Introduction
Systemic lupus erythematosus (SLE) is a common
autoimmune disease that can involve multiple organs and systems
(1). One of the most common
complications of SLE is lupus nephritis (LN) (2). The incidence rate of LN among
different nationalities is statistically significant, with a low
cumulative incidence rate of LN in Caucasian individuals (14% of
patients with SLE), compared with a relatively high incidence rate
among Asians (55% of patients with SLE), Africans (51% of patients
with SLE) and Hispanics (43% of patients with SLE) (2).
The main histological features of LN include
mesangial cell proliferation, followed by increased extracellular
matrix and inflammatory cell infiltration, as well as
glomerulosclerosis and renal interstitial fibrosis at the end stage
(3). Based on the disease
severity, 10-30% of patients with LN will progress to end-stage
renal disease (4). Although LN was
discovered centuries ago, its pathogenesis remains unclear.
Mesangial cells represent a type of glomerular
proper cell situated between the capillary loops of the glomerulus,
adjacent to endothelial cells or the basement membrane, that
exhibit an irregular shape (5).
Cellular processes can reach deep between endothelial cells and the
basement membrane, or extend into the capillary cavity through
endothelial cells. Moreover, mesangial cells have specialized
functions, including cytokine production, cell matrix secretion,
glomerular capillary network support, phagocytosis and the
clearance of macromolecular substances, which are important for the
maintenance of the normal glomeruli structure and function
(6). The excessive proliferation
of mesangial cells is one of the first pathological changes that
occur in LN (7). In particular,
the excessive proliferation of mesangial cells can stimulate the
release additional inflammatory factors, promote extracellular
matrix secretion, aggravate inflammation and cause
glomerulosclerosis (8). Therefore,
the elucidation of the genes associated with mesangial cell
proliferation is required to further reveal the pathological
mechanism of LN and develop novel therapeutic methods.
MicroRNAs (miRNAs/miRs) are a group of non-coding
short RNAs ranging from 19-25 nucleotides in length that are found
in eukaryotes (9). Moreover,
miRNAs exhibit highly conserved genetic characteristics and can
regulate gene expression at both the transcriptional and
post-transcriptional level. miRNAs have been revealed to be
involved in a variety of mammalian pathophysiological processes,
such as intracellular signal response, cell differentiation, cell
proliferation and apoptosis (10).
Therefore, the identification of miRNAs that can regulate the
proliferation or apoptosis of glomerular mesangial cells has
notable clinical significance to further reveal the pathological
mechanism of LN that could be used to develop strategies to delay
the onset of LN.
miR-98-5p is located on the X chromosome, is a
member of the let-7/miR-98-5p family with highly conserved
evolution and has a mature sequence that consists of 22 bases
(11). Recent studies have
revealed that miR-98-5p is involved in the pathogenesis of SLE.
Yuan et al (12) revealed
that miR-98-5p could ameliorate STAT3-mediated cellular
proliferation and inflammatory cytokine production in patients with
SLE via its target gene, IL-6. Another study conducted by Xie et
al (13) demonstrated that
miR-98-5p downregulation induced apoptosis by modulating the
Fas-mediated apoptotic signaling pathway in SLE CD4+ T
cells. Since miR-98-5p is involved in SLE pathogenesis, the present
study hypothesized that miR-98-5p may also be involved in LN
pathogenesis. The present study therefore aimed to elucidate the
effect of miR-98-5p on mesangial cell proliferation and its
specific mechanism in order to provide novel strategies for the
treatment of LN.
Materials and methods
Patients
A total of 38 patients (female, 21; male, 17; age,
49-66 years) with LN were enrolled between January 2015 and January
2020 from the Department of Rheumatology and Immunology at the
Affiliated Hospital of Hebei University (Hebei, China). The
classification of LN was based on the International Society of
Nephrology/Renal Pathology Society criteria (14), and the LN diagnosis was confirmed
by a pathologist which was independent from the study. The
exclusion criteria were as follows: i) Other rheumatic immune
diseases, tumors, or infectious diseases; and ii) pregnant women.
LN renal tissue was obtained during a renal biopsy. All patients
signed informed consent prior to surgery. As a control group,
carcinoma-adjacent renal tissues (5 cm away from tumor tissues)
were obtained from 38 patients (female, 18; male, 20; age, 48-69
years) who underwent nephrectomy for renal cell carcinoma, between
March 2017 and October 2019 at the Department of Urology, at the
Affiliated Hospital of Hebei University. The exclusion criteria
were as follows: i) Other rheumatic immune diseases, tumors, or
infectious diseases; ii) pregnant women; and iii) active
malignancies other than renal cell carcinoma. Written informed
consent was obtained from all patients prior to surgery. The
present study was approved by The Institute of Medical Ethics
Committee of Affiliated Hospital of Hebei University (approval no.
HBU201401221027).
Cell culture
A human mesangial cell line IP15 was purchased from
the Cell Resource Center, Shanghai Institutes for Biological
Sciences. The cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Hyclone; Cytiva), 100
IU/ml penicillin (Gibco; Thermo Fisher Scientific, Inc.) and 100
mg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.). All
cells were maintained in a humidified atmosphere containing 5%
CO2 at 37˚C. The NF-κB inhibitor BAY11-7082 (Selleck
Chemicals; cat. no. S2913) (5 µM) was added to the medium at 37˚C 1
h after transfection for 48 h.
