Introduction
Cardiovascular disease currently ranks as the most
common cause of illness and death worldwide, and atherosclerosis is
the main underlying pathogenetic mechanism of cardiovascular
diseases (1,2). Atherosclerosis is a pathological
disease characterized by lipid accumulation, inflammation,
fibroproliferation and formation of plaques with a necrotic core
that contains a variety of vessel wall cell types (3). Vascular smooth muscle cells (VSMCs)
are the primary components of the plaque from which extracellular
matrix (ECM) components are derived at all stages of
atherosclerosis (4,5). At the pre-atherosclerosis stage, VSMCs
in diffuse intimal thickening exhibit reduced expression of
contractile proteins and switch to a synthetic phenotype, which
leads to increased generation of ECM components rich in
proteoglycans; changes in ECM components lead to the retention of
apolipoproteins and recruitment of monocytes, triggering
inflammation (6,7). Progression from pre- to early
atherosclerosis is facilitated by a complex interaction between
lipid retention and oxidation, induction of inflammation and
proliferation of VSMCs, phenotypic transformation of VSMCs and VSMC
death (5,7). In the advanced plaque milieu, where
insufficient efferocytosis has occurred, the self-perpetuating
inflammatory response leads to an alteration in VSMC phenotypes and
changes in cell behavior, including apoptosis, senescence,
migration and proliferation; it is therefore critical to identify
the environmental cues that regulate phenotypic transitions of
VSMCs and the intrinsic mechanisms underlying these (4,5,8).
The anoxemia hypothesis suggests that a mismatch
between oxygen supply and demand in the arterial wall leads to the
development of lesions and plaques (9-11).
Hypoxia-inducible factors (HIFs) are a family of key regulator
proteins that are expressed in the hypoxic state, including HIF-1β
(whose activity is not affected by hypoxia) and HIF-1α (active
subunit with a half-life of 5 min and highly regulated by oxygen).
By activating the genes that alter energy metabolism, cell
proliferation, angiogenesis and vascular remodeling, HIF-1α allows
cells to adapt to a hypoxic environment (10). Hypoxia and particularly HIF-1α
expression is crucial for stimulating vascular remodeling through
the proliferation and migration of VSMCs (10).
Long non-coding RNAs (lncRNAs) represent a family of
non-protein coding transcripts of ~200 nucleotides in length
(12-15).
lncRNAs regulate gene expression through complex molecular
mechanisms at all levels, including the post-transcriptional,
transcriptional and chromatin levels, either in the nucleus or in
the cytoplasm (15-17).
As lncRNAs have high tissue specificity, they exist as vital
epigenetic regulators in almost every cellular process in which
gene expression is distinctly mediated under physiological and
pathological conditions (13,14,16).
It has been demonstrated that lncRNAs are involved in plasticity
and vascular dysfunction of VSMCs, and directly affect the
progression and development of atherosclerosis (16,17).
The emerging links between lncRNAs and
atherosclerosis have provided a new perspective to explore the
pathogenesis of atherosclerosis, and a new field of opportunities
to develop therapeutic and diagnostic approaches (16,18,19).
In the present study, the role of lncRNA RP11-531A24.3 in the
phenotypic regulation of VSMCs was identified, with a preliminary
investigation into its regulation mechanism conducted. Hypoxic
culture conditions were also used to evaluate the relationship
between RP11-531A24.3 and HIF-1α expression.
Materials and methods
Screening of differentially expressed
genes and bioinformatics prediction
By performing microarray analysis with an
Agilent-045997 Arraystar human lncRNA microarray V3 (Probe Name
Version; Agilent Technologies, Inc.), the lncRNA profile of three
advanced atherosclerosis samples and three normal intima tissues
was characterized (NCBI accession no. GSE97210; https://www.ncbi.nlm.nih.gov/geo/) (20,21).
Prediction of the non-coding sequence region of RP11-531A24.3 was
performed using the ORF prediction tools Coding Potential
Calculator (http://cpc2.gao-lab.org/) and Coding
Potential Assessing Tool (http://lilab.research.bcm.edu/cpat/index.php). The
Database for Annotation, Visualization and Integrated Discovery
bioinformatics resources, version 6.8 (https://david.ncifcrf.gov/tools.jsp) was used for
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (22) and Gene Ontology (GO) (23) enrichment analysis of the RNA-binding
proteins of RP11-531A24.3 with P<0.05 and gene count ≥1.
Reagents
Human aortic (HA-)VSMCs (cat. no. CRL-1999) were
obtained from the American Type Culture Collection and
authenticated via STR profiling. Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS), penicillin, streptomycin,
puromycin, bovine serum albumin and polybrene were purchased from
Gibco (Thermo Fisher Scientific, Inc.). Empty lentivirus vector
(anti-puromycin; LV-mock), LV-mediated lncRNA RP11-531A24.3
overexpression vector (anti-puromycin; LV-OE), small interfering
(si)RNA targeting annexin A2 (ANXA2) and its corresponding
si-negative control (si-NC; cat. no. SIGS0005567-1), and the siRNA
targeting HIF-1α and its corresponding si-NC (cat. no.
SIGS0002003-1) were constructed and purchased from Guangzhou
RiboBio Co., Ltd. An Annexin V-APC/7-aminoactinomyosin (7-AAD)
Apoptosis Detection kit (cat. no. KGA1023) and a Cell Cycle
Detection kit (cat. no. KGA512) were obtained from Nanjing KeyGen
Biotech. Co., Ltd. Cell Counting Kit-8 (CCK-8; cat. no. C0039) was
purchased from Beyotime Institute of Biotechnology and Transwell
chambers (cat. no. 3422) from BD Biosciences. Lipofectamine 3000
was purchased from Life Technologies. Co., Ltd. TRIzol®
was purchased from Invitrogen; Thermo Fisher Scientific, Inc. A
PrimeScript II 1st Strand cDNA Synthesis kit and a SYBR Green PCR
kit were purchased from Takara Biotechnology Co., Ltd. An
All-in-One First-Strand cDNA Synthesis kit was purchased from
GeneCopoeia, Inc. RIPA lysis buffer was purchased from Hangzhou
Fude Biological Technology Co., Ltd. Antibodies targeting ANXA2
(rabbit polyclonal; cat. no. ab235939) and β-tubulin (rabbit
polyclonal; cat. no. ab6046) were purchased from Abcam, while
antibody targeting HIF-1α (rabbit polyclonal; cat. no. AF1009) was
purchased from Affinity Biosciences. ECL Western Blotting Substrate
was purchased from Promega Corporation. RNA Antisense Purification
Kit (cat. no. Bes 5103-2; BersinBio) was used in the RNA antisense
purification (RAP) experiment (http://bersinbio.com/Product/detail/id/14.html). All
reagents were used according to the manufacturers' protocol.
Cell culture
HA-VSMCs were incubated in DMEM containing 100 U/ml
penicillin, 10% FBS and 100 mg/ml streptomycin in a humidified
atmosphere of 5% CO2 and 95% air at 37˚C. For standard
culture, the cells were exposed to co-mixture gas (75%
N2, 5% CO2 and 20% O2). For
hypoxic culture, the cells were exposed to a co-mixture of
hypoxia-inducing gases (94% N2, 5% CO2 and 1%
O2) in an impenetrable sealed modular incubator chamber
(Shel Lab Bactron series; Sheldon Manufacturing) at 37˚C for 6, 12,
and 24 h. Before hypoxic culture, cells had been transfected with
si-RNA targeting ANXA2 or HIF-1α.
