Introduction
Cerebral ischemia is one of the major leading causes
of morbidity and mortality worldwide (1). Axonal regeneration occurs in the
central nervous system (CNS) following cerebral ischemia (2). The re-establishment of appropriate
synaptic networks following axonal sprouting is necessary to
restore cognitive function following ischemia injury (3). Thus, current treatment strategies
target axonal regeneration following CNS injury (4).
Hydroxyapatite (HA) is a natural component that is
responsible for the strength and stability of the human skeletal
system (5). Owing to its notable
biocompatibility and bioactivity, HA has been extensively used as
reconstructive and prosthetic material for the repair and
regeneration of osseous tissue (6).
Ion exchange takes place between HA and body fluid following HA
implantation into the bone. In addition, HA releases calcium and
phosphorus to its surroundings, which along with other molecules,
enters the blood circulation and effects the CNS (5). To the best of our knowledge, there is
limited evidence denoting the effect of HA extract on the CNS.
HA is non-toxic to various cells, including human
monocyte-derived macrophages (7),
human osteosarcoma osteoblasts (8)
and mesenchymal stem cells (8). In
addition, a previous study has demonstrated the ability of HA to
promote the attachment of human osteoblast-like cells (7) to improve the adhesion and
proliferation of both human osteosarcoma osteoblasts (MG63 cells)
and mesenchymal stem cells (8). HA
exerts notable bioactivity, suggesting that it interacts with cells
and tissues, stimulating their repair and regeneration (5). Thus, the present study hypothesized
that HA may improve the growth of injured neurons in conditions of
axonal sprouting following CNS injury.
A transient ischemia/reperfusion rat model,
involving bilateral common carotid artery clamping, can decrease
blood flow in the brain to one-third of its normal value (9). CNS injury also affects the hippocampus
(10), which is associated with
several neuronal properties, including neuronal cell viability or
the electrophysiological behavior of neurons (11). The most-studied cellular model for
hippocampal learning and memory is the detection of long term
potential (LTP), which is a long lasting increase in the synaptic
transmission efficiency induced by high frequency stimulation
(12).
Ischemic brain insults, whether caused by global or
focal cerebral ischemia, may induce neurogenesis (13). Growth associated protein 43 (GAP43)
is a membrane-bound protein that is located in the axonal growth
cones of sprouting CNS axons (14);
it has been extensively used to quantitate sprouting axons during
neuroanatomical remodeling (15).
Previous evidence has demonstrated that the association between
astrocytes and neurons is important for protecting the CNS against
different types of insult, including cerebral ischemia and hypoxia,
or neurological disorders (16,17).
Glial fibrillary acidic protein (GFAP) is the major cytoskeletal
protein of astrocytes in the brain (18). Previous study reported that GFAP
expression is upregulated in different types of brain injury,
including trauma, demyelination and brain ischemia (18). Thus, GFAP was used as a marker for
the histological examination of brain tissue in the present
study.
The present study aimed to determine whether HA
extracts reverse the LTP deficit and improve axon regeneration in a
rat model of ischemia/reperfusion. The results of the present study
provide novel insights into LTP and the histological changes of
brain tissues, based on GFAP and GAP43 expression levels following
transient cerebral ischemia/reperfusion.
Materials and methods
Preparation of HA extracts
Wet precipitation was performed to prepare HA with a
Ca/P ratio of 1.67(7). Aqueous
extracts from HA (a gift from Professor Jie Huang, University
College of London) were prepared by adding 5 g HA in 100 ml
physiological saline at 37˚C for 72 h. All extracts were sterilized
via filtration through a 0.20 mm filter and the collected solution
was stored at 4˚C for 12 h for intraperitoneal injection.
Animal model and drug
administration
Male Wistar rats (purchased from the Laboratory
Animal Center of Academy of Military Medical Science of People's
Liberation Army, China; 6 weeks old; 270-300 g; n=24) were randomly
divided into three groups (n=8 per group): The sham-operated group,
the bilateral common carotid artery occlusion (2-VO) group and the
HA-treated group. Animals were housed with free access to water and
food under a 12 h light/dark cycle in a 22-25˚C with 40-60%
humidity. The animal care and experimental protocols were approved
by the Medical Ethics Committee of Tianjin Medical University
General Hospital (Tianjin, China).
