Introduction
Polycythemia vera (PV; polycythemia rubra or maladie
de Vaquez) is a chronic myeloproliferative disorder characterized
by the clonal proliferation of myeloid cells, which leads to an
increase in red-blood cell mass, increasing the risk of patients to
develop thrombosis (1). PV is
associated with a significant risk of developing thromboembolic
events; long-term complications are represented by myelofibrosis or
even leukemic transformation (2).
PV affects all ages in all populations, it can occur in early
adulthood, although it rarely affects children and adolescents
(3,4); the median age at diagnosis is 60 years
(5). As regards sex, PV has a
slightly higher incidence among males than females (2.8 vs. 1.3
cases/100,000 per year) (6) and a
lower incidence in Japan compared to Europe and the United States
(7). The incidence of PV exhibits a
tendency to increase with age among women, from an incidence of
0.04/100,000 cases in the age group of 20-34 years to an incidence
of 0.25/100,000 in the age group of 35-39 years (2). The life expectancy of patients with
symptomatic untreated PV has been found to be ~18 months; however,
in treated patients, the survival rate is >13 years (8,9).
PV, similar to other Philadelphia
chromosome-negative myeloproliferative disorders, is usually caused
by more than one genetic mutation (10). Major progress was made when the JAK2
(JAK2V617F) mutation was demonstrated to be the cause of PV
(11). Other theories concerning
the pathogenesis of PV have also been suggested, including the loss
of heterozygosity (LOH), with LOH on the short arm of chromosome 9
(9pLOH) being found in 50% of cases of PV and represents the most
common chromosomal lesion as a result of mitotic recombination
(12,13). Another mechanism is family
inheritance; there have been rare cases of familial PV reported,
with PV exhibiting an autosomal dominant trait with incomplete
penetrance. Clinical features in such cases present at a younger
age compared with sporadic PV (14).
JAK/STAT signaling is responsible for cellular
proliferation, as well as for cell survival, particularly via
erythropoietin-erythropoietin receptor gene implicated in
erythropoiesis. JAK2 mutation allows tyrosine kinase activity to
continue the STAT phosphorylation activity in the presence of
minimal or no erythropoietin levels (15,16).
In rare JAK2-negative PV cases, other JAK2 mutations have been
identified in exon 12(17). These
mutations are heterogenous and they are sometimes difficult to
detect by DNA sequencing or other conventional techniques due to
the low allele burden (18).
Pregnant patients with PV are at a high risk of
miscarriage, as well as other pregnancy-related complications, such
as pregnancy-induced hypertension, intrauterine growth restriction,
preeclampsia and placental abruption (19). The focus of treatment in pregnant
women with PV is to maintain hematocrit levels <45% or within
the normal pregnancy hematocrit range (30-39%), depending on which
levels are lower (20). The
preferred treatment among cytoreductive agents in fertile women or
in pregnant women is interferon-α due to the teratogenic potential
of the other agents, such as hydroxyurea, alkylating agents or
ruxolitinib (21). As a
complementary treatment, low-dose aspirin is strongly recommended
in the management of patients with PV (22).
The present study describes the case of a
38-year-old pregnant woman with JAK2-positive masked PV diagnosed
according to the World Health Organization (WHO) criteria (23) and associated pathologies and
discusses this condition with an aim to highlight the following: i)
Points of strategy for the management of PV during pregnancy; and
ii) the particularities of the course of pregnancy in the case of
PV.
Case report
The present study was conducted in accordance with
the World Medical Association Declaration of Helsinki and was
approved by the Ethical Board of the ‘Life Memorial Hospital’
Bucharest, Romania. The patient provided informed consent for
publication of the present case report.
A 38-year-old woman presented to the authors'
medical unit, Life Memorial Hospital (Bucharest, Romania), for an
early diagnosis of pregnancy. The patient was diagnosed with
JAK2-positive masked PV 3 years prior; the patient's medical
history included a diagnosis of stage IV peripheral venous
insufficiency, hepatic hemangioma, accessory spleen,
hyperhomocysteinemia and hereditary thrombophilia (MTHFR C667T
homozygote positive mutation).
The patient's haematological journey began at the
age of 28, when she was diagnosed with thrombocytosis [platelet
(PLT) count, 600,000-700,000/µl] with no other modified blood
tests, which was considered reactive until 2014 when the patient
was diagnosed with a JAK2-positive myeloproliferative neoplasm with
essential thrombocythemia (PLT count, 700,000/µl); however, the
patient refused to undergo a bone marrow biopsy. In 2015, the
patient presented to a medical unit complaining of vertigo and
spontaneous phosphenes. A hemogram revealed 616,000
thrombocytes/µl, hemoglobin (Hb) levels of 14.8 g/dl, hematocrit
(Ht) levels at 44.7% and a lymphocyte count of 10,720/µl. After
being subjected to several tests, the patient was diagnosed with
hyperhomocysteinemia in the context of a MTFR C667T
homozygote-positive mutation, marked poikilocytosis, ovalocytes,
teardrop erythrocytes and rouleaux formation. The bone marrow
biopsy revealed megakaryocyte hyperplasia with a secondary aspect.