Tissue samples
Plaque tissues and endothelial tissues (n=5 of each)
of suitable size were obtained from the carotid artery of patients
with atherosclerosis, and were isolated and placed in liquid
nitrogen. Patients (male vs. female, 3:2; age, 57-68 years, mean
age, 61.4 years) eligible for inclusion in the study were required
to have a confirmation of pathological diagnosis of primary
atherosclerosis, while those with metastatic tumors, congestive
heart failure, diabetes mellitus, hematologic disease and/or
communicable diseases were excluded. All patients were recruited
from Nanfang Hospital, Guangzhuo, China between April 2017 and
January 2020. The research study was approved by the Committee for
Ethical Review of Research Involving Human Subjects, Nanfang
Hospital, Southern Medical University, (Guangzhou, China; approval
no. NFEC-2018-142). Identifying information of patients were not
included in the study, so oral informed consent was obtained from
the participants
Lentivirus construction and cell
infection
RP11-531A24.3 cDNA was amplified by PCR and
subsequently cloned into a CMV-MCS-PGK-Puro vector, and the correct
sequence of the RP11-531A24.3 gene in this construct was validated
through sequencing by Guangzhou RiboBio Co., Ltd. Empty lentiviral
vector (LV-mock) and LV-lncRNA RP11-531A24.3 overexpression vector
(LV-OE) was used to infect HA-VSMCs at a multiplicity of infection
of 100 transducing units per cell in the presence of 8 mg/ml
polybrene. A 1 µg/ml quantity of puromycin was added into the cell
medium to select the stably transduced strain. Subsequent
experiments were carried out following 6 months, at 10 generations
after stable infection.
The siRNA was diluted as required. Before
transfection, cells were incubated on a 6-well plate at a density
of 1x105/well. On the next day, the cells were gently
washed with PBS and 750 µl Opti-MEM was added to each well, and the
cells were again incubated for 30 min. The working fluid containing
5 µl siRNA, 5 µl Lipofectamine 3000 and 240 µl Opti-MEM was mixed
and reacted for 20 min. The working fluid was then added to the
6-well plate and cultured for 8 h, before replacing the
transfection medium with fresh medium. The cells were divided into
three groups: i) Cell group infected with si-HIF-1α or si-ANXA2;
ii) Cell group infected with si-NC; and iii) Cell group used as a
blank control. Subsequent experiments were carried out after 3
days. The siRNA sequences (Guangzhou RiboBio Co., Ltd.) are listed
in Table I.
 | Table IsiRNA sequences of ANXA2 and
HIF-1α. |
Table I
siRNA sequences of ANXA2 and
HIF-1α.
| siRNA | Sequence
(5'-3') |
|---|
| si-ANXA2 |
GTCTGTCAAAGCCTATACT |
| si-HIF-1α |
CCAGCAACTTGAGGAAGTA |
Cell cycle analysis
Cells were seeded at a density of 1x106
in 6-well plates. Following fixation with 70% ice-cold ethanol
overnight at 4˚C, the samples were incubated with RNase A for 30
min at 37˚C and subsequently stained with propidium iodide for 10
min in room temperature. DNA in the labeled cells was identified
using a flow cytometer (ModFit LT 3.1; BD Biosciences).
Cell apoptosis analysis
Cells were seeded at a density of 1x106
in 6-well plates. Samples were incubated in serum-free medium for
48 h, trypsinized and washed with cold PBS once, followed by
Annexin V-APC staining for 10 min and 7-AAD staining for 5 min at
room temperature. The apoptotic rate (both early-stage and
late-stage apoptosis) was measured via flow cytometry (FlowJo 10;
BD Biosciences).
Cell migration assay
Transwell chambers were used to assess cell
migration. Briefly, cells were resuspended in serum-free medium at a
density 2-4x104/ml. A total of 100-200 µl of the cell
suspension (4x104/ml) was placed in the upper chamber
and 600 µl of DMEM with 20% FBS was added to the lower chamber. The
cells were incubated at 37˚C for 12 h. The cells from the upper
surface of the filter were removed. Next, cells in the lower
surface of the filter were fixed with methanol at room temperature
for 30 min and stained with crystal violet at room temperature for
15 min. The stained cells were observed using a Nikon Eclipse Ts2R
microscope in five randomly selected fields of each chamber
(magnification, x200). Cell counts were determined using ImageJ
v1.8.0 (National Institutes of Health).
Cell proliferation assay
Cell proliferation was evaluated using CCK-8. Cells
were suspended in 96-well plates for 12 h for adhesion at a density
of 2,000 cells/well (100 µl/well). At the beginning of this
experiment, we had laid 4 identical 96-wells plates. After cell
adhesion, CCK-8 solution (10 µl/well) was added to the medium for 2
h at 0, 12, 24 and 48 h time points, in four identical 96-wells
plates, respectively. The absorbance was read at 450 nm using a
microplate reader at each time point.
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA from cultured cells and tissues was
extracted using TRIzol reagent. The lncRNA RT was performed using
an All-in-One First-Strand cDNA Synthesis kit in a 20-µl reaction
mixture containing 2,000 ng total RNA. The reaction conditions for
RT were as follows: 10 µl RNA was heated to 70˚C for 10 min for
denaturation and mixed with 2 µl 10 mM dNTPs, 0.5 µl M-MLV reverse
transcriptase, 0.5 µl M-MLV RNase inhibitor, 4 µl 5X RT buffer, 0.5
µl reverse transcription primer for RP11-531A24.3 or 0.5 µl reverse
transcription primers for GAPDH (reference gene), and RNase-free
dH2O. After mixing, the mixture was incubated at 42˚C
for 60 min and at 85˚C for 5 min.
RT for mRNA used a PrimeScript II 1st Strand cDNA
Synthesis kit in a 20-µl reaction mixture containing 2,000 ng total
RNA. The reaction conditions were as follows: 4 µl 5X PrimeScript
buffer, 1 µl PrimeScript RT Enzyme Mix, 1 µl Random 6 mers (100
µM), 1 µl Oligo dT Primer (50 µM), 13 µl of RNA and RNase-free
dH2O; after uniform mixing, the mixture was incubated at
37˚C for 15 min and at 85˚C for 5 sec.
qPCR was performed using an SYBR Green PCR kit on an
ABI 7500 Fast Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) after cDNA was diluted five times. The
reaction system and the thermocycling conditions were as follows:
10 µl SuperMix, 0.5 µl forward primers, 0.5 µl reverse primers, 4
µl RNase-free dH2O and 5 µl cDNA were mixed, before
enzyme activation at 50˚C for 2 min, followed by 45 cycles of 95˚C
for 15 sec and 60˚C for 32 sec. Samples were then heated to 95˚C
for 1 min, 60˚C for 30 sec and 40˚C for 30 sec. GAPDH served as the
endogenous control for lncRNA and mRNA. Results were calculated
using the 2-ΔΔCq method (24). The primers used in the analysis are
listed in Table II.
 | Table IIPrimer sequences for RP11-531A24.3,
ANXA2 and GAPDH |
Table II
Primer sequences for RP11-531A24.3,
ANXA2 and GAPDH
| Primer name | Sequence
(5'-3') |
|---|
| RP11-531A24.3 | RT:
ATTTAGTGGGCTGGTGGG |
| | R:
CAAATAACCACCCGAAAA |
| | F:
ACCAAAGCAGATAGGCAC |
| GAPDH | RT:
TGGTGAAGACGCCAGTGGA |
| | R:
TGGTGAAGACGCCAGTGGA |
| | F:
GCACCGTCAAGGCTGAGAAC |
| ANXA2 | R:
ATTTAGTGGGCTGGTGGG |
| | F:
CAAATAACCACCCGAAAA |
Western blot analysis
Cells with or without hypoxia culture were used for
total protein extraction. Total protein was extracted using RIPA
buffer. Protein concentrations were determined using a Nanodrop
(NanoDrop 2000; Thermo Fisher Scientific, Inc.) and separated via
12% (ANXA2; 120 µg) or 10% (HIF-1α; 320 µg) SDS-PAGE before
transfer to a PVDF membrane. The membrane was blocked with 5%
bovine serum albumin for 1 h at room temperature and then incubated
overnight at 4˚C with primary antibodies (ANXA2, 1:1,000; HIF-1α,
1:500; tubulin, 1:10,000). After incubation with the Goat
anti-Rabbit IgG secondary antibody (1:10,000; cat. no. 31460;
Thermo Fisher Scientific, Inc.) at room temperature for 30 min,
immunoreactivity was detected by enhanced chemiluminescence using
Enhanced Western Blotting Substrate. The protein expression levels
were quantified with ImageJ software v1.8.0.
