Introduction
Acute coronary syndrome (ACS) is the most common
type of severe heart disease, which is mainly caused by damage to
major blood vessels, such as coronary vessels (1-3).
ACS defines a group of cardiovascular diseases, including unstable
angina pectoris (UAP) and acute myocardial infarction (AMI)
(3). Although current treatment
strategies of ACS, including percutaneous coronary angioplasty and
the tirofiban drug, have been developed for treating ACS, this
disease was responsible for >40% of all mortality of coronary
heart disease in China in 2017 (4,5).
Currently available diagnostic methods, such as coronary
angiography or echocardiography, fail to simultaneously achieve the
goals of convenience of application or high sensitivity,
significantly increasing the rate of misdiagnosis (6,7). It
has been previously reported that circulating brain natriuretic
peptide, high-sensitivity C-reactive protein (hs-CRP) and
myocardial enzymes can all predict the incidence of cardiovascular
events (8,9). However, an increase in the levels of
these biomarkers has also been found in other inflammatory
diseases, including periodontal disease and rheumatoid arthritis
(10,11). Therefore, identification of novel
biomarkers with high sensitivity and convenience for ACS diagnosis
would improve the outcome of this disease following treatment at
earlier stages.
Exosomes are small microvesicles with sizes of
30-150 nm that have the capacity to carry proteins, microRNAs (miR
or miRNA) and long noncoding RNAs (lncRNAs) (12,13).
Accumulating evidence has demonstrated that exosomes can serve as
messengers in mediating inflammation, vascular damage and apoptosis
(13,14). In particular, exosomes have also
been previously found to be involved in the pathophysiological
process of ACS (15,16). Yao et al (17) revealed that platelet-derived
exosomal miR-25-3p suppressed the inflammation of coronary artery
vascular endothelial cells by inhibiting the NF-κB pathway. Li
et al (18) reported that
platelet-derived exosomes activated by thrombin downregulated the
endothelial cell expression of intercellular cell adhesion
molecule-1 through miR-223 during the thrombosis-inflammatory
response. In addition, previous studies have demonstrated that both
exosomal surface molecules and its contents can be potentially
applied as biomarkers for ACS diagnosis (3,19,20).
Li et al (20) reported
that using serum-derived exosomal miR-146a levels conferred high
accuracy as a novel diagnostic biomarker for ACS. In addition,
serum exosomal miR-122-5p was found to be an independent biomarker
for ACS (19). Therefore, the
aforementioned observations suggest that exosomes can be used as
potentially non-invasive biomarkers for ACS.
Cysteine-rich protein 61 (Cyr61) has emerged as a
potential regulator of neovascularization, inflammation, thrombosis
and hemostasis, in addition to tissue remodeling (21,22).
Klingenberg et al (23)
reported that serum Cyr61 levels were significantly elevated in
patients with ST-elevation myocardial infarction compared with
those in patients with non-ST-elevation myocardial
infarction/unstable angina or stable ACS, irrespective of whether
coronary thrombi were present. Furthermore, Liu et al
(24) found that serum Cyr61
levels showed markedly accuracy in predicting the presence of ACS
by using receiver operating characteristic (ROC) curve analysis.
However, the potential role of exosomal Cyr61 in discriminating
patients with ACS from other similar conditions remains poorly
understood. Therefore, the present study was designed to assess the
potential of exosomal Cyr61 as a biomarker for ACS diagnosis and to
investigate the role of Cyr61 in vascular remodeling in
vitro.
Materials and methods
Patient samples
In total, 210 patients with ACS (male, 146; female,
54; age, 66.28±10.47) who received coronary stent implantation or
medical treatment at the Second Hospital of Hebei Medical
University (Shijiazhuang, China) between January 2016 and June 2020
were recruited for the present study. Recruited patients exhibited
symptoms compatible with angina pectoris (dyspnea and chest pain)
and fulfilled ≥ one of the following criteria (25): i) Rise in serial troponin levels
(the normal range of troponin being 0-0.04 ng/ml). The troponin
level was detected at the time of admission. It was checked every 4
h in the first 24 h, every 8 h in the second 24 h and once in the
third 24 h); ii) persistent ST-segment depression or elevation,
dynamic electrocardiogram (ECG) changes or T-inversion, new left
bundle branch block; and iii) diameter of coronary artery luminal
stenosis ≥50% as detected by coronary angiography. The exclusion
criteria were the following: i) Presence of inflammation,
infection, autoimmune diseases, progressive hepatic and renal
insufficiency, and tumor history; ii) history of ACS; and iii)
pregnancy. Based on their clinical diagnoses, 160 patients with ACS
were diagnosed with UAP and 50 patients with AMI. UAP is usually
triggered by physical exercise, emotional excitement or a cold
environment. The attack time is short and frequent. Nitroglycerin
treatment can significantly alleviate the symptoms. For AMI, there
is no obvious trigger. The attack time is long and up to several
hours. There is no remission after nitroglycerin treatment.
Patients often have fever, increased leukocytes and rapid
erythrocyte sedimentation rate. The patients display a necrotic Q
wave in ECG (26).
In addition, 50 healthy individuals with a mean age
of 64.56±9.61 years (male, 22; female, 28) who underwent physical
examination at the Second Hospital of Hebei Medical University
(Shijiazhuang, China) were recruited in the present study as the
control group between January 2016 and June 2020. All healthy
subjects had normal coronary arteries and history with normal ECG
characteristics and/or ECG treadmill test results. Participants
with severe concomitant diseases, including cardiomyopathy,
congenital heart disease, cerebrovascular or peripheral vascular
diseases, trauma or surgery within the last 3 months, chronic or
acute infection, malignant tumors, immune diseases, hepatic or
renal failure or a history of recent cardiopulmonary resuscitation
were excluded from this study.
The present study was approved by the Institutional
Ethics Committee of the Second Hospital of Hebei Medical
University. Written informed consent was obtained from every
participant in the present study. A total of 5 ml peripheral blood
samples were obtained from patients with ACS at the time of
diagnosis and healthy individuals. Cell-free plasma was isolated
within 6 h after collection by centrifugation at 4˚C (1,000 x g for
10 min), before being stored at -80˚C until further
experimentation.
Exosome isolation and
identification
Exosomes were collected from the plasma by
sequential ultracentrifugation. Plasma was centrifuged at 3,000 x g
for 20 min at 4˚C, followed by centrifugation at 12,000 x g for 20
min at 4˚C. Subsequently, exosomes were collected by
ultracentrifugation (100,000 x g for 70 min at 4˚C) in the pellet.
After suspension in 20 ml PBS, the exosomes were collected again by
ultracentrifugation (100,000 x g for 70 min at 4˚C) in the pellet.
The morphological characteristics of the exosomes were verified by
transmission electron microscopy. Nanoparticle tracking analysis
(NTA) using a Malvern Zetasizer Nano ZS90 (Malvern Panalytical) was
performed to measure the concentration and size of exosomes.
Transmission electron microscopy
Freshly prepared exosomes ml) were diluted in PBS
(20 ml) and fixed with 3.5% glutaraldehyde for 5 min at 4˚C.
Subsequently, exosome preparation was allowed to adsorb in a mesh
copper grid. The resulting grids were rinsed two times with wash
buffer and contrasted by 2% uranyl-oxalate solution (pH 7.0) for 5
min at 25˚C. The visualization of exosomes morphology was performed
using a H-9500 transmission electron microscope (Hitachi, Ltd.) at
300 kV, images were acquired using a F114 slow-scan CCD camera
(Tietz Video and Image Processing Systems GmbH) and analyzed using
EM-Menu v3.0 basic (Tietz Video and Image Processing Systems
GmbH).
