Introduction
The human endometrium (E) is a dynamic remodeling
tissue, which in response to the prevailing steroid environment of
sequential ovarian estrogen and progesterone exposure, undergoes
>400 cycles of regeneration, differentiation and shedding during
a woman's reproductive years (1,2).
Menstruation is the endometrial response to progesterone and
estrogen withdrawal that occurs with the decay of the corpus luteum
in the absence of pregnancy (1).
Chan et al (3) first
identified the endometrial epithelial and stromal cell clone
formation ability, suggesting that there are three types of cells
in the E: Epithelial, mesenchymal and endothelial cells. Smalley
and Clarke (4) observed that
endometrial epithelial cells had enhanced cloning activity during
the proliferative phase, and stromal cells had stronger activity
during the secretory phase.
Both epithelial and stromal cells are present in the
basal layer of the human E, and menstruation is formed after the
top two-thirds of the functional layer of the E (2). Therefore, there may be some
differences in biological characteristics between menstrual
blood-derived endometrial cells (MB-ECs) and E-derived ECs (E-ECs).
Ethical and practical considerations often limit the use of primary
human E-ECs for research purposes (5). The acquisition of MB is not invasive
and does not cause harm to the donor. MB-ECs isolated and cultured
in vitro demonstrated high proliferation potential and the
ability to differentiate into a variety of cells (6). Considering the easier access to MB,
ECs have become a convenient source of adult cells and are a
relevant cell source for the study of endometrial diseases
(7,8).
The present study compared MB-ECs and E-ECs to
investigate the isolation, identification, culture, expansion and
differentiation of ECs from two provenances, in order to identify
entry points and lay a foundation for the treatment of
gynecological diseases. The present study also provided important
experimental and theoretical guidance to obtain different ECs by
different means.
Materials and methods
Experimental materials
All samples were collected between March 2017 and
April 2017 in under a protocol approved by the institutional review
board of the Gynecology Center of The First Affiliated Hospital of
Xinjiang Medical University (Urumqi, China), and written informed
consent was obtained from each donor. Human endometrial tissue was
obtained from patients (Table I)
undergoing hysterectomy, and MB was obtained from healthy women.
The age range of the patients and healthy controls was 26-43 years.
The inclusion criteria were as follows: Childbearing age (30-45
years), benign gynecological diseases, no fertility requirements,
hysterectomy, no hormone treatment within 6 months, no or mild
anemia, and no liver, kidney, heart, brain or blood system
disorders. The exclusion criteria were as follows: Systemic or
reproductive malignant disease, endometrial or intrauterine
lesions, a history of hormone therapy within 3 months,
endometriosis and adenomyosis. The patients were matched according
to their age, menstrual history, fertility history, contraceptive
methods, uterine size, lack of hormone treatment within 3 months
and anemia. MB and endometrial tissues obtained from the resected
uterus were used as the research objects.
 | Table IInformation of samples of human
endometrium. |
Table I
Information of samples of human
endometrium.
| Patient no. | Age, years | Disease | Surgery or
operation check | Material
method | Sample
location |
|---|
| 1 | 36 | Uterine
fibroids | TCRM | Hysteroscope | Endometrium |
| 2 | 32 | Endometrial
polyps | TCRP | Hysteroscope | Endometrium |
| 3 | 43 | Abnormal uterine
bleeding associated with ovulatory dysfunction | TCRE | Hysteroscope | Endometrium |
| 4 | 42 | Ovarian cyst | Hysteroscopy | Hysteroscope | Endometrium |
| 5 | 33 | Ovarian serous
cystadenoma | Hysteroscopy | Hysteroscope | Endometrium |
| 6 | 26 | Endometrial
polyps | TCRP | Hysteroscope | Endometrium |
| 7 | 39 | Ovarian cyst | Hysteroscopy | Hysteroscope | Endometrium |
| 8 | 31 | Endometrial
polyps | TCRP | Hysteroscope | Endometrium |
| 9 | 38 | Pelvic inflammatory
disease, hydrosalpinx | Hysteroscopy | Hysteroscope | Endometrium |
Isolation and culture of MB-ECs and
E-ECs
For MB-EC isolation, 5 ml of MB samples from healthy
women were collected, and 0.2 ml amphotericin B (cat. no.
15290026), 0.2 ml penicillin-streptomycin (cat. no. 15140122), 0.1
ml Na2EDTA (cat. no. 15576028) and 9.5 ml PBS (cat. no.
20012050; all from Gibco; Thermo Fisher Scientific, Inc.) were
added; this mixture was transferred to a laboratory at 4˚C. The
cells were separated via centrifugation with 1.077 g/ml Ficoll
(cat. no. P8900; Beijing Solarbio Science & Technology Co.,
Ltd.) at 500 x g for 30 min at room temperature, and the middle
white membrane layer was collected. The separated cells were washed
twice with PBS and cultured in a 60-mm culture dish (cat. no.
430166; Corning Life Sciences) using complete DMEM/F12 (cat. no.
11320082; Gibco; Thermo Fisher Scientific, Inc.) + 10% FBS (cat.
no. FND500, Shanghai ExCell Biology, Inc.) + 1%
penicillin-streptomycin, followed by incubation at 37˚C with 5%
CO2. Cells were washed twice with PBS to remove
non-adherent cells after 3 days.
For E-EC isolation, the surgically removed human
endometrial tissues were washed in PBS to remove excess tissue and
blood. The endometrial tissues were cut into 1-mm3
pieces, then dissociated into single-cell suspensions using 200
µg/ml collagenase type IV (cat. no. C5138; Sigma-Aldrich; Merck
KGaA) in DMEM/F12 with 10% FBS and mechanical methods for 1-2 h at
37˚C. The undigested endometrial tissues were incubated with 0.25%
trypsin-EDTA (cat. no. 25200056, Gibco; Thermo Fisher Scientific,
Inc.) for 15 min at 37˚C. Single-cell suspensions were collected
using a cell strainer, then centrifuged at 200 x g for 8 min at
room temperature. The bottom cells were resuspended in complete
medium, plated into a 60-mm culture dish and incubated at 37˚C in
5% CO2. Cells were washed twice with PBS to remove
non-adherent cells after 24 h.