Fluorescence in situ hybridization
(FISH)
A green fluorescence-labeled lncRNA probe
recognizing RP11-531A24.3 was designed and synthesized according to
the sequence analysis and design principles (BersinBio). The probe
sequence is shown in Table
III.
 | Table IIIProbe sequence for RP11-531A24.3 used
for fluorescence in situ hybridization. |
Table III
Probe sequence for RP11-531A24.3 used
for fluorescence in situ hybridization.
| Gene name | Probe sequence |
|---|
| ENSG0000564832 |
CAGGCAGGTCTACATTGGCAATGGAAAATAAGCAATTATATGGGAAAATCAGTA
GATGTTTCTCTTTAGTTTGTTAGTAGGCAACACTTTTAAACTGAATTACTCAATGTATTTTGAC
TATGTAGATATGACACAGATATTTATTACAAGCCTGAAAAAACAGTTAAAAAATACTATTTCAG
TATTTACGGTAAAGAATACACAGATGTAAATGATTCCAACAGTGAGCCAGTTTGACTAAAAGC
GTTATTGCACTGCCTCAG |
Cells were fixed with 4% paraformaldehyde for 20 min
at room temperature, permeabilized with transparent liquid (10%
Triton X-100, 10X PBS, H2O) for 10 min at room
temperature, refixed with 1% paraformaldehyde for 10 min and
dehydrated using 70, 80, 95 and 100% ethanol for 5 min each. The
cells were transferred rapidly to a hybridization system containing
the probe and incubated overnight at 53˚C. DAPI (10 mg/ml) was
added for 10 min to stain the nucleus at room temperature. The
samples were imaged using a confocal laser microscope, and two
fields of view were captured (magnification, x200).
RAP and liquid chromatography-mass
spectrometry (LC-MS)
RAP was performed to separate lncRNA RP11-531A24.3
and its RBPs by designing biotin or streptomycin probes capable of
binding to the target lncRNA fragments. A total of 10 antisense DNA
probes (control group) and 10 sense DNA probes (experimental group,
which were divided into odd probes, including number 1, 3, 5, 7 and
9, and even probes, including number 2, 4, 6, 8 and 10) with
5'-biotin labels were ordered and purchased from BersinBio. The
probe sequences are shown in Table
IV.
 | Table IVProbe sequences for RP11-531A24.3
used for RNA antisense purification. |
Table IV
Probe sequences for RP11-531A24.3
used for RNA antisense purification.
| A, Odd probe |
|---|
| Probe sequence
(5'-3') | Probe location |
|---|
|
ATTCACATAATGACTAAGCATACTCAAATGTGTGGTAACAAGGAGGC | 224-270 |
|
CCTCAACAAACCAACCAAACAAATAAAATACCCCAAATAACCACCCGAAA | 917-966 |
|
CCAGGCAGGTCTACATTGGCAATGGAAAATAAGCAATTATATGGGAAAA | 1,624-1,672 |
|
TCCTCCACGCCAATCAACTTTTAGAGAATATACCATGCAAACTTTATTTA | 2,042-2,091 |
|
AAAGTCAATAATGCTTCATGCGACTGTACCTAGAAATCTAATACCATGTATA | 2,600-2,651 |
| B, Even probe |
| Probe sequence
(5'-3') | Probe location |
|
CTTGCTGCATTATATGTAGTCCTTGGTCATCACCCAGCTCCACTGCT | 627-673 |
|
ATTTAATACATTTAGTGGGCTGGTGGGCAGCATAAGGCTTTATCTGAATACTATTTCA | 1,169-1,226 |
|
TTCCATCCTTTCTTTTCCCTTCCCCACCAATGCTGGCCTT | 1,891-1,930 |
|
ACTTTGTGAATCTCCACAGAGCTGTCCTGAGTTTAACAATAATCTTCTAA | 2,249-2,298 |
|
TTTTCTATCTCGCTTATTCTACCAGACTGAAATGGAGAACAATGCCAGCAATTTTATA | 2,757-2,816 |
| C, Negative
probe |
| Probe sequence
(5'-3') | Probe location |
|
GAGCCACCACACCCAGCCCTTAAAATTATTAAGACTTCACATATACT | 154-200 |
|
TTCATAACCAGTTTGGTTCTAGATTTACTGTAATTGGCTATCTGCCAGAATAAACT | 339-394 |
|
GGGTGATGACCAAGGACTACATATAATGCAGCAAGCACGCT | 639-679 |
|
TTATTTGGGGTATTTTATTTGTTTGGTTGGTTTGTTGAGGGGTTTT | 927-972 |
|
AGATAAAGCCTTATGCTGCCCACCAGCCCACTAAATGTATTAAATACCTG | 1,182-1,231 |
|
GTTTAAAAGTGTTGCCTACTAACAAACTAAAGAGAAACATCTACTGATTTTCCCAT | 1,577-1,632 |
|
AAGGCCAGCATTGGTGGGGAAGGGAAAAGAAAGGATGGAA | 1,891-1,930 |
|
AAACTCAGGACAGCTCTGTGGAGATTCACAAAGTAATTTCATG | 2,265-2,307 |
|
TAAGTTATACATGGTATTAGATTTCTAGGTACAGTCGCATGAAGCATTATTGACTTT | 2,595-2,651 |
|
ATATGAGTTAGCATACTCGTGTTTGTTCAGCTGTCCATCCTGCATCG | 2,962-3,008 |
After performing cross-linking of cells, collection
of the cell pellet, homogenization and DNA removal processes
(25), the cell lysate was divided
into the following three groups: RAP odd, RAP even and RAP NC, and
the corresponding probes were added for hybridization (20 pmol
probes; denaturation for 10 min at 65˚C, hybridization for 180 min
at 45˚C). Magnetic beads were added to the three sample-probes for
enrichment. A magnetic rack was used to collect the beads, and the
supernatant was removed. Each group was divided into two parts:
Protein detection and RNA detection. The RNA detection group was
used to confirm whether the pulldown fragment of probes, including
both odd and even groups, were from RP11-531A24.3. A total of 30 µl
of each protein sample was collected for LC-MS detection.
The protein solution was subjected to the enzymatic
digestion step. The peptide was dissolved and centrifuged with a
sample solution (0.1% formic acid, 2% acetonitrile); the
supernatant was transferred, and mass spectrometry was performed (Q
Exactive; Thermo Fisher Scientific, Inc; positive; 320˚C; full scan
mode; 350-1,800 m/z, 300 nl/min). The original mass spectrometry
file was converted to an MGF file format, and the peptides were
searched against the Uniprot database using MASCOT (http://www.matrixscience.com/).