Western blotting
Exosomes and vascular smooth muscle cells (VSMCs)
were lysed using RIPA buffer containing protease inhibitors
(Beyotime Institute of Biotechnology). Each sample was quantified
by BCA protein quantitative kit (Beijing Solarbio Science &
Technology Co., Ltd.). Subsequently, 50 µg of protein per lane were
separated by 10% SDS-PAGE and transferred onto 0.22-µm PVDF
membranes. The membranes were then blocked with 5% non-fat milk for
60 min at 25˚C. The membranes were subsequently incubated with
primary antibodies against CD9 (dilution 1:1,000; cat. no.
ab223052; Abcam), CD63 (dilution 1:100; cat. no. ab216130; Abcam),
flotillin-1 (dilution 1:500; cat. no. ab13493; Abcam), Cyr61
(dilution 1:1,500; cat. no. ab228592; Abcam) and tumor
susceptibility 101 (TSG101; dilution 1:1,000; cat. no. ab30871;
Abcam) at 4˚C for 12 h, followed by incubation with an
HRP-conjugated secondary antibody (dilution 1:10,000; cat. no.
ab205718; Abcam) for 120 min at 25˚C. Bound antibodies were
detected using BM Chemiluminescence Western Blotting kit (cat. no.
11520709001; MilliporeSigma) and ImageJ v1.8 software (National
Institutes of Health).
Cyr61 analysis
Exosomes were lysed using RIPA buffer containing
protease inhibitors (Beyotime Institute of Biotechnology). Cyr61
levels were then measured using the CYR61 ELISA kit (cat. no.
SBJ-H0152; Nanjing SenBeiJia Biological Technology Co., Ltd.)
according to the manufacturer's instructions. Calibration and
standardization of the assay was performed according to the
manufacturer's protocol.
Cell culture and oxidized low-density
lipoprotein (ox-LDL) treatment
Human VSMCs from the National Infrastructure of Cell
Line Resource (cat. no. 4201HUM-CCTCC00632) were cultured in DMEM
(Thermo Fisher Scientific, Inc.) containing 10% FBS (Thermo Fisher
Scientific, Inc.) and incubated at 37˚C with 5% CO2. To
construct the atherosclerosis model in vitro, VSMCs were
treated with ox-LDL (Shanghai Yeasen Biotechnology Co., Ltd.) at
different concentrations (25, 50 and 100 mg/ml) for 24 h at 37˚C.
In addition, VSMCs were treated with ox-LDL (50 mg/ml) for
different times (12, 24 and 48 h) at 37˚C.
Cell transfection
Small interference RNA (siRNA) and negative control
(NC) for Cyr61 were synthesized by Sangon Biotech Co., Ltd. VSMCs
(1x106 cells/ml) were cultured in six-well plates.
According to the manufacturer's instructions,
Lipofectamine® 2000 reagent (Thermo Fisher Scientific,
Inc.) and siRNA (50 nmol/well) in serum-free DMEM medium were added
to cultured cells for 4 h at 37˚C. The medium was then replaced
with DMEM containing 10% FBS for 48 h at 37˚C. The siRNA sequences
used were as follows: NC sense, 5'-UUCUCCGAACGUGUCACGCTT-3' and
antisense, 5'-ACGUGACACGUUCGGAGAATT-3'; and siCyr61 sense,
5'-CAACGAGGACUGCAGCAAATT-3' and antisense,
5'-UUUGCUGCAGUCCUCGUUGAG-3'.
Cell Counting Kit-8 (CCK-8) assay
siCyr61-transfected VSMCs were treated with ox-LDL
(50 mg/ml). Subsequently, VSMCs (3,000 cells/well) were seeded into
96-well plates in triplicate wells. After 24 h at 37˚C, 10 µl CCK-8
solution (Beijing Biosynthesis Biotechnology Co., Ltd.) was added
and the VSMCs were cultured for 2 h at 37˚C. The optical density
value at 450 nm was detected in each well using a microplate reader
(Molecular Devices, LLC).
Flow cytometry
siCyr61-transfected VSMCs were treated with ox-LDL
(50 mg/ml). Subsequently, VSMCs were incubated in binding buffer
(1x106 cells/ml), followed by staining with Annexin
V-FITC (200 µl) and propidium iodide (PI) solutions (10 µl) at 25˚C
for 15 min using the Annexin V-FITC/PI Apoptosis Detection kit
(cat. no. A211-01; Vazyme Biotech Co., Ltd.) according to the
manufacturer's instructions. The apoptotic rate was detected using
a flow cytometer (FACSCanto III; BD Biosciences) and FlowJo v10.4
software (BD Biosciences).
Transwell assay
siCyr61-transfected VSMCs were treated with ox-LDL
(50 mg/ml). Subsequently, VSMCs resuspended in FBS-free DMEM were
added to the upper chamber of Transwell plates (Corning, Inc.) with
a cell density of 1x105 cell/ml. Subsequently, the
bottom chamber was filled with DMEM supplemented with 30% FBS.
After a 48-h culture at 37˚C, VSMCs that failed to migrate to the
lower chamber were removed. Next, the lower membrane was fixed with
4% paraformaldehyde for 30 min at 25˚C and the migratory cells were
stained with 0.1% crystal violet for 20 min at 25˚C. Migratory
cells from five randomly chosen fields (magnification, x40) from
each chamber were photographed using light microscopy and counted
using ImageJ v1.8 (National Institutes of Health).
Statistical analysis
Statistical analyses in the present study were
conducted using SPSS 22.0 software (IBM Corp.). χ2 test,
Fisher's exact test and t-test were performed for comparisons
between two groups. Differences among > two groups were
evaluated by one-way analysis of variance followed by Tukey's test.
The ROC curves were constructed to evaluate the diagnostic accuracy
of exosomal Cyr61. A multivariate analysis was applied to identify
independent predictors of the existence of ACS. A two-tailed
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological characteristics of
the study participants
The biochemical and clinical parameters of the 160
patients with UAP, 50 patients with AMI and 50 healthy individuals
are presented in Table I. No
significant difference was observed in the distribution of age,
body mass index (BMI), systolic blood pressure (SBP), diastolic
blood pressure (DBP) or history of hypertension, diabetes,
dyslipidemia or smoking. However, compared with those in healthy
subjects, the male sex was significantly more prevalent among
patients with ACS. Furthermore, between healthy UAP patients and
AMI patients, there were significant differences in ejection
fraction (EF), hemoglobin A1c (HbA1c), creatinine (CR), glucose and
high-sensitivity C-reactive protein (hs-CRP; all P<0.05;
Table I).
 | Table IDemographic characteristics of the
study subjects. |
Table I
Demographic characteristics of the
study subjects.
