Introduction
The ageing of the population is a global phenomenon.
The World Health Organization (WHO) reported that the number of
people over the age of 65 will exceed the number of children under
the age of 5 by 2020(1). Ageing is
a major risk factor for the development of metabolic disorders,
such as insulin resistance (IR) and obesity (2,3).
IR is a decrease in the ability of insulin to
maintain normal blood glucose levels, meaning that a certain
concentration of insulin will not produce the desired physiological
effect, and the response of tissues, such as liver tissue, adipose
tissue and skeletal muscle, to insulin decreases. Glucose toxicity
is a significant cause of IR (4).
In the IR state, the uptake and utilization of glucose by
peripheral tissues, including liver tissue, adipose tissue and
skeletal muscle) is significantly decreased (5,6).
Adipose tissue is considered to be one of the major peripheral
tissues that develops IR (7,8). In
particular, white adipose tissue serves an active role in IR by
secreting inflammatory factors, including tumor necrosis factor-α
(TNF-α), interleukin (IL)-6 and monocyte chemotactic protein
(MCP)1(9). Pro-inflammatory
cytokines, particularly TNF-α, could enhance the serine
phosphorylation of insulin receptor substrate-1 (IRS-1), which
induced insulin resistance (10).
The PI3K/Akt pathway serves a crucial role in
insulin signaling and insulin-mediated glucose metabolism pathways
(11-13).
In addition, the PI3K/Akt pathway is the primary signal
transduction pathway downstream of the insulin receptor, which is
closely associated with the development of diabetes and decreased
insulin sensitivity (14). In
adipocytes, activated PI3K and Akt promote translocation and fusion
of glucose transporter 4 (GLUT4)-containing vesicles to the
membrane, increasing GLUT4 transporter protein and glucose uptake
at the membrane surface (15,16).
Hence, impairment of PI3K/Akt signaling pathways may be the
underlying mechanism associated with IR.
Ursolic acid (UA) is a triterpenoid compound widely
found in natural plants, such as crataegus pinnatifida Bunge
and fructus mume. UA has gained attention due to its varied
effects including anti-inflammatory, antioxidant, anticarcinogenic,
antimicrobial, antiviral and hepatoprotective actions (17,18).
Previous studies have confirmed that UA can increase insulin
sensitivity, improve glucose and lipid metabolism disorders, and
hence alleviate the role of metabolic syndrome in high-fat
diet-induced non-alcoholic fatty liver disease rats (19,20).
Our previous study has also demonstrated that a mixture of malus
domestica and rosmarinus officinalis extract (rich in
UA) improved IR induced by high fructose in rats (21). However, to the best of our knowledge
there has been no study yet that has investigated the effects and
underlying mechanisms of UA on age-associated IR.
In the present study, the effect of UA on adipose
tissue IR was investigated in ageing rats. To explore its potential
mechanism, IRS-1/PI3K/Akt signaling pathway and inflammatory
factors (NF-κB, IL-6, IL-1β) in epididymis white adipose tissue
(eWAT) were examined. The results provided a molecular
pharmacological basis for UA to prevent and treat metabolic
diseases.
Materials and methods
Animals
Male young (3-month; body weight, 210±20 g) and old
(22-month; body weight, 550±20 g) Sprague-Dawley rats [Laboratory
animal certificate number, SCXK (Chongqing) 2018-0003] were
supplied by the Laboratory Animal Centre of Chongqing Medical
University (Chongqing, China). The rats were housed in a
temperature-controlled facility at 21±1˚C under a 12/12-h
light/dark cycle and a relative humidity of 55±5% in a specific
pathogen-free facility with free access to water and food. All
animal experiments were performed in accordance with the
institutional and national guidelines and regulations, and approved
by the Animal Ethics Committee of Chongqing Medical University.
Reagents
The following reagents were used in the present
study. Ursolic acid (donated by Pharmafood Institute; Kyoto,
Japan), arabic gum (cat. no. MC0124B1013J; Shanghai Shenggong
Biology Engineering Technology Service, Ltd.), isoflurane (cat. no.
217180801; Shenzhen Rui Wode Life Technology Co., Ltd.),
Triglyceride Reagent kit (TG; cat. no. 2017040017; Zhejiang Dongpu
Diagnostic Products Co., Ltd.), Glucose Reagent kit (cat. no.
20171004147; Shanghai Rongsheng Biological Pharmaceutical Co.,
Ltd.), non-esterified fatty acid (NEFA) Reagent kit (cat. no.
AR083; Fujifilm Wako Pure Chemical Corporation), Insulin Reagent
kit (cat. no. 13SERI049; Morinaga Institute of Biological Science,
Inc.) and RIPA buffer (Strong; cat. no. P0013B; Beyotime Institute
of Biotechnology).
Experimental protocol
Following 1 week of adaptable feeding, 25 ageing
model rats were divided initially into 3 groups: i) Untreated
ageing group (aged; n=9); ii) group supplemented with low UA 10
mg/kg (UA-L; n=8); and iii) high 50 mg/kg (UA-H; n=8) (21-23).
A total of 8 young rats were randomly selected as the young
untreated group (young). Animals in the UA-treated groups received
UA suspended in 5% arabic gum solution and administered via oral
gavage once daily for 7 weeks. The rats in the corresponding
untreated aged and young groups received vehicle (5% arabic gum)
alone for 7 weeks. All rats had free access to a standard diet. The
consumed goods and body weight of the animals was measured every 2
days and the behavior of the rats was monitored. The study was
scheduled for 7 weeks. The day before the experiment was
terminated, the rats were deprived of food, but still had free
access to water overnight (14 h). Subsequently, all the animals
were weighed, anesthetized with inhaled isoflurane at a
concentration of 3-4% and sacrificed via prompt cervical
dislocation. Death was confirmed in the animals by the
disappearance of breathing and heartbeat. Subsequently, eWAT were
collected and weighed and immediately frozen in liquid nitrogen and
stored at -80˚C until further use.
Biochemical determination
The day before the experiment was terminated, all
rats were deprived of food, but still had free access to water for
14 h. Blood samples (~700 µl) were collected via retro-orbital
venous puncture under isoflurane anesthesia, then plasma was
extracted by centrifugation at 1,200 x g for 15 min at 4˚C for
determination of concentrations of glucose. All the kits were used
according to the manufacturer's instructions.
Detection of homeostasis model
assessment of insulin resistance (HOMA-IR) and adipose tissue
insulin resistance (Adipo-IR) indices
The following formulae were used to determine
HOMA-IR and the Adipo-IR indices: HOMA-IR index=[fasted insulin
level (mIU/ml) x fasted glucose level (mM)]/22.5(24); Adipo-IR=fasted insulin (mmol/l) x
fasted NEFA (pmol/l) (25-29).
Western blotting
Total, plasma and membrane protein was prepared from
eWAT using the Minute™ Plasma Membrane Protein Isolation
kit (cat. no. SM-005 Minute™ Plasma Membrane Protein
Isolation kit (cat. no. SM-005; Invent Biotechnologies, Inc.)
according to the manufacturer's instructions. The epididymal
adipose tissue was homogenized with RIPA buffer supplemented with a
1:100 dilution of protease and phosphatase inhibitors (PMSF) (cat.
no. P8340 and cat. no. P5726, respectively; Sigma-Aldrich; Merck
KGaA). The protein concentration was determined using a
bicinchoninic acid (BCA) protein concentration assay kit (Enhanced;
cat. no. P0010S; Beyotime Institute of Biotechnology). Proteins (25
µg) were separated via 8% SDS-PAGE under reducing conditions and
transferred to PVDF membranes (GE Healthcare). Membranes were
incubated in blocking buffer (5% skimmed milk) for 2 h at room
temperature and immunoblotted with the following primary antibodies
for 14 h at 4˚C: Rabbit polyclonal anti-IRS-1 antibody (1:1,000;
cat. no. TA312859; Origene Technologies, Inc.), rabbit polyclonal
anti-phosphorylated(p)-IRS-1Ser307 antibody (1:1,000;
cat. no. TA325573; Origene Technologies, Inc.), rabbit monoclonal
anti-Akt antibody (1:1,000; cat. no. ab179463; Abcam), rabbit
monoclonal anti-p-AktSer473 antibody (1:1,000; cat. no.