In the sham-operated group, the carotid arteries
were exposed but not occluded. Rats were subjected to transient
cerebral ischemia/reperfusion in both the 2-VO and HA-treated
groups. Prior to induction of transient cerebral ischemia, rats
were anaesthetized with 10% chloralhydrate (intraperitoneal, 300
mg/kg), and exhibited no signs of peritonitis following
administration. The common carotid arteries were isolated and
clamped using non-traumatic aneurysm clips for 30 min. The clips
were subsequently released for 10 min and a second ischemia was
applied for 30 min.
On day 1 after surgery, rats of the HA-treated group
were administered HA saline solution via intraperitoneal injection
at 18 mg/100 g body weight dissolved in 1 ml PBS per day. This
regimen was performed for a total of 14 days. In the sham and 2-VO
groups, rats were injected with an equal volume of PBS.
Electrophysiological recordings
Electrophysiological recordings were obtained at 14
days post-surgery. Rats were anesthetized with urethane (1 g/kg)
and subsequently placed in a stereotaxic frame (Narishige
International, Ltd.). Small holes were drilled into the skull to
insert stimulatory and recording electrodes (A.M.P.I., Jerusalem,
Israel). The tip of the recording electrode was positioned in the
stratum radiatum of the cornu ammonis region 1 (CA1) area (3.5 mm
posterior to the bregma and 2.5 mm lateral to the midline). The
stimulating electrode was inserted into the CA3 region (4.2 mm
posterior to the bregma and 3.5 mm to the midline). The test
stimuli (0.3-0.5 mA) were delivered to the CA3 region every 30 sec
at an intensity that evoked a response of 50% of its maximum. After
recording every 20 sec for 20 min to produce stable baseline data,
a high frequency stimulation consisting of 10 trains of 10 stimuli
at 100 Hz with 2 sec intertrain intervals was delivered to induce
LTP. The field excitatory postsynaptic potential (fEPSP)was
subsequently recorded at 40 kHz every 20 sec for 1 h using Scope
software (PowerLab; AD Instruments). Finally, the fEPSP slope was
normalized and analyzed using Clampfifit 9.0 (Molecular Devices,
LLC).
Histology and immunohistochemistry
(IHC) analyses
Following electrophysiological analysis, the rats
were deeply anesthetized with urethane (1 g/kg) and perfused
through the left cardiac ventricle with PBS (pH 7.2), followed by
4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2). The brain
of each rat was removed, fixed by immersion in the same solution
and embedded in paraffin. Paraffin-embedded tissue sections were
then cut into 5-µm-thick sections. Tissue sections were placed onto
uncoated slides prior to hematoxylin and eosin (H&E)staining,
while sections intended for IHC analysis were placed onto coated
slides (ZSJQ-BIO). H&E staining using light microscopy was
routinely performed for the assessment of histomorphology.
Immunohistochemical procedures for
GFAP and GAP-43
Tissue sections intended for immunoreactivity
analysis were placed onto coated slides (OriGene Technologies,
Inc.). Labeled dextran polymer IHC was used to detect the
expression levels of GAP43 and GFAP in CA1 subfields.
Deparaffinized tissue sections were boiled in citrate buffer for
antigen retrieval and incubated with 3% H2O2
solution for 30 min at room temperature to inhibit endogenous
peroxidase activity. Tissue sections were incubated with rabbit
polyclonal anti-GFAP (1:100; ZSJQ-BIO) or rabbit polyclonal
anti-GFAP (1:100; Newmarker Biotechnology) antibodies overnight at
4˚C. PBS replaced the antibodies as the negative controls.
Following the primary incubation, tissue sections were incubated
with En Vision-Systems polymer-conjugated PV-9000 secondary
antibody (GBI), and subsequently incubated with DAB at room
temperature for 3 min. Between steps, the slides were washed twice
with PBS. Tissue sections were observed and imaged using an Olympus
YS100 microscope (Olympus Corporation) with a CCD camera (JVC).
Statistical analysis
SPSS software (version 16.0; SPSS, Inc.) was used
for statistical analysis. Data are presented as the mean ± standard
error of the mean. One-way ANOVA followed by Bonferroni's post hoc
test was used to analyze the data. P<0.05 was considered to
indicate a statistically significant difference.