| Variables | Healthy (n=50) | Unstable angina
pectoris (n=160) | Acute myocardial
infarction (n=50) | F-value | P-value |
|---|
| General
information | | | | | |
|
Age,
years | 64.56±9.61 | 67.34±9.96 | 64.6±12.46 | 2.163 | 0.117 |
|
Sex,
male/female | 22/28 | 106/54 | 40/10 | 14.657 | 0.001 |
|
Body mass
index, kg/m2 | 23.37±2.20 | 23.62±2.79 | 23.75±2.47 | 0.285 | 0.752 |
|
Systolic
blood pressure, mmHg | 143.42±19.19 | 145.53±88.40 | 125.76±24.97 | 1.509 | 0.223 |
|
Diastolic
blood pressure, mmHg | 78.74±12.58 | 77.69±10.88 | 78.64±13.89 | 0.219 | 0.804 |
| Medical
history | | | | | |
|
Hypertension,
N (%) | 35(70) | 117 (73.13) | 34(68) | 0.563 | 0.755 |
|
Diabetes, N
(%) | 7(14) | 40(25) | 14(28) | 5.462 | 0.065 |
|
Dyslipidemia,
N (%) | 12(24) | 47 (29.38) | 13(26) | 0.638 | 0.727 |
|
Smoking, N
(%) | 9(18) | 46 (28.75) | 20(40) | 5.897 | 0.052 |
|
Family
history of ACS, N (%) | 0 | 17 (10.63) | 7(14) | 11.230 | 0.004 |
| Cardiac ultrasound
indices | | | | | |
|
LVID at
diastole, cm | 4.75±0.50 | 4.94±0.54 | 4.88±0.62 | 2.257 | 0.107 |
|
LVID at
systole, cm | 2.93±0.51 | 3.12±0.65 | 3.14±0.65 | 2.068 | 0.129 |
|
Left atrial,
cm | 3.74±0.62 | 3.91±0.61 | 3.91±0.52 | 1.676 | 0.189 |
|
Fractional
shortening, % | 38.52±6.47 | 37.66±7.41 | 35.39±7.54 | 2.609 | 0.076 |
|
Ejection
fraction, % | 68.15±8.72 | 66.76±10.4 | 61.14±11.87 | 6.942 | 0.001 |
| Laboratory
indices | | | | | |
|
Hemoglobin
A1c, % | 5.75±0.99 | 5.97±0.99 | 6.31±1.46 | 3.346 | 0.037 |
|
Creatinine,
µmol/l | 73.4±14.55 | 82.55±17.41 | 89.48±27.46 | 8.796 | <0.001 |
|
Estimated
glomerular filtration rate, ml/min | 81.81±14.71 | 76.28±15.50 | 75.75±19.02 | 2.512 | 0.083 |
|
Glucose,
mmol/l | 5.63±1.72 | 5.67±1.44 | 7.11±3.12 | 11.473 | <0.001 |
|
Triglyceride,
mmol/l | 1.42±0.62 | 1.59±0.93 | 1.66±1.08 | 0.903 | 0.407 |
|
Total
cholesterol, mmol/l | 4.2±0.94 | 4.22±1.13 | 4.43±1.24 | 0.752 | 0.472 |
|
High-density
lipoprotein cholesterol, mmol/l | 1.23±0.31 | 1.23±0.29 | 1.12±0.28 | 2.685 | 0.070 |
|
Low-density
lipoprotein cholesterol, mmol/l | 2.29±0.69 | 2.32±0.79 | 2.48±0.92 | 0.866 | 0.422 |
|
High-sensitivity
C-reactive protein, mg/l | 6.96±6.98 | 6.28±6.41 | 25.72±35.77 | 26.960 | <0.001 |
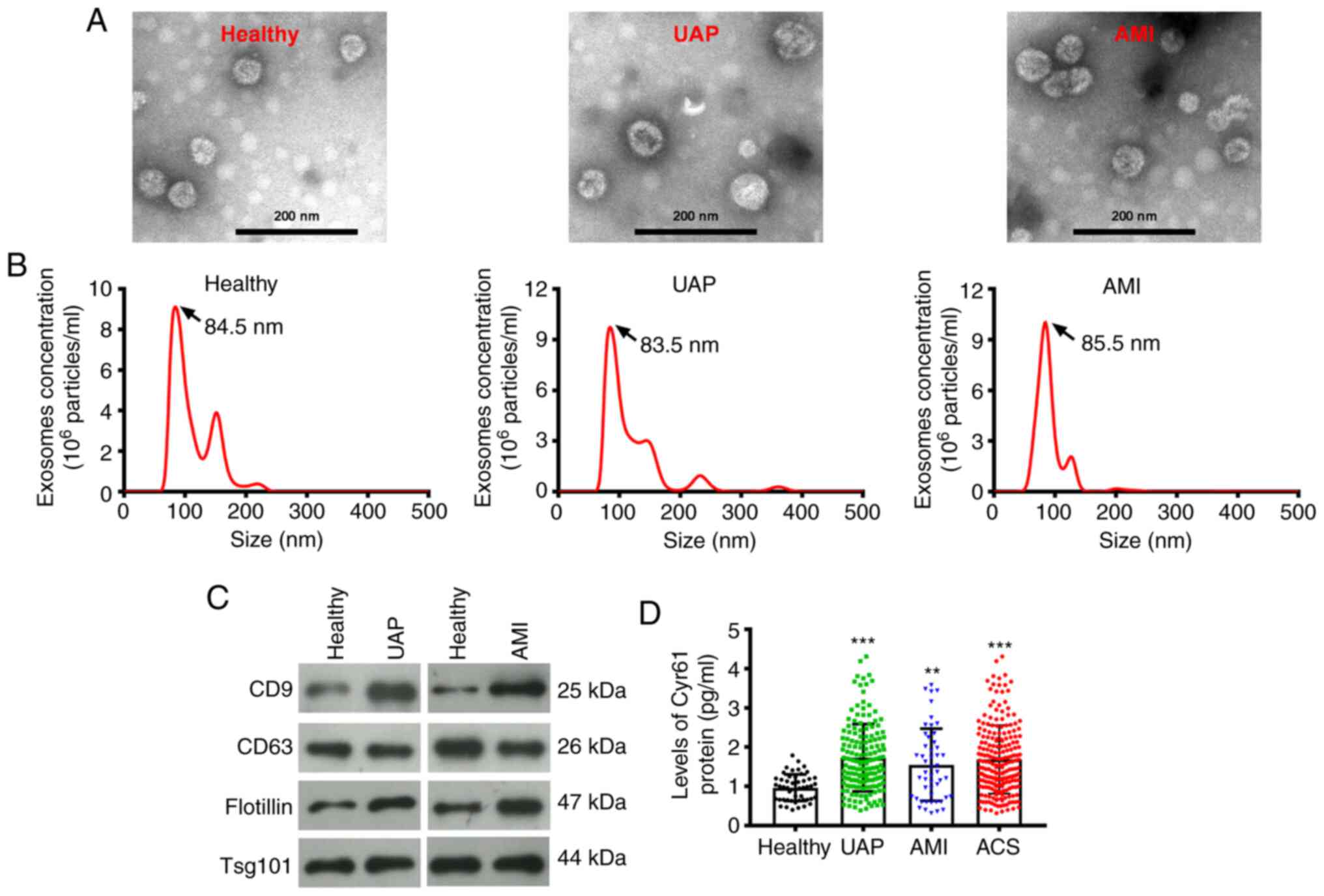
Expression pattern of exosomal Cyr61
in patients with ACS
Firstly, plasma exosomes were isolated from blood
samples collected from healthy individuals, patients with UAP and
patients with AMI. Transmission electron microscopy and
nanoparticle tracking analysis revealed that the plasma exosomes
displayed a round-shaped morphology with diameters ranging from 60
to 110 nm (Fig. 1A). The size
distribution of the exosomes isolated from healthy individuals,
UAP, and AMI plasma samples was 85.73±10.13, 89.7±10.81 and
87.56±13.92 nm, respectively (Fig.
1B). Furthermore, the expression of exosome markers CD9, CD63,
flotillin and TSG101 were examined by western blotting. Exosomes
isolated from healthy individuals, patients with UAP and patients
with AMI were tested positive for CD9 expression in addition to
CD63, flotillin and TSG101 (Fig.
