Introduction
In recent years, environmental pollution has become
a major threat to human health. Increased attention has been paid
to the effect of environmental factors on the occurrence and
development of respiratory diseases. Fine particular matter ≤2.5 µm
in diameter (PM2.5) is the main pollutant in the
atmosphere. It refers to fine particulate matter with an
aerodynamic diameter under 2.5 µm. PM2.5 inhalation can
induce respiratory tract injury directly and aggravate a variety of
diseases (1,2). A large number of epidemiological
studies have revealed that PM2.5 exposure is closely
related to the morbidity and mortality of cardiovascular and
respiratory systems as well as other diseases, such as arterial
embolism, asthma, pneumonia, and lung cancer (3,4).
Flavonoids are natural compounds with a wide range
of molecular diversity. Fruits, vegetables, herbs and other plant
foods are the sources of flavonoids (5). Flavonoids have received increasing
attention due to their anti-inflammatory, antimicrobial and
anticancer activities (6). The
important and beneficial effect of flavonoids is antioxidant
activity for human body health, which is related with scavenging of
reactive oxygen species (ROS) (7).
Flavonoids can adjust the expression of inflammatory mediators
including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β)
and cyclooxygenase-2 (COX-2) through regulation of the Toll
receptor (TLR)/NF-κB axis (8). The
large number of natural compounds containing flavonoids used to
treat, manage, and prevent human diseases have been widely studied.
A typical example is usage of licorice derived from the dried roots
and rhizomes of the Glycyrrhiza species. Licorice has been used to
treat diabetes, tuberculosis, and other inflammatory disorders
(9). In addition, some flavonoids
(quercetin, apigenin and luteolin) have been revealed to reduce the
expression and secretion of cytokines (10). However, the anti-inflammatory effect
of isosinensetin (ISO) has not been reported. Therefore, in the
present study, the intervention effect and possible mechanism of
ISO on human bronchial epithelial cells injured by PM2.5
were investigated.
Materials and methods
Materials
The human bronchial epithelial cell line, 16-HBE,
was obtained from the American Type Culture Collection (ATCC). DMEM
medium was purchased from Hyclone; Cytiva. Epithelial cell medium
(ECM; cat. no. 4101) containing fetal bovine serum (FBS; cat. no.
0010) and epithelial cell growth supplements (ECGs; cat. no. 4152)
were purchased from ScienCell Research Laboratories, Inc.
Anti-proliferating cell nuclear antigen (PCNA; product no. 2586)
antibody was purchased from Cell Signaling Technology, Inc.
Anti-nuclear factor erythroid 2-related factor 2 (Nrf2; cat. no.
sc-365949), anti-p65 (cat. no. sc-8008), anti-IκB kinase (IKK; cat.
no. sc52932) and anti-actin (cat. no. sc-81178) antibodies were
purchased from Santa Cruz Biotechnology, Inc.
2',7'-Dicholorofluorescein-diacetate (DCFH-DA) probe (product no.
D6883) was obtained from Sigma-Aldrich; Merck KGaA. Cell Counting
Kit-8 (CCK-8; cat. no. AR1199) was purchased from Wuhan Boster
Biological Technology, Ltd. IL-6, IL-1β and TNF-α ELISA kits (cat.
nos. BMS213-2, BMS224-2 and BMS223-4, respectively) were purchased
from Thermo Fisher Scientific, Inc. N-acetyl-L-cysteine (NAC;
product no. S0077), trypsin (product no. C0202), PBS solution
(product no. C0221A), and RIPA lysis solution (product no. P0013B)
were obtained from Shanghai Beyotime Institute of Biotechnolgy.
Drug
Isosinensetin (99.5% purity) was purchased from the
National Institute for the Control of Pharmaceutical and Biological
Products (NICPBP; Beijing, China). ISO (2 mg) was firstly dissolved
in 0.1 ml absolute ethyl alcohol. The concentrations used were 0,
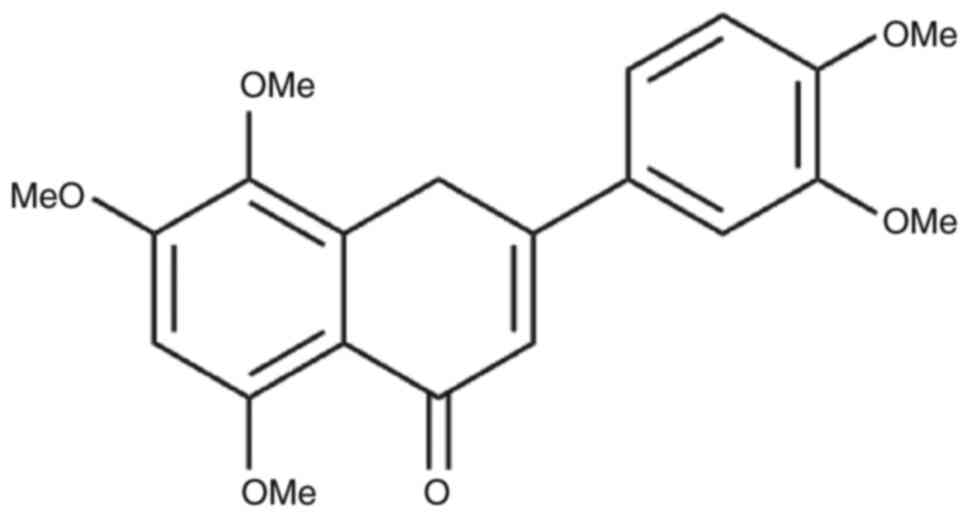
20, 40, 80 or 160 µg/ml and the drug was stored at 4˚C (Fig. 1; structure of ISO). NAC (2 mg) was
dissolved in 2 ml sterile deionized water and stored at 4˚C.