Results
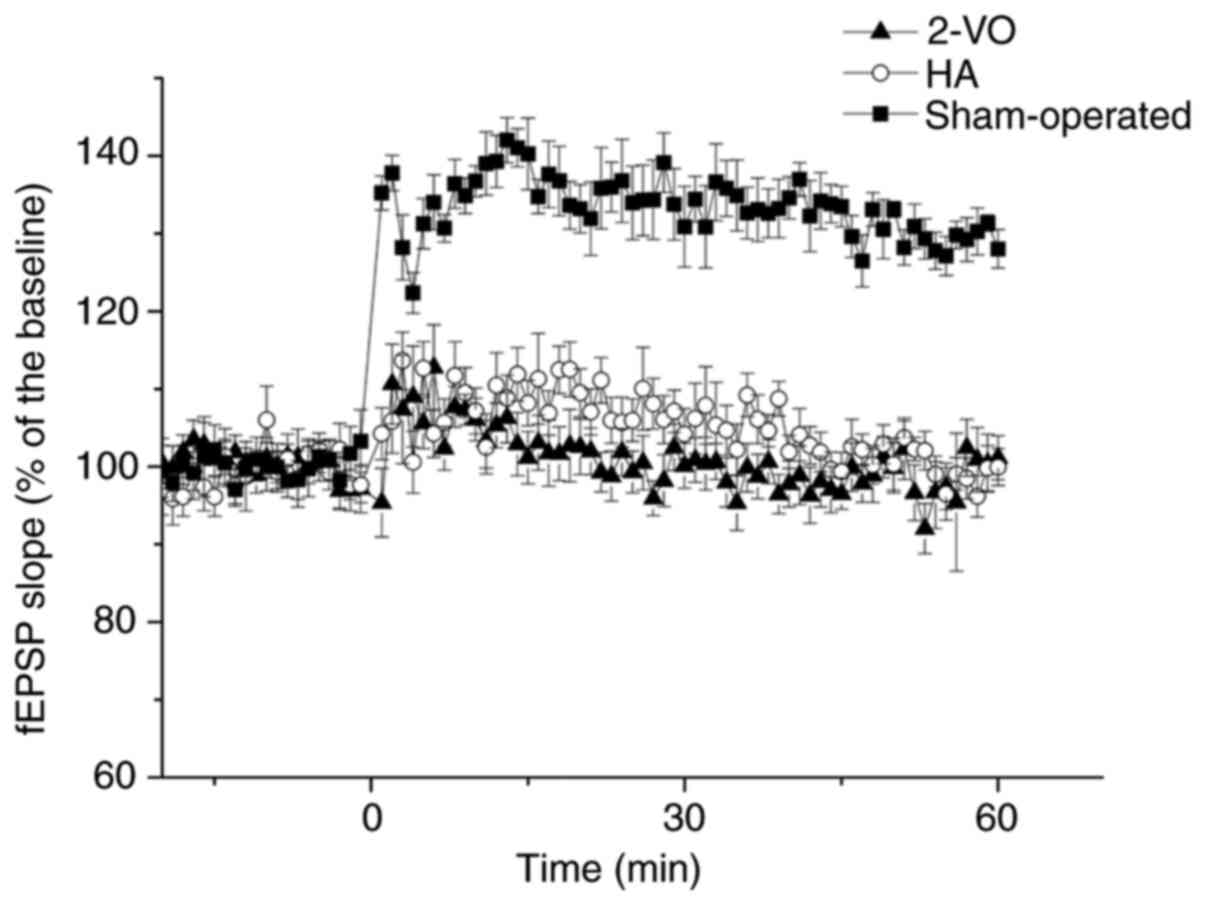
Change of LTP
The mean normalized slope of the fEPSP across the
60-min assessment period for each animal was significantly
decreased in the 2-VO group compared with that in the sham-operated
group (2-VO, 100.9±3.4%; sham-operated, 133.4±3.3%; P<0.05;
Fig. 1). Notably, this change was
partially recovered following treatment with HA (HA, 105.2±3.1%;
P<0.05 vs. sham or 2-VO; Fig.
1).