When cultures reached 90% confluence, cells were
digested with warmed 0.25% trypsin-EDTA at 37˚C for 3 min, then
subcultured onto fresh culture plates. To purify the cells, the
isolated cells were screened using different time digestion
methods. The cells were digested with warmed 0.25% trypsin-EDTA at
37˚C for 1-2 min, then digestion was stopped and the digested cells
were removed. Subsequently, the remaining cells in the plate were
digested again and cultured. This step was repeated between three
and five times.
Proliferation kinetics
Cells from passage 5 were used to analyze
proliferation kinetics. MB-ECs and E-ECs were seeded in 24-well
plates (cat. no. 3524; Corning Life Sciences) at a density of
1x104 cells/well. The cells from three random wells were
counted each day for 7 days. Proliferation curves were plotted
according to mean values. The population doubling time (PDT) was
calculated as follows:
PDT=(t-t0)lg2/(lgNt-lgN0), where
t0 is the start time of the logarithmic proliferation
period, t is the termination time of the logarithmic proliferation
period, N0 is the initial number of cells in the
logarithmic proliferation period and Nt the final number
of cells in the logarithmic proliferation period.
In vitro scratch assay
MB-ECs and E-ECs were seeded and grown to confluence
in a 6-well plate (cat. no. 3516; Corning Life Sciences). Both cell
types were starved for 12 h in DMEM/F12. The scratch was performed
with a pipette tip running through the dish. The plates were washed
twice with fresh medium to remove non-adherent cells, and the cells
were subsequently cultured in DMEM/F12 at 37˚C for 48 h. Cell
migration was evaluated by measuring the wound area under a light
microscope.
Epithelial-mesenchymal
transdifferentiation (EMT) assay in vitro
MB-ECs and E-ECs were seeded and grown to confluence
in a six-well plate. All cells were cultured for 24 h in DMEM/F12 +
10% FBS. The medium was changed to DMEM/F12 + 10% FBS + 3 ng/ml
TGF-β1 (dissolved in ddH2O; cat. no. 96-100-21;
PeproTech, Inc.). The cells were cultured at 37˚C for 6, 12 or 24
h. As a control group, cells were cultured in DMEM/F12 + 10%
FBS.
RNA extraction and reverse
transcription-quantitative PCR assay (qPCR)
Total cellular RNA was isolated using
TRIzol® Reagent (cat. no. 15596026; Invitrogen; Thermo
Fisher Scientific, Inc.) with genomic DNA removed. RNA quality was
assessed by spectrophotometry. RNA (1-µg aliquots) was
reverse-transcribed into complementary DNA (cDNA) using a RevertAid
First Strand cDNA Synthesis kit (cat. no. K1621; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
cDNA was amplified using a Taq PCR Master Mix kit (cat. no. KT201;
Tiangen Biotech Co., Ltd.) in a PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The following thermocycling
conditions were used for PCR (total volume per reaction, 10 µl):
Initial denaturation at 95˚C for 5 min; 30 cycles of 95˚C for 30
sec, 60˚C for 30 sec and 72˚C for 30 sec; and final extension at
72˚C for 10 min. The reaction products were analyzed using 2.0%
agarose gel electrophoresis and GelRed (cat. no. 41003; Biotium,
Inc.). The sequence of each product was confirmed using DNA marker
I ladder (cat. no. MD101; Tiangen Biotech Co., Ltd.).
qPCR was performed in duplicates using
SYBR® Premix Ex Taq™ (cat. no. RR420A; Takara
Biotechnology Co., Ltd.) and performed using a 7500 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR
reactions of 20 µl for each sample consisted of cDNA (200 µg cDNA
after dilution), 2X SYBR Premix Ex Taq, 50X ROX Reference Dye,
dH2O and 10 µM of each gene-specific primer. Primer
sequences are shown in Table II.
The following thermocycling conditions were used for qPCR: Initial
denaturation at 95˚C for 30 sec; 40 cycles of 95˚C for 5 sec, 60˚C
for 30 sec and 72˚C for 30 sec; and final extension at 72˚C for 10
min. Relative mRNA abundance was calculated using the
2-ΔΔCq method (9) and
normalized to the expression levels of GAPDH. The primer pairs used
for qPCR are listed in Table
II.
 | Table IIPrimer sequences used for reverse
transcription-quantitative PCR. |
Table II
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Primer
sequence | Melting
temperature, ˚C | Product length,
bp |
|---|
| Vim | F:
5'-GGACCAGCTAACCAACGACA-3' | 60.0 | 116 |
| | R:
5'-CGGCTTCCTCTCTCTGAAGC-3' | 60.2 | |
| CK18 | F:
5'-CCTACAAGCCCAGATTGCCA-3' | 60.0 | 115 |
| | R:
5'-CCGAGCCAGCTCGTCATATT-3' | 60.0 | |
| GAPDH | F:
5'-TATGACAACAGCCTCAAGAT-3' | 54.1 | 104 |
| | R:
5'-AGTCCTTCCACGATACCA-3' | 54.4 | |
Flow cytometry
Following digestion into single cells with warmed
0.25% trypsin-EDTA, MB-ECs and E-ECs were incubated with
anti-CD90-PE (1:200; cat. no. bs-10430R-PE; BIOSS), anti-CD34-PE
(1:200; cat. no. bs-0646R-PE; BIOSS), unconjugated antibodies or
matched-isotype control IgG (1:200; cat. no. bs-0295P-PE; BIOSS)
for 1 h at room temperature and analyzed via flow cytometry using a
BD Accuri™ C6 flow cytometer (BD Biosciences). Data were
analyzed using CFlow Plus software (v1.0.264.15; Accuri Cytometers,
Inc.). Events with forward scatter (FSC) <12,500,000 and side
scatter (SSC) <5,000,000 were gated in P1, and the events in P1
with FSC >2,000,000 and SSC >100,000 were gated in P2 to
remove large cell adhesion bodies and small cell debris.