Cell transfection
The negative control (5'-UCGCUUGG UGCAGGUCGGG-3'),
miR-98-5p mimic (5'-UGAGG UAGUAAGUUGUAUUGUU-3'), miR-98-5p
inhibitor (5'-AAAUAUGCUGUAUGUCAUGUGUU-3'), BTB and CNC homology 1
(BACH1) small interfering RNA (siRNA; 5'-GGAGGTGGATGTGAAAGAT-3'),
miR-98-5p mimic control (5'-UCACAACCUCCUAGAAAGAGUAGA-3'), miR-98-5p
inhibitor control (5'-GUCCAGUGAAUUCC CAG-3') and BACH1 siRNA
control (5'-GGCCTTGCGAT AGCGTAGC-3'), as well as the
BACH1-overexpression plasmid and empty vector were designed and
synthesized by Shanghai GenePharma Co., Ltd. Human mesangial IP15
cells were seeded into 24-well plates at a density of
5x105 cells/well and incubated overnight at 37˚C. The
negative control (NC) (20 µM), miR-98-5p mimic control (20 µM),
miR-98-5p mimic (20 µM), miR-98-5p inhibitor control (20 µM),
miR-98-5p inhibitor (20 µM), BACH1 siRNA control (20 µM), BACH1
siRNA (20 µM), empty vector (1,000 ng) and BACH1-overexpression
plasmid (1,000 ng) were transfected into human mesangial cells
using Lipofectamine® 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol
at 37˚C for 48 h. Total RNA and protein were extracted from the
human mesangial cells at 48 h post-transfection for reverse
transcription-quantitative PCR (RT-qPCR) and western blotting,
respectively.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 kit (Dojindo Molecular Technologies, Inc.)
was used to evaluate the proliferative ability of human mesangial
cells. Human mesangial cells were seeded into 96-well plates at a
density of 2x103 cells/well and incubated for 24, 48, 72
or 96 h at 37˚C. Following this incubation, 20 µl of 10% CCK-8
reagent was added per well and incubated at 37˚C for 2 h. The
optical density was measured at 450 nm using an Enzyme Immunoassay
Analyzer (Bio-Rad Laboratories, Inc.).
ELISA
Human mesangial cells were transfected with the
miR-98-5p mimic, miR-98-5p mimic control, miR-98-5p inhibitor or
miR-98-5p inhibitor control for 48 h. The culture supernatant was
collected and the concentrations of IL-1β (cat. no. DLB50) and
TNF-α (cat. no. DTA00D) were detected using commercial ELISA kits
(R&D Systems, Inc.) in accordance with the manufacturer's
instructions. The optical density at 450 nm was measured using an
ELISA reader (Molecular Devices, LLC).
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR analysis was performed as previously
described (15). Total RNA was
extracted from renal tissues or transfected IP15 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) in accordance with the manufacturer's protocol. Reverse
transcription was performed using a PrimeScript™ RT reagent kit
(for BACH1 and β-actin; Takara Bio, Inc.) and an Mir-X miRNA
RT-qPCR TB Green® kit (for miR-98-5p and U6; Takara Bio,
Inc.) based on the manufacturer's instructions. Quantitative PCR
was performed using TB Green® Premix Ex Taq™ II (Tli
RNaseH Plus; Takara Bio, Inc.) in accordance with the
manufacturer's instructions with a StepOnePlus Real-time PCR System
(Thermo Fisher Scientific, Inc.). The thermocycling conditions were
as follows: Initial denaturation at 95˚C for 30 sec, followed by 40
cycles of 95˚C for 5 sec and 60˚C for 31 sec. BACH1 forward,
5'-GCCCGTATGCTTGTGTGATT-3' and reverse, 5'-CGTGAGAGCGAAATTATCCG-3';
β-actin forward, 5'-CACCCACACTGTGCCCATCT-3' and reverse,
5'-GAACCGCTCATTGCCAATGG-3'; miR-98-5p forward,
5'-TGAGGTAGTAAGTTGTATTGTT-3' and reverse, 5'-GTGCAGGGTCCGAGGT-3';
and U6 forward, 5'-GCTTCGGCAGCACATATACTAAAAT-3' and reverse,
5'-CGCTTCACGAAT TTGCGTGTCAT-3'. All reactions were performed in
triplicate. Relative expression levels were quantified using the
2-∆∆Cq method (16).
Western blotting
Total protein was extracted from renal tissues or
transfected IP15 cells using RIPA lysis buffer (Beyotime Institute
of Biotechnology, Cat. No. P0013B) according to the manufacturer's
protocol. The BCA Protein assay kit (Beyotime Institute of
Biotechnology) was used to measure the protein concentration. Total
protein (60 µg/lane) was quantified and separated via 10% SDS-PAGE.
The separated proteins were subsequently transferred onto PVDF
membranes and blocked with 5% skimmed milk for 1 h at room
temperature. The membranes were then incubated with the following
primary antibodies in 5% fat-free milk overnight at 4˚C: Anti-BACH1
(cat. no. PA5-103060; 1:1,000; Invitrogen; Thermo Fisher
Scientific, Inc.) and anti-β-actin (cat. no. ab8227; 1:1,000;
Abcam). Following incubation with the primary antibody, membranes
were washed three times in TBS-Tween (0.5% Tween) and incubated
with an anti-mouse horseradish peroxidase-conjugated secondary
antibody (cat. no. ab7090; 1:5,000; Abcam) for 1 h at room
temperature. Protein bands were visualized using the enhanced
chemiluminescence reagent (Pierce; Thermo Fisher Scientific, Inc.)
and visualized on an iBright FL1500 imaging system (Invitrogen;
Thermo Fisher Scientific, Inc.).
