
peroxidase and peroxiredoxins (22). Among them, CAT contains a
H2O2 dismutase, which is localized in the
peroxisome and removes the extra H2O2 or
prevents H2O2 leakage (23). TRX is a protein that is
characterized by a catalytically active dithiol site, and widely
exists in bacteria, plants and animals (24). TRX catalyzes the cleavage of
disulfide bonds of downstream proteins and converts them into the
oxidized form. TRX can also quench ROS via cooperation with
TRX-dependent peroxidases or PRX (25). TRX reductase can transfer electrons
from NADPH to TRX to decrease disulfide linkage formation in PRX
through peroxidase reaction (26).
SOD1 is also a critical enzyme in the antioxidative system; in the
cytoplasm, SOD1 dismutates the superoxide anion radical into
H2O2, which is further reduced by CAT,
glutathione peroxidases or PRX into H2O (27). SOD1 performs a similar protective
role in the regulation of superoxide in the intermembrane space as
in the cytosol (28). The present
study demonstrated that the increased oxidative level in the PE
mouse model was decreased after low-dose aspirin treatment. Hence,
low-dose aspirin administration may serve as a therapeutic strategy
for PE.
ROS also participates in the production of multiple
genes involved in cell differentiation and proliferation, affecting
the cellular defense system and mitogenic pathway (29). A low level of ROS promotes the
angiogenesis process in the placenta during pregnancy via
upregulation of transcription factor E26 transformation-specific
oncogene homolog 1, resulting in the upregulation of VEGF.
Moreover, a low level of ROS can also promote the invasion of cells
via upregulation of kruppel-like factor 8 and activation of matrix
metalloproteinase 9(30). The
PI3K/AKT/mTOR signaling pathway serves an important role in the
regulation of cellular proliferation via multiple mechanisms. A
high level of ROS can elevate proproliferative signaling with
inhibition of growth suppressors via activation of the PI3K/AKT
signaling pathway through inhibition of phosphatases of PTEN
(31). PTEN negatively regulates
the PI3K/AKT signaling pathway via dephosphorylation of
phosphatidylinositol (3,4,5)-trisphosphate to phosphatidylinositol
(4,5)-bisphosphate (32). PI3K/AKT further promotes cell
proliferation via the mTORC1-dependent signaling pathway (33). A previous study demonstrated that
AKT could directly phosphorylate mitochondrial GSK-3β, leading to
decreases in GSK-3β activity and alleviation of negative regulation
mediated by pyruvate dehydrogenase and α-ketoglutarate
dehydrogenase complexes, which results in the generation of
superoxide and H2O2 (34). The present study demonstrated that,
compared with the PE group, activation of the AKT/mTOR signaling
pathway was significantly suppressed in the low-aspirin treatment
group, and AKT signaling was significantly promoted in the
high-aspirin treatment group, indicating that the oxidative stress
process was activated after high-dose aspirin treatment.
Furthermore, compared with the PE group, the expression of PTEN was
not significantly increased in the low-dose aspirin treatment
group, but significantly decreased in the high-dose aspirin
treatment group, also resulting in the activation of the AKT/mTOR
signaling pathway.