PM2.5 sample
The collection and preparation of PM2.5
sample were conducted according to our previously reported methods
(11). Briefly, samples were
collected on nitrocellulose filters using a high-volume sampler
particle collector in the center of Shenyang city. PM2.5
was extracted from the nitrocellulose filters by immersing in
deionized water and then sonicating for 30 min. The
PM2.5 sample was stored at -80˚C.
Cell culture
16-HBE cells were cultured in ECM supplemented with
10% (v/v) heat-inactivated fetal bovine serum (FBS) and 1%
penicillin/ECGs at 37˚C in an atmosphere of 5% CO2 and
95% air. The cells were routinely sub-cultured every 2-3 days and
it was confirmed that all of the cells were in the logarithmic
phase before subsequent experimentation.
Cell viability assay
16-HBE cells were seeded at a density of
1x104 cells/ml in a 96-well plate. When confluence
reached 70-80%, the cells were treated with serum-free medium for
12 h at 37˚C. Subsequently, the cells were incubated with
PM2.5 solution or PM2.5 solution plus ISO for
6, 12, or 24 h at 37˚C. The cell viability was assessed by the
CCK-8 assay. A total of 10 µl of CCK-8 reagent was added to each
well, and incubated at 37˚C for 1 h. The absorbance was measured at
540 nm using a microplate spectrophotometer (Thermo MK3; Thermo
Fisher Scientific, Inc.).
Contents of pro-inflammatory factors
determination
The concentrations of IL-1β, IL-6 or TNF-α were
detected by ELISA kits. 16-HBE cells were seeded at a density of
2x105 cells/ml in 6-well plates and cultured as
aforementioned, followed by exposure to PM2.5 solution
or PM2.5 solution plus ISO for 24 h. The supernatants
were collected. Inflammatory factors were detected using IL-6,
IL-1β and TNF-α ELISA kits (eBioscience; Thermo Fisher Scientific,
Inc.) by measuring the absorbance at a 460-nm wavelength.
Assessment of ROS production
16-HBE cells were cultured and treated as
aforementioned. A DCFH-DA probe was used to determine the level of
intracellular ROS production. A total of 10 µM DCFH-DA probe was
added for 30 min at 37˚C in the dark. After incubation, the cells
were washed three times with ice-cold PBS. The fluorescence was
observed with an immunofluorescence microscope (excitation
wavelength, 488 nm; emission wavelength, 525 nm) (Leica CTR 4000;
Leica Microsystems GmbH).
Western blot analysis
The procedures and quantification methods were the
same as those previously described (12). Briefly, the proteins in cell lysates
were separated using sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to the
polyvinylidenefluoride (PVDF) membranes. The membranes were
incubated with primary antibodies (anti-actin, 1:1,000 dilution;
anti-PCNA, 1:1,000 dilution; anti-Nrf2, 1:800 dilution; anti-p65,
1:800 dilution; and anti-IKK, 1:800 dilution, respectively) for 12
h at 4˚C. Subsequently, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (1:5,000
dilution; cat. no. 62-6520; Pierce; Thermo Fisher Scientific,
Inc.). The protein bands were detected using an enhanced
chemiluminescence western blotting detection kit (Amersham;
Cytiva). The data were analyzed using the Quantity One software
(v.4.6.7; Bio-Rad Laboratories, Inc.).
Statistical analysis
All data are presented as the mean ± standard
deviation (SD) from three or more independent experiments.
Differences between the groups were evaluated for significance by
one-way ANOVA followed by Tukey's post hoc test for pairwise
comparisons or by Dunnett's test for comparisons vs. a control,
where appropriate. The statistical graphs were constructed using
GraphPad Prism (version 5; GraphPad Software, Inc.) and photoshop
(version 8.0; Adobe systems, Inc). P<0.05 was considered to
indicate a statistically significant difference.