1C). To assess the expression pattern of Cyr61 derived from the
exosomes, ELISA was performed. As shown in Fig. 1D, the level of Cyr61 in exosomes
isolated from patients with ACS was significantly higher compared
with that in healthy individuals (P<0.001). Subsequently,
changes in exosomal Cyr61 levels among the three ACS subtypes were
evaluated. Compared with those in healthy individuals,
exosome-derived Cyr61 levels in the UAP subgroup (P<0.001;
Fig. 1D) or AMI subgroup
(P<0.01) were also significantly higher. However, there was no
significant difference in exosomal Cyr61 levels between those in
the UAP and AMI subgroups (Fig.
1D).
Association of exosomal Cyr61 levels
with clinical characteristics
Based on the median value of exosomal Cyr61 levels
obtained from ELISA experiments, the 210 patients with ACS were
equally divided into two groups, the high Cyr61 group (exosomal
Cyr61 >1.545 pg/ml) and the low Cyr61 group (exosomal Cyr61
<1.545 pg/ml). Association analysis of exosomal Cyr61 levels
with the clinical characteristics of the 210 patients with ACS were
then performed. As shown in Table
II, high exosomal Cyr61 levels were significantly associated
with sex (P=0.016), family history of ACS (P=0.002) and glucose
levels (P=0.001). By contrast, there is no significant association
between exosomal Cyr61 levels and other parameters, namely age,
BMI, SBP, DBP, left ventricular internal diameter (LVID) at
diastole, LVID at systole, left atrial, fractional shortening, EF,
HbA1c, CR, estimated glomerular filtration rate, TG, TC,
high-density lipoprotein cholesterol, low-density lipoprotein
cholesterol and hs-CRP (Table
II).
 | Table IIAssociation of exosomal Cyr61 levels
with clinical and biochemical parameters. |
Table II
Association of exosomal Cyr61 levels
with clinical and biochemical parameters.
| Parameters | Low Cyr61 group
(n=105) | High Cyr61 group
(n=105) |
t/χ2-value | P-value |
|---|
| Age, years | 66.48±9.95 | 66.9±11.34 | 0.291 | 0.771 |
| Sex,
male/female | 81/24 | 65/40 | 5.753 | 0.016 |
| Body mass index,
kg/m2 | 23.94±3.04 | 23.36±2.32 | 1.557 | 0.121 |
| Systolic blood
pressure, mmHg | 144.21±108.79 | 137.44±22.94 | 0.624 | 0.533 |
| Diastolic blood
pressure, mmHg | 77.37±11.20 | 78.47±12.09 | 0.681 | 0.497 |
| Hypertension, N
(%) | 74 (70.48) | 77 (73.33) | 0.212 | 0.645 |
| Diabetes, N
(%) | 28 (26.67) | 26 (24.76) | 0.1 | 0.752 |
| Dyslipidemia, N
(%) | 33 (31.43) | 27 (25.71) | 0.840 | 0.359 |
| Smoking, N (%) | 38 (36.19) | 28 (26.67) | 2.210 | 0.137 |
| Family history, N
(%) | 5 (4.76) | 19 (18.10) | 9.220 | 0.002 |
| LVID at diastole,
cm | 5±0.50 | 4.86±0.61 | 1.837 | 0.068 |
| LVID at systole,
cm | 3.14±0.63 | 3.11±0.68 | 0.376 | 0.707 |
| Left atrial,
cm | 3.96±0.60 | 3.86±0.58 | 1.151 | 0.251 |
| Fractional
shortening, % | 37.25±7.47 | 36.99±7.53 | 0.246 | 0.806 |
| Ejection fraction,
% | 65.25±11.23 | 65.59±10.83 | 0.225 | 0.822 |
| Hemoglobin A1c,
% | 6.13±1.30 | 5.97±0.91 | 1.019 | 0.309 |
| Creatinine,
µmol/l | 86.3±21.92 | 82.1±18.63 | 1.493 | 0.137 |
| Estimated
glomerular filtration rate, ml/min | 75.92±16.29 | 76.39±16.51 | 0.208 | 0.835 |
| Glucose,
mmol/l | 6.48±2.61 | 5.54±1.12 | 3.388 | 0.001 |
| Triglyceride,
mmol/l | 1.58±0.89 | 1.62±1.04 | 0.300 | 0.765 |
| Total cholesterol,
mmol/l | 4.27±1.17 | 4.27±1.14 | 0.009 | 0.993 |
| High-density
lipoprotein cholesterol, mmol/l | 1.23±0.31 | 1.17±0.26 | 1.493 | 0.137 |
| Low-density
lipoprotein cholesterol, mmol/l | 2.32±0.83 | 2.4±0.82 | 0.716 | 0.475 |
| High-sensitivity
C-reactive protein, mg/l | 12.18±22.24 | 9.64±17.5 | 0.921 | 0.358 |
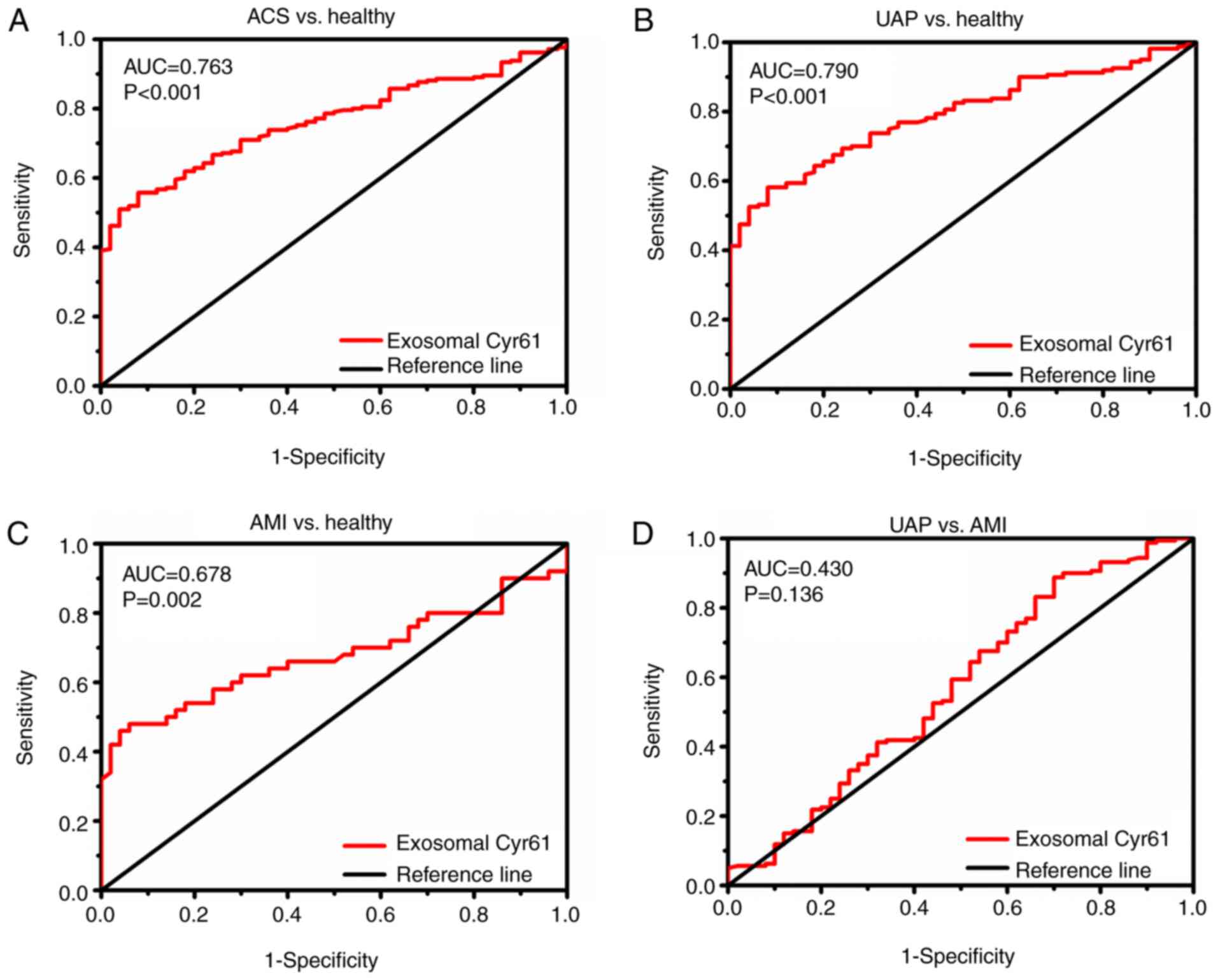
Evaluation of exosomal Cyr61 as a
diagnostic marker
Given that Cyr61 levels were significantly higher in
plasma exosomes of patients with ACS, the diagnostic accuracy of
exosomal Cyr61 as a biomarker to discriminate patients with ACS
from individuals was evaluated further. As shown in Fig. 2A and Table III, exosomal Cyr61 levels could
differentiate between patients with ACS and healthy subjects with
high accuracy [area under the curve (AUC), 0.763; 95% confidence
interval (CI), 0.705-0.822; P<0.01]. At the cut-off value of
1.435 pg/ml for exosomal Cyr61, the optimal sensitivity and
specificity were 55.2 and 92%, respectively. Subsequently, the
efficiency of using exosomal Cyr61 as a diagnostic marker for
identifying the ACS subgroups was analyzed.