4060; Cell Signaling Technology, Inc.), rabbit polyclonal PI3K
antibody (1:6,000; cat. no. 2225185; EMD Millipore), rat monoclonal
GLUT4 antibody (1:1,000; cat. no. 10613; Santa Cruz Biotechnology,
Inc.), rabbit polyclonal NF-κB antibody (1:2,000; cat. no. ab16502;
Abcam), rabbit polyclonal IL-1β antibody (1:1,000; cat. no. ab9722;
Abcam), rat monoclonal IL-6 antibody (1:1,000; cat. no. ab9324;
Abcam), and rabbit polyclonal anti-sodium/potassium-transporting
ATPase subunit alpha-1 (ATP-1a1) antibody (1:1,000; cat. no.
TA326844; Origene Technologies, Inc.). Polyclonal rabbit GAPDH
antibody (1:25,000; cat. no. sc-AP0063; Bioworld Technology, Inc.)
was used as a loading control to normalize the signal that was
obtained. Subsequent to washing (4 times, 10 min/time) with
TBS-0.1% Tween 20 (TBST), horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG secondary antibody (1:5,000; cat. no. BA1054;
Boster Biological Technology) or goat anti-mouse IgG secondary
antibody (1:5,000; cat. no. BA1050; Boster Biological Technology)
diluted in phosphate buffered saline were applied to the membrane,
followed by incubation for 1.5 h at room temperature. After washing
4 times with TBST solution for 10 min each time, the immunoreactive
bands were visualized via autoradiography and the density was
evaluated using ImageJ v.1.43 (National Institutes of Health).
Levels in young rats were arbitrarily assigned a value of 1.
Statistical analysis
All results are expressed as the mean ± SEM. At
least eight biological replicates were performed. Data were
analyzed using StatView software v.5.0 (SAS Institute, Inc.). Data
were analyzed by one-way ANOVA followed by the post hoc Student
Newman-Keuls and Bonferroni tests to determine differences between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Characteristics of rats
The chow intake and body weight of the rats were
measured every 2 days. Body weight, food intake, eWAT weight and
the ratio of eWAT weight/body weight significantly increased, while
body weight gain decreased in the aged group compared with the
young normal group (Fig. 1A-E).
Treatment with UA-H decreased the weight of eWAT and the ratio of
eWAT weight/body weight (Fig. 1D
and E); however, UA-L did not
(Fig. 1D and E). UA administration did not significantly
alter other parameters (Fig. 1A-C).
Therefore, the aged rat model has been initially established, and
UA may improve the phenomenon of fat accumulation in the epididymis
of the aged rats.
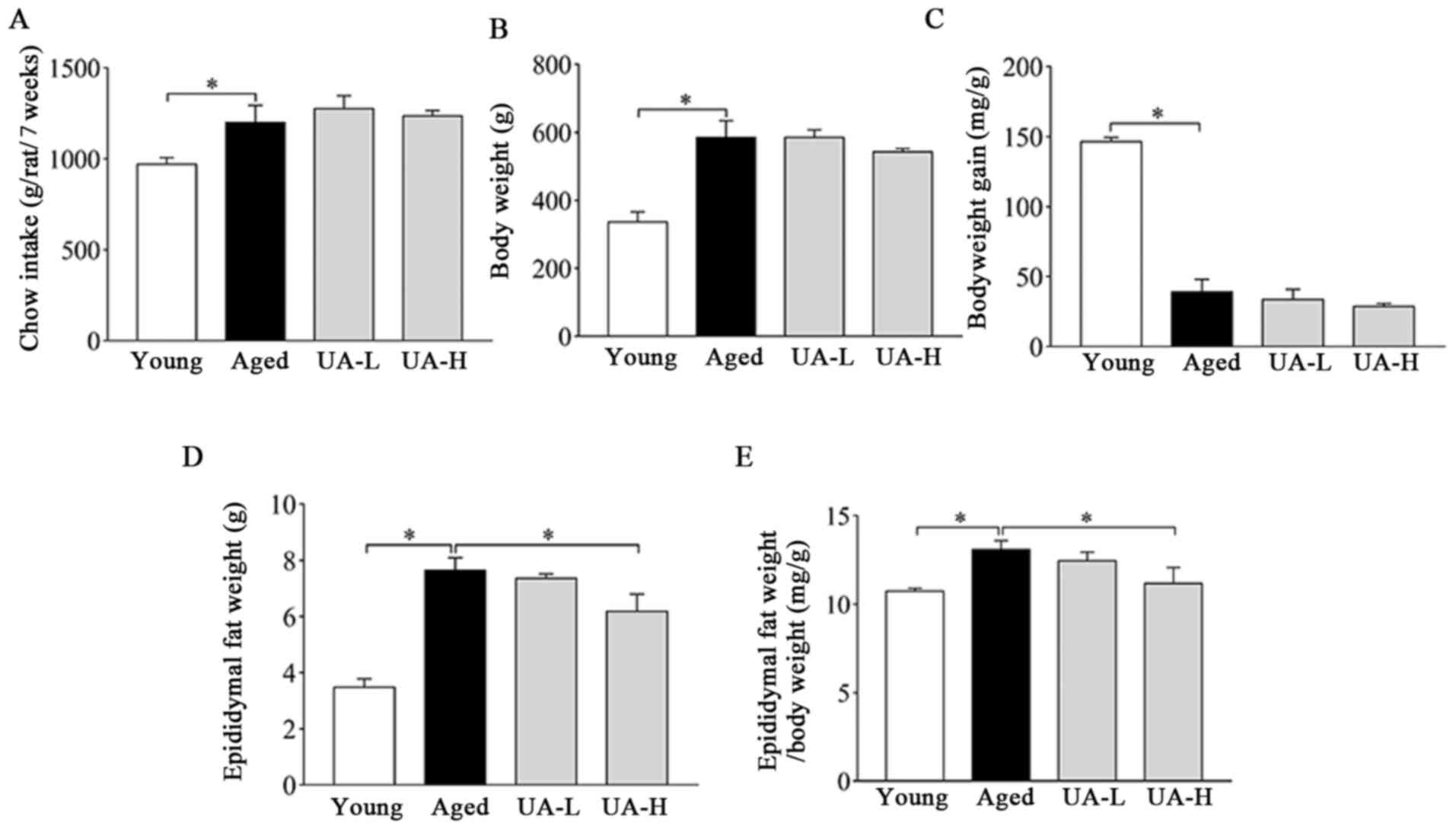 | Figure 1General characteristics of rats
included in the present study. (A) Food (chow) intake, (B) body
weight, (C) body weight gain, (D) epididymal fat weight and (E)
ratio of epididymal fat weight/body weight in rats. Treatment
groups were: Young (untreated, n=8), aged (untreated, n=9), treated
with 10 mg/kg UA (UA-L, n=8) or 50 mg/kg UA (UA-H, n=9) for 7
weeks. All animals had free access to a standard diet.