Serum erythropoietin or leucocyte alkaline phosphatase levels were
not tested. Therefore, the patient was considered to have essential
thrombocythemia (ET). The patient received treatment consisting of
low-dose aspirin (150 mg daily), alopurinol, folic acid and
Diosminum. In 2016, during a specialized control, due to further
alterations in blood tests results (Hb levels, 17 g/dl; Ht, 51.4%;
lymphocyte count, 12,990/µl; PLT count, 760,000/µl), the patient
was advised to commence interferon or hydroxyurea therapy; however,
she refused and continued treatment with low-dose aspirin,
Diosminum and folic acid. The following year, the patient's blood
tests revealed further modifications: Hb levels, 18.3 g/dl; Ht,
53.3%; lymphocyte count, 26,500/µl; PLT count, 1,048,000/µl,
leucocyte alkaline phosphatase score of 42; and serum
erythropoietin levels, 1.98 mU/ml. Another bone marrow examination
was performed and revealed panmyelosis, a small reactive lymphoid
nodule, CD34+ cells (2-3%) and sinus dilation,
suggestive of PV. The patient accepted immunomodulatory treatment
(3 MU pegylated interferon-α subcutaneously administered three
times/week), a short time before she became pregnant. From her
family medical history, it was noted that her father was suffering
from insulin-dependent diabetes mellitus and renal cancer. Her
gynecological anamnesis consisted of an abortion with no further
complications.
The ultrasound evaluation of the incipient pregnancy
was normal for the gestational age. The patient was counseled about
the possible risks and complications during pregnancy, considering
the associated hematological pathology and received the
recommendation for proper hydration and to wear compression
stockings. During the pregnancy, an obstetrician and hematologist
closely monitored the patient, and anti-coagulant treatment (4,000
UI enoxaparin/day) was introduced in addition to the patient's
previous medication, considering the cumulative thrombotic risk due
to pregnancy, thrombocytosis, chronic venous disease and the
advanced maternal age. The patient was subjected to a non-invasive
prenatal screening test for the major chromosomal abnormalities,
the result of which was negative. Due to the favorable evolution of
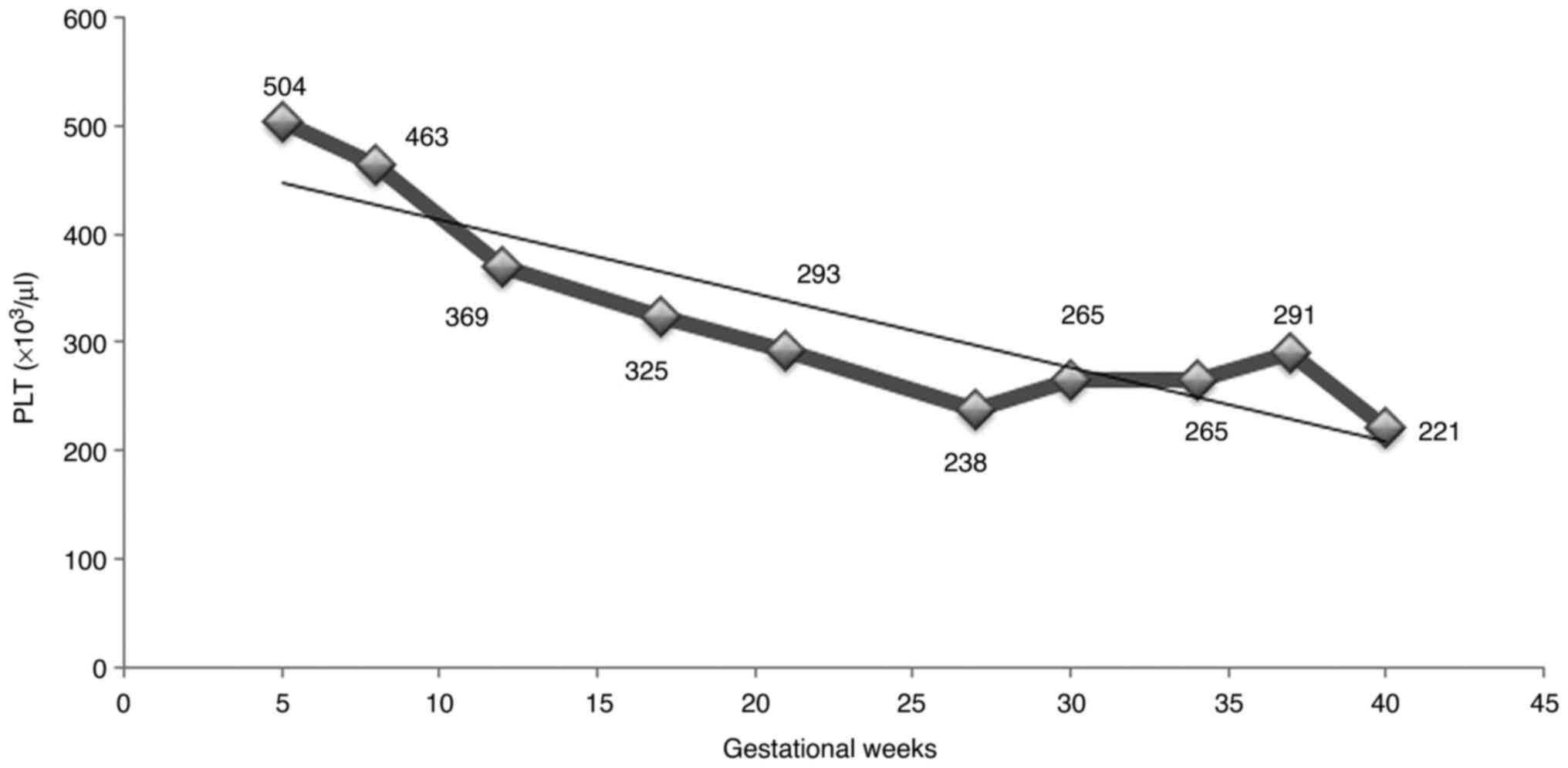
PV, consisting of the normalization of the hemogram and
particularly of the thrombocyte value (Fig. 1), the cytoreductive treatment was
reduced to two administrations per week of pegylated interferon-α,
commencing on the 23th week of gestation.