Dual luciferase reporter assay
StarBase 3.0 (http://starbase.sysu.edu.cn/) was used to predict
whether miR-98-5p could directly bind to BACH1 mRNA. Subsequently,
wild-type (WT) or mutant (MUT) BACH1, which contained miR-98-5p
binding sites, were cloned into pmirGLO reporter vectors (Promega
Corporation) to generate pmirGLO-BACH1-WT and pmirGLO-BACH1-MUT
vectors, respectively, using a site-directed mutagenesis kit (cat.
no. 200518; Agilent Technologies, Inc.). Subsequently, human
mesangial cells were seeded into 24-well plates at a density of
5x105 cells/well until they reached 70% confluence. The
cells were subsequently co-transfected with synthesized reporter
vectors (0.8 µg), an miR-98-5p mimic (0.8 µg) or miR-98-5p mimic
control (0.8 µg) using Lipofectamine 2000 reagent. Following a 48 h
transfection at 37˚C, the level of Renilla luciferase
activity was measured using a Dual-Luciferase Reporter Assay system
(Promega Corporation) according to the manufacturer's protocol.
Statistical analysis
Statistical analysis was performed using SPSS
version 19 software (IBM Corp.) and the data are presented as the
mean ± standard deviation (unless otherwise shown). Statistical
differences between groups were determined using a paired Student's
t-test or a one-way ANOVA followed by a Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-98-5p expression is downregulated
in LN renal tissue
The levels of miR-98-5p expression in LN and control
renal tissues were analyzed using RT-qPCR. The level of miR-98-5p
expression was significantly downregulated in the LN renal tissues
compared with control renal tissues (Fig. 1). This result suggests that
miR-98-5p inhibited LN.
miR-98-5p inhibits the proliferation
of human mesangial cells and the secretion of TNF-α and IL-6
Human mesangial cells were transfected with either a
miR-98-5p mimic or miR-98-5p inhibitor. Transfection with the
miR-98-5p mimic significantly upregulated the expression level of
miR-98-5p compared with the miR-98-5p mimic control group, whereas
transfection with the miR-98-5p inhibitor significantly
downregulated the level of miR-98-5p expression compared with the
miR-98-5p inhibitor control (Fig.
2A). The effects of miR-98-5p on the proliferation of human
mesangial cells was investigated using a CCK-8 assay. The results
of the CCK-8 proliferation assay revealed that, after 72 h,
transfection with the miR-98-5p mimic significantly inhibited the
proliferation of human mesangial cells compared with the mimic
control; whereas transfection with the miR-98-5p inhibitor
significantly increased the proliferation of human mesangial cells
compared with that of the inhibitor control group (Fig. 2B). The results of the ELISA
revealed no significant differences in cytokine secretion between
the NC, miR-98-5p mimic control and miR-98-5p inhibitor control
groups. Furthermore, compared with miR-98-5p mimic control, the
miR-98-5p mimic significantly inhibited the secretion of TNF-α and
IL-6. Whereas, compared with the miR-98-5p inhibitor control, the
miR-98-5p inhibitor significantly promoted the secretion of TNF-α
and IL-6 (Fig. 2C). These results
suggest that miR-98-5p inhibited the proliferation and the
inflammatory factors secretion of human mesangial cells.
miR-98-5p downregulates the expression
level of BACH1 by directly targeting BACH1
To determine the downstream targets of miR-98-5p, a
bioinformatics analysis was performed using StarBase 3.0. BACH1 was
identified as a putative miR-98-5p target gene (Fig. 3A. Furthermore, BACH1 gene (Fig. 3B) and protein expression levels
(Fig. 3C) in LN tissues were
significantly higher compared with control renal tissues. RT-qPCR
revealed that, compared with the mimic control group, transfection
with the miR-98-5p mimic significantly downregulated the expression
level of BACH1, whereas transfection with the miR-98-5p inhibitor
significantly upregulated the expression level of BACH1 in human
mesangial cells when compared with the miR-98-5p inhibitor control
(Fig. 3D). The western blotting
results confirmed those of RT-qPCR (Fig. 3E). To validate whether miR-98-5p
directly targeted BACH1, dual luciferase reporter assays were
performed. The relative luciferase activity was significantly
different between the MUT plasmid + miR-98-5p mimic group and WT
plasmids + miR-98-5p mimic group (Fig.
3F). These results indicate that miR-98-5p may downregulate the
level of BACH1 expression by directly targeting the BACH1
3'-untranslated region (UTR). These results suggest that miR-98-5p
directly inhibited the expression of BACH1.