*P<0.05 compared with aged group. UA, ursolic acid;
UA-L, low ursolic acid; UA-H, high ursolic acid. |
Effect of UA on glucose and lipid
metabolism disorders in ageing rats
At the end of the experiment, blood was collected
from the rats after overnight fasting. As indicated in Fig. 2, aged rats had increased fasting
plasma TG, glucose and insulin concentrations, compared with young
rats (Fig. 2A-C). Treatment with
UA-H partially reversed the increase in plasma TG and insulin
concentration (Fig. 2A and C) but had no effect on glucose
concentrations (Fig. 2B). In
addition, compared with young rats, the HOMA-IR index and Adipo-IR
index increased ~2 fold in ageing rats (Fig. 2D and F), indicating increase of systemic and
adipose tissue IR with ageing. Oral administration of UA-H reversed
the increase in the HOMA-IR index and Adipo-IR index (Fig. 2D and F), but the UA-L group demonstrated minor
changes (Fig. 2D and F). Plasma NEFA had no significant
differences between all the rat groups tested (Fig. 2E). Taken together, these results
suggested that treatment with UA-H improves metabolic effects
compared with UA-L treatment.
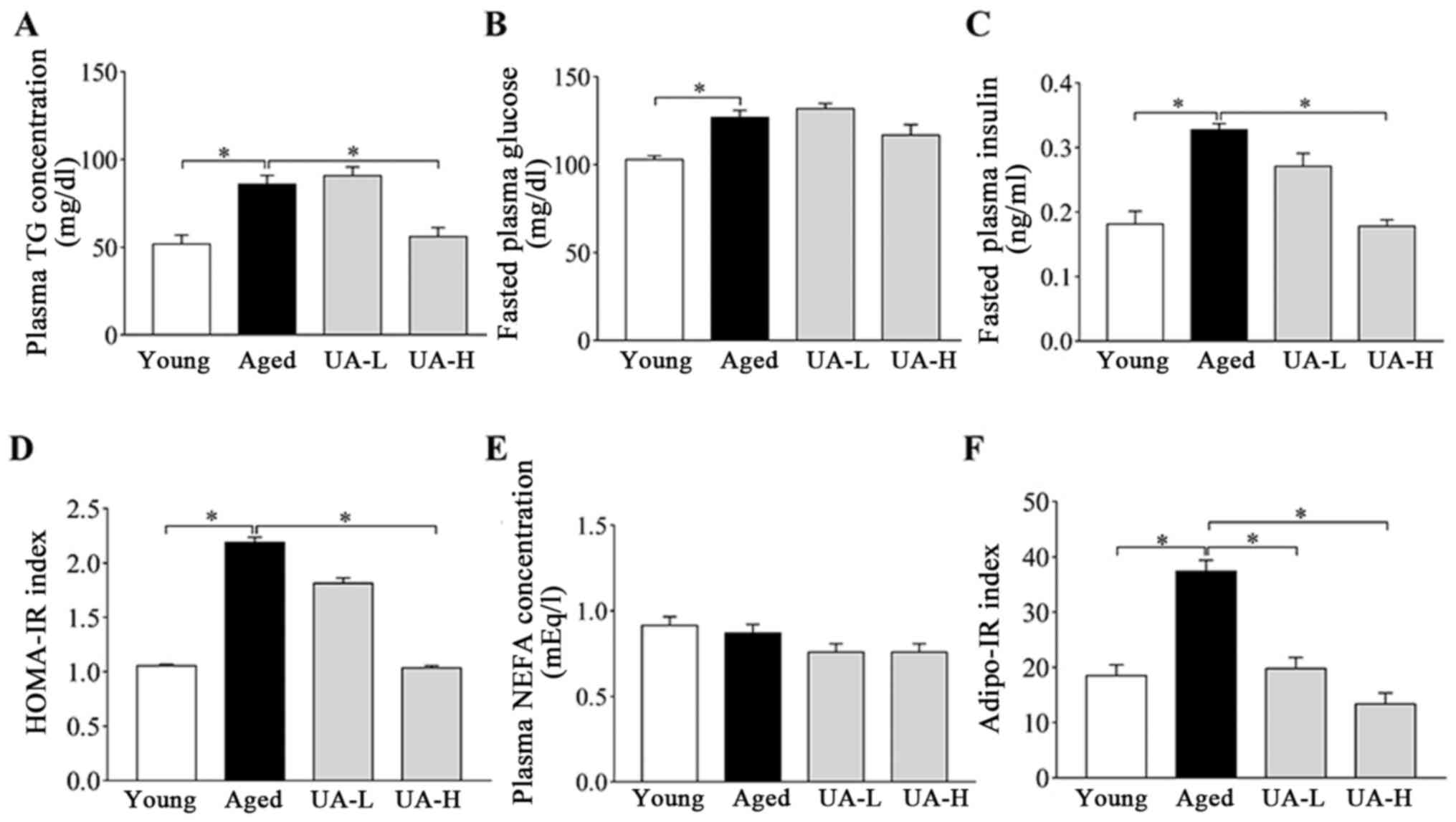 | Figure 2Effect of UA on glucose and lipid
metabolism disorders in aged rats. (A) Plasma TG concentration, (B)
fasted plasma glucose, (C) fasted plasma insulin, (D) HOMA-IR
index, (E) plasma NEFA concentration and (F) adipo-IR index in
rats. Treatment groups were: Young (untreated, n=8), aged
(untreated, n=9), treated with 10 mg/kg UA (UA-L, n=8) or 50 mg/kg
UA (UA-H, n=9) for 7 weeks. All animals had free access to a
standard diet. *P<0.05 compared with aged group. TG,
triglyceride; UA-L, low ursolic acid; UA-H, high ursolic acid;
HOMA-IR, the homeostasis model assessment of insulin resistance;
NEFA, non-esterified fatty acid; Adipo-IR, adipose tissue insulin
resistance. |
Effect of UA on the IRS-1/PI3K/Akt
signaling pathway in eWAT of ageing rats
Subsequently, the effect of UA on insulin signaling
pathways was further investigated in the UA-H group. Western
blotting was used to investigate the adipose tissue proteins of the
epididymis of rats in each group. The expression of IRS-1, p-IRS-1
(ser307) and p-Akt (ser473), and the ratio of p-Akt (ser473) to Akt
were all significantly decreased in the eWAT of ageing rats,
compared with young normal rats (Fig.
3A). Treatment with UA-H significantly reversed all these
changes, except for the ratio of p-IRS-1 (ser307) to IRS-1 protein
(Fig. 3A). In addition, the
expression of PI3K and Akt did not differ between the young, aged
and UA-H groups (Fig. 3A and
B). The aforementioned results
indicated that UA ameliorated eWAT IR in ageing rats, which was
associated with the Akt signaling pathway.