 | Table IIIData obtained from the receiver
operating characteristic curve corresponding to results shown in
Fig. 2, including AUC, 95% CI,
sensitivity, specificity and Youden index. |
Table III
Data obtained from the receiver
operating characteristic curve corresponding to results shown in
Fig. 2, including AUC, 95% CI,
sensitivity, specificity and Youden index.
| Indices | AUC | 95% CI
(Down-up) | P-value | Sensitivity
(%) | Specificity
(%) | Yoden index | Cut-off value |
|---|
| ACS vs.
healthy | 0.763 | 0.705-0.822 | <0.001 | 55.2 | 92 | 0.472 | 1.435 |
| UAP vs.
Healthy | 0.790 | 0.730-0.851 | <0.001 | 57.5 | 92 | 0.495 | 1.435 |
| AMI vs.
Healthy | 0.678 | 0.567-0.788 | 0.002 | 48 | 94 | 0.420 | 1.485 |
| UAP vs. AMI | 0.430 | 0.333-0.527 | 0.136 | 90 | 93.7 | 0.038 | 3.155 |
ROC curve analyses (Fig. 2B and Table III) revealed that exosomal Cyr61
could discriminate patients with UAP from healthy individuals with
AUC values of 0.790 (95% CI, 0.730-0.851; P<0.001). The optimal
cut-off values of exosomal Cyr61 were 1.435 pg/ml (57.5%
sensitivity and 92% specificity) to detect UAP. Furthermore,
exosomal Cyr61 was also found to be an accurate marker for
discriminating patients with AMI from healthy individuals, with AUC
values of 0.678 (95% CI, 0.567-0.788; P=0.002). At the cut-off
value of 1.485 pg/ml for exosomal Cyr61, the optimal sensitivity
and specificity were 48 and 94%, respectively (Fig. 2C and Table III). However, the ability of
exosomal Cyr61 in differentiating patients with UAP from patients
with AMI was not satisfactory (P=0.136; Fig. 2D and Table III).
Multivariable logistic regression analyses were
subsequently performed to identify potential independent
contribution of each parameter to the presence of ACS. The results
(Table IV) revealed that exosomal
Cyr61 (P<0.001) and CR (P=0.001) were independently associated
with the presence of ACS. However, EF, HbA1c, glucose and hs-CRP
levels were not significantly associated with ACS (Table IV).
 | Table IVMultivariate analysis of the
association between exosomal Cyr61 levels and acute coronary
syndrome. |
Table IV
Multivariate analysis of the
association between exosomal Cyr61 levels and acute coronary
syndrome.
| | 95% confidence
interval for Exp (β) |
|---|
| Variables | β value | Standard error | Wald | P-value | Exp (β) | Lower | Upper |
|---|
| Ejection
fraction | -0.009 | 0.020 | 0.204 | 0.652 | 0.991 | 0.952 | 1.031 |
| Hemoglobin A1c | 0.249 | 0.238 | 1.100 | 0.294 | 1.283 | 0.805 | 2.045 |
| Creatinine | 0.056 | 0.016 | 11.815 | 0.001 | 1.058 | 1.024 | 1.092 |
| Glucose | 0.096 | 0.145 | 0.443 | 0.506 | 1.101 | 0.829 | 1.462 |
| High-sensitivity
C-reactive protein | 0.018 | 0.019 | 0.852 | 0.356 | 1.018 | 0.980 | 1.057 |
| Exosomal Cyr61 | 1.980 | 0.384 | 26.619 | <0.001 | 7.242 | 3.414 | 15.365 |
Cyr61 regulates proliferation and
migration in ox-LDL-stimulated VSMCs
To investigate the role of Cyr61 in atherosclerosis,
the expression levels of Cyr61 were measured in VSMCs after
stimulation with ox-LDL. As shown in Fig. 3A and B, Cyr61 levels were significantly
increased in VSMCs following a 24-h exposure to ox-LDL in a
concentration-dependent manner. Ox-LDL treatment also induced a
significant increase in Cyr61 expression in a time-dependent manner
(Fig. 3C and D). Subsequently, the efficiency of Cyr61
knockdown was confirmed after using siRNAs in VSMCs: siCyr61
transfection significantly decreased the expression level of Cyr61
compared with the NC group (P<0.01; Fig. 3E). Moreover, the increased
expression of Cyr61 induced by ox-LDL was partially inhibited by
siCyr61 transfection (P<0.01; Fig.
3F and G). Cell viability
analysis revealed that, when compared with that in the control
group, exposure to ox-LDL (50 µg/ml) significantly enhanced cell
viability (P<0.01), whilst knocking down Cyr6 expression
significantly reduced cell viability in the presence of ox-LDL at
48 and 72 h, compared with the ox-LDL group (P<0.01; Fig. 3H). Furthermore, following treatment
with 50 µg/ml ox-LDL for 24 h, the levels of cell apoptosis were
significantly reduced (P<0.01; Fig.
3I and J). By contrast, Cyr61
knockdown significantly increased the apoptosis rate of VSMCs
compared with that in the ox-LDL group (P<0.01; Fig. 3I and J). In addition, a significant increase in
the number of migratory cells was observed in response to ox-LDL
treatment, then was partially but significantly reversed by Cyr61
knockdown (P<0.01; Fig. 3K and
L).