Statistical analysis
Each experiment was performed in triplicate.
Statistical analysis was performed using SPSS 22.0 program (IBM,
Corp.). Statistical evaluation was carried out using Student's
t-test for comparison between the two groups and ANOVA followed by
the Bonferroni method was used for comparison of >2 groups. A
paired t-test was used between atherosclerotic plaque tissues and
normal intima tissues which were obtained from the same
individuals. P<0.05 was considered to indicate a statistically
significant difference.
Results
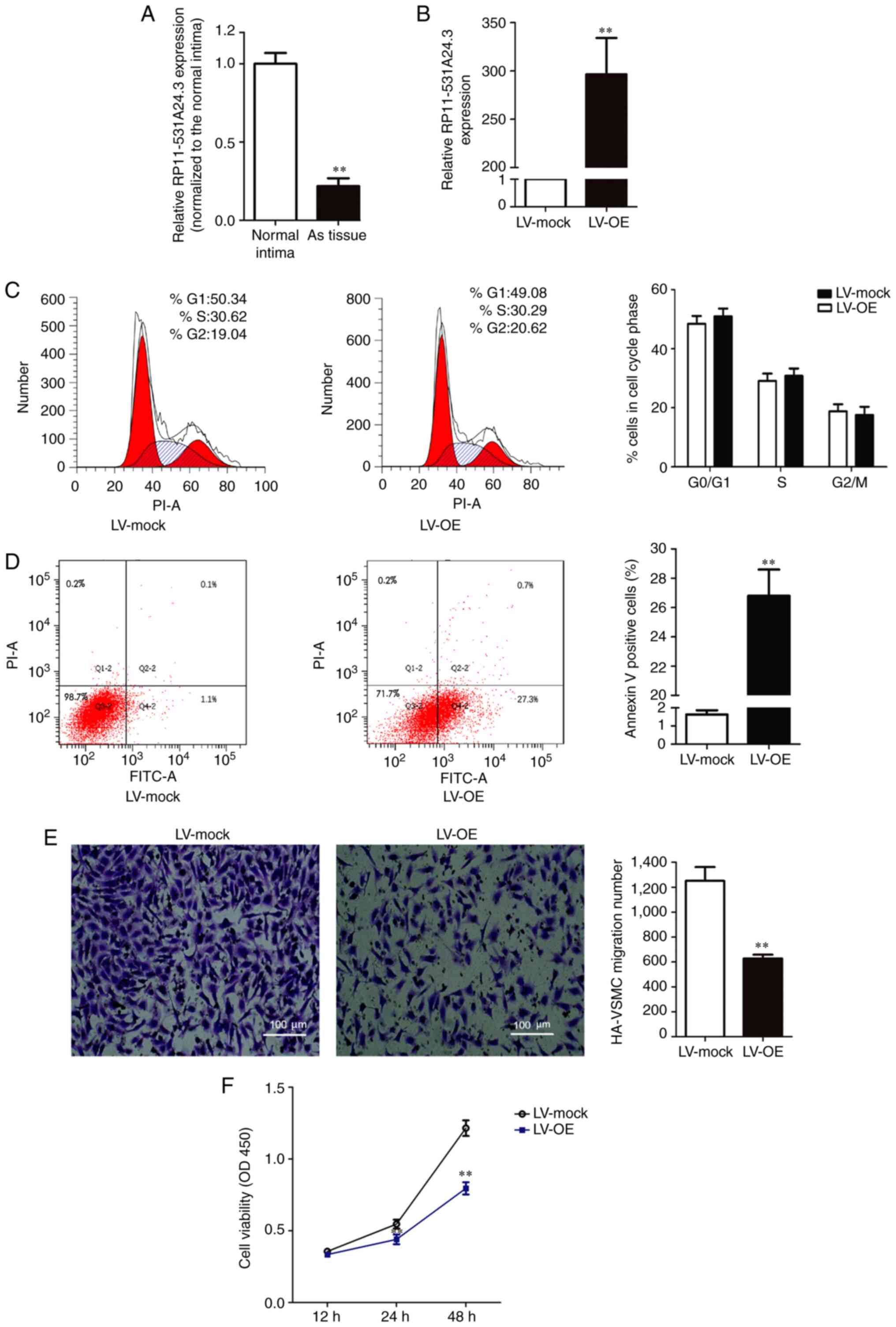
Expression of RP11-531A24.3 is reduced
in advanced atherosclerotic lesions, and RP11-531A24.3 inhibits the
proliferation and migration of HA-VSMCs
Microarray analysis indicated that RP11-531A24.3
(https://ensembl.org/, ENST00000564832.1)
expression was reduced in the advanced atherosclerosis plaque, with
a 222.5-fold reduction in expression compared with control tissues
(n=3). RP11-531A24.3 was further confirmed as a non-coding or less
coding transcript by the Coding Potential Calculator. To validate
the varying expression levels of RP11-531A24.3, five paired
atherosclerotic plaque tissues and normal intima tissues were
examined via RT-qPCR (Fig. 1A). The
results indicated that RP11-531A24.3 was downregulated in
atherosclerotic plaque tissues compared with normal tissues (fold
change =0.179).
HA-VSMCs were infected with LV-mock or LV-OE
(Fig. 1B). A series of
gain-of-function studies were performed in HA-VSMCs to investigate
the regulatory effect of RP11-531A24.4 on the phenotypes of VSMCs
(Fig. 1C-F). The first study was
conducted to investigate whether RP11-531A24.3 was involved in cell
cycle distribution and apoptosis. The flow cytometry analysis
showed no significant difference in the number of cells in the
G0/G1 phase, S phase and G2/M phase between the LV-OE and LV-mock
groups (Fig. 1C). The cell
apoptosis test showed that the apoptotic rate was significantly
increased in the LV-OE group compared with in LV-mock group
(Fig. 1D). The Transwell assay
indicated that overexpression of RP11-531A24.3 significantly
inhibited the migration of HA-VSMCs compared with LV-mock (Fig. 1E). The CCK-8 assay showed that
overexpression of RP11-531A24.3 reduced the viability of the cells
in comparison with a control (Fig.
1F). Taken together, these results demonstrated that
RP11-531A24.3 inhibited migration and increased apoptosis, while it
had no effect on the cell cycle distribution of these cells.