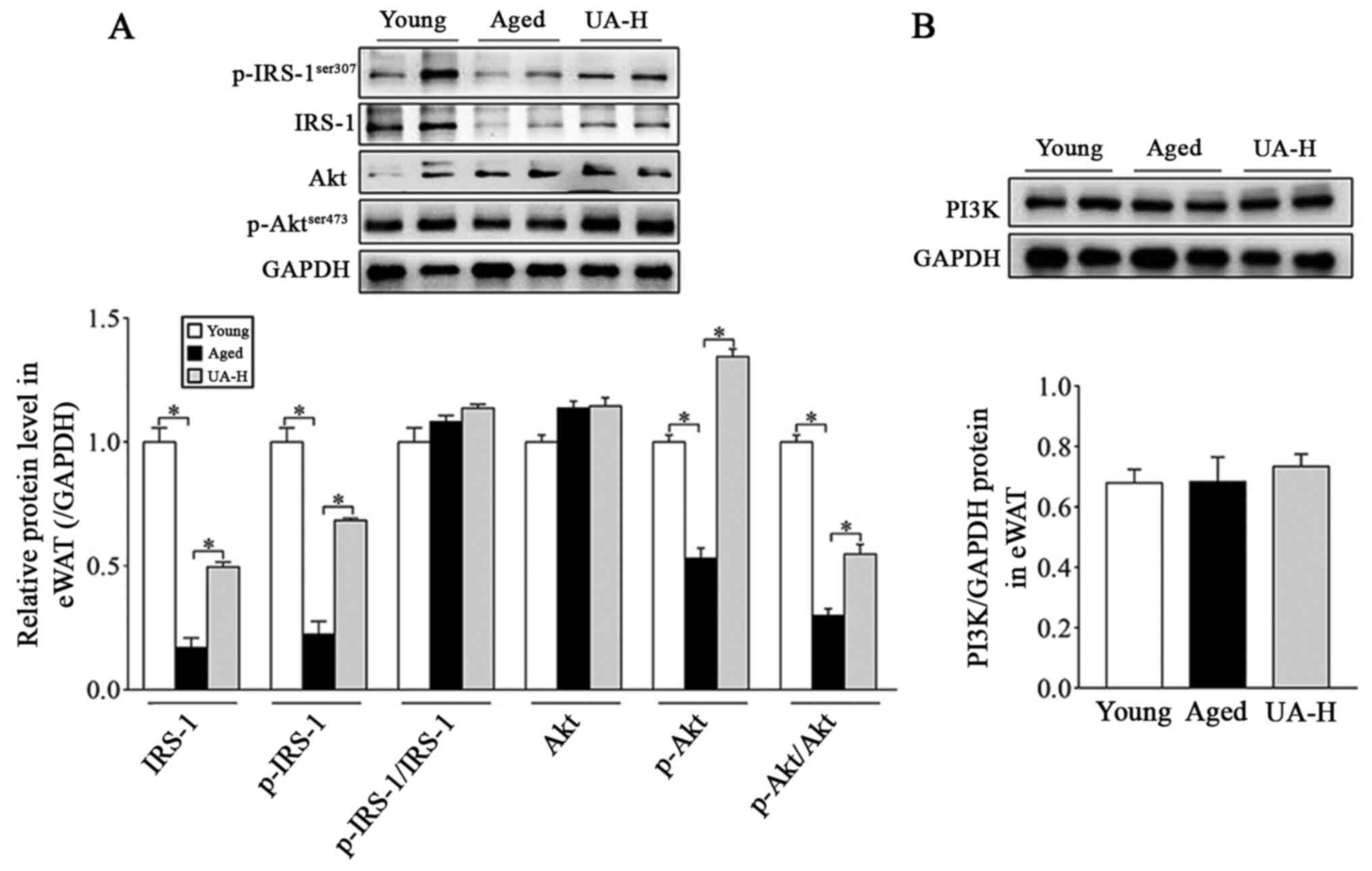 | Figure 3Effect of UA on IRS-1/PI3K/Akt
signaling pathway in eWAT of aged rats. (A) Expression of IRS-1,
p-IRS-1 (Ser307), p-IRS-1 (Ser307)/IRS-1, Akt, p-Akt (Ser473) and
p-Akt (Ser473)/Akt protein levels. (B) PI3K protein levels.
Treatment groups were: Young (untreated, n=8), aged (untreated,
n=9), treated with 10 mg/kg UA (UA-L, n=8) or 50 mg/kg UA (UA-H,
n=9) for 7 weeks. All animals had free access to a standard diet.
*P<0.05 compared with aged group. UA-H, high ursolic
acid; eWAT, epididymis white adipose tissue; IRS-1, insulin
receptor substrate-1; p, phosphorylated; Akt, protein kinase B;
PI3K, phosphatidylinositol 3-kinase. |
Effect of UA on GLUT4 translocation in
eWAT of ageing rats
Membrane proteins and total proteins were extracted
and detected via western blotting. GLUT4 total protein expression
was significantly decreased in eWAT of aged rats compared with the
young normal group (Fig. 4A).
Treatment with UA-H significantly upregulated GLUT4 expression in
the aged rats (Fig. 4A). Due to
GLUT4 primarily exerting its effects in the plasma membrane, the
impact of UA on GLUT4 on the plasma membrane and cytoplasmic Glut4
in eWAT of ageing rats was evaluated. As expected, UA-H
significantly inhibited the decrease of GLUT4 on the plasma
membrane and cytoplasmic Glut4 in the adipocytes of ageing rats
(Fig. 4B and C). These results indicated that ageing may
result in impaired glucose transport and UA may regulate the
translocation of GLUT4 to facilitate glucose intake in eWAT.
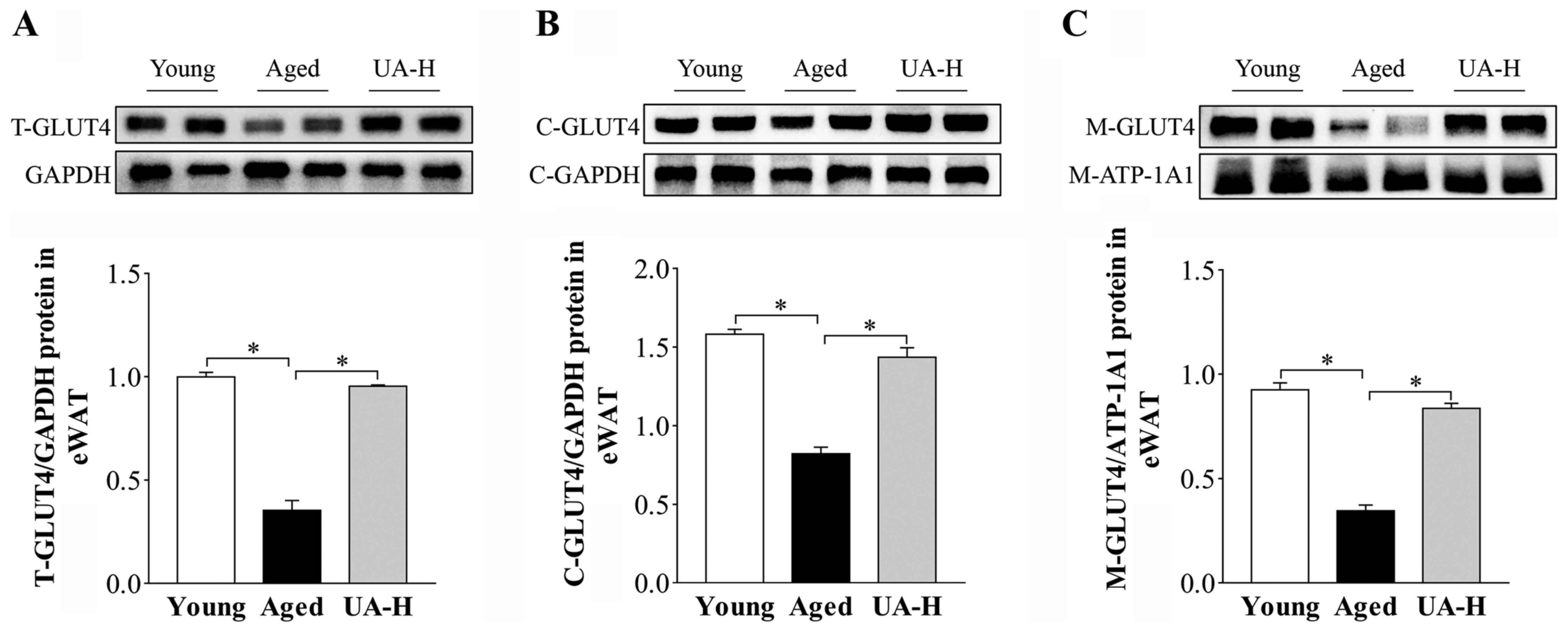 | Figure 4Effect of UA on Glut4 translocation
in eWAT of aged rats. Protein expression of (A) T-Glut4 protein
levels, (B) C-Glut4 protein levels and (C) M-Glut4 protein levels
in eWAT of rats. Treatment groups were: Young (untreated, n=8),
aged (untreated, n=9), treated with 10 mg/kg UA (UA-L, n=8) or 50
mg/kg UA (UA-H, n=9) for 7 weeks. All animals had free access to a
standard diet. *P<0.05 compared with aged group.