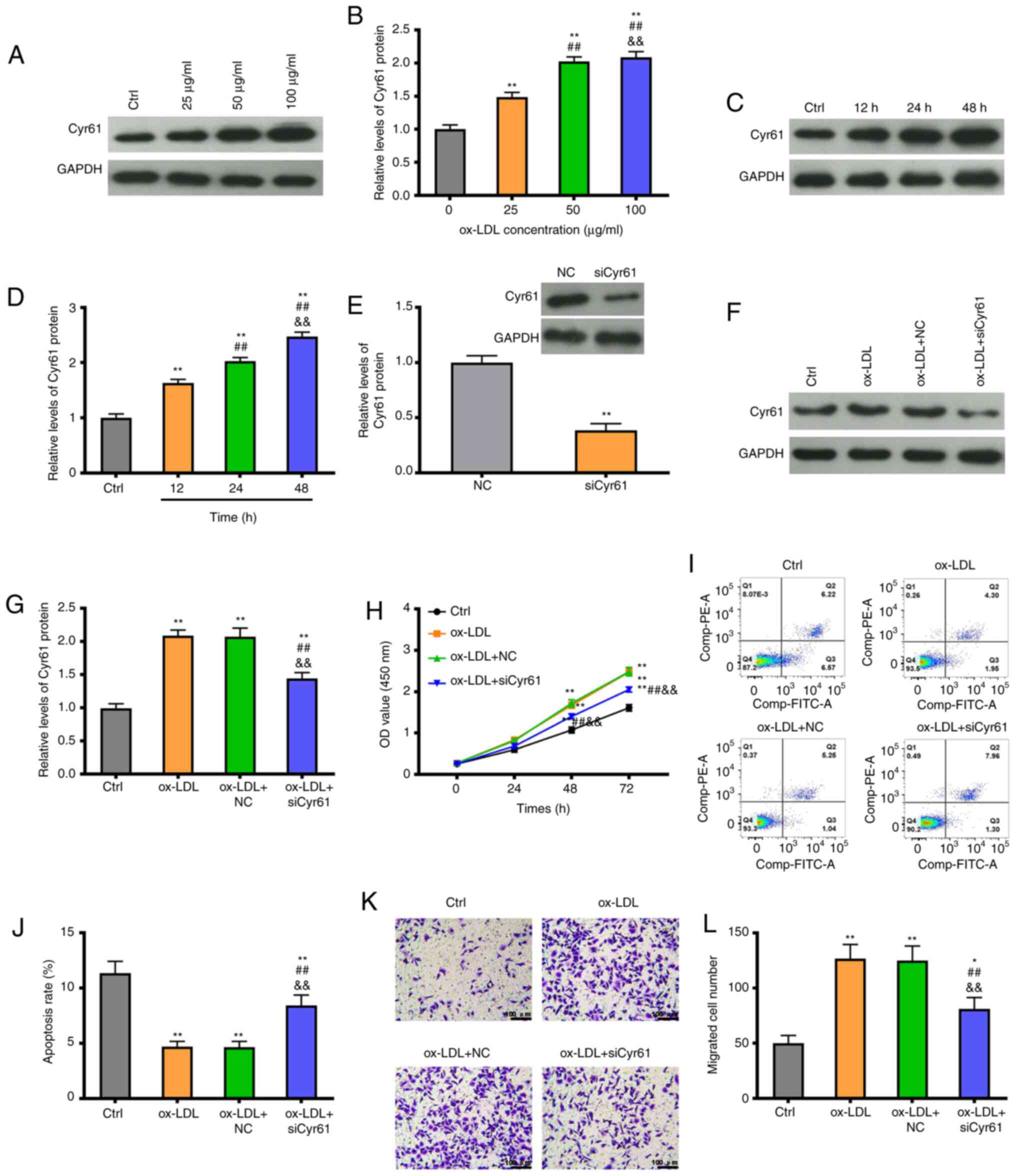 | Figure 3Knockdown of Cyr61 expression
inhibits cell proliferation and migration whilst promoting
apoptosis in the ox-LDL-induced atherosclerosis VSMC model in
vitro. (A) Representative western blotting images, (B) showing
that Cyr61 expression is increased in VSMCs after treatment with
ox-LDL at different concentrations observed at 24 h. (C)
Representative western blotting images, (D) showing that Cyr61
expression is increased in VSMCs treated with 50 µg/ml ox-LDL at
different time points. (E) Cyr61 expression levels were measured by
western blotting in VSMCs transfected with siCyr61 or NC. (F) Cyr61
expression levels were measured by western blotting in VSMCs
transfected with siCyr61 or NC following treatment with ox-LDL (50
µg/ml) or in controls, (G) which were quantified. (H) Cell
viability was detected by the Cell Counting Kit-8 assay in VSMCs
transfected with siCyr61 or NC following treatment with or without
ox-LDL (50 µg/ml). (I) Cell apoptosis was determined by flow
cytometry in VSMCs transfected with siCyr61 or NC after treatment
following treatment with ox-LDL (50 µg/ml) or in controls, (J)
which was quantified. (K) Cell migration was measured using
Transwell assays in VSMCs transfected with siCyr61 or NC following
treatment with ox-LDL (50 µg/ml) or in controls, (L) and was
quantified. Scale bars, 100 µm. *P<0.05 and
**P<0.01 vs. Ctrl or NC; ##P<0.01 vs.
ox-LDL 25 µg/ml or 12 h; &&P<0.01 vs. ox-LDL
+ NC 50 µg/ml or 24 h. si, small interfering; ox-LDL, oxidized
low-density lipoprotein; NC, negative control; Cyr61, cysteine-rich
protein 61; VSMCs, vascular smooth muscle cells. |
Discussion
ACS remains a major health concern worldwide despite
advances in treatment strategies (27). There is a demand for highly
sensitive and specific biomarkers for ACS to facilitate ACS
diagnosis. In the present study, Cyr61 expression was first
profiled in plasma-derived exosomes, which found that the levels of
exosomal Cyr61 were increased in patients with UAP and AMI compared
with those in healthy individuals. In addition, the levels of
exosomal Cyr61 were found to be significantly associated with sex
and glucose levels. Subsequently, the results of ROC curve analyses
indicated that exosomal Cyr61 could effectively differentiate
patients with UAP, AMI and ACS from healthy individuals.
Multivariable logistic regression analyzes showed that high
exosomal Cyr61 levels was independently associated with the
presence of ACS. The role of Cyr61 in vitro was then
explored, which found that the elevated cell viability and
migration induced by ox-LDL was reversed by Cyr61 knockdown, which
also significantly increased the rate of apoptosis in VSMCs
compared with cells exposed to ox-LDL alone. These results suggest
that increased Cyr61 levels were associated with atherosclerosis,
which may provide a rationale for the application of exosomal Cyr61
levels in the clinical diagnosis of ACS.
Previous studies have reported the value of using
Cyr61 for the diagnosis and prognosis of ACS (23,24,28).
Klingenberg et al (23)
reported that the diagnostic accuracy of Cyr61 was superior to that
of high sensitivity-troponin T for detecting coronary thrombi in a
subset of 1641 patients with available data, which was subsequently
confirmed by angiography. However, these authors found that the
sensitivity (55%) and specificity (70%) of Cyr61 for ACS diagnosis
was moderate (23). Exosomes
containing nucleic acids, proteins and lipids are secreted by
membrane-enclosed vesicles (extracellular vesicles) and can be
released into a variety of bodily fluids, including plasma, saliva
and urine (29,30). Accumulating evidence supports the
notion that exosomal cargos can be used as a diagnostic biomarker
of ACS (16,31). For instance, Ling et al
(3) found that serum exosomal
miRNA-21, miRNA-126 and PTEN are novel biomarkers for the diagnosis
of ACS. Therefore, for the present study the potential of exosomal
Cyr61 for ACS diagnosis was explored.
It was found that exosomal Cyr61 levels were higher
in patients with UAP and AMI compared with those in healthy
individuals. These findings are consistent with those in a recent
study. where serum Cyr61 levels were found to be significantly
increased among ACS patients compared with healthy volunteers
(23). Choi et al (32) previously revealed that Cyr61
synthesis is induced by IL-6 in fibroblast-like synoviocytes.
