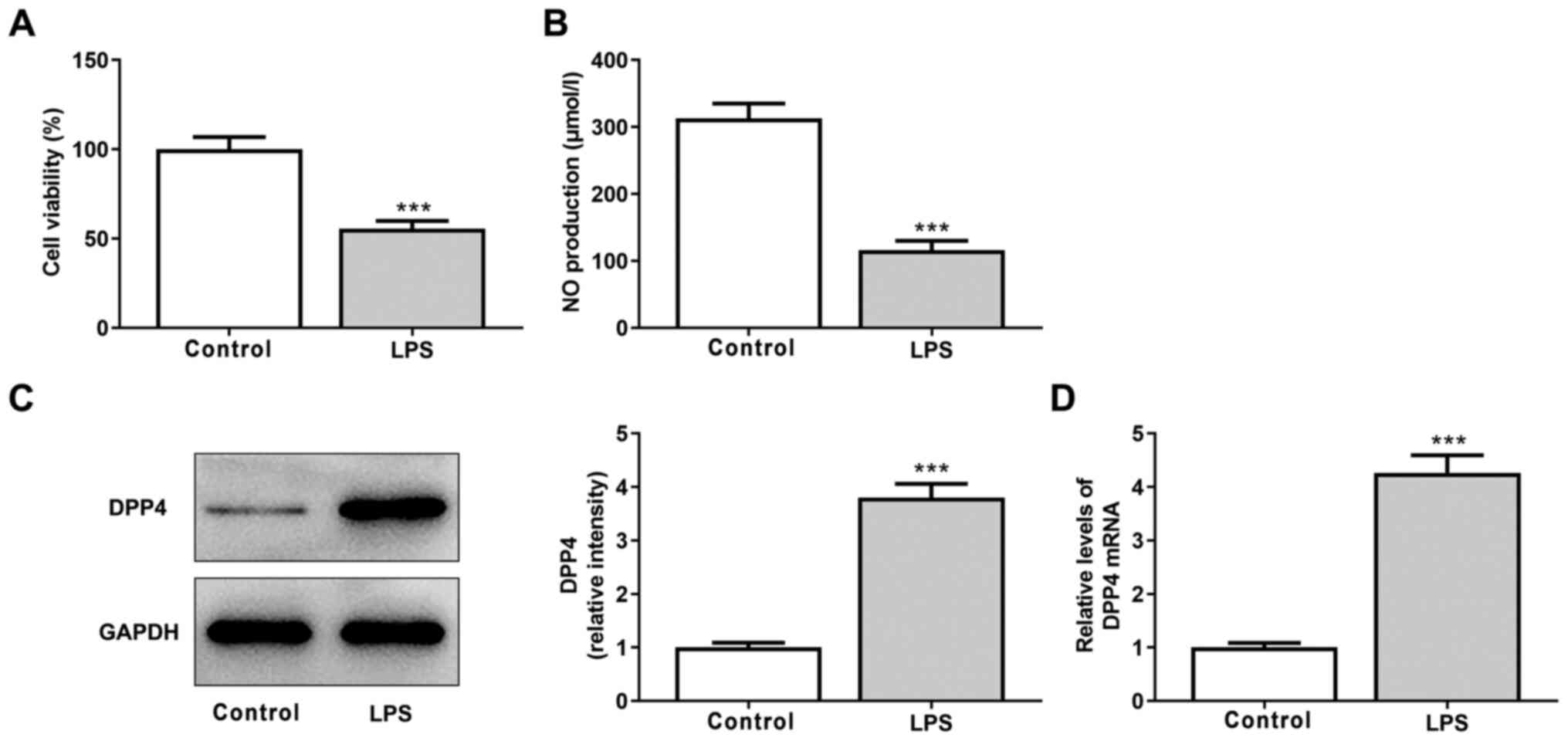
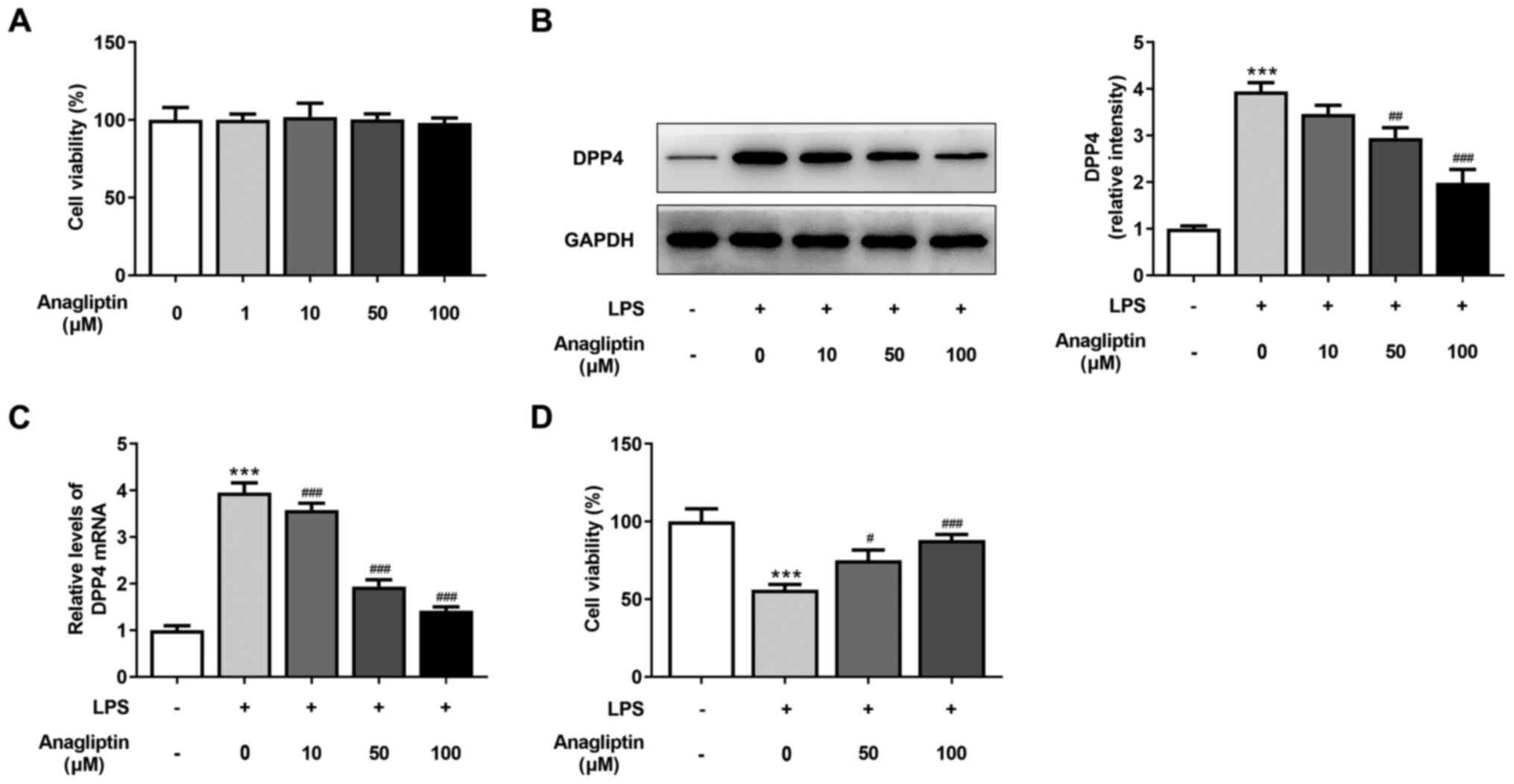
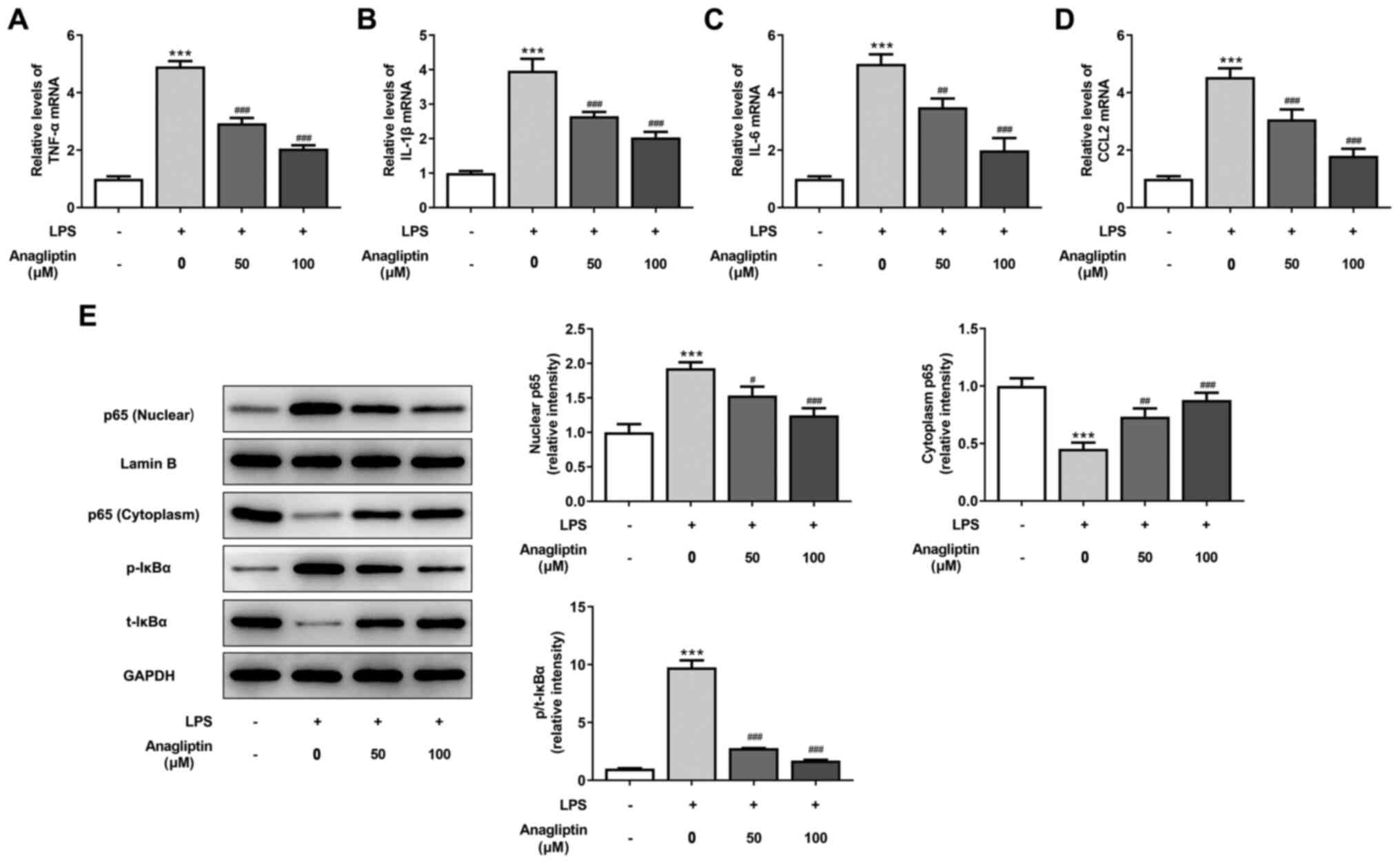
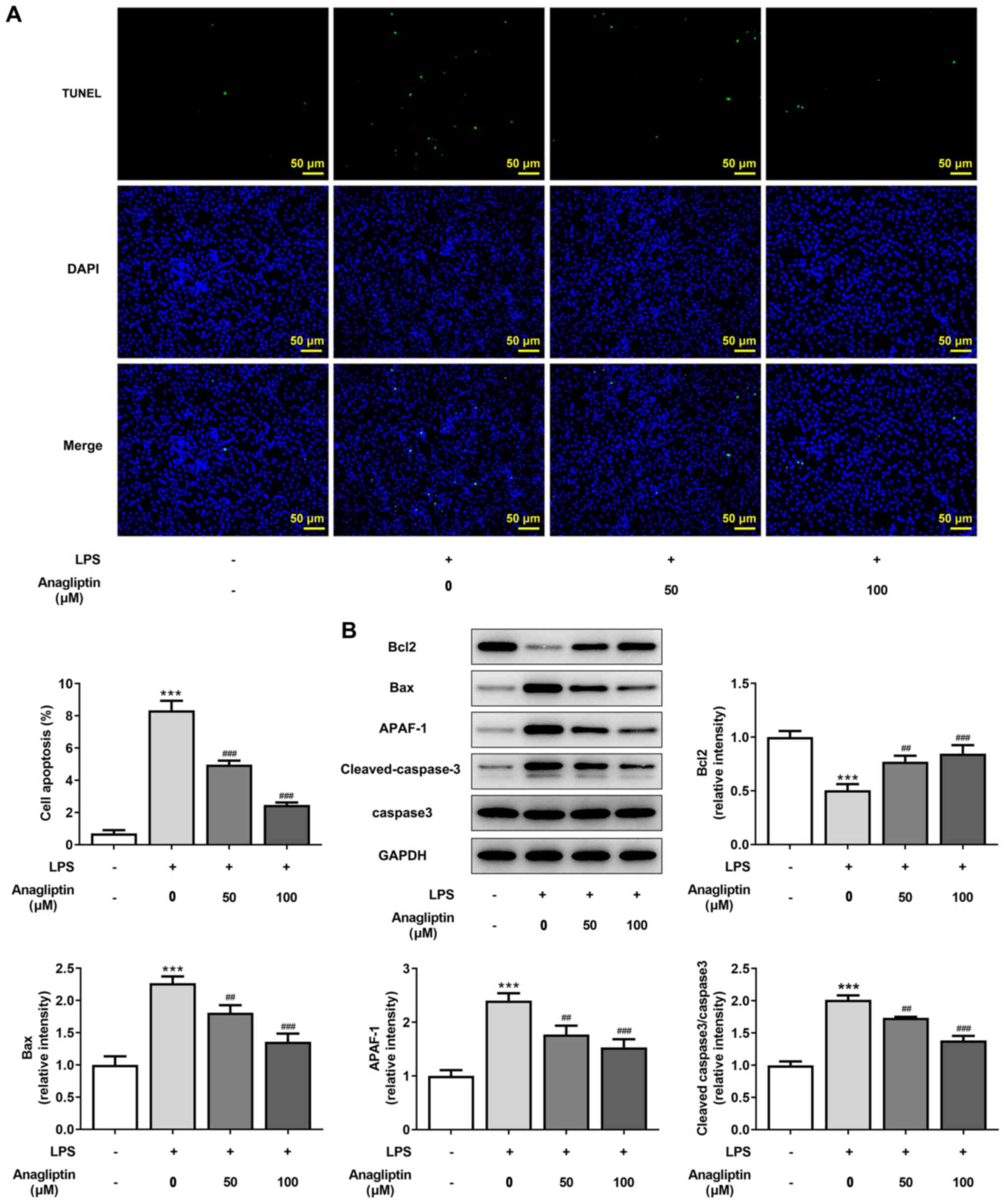
|
1
|
Dushianthan A, Grocott MP, Postle AD and
Cusack R: Acute respiratory distress syndrome and acute lung
injury. Postgrad Med J. 87:612–622. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ali H, Khan A, Ali J, Ullah H, Khan A, Ali
H, Irshad N and Khan S: Attenuation of LPS-induced acute lung