Cellular immunofluorescence assay
Cultures of MB-ECs and E-ECs at passage 3 were
seeded (0.5x105 cells/1-cm2 glass coverslip)
on glass coverslips coated with poly-L-lysine. For
immunofluorescent assay analysis, the MB-ECs and E-ECs were fixed
with 4% paraformaldehyde for 15 min at room temperature and washed
three times (5 min/wash) with PBS. For vimentin (Vim) staining,
cells were permeabilized with 0.25% Triton X-100 (cat. no. X100;
Sigma-Aldrich; Merck KGaA) for 10 min. Cells were washed three
times (5 min/wash) with PBS, then blocked with 10% goat serum (cat.
no. ZLI-9021; OriGene Technologies, Inc.) for 1 h at room
temperature prior to incubation with primary antibodies. The cells
were incubated with the following primary antibodies: Anti-Vim
(1:250; cat. no. ab92547; Abcam) and anti-cytokeratin 18 (CK18;
1:250; cat. no. ab133263; Abcam) at 4˚C overnight. Cells were
washed three times (5 min per wash) with PBS, followed by
incubation with Alexa Fluor® 594-conjugated goat
anti-rabbit secondary antibody (1:100; cat. no. ZF-0516; OriGene
Technologies, Inc.) in the dark for 1 h at room temperature.
Finally, 10 µg/ml of DAPI (cat. no. D9542; Sigma-Aldrich; Merck
KGaA) were used to label cell nuclei for 15 min, and images were
captured using a fluorescence microscope (TE-2000-E; Nikon
Corporation). PBS was used as a substitute for primary antibodies
as a technical control.
Western blotting
Cells were prepared and lysed in ice-cold RIPA
buffer (cat. no. 89900; Thermo Fisher Scientific, Inc.) containing
freshly added Halt Phosphatase Inhibitor Cocktail and Protease
Inhibitor Cocktail, and protein concentrations were determined
using the Bradford protein assay (Bio-Rad Laboratories, Inc.).
Samples of 20 µg proteins were separated via SDS-PAGE on a 12% gel
(Bio-Rad Laboratories, Inc.) and proteins were immunoblotted onto
nitrocellulose membranes. The membranes were blocked in 5% non-fat
milk for 1 h at room temperature and probed with primary antibodies
[anti-β-actin (1:1,000; cat. no. 60008-1-Ig; ProteinTech Group,
Inc.), anti-Vim (1:1,000; cat. no. ab92547; Abcam) or anti-CK18
(1:1,000; cat. no. ab133263; Abcam)] at 4˚C overnight, followed by
HRP-conjugated secondary antibodies [HRP-conjugated Affinipure Goat
Anti-Mouse IgG (H + L) (cat. no. SA00001-1; 1:2,500; ProteinTech
Group, Inc.) and HRP-conjugated Affinipure Goat Anti-Rabbit IgG (H
+ L) (cat. no. SA00001-2; 1:2,500; ProteinTech Group, Inc.)] for 2
h at room temperature. The blots were visualized using Immobilon
Western Chemiluminescent HRP Substrate (cat. no. WBKLS0500;
MilliporeSigma), and images were captured using the ECL Tanon 5500
system (Tanon Science and Technology Co., Ltd.). Protein expression
was normalized to the expression levels of β-actin.
Statistical analysis
Data are presented as the mean ± SEM for n=3
samples. Statistical differences between two groups were analyzed
using Student's t-test (two-tailed unpaired). Comparisons between
multiple groups were performed using one-way ANOVA followed by
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Isolation, culture and morphology of
MB-ECs and E-ECs
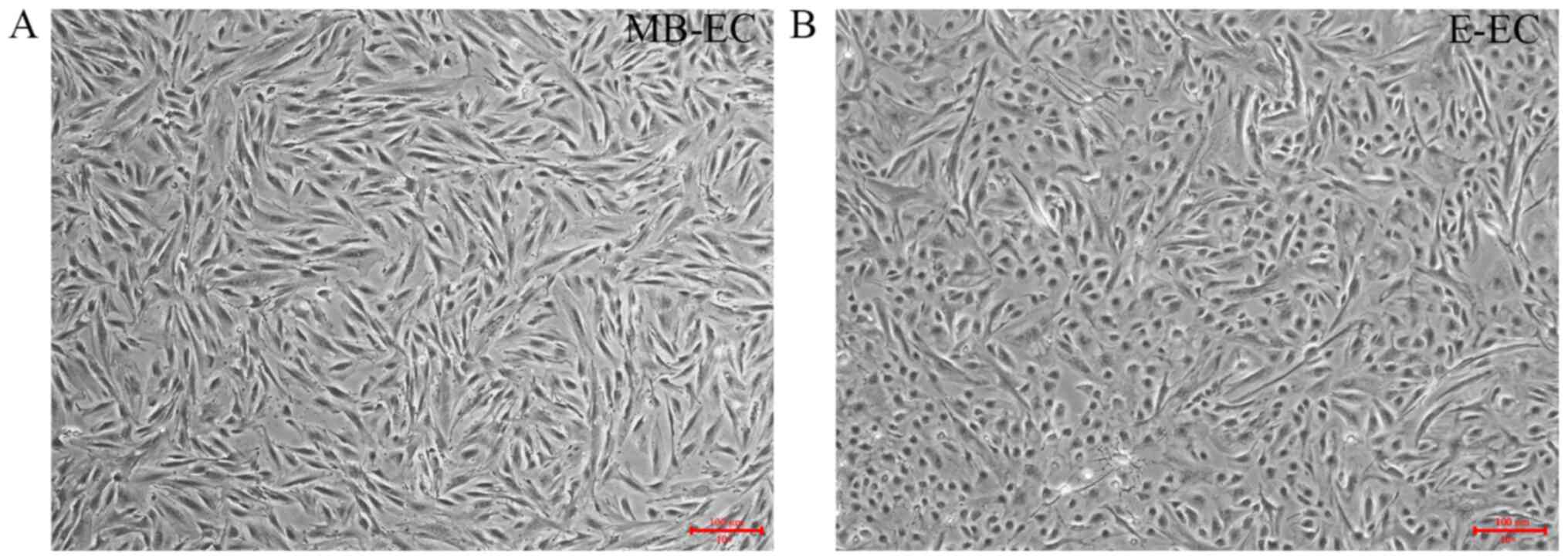
MB-ECs were isolated from MB by density gradient
centrifugation. MB-ECs demonstrated a flat two-dimensional
morphology after three generations of subculture (Fig. 1A). E-ECs were isolated from
endometrial tissue by enzymatic digestion. E-ECs demonstrated a
round and flattened shape morphology after three generations of
subculture (Fig. 1B), distinct from
the MB-EC cell morphology of small spindle-like cells (Fig. 1A).