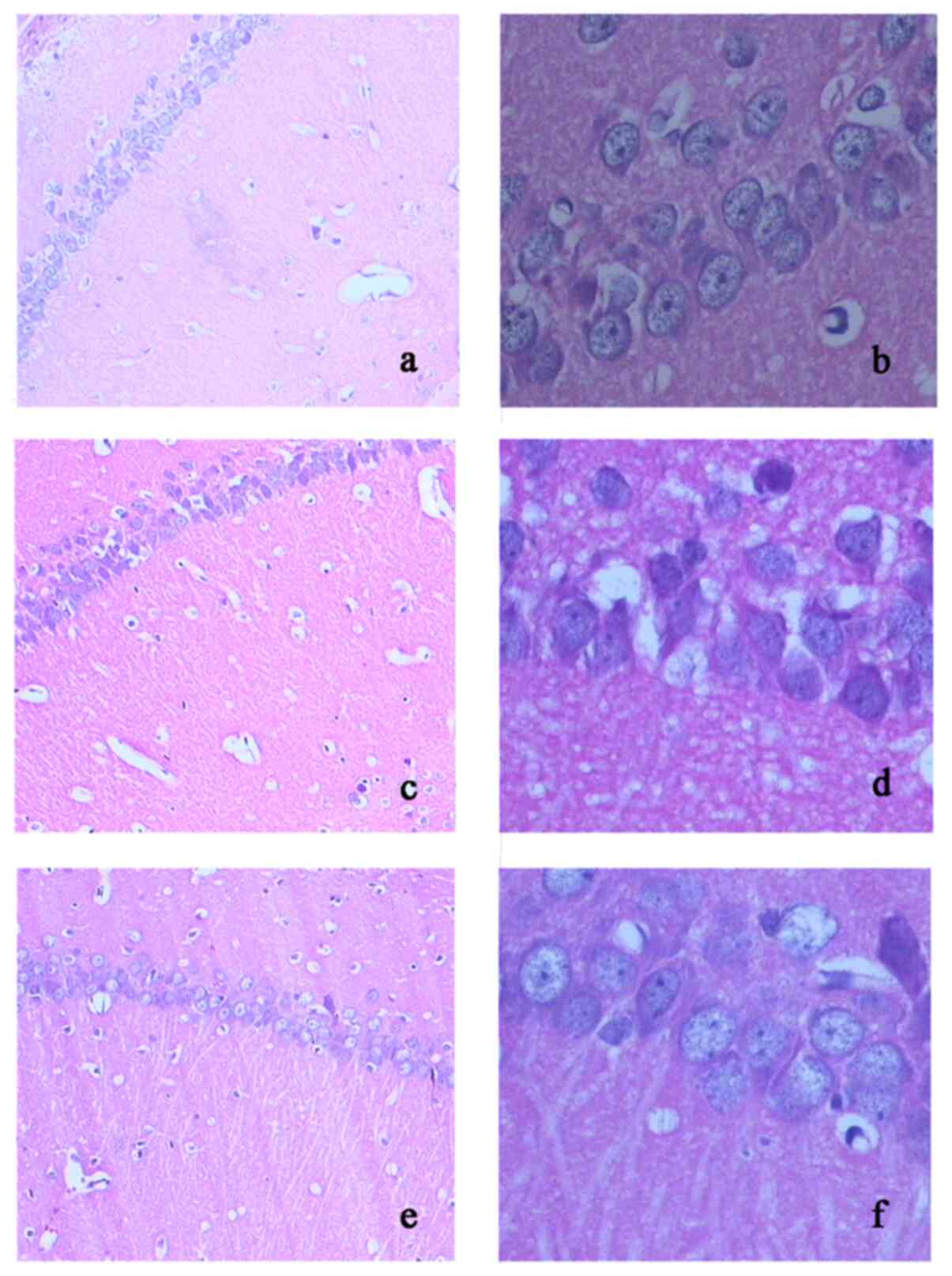
H&E staining
In the hippocampus, marked morphological changes
were detected in the ischemic group, including neuronal cell loss,
glial proliferation, nuclei shrinkage, cerebral edema and dark
staining of neurons, all of which were observed in the hippocampal
CA1 region. Treatment with HA markedly decreased these pathological
changes (Fig. 2a and f).