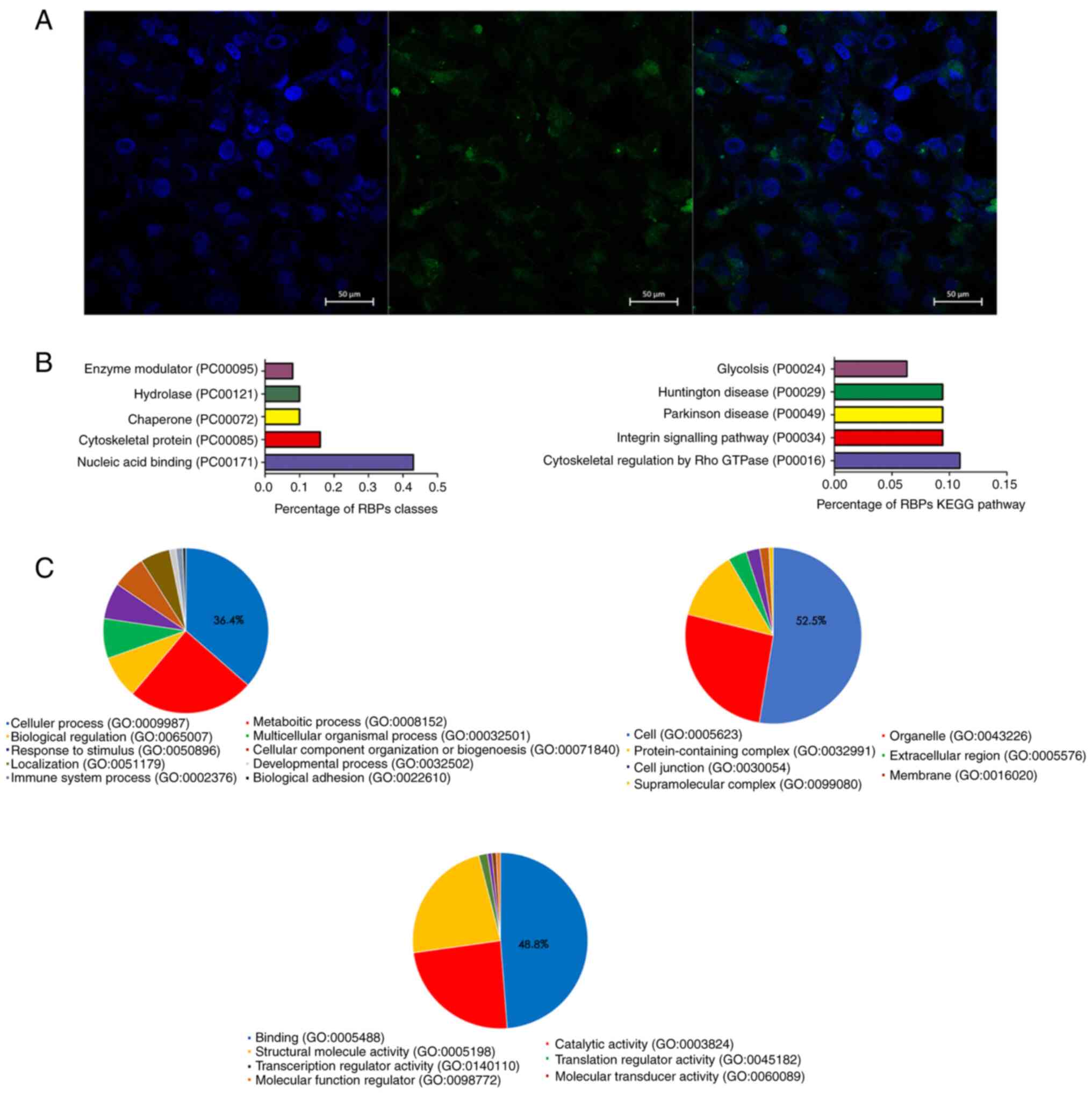
RP11-531A24.3 is located in the
cytoplasm of HA-VSMCs and is associated with the KEGG term
‘cytoskeletal regulation by Rho GTPase’
The cellular localization of RP11-531A24.3 in
HA-VSMCs was assessed via FISH. The result indicated that
RP11-531A24.3 was localized in the cytoplasm of HA-VSMCs (Fig. 2A).
The RAP-MS experimental results showed that there
were 141 proteins that bound with RP11-531A24.3 directly. KEGG
pathway enrichment (Fig. 2B) and GO
analysis (Fig. 2C) were conducted
to investigate the biological function of those RBPs. The most
commonly associated terms were ‘nucleic acid binding’,
‘cytoskeletal protein’, ‘hydrolase’ and ‘chaperone’ (Fig. 2B). The top three molecular function
terms from the GO analysis of the RBPs were ‘binding (GO:0005488)’,
‘catalytic activity (GO:0003824)’ and ‘structural molecule activity
(GO:0005198)’ (Fig. 2C).
‘Cytoskeletal regulation by Rho GTPase (P00016)’ ranked the highest
in the enrichment analysis of the KEGG pathway (Fig. 2B). Translocation of VSMCs is driven
by a dynamic reorganization of the actin cytoskeleton (26). The regulatory mechanism of actin
recombination is unclear; however, it is thought that complex
interactions between actin cytoskeleton-related proteins and
GTPases may play a role in this mechanism (26,27).
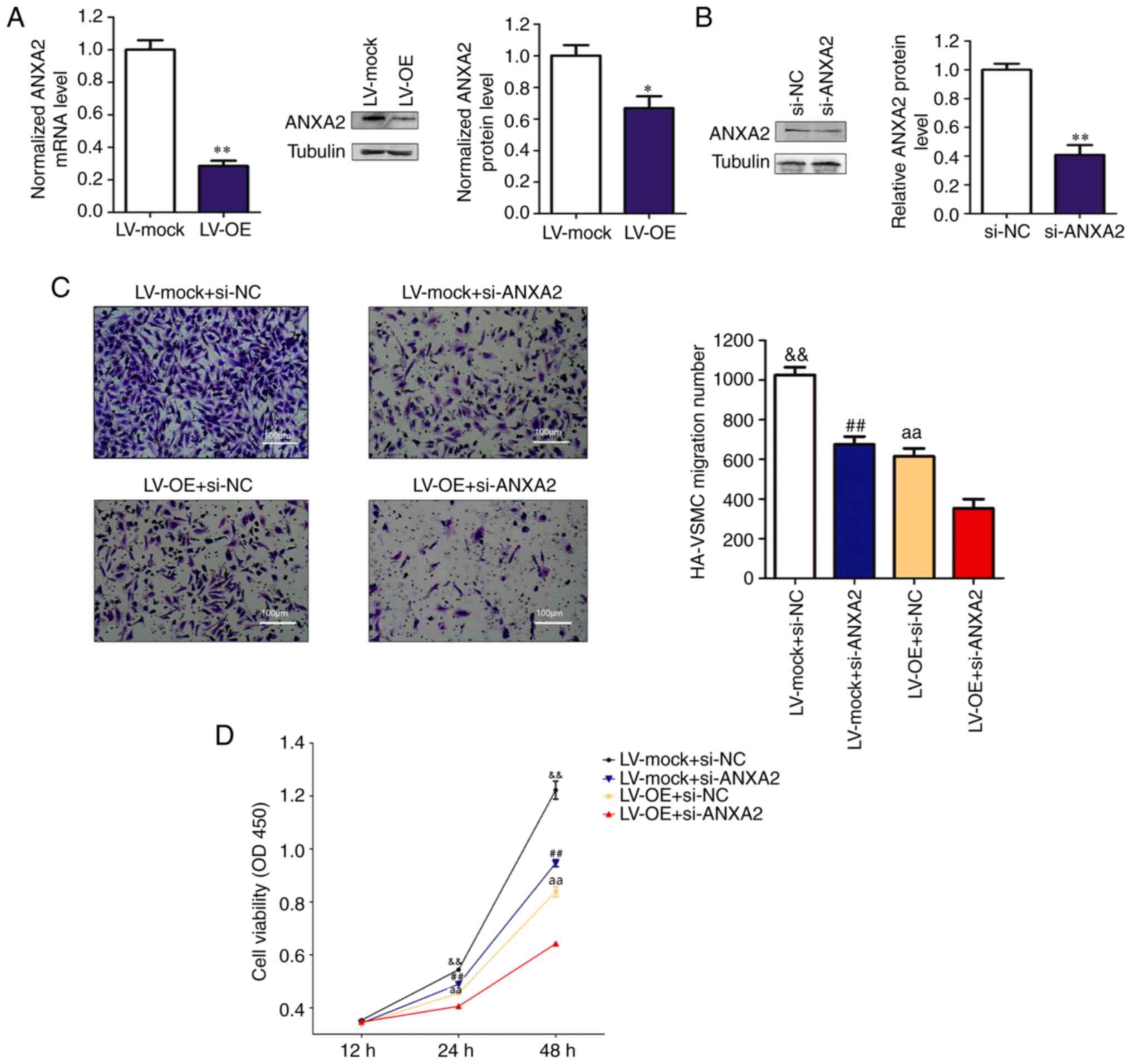
RP11-531A24.3 suppresses the migration
and proliferation of HA-VSMCs via inhibition of ANXA2
expression
Among the RBPs, ANXA2, whose enhanced expression has
been reported to potentiate the migration, invasion and
proliferation of various cancers (28), was identified. ANXA2 is a member of
the calcium-dependent phospholipid-binding protein family and is
related to the smooth muscle contraction pathway (29,30). A
previous study using Affinity Capture-MS showed that ANXA2 binds to
myosin heavy chain 9 protein, which is one of the downstream
proteins in the Rho GTPase regulatory pathway (31). Overexpression of RP11-531A24.3
suppressed ANXA2 expression at both mRNA and protein levels
compared with controls (Fig. 3A).