Characterization of MB-ECs and
E-ECs
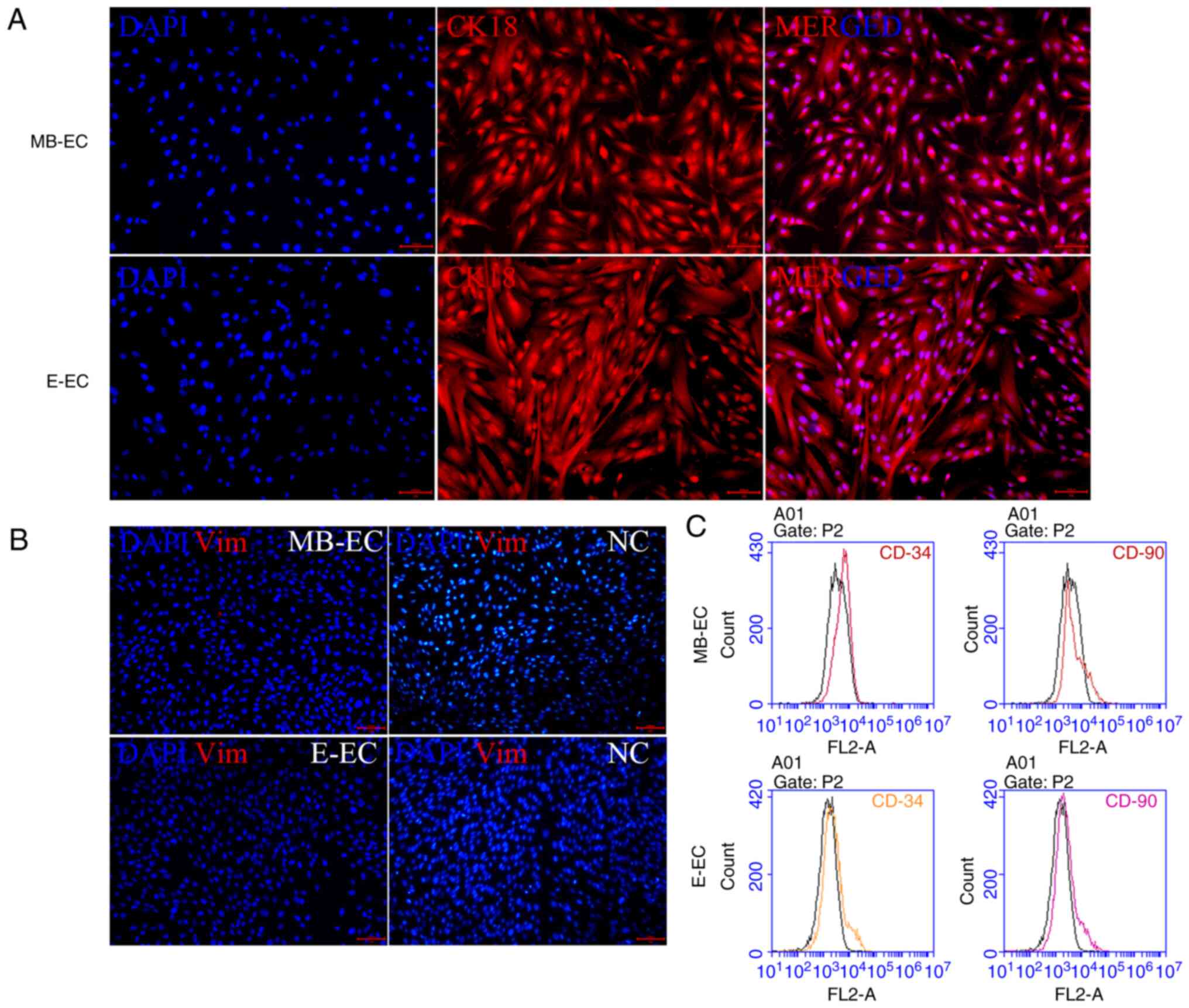
In order to confirm that the isolated and cultured
cells were not mesenchymal stem cells or hematopoietic stem cells,
the cell expression of CK18 (a marker of epithelial cells), Vim (a
marker of mesenchymal stem cells), CD90 (a marker of mesenchymal
stem cells) and CD34 (a marker for hematopoietic stem cells) was
quantified (10,11). Specific markers of MB-ECs and E-ECs
were detected via immunofluorescence and flow cytometry.
Immunofluorescence assays indicated that MB-ECs and E-ECs both
expressed CK18 (Fig. 2A), but
neither expressed Vim (Fig. 2B).
Flow cytometry assays demonstrated that neither MB-ECs nor E-ECs
expressed CD90 and CD34 (Fig.
2C).