Furthermore, atherosclerotic arteries have also been reported as
having significantly higher IL-6 levels compared with their
non-atherosclerotic counterparts (33). Therefore, it is reasonable to
suggest that increases in IL-6 levels can lead to an increase in
Cyr61 levels in the plasma exosomes of patients with ACS. The
present study found that the levels of exosomal Cyr61 were
significantly associated with sex, family history of ACS and
glucose levels in patients with ACS. Similar patterns were also
observed in a study by Feng et al (34), in which Cyr61 was significantly
associated with the risk of peripheral artery disease in both the
crude and adjusted models, including age, sex, diabetes duration,
fasting glucose and hypertension. In addition, a previous study has
demonstrated that serum Cyr61 levels were positively correlated
with CRP levels in coronary artery disease (23). However, no significant association
was found between exosomal Cyr61 levels and hs-CRP. This may be due
to differences between exosomal and serum expression levels. The
present data suggested that exosomal Cyr61 could be a candidate for
ACS diagnosis.
ROC curve analysis in the present study revealed a
high accuracy of exosomal Cyr61 in differentiating patients with
ACS from healthy individuals, which was notably higher in
sensitivity and specificity compared with that reported by
Klingenberg et al (23),
although the AUC was lower compared with that reported by Deng
et al (25). These
differences may be attributed to differences among the patients
selected (ACS vs. coronary artery disease). Therefore, large-scale,
multicenter studies are required to elucidate whether Cyr61 derived
from exosomes can represent an accurate biomarker for ACS
diagnosis. However, multivariate logistic regression analyses
performed in the present study showed that exosomal Cyr61 levels
were independently associated with the presence of ACS, supporting
the possible role of exosomal Cyr61 as a potential biomarker for
ACS diagnosis. In addition, the present study revealed that
exosomal Cyr61 can be used for discriminating patients with UAP or
AMI from healthy individuals, although the ability of exosomal
Cyr61 in differentiating patients with UAP from patients with AMI
was not satisfactory. Therefore, additional indicators in
combination with exosomal Cyr61 may be needed to make this
distinction.
Exosomes are derived from blood vessels and other
tissues, which can be used to reflect molecular information about
ACS (12-15).
Some cell types, such as tumor cells and dendritic cells, can
secrete larger quantities of exosomes compared with normal cells
(35,36). It was reported that tumor
cell-derived exosomes have a relatively stable structure, which can
protect its cargos from destruction (37). These reported characteristics
suggest that the detection of exosomal Cyr61 can be more specific
than serum Cyr61. At present, exosomes can be rapidly extracted,
which can be exploited to monitor changes in the expression of
molecular markers during the process of ACS pathogenesis (38). The advantage of exosomes and
findings from the present study suggest that exosomal Cyr61 is an
able biomarker for ACS diagnosis.
Atherosclerosis is one of the most frequently
observed pathological process underlying cardiovascular diseases
(39). It is a multistep process
that involves the production of proinflammatory cytokines,
accumulation of macrophages and dysfunction of endothelial and
VSMCs (40-42).
Vascular remodeling is closely associated with atherosclerosis
progression, where the main cause is aberrant VSMC proliferation
and migration, which ultimately result in neointimal hyperplasia
(42-44).
Therefore, understanding the role of Cyr61 in vascular remodeling
will contribute to improving the clinical diagnostic strategy of
ACS.
In the present study, an in vitro model of
atherosclerosis was established using ox-LDL-stimulated VSMCs. A
significant increase in Cyr61 levels was found in VSMCs after
ox-LDL treatment, suggesting that Cyr61 is involved in vascular
remodeling. Subsequently, it was found that Cyr61 knockdown
reversed the increases in cell viability whilst also reversing the
reduction in VSMC apoptosis induced by ox-LDL. These findings
concur with those from a previous study, where Cyr61 upregulated
the migration and proliferation of mouse aortic smooth muscle cells
(45). These data suggest that
Cyr61 is involved in vascular remodeling in vitro, which
also supports the reported association between Cyr61 levels and
ACS. Furthermore, the present study provided a theoretical basis
for the clinical application of exosomal Cyr61 for ACS
diagnosis.
In conclusion, the present study reported an
increase in the expression of exosomal Cyr61, which associated with
sex, family history and glucose levels. Exosomal Cyr61 was found to
be a promising biomarker for discriminating patients with UAP and
AMI, providing initial insights into the role of using exosomal
Cyr61 levels for the diagnosis of ACS. Functionally, Cyr61
participates in vascular remodeling in vitro. These results
suggest that exosomal Cyr61 can be translated into a blood-based
biomarker for clinical application.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL conceived and designed the study. WL, YL, WZ, CL,
WF, QM and XG provided study materials or patients. WL, WF and QM
performed the experiments. WL and XG confirm the authenticity of
all the raw data. WL, YL, WZ, CL, WF and QM collected and assembled
data. WL, YL, WZ, CL and XG analyzed and interpreted data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Ethics
Committee of the Second Hospital of Hebei Medical University
(Shijiazhuang, China). Written informed consent was obtained from
every participant in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Malakar AK, Choudhury D, Halder B, Paul P,
Uddin A and Chakraborty S: A review on coronary artery disease, its
risk factors, and therapeutics. J Cell Physiol. 234:16812–16823.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khera AV and Kathiresan S: Genetics of
coronary artery disease: Discovery, biology and clinical
translation. Nat Rev Genet. 18:331–344. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ling H, Guo Z, Shi Y, Zhang L and Song C:
Serum exosomal MicroRNA-21, MicroRNA-126, and PTEN are novel
biomarkers for diagnosis of acute coronary syndrome. Front Physiol.
11(654)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kandaswamy E and Zuo L: Recent advances in
treatment of coronary artery disease: Role of science and
technology. Int J Mol Sci. 19(424)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Head SJ, Milojevic M, Daemen J, Ahn JM,
Boersma E, Christiansen EH, Domanski MJ, Farkouh ME, Flather M,
Fuster V, et al: Mortality after coronary artery bypass grafting
versus percutaneous coronary intervention with stenting for
coronary artery disease: a pooled analysis of individual patient
data. Lancet. 391:939–948. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Linde JJ, Kelbæk H, Hansen TF, Sigvardsen
PE, Torp-Pedersen C, Bech J, Heitmann M, Nielsen OW, Høfsten D,
Kühl JT, et al: Coronary CT angiography in patients with
non-ST-segment elevation acute coronary syndrome. J Am Coll
Cardiol. 75:453–463. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Garg P, Morris P, Fazlanie AL, Vijayan S,
Dancso B, Dastidar AG, Plein S, Mueller C and Haaf P: Cardiac
biomarkers of acute coronary syndrome: From history to
high-sensitivity cardiac troponin. Intern Emerg Med. 12:147–155.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Omland T: Can circulating B-type
natriuretic peptide concentrations guide treatment of obstructive
left main coronary artery disease? Circulation. 138:479–482.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhu M, Lin J, Wang C, Yang M, Lv H, Yang
M, Xu B, Chen X and Jiang J: The relationship among angiotensinogen
genes polymorphisms and hs-CRP and coronary artery disease. J Clin
Lab Anal. 33(e22881)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Miki K, Kitamura M, Hatta K, Kamide K,
Gondo Y, Yamashita M, Takedachi M, Nozaki T, Fujihara C, Kashiwagi
Y, et al: Periodontal inflamed surface area is associated with
hs-CRP in septuagenarian Japanese adults in cross-sectional
findings from the SONIC study. Sci Rep. 11(14436)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yeh JC, Wu CC, Choy CS, Chang SW, Liou JC,
Chen KS, Tung TH, Lin WN, Hsieh CY, Ho CT, et al: Non-hepatic
alkaline phosphatase, hs-CRP and progression of vertebral fracture
in patients with rheumatoid arthritis: A population-based
longitudinal study. J Clin Med. 7(439)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367(eaau6977)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang Y, Xie Y, Zhang A, Wang M, Fang Z and
Zhang J: Exosomes: An emerging factor in atherosclerosis. Biomed
Pharmacother. 115(108951)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chan BD, Wong WY, Lee MM, Cho WC, Yee BK,
Kwan YW and Tai WC: Exosomes in inflammation and inflammatory
disease. Proteomics. 19(e1800149)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gao XF, Wang ZM, Wang F, Gu Y, Zhang JJ
and Chen SL: Exosomes in coronary artery disease. Int J Biol Sci.