The reduced migration and viability mediated by RP11-531A24.3
overexpression were more significantly suppressed after ANXA2
depletion in RP11-531A24.3-overexpressing cells compared with in
controls (Fig. 3B-D). Collectively,
these data suggested that RP11-531A24.3-mediated depletion of the
expression and function of ANXA2 inhibited the migration and
viability of HA-VSMCs.
Hypoxia regulates the expression of
RP11-531A24.3 and ANXA2 in HA-VSMCs
HA-VSMCs transfected with LV-OE or LV-mock and
normal HA-VSMCs were cultured in normal conditions (20%
O2) or hypoxic conditions (1% O2) for 6-24 h.
Next, the expression levels of RP11-531A24.3, ANXA2 and HIF-1α were
analyzed. The results indicated that the expression of
RP11-531A24.3 was reduced (Fig.
4A), while that of ANXA2 and HIF-1α was augmented, by hypoxia
compared with controls (Fig. 4B).
Of note, overexpression of RP11-531A24.3 suppressed HIF-1α
expression at the protein level (Fig.
4C). Under hypoxic conditions, transfection with siRNA
targeting HIF-1α exerted no significant effects on ANXA2
expression, while an siRNA targeting ANXA2 decreased the levels of
HIF-1α expression in HA-VSMCs (Fig.
4D and E). These results
indicated that hypoxia may regulate the expression of RP11-531A24.3
and ANXA2, and facilitate the expression of HIF-1α via ANXA2.
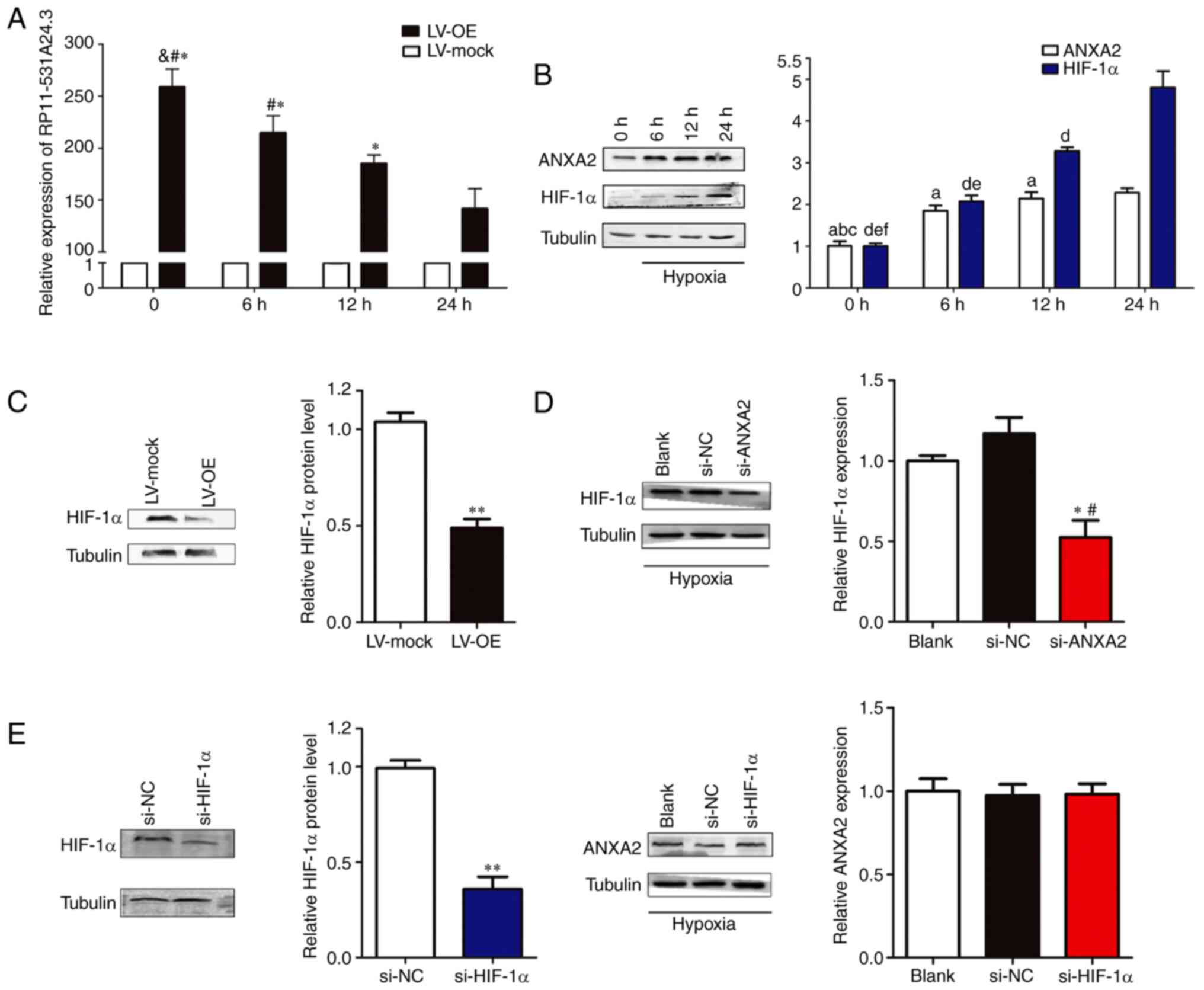 | Figure 4Hypoxia regulates the expression of
RP11-531A24.3, ANXA2 and HIF-1α in HA-VSMCs. Effect of hypoxia on
the expression of (A) RP11-531A24.3 *P<0.05 vs. 24 h;
#P<0.05 vs. 12 h; &P<0.05 vs. 6 h
and (B) ANXA2, aP<0.05 vs. 24 h;
bP<0.05 vs. 12 h; cP<0.05 vs. 6 h; and
HIF-1α, dP<0.05 vs. 24 h; eP<0.05 vs.
12 h; fP<0.05 vs. 6 h. (C) HIF-1α expression at
protein levels under hypoxic conditions was detected in HA-VSMCs
transfected with LV-OE or LV-mock. **P<0.01 vs.
LV-mock. (D) HIF-1α expression at the protein level was detected in
HA-VSMCs transfected with si-NC, si-ANXA2 and blank control.
*P<0.05 vs. si-NC; #P<0.05 vs. Blank.
(E) HIF-1α and ANXA2 expression levels were detected in HA-VSMCs
transfected with si-NC, si-HIF-1α and blank control.