Cell proliferation and migration
capacity assays
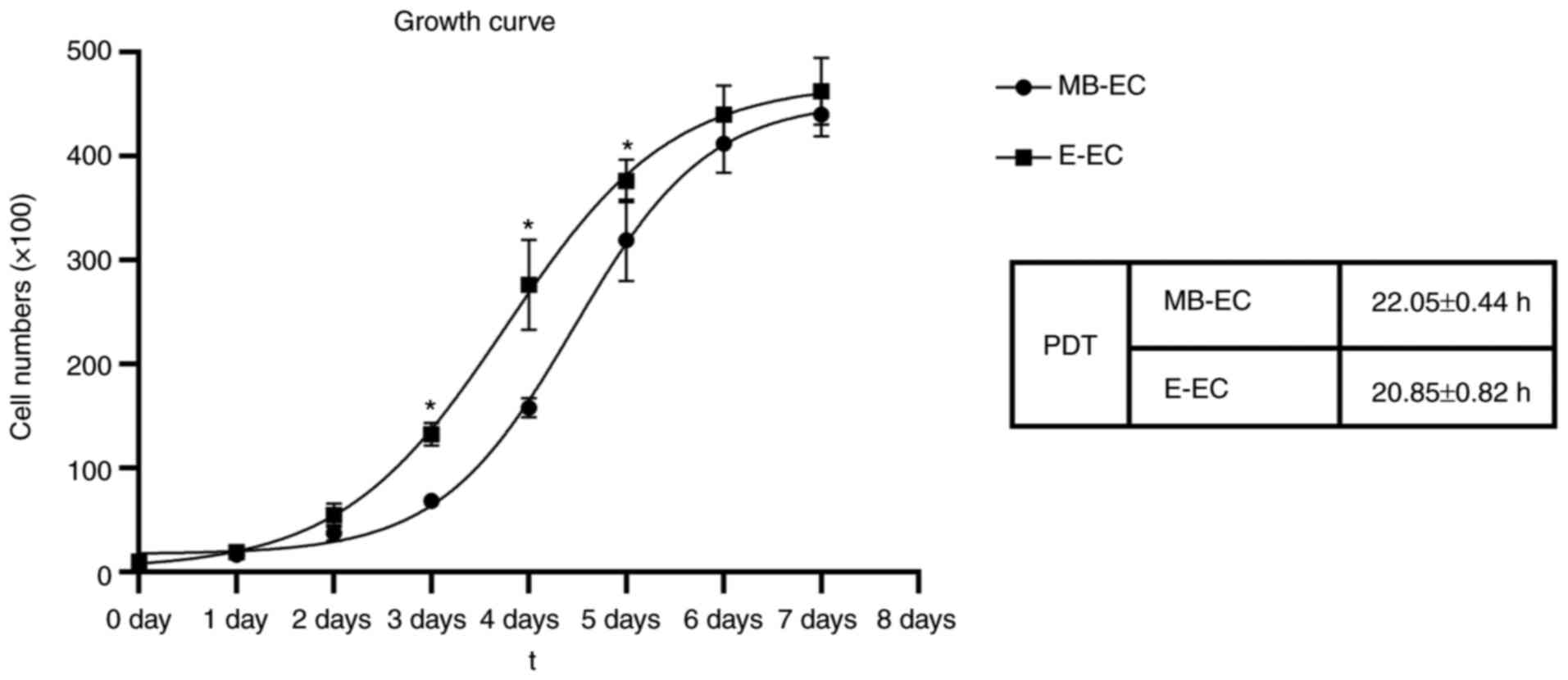
The proliferation curves of both MB-ECs and E-ECs
appeared as typically sigmoidal, which consisted of a latent phase,
a logarithmic phase and a plateau phase (Fig. 3). The PDT of MB-ECs was 22.05±0.44 h
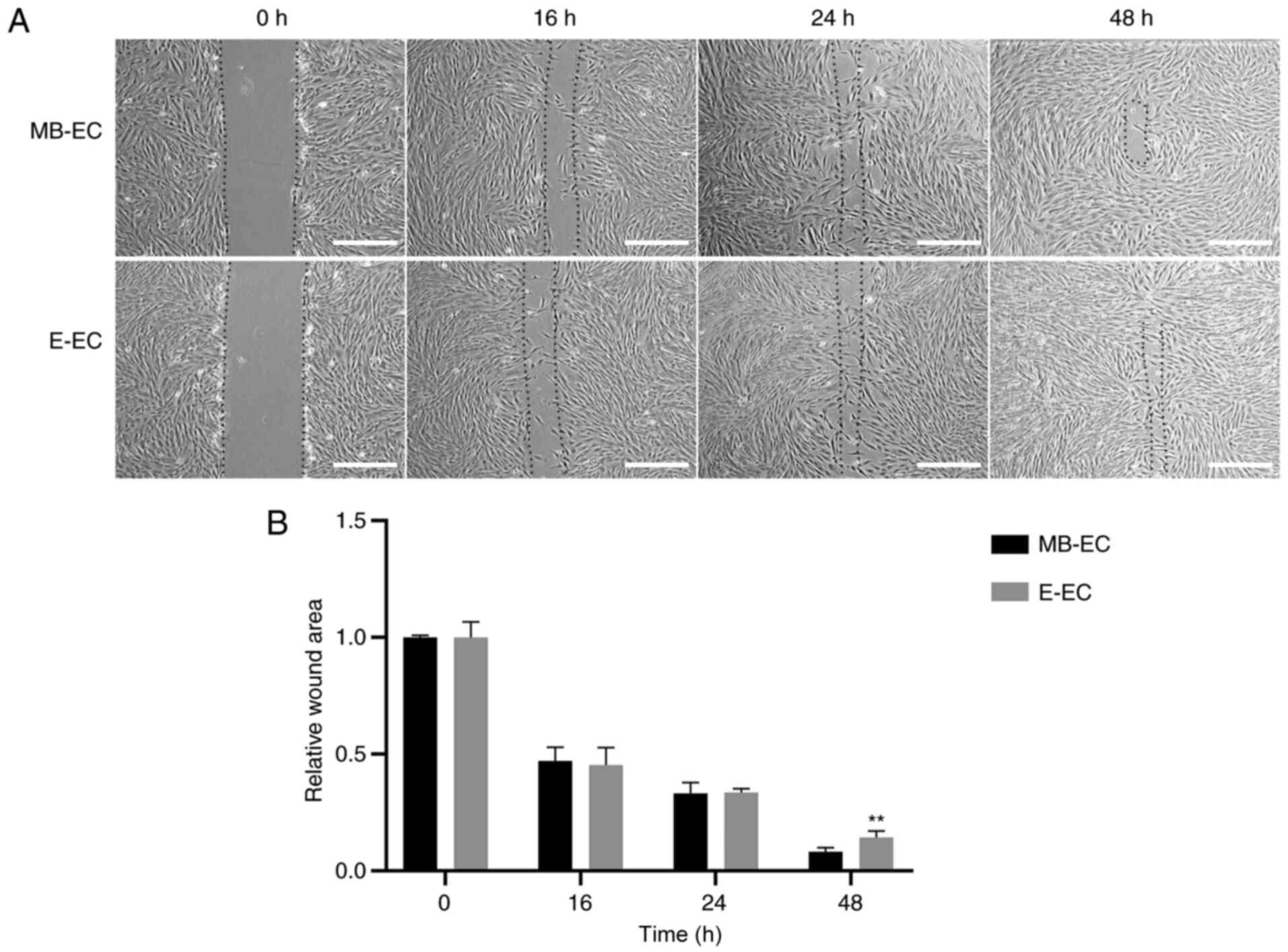
and that of E-ECs was 20.85±0.82 h (Fig. 3; P<0.05). The migration capacity
assay results indicated that the wounds in confluent MB-ECs and
E-ECs healed after 48 h, but the wound area of MB-ECs was
significantly smaller than that of E-ECs at 48 h (P<0.01;
Fig. 4).