injury by continentalic acid in rodents through inhibition of
inflammatory mediators correlates with increased Nrf2 protein
expression. BMC Pharmacol Toxicol. 21(81)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Butt Y, Kurdowska A and Allen TC: Acute
Lung Injury: A Clinical and Molecular Review. Arch Pathol Lab Med.
140:345–350. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hughes KT and Beasley MB: Pulmonary
Manifestations of Acute Lung Injury: More Than Just Diffuse
Alveolar Damage. Arch Pathol Lab Med. 141:916–922. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hu Q, Wang Q, Han C and Yang Y: Sufentanil
attenuates inflammation and oxidative stress in sepsis-induced
acute lung injury by downregulating KNG1 expression. Mol Med Rep.
22:4298–4306. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schmidt GA: Managing Acute Lung Injury.
Clin Chest Med. 37:647–658. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Piao L, Li Y, Narisawa M, Shen X and Cheng
XW: Role of Dipeptidyl Peptidase-4 in Atherosclerotic
Cardiovascular Disease in Humans and Animals with Chronic Stress.
Int Heart J. 62:470–478. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yip HK, Lee MS, Li YC, Shao PL, Chiang JY,
Sung PH, Yang CH and Chen KH: Dipeptidyl Peptidase-4 deficiency
effectively protects the brain and neurological function in rodent
after acute Hemorrhagic Stroke. Int J Biol Sci. 16:3116–3132.