Results
PM2.5 induces damage of
human bronchial epithelial cells
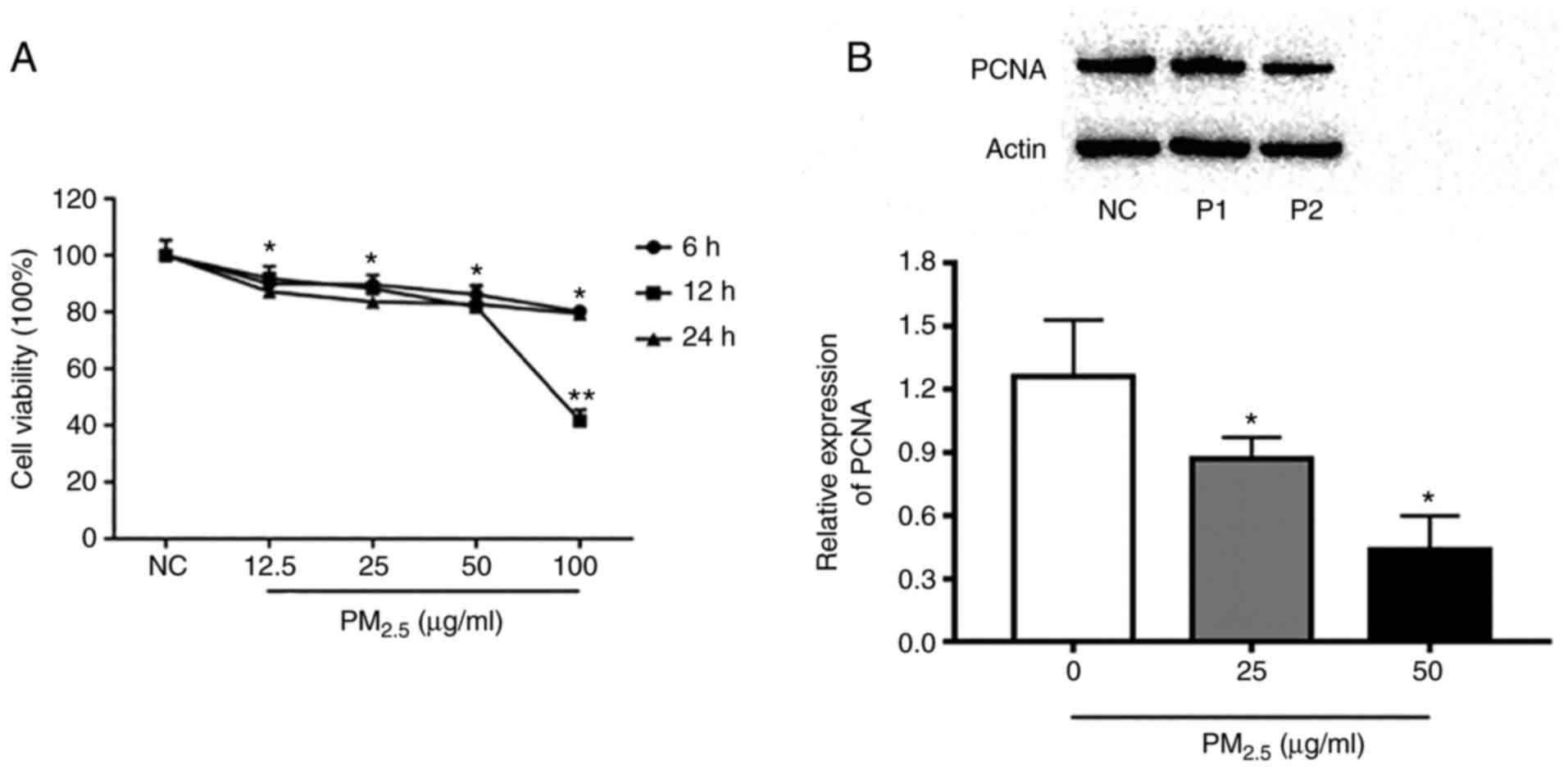
A CCK-8 assay was used to assess the viability of
16-HBE cells in order to determine the effective dose of
PM2.5 on 16-HBE cells after PM2.5 exposure
for 6, 12 and 24 h, respectively. As revealed in Fig. 2, a dose greater than 25 µg/ml
PM2.5 posed a threat to cell viability of 16-HBE cells
(P<0.05).
PCNA is a type of nucleoprotein, whose level can
reflect the condition of cell growth and proliferation (13). Therefore, the expression of PCNA
protein in 16-HBE cells was detected by western blotting. The
results revealed that after exposure to 25 and 50 µg/ml
PM2.5, the expression of PCNA was significantly
decreased compared with the control group of 16-HBE cells.
ISO increases cell viability of 16-HBE
cells injured by PM2.5
In order to investigate the role of ISO in
alleviating PM2.5-induced cell damage and select an
optimal concentration to perform further experiments, the effects
of various concentrations of ISO (0, 20, 40, 80 and 160 µg/ml) were
investigated regarding the viability of 16-HBE cells exposed to
PM2.5 at 25 µg/ml which caused a 20-30%-decrease in cell
viability (Fig. 3). The results
indicated that the addition of ISO could attenuate the toxicity
caused by PM2.5 on 16-HBE cells. The optimum
concentration observed in cells treated with ISO was 80 µg/ml.
Compared with the cells exposed to PM2.5 for 24h, ISO
(80 µg/ml) significantly increased the expression of PCNA protein
in 16-HBE cells.