UA-H, high ursolic acid; eWAT, epididymis white adipose tissue;
T-Glut4, Glut4 total protein; C-Glut4, cytoplasmic Glut4; M-Glut4,
Glut4 on the plasma membrane; GLUT4, glucose transporter 4. |
Effect of UA on inflammation in eWAT
of ageing rats
Western blotting results revealed that the protein
expression of NF-κB and proinflammatory cytokines IL-6 and IL-1β
were higher in aged rats compared with young normal rats (Fig. 5A-D). Treatment with UA-H
significantly decreased these factors (Fig. 5A-D) indicating that UA may alleviate
age-related adipose tissue inflammatory.
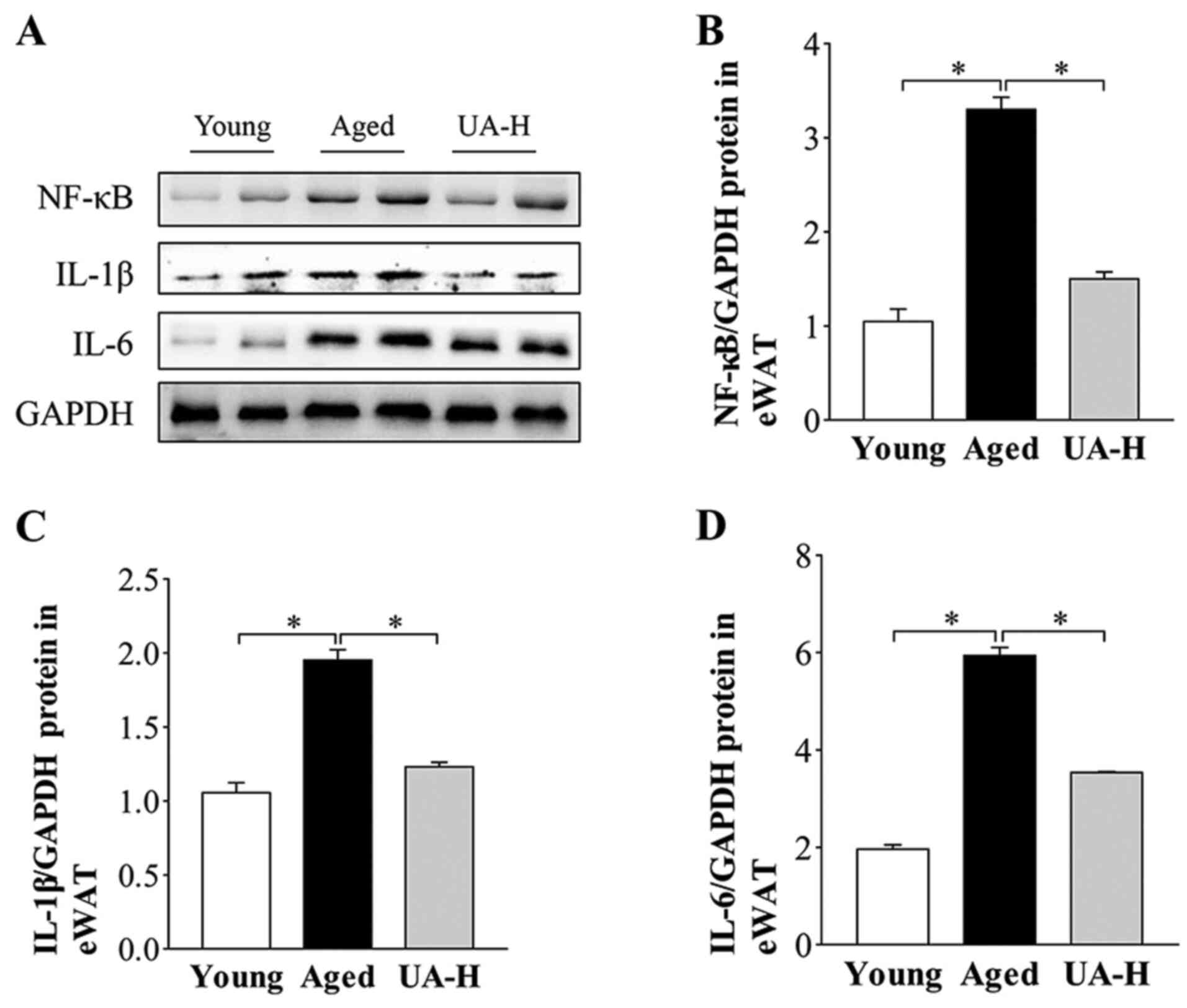 | Figure 5Effect of UA on inflammatory markers
in eWAT of aged rats. (A) Representative images of western blot
analysis of NF-κB, IL-6 and IL-1β in eWAT. (B) NF-κB (C) IL-1β and
(D) IL-6 protein expression levels in eWAT of rats. Treatment
groups were: Young (untreated, n=8), aged (untreated, n=9), treated
with 10 mg/kg UA (UA-L, n=8) or 50 mg/kg UA (UA-H, n=9) for 7
weeks. All animals had free access to a standard diet.
*P<0.05 compared with aged group. UA-H, high ursolic
acid; eWAT, epididymis white adipose tissue; NF-κB, nuclear factor
κB; IL, interleukin. |
Discussion
During ageing, the body's metabolic changes are
closely associated with several prevalent medical conditions, such
as obesity, IR, type 2 diabetes, cardiovascular diseases, such as
hyperlipidemia and coronary atherosclerosis, and neurodegenerative
disorders, such as Alzheimer's disease and Parkinson's disease
(30). Of these, IR is the common
pathophysiological basis and risk factor of multiple metabolic
diseases, which is the premise of the present study (31,32).
HOMA-IR is a simple and useful indicator in the assessment of IR in
epidemiological studies (33) and
is widely used for the determination of glucose and insulin levels
to reflect systemic IR (34).
Adipo-IR is often used as an effective indicator to measure insulin
sensitivity in adipose tissue (35). It has been reported that adipose
tissue is a tissue type in which inflammation and IR are
established particularly early during ageing (36). Epididymal fat is a representative
visceral adipose tissue, which influences metabolic processes due
to its active secretion of cytokines (visfatin, adiponectin) and
chemokines (C-C motif chemokine 2, IL-8), and is widely used in
metabolic studies (37). In the
present study, compared with young rats, the aged rats had
significantly higher eWAT weight, plasma glucose, insulin, TG
concentration, HOMA-IR index and Adipo-IR, which indicated
ageing-associated adipose tissue IR. It has been reported that UA
regulates the ageing process via enhancement of metabolic sensor
protein levels (including sirtuin 1, sirtuin 6 and
proliferator-activated receptor gamma coactivator-1β) (38). Previous studies have demonstrated
that dietary UA improves health and life span in male Drosophila
melanogaster (39), and exerts an
antiaging effect by promoting mice skeletal muscle rejuvenation in
C57BL/6(40). Notably, in the
present study, UA treatment significantly decreased the
aforementioned parameters (eWAT weight, plasma glucose, insulin, TG
concentration, HOMA-IR index and Adipo-IR) in aged rats. The
findings of the present study suggested that UA may improve
ageing-related adipose tissue IR; however, the possible mechanism
for this needs further verification.