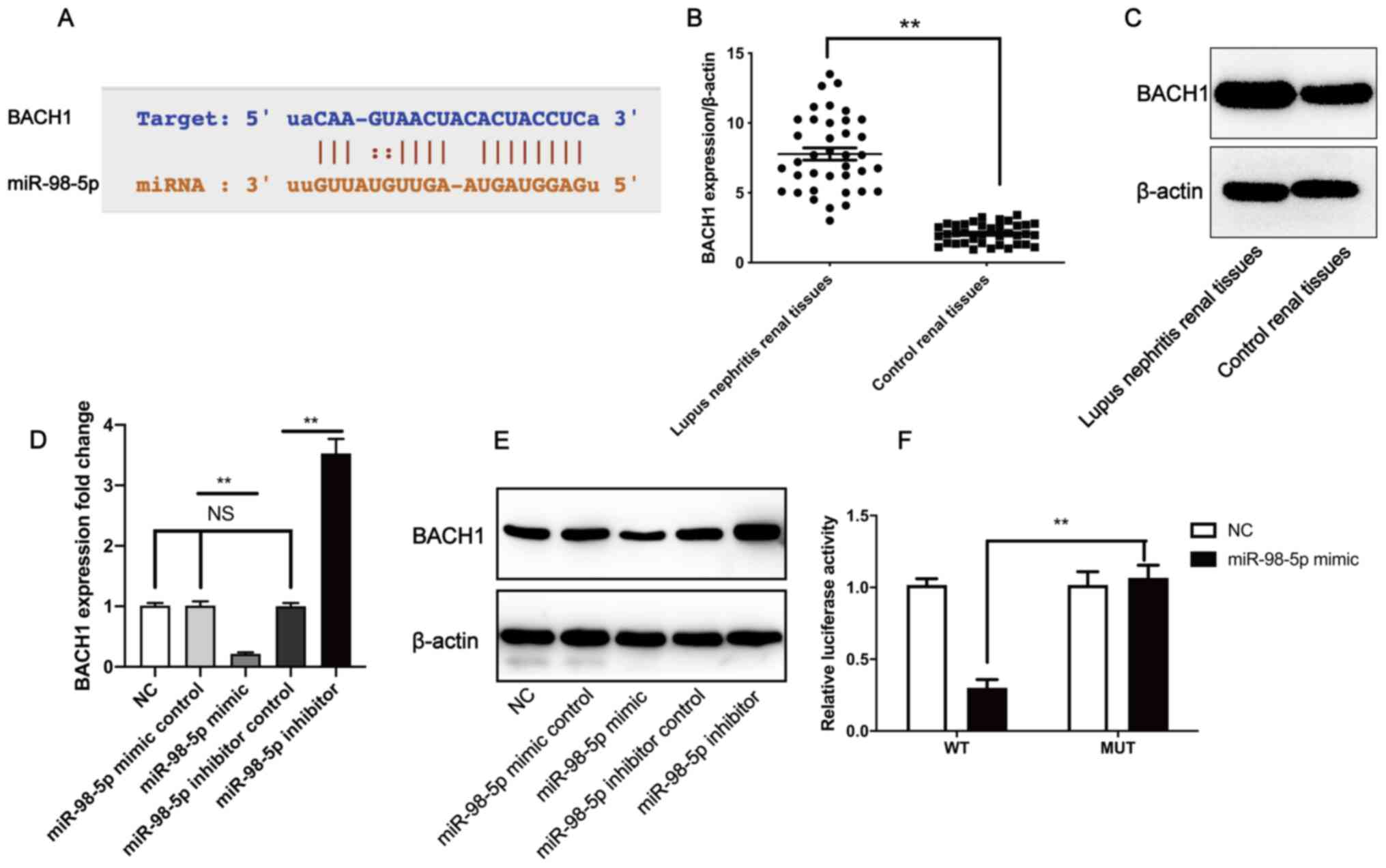 | Figure 3miR-98-5p directly binds to the 3'-UTR
of BACH1 to downregulate its expression. (A) Putative binding site
of miR-98-5p in the 3'-UTR of BACH1. (B) BACH1 gene expression and
(C) protein level in lupus nephritis tissue compared with control
renal tissues. Human mesangial cells were treated with a miR-98-5p
inhibitor, mimic and respective controls, after which (D) BACH1
gene and (E) protein expression were measured. (F) Human mesangial
cells were co-transfected with either a miR-98-5p mimic and
pmirGLO-BACH1-MUT or co-transfected with a miR-98-5p mimic and
pmirGLO-BACH1-WT, after which relative luciferase activity was
measured. All experiments were repeated three times and the data
are expressed as the mean ± standard deviation. Data in panel B and
F were analyzed by a paired Student's t-test. Data in panel D and E
were analyzed by one-way ANOVA followed by a Tukey's post hoc test.
**P<0.01. miR/miRNA, microRNA; UTR, untranslated
region; BACH1, BTB and CNC homology 1; MUT, mutant; WT, wild-type;
NC, negative control; NS, no significance. |
BACH1 promotes human mesangial cell
proliferation and the secretion of TNF-α and IL-6 through
NF-κB
To further elucidate the role of BACH1, the present
study overexpressed or knocked-down BACH1 in human mesangial cells.
The knockdown of BACH1 using siRNA was confirmed to significantly
downregulate BACH1 expression compared with the BACH1 siRNA
control. Furthermore, the BACH1-overexpression plasmid
significantly upregulated the level of BACH1 expression compared
with the empty vector in human mesangial cells (Fig. 4A and B). According to western blotting,
transfection with the BACH1 siRNA decreased p-p65 protein levels
compared with the NC group, whereas the BACH1-overexpression
plasmid increased the level of p-p65 protein. Moreover, the
BACH1-overexpression plasmid + BAY11-7082 (an inhibitor of NF-κB, 5
µM) group displayed a lower p-p65 protein expression level compared
with the BACH1-overexpression plasmid group (Fig. 4C). CCK-8 and ELISA assays were
performed to investigate the effects of BACH1 overexpression and
knockdown on cell proliferation and the secretion of TNF-α and IL-6
by human mesangial cells. After 72 h, treatment with the
BACH1-overexpression plasmid significantly promoted human mesangial
cell proliferation compared with the NC. However, treatment with
the BACH1-overexpression plasmid and BAY11-7082 significantly
inhibited human mesangial cell proliferation compared with the
BAY11-7082 control group. Treatment with BACH1 siRNA significantly
inhibited human mesangial cell proliferation compared with the
BACH1 siRNA control group after 72 h (Fig. 4D). The BACH1-overexpression plasmid
significantly promoted human mesangial cell secretion of TNF-α and
IL-6 compared with the empty vector; while treatment with the
BACH1-overexpression plasmid and BAY11-7082 inhibited human
mesangial cell secretion of TNF-α and IL-6 compared with the
BAY11-7082 control group. In addition, compared with BACH1 siRNA
control, BACH1 siRNA inhibited human mesangial cell secretion of
TNF-α and IL-6 (Fig. 4E). These
results suggest that BACH1 promoted the proliferation and
inflammatory factor secretion of human mesangial cells.