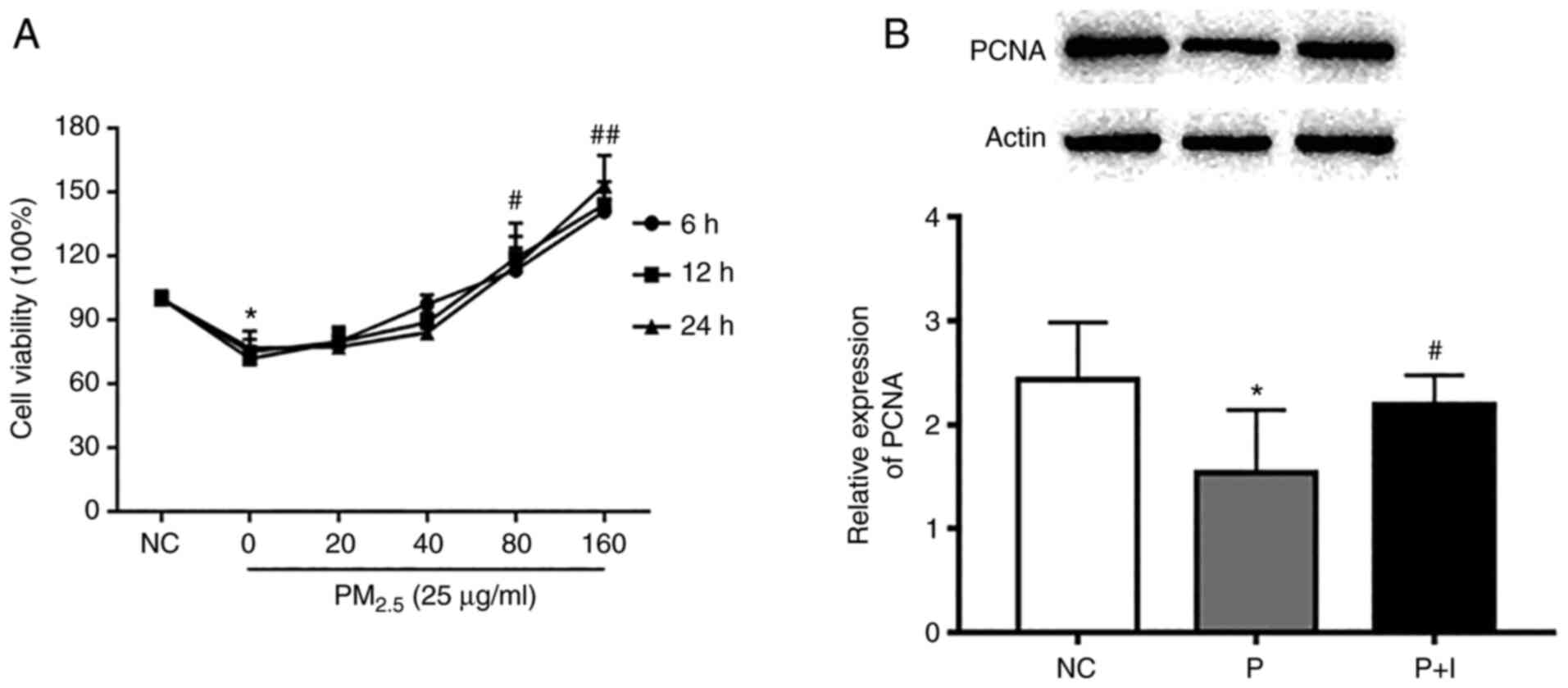 | Figure 3ISO treatment increases cell
viability in human bronchial epithelial cells induced by
PM2.5 exposure. (A) 16-HBE cells were exposed to
PM2.5 (25 µg/ml, 24 h) or PM2.5 (25 µg/ml)
plus different doses of ISO (0, 20, 40, 80, or 160 µg/ml) for 6, 12
and 24 h and the cell viability was detected by Cell Counting Kit-8
method. (B) 16-HBE cells were exposed to PM2.5 (25
µg/ml) solution or PM2.5 (25 µg/ml) plus ISO (80 µg/ml)
for 24 h. The intracellular proliferating cell nuclear antigen
expression levels were detected by western blot analysis. P
indicates 25 µg/ml PM2.5 solution treatment. P + I
indicates 25 µg/ml PM2.5 solution + 80 µg/ml ISO
treatment. *P<0.05 compared with NC group;
#P<0.05 and ##P<0.01 compared with the
P group. ISO, isosinensetin; PM2.5, fine particular
matter ≤2.5 µm in diameter; PCNA, proliferating cell nuclear
antigen; NC, negative control. |
ISO decreases the contents of
pro-inflammatory factors
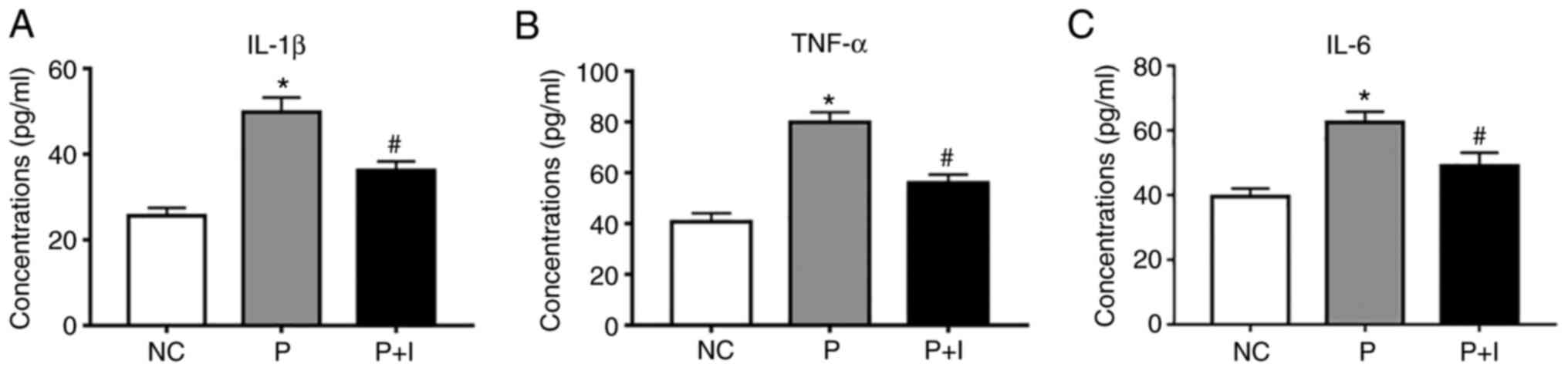
The concentrations of IL-1β, TNF-α and IL-6 were
detected by ELISA kits. The concentrations of IL-1β, TNF-α and IL-6
in the PM2.5 group were significantly higher compared
with the negative control (NC) group (from 49.8±3.4, 79.8±3.9 and
62.5±3.3 to 25.6±1.8, 40.6±3.5 and 39.6±2.5, respectively).