**P<0.01 vs. si-NC. Data are presented as the mean ±
SD (n=3). HA-VSMCs, human aorta-vascular smooth muscle cells;
ANXA2, annexin A2; HIF-1α, hypoxia inducible factor-1α; LV-mock,
empty lentiviral vector; LV-OE, lentiviral RP11-531A24.3
overexpression vector; si, small interfering; NC, negative
control. |
Discussion
The major clinical consequences of atherosclerosis
are not due to the diminishing blood supply in the gradually
narrowing lumen caused by stable plaques, but rather due to a
sudden blockage of blood flow caused by acute rupture or
decomposition of an unstable plaque in a thrombotic event (5,16). The
development of new therapies to treat atherosclerosis and minimize
its clinical consequences will be contingent on a rigorous
understanding of the biology of VSMCs, which contribute to the
pathogenesis of advanced lesions. In advanced lesions, plaque
disruption is inversely proportional to the number of VSMCs, which
is closely related to the proliferation, migration, and death of
VSMCs (4). In humans, advanced
atherosclerotic lesions demonstrate little VSMC proliferation
(4,5,16). The
proliferation of VSMCs in the development of atherosclerosis
appears to be predominantly restorative, rather than as a major
driver of plaque formation (5).
Human VSMCs can migrate in response to varying stimuli in
vitro; however, it remains unclear how VSMC migration affects
atherosclerosis and whether migration occurs independent of
proliferation (4,5). In present study, RP11-531A24.3 was
identified through screening of differentially expressed genes in
an lncRNA microarray in three advanced atherosclerosis samples and
in three normal intima tissue. Next, a series of gain-of-function
assays were performed to demonstrate that RP11-531A24.3 was
associated with migration and proliferation phenotypes in HA-VSMCs.
KEGG pathway analysis indicated that the RBPs were enriched in the
pathway termed ‘cytoskeletal regulation by Rho GTPase’, which has
been reported to be closely associated with cell migration and
invasion in tumor cell metastasis (32).
ANXA2 is one of the RBPs of RP11-531A24.3, and is a
member of the calcium-dependent phospholipid-binding protein
family. ANXA2 plays a key role in tumorigenesis and cancer
progression, and is involved in the positive regulation of
low-density lipoprotein receptor activity, lipoprotein receptor
binding and lipoprotein clearance (28-30,33,34).
Given the biological function of ANXA2 in cancers and
atherosclerosis (28,29,33,34)
and based on the association between RP11-531A24.3 and ANXA2, it
was investigated and demonstrated that RP11-531A24.3 inhibited the
migration and proliferation of HA-VSMCs through binding to ANXA2
directly in the cytoplasm to reduce its expression at both mRNA and
protein levels. Collectively, the results of the present study may
advance understanding of the biological behavior of lncRNA
RP11-531A24.3 in HA-VSMCs, and may serve as a preliminarily
investigation into the mechanism by which RP11-531A24.3 regulates
the migration and proliferation of VSMCs via ANXA2.
Non-coding RNAs, including microRNAs, circular RNAs
and lncRNAs, are considered to serve central roles in the
regulation of phenotypic transformation of SMCs, involving changes
in migration, proliferation, apoptosis, and ECM-generating capacity
(16,35,36).
The subcellular localization of lncRNA is likely to define its
network of interactions with other macromolecules and their
functions (15). In the present
study, FISH indicated the cytoplasmic localization of
RP11-531A24.3, while RAP and LC-MS indicated its RBPs were enriched
in the pathway terms closely related to migration. These results
were consistent with phenotypic changes caused by
RP11-531A24.3.
ANXA2, a Ca2+-dependent
phospholipid-binding protein that directly binds to RP11-531A24.3,
has a unique N-terminus tail domain that contains a nuclear export
signal and multiple phosphorylation sites (28,29).
It forms a heterotetramer and drives the conversion of plasminogen
to plasmin at the cell surface (33,37);
this further activates metalloproteinases and degrades EMC
components, which is an essential process for cell metastatic
progression (28,37). ANXA2 is upregulated in various
cancers and is related to cell angiogenesis, proliferation,
invasion, and adhesion (28,30).
Upregulation of ANXA2 is reported to be related to a more
proangiogenic phenotype in macrophages and more migratory phenotype
in VSMCs (34,38). In the present study, a direct
interaction between ANXA2 and RP11-531A24.3 that regulates the
proliferation and migration of HA-VSMCs was indicated. However, the
binding site and the binding mechanism need to be further
investigated.
The anoxemia hypothesis proposes that an unbalanced
supply and demand for oxygen in the arterial wall is one of the key
factors in the development of atherosclerosis (9,10). The
presence of hypoxic areas in developing atherosclerotic plaques has
been widely reported to alter the function, metabolism, and
responses of various cell types, and to determine whether the
plaque will evolve into an unstable phenotype (9,11). In
SMCs, HIF-1α plays an important role in cell proliferation and
migration, two important features of foam cell formation, and also
mediates multiple mechanisms, such as macrophage outflow pathways
(10). In esophageal squamous cell
carcinoma, phosphorylated ANXA2 inhibited ubiquitin-dependent
proteasomal degradation of MYC proto-oncogene protein, which
potentiated HIF-1α transcription and promoted migration, invasion,
and metastasis (30). ANXA2
expression was delineated in RAW264.7 macrophages under hypoxic
condition (34). The present study
indicated that the expression levels of ANXA2 and HIF-1α were
markedly augmented in hypoxic conditions and that knockdown of
ANXA2 decreased HIF-1α expression, but the mechanism related to
these aspects remain to be elucidated. lncRNAs are also involved in
hypoxia-induced pulmonary hypertension-related vascular remodeling
by acting as a sponge or through the regulation of genes involved
in cell proliferation, migration and apoptosis in pulmonary VSMCs
(39). The results of the present
study demonstrated that lncRNA RP11-531A24.3 was downregulated in
HA-VSMCs in hypoxia. Further studies on how hypoxia manipulates
RP11-531A24.3, as well as the interaction between RP11-531A24.3 and
ANXA2, are required in the future.
In conclusion, the present study suggested that
lncRNA RP11-531A24.4 regulated the phenotypes of migration and
proliferation in HA-VSMCs through ANXA2. The effect of hypoxia on
the expression levels of RP11-531A24.3, ANXA2 and HIF-1α was
assessed, however, HIF-1α expression was not identified by RAP-MS.
It may be hypothesized that HIF-1α was not easy to detect due to
its low expression abundance in the cytoplasm under a constant
oxygen environment. Further research is required into how
RP11-531A24.3 plays a role in phenotypic switching of VSMCs during
atherosclerosis and the mechanism via which hypoxia regulates these
molecules. This research may facilitate the development of new
therapeutic strategies for the treatment of cardiovascular
diseases.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National Natural
Science Foundation of China (grant no. 81772244) and the Natural
Science Fund of Guangdong (grant no. 2020B1515020013).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW carried out the experiments and wrote the
manuscript. FC and YL performed the statistical analysis and
created the figures. QW and YH designed the study and revised the
manuscript. YW and YL confirmed the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Committee for
Ethical Review of Research Involving Human Subjects, Nanfang
Hospital, Southern Medical University, Guangzhou, China (approval
no. NFEC-2018-142). Oral informed consent was obtained from the
participants or their relatives.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roth GA, Johnson C, Abajobir A, Abd-Allah
F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al:
Global, Regional, and National Burden of Cardiovascular Diseases
for 10 Causes, 1990 to 2015. J Am Coll Cardiol. 70:1–25.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Song P, Zha M, Yang X, Xu Y, Wang H, Fang
Z, Yang X, Xia W and Zeng C: Socioeconomic and geographic
variations in the prevalence, awareness, treatment and control of
dyslipidemia in middle-aged and older Chinese. Atherosclerosis.
