Introduction
Osteoarthritis (OA) is a type of degenerative
arthropathy that commonly occurs in weight-bearing joints and is
characterized by articular cartilage degeneration (1). Post-traumatic osteoarthritis (PTOA) is
a complication of joint injury, accounting for ~12% of OA, in which
the knee joint is one of the most commonly affected joints
(2). Due to recent societal
technological advancements, traffic accidents and sports injuries
have increased (3). The incidence
rate of PTOA is also increasing (4). At present, the early diagnosis of PTOA
is difficult (5). Due to a lack of
specific interventions, PTOA seriously affects the daily life of
patients (6). Therefore,
investigation into potential biomarkers of PTOA will aid in
improving joint function and the quality of life of patients with
PTOA.
MicroRNAs (miRNAs) are important regulators of
various diseases via interactions with the 3'-untranslated regions
(UTRs) of mRNAs (7). Increasing
evidence has demonstrated the key roles that miRNAs serve in the
progression of OA (8,9). For example, the level of miR-16-5p is
significantly increased in the chondrocytes of patients with OA
compared with those of healthy controls (10). Additionally, miR-26a has been
demonstrated to inhibit the activation of the NF-κB signaling
pathway, thereby alleviating synovial inflammation and cartilage
injury in rats with OA (11). These
findings suggested that miRNAs serve a critical role in cartilage
homeostasis. In addition, previous studies have indicated an
important role of miR-519 in the progression of various types of
cancer, including breast cancer, gastric cancer and nasopharyngeal
carcinoma (12-14).
However, whether miR-519d-3p is dysregulated in patients with PTOA
has not been previously reported.
In the present study, novel data are provided
demonstrating that decreased levels of miR-519d-3p in the synovium
and joint fluid promotes the progression of PTOA by inhibiting
VEGF. The diagnostic values of miR-51d-3p and VEGF were further
evaluated, which may shed light on the early prediction and
intervention of PTOA.
Materials and methods
Patient samples
Between January 2018 and December 2019, 104 patients
with PTOA were enrolled at the Affiliated Jianhu Hospital of
Nantong University, with an average age of 52.30±9.97 (age range,
43-63 years; 55 males and 49 females) years. The inclusion criteria
for patients with PTOA were as follows: i) all patients met the
diagnostic criteria of PTOA according to clinical manifestations
and imaging results and ii) all patients had at least one injured
knee joint (left or right). The Kellgren-Lawrence (KL) scale
(grades 0-4) was used to determine the severity: grade 0, no
radiographic features of OA are present; grade 1, possible
narrowing of the joint space and osteophytic lipping; grade 2,
definite osteophyte formation and possible narrowing of the joint
space; grade 3, moderate osteophyte formation, definite narrowing
of the joint space, some sclerosis and possible bone contour
deformity; and grade 4, large osteophytes, marked narrowing of the
joint space, severe sclerosis and definite bone contour deformity
(15). Only knee OA patients with
KL grades of ≥2 were considered to be significant and were
subsequently enrolled. If a patient had bilateral knee OA, the more
affected knee was defined as the study knee. The X-ray was read by
two orthopedic experts in our hospital in a blinded manner. KL
scale classification was used to evaluate the imaging severity. The
exclusion criteria were as follows: non-traumatic arthritis,
rheumatoid arthritis, suppurative arthritis, gout, diabetes or knee
mechanical injury. Synovial fluid (SF) was obtained from 106 knees
of patients with PTOA at the time of knee arthroscopy or total knee
replacement surgery. Human synovium was harvested from the knee
joints of patients during total knee arthroplasty. The synovium and
synovial tissue from knee joints donated voluntarily by 60 patients
undergoing amputations (51.40±10.69 years; age range, 40-63 years;
33 males and 27 females) were obtained during the same period.
There was no previous history of joint pain, joint activity
restriction or joint swelling, no disease affecting the synthesis
or metabolism of bone or joints. Synovitis and articular cartilage
degeneration were excluded by the knee joint examination before,
during and after surgery. There were no significant differences
between the two groups regarding age, sex or other general
information. The present study was approved by the Research Ethics
Committee of the Affiliated Jianhu Hospital of Nantong University,
and all the participants provided written informed consent to
participate in this study.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
SF was obtained from 106 knees of patients with PTOA
at the time of knee arthroscopy or total knee replacement surgery.
Human synovium was harvested during total knee arthroplasty from
the knee joints of patients.
Next, 4 ml joint fluid was centrifuged at 2,000 x g
for 12 min at 4˚C. The supernatant was collected and stored at
-80˚C for examination. Total RNA was isolated from the joint fluid
samples (4 ml) or the synovium using RNAVzol LS (Vigorous
Biotechnology Beijing Co., Ltd.) according to the manufacturer's
protocol. The concentrations and purities of the RNA samples were
determined by measuring the optical density (OD) 260/OD280. RT-qPCR
was performed using the TaqMan™ MicroRNA Reverse Transcription kit
(Thermo Fisher Scientific, Inc.) The PCR amplifications were
performed in a 12-µl reaction system containing 6.0 µl custom RT
primer pool, 0.3 µl dNTP, 3.0 µl MultiScribe Reverse Transcriptase,
1.5 µl 10X RT buffer, 0.19 µl RNase Inhibitor, 1.01 µl
Nuclease-free water. The temperature protocol used for RT was as
follows: 16˚C for 30 min, 42˚C for 30 min and 85˚C for 5 min,
followed by storage at 4˚C. qPCR was performed using SYBR-Green
Supermix (Bio-Rad Laboratories, Inc.). The PCR amplifications were
performed in a 10 µl reaction system containing 5 µl SYBR-Green
Supermix, 0.4 µl forward primer, 0.4 µl reverse primer, 2.2 µl
double distilled H2O and 2 µl template cDNA.
Thermocycling conditions were as follows: 95˚C for 10 min followed
by 40 cycles of 95˚C for 15 sec and 60˚C for 1 min. Relative miRNA
expression was normalized to U6 using the 2-∆∆Cq method
(16). The primers used were
designed (17) and listed as
follows: Universal stem-loop miR-545 reverse transcription primer,
5'-GAAAGAAGGCGAGGAGC AGATCGAGGAAGAAGACGGAAGAATGTGCGTCTCGC
CTTCTTTCAGTCATT-3'; U6 reverse transcription primer,
5'-CGCTTCACGAATTTGCGTGTCAA-3'; miR-545 PCR primer forward,
5'-GCTCAGTAAATGTTTATTAG-3' and reverse,
5'-CGAGGAAGAAGACGGAAGAAT-3'; U6 PCR primer forward,
5'-CTCGCTTCGGCAGCACATATACT-3' and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'.