However, after treatment with 80 µg/ml ISO, the concentrations of
IL-1β, TNF-α and IL-6 were decreased (36.3±2.1, 56.1±3.3 and
49.1±4.1, respectively) (Fig. 4)
(P<0.05). These results indicated that ISO could alleviate the
inflammatory effect induced by PM2.5 in 16-HBE
cells.
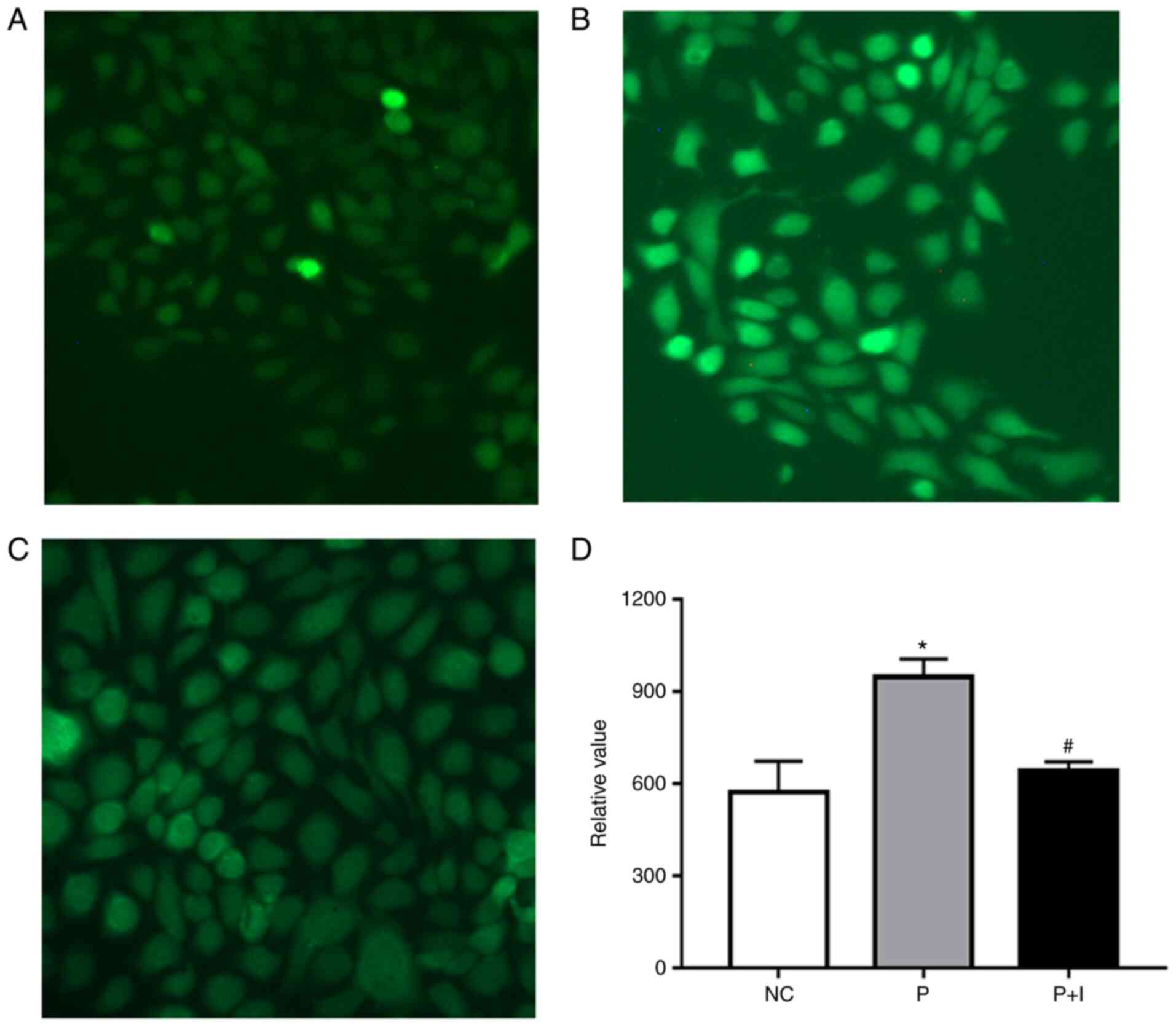
ISO inhibits the ROS generation
induced by PM2.5
ROS acts as a contributor to the toxicity of
PM2.5 and flavonoids are the antioxidants of nature
(14). Fluorescence microscopy
revealed that ISO significantly inhibited the ROS content caused by
PM2.5 (Fig. 5). The
level of ROS was significantly decreased in the PM2.5 +
ISO co-incubation group in comparison with the PM2.5
group. These data demonstrated that ISO played a restraining role
against the toxic effect of PM2.5 by reducing ROS.