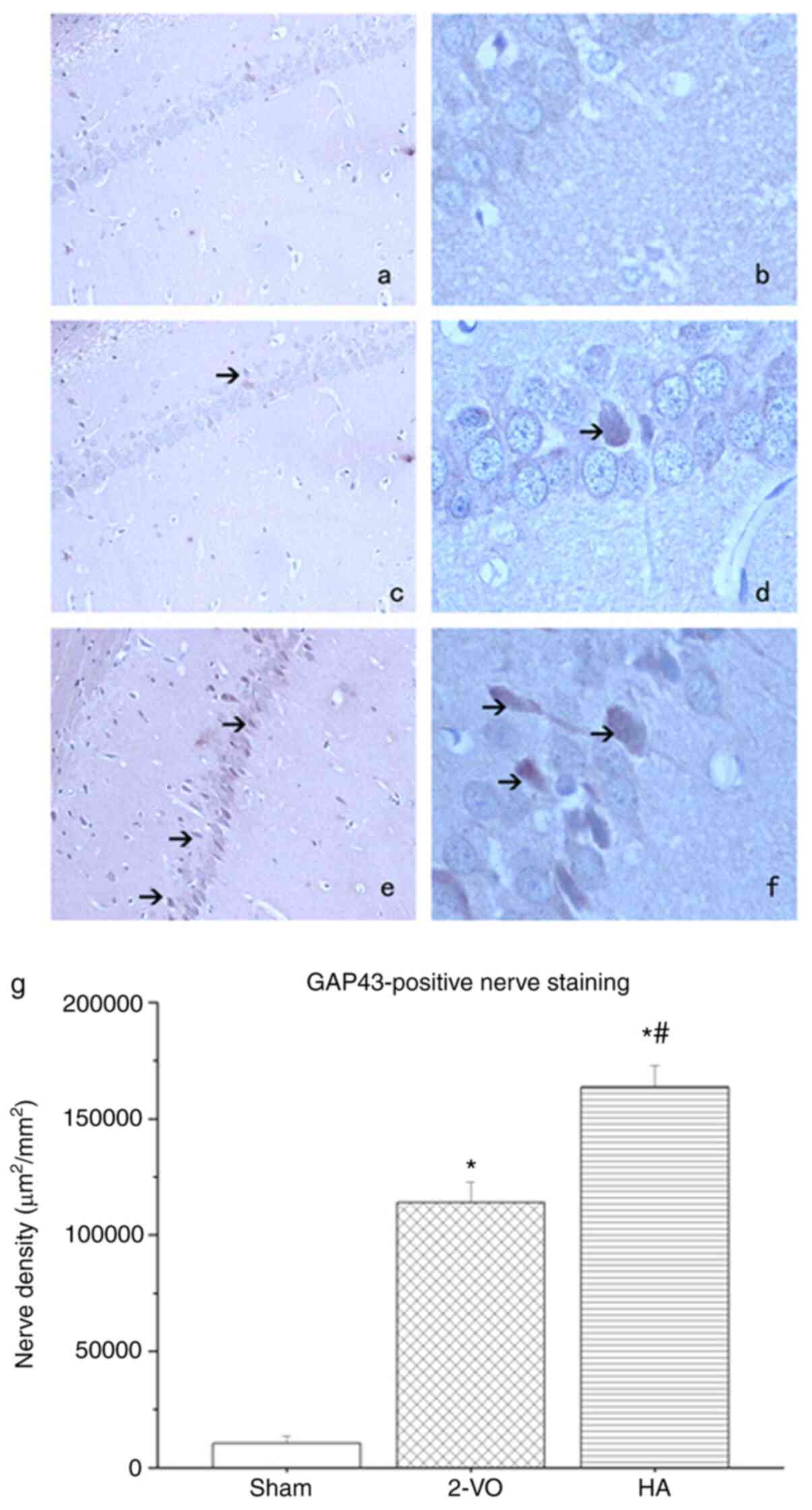
IHC analysis of GAP43 and GFAP
To investigate the effect of HA on axonal growth
following transient cerebral ischemia/reperfusion, the expression
levels of GAP43 and GFAP in the hippocampus of rat brains were
compared. As presented in Fig. 3,
GAP43 had more prominent localization in the HA-treated group
compared with that in the 2-VO group (163,596±9,263 vs.
113,892±8,972 m2/mm2; P<0.05).
Additionally, when compared with the 2-VO and HA groups, the lowest
expression of GAP43 was observed in the sham-operated group
(10,364±3,298 m2/mm2; P<0.05 in the 2-VO
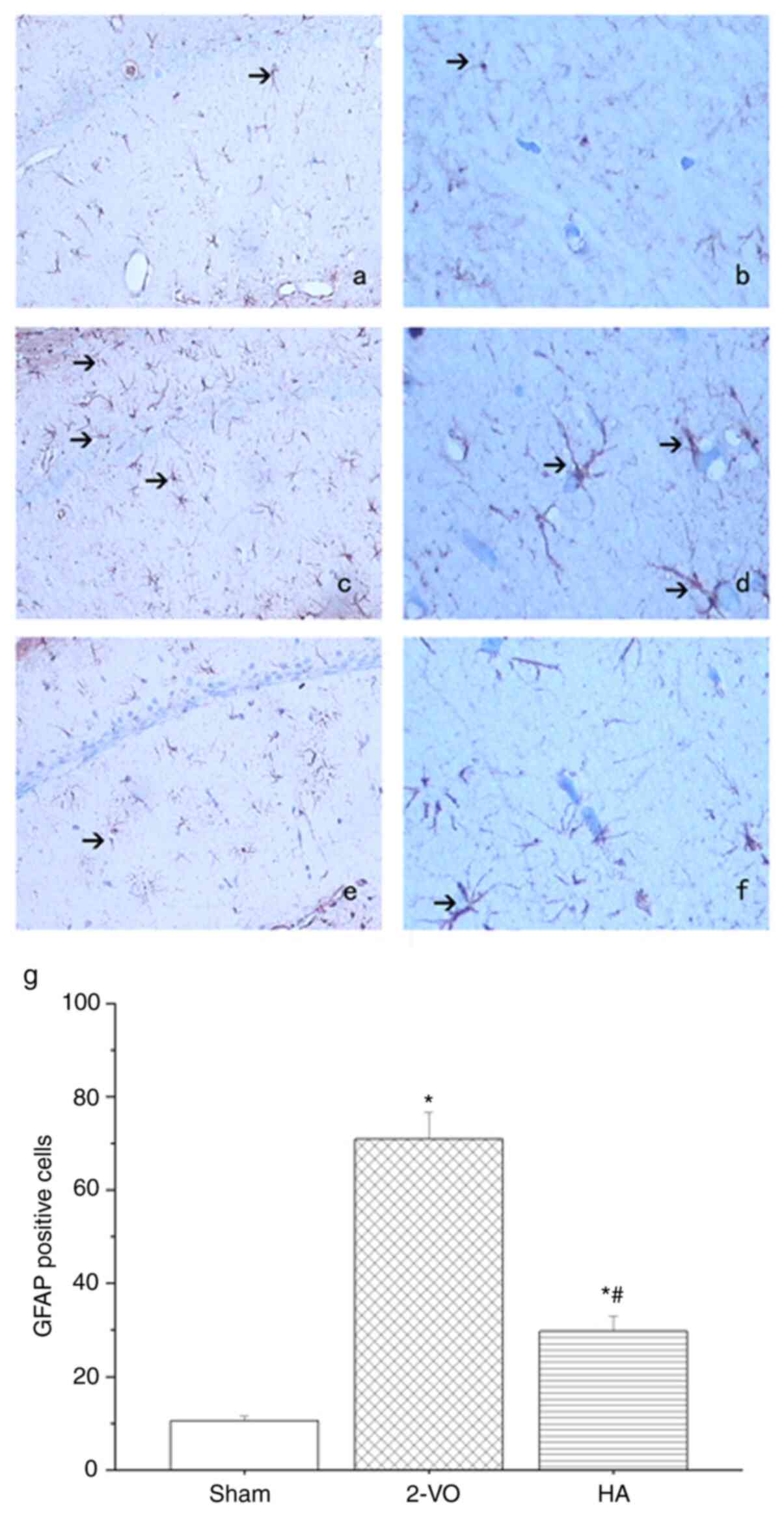
and HA groups vs. the sham-operated group). As presented in
Fig. 4, lower GFAP positive signals
were expressed in the hippocampus of the HA-treated group compared
with those in the 2-VO group (29.73±3.28 vs. 70.95±5.68;
P<0.05), with the lowest expression in the sham-operated group
(10.5±1.03; P<0.05 in the 2-VO and HA groups vs. the
sham-operated group).
Discussion
Bilateral common carotid artery clamping decreases
blood flow in the brain to one-third of its normal value in rats
(9). Given that hypoperfusion also
affects the hippocampus (10), it
may exert effects on several neuronal properties, including
neuronal cell viability or the electrophysiological behavior of
neurons (11). Thus, LTP impairment
may be expected to occur in rat hippocampal Schaffer collateral-CA1
synapses.
The results of the present study demonstrated that
LTP was inhibited in both the HA-treated and 2-VO groups following
transient cerebral ischemia/reperfusion when compared with the
sham-operated group. These results confirmed the utility of this
model in the investigation of HA effects. The reconstruction of
synapses is essential in developing LTP following injury (19), and the results of the present study
demonstrated that administration of HA extracts decreases transient
cerebral ischemia/reperfusion-induced LTP impairment, suggesting
that HA extracts may be used to improve the reconstruction of
synapses following transient cerebral ischemia/reperfusion.
A previous study demonstrated that a reduction in
cerebral blood flow, and the concomitant abnormalities of energy
metabolism that arise in chronic cerebral ischemia can lead to the
selective neuronal injuries in vulnerable regions of the brain,
particularly the hippocampus and cerebral cortex (20). The results of the present study were
consistent with these findings, as H&E staining exhibited
neuronal cell loss, glial proliferation, nuclei shrinkage, cerebral
edema and dark staining of neurons in the hippocampus of both the
HA-treated and 2-VO groups following transient cerebral
ischemia/reperfusion. Treatment with HA extracts markedly decreased
these pathological changes, which initially indicated the
neuroprotective effects of HA extracts on the CNS following
ischemia.