2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee M, Shin E, Bae J, Cho Y, Lee JY, Lee
YH, Lee BW, Kang ES and Cha BS: Dipeptidyl peptidase-4 inhibitor
protects against non-alcoholic steatohepatitis in mice by targeting
TRAIL receptor-mediated lipoapoptosis via modulating hepatic
dipeptidyl peptidase-4 expression. Sci Rep.
10(19429)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Klemann C, Wagner L, Stephan M and von
Hörsten S: Cut to the chase: A review of CD26/dipeptidyl
peptidase-4's (DPP4) entanglement in the immune system. Clin Exp
Immunol. 185:1–21. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao Y: CD26 in autoimmune diseases: The
other side of ‘moonlight protein’. Int Immunopharmacol.
75(105757)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang X, Ke J, Zhu YJ, Cao B, Yin RL, Wang
Y, Wei LL, Zhang LJ, Yang LY and Zhao D: Dipeptidyl peptidase-4
(DPP4) inhibitor sitagliptin alleviates liver inflammation of
diabetic mice by acting as a ROS scavenger and inhibiting the NFκB
pathway. Cell Death Discov. 7(236)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kawasaki T, Chen W, Htwe YM, Tatsumi K and
Dudek SM: DPP4 inhibition by sitagliptin attenuates LPS-induced
lung injury in mice. Am J Physiol Lung Cell Mol Physiol.
315:L834–L845. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Solerte SB, Di Sabatino A, Galli M and
Fiorina P: Dipeptidyl peptidase-4 (DPP4) inhibition in COVID-19.
Acta Diabetol. 57:779–783. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Strollo R and Pozzilli P: DPP4 inhibition:
Preventing SARS-CoV-2 infection and/or progression of COVID-19?
Diabetes Metab Res Rev. 36(e3330)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zou H, Zhu N and Li S: The emerging role
of dipeptidyl-peptidase-4 as a therapeutic target in lung disease.
Expert Opin Ther Targets. 24:147–153. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Seys LJM, Widagdo W, Verhamme FM, Kleinjan
A, Janssens W, Joos GF, Bracke KR, Haagmans BL and Brusselle GG:
DPP4, the Middle East Respiratory Syndrome Coronavirus Receptor, is
Upregulated in Lungs of Smokers and Chronic Obstructive Pulmonary
Disease Patients. Clin Infect Dis. 66:45–53. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Guo K and Jin F: Dipeptidyl peptidase-4
(DPP-4) inhibitor saxagliptin alleviates lipopolysaccharide-induced
acute lung injury via regulating the Nrf-2/HO-1 and NF-κB pathways.
J Invest Surg. 34:695–702. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Suzuki T, Tada Y, Gladson S, Nishimura R,
Shimomura I, Karasawa S, Tatsumi K and West J: Vildagliptin
ameliorates pulmonary fibrosis in lipopolysaccharide-induced lung
injury by inhibiting endothelial-to-mesenchymal transition. Respir
Res. 18(177)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Roussel R, Duran-García S, Zhang Y, Shah
S, Darmiento C, Shankar RR, Golm GT, Lam RLH, O'Neill EA, Gantz I,
et al: Double-blind, randomized clinical trial comparing the
efficacy and safety of continuing or discontinuing the dipeptidyl
peptidase-4 inhibitor sitagliptin when initiating insulin glargine
therapy in patients with type 2 diabetes: The CompoSIT-I Study.
Diabetes Obes Metab. 21:781–790. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao X, Sun J, Chen Y, Su W, Shan H, Li Y,
Wang Y, Zheng N, Shan H and Liang H: lncRNA PFAR Promotes Lung
Fibroblast Activation and Fibrosis by Targeting miR-138 to Regulate
the YAP1-Twist Axis. Mol Ther. 26:2206–2217. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jiang T, Jiang D, Zhang L, Ding M and Zhou
H: Anagliptin ameliorates high glucose- induced endothelial
dysfunction via suppression of NLRP3 inflammasome activation
mediated by SIRT1. Mol Immunol. 107:54–60. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li Q, Zhang M, Xuan L, Liu Y and Chen C:
Anagliptin inhibits neointimal hyperplasia after balloon injury via
endothelial cell-specific modulation of SOD-1/RhoA/JNK signaling in
the arterial wall. Free Radic Biol Med. 121:105–116.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pantanetti P, Cangelosi G and Ambrosio G:
Potential role of incretins in diabetes and COVID-19 infection: A
hypothesis worth exploring. Intern Emerg Med. 15:779–782.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang S, Li P, Xin M, Jin X, Zhao L, Nan Y
and Cheng XW: Dipeptidyl peptidase-4 inhibition prevents lung
injury in mice under chronic stress via the modulation of oxidative
stress and inflammation. Exp Anim. 21-0067:2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer.