TGF-β1 effect on EMT gene
expression
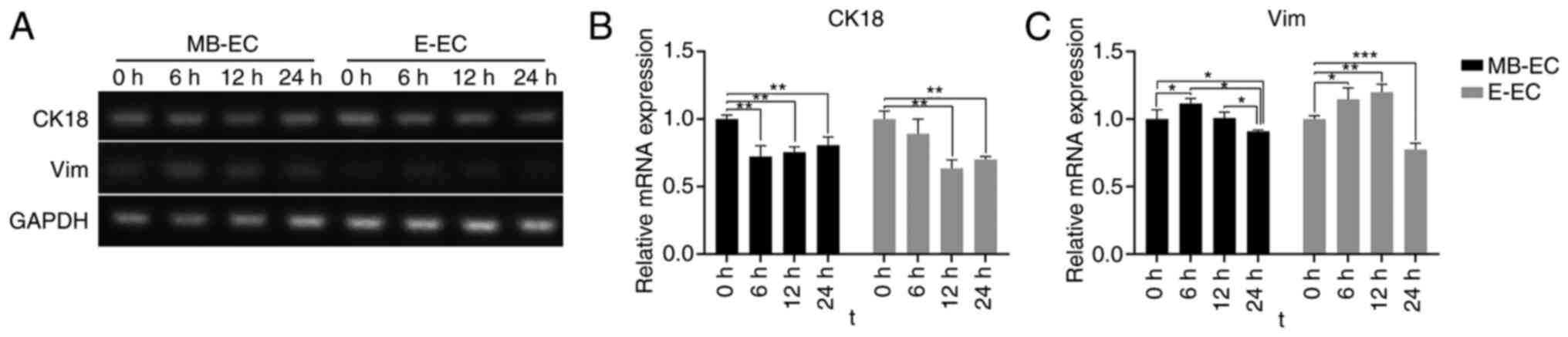
Following TGF-β1 treatment, CK18 mRNA expression in
MB-ECs and E-ECs decreased, and Vim mRNA expression in MB-ECs and
E-ECs increased. The CK18 mRNA expression level in MB-ECs decreased
significantly after 6 h (P<0.01 vs. 0 h) and in E-ECs after 12 h
(P<0.01 vs. 0 h; Fig. 5A). The
CK18 mRNA expression level gradually increased but remained
significantly lower than its initial level after 24 h (P<0.01
vs. 0 h; Fig. 5A and B). Vim mRNA expression level in MB-ECs and
E-ECs significantly increased after 6 h (P<0.05 vs. 0 h;
Fig. 5A and C), but was significantly lower than its
initial level after 24 h (MB-ECs, P<0.05 vs. 0 h; E-ECs,
P<0.001 vs. 0 h; Fig. 5A and
C).
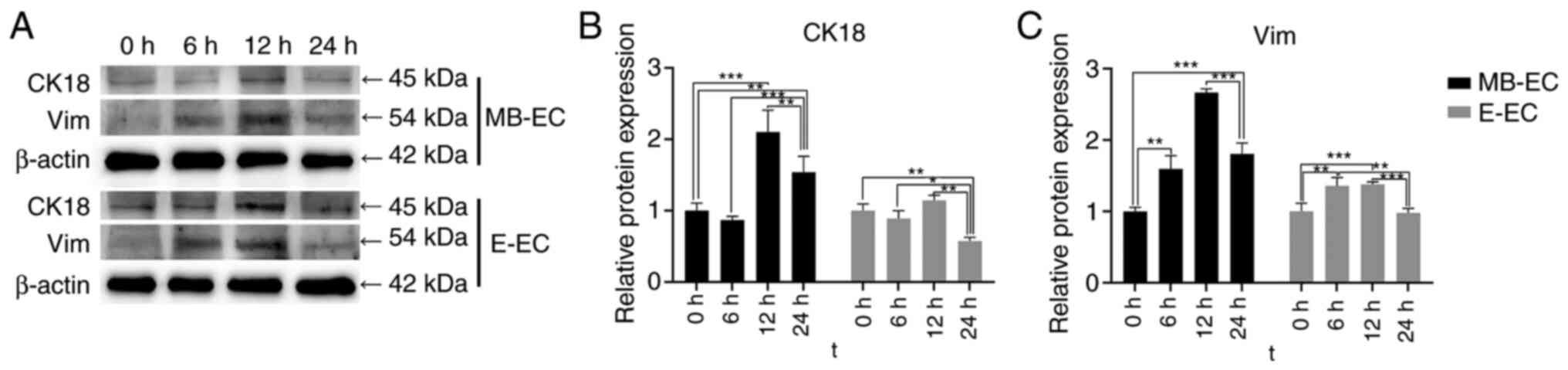
TGF-β1 effect on EMT protein
expression
Following TGF-β1 treatment, CK18 protein expression
in E-ECs significantly decreased at 24 h (P<0.01 vs. 0 h), but
significantly increased in MB-ECs at 12 h (P<0.001 vs. 0 h) and
24 h (P<0.01 vs. 0 h; Fig. 6A
and B). Vim protein expression in
MB-ECs was significantly upregulated at 6 h (P<0.01 vs. 0 h),
reached its highest level at 12 h (P<0.001 vs. 0 h), and then
decreased slightly at 24 h, while significantly higher than that at
0 h (P<0.001 vs. 0 h; Fig. 6A
and C). Vim protein expression in
E-ECs was significantly upregulated at 6 and 12 h (P<0.05 vs. 0
h), then decreased to initial levels at 24 h (Fig. 6A and C).
Discussion
Endometrial epithelial cells are a functional layer
of the uterine surface and play an important role in the
implantation of fertilized eggs (12). Wounding or bacterial infection may
result in severe uterine diseases and even infertility (8). Therefore, studying endometrial
epithelial cell biological function is of great relevance and
provides a scientific basis for the potential treatment of
endometrial epithelial defects (13). MB-ECs and E-ECs are two types of
endometrial cells at different developmental stages. MB, the top
two-thirds of the functional layer of the E, is shed during
menstruation, and contains cells (a large number of epithelial and
stromal cells) and tissues from the functional layer of the E
(14,15). This course of events is the process
of E-ECs forming MB-ECs. Therefore, it was hypothesized that MB-ECs
and E-ECs had different morphological and biological
characteristics. As it requires surgery to be sampled, endometrial
tissue is difficult to obtain from healthy women. Although
endometrial tissues were obtained from patients undergoing
hysterectomy and MB from healthy women, the biological
characteristics of the two cell types were comparable. Isolated
MB-ECs and E-ECs expressed CK18 and not Vim, CD34 (a surface marker
of hematopoietic stem cells) (10)
nor CD90 (a surface marker of mesenchymal cells) (11). The two cell types demonstrated
different cell morphology, proliferation and migration ability.