 | Figure 4BACH1 promotes human mesangial cell
proliferation. The effect of transfection with BACH1 siRNA on the
levels of BACH1 expression at the (A) gene and (B) protein level
were assessed. (C) Effect of transfection with BACH1 siRNA,
BACH1-overexpression plasmid and BACH1-overexpression plasmid +
BAY11-7082 (an inhibitor of NF-κB) on p-p65 and t-p65 protein
levels. (D) Human mesangial cell proliferation was measured
following transfection with the BACH1-overexpression plasmid,
BACH1-overexpression plasmid + BAY11-7082, BACH1 siRNA and their
respective controls. (E) Human mesangial cell secretion of TNF-α
and IL-6 were measured after transfection with the
BACH1-overexpression plasmid, BACH1-overexpression plasmid +
BAY11-7082, BACH1 siRNA and their respective controls. All
experiments were repeated three times and the data are expressed as
the mean ± standard deviation. Data were analyzed by one-way ANOVA
followed by a Tukey's post hoc test. **P<0.01. BACH1,
BTB and CNC homology 1; siRNA, small interfering RNA; p-,
phosphorylated; t, total; NS, non-significant; OD, optical density;
NC, negative control. |
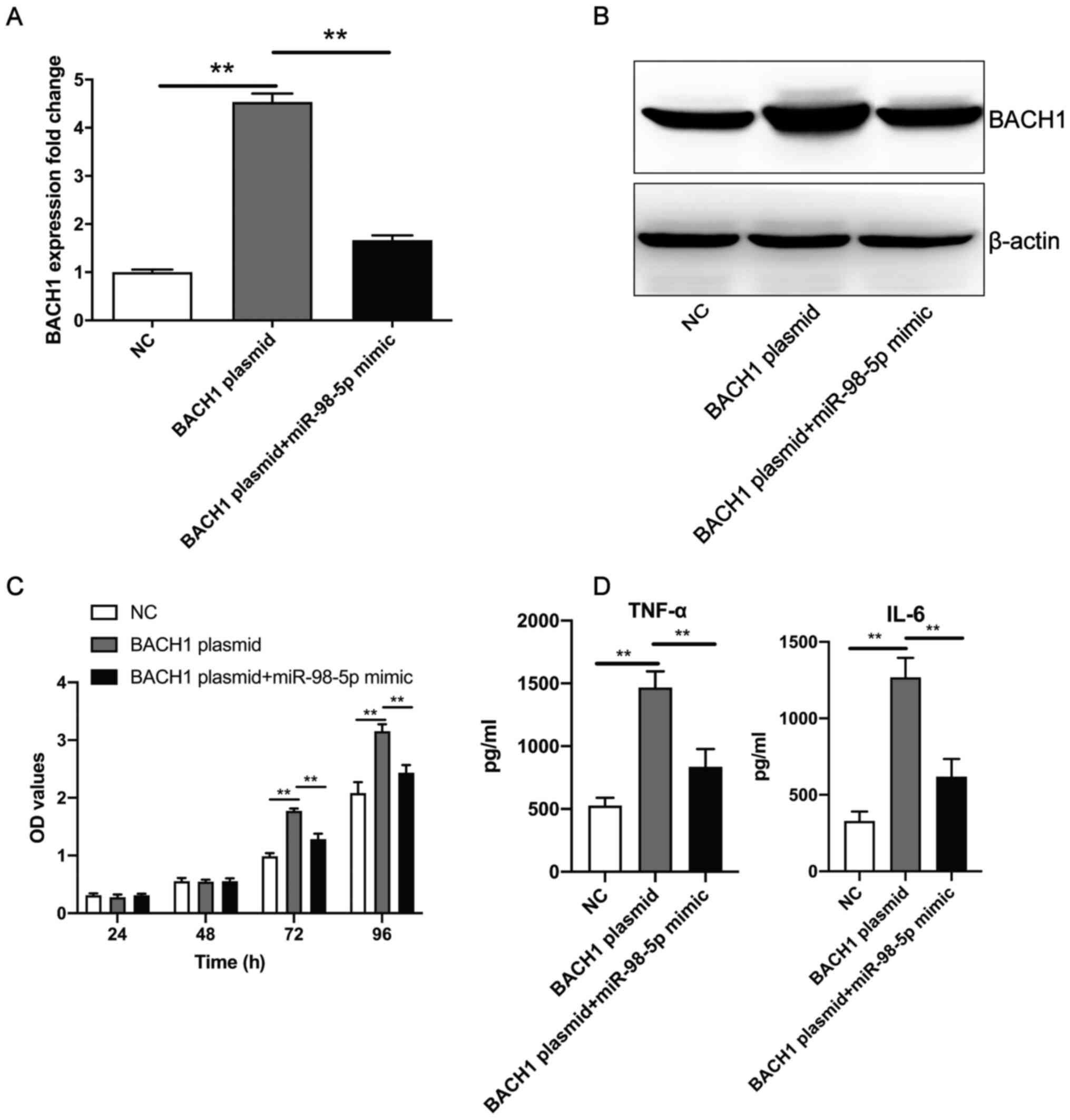
miR-98-5p mimic inhibits the
BACH1-overexpression-induced increase of human mesangial cell
proliferation and the secretion of TNF-α and IL-6
The aforementioned experiments revealed that the
overexpression of BACH1 promoted human mesangial cell proliferation
and the secretion of TNF-α and IL-6. Compared with
BACH1-overexpression plasmid transfection, co-transfection with the
miR-98-5p mimic and BACH1-overexpression plasmid resulted in the
significant downregulation of BACH1 expression levels at both the
mRNA (Fig. 5A) and protein
(Fig. 5B) level. In addition,
after 72 h, the co-transfection of the miR-98-5p mimic and
BACH1-overexpression plasmid significantly inhibited human
mesangial cell proliferation and secretion of TNF-α and IL-6 when
compared with transfection of the BACH1-overexpression plasmid
alone (Fig. 5C and D). These results suggest that miR-98-5p
mimics reversed the BACH1-overexpression-induced increase of human
mesangial cell proliferation and the secretion of inflammatory
factors.