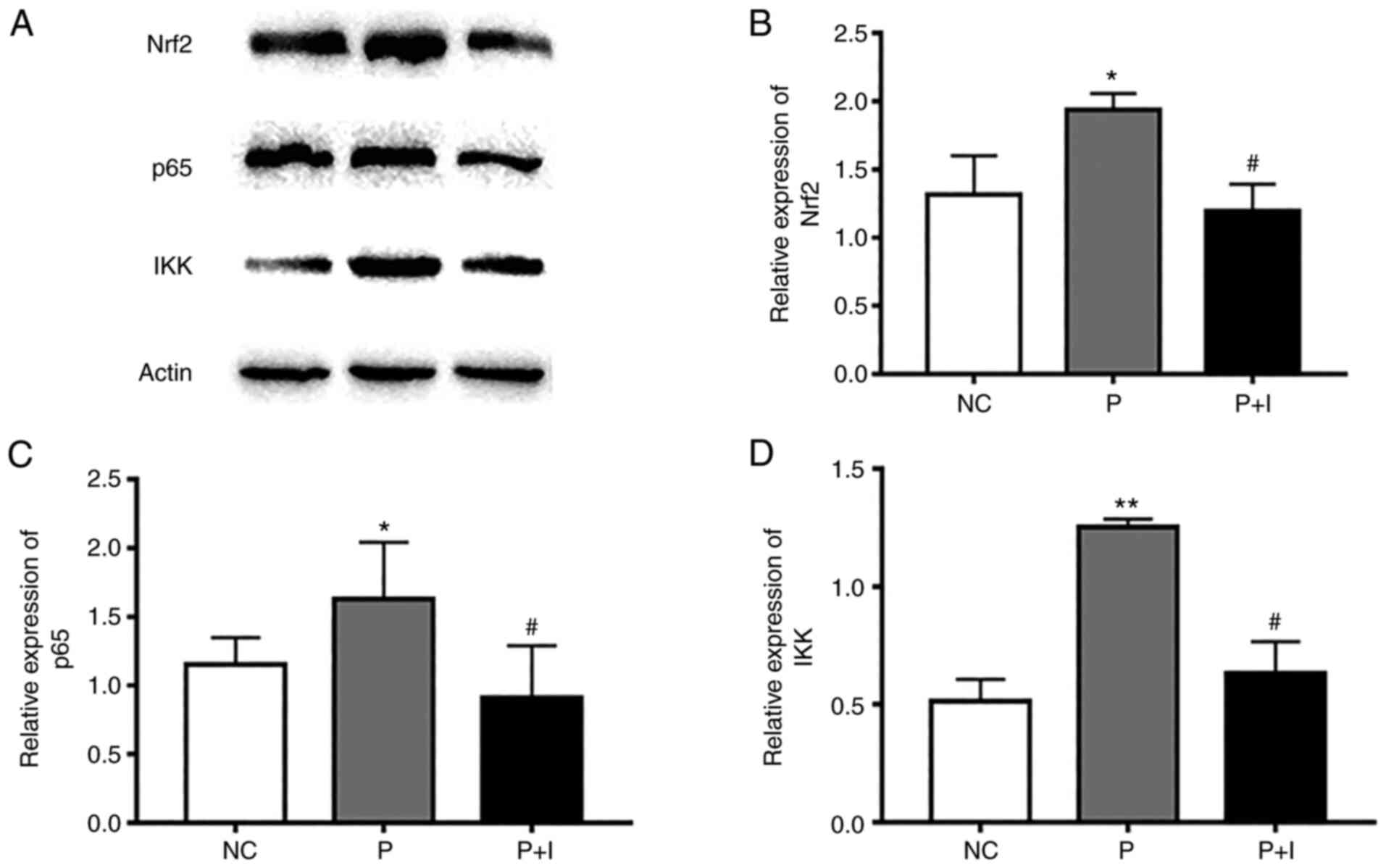
ISO downregulates the expression of
inflammatory-related proteins
The transcription factor NF-κB may be related with
the anti-inflammatory properties of PM2.5 exposure. The
proteins p65 and IKK are the important subunits of the NF-κB family
and their expression is correlated with NF-κB activity (15). Our results revealed that the
expression levels of p65 and IKK in the PM2.5 group were
markedly higher than that in the NC group. However, the expression
levels of p65 and IKK were significantly decreased after treatment
with 80 µg/ml ISO.
Nrf2 protein is a critical signal molecule that
regulates immunity and inflammation (16). The expression of Nrf2 is associated
with inflammatory molecules. The results revealed that ISO
downregulated the expression of Nrf2 protein. These data
illustrated that ISO played a restraining role against the toxic
effect of PM2.5 through the ROS-Nrf2/NF-κB signaling
pathway (Fig. 6).
Moreover, the results revealed after inhibiting ROS
generation through ROS inhibitor NAC treatment, the expression
levels of Nrf2, p65 and IKK proteins were downregulated compared
with the PM2.5 group. However, there was no statistical
significant difference between the NAC + PM2.5 group and
NAC + PM2.5 + ISO group (Fig. S1).
Discussion
In the present study, it was firstly indicated that
ISO had protective effects on oxidative damage and decreased the
expression levels of inflammatory factors induced by
PM2.5. ISO could alleviate the injury of human bronchial
epithelial cells induced by PM2.5.