MB-ECs exhibited a small, flat, spindle-like cell morphology
(15), and E-ECs appeared rounder
and flattened cells.
Cell proliferation is closely related to the cell
development stage. A previous study indicated that the development
of the terminal end of the cells and cell cycle exit substantially
reduced their proliferation ability (8). MB-ECs were derived from the
development of the terminal end of E-ECs, and the PDT of MB-ECs was
greater than that of E-ECs. Cell migration happens throughout life
and is a coordinated physiological process used by normal cells
during embryonic morphogenesis, wound healing and cell transport,
while tumor cells spread within tissues (16-18).
The in vitro scratch assay is a simple, economical and
well-developed method that mimics the migration of cells in
vivo to study cell migration in vitro (19). The present study demonstrated that
MB-ECs had a higher migration ability than E-ECs. Cell movement is
a complex process involving a number of steps, including the
disruption of cell-cell junctions, cytoskeletal rearrangements and
constant remodeling of adhesive contacts with the extracellular
matrix (18). A previous study
indicated that, at the end of the menstrual cycle, with withdrawal
of steroid hormone support, the activity of matrix-degrading
enzymes induced endometrial destruction (20), which may lead to enhanced cell
migration. Implantation of the human embryo into the uterine wall
during the early stages involves embryo apposition and adhesion to
the endometrial epithelium, followed by penetration through the
epithelium and invasion of the embryonic trophoblast through the
endometrial stroma (21). Estradiol
administration has been previously demonstrated to have a marked
effect on migration kinetics, induced non-dividing gland cells to
enter the cell cycle and decreased the loss of epithelial cells
(22). Decreased cell death in the
mouse uterine epithelium has been observed after repeated estrogen
administration (23). However, in
intact rabbits with induced ovulation, the uterine epithelium is
desensitized to estrogens (22).
MB-ECs and E-ECs are two cell types at different developmental
stages; MB-ECs are derived from the development of the terminal end
of E-ECs (24). In the present
study, MB-ECs were isolated from menstrual blood, and E-ECs were
isolated from endometrial tissue. The cell proliferation curve and
migration ability in MB-ECs and E-ECs were found to be
different.
EMT is an organized process in which epithelial
cells are induced to differentiate into a mesenchymal phenotype,
and has been previously recognized in developmental biology as a
means to achieve morphogenetic change (25). The maternal endometrial epithelium
undergoes EMT-related changes during the embryo implantation period
(26). Molecular signals from
within the maternal E regulate the EMT process; a number of these
signals have been implicated in embryo implantation failure
(26). Human endometrial stromal
cells can maintain endometrial homeostasis and play a critical role
in repairing endometrial injury, and mesenchymal cells can increase
the proliferation of damaged endometrial stromal cells (27). A previous study indicated that
TGF-β1 mRNA and protein expression increased around menstruation to
contribute to tissue repair following endometrial shedding
(28). TGF-β1 is secreted
abundantly in the E, specifically in patients with endometriosis
(29,30). TGF-β1 not only induces apoptosis but
also simultaneously induces EMT (31), and endometrial epithelial cells can
induce EMT through TGF-β1(32). The
acquisition of mesenchymal markers (such as N-cadherin and Vim) and
the loss of epithelial markers (such as E-cadherin) are hallmarks
of EMT in endometriosis (33) and
adenomyosis (34). In addition,
TGF-β1-induced EMT was previously demonstrated to lead to the
migration and invasion of local epithelial cells (31). The present results indicated that
MB-ECs and E-ECs may induce EMT through TGF-β1; CK18 gene
expression was downregulated and Vim expression upregulated. A
previous study indicated that TGF-β1 treatment induced EMT in a
dose- and time-dependent manner (31). CK18 and Vim expression returned to
their initial levels after 24 h, which may indicate that TGF-β1 was
absorbed and removed by the cells.
In conclusion, the present study isolated human
MB-ECs and E-ECs, which demonstrated differences in morphology and
cell biology; however, both cell types could exhibit EMT via TGF-β1
induction in vitro. Therefore, MB-ECs could be used in place
of E-ECs for cell biology research and potential bioengineering
applications. MB-ECs were isolated from menstrual blood, a
convenient source with no harm to the human body. E-ECs were
isolated from endometrial tissue, which is an inconvenient source
that damages women's uterine tissue. Although some differences were
noticed between the two sources of endometrial cells (such as PDT
and migration ability), their functions were found to be
similar.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of The Xinjiang Uygur Autonomous Region funded projects
(grant no. 2016D01C280).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and YD conceived and designed the experiments of
the current study. MJ and ZC performed the experiments. LZ and MJ
wrote the manuscript. MJ and LY analyzed the data. LZ and MJ
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All samples were collected under a protocol approved
by the institutional review board of the Gynecology Center of The
First Affiliated Hospital of Xinjiang Medical University (China),
and written informed consent was obtained from each donor.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jabbour HN, Kelly RW, Fraser HM and
Critchley HO: Endocrine regulation of menstruation. Endocr Rev.
27:17–46. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gargett CE, Schwab KE, Zillwood RM, Nguyen
HP and Wu D: Isolation and culture of epithelial progenitors and
mesenchymal stem cells from human endometrium. Biol Reprod.
80:1136–1145. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chan RW, Schwab KE and Gargett CE:
Clonogenicity of human endometrial epithelial and stromal cells.
Biol Reprod. 70:1738–1750. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Smalley MJ and Clarke RB: The mammary
gland ‘side population’: A putative stem/progenitor cell marker? J
Mammary Gland Biol Neoplasia. 10:37–47. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hennes A, Held K, Boretto M, De Clercq K,
Van den Eynde C, Vanhie A, Van Ranst N, Benoit M, Luyten C, Peeraer
K, et al: Functional expression of the mechanosensitive PIEZO1
channel in primary endometrial epithelial cells and endometrial
organoids. Sci Rep. 9(1779)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Meng X, Ichim TE, Zhong J, Rogers A, Yin
Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, et al: Endometrial
regenerative cells: A novel stem cell population. J Transl Med.