Dual-luciferase reporter assay
The possible target gene of miR-519d-3p was
predicted by TargetScan (http://www.targetscan.org/vert_72/). The 3'-UTR of
VEGF was amplified from the genomic DNA of human primary
chondrocytes using Platinum™ II Hot-Start Green PCR Master Mix (2X;
Invitrogen; Thermo Fisher Scientific, Inc.). The PCR amplifications
were performed in a 20 µl reaction system containing 4 µl 5X
Platinum II PCR Buffer, 0.4 µl forward primer, 0.4 µl reverse
primer, 0.4 µl dNTP, 2.2 µl double-distilled H2O and 2
µl template cDNA, 0.32 µl Platinum II Taq Hot-Start DNA Polymerase.
Thermocycling conditions were as follows: Initial denaturation at
94˚C for 2 min, followed by 30 cycles of denaturation at 94˚C for
15 sec, annealing at 60˚C for 30 sec, extension at 68˚C for 30 sec
and storage at 4˚C. The mutant was cloned using the Fast
Mutagenesis System (TransGen Biotech). The PCR amplifications were
performed in a 50 µl reaction system containing 10 ng genomic DNA
(2 µl), 1 µl forward primer, 1 µl reverse primer, 25 µl 2X
TransStart FastPfu Fly PCR SuperMix, 21 µl Nuclease-free water.
Thermocycling conditions were as follows: Initial denaturation at
94˚C for 2 min, followed by 20 cycles of denaturation at 94˚C for
20 sec, annealing at 55˚C for 20 sec, extension at 72˚C for 30 sec
and 4˚C forever. Next, the amplified PCR fragment of VEGF-3'-UTR or
VEGF-3'-UTR-Mut was cloned into the pmirGLO plasmid (Promega
Corporation). The PCR products were ligated with
EcoRI/XhoI-linearized pmiRGLO plasmid (Promega
Corporation) to construct plasmids (pmirGLO-VEGF-3'-UTR,
pmirGLO-VEGF-3'-UTR-Mut). All the expression plasmids were
transformed into E. coli BL21(DE3) competent cells. The
plasmid was isolated using GenElute™ Plasmid small quantity
preparation kit (Thermo Fisher Scientific, Inc.). 293 cells were
co-transfected with miR-519d-3p mimic and pmirGLO-VEGF-3'-UTR,
pmirGLO-VEGF-3'-UTR-Mut using Vigofect transfection reagent
(Vigorous Biotechnology Beijing Co., Ltd.), according to the
manufacturer's protocol. 293 cells were seeded into 6-well plates
at a density of 106 cells/well. In brief, 10 µl Vigofect
transfection reagent was mixed with 100 µl serum-free culture.
Meanwhile, 10 µl miR-519d-3p mimic and pmirGLO-VEGF-3'-UTR,
pmirGLO-VEGF-3'-UTR-Mut was mixed with the aforementioned mixture.
Next, the two mixtures were mixed and incubated at room temperature
for 10 min. Subsequently, the mixture was added to the 6-well plate
at a final concentration of 20 nM. Following transfection for 48 h,
the cells were collected for subsequent experiments. A
dual-luciferase reporter assay was performed using a
Dual-Luciferase Reporter Kit (Promega Corporation), according to
the manufacturer's protocol (18).
The relative luciferase activity was normalized to Renilla
luciferase.
Enzyme-linked immunosorbent assay
(ELISA)
The levels of VEGF in the SF samples of all subjects
were analyzed using a Human VEGF ELISA Kit (cat. no. ab222510;
Abcam).
Cell culture and cell
transfection
Human primary chondrocytes (HC-a; cat. no. 4650)
were purchased from ScienCell Research Laboratories. The cells were
cultured in a chondrocyte medium (ScienCell Research Laboratories;
cat. no. 4651), supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.), streptomycin (100
mg/ml) and penicillin (100 U/ml) at 37˚C in a humidified atmosphere
containing 5% CO2. Only cells within the fifth passage
were used for the subsequent experiments.
The siRNA, miR-519d-3p mimic, miR-519d-3p inhibitor
and respective negative control (NC) was purchased from Shanghai
GenePharma Co., Ltd. The sequences for siRNA, miR mimics and miR
inhibitors were as follows: si-VEGF, 5'-GGCCGC CCAGGCUCCUG-3';
si-VEGF NC, 5'-CCGAUAGGUUUAC UGCCAAUU-3'; miR-519d-3p mimic,
5'-CCUCCAAAGGG AAGCGCUUUCUGUU-3'; miR-519d-3p mimic NC, 5'-UUC
UCCGAACGUGUCACGU-3'; miR-519d-3p inhibitor, 5'-AAC
AGAAAGCGCUUCCCUUUGGAGG-3'; miR-519d-3p inhibitor NC,
5'-CGAUAGGUUUACUGCCAAU-3'.
In brief, human primary chondrocytes cells were
seeded into 6-well plates at a density of 5x106
cells/well. Subsequently, the cells were transfected with the 20 nM
siRNA, 20 nM miR-519d-3p mimic, 20 nM miR-519d-3p inhibitor or
respective 20 nM NC for 48 h using Hiperfect Transfection Reagent
(Qiagen) according to the manufacturer's protocols. In brief, 12 µl
Hiperfect Transfection Reagent was mixed with 100 µl serum-free
chondrocyte medium (ScienCell Research Laboratories; cat. no.
4651). Additionally, 10 µl siRNA, miR-519d-3p mimic, miR-519d-3p
inhibitor or respective NC was mixed with serum-free chondrocyte
medium. Next, the two mixtures were mixed and incubated at room
temperature for 15 min. Subsequently, the mixture was added to the
6-well plate at a final concentration of 20 nM. Following
transfection for 48 h, the cells were collected for subsequent
experiments.
Cell apoptosis assay
Flow cytometry was performed using a FITC/Annexin V
Apoptosis Detection Kit I (BD Pharmingen™) according to the
manufacturer's protocols. In brief, following transfection for 48
h, cells were washed with PBS and resuspended in 100 µl annexin
binding buffer (1X), followed by incubation with 5 µl FITC Annexin
V and 5 µl PI working solution at room temperature for 15 min.
Subsequently, 400 µl annexin binding buffer (1X) was added to the
mixture. Finally, the apoptosis rate of cells was analyzed by flow
cytometry using a BD FACSCalibur system (LSRFortessa X-20; BD
Biosciences). Data were analyzed using FCSalyzer v16.0.153 software
(Huajun Software Park).
Cell cycle distribution assay
HC-a cells (~1x106) were washed twice
with PBS and were fixed in 70% ice-cold ethanol for 1 h at room
temperature. The samples were centrifuged at 300 x g for 5 min at
4˚C. The ethanol was removed, and the cells were exposed to 100
mg/ml RNaseA (Sigma-Aldrich; Merck KGaA) for 30 min at 37˚C.
Cellular DNA was stained with propidium iodide (Nanjing KeyGen
Biotech Co., Ltd.) for 15 min at room temperature. Cell cycle
distributions were determined by flow cytometry using a BD
FACSCalibur system (BD Biosciences), and data were analyzed using
the ModFit software version 4.1 (Verity Software House, Inc.).