282:57–66. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Bennett MR, Sinha S and Owens GK: Vascular
Smooth Muscle Cells in Atherosclerosis. Circ Res. 118:692–702.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Basatemur GL, Jørgensen HF, Clarke MCH,
Bennett MR and Mallat Z: Vascular smooth muscle cells in
atherosclerosis. Nat Rev Cardiol. 16:727–744. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Williams KJ and Tabas I: The
response-to-retention hypothesis of early atherogenesis.
Arterioscler Thromb Vasc Biol. 15:551–561. 1995.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tabas I, Williams KJ and Borén J:
Subendothelial lipoprotein retention as the initiating process in
atherosclerosis: Update and therapeutic implications. Circulation.
116:1832–1844. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801.
2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ferns GAA and Heikal L: Hypoxia in
Atherogenesis. Angiology. 68:472–493. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jain T, Nikolopoulou EA, Xu Q and Qu A:
Hypoxia inducible factor as a therapeutic target for
atherosclerosis. Pharmacol Ther. 183:22–33. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Luque A, Turu M, Juan-Babot O, Cardona P,
Font A, Carvajal A, Slevin M, Iborra E, Rubio F, Badimon L, et al:
Overexpression of hypoxia/inflammatory markers in atherosclerotic
carotid plaques. Front Biosci. 13:6483–6490. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Consortium EP: ENCODE Project Consortium.
An integrated encyclopedia of DNA elements in the human genome.
Nature. 489:57–74. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mishra K and Kanduri C: Understanding Long
Noncoding RNA and Chromatin Interactions: What We Know So Far.
Noncoding RNA. 5(5)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
McDonel P and Guttman M: Approaches for
Understanding the Mechanisms of Long Noncoding RNA Regulation of
Gene Expression. Cold Spring Harb Perspect Biol.
11(11)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sauvageau M: Diverging RNPs: Toward
Understanding lncRNA-Protein Interactions and Functions. Adv Exp
Med Biol. 1203:285–312. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Leeper NJ and Maegdefessel L: Non-coding
RNAs: Key regulators of smooth muscle cell fate in vascular
disease. Cardiovasc Res. 114:611–621. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kumar S, Williams D, Sur S, Wang JY and Jo
H: Role of flow-sensitive microRNAs and long noncoding RNAs in
vascular dysfunction and atherosclerosis. Vascul Pharmacol.
114:76–92. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Holdt LM, Kohlmaier A and Teupser D: Long
Noncoding RNAs of the Arterial Wall as Therapeutic Agents and
Targets in Atherosclerosis. Thromb Haemost. 119:1222–1236.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu S, Kamato D, Little PJ, Nakagawa S,
Pelisek J and Jin ZG: Targeting epigenetics and non-coding RNAs in
atherosclerosis: From mechanisms to therapeutics. Pharmacol Ther.
196:15–43. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hu YW, Guo FX, Xu YJ, Li P, Lu ZF, McVey
DG, Zheng L, Wang Q, Ye JH, Kang CM, et al: Long noncoding RNA
NEXN-AS1 mitigates atherosclerosis by regulating the actin-binding
protein NEXN. J Clin Invest. 129:1115–1128. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Bai HL, Lu ZF, Zhao JJ, Ma X, Li XH, Xu H,
Wu SG, Kang CM, Lu JB, Xu YJ, et al: Microarray profiling analysis
and validation of novel long noncoding RNAs and mRNAs as potential
biomarkers and their functions in atherosclerosis. Physiol
Genomics. 51:644–656. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kanehisa M, Furumichi M, Sato Y,
Ishiguro-Watanabe M and Tanabe M: KEGG: Integrating viruses and
cellular organisms. Nucleic Acids Res. 49D:D545–D551.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gene Ontology C: Gene Ontology Consortium.
The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids
Res. 49D:D325–D334. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nan A, Chen L, Zhang N, Liu Z, Yang T,
Wang Z, Yang C and Jiang Y: A novel regulatory network among
lncRpa, circRar1, miR-671 and apoptotic genes promotes lead-induced
neuronal cell apoptosis. Arch Toxicol. 91:1671–1684.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fukata Y, Amano M and Kaibuchi K:
Rho-Rho-kinase pathway in smooth muscle contraction and
cytoskeletal reorganization of non-muscle cells. Trends Pharmacol
Sci. 22:32–39. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Webb RC: Smooth muscle contraction and
relaxation. Adv Physiol Educ. 27:201–206. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lokman NA, Ween MP, Oehler MK and
Ricciardelli C: The role of annexin A2 in tumorigenesis and cancer
progression. Cancer Microenviron. 4:199–208. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mayer G, Poirier S and Seidah NG: Annexin
A2 is a C-terminal PCSK9-binding protein that regulates endogenous
low density lipoprotein receptor levels. J Biol Chem.
283:31791–31801. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ma S, Lu CC, Yang LY, Wang JJ, Wang BS,
Cai HQ, Hao JJ, Xu X, Cai Y, Zhang Y, et al: ANXA2 promotes
esophageal cancer progression by activating MYC-HIF1A-VEGF axis. J
Exp Clin Cancer Res. 37(183)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hussain S, Saxena S, Shrivastava S,
Mohanty AK, Kumar S, Singh RJ, Kumar A, Wani SA, Gandham RK, Kumar
N, et al: Gene expression profiling of spontaneously occurring
canine mammary tumours: Insight into gene networks and pathways
linked to cancer pathogenesis. PLoS One.
13(e0208656)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tang Y, He Y, Zhang P, Wang J, Fan C, Yang
L, Xiong F, Zhang S, Gong Z, Nie S, et al: lncRNAs regulate the
cytoskeleton and related Rho/ROCK signaling in cancer metastasis.
Mol Cancer. 17(77)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Saiki Y and Horii A: Multiple functions of
S100A10, an important cancer promoter. Pathol Int. 69:629–636.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Z, Wei Q, Han L, Cao K, Lan T, Xu Z,
Wang Y, Gao Y, Xue J, Shan F, et al: Tenascin-c renders a
proangiogenic phenotype in macrophage via annexin II. J Cell Mol
Med. 22:429–438. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ding P, Ding Y, Tian Y and Lei X: Circular
RNA circ_0010283 regulates the viability and migration of oxidized
low density lipoprotein induced vascular smooth muscle cells via an
miR 370 3p/HMGB1 axis in atherosclerosis. Int J Mol Med.
46:1399–1408. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yan Z, Wang H, Liang J, Li Y and Li X:
MicroRNA-503-5p improves carotid artery stenosis by inhibiting the
proliferation of vascular smooth muscle cells. Exp Ther Med.
20(85)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Deryugina EI and Quigley JP: Cell surface
remodeling by plasmin: A new function for an old enzyme. J Biomed
Biotechnol. 2012(564259)2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li L, Li X, The E, Wang LJ, Yuan TY, Wang
SY, Feng J, Wang J, Liu Y, Wu YH, et al: Low expression of
lncRNA-GAS5 is implicated in human primary varicose great saphenous
veins. PLoS One. 10(e0120550)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhu B, Gong Y, Yan G, Wang D, Qiao Y, Wang
Q, Liu B, Hou J, Li R and Tang C: Down-regulation of lncRNA MEG3
promotes hypoxia-induced human pulmonary artery smooth muscle cell
proliferation and migration via repressing PTEN by sponging miR-21.
Biochem Biophys Res Commun. 495:2125–2132. 2018.PubMed/NCBI View Article : Google Scholar
|