GAP43 was selected as an indicator of axonal
regeneration in the present study. Neurons can extend axon branches
and form new connections following injury (21). It is also known that axonal
regeneration occurs in the CNS following cerebral ischemia
(2). During axon sprouting, GAP43
expression increases, thus GAP43 has been used as a marker of axon
growth and/or terminal sprouting in stroke models (22). The results of the current study
demonstrated high GAP43 protein expression in the HA-treated group
compared with that in the 2-VO group, which suggests that sprouting
of the injured axon was more active in the HA group following
ischemia. Based on the results of H&E staining, it was
hypothesized that the neuroprotective effects of HA extracts may
include improving axon regeneration following ischemia.
Accompanied with several pathological conditions,
including trauma, neuroinflammation and ischemic damage, reactive
astrogliosis affects the CNS (18).
Reactive astrocytes increase the expression of their structural
proteins, such as GFAP and vimentin (23). A previous study has demonstrated
that GFAP expression is upregulated in several types of brain
injury, including trauma, demyelination and brain ischemia
(18). The results of the present
study were consistent with these results, demonstrating upregulated
GFAP protein expression in the 2-VO group compared with that in the
sham-operated group.
A notable role of reactive astrocytes in late-stage
neurotrauma is the facilitation of the formation of post-traumatic
glial scars and the inhibition of CNS regeneration (18). In particular, they appear to
compromise neural graft survival and integration, decreasing the
extent of synaptic regeneration, inhibiting neurogenesis in old age
and inhibiting the regeneration of severed CNS axons (24). The current study demonstrated that
treatment with HA significantly decreased
ischemia-reperfusion-induced GFAP expression. Thus, low GFAP
expression in the HA-treated group compared with that in the 2-VO
group indicated that the HA-treated group demonstrated less
inhibition and improved axonal regeneration, which was consistent
with the results of LTP and GAP43.
There is a limitation in the present study due to
the absence of a sham-operated group receiving HA treatment.
However, the current study did demonstrate that HA extract reversed
the LTP deficit and improved axon regeneration induced by
ischemia/reperfusion, which may indicate a potential safe and
effective method of using HA in vivo.
Taken together, the results of the present study
revealed that HA extracts reversed the LTP deficit and improved
axon regeneration induced by ischemia/reperfusion, which may be
useful in understanding the molecular mechanism underlying the
neuroprotective effects of HA.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science
Foundation of China (grant nos. 81601041 and 81601411), the Medical
Foundation of Jieping Wu (grant no. 320.6750.19089-56) and the
Youth Incubation Fund of the General Hospital of Tianjin Medical
University (grant no. zyyfy2019007).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JX, CW and PX performed the experiments, collected
the results and wrote the manuscript. JX and CW analyzed the data
and wrote the manuscript. JX and CW confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The current study was approved by the Medical Ethics
Committee of Tianjin Medical University General Hospital (Tianjin,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mozaffarian D, Benjamin EJ, Go AS, Arnett
DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP,
Fullerton HJ, et al: Writing Group Members; American Heart
Association Statistics Committee; Stroke Statistics Subcommittee:
Heart Disease and Stroke Statistics-2016 Update: A Report From the
American Heart Association. Circulation. 133:e38–e360.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang AR, Hu MZ, Zhang ZL, Zhao ZY, Li YB
and Liu B: Fastigial nucleus electrostimulation promotes axonal
regeneration after experimental stroke via cAMP/PKA pathway.
Neurosci Lett. 699:177–183. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gondard E, Teves L, Wang L, McKinnon C,
Hamani C, Kalia SK, Carlen PL, Tymianski M and Lozano AM: Deep
Brain Stimulation Rescues Memory and Synaptic Activity in a Rat
Model of Global Ischemia. J Neurosci. 39:2430–2440. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ueno R, Takase H, Suenaga J, Kishimoto M,
Kurihara Y, Takei K, Kawahara N and Yamamoto T: Axonal regeneration
and functional recovery driven by endogenous Nogo receptor
antagonist LOTUS in a rat model of unilateral pyramidotomy. Exp
Neurol. 323(113068)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ramesh N, Moratti SC and Dias GJ:
Hydroxyapatite-polymer biocomposites for bone regeneration: A
review of current trends. J Biomed Mater Res B Appl Biomater.