Statistical analysis
Data were analyzed using SPSS software (version
20.0; IBM Corp.). The relevant data were expressed as the mean ±
standard deviation. A Mann-Whitney U test was used to examine
differences between two groups. One-way analysis of variance,
followed by the Tukey's post hoc test, was used to examine
differences among multiple groups. Receiver operating
characteristic (ROC) curves were created, and the areas under the
curve (AUCs) were measured to further assess the specificity and
sensitivity of miR-519d-3p as a diagnostic biomarker. Pearson's
correlation was used to analyze the levels of miR-519d-3p and VEGF
in the synovium and SF of patients with PTOA. P<0.05 was
considered to indicate a statistically significant difference.
Results
Demographic characteristics of
patients with PTOA and healthy controls
To begin with, the demographic data of patients with
PTOA and the healthy controls (HCs) were analyzed. As shown in
Table I, there were no significant
differences in age, sex or body mass index (BMI) between patients
with PTOA and HCs. Furthermore, the differences among PTOA patients
with K-L grade I, grade II, grade III and grade IV disease were
compared. The results demonstrated that no significant differences
in age, sex or BMI were identified among these groups (Table II).
 | Table IDemographic characteristics of
patients with PTOA and HCs. |
Table I
Demographic characteristics of
patients with PTOA and HCs.
| Variable | PTOA (n=104) | HC (n=60) | P-value |
|---|
| Age, years | 52.30±9.67 | 51.40±10.69 | 0.21 |
| Sex, M/F | 55/49 | 33/27 | 0.37 |
| BMI,
kg/m2 | 25.30±1.50 | 24.68±1.33 | 0.43 |
 | Table IIDemographic characteristics of
patients with PTOA with different KL grades. |
Table II
Demographic characteristics of
patients with PTOA with different KL grades.
| Variable | KL grade I
(n=22) | KL grade II
(n=28) | KL grade III
(n=29) | KL grade IV
(n=25) | P-value |
|---|
| Age, years | 54.73±11.07 | 51.57±10.33 | 52.16±11.08 | 51.60±7.69 | 0.56 |
| Sex, M/F | 11/11 | 13/15 | 14/15 | 12/13 | 0.47 |
| BMI,
kg/m2 | 25.52±2.02 | 25.19±1.94 | 25.27±1.96 | 25.29±1.85 | 0.58 |
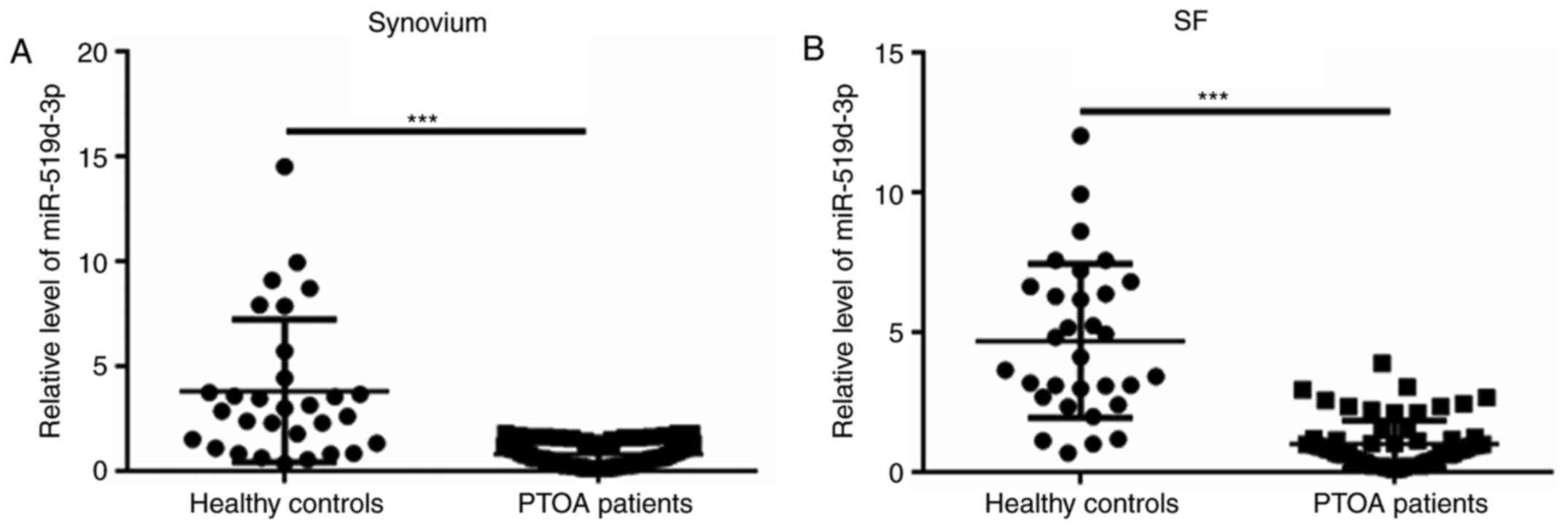
Decreased level of miR-519d-3p in the
synovium and SF of patients with PTOA
Next, the levels of miR-519d-3p were analyzed in
patients with PTOA and in HCs. Compared with the control group, the
level of miR-519d-3p in the synovium and SF in the PTOA group was
significantly decreased (Fig. 1A
and B).
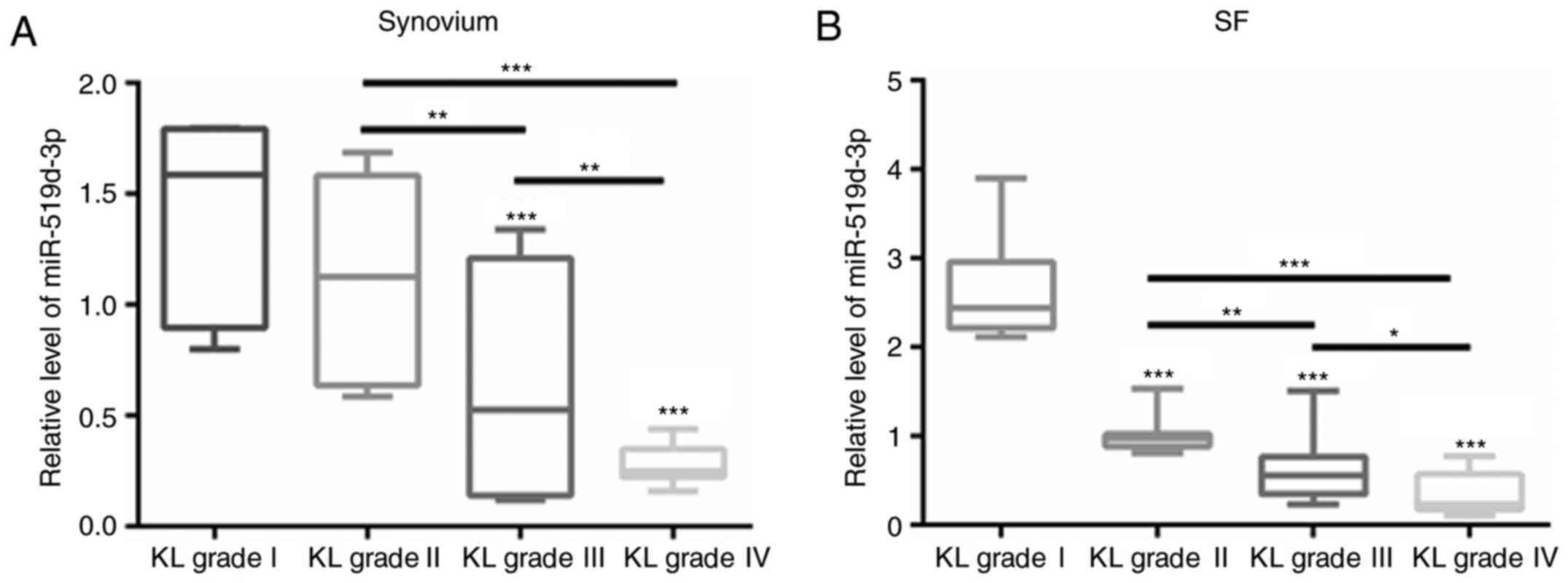
Decrease in miR-519d-3p in the
synovium and SF of PTOA patients with increased K-L grades
The levels of miR-519d-3p were also compared among
PTOA patients with different K-L grades. As demonstrated in
Fig. 2A, the levels of miR-519d-3p
in the synovium of patients in the grade III and IV groups were
significantly lower than those in the grade I and II groups
(Fig. 2A). By contrast, compared
with the grade I group, the levels of miR-519d-3p in the SF of the
grade II, III and IV groups were significantly lower (Fig. 2B).