The key mechanism of IR is hypothesized to be the
disturbance of the insulin signaling pathway (41). In addition, the PI3K/Akt signaling
pathway serves an important role in the metabolic function of
insulin, which regulates the absorption and metabolism of glucose
(42,43). In addition, in adipocytes, IR has
been attributed to post-insulin receptor defects, making IRS-1 and
Akt appropriate candidates to study insulin pathway alterations
(44). Ser307 in IRS-1 has been
revealed as a molecular indicator of IR, when the serine residues
of IRS-1 are phosphorylated in response to stimuli (TNF-α, insulin)
(45). There is a progressive
decline of insulin-stimulated phosphorylation associated with
ageing, such as insulin-induced IR and IRS-1 phosphorylation
(46). Similarly, the findings of
the present study demonstrated that the expression of IRS-1 and
p-IRS-1(ser307) was inhibited in eWAT of ageing rats compared with
the young normal group. In the present study, oral administration
of UA reversed these changes, but it did not alter the ratio of
p-IRS-1 (ser307)/IRS-1. IRS-1 couples insulin receptor signaling to
a PI3K-dependent pathway and activated PI3K regulates insulin
metabolism primarily via IRS-1(47). Other molecules downstream of PI3K,
such as Akt/mTOR and Akt/NF-κB, are also altered (46). Akt is a key protein located
downstream of PI3K, which stimulates the GLUT4 transmembrane
receptor to transport glucose, accelerating glycometabolism
(48). Several studies have
suggested that ageing deteriorates both systemic insulin
sensitivity in aged ovariectomized female rats (49,50),
and GLUT4 total expression levels in aged female rats (51,52) in
the cerebral cortex. In addition, in adipose tissue of transgenic
mice, insulin-stimulated transport of glucose was primarily
mediated by GLUT4(51). Under the
stimulation of insulin, GLUT4 promoted the translocation of glucose
to the cell membrane, promoting glucose uptake and metabolism in
3T3-L1 adipocytes (53). Hence, the
decrease in GLUT4 activity associated with ageing may result in a
barrier to glucose uptake from fat and result in a decrease in
insulin sensitivity. Similarly, the findings of the present study
revealed that the protein expression of IRS-1 (ser307)/p-IRS-1,
p-Akt (Ser473)/Akt, and both total GLUT4 and cellular membrane
GLUT4 were inhibited in eWAT of aged rats compared with the young
normal group, indicating the age-related impairment of the
IRS-1-PI3K-Akt/GLUT4 pathway. In the present study, although UA had
no effect on p-IRS-1 (ser307)/IRS-1, PI3K and total Akt, it
significantly reversed the decrease in the ratio of p-Akt
(Ser473)/Akt and the level of GLUT4 on the plasma membrane of
adipocytes. The findings of the present study suggested that UA may
improve adipose tissue insulin sensitivity to facilitate glucose
uptake and metabolism, which is associated with Akt activation and
GLUT4 membrane translocation.
Although, all tissues exhibit signs of inflammation
and IR with ageing, epididymal fat is the first to develop
age-associated signs of inflammation and IR of the white fat
tissues (35). Chronic systemic
inflammation without apparent infection in elderly humans is often
referred to as the ‘inflammageing’ phenotype and is primarily
characterized by elevated levels of pro-inflammatory cytokines
(IL-6, TNF-α) (54-59).
The present study demonstrated that the levels of pro-inflammatory
cytokines IL-6 and IL-1β are significantly elevated in the eWAT of
ageing rats compared with the young normal group. Previous studies
have demonstrated that NF-κB serves an important role in
obesity-related inflammation, by activating the production of
pro-inflammatory factors, such as activator protein-1, TNF-α and
IL-6 (60-63).
In addition, it has been reported that in mice with
diabetes-induced nephropathy, UA alleviated the levels of
pro-inflammatory factors TNF-α, IL-1β, IL-6 and IL-8(64). Concordantly, the present study
revealed that UA significantly decreased expression levels of
pro-inflammatory factors, such as IL-6 and IL-1β in the eWAT of
aged rats compared with aged group, which may be associated with UA
regulation of NF-κB signaling. It has been demonstrated that the
insulin receptor signaling pathway and inflammatory cytokines
(TNF-α, IL-1β, IL-6) ross-talk with each other (65). Hence, the amelioration of insulin
sensitivity induced by UA may be associated with inhibiting
proinflammatory factor release in adipose tissue of aged rats. The
limitation of the present study is that in vitro experiments
were not performed. Future work will verify the current conclusions
through in vitro cell experiments.
In conclusion, the present study demonstrated that
UA had favorable effects on hyperinsulinemia, hypertriglyceridemia
and pro-inflammatory cytokine expression in eWAT of aged rats,
indicating that UA may improve age-related IR and inflammation in
adipose tissue. The findings of the present study indicated that UA
has potential as a therapeutic agent for IR and metabolic syndromes
associated with ageing, and provides a basis for the development of
effective and safe drugs or functional foods for the prevention and
treatment of metabolic diseases, including hypertension, coronary
heart disease and obesity.
Acknowledgements
The authors would like to thank Dr Chun-Li Li
(Institute of Life Sciences, Chongqing Medical University,
Chongqing, China) and Dr Da-Zhi Ke (The Second Affiliated Hospital
of Chongqing Medical University, Chongqing, China) for their
critical revisions and constructive comments on the content of the
present study.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81374033 and 81673659), Chongqing
Postgraduate Research and Innovation Project Fund (grant no.
CYS18210), Project of Chongqing Education Commission (grant. no.
KJ1600233) and Cultivation Fund of Chongqing medical university
(grant no. X12100).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TZW, GWZ and JWW conceived and designed the
experiments of the current study. TZW, LY, CLY, HFL, YL, ZWC, YQJ
and JZ performed the experiments. TZW, CLY, HFL and GWZ analyzed
the data. JY drafted the work and reviewed the research design.
DZK, CLL and JY revised the manuscript critically for important
intellectual content. JWW provided reagents, materials and analysis
tools and finally approved the version to be released. TZW wrote
the manuscript. GWZ and JWW were responsible for reviewing the
manuscript for important intellectual content. TZW, GWZ and JWW
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with the institutional and national guidelines and regulations, and
approved by the Chongqing Medical University Animal Care and Use
Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bai Y, Bian F, Zhang L and Cao Y: The
impact of social support on the health of the rural elderly in
China. Int J Environ Res Public Health. 17(2004)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Evans JL and Goldfine ID: Aging and
insulin resistance: Just say iNOS. Diabetes. 62:346–348.
2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hanson RL, Imperatore G, Bennett PH and
Knowler WC: Components of the ‘metabolic syndrome’ and incidence of
type 2 diabetes. Diabetes. 51:3120–3127. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Joannides CN, Mangiafico SP, Waters MF,
Lamont BJ and Andrikopoulos S: Dapagliflozin improves insulin
resistance and glucose intolerance in a novel transgenic rat model
of chronic glucose overproduction and glucose toxicity. Diabetes
Obes Metab. 19:1135–1146. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jeong JH and Kang EB: Effects of treadmill
exercise on PI3K/AKT/GSK-3β pathway and tau protein in high-fat
diet-fed rats. J Exerc Nutrition Biochem. 22:9–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li Y, Wang J, Gu T, Yamahara J and Li Y:
Oleanolic acid supplement attenuates liquid fructose-induced
adipose tissue insulin resistance through the insulin receptor
substrate-1/phosphatidylinositol 3-kinase/Akt signaling pathway in
rats. Toxicol Appl Pharmacol. 277:155–163. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Luan G, Li G, Ma X, Jin Y, Hu N, Li J,
Wang Z and Wang H: Dexamethasone-induced mitochondrial dysfunction
and insulin resistance-study in 3T3-L1 adipocytes and mitochondria
isolated from mouse liver. Molecules. 24(1982)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang F, Han L, Qin RR, Zhang YY, Wang D,
Wang ZH, Tang MX, Zhang Y, Zhong M and Zhang W: Overexpressing
STAMP2 attenuates adipose tissue angiogenesis and insulin
resistance in diabetic ApoE -/- /LDLR -/-
mouse via a PPARγ/CD36 pathway. J Cell Mol Med. 21:3298–3308.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee BC and Lee J: Cellular and molecular
players in adipose tissue inflammation in the development of
obesity-induced insulin resistance. Biochim Biophys Acta.