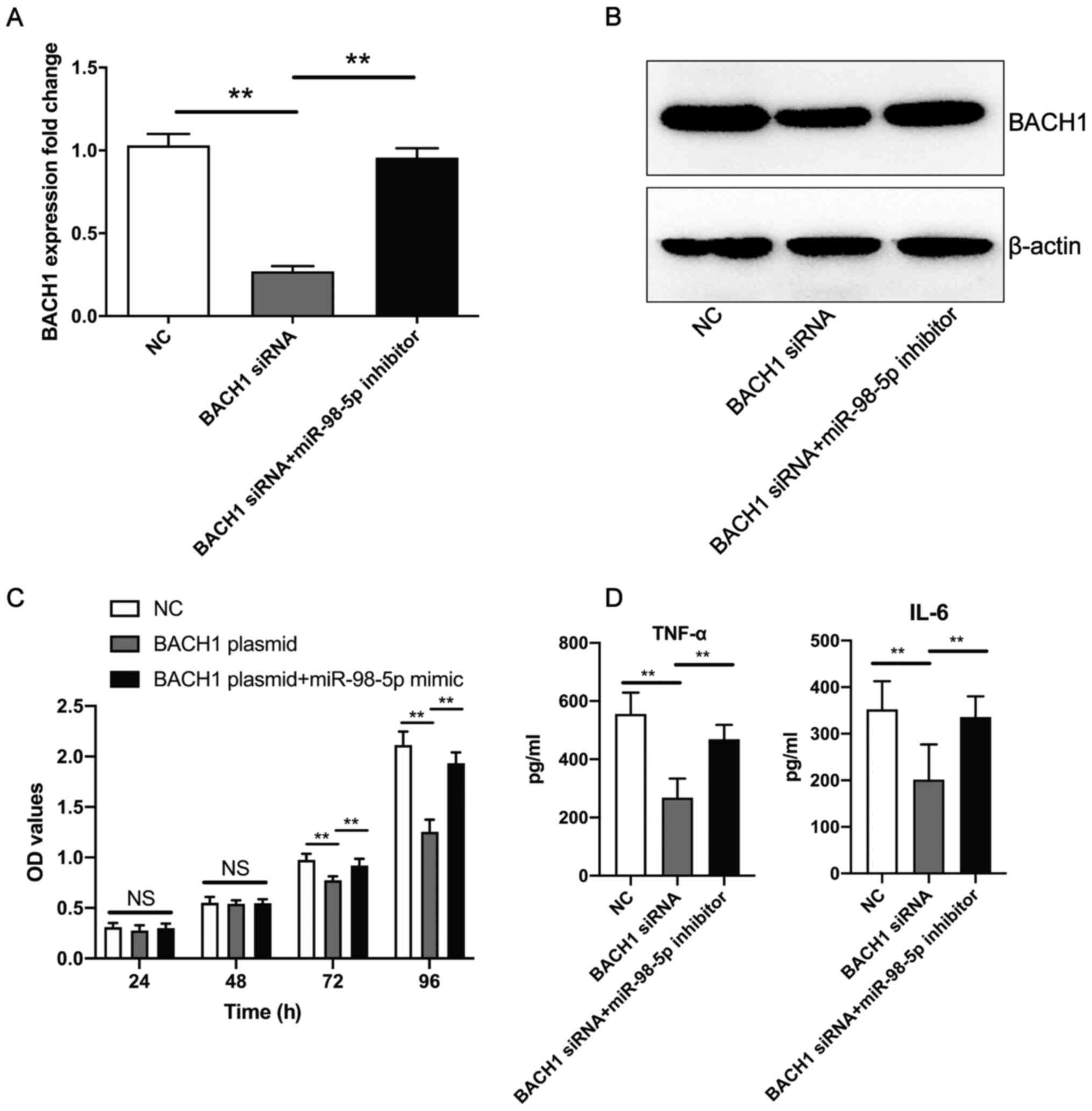
miR-98-5p inhibitor promotes the BACH1
siRNA-induced decrease in human mesangial cell proliferation and
the secretion of TNF-α and IL-6
Co-transfection of the miR-98-5p inhibitor and BACH1
siRNA resulted in a significant upregulation of BACH1 expression at
the mRNA (Fig. 6A) and protein
(Fig. 6B) level in human mesangial
cells compared with BACH1 siRNA transfection. Furthermore, compared
with BACH1 overexpression plasmid transfection, the co-transfection
of the BACH1 overexpression plasmid + miR-98-5p mimic promoted
human mesangial cell proliferation (Fig. 6C), as well as the secretion of
TNF-α and IL-6 (Fig. 6D) induced
by BACH1 siRNA. These results suggest that miR-98-5p inhibitor
reversed the BACH1 siRNA-induced decrease in human mesangial cell
proliferation and the secretion of inflammatory factors.
Discussion
miRNAs have been reported to play a variety of
important regulatory roles, including cell development,
differentiation and proliferation, and a single miRNA can have
multiple regulatory target genes (17). The relationship between miRNA and
LN has been studied. For example, miR-124 serves as a novel
diagnostic marker in human LN and can inhibit the proliferation and
inflammation of renal mesangial cells by targeting TNF
receptor-associated factor 6(18).
However, to the best of our knowledge, the relationship between
miR-98-5p and LN yet to be fully elucidated.