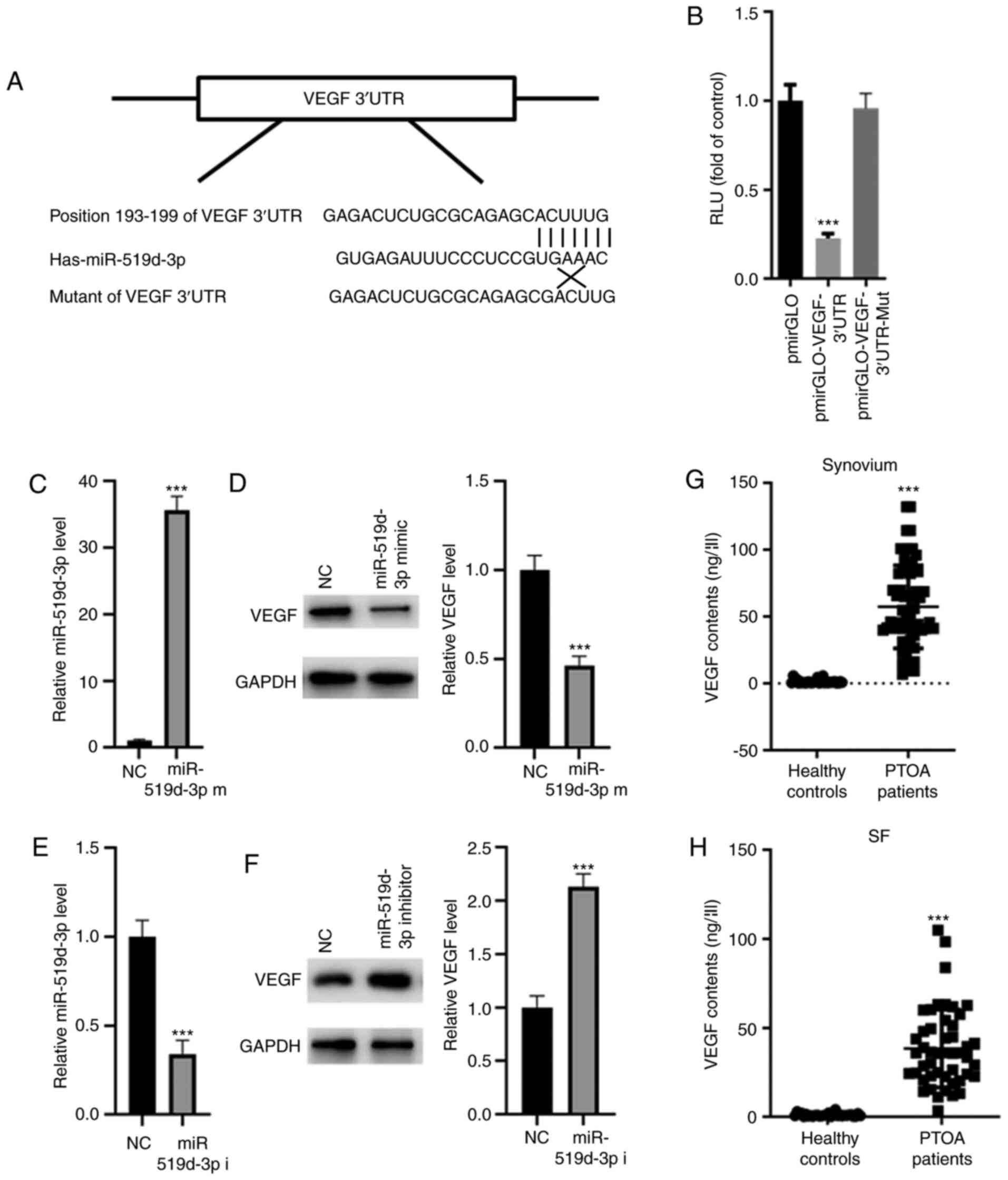
VEGF is a target gene of
miR-519d-3p
The target gene of miR-519d-3p was subsequently
analyzed based on TargetScan (http://www.targetscan.org/vert_72/). A conserved
binding site was identified in the 3'-UTR of enhanced VEGF
(Fig. 3A), which has been suggested
to be associated with OA progression (19). Dual-luciferase assays demonstrated
that miR-519d-3p significantly suppressed the relative luciferase
activity of pmirGLO-VEGR-3'-UTR, but no change in
pmirGLO-VEGR-3'-UTR-Mut was observed (Fig. 3B). Next, miR-519d-3p mimic was
transfected into human primary chondrocytes and RT-qPCR
demonstrated that the level of miR-519d-3p was significantly
increased following transfection with miR-519d-3p mimic, compared
with NC (Fig. 3C), indicating
successful transfection of miR-519d-3p mimic. Western blot analysis
demonstrated that overexpression of miR-519d-3p significantly
suppressed the expression of VEGF (Fig.
3D). By contrast, transfection with miR-519d-3p inhibitor
significantly suppressed the level of miR-519d-3p compared with
transfection with the NC (Fig. 3E).
Meanwhile, transfection with an miR-519d-3p inhibitor significantly
enhanced the expression of VEGF (Fig.
3F). Furthermore, the levels of VEGF were revealed to be
increased in the synovium and SF of the PTOA group, compared with
that of the HC group (Fig. 3G and
H).