In the present study, a model of PE in C57/BL mice
was established. Subsequently, the blood pressure and proteinuria
concentrations were measured to confirm successful establishment of
the model. The expression levels and concentrations of
antioxidative stress enzymes in the placental tissues and
circulating serum samples were determined by performing western
blotting and ELISAs, respectively. The results indicated that,
compared with the NC group, the expression of antioxidative stress
enzymes was decreased in the PE model and further decreased after
high-dose aspirin treatment, but these PE-induced effects were
partially reversed after low-dose aspirin treatment. Furthermore,
the results suggested that aspirin-induced effects might be
mediated via the PI3K/AKT/mTOR signaling pathway and regulation of
ROS production. Therefore, low-dose aspirin administration may
serve as a therapeutic strategy for PE.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WX designed the study and drafted and revised the
manuscript. YW and XZ collected, analyzed and interpreted data. WX
and XZ confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Chengdu Women's and Children's Central Hospital
(approval no. SCXK 2009-0017; Chengdu, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
No authors listed. Hypertension in
pregnancy. Report of the American College of Obstetricians and
Gynecologists' Task Force on Hypertension in Pregnancy. Obstet
Gynecol. 122:1122–1131. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Abalos E, Cuesta C, Grosso AL, Chou D and
Say L: Global and regional estimates of preeclampsia and eclampsia:
A systematic review. Eur J Obstet Gynecol Reprod Biol. 170:1–7.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kuklina EV, Ayala C and Callaghan WM:
Hypertensive disorders and severe obstetric morbidity in the United
States. Obstet Gynecol. 113:1299–1306. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Young J: The AEtiology of eclampsia and
albuminuria and their relation to accidental haemorrhage: (An
anatomical and experimental investigation). Proc R Soc Med.
7:307–348. 1914.PubMed/NCBI
|
|
5
|
Phipps E, Prasanna D, Brima W and Jim B:
Preeclampsia: Updates in pathogenesis, definitions, and guidelines.
Clin J Am Soc Nephrol. 11:1102–1113. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Crocker IP, Tansinda DM and Baker PN:
Altered cell kinetics in cultured placental villous explants in
pregnancies complicated by pre-eclampsia and intrauterine growth
restriction. J Pathol. 204:11–18. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pereira RD, De Long NE and Wang RC:
Angiogenesis in the placenta: The role of reactive oxygen species
signaling. Biomed Res Int. 2015(814543)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang Z, Wei C, Zhou Y, Yan T, Wang Z, Li
W and Zhao L: Homocysteine induces apoptosis of human umbilical
vein endothelial cells via mitochondrial dysfunction and
endoplasmic reticulum stress. Oxid Med Cell Longev.
2017(5736506)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wu F, Tian FJ and Lin Y: Oxidative stress
in placenta: Health and diseases. Biomed Res Int.
2015(293271)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vane JR and Botting RM: The mechanism of
action of aspirin. Thromb Res. 110:255–258. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schrottmaier WC, Kral JB, Badrnya S and
Assinger A: Aspirin and P2Y12 inhibitors in platelet-mediated
activation of neutrophils and monocytes. Thromb Haemost.
114:478–489. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Muzaffar S, Shukla N, Massey Y, Angelini
GD and Jeremy JY: NADPH oxidase 1 mediates upregulation of
thromboxane A2 synthase in human vascular smooth muscle cells:
Inhibition with iloprost. Eur J Pharmacol. 658:187–192.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Henderson JT, Whitlock EP, O'Connor E,
Senger CA, Thompson JH and Rowland MG: Low-dose aspirin for
prevention of morbidity and mortality from preeclampsia: A
systematic evidence review for the U.S. Preventive Services Task
Force. Ann Intern Med. 160:695–703. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Rey E and Rivard GE: Is testing for
aspirin response worthwhile in high-risk pregnancy? Eur J Obstet
Gynecol Reprod Biol. 157:38–42. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
National Research Council; Division on
Earth and Life Studies; Institute for Laboratory Animal Research;
Committee for the Update of the Guide for the Care and Use of
Laboratory Animals Washington, D.C: National Academies Press, Guide
for the Care and Use of Laboratory Animals: Eighth edition-Thai
version, 2011.
|
|
16
|
Chaiworapongsa T, Chaemsaithong P, Yeo L
and Romero R: Pre-eclampsia part 1: Current understanding of its
pathophysiology. Nat Rev Nephrol. 10:466–480. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wallis AB, Saftlas AF and Hsia J: Secular
trends in the rates of preeclampsia, eclampsia, and gestational
hypertension, United States, 1987-2004. Am J Hypertens. 21:521–526.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bussolino F, Benedetto C and Massobrio M:
Maternal vascular prostacyclin activity in pre-eclampsia. Lancet.