1842:446–462. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cui X, Qian DW, Jiang S, Shang EX, Zhu ZH
and Duan JA: Scutellariae radix and coptidis rhizoma improve
glucose and lipid metabolism in T2DM rats via regulation of the
metabolic profiling and MAPK/PI3K/Akt signaling pathway. Int J Mol
Sci. 19(3634)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cusi K, Maezono K, Osman A, Pendergrass M,
Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR and Mandarino LJ:
Insulin resistance differentially affects the PI 3-kinase- and MAP
kinase-mediated signaling in human muscle. J Clin Invest.
105:311–320. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Brachmann SM, Ueki K, Engelman JA, Kahn RC
and Cantley LC: Phosphoinositide 3-kinase catalytic subunit
deletion and regulatory subunit deletion have opposite effects on
insulin sensitivity in mice. Mol Cell Biol. 25:1596–1607.
2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kanai F, Ito K, Todaka M, Hayashi H,
Kamohara S, Ishii K, Okada T, Hazeki O, Ui M and Ebina Y:
Insulin-stimulated GLUT4 translocation is relevant to the
phosphorylation of IRS-1 and the activity of PI3-kinase. Biochem
Biophys Res Commun. 195:762–768. 1993.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kohn AD, Summers SA, Birnbaum MJ and Roth
RA: Expression of a constitutively active Akt Ser/Thr kinase in
3T3-L1 adipocytes stimulates glucose uptake and glucose transporter
4 translocation. J Biol Chem. 271:31372–31378. 1996.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gandhi GR, Stalin A, Balakrishna K,
Ignacimuthu S, Paulraj MG and Vishal R: Insulin sensitization via
partial agonism of PPARγ and glucose uptake through translocation
and activation of GLUT4 in PI3K/p-Akt signaling pathway by embelin
in type 2 diabetic rats. Biochim Biophys Acta. 1830:2243–2255.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu J: Oleanolic acid and ursolic acid:
Research perspectives. J Ethnopharmacol. 100:92–94. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ikeda Y, Murakami A and Ohigashi H:
Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol Nutr
Food Res. 52:26–42. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li S, Liao X, Meng F, Wang Y, Sun Z, Guo
F, Li X, Meng M, Li Y and Sun C: Therapeutic role of ursolic acid
on ameliorating hepatic steatosis and improving metabolic disorders
in high-fat diet-induced non-alcoholic fatty liver disease rats.
PLoS One. 9(e86724)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chu X, He X, Shi ZP, Li CJ, Guo FC, Li ST,
Li Y, Na LX and Sun CH: Ursolic acid increases energy expenditure
through enhancing free fatty acid uptake and β-oxidation via an
UCP3/AMPK-dependent pathway in skeletal muscle. Mol Nutr Food Res.
59:1491–1503. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ma P, Yao L, Lin X, Gu T, Rong X, Batey R,
Yamahara J, Wang J and Li Y: A mixture of apple pomace and rosemary
extract improves fructose consumption-induced insulin resistance in
rats: Modulation of sarcolemmal CD36 and glucose transporter-4. Am
J Transl Res. 8:3791–3801. 2016.PubMed/NCBI
|
|
22
|
Jäger S, Trojan H, Kopp T, Laszczyk MN and
Scheffler A: Pentacyclic triterpene distribution in various
plants-rich sources for a new group of multi-potent plant extracts.
Molecules. 14:2016–2031. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jia YY, Kim S, Kim J, Kim B, Wu CY, Lee
JH, Jun HJ, Kim N, Lee D and Lee SJ: Ursolic acid improves lipid
and glucose metabolism in high-fat-fed C57BL/6J mice by activating
peroxisome proliferator-activated receptor alpha and hepatic
autophagy. Mol Nutr Food Res. 59:344–354. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gastaldelli A, Harrison SA,
Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S and Cusi K:
Importance of changes in adipose tissue insulin resistance to
histological response during thiazolidinedione treatment of
patients with nonalcoholic steatohepatitis. Hepatology.
50:1087–1093. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bugianesi E, Gastaldelli A, Vanni E,
Gambino R, Cassader M, Baldi S, Ponti V, Pagano G, Ferrannini E and
Rizzetto M: Insulin resistance in non-diabetic patients with
non-alcoholic fatty liver disease: Sites and mechanisms.
Diabetologia. 48:634–642. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gastaldelli A, Cusi K, Pettiti M, Hardies
J, Miyazaki Y, Berria R, Buzzigoli E, Sironi AM, Cersosimo E,
Ferrannini E and Defronzo RA: Relationship between hepatic/visceral
fat and hepatic insulin resistance in nondiabetic and type 2
diabetic subjects. Gastroenterology. 133:496–506. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lomonaco R, Ortiz-Lopez C, Orsak B, Webb
A, Hardies J, Darland C, Finch J, Gastaldelli A, Harrison S, Tio F
and Cusi K: Effect of adipose tissue insulin resistance on
metabolic parameters and liver histology in obese patients with
nonalcoholic fatty liver disease. Hepatology. 55:1389–1397.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Armstrong MJ, Hazlehurst JM, Hull D, Guo
K, Borrows S, Yu J, Gough SC, Newsome PN and Tomlinson JW:
Abdominal subcutaneous adipose tissue insulin resistance and
lipolysis in patients with non-alcoholic steatohepatitis. Diabetes
Obes Metab. 16:651–660. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bell LN, Wang J, Muralidharan S, Chalasani
S, Fullenkamp AM, Wilson LA, Sanyal AJ, Kowdley KV,
Neuschwander-Tetri BA, Brunt EM, et al: Relationship between
adipose tissue insulin resistance and liver histology in
nonalcoholic steatohepatitis: A pioglitazone versus vitamin E
versus placebo for the treatment of nondiabetic patients with
nonalcoholic steatohepatitis trial follow-up study. Hepatology.
56:1311–1318. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dagdeviren S, Jung DY, Friedline RH, Noh
HL, Kim JH, Patel PR, Tsitsilianos N, Inashima K, Tran DA, Hu X, et
al: IL-10 prevents ageing-associated inflammation and insulin
resistance in skeletal muscle. FASEB J. 31:701–710. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chiyanika C, Chan DFY, Hui SCN, So HK,
Deng M, Yeung DKW, Nelson EAS and Chu WCW: The relationship between
pancreas steatosis and the risk of metabolic syndrome and insulin
resistance in Chinese adolescents with concurrent obesity and
non-alcoholic fatty liver disease. Pediatr Obes.
15(e12653)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yan H, Li T, Wang Y, Li H, Xu J and Lu X:
Insulin-like growth factor binding protein 7 accelerates hepatic
steatosis and insulin resistance in non-alcoholic fatty liver
disease. Clin Exp Pharmacol Physiol. 46:1101–1110. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Antunes LC, Elkfury JL, Jornada MN,
Foletto KC and Bertoluci MC: Validation of HOMA-IR in a model of
insulin-resistance induced by a high-fat diet in Wistar rats. Arch
Endocrinol Metab. 60:138–142. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Matthews DR, Hosker JP, Rudenski AS,
Naylor BA, Treacher DF and Turner RC: Homeostasis model assessment:
Insulin resistance and beta-cell function from fasting plasma
glucose and insulin concentrations in man. Diabetologia.