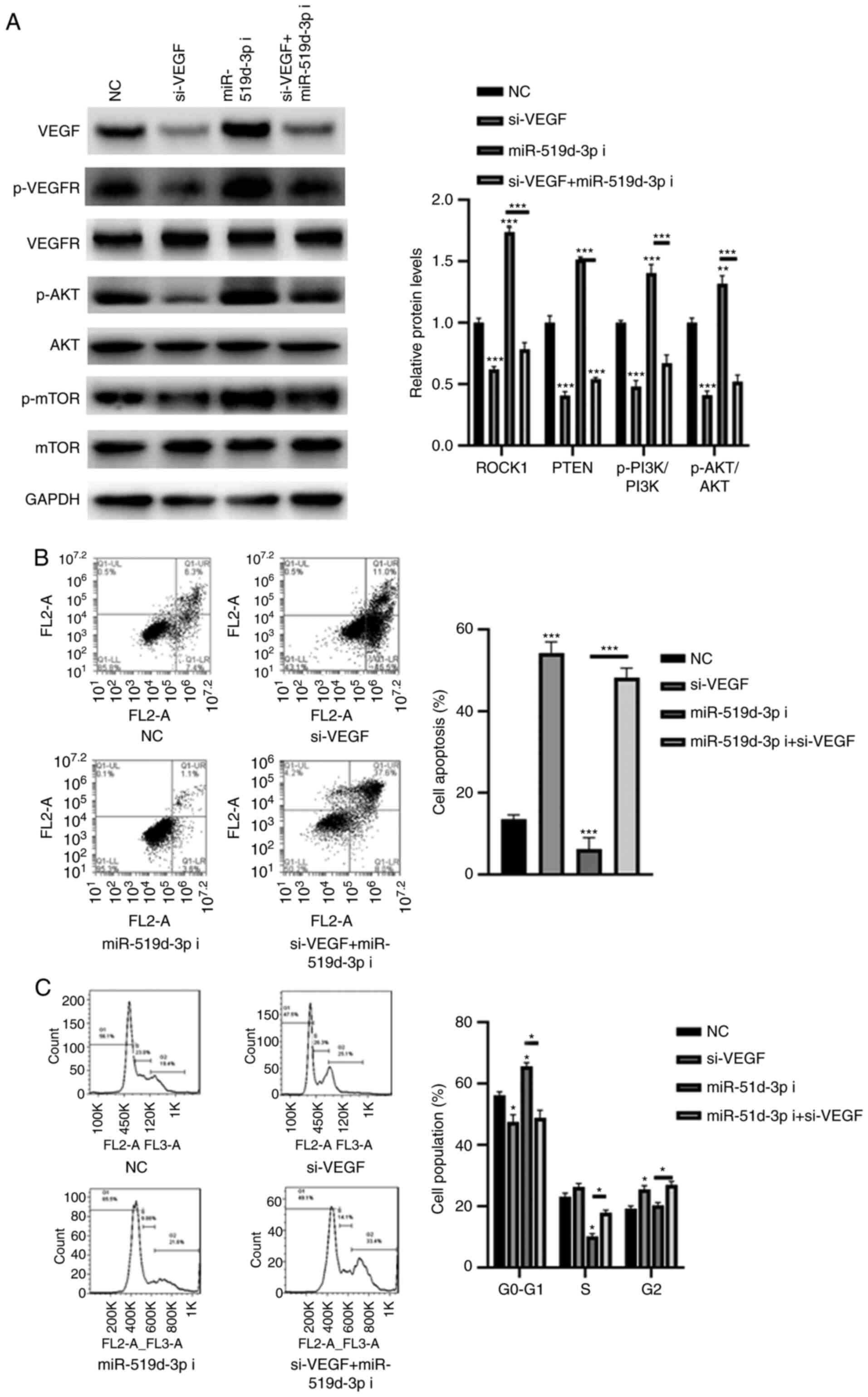
miR-519d-3p inhibitor induces cell
apoptosis, and cell cycle arrest is mediated via VEGF
Articular cartilage is a tissue that is nourished by
SF (20). Increasing evidence has
demonstrated an interaction between the SF microenvironment and
chondrocytes and its role in cartilage degradation in OA (21,22).
It is suggested that inflammatory synovial fluids of patients with
reactive arthritis result in apoptosis and cell death of
chondrocytes (21). Therefore, the
effect of miR-519d-3p on the apoptosis and cell cycle arrest of
chondrocytes was investigated. To further investigate whether
miR-519d-3p is involved in the development of OA via VEGF, a
specific siRNA targeting VEGF was selected. As demonstrated in
Fig. 4A, transfection with si-VEGF
significantly decreased the expression of VEGF compared with the
NC. Meanwhile, the silencing of VEGF significantly decreased the
phosphorylation levels of VEGFR, AKT and mTOR, even in HC-a cells
transfected with the miR-519d-3p inhibitor. Furthermore, the
silencing of VEGF decreased HC-a cell apoptosis and induced cell
division (Fig. 4B and C). By contrast, inhibition of miR-519d-3p
resulted in more cell apoptosis and in the initiation of cell cycle
arrest in G0 phase, compared with transfection with the NC
(Fig. 4B and C). However, such effects could be reversed
in HC-a cells transfected with si-VEGF (Fig. 4B and C).