Previous research has highlighted the importance of
miR-98-5p in several diseases; for example, miR-98-5p has been
reported to protect cardiomyocytes from DOX-induced injury through
regulation of the caspase-8-dependent Fas/RIP3 pathway (19). Another study demonstrated that
miR-98-5p inhibits hepatic stellate cell activation and attenuates
liver fibrosis by regulating hepatic leukemia factor expression
(20). Additionally, the
diminution of miR-98-5p has been demonstrated to alleviate renal
fibrosis in diabetic nephropathy by elevating E3 ubiquitin-protein
ligase NEDD4-like and inactivating the TGF-β/Smad2/3 pathway
(21). Furthermore, miR-98-5p was
reported to ameliorate oxygen-glucose
deprivation/reoxygenation-induced neuronal injury by inhibiting
BACH1(22). However, to the best
of our knowledge, the relationship between miR-98-5p expression
levels and patients with LN has not yet been reported. The results
of the present study demonstrated that the expression levels of
miR-98-5p were downregulated in LN renal tissues compared with
control renal tissues. This suggested that miR-98-5p may be
associated with LN.
The current study also indicated that the miR-98-5p
mimic inhibited human mesangial cell proliferation, while the
miR-98-5p inhibitor promoted the same process. Moreover, miRNAs
modulate the expression levels of their target genes by targeting
the 3'-UTRs of mRNA, with ~2/3 of human mRNAs being regulated by
miRNAs in this manner (23).
According to bioinformatics predictions, the present study
hypothesized that miR-98-5p may affect the biological behavior of
human mesangial cells, at least in part, by targeting BACH1 mRNA to
regulate the expression levels of BACH1.
BACH1 is a heme binding protein composed of 739
amino acids with a molecular weight of 81 kDa, constituting the
relationship between heme content, redox and transcription
(24). BACH1 plays a key role in
the physiological regulation of oxidative stress by inhibiting its
important target, heme oxygenase 1(24). In addition, BACH1 is widely
expressed in human tissues (25).
Moreover, certain studies have demonstrated that BACH1 is closely
associated with LN. For example, the study by Kishimoto et
al (26) suggested that
dysregulated M2-like macrophages play a proinflammatory role in LN,
and that BACH1 represents a potential therapeutic target that can
restore the anti-inflammatory properties of M2 macrophages.
Furthermore, altered B cell subsets and cellular signatures of
BACH1 may be associated with distinct patient phenotypes associated
with the risk of LN relapse (27).
In the present study, the relative luciferase
activity of the WT plasmid + miR-98-5p mimic group was lower
compared with the NC and WT plasmid groups. The results of the dual
luciferase reporter assay suggested that miR-98-5p could directly
inhibit the expression of BACH1. Moreover, the present study
revealed high levels of BACH1 expression in LN renal tissue
compared with control tissues. BACH1-overexpression also promoted
human mesangial cell proliferation, as well as the secretion of
TNF-α and IL-6.
Further results of the present study showed that
BACH1 increased the p-NF-κB level, and that cell proliferation and
inflammatory factor secretion were inhibited by an NF-κB inhibitor.
These results revealed that BACH1 promoted cell proliferation and
inflammatory factor secretion through NF-κB. NF-κB is an important
signal pathway that promotes LN, including promoting mesangial cell
proliferation (28) and promoting
the secretion of inflammatory factors (29).
However, the study was only a preliminary in
vitro investigation on the role of miR-98-5p in human mesangial
cells; the relationship between miR-98-5p and LN could not be
confirmed. Furthermore, the internal environment is more complex,
and this in vitro study could not represent the true
situation in vivo. In the future, animal models should be
used to investigate the relationship between miR-98-5p and LN.
In conclusion, the results of the present study
indicated that the expression of miR-98-5p may be significantly
downregulated in human LN renal tissue. Mechanistically,
miR-98-5p-overexpression suppressed the proliferation of human
mesangial cells by targeting BACH1. Overall, these data indicated
that miR-98-5p and BACH1 may represent novel therapeutic strategies
for LN.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YD, JL and YJ made substantial contributions to the
conception and design of the study, as well as drafted and revised
the manuscript for important intellectual content. YD, JL, XS and
YJ acquired the data, and performed data analysis and
interpretation. YD and YJ confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript and
agree to be accountable for the accuracy and integrity of the
study.
Ethics approval and consent to
participate
The present study was approved by The Institute of
Medical Ethics Committee of Affiliated Hospital of Hebei University
(approval no. HBU201401221027; Hebei, China). Written informed
consent was obtained from all patients prior to surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Talotta R, Atzeni F and Laska MJ:
Therapeutic peptides for the treatment of systemic lupus
erythematosus: A place in therapy. Expert Opin Investig Drugs.
29:845–867. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ramanujam M, Steffgen J, Visvanathan S,
Mohan C, Fine JS and Putterman C: Phoenix from the flames:
Rediscovering the role of the CD40-CD40L pathway in systemic lupus
erythematosus and lupus nephritis. Autoimmun Rev.
19(102668)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang SF, Chen YH, Chen DQ, Liu ZZ, Xu F,
Zeng CH and Hu WX: Mesangial proliferative lupus nephritis with
podocytopathy: A special entity of lupus nephritis. Lupus.
27:303–311. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wofsy D, Hillson JL and Diamond B:
Comparison of alternative primary outcome measures for use in lupus
nephritis clinical trials. Arthritis Rheum. 65:1586–1591.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wright RD, Dimou P, Northey SJ and
Beresford MW: Mesangial cells are key contributors to the fibrotic
damage seen in the lupus nephritis glomerulus. J Inflamm (Lond).
16(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sung SJ and Fu SM: Interactions among
glomerulus infiltrating macrophages and intrinsic cells via
cytokines in chronic lupus glomerulonephritis. J Autoimmun.