15:2461–2470. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li X, He X, Wang J, Wang D, Cong P, Zhu A
and Chen W: The regulation of exosome-derived miRNA on
heterogeneity of macrophages in atherosclerotic plaques. Front
Immunol. 11(2175)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yao Y, Sun W, Sun Q, Jing B, Liu S, Liu X,
Shen G, Chen R and Wang H: Platelet-derived exosomal MicroRNA-25-3p
inhibits coronary vascular endothelial cell inflammation through
adam10 via the NF-κB signaling pathway in ApoE(-/-) mice. Front
Immunol. 10(2205)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li J, Tan M, Xiang Q, Zhou Z and Yan H:
Thrombin-activated platelet-derived exosomes regulate endothelial
cell expression of ICAM-1 via microRNA-223 during the
thrombosis-inflammation response. Thromb Res. 154:96–105.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ling H, Guo Z, Du S, Liao Y, Li Y, Ding C
and Song C: Serum exosomal miR-122-5p is a new biomarker for both
acute coronary syndrome and underlying coronary artery stenosis.
Biomarkers. 25:539–547. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li LJ, Gu YJ, Wang LQ, Wan W, Wang HW,
Yang XN, Ma LL, Yang LH and Meng ZH: Serum exosomal microRNA-146a
as a novel diagnostic biomarker for acute coronary syndrome. J
Thorac Dis. 13:3105–3114. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang Y, Qi Q, Wang Y, Shi Y, Yang W, Cen
Y, Zhu E, Li X, Chen D and Wang B: Cysteine-rich protein 61
regulates adipocyte differentiation from mesenchymal stem cells
through mammalian target of rapamycin complex 1 and canonical Wnt
signaling. FASEB J. 32:3096–3107. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Song Y, Lin Q, Cai Z, Hao T, Zhang Y and
Zhu X: Cysteine-rich protein 61 regulates the chemosensitivity of
chronic myeloid leukemia to imatinib mesylate through the nuclear
factor kappa B/Bcl-2 pathway. Cancer Sci. 110:2421–2430.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Klingenberg R, Aghlmandi S, Liebetrau C,
Räber L, Gencer B, Nanchen D, Carballo D, Akhmedov A, Montecucco F,
Zoller S, et al: Cysteine-rich angiogenic inducer 61 (Cyr61): A
novel soluble biomarker of acute myocardial injury improves risk
stratification after acute coronary syndromes. Eur Heart J.
38:3493–3502. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu C, Cao Y, He X, Zhang C, Liu J, Zhang
L, Wu D, Zhuang X, Xue R, Huang H, et al: Association of
Cyr61-cysteine-rich protein 61 and short-term mortality in patients
with acute heart failure and coronary heart disease. Biomark Med.
13:1589–1597. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Deng J, Qian X, Li J, Li Y, Li Y and Luo
Y: Evaluation of serum cysteine-rich protein 61 levels in patients
with coronary artery disease. Biomark Med. 12:329–339.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rajpurohit N, Ayaz SZ, Yee J, Khan MA and
Stys A: Review of acute coronary syndromes: Diagnosis and
management of unstable angina and non ST-elevation myocardial
infarction. S D Med. 68:71–73. 2015.PubMed/NCBI
|
|
27
|
Ferrari R and Fox K: Heart rate reduction
in coronary artery disease and heart failure. Nat Rev Cardiol.
13:493–501. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhao J, Zhang C, Liu J, Zhang L, Cao Y, Wu
D, Yao F, Xue R, Huang H, Jiang J, et al: Prognostic significance
of serum cysteine-rich protein 61 in patients with acute heart
failure. Cell Physiol Biochem. 48:1177–1187. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Koritzinsky EH, Street JM, Star RA and
Yuen PS: Quantification of exosomes. J Cell Physiol. 232:1587–1590.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Meldolesi J: Exosomes and ectosomes in
intercellular communication. Curr Biol. 28:R435–R444.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kumar D, Narang R, Sreenivas V, Rastogi V,
Bhatia J, Saluja D and Srivastava K: Circulatory miR-133b and
miR-21 as novel biomarkers in early prediction and diagnosis of
coronary artery disease. Genes (Basel). 11(164)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Choi C, Jeong W, Ghang B, Park Y, Hyun C,
Cho M and Kim J: Cyr61 synthesis is induced by interleukin-6 and
promotes migration and invasion of fibroblast-like synoviocytes in
rheumatoid arthritis. Arthritis Res Ther. 22(275)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Seino Y, Ikeda U, Ikeda M, Yamamoto K,
Misawa Y, Hasegawa T, Kano S and Shimada K: Interleukin 6 gene
transcripts are expressed in human atherosclerotic lesions.
Cytokine. 6:87–91. 1994.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Feng B, Xu G, Sun K, Duan K, Shi B and
Zhang N: Association of serum Cyr61 levels with peripheral arterial
disease in subjects with type 2 diabetes. Cardiovasc Diabetol.
19(194)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhao L, Yu J, Wang J, Li H, Che J and Cao
B: Isolation and identification of miRNAs in exosomes derived from
serum of colon cancer patients. J Cancer. 8:1145–1152.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li X, Li X, Lin J, Sun X and Ding Q:
Exosomes derived from low-intensity pulsed ultrasound-treated
dendritic cells suppress tumor necrosis factor-induced endothelial
inflammation. J Ultrasound Med. 38:2081–2091. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ren J, He W, Zheng L and Duan H: From
structures to functions: Insights into exosomes as promising drug
delivery vehicles. Biomater Sci. 4:910–921. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
De Toro J, Herschlik L, Waldner C and
Mongini C: Emerging roles of exosomes in normal and pathological
conditions: New insights for diagnosis and therapeutic
applications. Front Immunol. 6(203)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Geovanini GR and Libby P: Atherosclerosis
and inflammation: Overview and updates. Clin Sci (Lond).
132:1243–1252. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Grootaert MOJ, Moulis M, Roth L, Martinet
W, Vindis C, Bennett MR and De Meyer GRY: Vascular smooth muscle
cell death, autophagy and senescence in atherosclerosis. Cardiovasc
Res. 114:622–634. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liu D, Zeng X, Li X, Mehta JL and Wang X:
Role of NLRP3 inflammasome in the pathogenesis of cardiovascular
diseases. Basic Res Cardiol. 113(5)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Basatemur GL, Jørgensen HF, Clarke MCH,
Bennett MR and Mallat Z: Vascular smooth muscle cells in
atherosclerosis. Nat Rev Cardiol. 16:727–744. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Frismantiene A, Philippova M, Erne P and
Resink TJ: Smooth muscle cell-driven vascular diseases and
molecular mechanisms of VSMC plasticity. Cell Signal. 52:48–64.
2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Serino A and Salazar G: Protective role of
polyphenols against vascular inflammation, aging and cardiovascular
disease. Nutrients. 11(53)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Imhof BA, Jemelin S, Ballet R, Vesin C,
Schapira M, Karaca M and Emre Y: CCN1/CYR61-mediated meticulous
patrolling by Ly6Clow monocytes fuels vascular inflammation. Proc
Natl Acad Sci USA. 113:E4847–E4856. 2016.PubMed/NCBI View Article : Google Scholar
|