12(86)2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Marcelo KL, Goldie LC and Hirschi KK:
Regulation of endothelial cell differentiation and specification.
Circ Res. 112:1272–1287. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pan X, Xu S, Zhou Z, Wang F, Mao L, Li H,
Wu C, Wang J, Huang Y, Li D, et al: Fibroblast growth factor-2
alleviates the capillary leakage and inflammation in sepsis. Mol
Med. 26(108)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang T, Yegambaram M, Gross C, Sun X, Lu
Q, Wang H, Wu X, Kangath A, Tang H, Aggarwal S, et al: RAC1
nitration at Y32 IS involved in the endothelial barrier
disruption associated with lipopolysaccharide-mediated acute lung
injury. Redox Biol. 38(101794)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu X, Wang D, Zhang X, Lv M, Liu G, Gu C,
Yang F and Wang Y: Effect and mechanism of phospholipid scramblase
4 (PLSCR4) on lipopolysaccharide (LPS)-induced injury to human
pulmonary microvascular endothelial cells. Ann Transl Med.
9(159)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lin J, Lin Z and Lin L: miR-490 alleviates
sepsis-induced acute lung injury by targeting MRP4 in new-born
mice. Acta Biochim Pol. 68:151–158. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang Y, Chen H, Li H, Zhang J and Gao Y:
Effect of angiopoietin-like protein 4 on rat pulmonary
microvascular endothelial cells exposed to LPS. Int J Mol Med.
32:568–576. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shakeri R, Kheirollahi A and Davoodi J:
Apaf-1: Regulation and function in cell death. Biochimie.
135:111–125. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cyr AR, Huckaby LV, Shiva SS and
Zuckerbraun BS: Nitric Oxide and Endothelial Dysfunction. Crit Care
Clin. 36:307–321. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zheng X, Zhang W and Wang Z: Simvastatin
preparations promote PDGF-BB secretion to repair LPS-induced
endothelial injury through the PDGFRβ/PI3K/Akt/IQGAP1 signalling
pathway. J Cell Mol Med. 23:8314–8327. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Everaert BR, Van Craenenbroeck EM, Hoymans
VY, Haine SE, Van Nassauw L, Conraads VM, Timmermans JP and Vrints
CJ: Current perspective of pathophysiological and interventional
effects on endothelial progenitor cell biology: Focus on
PI3K/AKT/eNOS pathway. Int J Cardiol. 144:350–366. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kang SM, Jung HS, Kwon MJ, Lee SH and Park
JH: Effects of anagliptin on the stress induced accelerated
senescence of human umbilical vein endothelial cells. Ann Transl
Med. 9(750)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Steven S, Jurk K, Kopp M, Kröller-Schön S,
Mikhed Y, Schwierczek K, Roohani S, Kashani F, Oelze M, Klein T, et
al: Glucagon-like peptide-1 receptor signalling reduces
microvascular thrombosis, nitro-oxidative stress and platelet
activation in endotoxaemic mice. Br J Pharmacol. 174:1620–1632.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ma Y, Wang J, Wang C, Zhang Q, Xu Y, Liu
H, Xiang X and Ma J: DPP-4 inhibitor anagliptin protects against
hypoxia-induced cytotoxicity in cardiac H9C2 cells. Artif Cells
Nanomed Biotechnol. 47:3823–3831. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sato A, Suzuki S, Watanabe S, Shimizu T,
Nakamura Y, Misaka T, Yokokawa T, Shishido T, Saitoh SI, Ishida T,
et al: DPP4 Inhibition Ameliorates Cardiac Function by Blocking the
Cleavage of HMGB1 in Diabetic Mice After Myocardial Infarction. Int
Heart J. 58:778–786. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu Z, Wang J, Xing W, Peng Y, Quan J and
Fan X: LPS binding to HMGB1 promotes angiogenic behavior of
endothelial cells through inhibition of p120 and CD31 via
ERK/P38/Src signaling. Eur J Cell Biol. 96:695–704. 2017.PubMed/NCBI View Article : Google Scholar
|