Negative correlation between the level
of miR-519d-3p and VEGF in the synovium and SF of patients with
PTOA
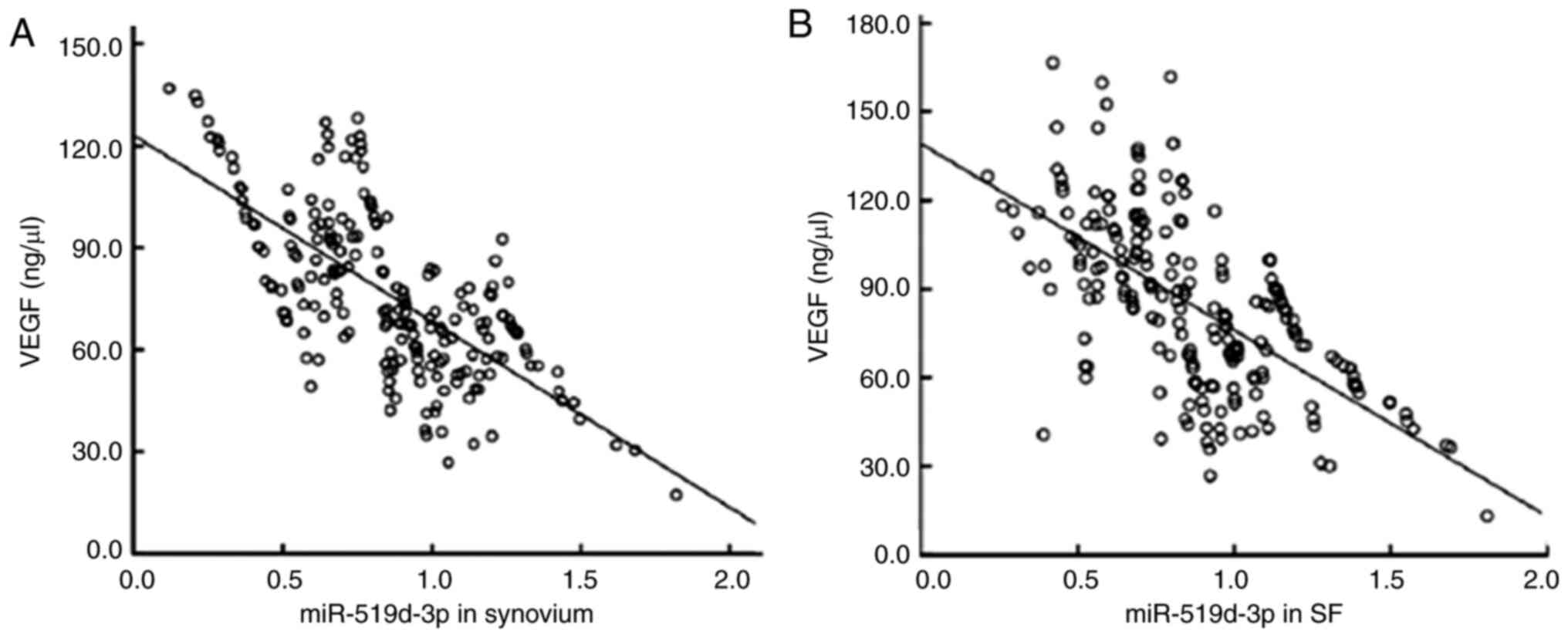
Pearson's correlation analysis demonstrated that the
levels of miR-519d-3p in the synovium and joint fluid of patients
with PTOA were negatively correlated with the contents of VEGF (r
=-0.701, -0.686, P<0.05; Fig. 5A
and B).
Diagnostic value of miR-519d-3p and
VEGF in the synovium and SF of patients with PTOA
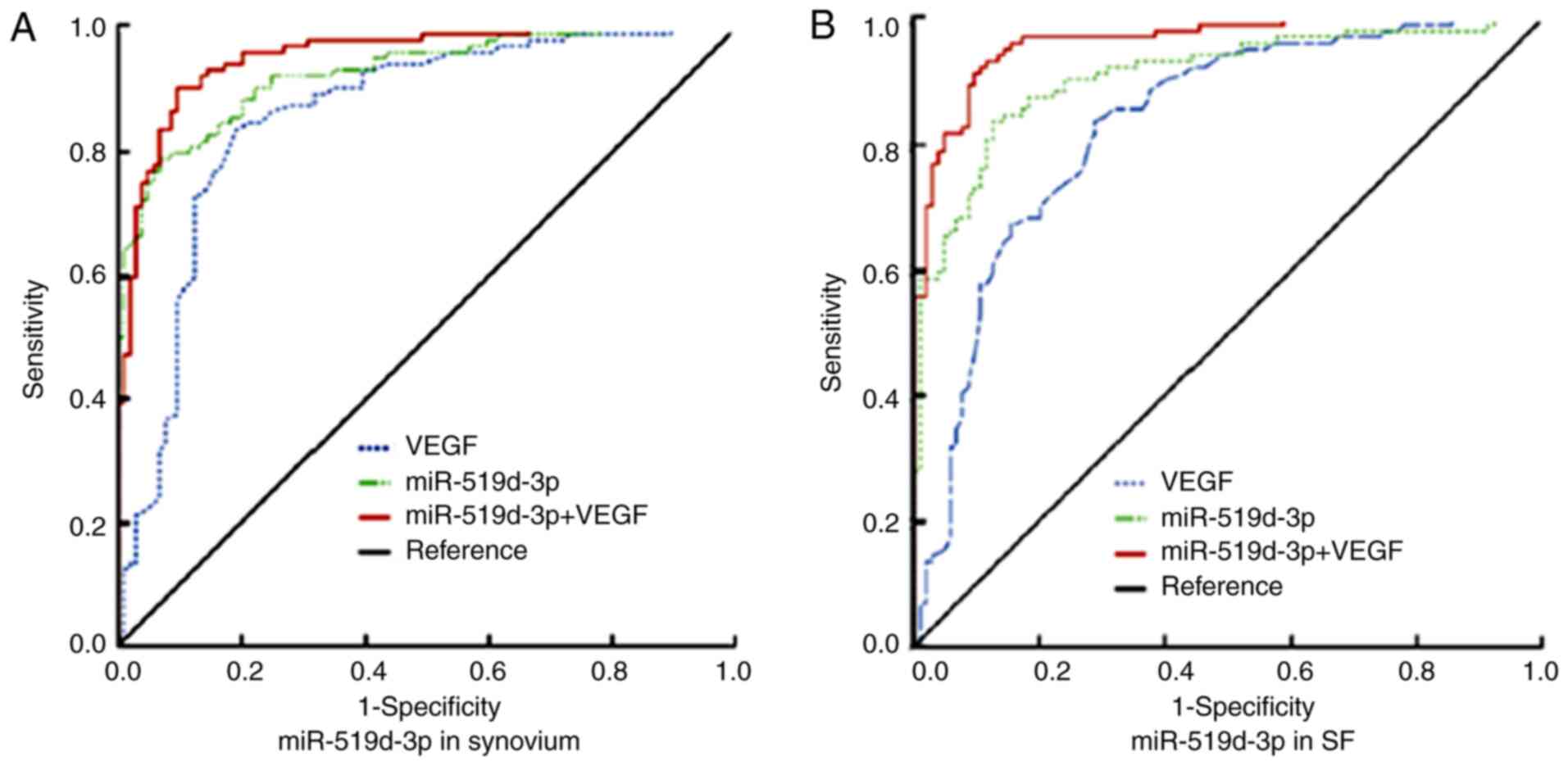
The ROC curve analysis demonstrated that the AUC of
VEGF in the synovium was 0.857 (95% CI: 0.804-0.911). When the
cut-off value was 0.855, the sensitivity and specificity were 83.7
and 71.8%, respectively (Fig. 5A).
The AUC of miR-519d-3p in the diagnosis of PTOA was 0.928 (95% CI:
0.894-0.962). When the cut-off value was 1.306, the sensitivity and
specificity were 78.8 and 93.3%, respectively. The AUC of the
combined use of VEGF and miR-519d-3p was 0.954 (95% CI:
0.928-0.981), with a sensitivity and specificity of 90.4 and 90.4%,
respectively (Fig. 6A).
By contrast, the AUC of miR-519d-3p in SF was 0.896
(95% CI: 0.869-0.950). When the cut-off value was 1.492, the
sensitivity and specificity were 83.7 and 87.5%, respectively
(Fig. 6B). The AUC of VEGF was
0.830 (95% CI: 0.774-0.886). When the cutoff value was 0.785, the
sensitivity and specificity were 67.3 and 84.6%, respectively.