28:412–419. 1985.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Søndergaard E, Espinosa De Ycaza AE,
Morgan-Bathke M and Jensen MD: How to measure adipose tissue
insulin sensitivity. J Clin Endocrinol Metab. 102:1193–1199.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sierra Rojas JX, García-San Frutos M,
Horrillo D, Lauzurica N, Oliveros E, Carrascosa JM,
Fernández-Agulló T and Ros M: Differential development of
inflammation and insulin resistance in different adipose tissue
depots along ageing in Wistar rats: Effects of caloric restriction.
J Gerontol A Biol Sci Med Sci. 71:310–322. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Altintas MM, Azad A, Nayer B, Contreras G,
Zaias J, Faul C, Reiser J and Nayer A: Mast cells, macrophages, and
crown-like structures distinguish subcutaneous from visceral fat in
mice. J Lipid Res. 52:480–488. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bahrami SA and Bakhtiari N: Ursolic acid
regulates ageing process through enhancing of metabolic sensor
proteins level. Biomed Pharmacother. 82:8–14. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Staats S, Wagner AE, Lüersen K, Künstner
A, Meyer T, Kahns AK, Derer S, Graspeuntner S, Rupp J, Busch H, et
al: Dietary ursolic acid improves health span and life span in male
Drosophila melanogaster. Biofactors. 45:169–186. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bakhtiari N, Hosseinkhani S, Tashakor A
and Hemmati R: Ursolic acid ameliorates ageing-metabolic phenotype
through promoting of skeletal muscle rejuvenation. Med Hypotheses.
85:1–6. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhou X, Li JQ, Wei LJ, He MZ, Jia J, Zhang
JY, Wang SS and Feng L: Silencing of DsbA-L gene impairs the PPARγ
agonist function of improving insulin resistance in a high-glucose
cell model. J Zhejiang Univ Sci B. 21:990–998. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Jiang S, Ren D, Li J, Yuan G, Li H, Xu G,
Han X, Du P and An L: Effects of compound K on hyperglycemia and
insulin resistance in rats with type 2 diabetes mellitus.
Fitoterapia. 95:58–64. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Vareda PM, Saldanha LL, Camaforte NA,
Violato NM, Dokkedal AL and Bosqueiro JR: Myrcia bella leaf extract
presents hypoglycemic activity via PI3k/Akt insulin signaling
pathway. Evid Based Complement Alternat Med.
2014(543606)2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ormazabal P, Scazzocchio B, Varì R,
Santangelo C, D'Archivio M, Silecchia G, Iacovelli A, Giovannini C
and Masella R: Effect of protocatechuic acid on insulin
responsiveness and inflammation in visceral adipose tissue from
obese individuals: Possible role for PTP1B. Int J Obes (Lond).
42:2012–2021. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Aguirre V, Werner ED, Giraud J, Lee YH,
Shoelson SE and White MF: Phosphorylation of Ser307 in insulin
receptor substrate-1 blocks interactions with the insulin receptor
and inhibits insulin action. J Biol Chem. 277:1531–1537.
2002.PubMed/NCBI View Article : Google Scholar
|
|
46
|
García-San Frutos M, Fernández-Agulló T,
De Solís AJ, Andrés A, Arribas C, Carrascosa JM and Ros M: Impaired
central insulin response in aged Wistar rats: Role of adiposity.
Endocrinology. 148:5238–5247. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Aziz AUR, Farid S, Qin K, Wang H and Liu
B: Regulation of insulin resistance and glucose metabolism by
interaction of PIM kinases and insulin receptor substrates. Arch
Physiol Biochem. 126:129–138. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Gandhi GR, Jothi G, Antony PJ, Balakrishna
K, Paulraj MG, Ignacimuthu S, Stalin A and Al-Dhabi NA: Gallic acid
attenuates high-fat diet fed-streptozotocin-induced insulin
resistance via partial agonism of PPARγ in experimental type 2
diabetic rats and enhances glucose uptake through translocation and
activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur J
Pharmacol. 745:201–216. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Alonso A, González-Pardo H, Garrido P,
Conejo NM, Llaneza P, Díaz F, Del Rey CG and González C: Acute
effects of 17 β-estradiol and genistein on insulin sensitivity and
spatial memory in aged ovariectomized female rats. Age (Dordr).
32:421–434. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Alonso A, Fernández R, Moreno M, Ordóñez
P, González-Pardo H, Conejo NM, Díaz F and González C: Positive
effects of 17beta-estradiol on insulin sensitivity in aged
ovariectomized female rats. J Gerontol A Biol Sci Med Sci.
61:419–426. 2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Shaohui H and Michael PC: The GLUT4
glucose transporter. Cell Metab. 5:237–252. 2007.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Morán J, Garrido P, Alonso A, Cabello E
and González C: 17β-estradiol and genistein acute treatments
improve some cerebral cortex homeostasis aspects deteriorated by
ageing in female rats. Exp Gerontol. 48:414–421. 2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lu Y, Ma X, Kong Q, Xu Y, Hu J, Wang F,
Qin W, Wang L and Xiong W: Novel dual-color drug screening model
for GLUT4 translocation in adipocytes. Mol Cell Probes. 43:6–12.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Day MJ: Ageing, immunosenescence and
inflammageing in the dog and cat. J Comp Pathol. 142 (Suppl
1):S60–S69. 2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Franceschi C, Bonafè M, Valensin S,
Olivieri F, De Luca M, Ottaviani E and De Benedictis G:
Inflamm-ageing. An evolutionary perspective on immunosenescence.
Ann N Y Acad Sci. 908:244–254. 2000.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ahmad A, Banerjee S, Wang Z, Kong D,
Majumdar AP and Sarkar FH: Ageing and inflammation: Etiological
culprits of cancer. Curr Ageing Sci. 2:174–186. 2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Fulop T, Larbi A, Kotb R, de Angelis F and
Pawelec G: Ageing, immunity, and cancer. Discov Med. 11:537–550.
2011.PubMed/NCBI
|
|
58
|
Khatami M: Inflammation, ageing, and
cancer: Tumoricidal versus tumorigenesis of immunity: A common
denominator mapping chronic diseases. Cell Biochem Biophys.
55:55–79. 2009.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Morrisette-Thomas V, Cohen AA, Fülöp T,
Riesco É, Legault V, Li Q, Milot E, Dusseault-Bélanger F and
Ferrucci L: Inflamm-aging does not simply reflect increases in
pro-inflammatory markers. Mech Ageing Dev. 139:49–57.
2014.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hirosumi J, Tuncman G, Chang L, Görgün CZ,
Uysal KT, Maeda K, Karin M and Hotamisligil GS: A central role for
JNK in obesity and insulin resistance. Nature. 420:333–336.
2002.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Maekawa T, Jin W and Ishii S: The role of
ATF-2 family transcription factors in adipocyte differentiation:
Antiobesity effects of p38 inhibitors. Mol Cell Biol. 30:613–625.
2010.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Yuan M, Konstantopoulos N, Lee J, Hansen
L, Li ZW, Karin M and Shoelson SE: Reversal of obesity- and
diet-induced insulin resistance with salicylates or targeted
disruption of Ikkbeta. Science. 293:1673–1677. 2001.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Arkan MC, Hevener AL, Greten FR, Maeda S,
Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J and Karin M:
IKK-beta links inflammation to obesity-induced insulin resistance.
Nat Med. 11:191–198. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
64
|
Li J, Li N, Yan S, Liu M, Sun B, Lu Y and
Shao Y: Ursolic acid alleviates inflammation and against
diabetes-induced nephropathy through TLR4-mediated inflammatory
pathway. Mol Med Rep. 18:4675–4681. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Liu S, Li X, Wu Y, Duan R, Zhang J, Du F,
Zhang Q, Li Y and Li N: Effects of vaspin on pancreatic β cell
secretion via PI3K/Akt and NF-κB signaling pathways. PLoS One.
12(e0189722)2017.PubMed/NCBI View Article : Google Scholar
|



