5(57)2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen L, Qu J, Cheng T, Chen X and Xiang C:
Menstrual blood-derived stem cells: Toward therapeutic mechanisms,
novel strategies, and future perspectives in the treatment of
diseases. Stem Cell Res Ther. 10(406)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hu J, Song K, Zhang J, Zhang Y and Tan BZ:
Effects of menstrual blood-derived stem cells on endometrial injury
repair. Mol Med Rep. 19:813–820. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Krause DS, Ito T, Fackler MJ, Smith OM,
Collector MI, Sharkis SJ and May WS: Characterization of murine
CD34, a marker for hematopoietic progenitor and stem cells. Blood.
84:691–701. 1994.PubMed/NCBI
|
|
11
|
L Ramos T, Sánchez-Abarca LI, Muntión S,
Preciado S, Puig N, López-Ruano G, Hernández-Hernández Á, Redondo
A, Ortega R, Rodríguez C, et al: MSC surface markers (CD44, CD73,
and CD90) can identify human MSC-derived extracellular vesicles by
conventional flow cytometry. Cell Commun Signal.
14(2)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Salamonsen LA, Evans J, Nguyen H and
Edgell TA: The microenvironment of human implantation: Determinant
of reproductive success. Am J Reprod Immunol. 75:218–225.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liang L, Wang L, Zhou S, Li J, Meng L,
Zhang H, Cui C and Zhang C: Exosomes derived from human umbilical
cord mesenchymal stem cells repair injured endometrial epithelial
cells. J Assist Reprod Genet. 37:395–403. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Padykula HA: Regeneration in the primate
uterus. In: Biology of the Uterus. Springer, pp279-288, 1989.
|
|
15
|
Toyoda M, Cui Ch and Umezawa A: Myogenic
transdifferentiation of menstrual blood-derived cells. Acta Myol.
26:176–178. 2007.PubMed/NCBI
|
|
16
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: Integrating signals from front to back. Science.
302:1704–1709. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Oh JM, Venters CC, Di C, Pinto AM, Wan L,
Younis I, Cai Z, Arai C, So BR, Duan J and Dreyfuss G: U1 snRNP
regulates cancer cell migration and invasion in vitro. Nat Commun.
11(1)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gentilini D, Busacca M, Di Francesco S,
Vignali M, Viganò P and Di Blasio AM: PI3K/Akt and ERK1/2
signalling pathways are involved in endometrial cell migration
induced by 17beta-estradiol and growth factors. Mol Hum Reprod.
13:317–322. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Salamonsen LA: Tissue injury and repair in
the female human reproductive tract. Reproduction. 125:301–311.
2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Grewal S, Carver JG, Ridley AJ and Mardon
HJ: Implantation of the human embryo requires Rac1-dependent
endometrial stromal cell migration. Proc Natl Acad Sci USA.
105:16189–16194. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Conti CJ, Gimenez-Conti IB, Conner EA,
Lehman JM and Gerschenson LE: Estrogen and progesterone regulation
of proliferation, migration, and loss in different target cells of
rabbit uterine epithelium. Endocrinology. 114:345–351.
1984.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Martin L, Pollard JW and Fagg B: Oestriol,
oestradiol-17beta and the proliferation and death of uterine cells.
J Endocrinol. 69:103–115. 1976.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kato K, Yoshimoto M, Kato K, Adachi S,
Yamayoshi A, Arima T, Asanoma K, Kyo S, Nakahata T and Wake N:
Characterization of side-population cells in human normal
endometrium. Hum Reprod. 22:1214–1223. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kokkinos MI, Murthi P, Wafai R, Thompson
EW and Newgreen DF: Cadherins in the human
placenta-epithelial-mesenchymal transition (EMT) and placental
development. Placenta. 31:747–755. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Owusu-Akyaw A, Krishnamoorthy K, Goldsmith
LT and Morelli SS: The role of mesenchymal-epithelial transition in
endometrial function. Hum Reprod Update. 25:114–133.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang J, Hu R, Xing Q, Feng X, Jiang X, Xu
Y and Wei Z: Exosomes derived from umbilical cord mesenchymal stem
cells alleviate mifepristone-induced human endometrial stromal cell
injury. Stem Cells Int. 2020(6091269)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Omwandho CO, Konrad L, Halis G, Oehmke F
and Tinneberg HR: Role of TGF-betas in normal human endometrium and
endometriosis. Hum Reprod. 25:101–109. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Young VJ, Brown JK, Saunders PT, Duncan WC
and Horne AW: The peritoneum is both a source and target of TGF-β
in women with endometriosis. PLoS One. 9(e106773)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ibrahim MG, Elghonaimy EA, Schäfer S,
Vennemann M, Kliesch S, Kiesel L, Götte M and Schüring AN: Seminal
plasma (SP) induces a rapid transforming growth factor beta 1
(TGFβ1)-independent up-regulation of epithelial-mesenchymal
transdifferentiation (EMT) and myofibroblastic metaplasia-markers
in endometriotic (EM) and endometrial cells. Arch Gynecol Obstet.
299:173–183. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Song J: EMT or apoptosis: A decision for
TGF-beta. Cell Res. 17:289–290. 2007.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yao Y, Chen R, Wang G, Zhang Y and Liu F:
Exosomes derived from mesenchymal stem cells reverse EMT via
TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem
Cell Res Ther. 10(225)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Bartley J, Jülicher A, Hotz B, Mechsner S
and Hotz H: Epithelial to mesenchymal transition (EMT) seems to be
regulated differently in endometriosis and the endometrium. Arch
Gynecol Obstet. 289:871–881. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen YJ, Li HY, Huang CH, Twu NF, Yen MS,
Wang PH, Chou TY, Liu YN, Chao KC and Yang MH: Oestrogen-induced
epithelial-mesenchymal transition of endometrial epithelial cells
contributes to the development of adenomyosis. J Pathol.
222:261–270. 2010.PubMed/NCBI View Article : Google Scholar
|