106:2046–2057. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wei G, Gong C, Hu K, Wang Y and Zhang Y:
Biomimetic Hydroxyapatite on Graphene Supports for Biomedical
Applications: A Review. Nanomaterials (Basel).
9(1435)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huang J, Best SM, Bonfield W, Brooks RA,
Rushton N, Jayasinghe SN and Edirisinghe MJ: In vitro assessment of
the biological response to nano-sized hydroxyapatite. J Mater Sci
Mater Med. 15:441–445. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liuyun J, Yubao L and Chengdong X:
Preparation and biological properties of a novel composite scaffold
of nano-hydroxyapatite/chitosan/carboxymethyl cellulose for bone
tissue engineering. J Biomed Sci. 16(65)2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Farkas E, Luiten PG and Bari F: Permanent,
bilateral common carotid artery occlusion in the rat: A model for
chronic cerebral hypoperfusion-related neurodegenerative diseases.
Brain Res Rev. 54:162–180. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Todd NV, Crockard HA, Russel RW and
Picozzi P: Cerebral blood flow in the four-vessel occlusion rat
model. Stroke. 15(579)1984.PubMed/NCBI
|
|
11
|
Yu L, Duan Y, Zhao Z, He W, Xia M, Zhang Q
and Cao X: Hydroxysafflor Yellow A (HSYA) Improves Learning and
Memory in Cerebral Ischemia Reperfusion-Injured Rats via Recovering
Synaptic Plasticity in the Hippocampus. Front Cell Neurosci.
12(371)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bliss TV and Lomo T: Long-lasting
potentiation of synaptic transmission in the dentate area of the
anaesthetized rabbit following stimulation of the perforant path. J
Physiol. 232:331–356. 1973.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li W, Ye A, Ao L, Zhou L, Yan Y, Hu Y,
Fang W and Li Y: Protective Mechanism and Treatment of Neurogenesis
in Cerebral Ischemia. Neurochem Res. 45:2258–2277. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen MK, Peng CC, Maner RS, Zulkefli ND,
Huang SM and Hsieh CL: Geniposide ameliorated fluoxetine-suppressed
neurite outgrowth in Neuro2a neuroblastoma cells. Life Sci.
226:1–11. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang M, Yao M, Liu J, Takagi N, Yang B,
Zhang M, Xu L, Ren J, Fan X and Tian F: Ligusticum
chuanxiong exerts neuroprotection by promoting adult
neurogenesis and inhibiting inflammation in the hippocampus of ME
cerebral ischemia rats. J Ethnopharmacol.
249(112385)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen J, Zhang DM, Feng X, Wang J, Qin YY,
Zhang T, Huang Q, Sheng R, Chen Z, Li M, et al: TIGAR inhibits
ischemia/reperfusion-induced inflammatory response of astrocytes.
Neuropharmacology. 131:377–388. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang JL and Xu CJ: Astrocytes autophagy in
aging and neurodegenerative disorders. Biomed Pharmacother.
122(109691)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Brenner M: Role of GFAP in CNS injuries.
Neurosci Lett. 565:7–13. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lisman J: Glutamatergic synapses are
structurally and biochemically complex because of multiple
plasticity processes: Long-term potentiation, long-term depression,
short-term potentiation and scaling. Philos Trans R Soc Lond B Biol
Sci. 372(20160260)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Farhadi Moghadam B and Fereidoni M:
Neuroprotective effect of menaquinone-4 (MK-4) on transient global
cerebral ischemia/reperfusion injury in rat. PLoS One.
15(e0229769)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stokowska A, Atkins AL, Morán J, Pekny T,
Bulmer L, Pascoe MC, Barnum SR, Wetsel RA, Nilsson JA, Dragunow M,
et al: Complement peptide C3a stimulates neural plasticity after
experimental brain ischaemia. Brain. 140:353–369. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Holahan MR: A Shift from a Pivotal to
Supporting Role for the Growth-Associated Protein (GAP-43) in the
Coordination of Axonal Structural and Functional Plasticity. Front
Cell Neurosci. 11(266)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Erfani S, Moghimi A, Aboutaleb N and
Khaksari M: Protective Effects of Nucleobinding-2 After Cerebral
Ischemia Via Modulating Bcl-2/Bax Ratio and Reducing Glial
Fibrillary Acid Protein Expression. Basic Clin Neurosci.
10:451–459. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guillamón-Vivancos T, Gómez-Pinedo U and
Matías-Guiu J: Astrocytes in neurodegenerative diseases (I):
function and molecular description. Neurologia. 30:119–129.
2015.PubMed/NCBI View Article : Google Scholar
|