Furthermore, the combined AUC of the two was 0.963 (95% CI: 0.941,
0.986), with a sensitivity and specificity of 93.3 and 88.5%,
respectively (Fig. 6B). These
observations suggested that miR-519d-3p in the synovium and SF may
be a useful biomarker in the diagnosis of patients with PTOA.
Discussion
PTOA is mainly caused by trauma and is characterized
by joint pain and biomechanical dysfunction (23). With the development of PTOA,
disability develops and may seriously affect the quality of life of
patients (1). At present, it
remains difficult to diagnose and evaluate the disease in the early
stages (24).
MiRNAs are suggested to be associated with the
processes involved in OA pathogenesis, including cartilage
homeostasis, extracellular matrix regulation, endochondral
ossification, bone metabolism and angiogenesis (25,26).
In the present study, the levels of miR-519d-3p in the synovium and
SF of patients with PTOA were significantly lower than those of the
control group. Furthermore, the levels of miR-519d-3p in the
synovium and SF of patients with PTOA decreased with an increasing
KL grade. The results indicated that the downregulation of
miR-519d-3p may contribute toward the development and progression
of PTOA. Therefore, the upregulation of endogenous miR-519d-3p
function could be an alternative therapeutic approach for PTOA
prevention and treatment.
Abnormal expression of miR-519 has been widely
reported in various tumor types (14,27).
For example, in several human carcinoma cell lines, including HeLa
(cervical), HCT116 (colon), RKO (colon) and A2780 (ovarian),
miR-519 has been shown to suppress cell proliferation by targeting
HuR (27). In nasopharyngeal
carcinoma cells, miR-519 suppresses cell proliferation by
inhibiting URG4(14). The present
study provided novel data suggesting that VEGF is a target gene of
miR-519d-3p. VEGF is a typical cytokine that may serve a key role
in a variety of inflammatory pathways and angiogenesis (19). The overexpression of VEGF may cause
long-term inflammatory effects and cartilage destruction of the
synovium (28). In addition, VEGF
may participate in rheumatoid arthritis, ankylosing spondylitis, OA
and other inflammatory conditions (29). In support of these observations, the
levels of VEGF in the synovium and SF of patients with PTOA were
significantly higher than in the controls, suggesting that VEGF in
the synovium and SF may participate in the occurrence and
development of PTOA. Furthermore, miR-519d-3p was negatively
associated with VEGF expression in the synovium and SF of patients
with PTOA, suggesting the possible involvement of miR-519d-3p and
VEGF in the pathogenesis of PTOA.
Apoptosis is a highly regulated cell death process
that is extensively involved in the development, homeostasis and
aging of various processes (30,31).
As the most common chronic joint disease in the elderly population,
OA is characterized by the progressive destruction of articular
cartilage (32,33). Previous studies have indicated that
chondrocyte apoptosis is correlated with the severity of OA
(34,35). In the present study, the effects of
miR-519d-3p on chondrocyte apoptosis via VEGF were evaluated. The
results demonstrated that silencing VEGF decreases chondrocyte
apoptosis and induces cell division compared with the NC. By
contrast, inhibition of miR-519d-3p induces HC-a cell apoptosis and
cell cycle arrest. However, such effects could be eliminated by the
knockdown of VEGF. These results suggested that the inhibition of
miR-519d-3p induces chondrocyte apoptosis and cell cycle arrest by
targeting VEGF.
To evaluate the diagnostic role of miR-519d-3p in
PTOA, an ROC curve was constructed. The AUCs of miR-519d-3p in the
synovium and SF were 0.954 and 0.963, respectively, with
sensitivities of 90.4 and 93.3%, respectively. This suggests that
miR-519d-3p and VEGF levels in the synovium and SF have diagnostic
value for PTOA. However, there are certain limitations to the
present study. To begin with, the sample size of this study was
small, and the results need to be further confirmed with a larger
sample size. Additionally, the present study did not investigate
other possible target genes of miR-519d-3p that may also affect the
pathogenesis of PTOA. Furthermore, whether the dose of miR-519d-3p
under pathological conditions (reduction of ~0.5-fold) can inhibit
the progression of PTOA has not been confirmed. In future studies,
an animal model of PTOA will be established and administrate normal
doses of miR-519d-3p mimic to elucidate this issue. Additionally,
the present study investigated the decrease in miR-519d-3p in the
apoptosis and cell cycle arrest of human primary chondrocytes. To
further prove that the decrease in miR-519d-3p in synovial membrane
and SF resulted in the upregulation of VEGF of patients with PTOA,
it is necessary to investigate the effect of miR-519d-3p on
synoviocytes and to evaluate its functional role in the SF
microenvironment.
In conclusion, the present study demonstrated that
the decrease in miR-519d-3p levels in the synovium and SF results
in the upregulation of VEGF in patients with PTOA, and the combined
use of miR-519d-3p and VEGF may improve the early diagnosis of
PTOA.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the NANTONG
supporting fund of The Affiliated Jianhu Hospital of Nantong
University (NTJH-20190825).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG performed the experiments, analyzed the data and
wrote the manuscript. SX designed the experiments and analyzed the
data. JG and SX confirmed the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of The Affiliated Jianhu Hospital of Nantong
University (Nantong, China), and all the participants provided
written informed consent for participation in this study.
Patient consent for publication
All patients provided written informed consent for
the publication of data in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Takada S, Nakamura E, Sabanai K, Tsukamoto
M, Otomo H, Kanoh S, Murai T, Fukuda H, Okada Y, Uchida S, et al:
Attenuation of post-traumatic osteoarthritis after anterior
cruciate ligament injury via inhibition of hedgehog signaling. J
Orthop Res. 38:609–619. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bodkin SG, Werner BC, Slater LV and Hart
JM: Post-traumatic osteoarthritis diagnosed within 5 years
following ACL reconstruction. Knee Surg Sports Traumatol Arthrosc.
28:790–796. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Brown SB, Hornyak JA, Jungels RR, Shah YY,
Yarmola EG, Allen KD and Sharma B: Characterization of
post-traumatic osteoarthritis in rats following anterior cruciate
ligament rupture by non-invasive knee injury (NIKI). J Orthop Res.
38:356–367. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cornelis FMF, de Roover A, Storms L, Hens
A, Lories RJ and Monteagudo S: Increased susceptibility to develop
spontaneous and post-traumatic osteoarthritis in Dot1l-deficient
mice. Osteoarthritis Cartilage. 27:513–525. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Havryliuk H and Khimion L: Platelet
autologous plasma in post-traumatic knee osteoarthritis treatment.