106(102331)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kong J, Li L, Lu Z, Song J, Yan J, Yang J,
Gu Z and Da Z: Microrna-155 suppresses mesangial cell proliferation
and tgf-β1 production via inhibiting cxcr5-erk signaling pathway in
lupus nephritis. Inflammation. 42:255–263. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen CC, Chang ZY, Tsai FJ and Chen SY:
Resveratrol pretreatment ameliorates concanavalin a-induced
advanced renal glomerulosclerosis in aged mice through upregulation
of sirtuin 1-mediated klotho expression. Int J Mol Sci.
21(21)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Honarpisheh M, Köhler P, von Rauchhaupt E
and Lech M: The involvement of micrornas in modulation of innate
and adaptive immunity in systemic lupus erythematosus and lupus
nephritis. J Immunol Res. 2018(4126106)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Wang Y, Bao W, Liu Y, Wang S, Xu S, Li X,
Li Y and Wu S: miR-98-5p contributes to cisplatin resistance in
epithelial ovarian cancer by suppressing miR-152 biogenesis via
targeting Dicer1. Cell Death Dis. 9(447)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yuan S, Tang C, Chen D, Li F, Huang M, Ye
J, He Z, Li W, Chen Y, Lin X, et al: Mir-98 modulates cytokine
production from human PBMCS in systemic lupus erythematosus by
targeting IL-6 mRNA. J Immunol Res. 2019(9827574)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xie L and Xu J: Role of mir-98 and its
underlying mechanisms in systemic lupus erythematosus. J Rheumatol.
45:1397–1405. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang Y, Yu F, Song D, Wang SX and Zhao MH:
Podocyte involvement in lupus nephritis based on the 2003 ISN/RPS
system: A large cohort study from a single centre. Rheumatology
(Oxford). 53:1235–1244. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu J, Xu G, Zhang T, Chen T, Zhao W and
Wang G: NFIL3 Acts as a Nuclear Factor to Increase Osteosarcoma
Progression. BioMed Res Int. 2019(4068521)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mohr AM and Mott JL: Overview of microRNA
biology. Semin Liver Dis. 35:3–11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang L, Zhang X and Si F: MicroRNA-124
represents a novel diagnostic marker in human lupus nephritis and
plays an inhibitory effect on the growth and inflammation of renal
mesangial cells by targeting TRAF6. Int J Clin Exp Pathol.
12:1578–1588. 2019.PubMed/NCBI
|
|
19
|
Pan Y, Pan YM, Liu FT, Xu SL, Gu JT, Hang
PZ and Du ZM: MicroRNA-98 ameliorates doxorubicin-induced
cardiotoxicity via regulating caspase-8 dependent Fas/RIP3 pathway.
Environ Toxicol Pharmacol. 85(103624)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Q, Wei S, Zhou H, Li L, Zhou S, Shi
C, Shi Y, Qiu J and Lu L: MicroRNA-98 Inhibits Hepatic Stellate
Cell Activation and Attenuates Liver Fibrosis by Regulating HLF
Expression. Front Cell Dev Biol. 8(513)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zeng Y, Feng Z, Liao Y, Yang M, Bai Y and
He Z: Diminution of microRNA-98 alleviates renal fibrosis in
diabetic nephropathy by elevating Nedd4L and inactivating
TGF-β/Smad2/3 pathway. Cell Cycle. 19:3406–3418. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun X, Li X, Ma S, Guo Y and Li Y:
MicroRNA-98-5p ameliorates oxygen-glucose deprivation/reoxygenation
(OGD/R)-induced neuronal injury by inhibiting Bach1 and promoting
Nrf2/ARE signaling. Biochem Biophys Res Commun. 507:114–121.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Patil SL, Palat A, Pan Y, Rajapakshe K,
Mirchandani R, Bondesson M, Yustein JT, Coarfa C and Gunaratne PH:
MicroRNA-509-3p inhibits cellular migration, invasion, and
proliferation, and sensitizes osteosarcoma to cisplatin. Sci Rep.
9(19089)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kanezaki R, Toki T, Yokoyama M, Yomogida
K, Sugiyama K, Yamamoto M, Igarashi K and Ito E: Transcription
factor BACH1 is recruited to the nucleus by its novel alternative
spliced isoform. J Biol Chem. 276:7278–7284. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Warnatz HJ, Schmidt D, Manke T, Piccini I,
Sultan M, Borodina T, Balzereit D, Wruck W, Soldatov A, Vingron M,
et al: The BTB and CNC homology 1 (BACH1) target genes are involved
in the oxidative stress response and in control of the cell cycle.
J Biol Chem. 286:23521–23532. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kishimoto D, Kirino Y, Tamura M, Takeno M,
Kunishita Y, Takase-Minegishi K, Nakano H, Kato I, Nagahama K,
Yoshimi R, et al: Dysregulated heme oxygenase-1low M2-like
macrophages augment lupus nephritis via Bach1 induced by type I
interferons. Arthritis Res Ther. 20(64)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yap DYH, Yung S, Lee P, Yam IYL, Tam C,
Tang C and Chan TM: B Cell Subsets and Cellular Signatures and
Disease Relapse in Lupus Nephritis. Front Immunol.
11(1732)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu Y, Yu C, Ji K, Wang X, Li X, Xie H,
Wang Y, Huang Y, Qi D and Fan H: Quercetin reduces TNF-α-induced
mesangial cell proliferation and inhibits PTX3 production:
Involvement of NF-κB signaling pathway. Phytother Res.
33:2401–2408. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Sun J, Guo S, Niu F, Liu D and Zhuang Y:
Complement 1q protects MRL/lpr mice against lupus nephritis via
inhibiting the nuclear factor-κB pathway. Mol Med Rep.
22:5436–5443. 2020.PubMed/NCBI View Article : Google Scholar
|