2(702)1980.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Broughton Pipkin F, Crowther C, de Swiet
M, Duley L, Judd A, Lilford RJ, Onwude J, Prentice C, Redman CW,
Roberts J, et al: Where next for prophylaxis against pre-eclampsia?
Br J Obstet Gynaecol. 103:603–607. 1996.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Burton GJ and Jauniaux E: Oxidative
stress. Best Pract Res Clin Obstet Gynaecol. 25:287–299.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mikhail MS, Anyaegbunam A and Garfinkel D:
Preeclampsia and antioxidant nutrients: decreased plasma levels of
reduced ascorbic acid, alpha-tocopherol, and beta-carotene in women
with preeclampsia. Am J Obstet Gynecol. 171:150–157.
1994.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rocha S, Gomes D, Lima M, Bronze-da-Rocha
E and Santos-Silva A: Peroxiredoxin 2, glutathione peroxidase, and
catalase in the cytosol and membrane of erythrocytes under
H2O2-induced oxidative stress. Free Radic
Res. 49:990–1003. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Putnam CD, Arvai AS, Bourne Y and Tainer
JA: Active and inhibited human catalase structures: Ligand and
NADPH binding and catalytic mechanism. J Mol Biol. 296:295–309.
2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mahmood DF, Abderrazak A, El Hadri K,
Simmet T and Rouis M: The thioredoxin system as a therapeutic
target in human health and disease. Antioxid Redox Signal.
19:1266–1303. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rhee SG, Chae HZ and Kim K:
Peroxiredoxins: A historical overview and speculative preview of
novel mechanisms and emerging concepts in cell signaling. Free
Radic Biol Med. 38:1543–1552. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mustacich D and Powis G: Thioredoxin
reductase. Biochem J. 346:1–8. 2000.PubMed/NCBI
|
|
27
|
Rhee SG, Yang KS, Kang SW, Woo HA and
Chang TS: Controlled elimination of intracellular H(2)O(2):
Regulation of peroxiredoxin, catalase, and glutathione peroxidase
via post-translational modification. Antioxid Redox Signal.
7:619–626. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fischer LR, Igoudjil A, Magrané J, Li Y,
Hansen JM, Manfredi G and Glass JD: SOD1 targeted to the
mitochondrial intermembrane space prevents motor neuropathy in the
Sod1 knockout mouse. Brain. 134:196–209. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pizzino G, Irrera N, Cucinotta M, Pallio
G, Mannino F, Arcoraci V, Squadrito F, Altavilla D and Bitto A:
Oxidative stress: Harms and benefits for human health. Oxid Med
Cell Longev. 2017(8416763)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Travaglino A, Raffone A, Saccone G,
Migliorini S, Maruotti GM, Esposito G, Mollo A, Martinelli P, Zullo
F and D'Armiento M: Placental morphology, apoptosis, angiogenesis
and epithelial mechanisms in early-onset preeclampsia. Eur J Obstet
Gynecol Reprod Biol. 234:200–206. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Keum D, Kruse M, Kim DI, Hille B and Suh
BC: Phosphoinositide 5- and 3-phosphatase activities of a
voltage-sensing phosphatase in living cells show identical voltage
dependence. Proc Natl Acad Sci USA. 113:E3686–E3695.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fruman DA, Chiu H, Hopkins BD, Bagrodia S,
Cantley LC and Abraham RT: The PI3K pathway in human disease. Cell.
170:605–635. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li C, Li Y, He L, Agarwal AR, Zeng N,
Cadenas E and Stiles BL: PI3K/AKT signaling regulates bioenergetics
in immortalized hepatocytes. Free Radic Biol Med. 60:29–40.
2013.PubMed/NCBI View Article : Google Scholar
|