PM2.5, as one of the toxic and harmful
components in the air, is correlated with various systemic
diseases, including respiratory and cardiovascular diseases
(17). Fine particles can reach and
be retained at the alveolar walls of the lungs, which results in
allergies, asthma and lung emphysema. In previous studies, it has
been demonstrated that PM2.5 exposure caused significant
formation of ROS and an inflammatory reaction. The mechanisms
mainly involved PM2.5 triggering systemic oxidative
stress and inflammation (18-20).
Various studies have revealed that flavonoids are
the main bioactive components in plants which possess
health-promoting properties. Numerous flavonoids such as
hesperidin, quercetin and apigenin have anti-nociceptive and
anti-inflammatory properties (21,22).
Nobiletin, as a classical polymethoxyflavone, has been reported to
have an anticancer effect by inhibiting the migration ability of
breast cancer cells (23). ISO is a
compound of flavonoids, found in fruits and vegetables. In the
present study, our results indicated that ISO could alleviate the
injury of human bronchial epithelial cells and had a protective
effect on 16-HBE by reducing the release of inflammatory cytokines
and relieving the oxidative stress under PM2.5
exposure.
It has been reported that the activators of Nrf2 and
NF-κB proteins can act with strong antioxidative and
anti-inflammatory activities after harmful chemical stimulation in
a cell culture model (24,25). Nrf2 is a transcription factor which
plays a vital role in activating antioxidant response by decreasing
ROS production (26). NF-κB plays
an important role in regulating the inflammatory response. The
increase of ROS can lead to continuous activation of NF-κB and
promote the release of cytokines (27). Our data revealed that ISO could
downregulate the expression of Nrf2 and NF-кB in 16-HBE cells
induced by PM2.5. Moreover, after inhibiting the release
of ROS induced by PM2.5, the expression levels of Nrf2,
p65 and IKK proteins exhibited no obvious differences between the
PM2.5 group and PM2.5 + ISO group. These
results indicated that ISO could alleviate the injury of 16-HBE
cells induced by PM2.5 through the ROS-Nrf2/NF-кB
signaling pathway. However, a limitation of the present study was
that further investigation of the mechanism of Nrf2/NF-кB after
PM2.5 or ISO treatment was not performed in 16-HBE
cells. It will be conducted in subsequent experiments.
The apparent effect of ISO has been revealed to be
presumably dependent on the concentration. Nonetheless, the
anti-inflammatory and oxidative mechanisms of ISO have not yet been
clearly determined. Among the different molecules produced
intracellularly, the transcription factor Nrf2 plays a vital role
in the prevention of cell dysfunction in response to oxidative
stress and in protection against exposure to toxins and carcinogens
through the ARE-mediated expression of a battery of cytoprotective
genes (28). It is anticipated that
in future studies, ISO may be used to evaluate the therapeutic
interventions on the cellular injury induced by
PM2.5.
Supplementary Material
ROS inhibitor NAC downregulates the
expression levels of Nrf2, p65 and IKK proteins induced by PM2.5 or
PM2.5 plus ISO. 16-HBE cells were cultured and treated with ROS
inhibitor (5 mM) for 2 h. Subsequently, the cells were exposed to
PM2.5 (25 μg/ml) or PM2.5 plus ISO (80 μg/ml) for 24
h. (A) The expression levels of Nrf2, p65 and IKK proteins were
detected by western blot analysis. Statistical graphs of the
expression of (B) Nrf2, (C) p65 and (D) IKK. Results are expressed
as the mean ± SD, (n=6). P indicates PM2.5. NAC + P indicates NAC +
PM2.5. NAC + P + I indicates NAC + PM2.5 + ISO.
*P<0.05 compared with the NC group, NAC + P group or
NAC + P + I group. ROS, reactive oxygen species; Nrf2, nuclear
factor erythroid 2-related factor 2; IKK, IκB kinase; PM2.5, fine
particular matter ≤2.5 μm in diameter.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Liaoning Province (grant no. 2018010874-301) and the
Shenyang Medical College Scientific Research Fund (grant nos.
20182033 and 20191038).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ, SL, XL and CX conceptualized and designed the
present study. YZ and SL performed all of the experiments and
confirmed the authenticity of all the raw data. YZ, SL, YS, MM, HT,
NW and JY were responsible for the data collection and analysis. XL
and YS interpreted the data and drafted the manuscript. YZ and CX
revised the manuscript for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aaron CP, Chervona Y, Kawut SM, Diez Roux
AV, Shen M, Bluemke DA, Van Hee VC, Kaufman JD and Barr RG:
Particulate matter exposure and cardiopulmonary differences in the
multi-ethnic study of atherosclerosis. Environ Health Perspect.
124:1166–1173. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Thurston G and Lippmann M: Ambient
particulate matter air pollution and cardiopulmonary diseases.
Semin Respir Crit Care Med. 36:422–432. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fuentes-Mattei E, Rivera E, Gioda A,
Sanchez-Rivera D, Roman-Velazquez FR and Jimenez-Velez BD: Use of
human bronchial epithelial cells (BEAS-2B) to study immunological
markers resulting from exposure to PM(2.5) organic extract from
Puert Rico. Toxicol Appl Pharmacol. 243:381–389. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu ST, Liao CY, Kuo CY and Kuo HW: The
Effects of PM2.5 from Asian dust storms on emergency
room visits for cardiovascular and respiratory diseases. Int J
Environ Res Public Health. 14(428)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Babu PV, Liu D and Gilbert ER: Recent
advances in understanding the anti-diabetic actions of dietary
flavonoids. J Nutr Biochem. 24:1777–1789. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jiang N, Doseff AI and Grotewold E:
Flavones: From biosynthesis to health benefits. Plants (Basel).