J Clin Orthop Trauma. 10:42–45. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rhon DI, Perez KG and Eskridge SL: Risk of
post-traumatic knee osteoarthritis after knee injury in military
service members. Musculoskelet Care. 17:113–119. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Lai Z and Cao Y: Plasma miR-200c-3p,
miR-100-5p, and miR-1826 serve as potential diagnostic biomarkers
for knee osteoarthritis: Randomized controlled trials. Medicine
(Baltimore). 98(e18110)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ma F, Li G, Yu Y, Xu J and Wu X:
miR-33b-3p promotes chondrocyte proliferation and inhibits
chondrocyte apoptosis and cartilage ECM degradation by targeting
DNMT3A in osteoarthritis. Biochem Biophys Res Commun. 519:430–437.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ma Y, Wu Y, Chen J, Huang K, Ji B, Chen Z,
Wang Q, Ma J, Shen S and Zhang J: miR-10a-5p promotes chondrocyte
apoptosis in osteoarthritis by targeting HOXA1. Mol Ther Nucleic
Acids. 14:398–409. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li L, Jia J, Liu X, Yang S, Ye S, Yang W
and Zhang Y: MicroRNA-16-5p controls development of osteoarthritis
by targeting SMAD3 in chondrocytes. Curr Pharm Des. 21:5160–5167.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao Z, Dai XS, Wang ZY, Bao ZQ and Guan
JZ: MicroRNA-26a reduces synovial inflammation and cartilage injury
in osteoarthritis of knee joints through impairing the NF-κB
signaling pathway. Biosci Rep. 39(39)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ren L, Li Y, Zhao Q, Fan L, Tan B, Zang A
and Yang H: miR-519 regulates the proliferation of breast cancer
cells via targeting human antigen R. Oncol Lett. 19:1567–1576.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xu J, You Q, Wei Z, Fu H, Zhang Y, Hu Z
and Cai Q: miR-519 inhibits epithelial-mesenchymal transition and
biologic behavior of gastric cancer cells by down-regulating FOXQ1.
Int J Clin Exp Pathol. 13:425–436. 2020.PubMed/NCBI
|
|
14
|
Yu G, Zhang T, Jing Y, Bao Q, Tang Q and
Zhang Y: miR-519 suppresses nasopharyngeal carcinoma cell
proliferation by targeting oncogene URG4/URGCP. Life Sci.
175:47–51. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kellgren JH and Lawrence JS: Radiological
assessment of rheumatoid arthritis. Ann Rheum Dis. 16:485–493.
1957.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang LH, Wang SL, Tang LL, Liu B, Ye WL,
Wang LL, Wang ZY, Zhou MT and Chen BC: Universal stem-loop primer
method for screening and quantification of microRNA. PLoS One.
9(e115293)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fang W, Guo J, Cao Y, Wang S, Pang C, Li
M, Dou L, Man Y, Huang X, Shen T, et al: MicroRNA-20a-5p
contributes to hepatic glycogen synthesis through targeting p63 to
regulate p53 and PTEN expression. J Cell Mol Med. 20:1467–1480.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hamilton JL, Nagao M, Levine BR, Chen D,
Olsen BR and Im HJ: Targeting VEGF and its receptors for the
treatment of osteoarthritis and associated Pain. J Bone Miner Res.
31:911–924. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Carballo CB, Coelho TR, de Holanda Afonso
RC, Faria JC, Alves T, Monte SM, Ventura Matioszek GM, Moura-Neto V
and Brito JM: Osteoarthritic synovial fluid and TGF-β1 induce
interleukin-18 in articular chondrocytes. Cartilage. 11:385–394.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hoff P, Buttgereit F, Burmester GR,
Jakstadt M, Gaber T, Andreas K, Matziolis G, Perka C and Röhner E:
Osteoarthritis synovial fluid activates pro-inflammatory cytokines
in primary human chondrocytes. Int Orthop. 37:145–151.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pearson MJ, Herndler-Brandstetter D, Tariq
MA, Nicholson TA, Philp AM, Smith HL, Davis ET, Jones SW and Lord
JM: IL-6 secretion in osteoarthritis patients is mediated by
chondrocyte-synovial fibroblast cross-talk and is enhanced by
obesity. Sci Rep. 7(3451)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Singleton Q, Bapat S and Fulzele S:
Post-traumatic osteoarthritis (PTOA) animal model to understand
pathophysiology of osteoarthritis. Ann Transl Med. 7 (Suppl
3)(S81)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zou YC, Li HH, Yang GG, Yin HD, Cai DZ and
Liu G: Attenuated levels of ghrelin in synovial fluid is related to
the disease severity of ankle post-traumatic osteoarthritis.
Biofactors. 45:463–470. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
van Meurs JB: Osteoarthritis year in
review 2016: Genetics, genomics and epigenetics. Osteoarthritis
Cartilage. 25:181–189. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sondag GR, Mbimba TS, Moussa FM, Novak K,
Yu B, Jaber FA, Abdelmagid SM, Geldenhuys WJ and Safadi FF:
Osteoactivin inhibition of osteoclastogenesis is mediated through
CD44-ERK signaling. Exp Mol Med. 48(e257)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Abdelmohsen K, Srikantan S, Kuwano Y and
Gorospe M: miR-519 reduces cell proliferation by lowering
RNA-binding protein HuR levels. Proc Natl Acad Sci USA.
105:20297–20302. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yuan Q, Sun L, Li JJ and An CH: Elevated
VEGF levels contribute to the pathogenesis of osteoarthritis. BMC
Musculoskelet Disord. 15(437)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nagao M, Hamilton JL, Kc R, Berendsen AD,
Duan X, Cheong CW, Li X, Im HJ and Olsen BR: Vascular endothelial
growth factor in cartilage development and osteoarthritis. Sci Rep.
7(13027)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang WT, Huang ZP, Sui S, Liu JH, Yu DM
and Wang WB: microRNA-1236 promotes chondrocyte apoptosis in
osteoarthritis via direct suppression of PIK3R3. Life Sci.
253(117694)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Feng L, Feng C, Wang CX, Xu DY, Chen JJ,
Huang JF, Tan PL and Shen JM: Circulating microRNA let 7e is
decreased in knee osteoarthritis, accompanied by elevated apoptosis
and reduced autophagy. Int J Mol Med. 45:1464–1476. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li GS, Cui L and Wang GD: miR-155-5p
regulates macrophage M1 polarization and apoptosis in the synovial
fluid of patients with knee osteoarthritis. Exp Ther Med.
21(68)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li L, Lv G, Wang B and Kuang L:
XIST/miR-376c-5p/OPN axis modulates the influence of
proinflammatory M1 macrophages on osteoarthritis chondrocyte
apoptosis. J Cell Physiol. 235:281–293. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sun Y, Kang S, Pei S, Sang C and Huang Y:
MiR93-5p inhibits chondrocyte apoptosis in osteoarthritis by
targeting lncRNA CASC2. BMC Musculoskelet Disord.
21(26)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tian J, Cheng C, Kuang SD, Su C, Zhao X,
Xiong YL, Li YS and Gao SG: OPN Deficiency increases the severity
of osteoarthritis associated with aberrant chondrocyte senescence
and apoptosis and upregulates the expression of
osteoarthritis-associated genes. Pain Res Manag.
2020(3428587)2020.PubMed/NCBI View Article : Google Scholar
|