5(27)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang WL, Chen SY, Ho CY and Yen GC: Citrus
flavonoids suppress IL-5 and ROS through distinct pathways in
PMA/ionomycin-induced EL-4 cells. Food Funct. 11:824–833.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Akira S, Takeda K and Kaisho T: Toll-like
receptors: Critical proteins linking innate and acquired immunity.
Nat Immunol. 2:675–680. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Fu Y, Chen J, Li YJ, Zheng YF and Li P:
Antioxidant and anti-inflammatory activities of six flavonoids
separated from licorice. Food Chem. 141:1063–1071. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li Y, Yao J, Han C, Yang J, Chaudhry MT,
Wang S, Liu H and Yin Y: Quercetin, inflammation and immunity.
Nutrients. 8(167)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ma M, Li S, Jin H, Zhang Y, Xu J, Chen D,
Kuimin C, Yuan Z and Xiao C: Characteristics and oxidative stress
on rats and traffic policemen of ambient fine particulate matter
from Shenyang. Sci Total Environ. 526:110–115. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang D, Ma M, Zhou W, Yang B and Xiao C:
Inhibition of miR-32 activity promoted EMT induced by PM2.5
exposure through the modulation of the Smad1-mediated signaling
pathways in lung cancer cells. Chemosphere. 184:289–298.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cardano M, Tribioli C and Prosperi E:
Targeting proliferating cell nuclear antigen (PCNA) as an effective
strategy to inhibit tumor cell proliferation. Curr Cancer Drug
Targets. 20:240–252. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ren X, Tang Y, Sun J, Feng J, Chen L, Chen
H, Zeng S, Chen C, Li X, Zhu H and Zeng Z: Flavone protects HBE
cells from DNA double-strand breaks caused by PM2.5. Hum Cell.
31:116–126. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mora-Ramiro B, Jiménez-Estrada M,
Zentella-Dehesa A, Ventura-Gallegos JL, Gomez-Quiroz LE,
Rosiles-Alanis W, Alarcón-Aguilar FJ and Almanza-Pérez JC: Cacalol
acetate, a sesquiterpene from psacalium decompositum, exerts an
anti-inflammatory effect through LPS/NF-KB signaling in Raw 264.7
macrophages. J Nat Prod. 83:2447–2455. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Saha S, Buttari B, Panieri E, Profumo E
and Saso L: An overview of Nrf2 signaling pathway and its role in
inflammation. Molecules. 25(5474)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cui Y, Sun Q and Liu Z: Ambient
particulate matter exposure and cardiovascular diseases: A focus on
progenitor and stem cells. J Cell Mol Med. 20:782–793.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
An Z, Jin Y, Li J, Li W and Wu W: Impact
of particulate air pollution on cardiovascular health. Curr Allergy
Asthma Rep. 18(15)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Feng S, Gao D, Liao F, Zhou F and Wang X:
The health effects of ambient PM2.5 and potential mechanisms.
Ecotoxicol Environ Saf. 128:67–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Y, Li D, Song L and Ding H:
Ophiopogonin D attenuates PM2.5-induced inflammation via
suppressing the AMPK/NF-κB pathway in mouse pulmonary epithelial
cells. Exp Ther Med. 20(139)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yamanishi R, Yoshigai E, Okuyama T, Mori
M, Murase H, Machida T, Okumura T and Nishizawa M: The
anti-inflammatory effects of flavanol-rich lychee fruit extract in
rat hepatocytes. PLoS One. 9(e93818)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nishizuka Y: The molecular heterogeneity
of protein kinase C and its implications for cellular regulation.
Nature. 334:661–665. 1988.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Baek SH, Kim SM, Nam D, Lee JH and Ahn KS,
Choi SH, Kim SH, Shim BS, Chang IM and Ahn KS: Antimetastatic
effect of nobiletin through the down-regulation of CXC chemokine
receptor type 4 and matrix metallopeptidase-9. Pharm Biol.
50:1210–1218. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Du Y, Villeneuve NF, Wang XJ, Sun Z, Chen
W, Li J, Lou H, Wong PK and Zhang DD: Oridonin confers protection
against arsenic-induced toxicity through activation of the
Nrf2-mediated defensive response. Environ Health Perspect.
116:1154–1161. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Morgan MJ and Liu ZG: Crosstalk of
reactive oxygen species and NF-κB signaling. Cell Res. 21:103–115.
2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sporn MB and Liby KT: NRF2 and cancer: The
good, the bad and the importance of context. Nat Rev Cancer.
12:564–571. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Kovac S, Angelova PR, Holmström KM, Zhang
Y, Dinkova-Kostova AT and Abramov AY: Nrf2 regulates ROS production
by mitochondria and NADPH oxidase. Biochim Biophys Acta.
1850:794–801. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lee HS, Lee GS, Kim SH, Kim HK, Suk DH and
Lee DS: Anti-oxidizing effect of the dichloromethane and hexane
fractions from Orostachys japonicus in LPS-stimulated RAW 264.7
cells via upregulation of Nrf2 expression and activation of MAPK
signaling pathway. BMB Rep. 47:98–103. 2014.PubMed/NCBI View Article : Google Scholar
|