The clinical and ultrasound evaluation during
pregnancy revealed a normal fetal growth and a normal amniotic
fluid index. There were no thrombotic events and the thrombocyte
count exhibited an unexpected decreasing trend, being maintained
within safety limits during the entire pregnancy. The pregnancy was
finalized at 38 weeks and 5 days and a male fetus weighing 3,190 g,
with an Apgar score of 8 at 1 min, was delivered by cesarean
section, with a slightly poor adaptation to the extra-uterine life.
The further evolution of the offspring was favorable, with no other
neonatal complications.
Discussion
The particularity of the case of PV in pregnancy
presented herein embodies the difficulties in diagnosis of this
pathology in a woman of fertile age, and the need for
interdisciplinary collaboration in order to obtain a good and safe
outcome for both mother and child. A young age for PV, but an
advanced maternal age is the most challenging background for a
high-risk pregnancy with multiple and complex associated diseases.
The authors' experience in the management of myeloproliferative
diseases was demonstrated in a previous study published 3 years ago
(24); the pregnancy associated
with primary myelofibrosis had a good outcome under combined
treatment with interferon-α and low-dose aspirin, and even an
improved hematological evolution marked by the same decrease in
platelet counts following low molecular-weight heparin (LMWH)
administration in the third trimester (24).
The evolution of latent PV with thrombocytosis
mimicking essential thrombocythemia was previously described in a
series of 23 cases of masked PV by Thiele et al (25), revealing the explanation for the
long journey to the final diagnosis with four-field 2016 WHO
criteria for essential thrombocythemia (26) and the special pattern of the second
bone marrow histopathology of the patient described herein.
The preferred drug in the initial treatment of PV is
hydroxyurea, as a result of its efficacy, low-cost, comfort in
administration and limited associated toxicity (27-29).
However, for young patients (<40 years of age) and pregnant
patients, the elective drug is interferon-α due to its possibility
in achieving cytogenetic remission and its safety in pregnancy in
contrast to the teratogenicity of hydroxyurea (20). Interferon-α therapy is quite a
relatively recent acquisition in the treatment of
myeloproliferative disorders, exhibiting its effectiveness in
normalizing the platelet count (30). The fetal safety of interferon-α was
previously investigated by Yazdani Brojeni et al (31), who concluded that interferon-α does
not significantly increase the risk of major malformation,
spontaneous abortion, fetal demise, or preterm delivery when
compared with the general population rate.
To the best of our knowledge, there are limited
studies available in the literature debating the management of PV
in pregnancy. A recent study by Robinson et al (2) concluded that it is essential for the
optimal fetal prognosis to act actively in the hemogram balance.
The aspirin administration is mainly based on the results of the
European Collaboration on Low-Dose Aspirin in Polycythemia Vera
(ECLAP) trial, which demonstrated that 100 mg aspirin daily reduced
the risk of the combined endpoint of non-fatal myocardial
infarction, non-fatal stroke, pulmonary embolism, major venous
thrombosis, or death from cardiovascular causes (relative risk,
0.40; 95% CI 0.18-0.91) (22). The
mechanism of the efficacy of low-dose aspirin is the irreversible
inhibition of the enzyme, COX-1, in the vascular endothelium,
necessary for the production of thromboxane A2, the factor
responsible for platelet aggregation and vasoconstriction. As
regards the administration of LMWH, it is not prophylactically
recommended for women without active thrombosis or a history of a
major thrombotic event (20,29,30).
For the case in the present study, the use of a 150 mg daily dose
of aspirin was considered, based on the authors' experience in
screening and preventing, first and foremost preeclampsia, but also
other fetal and maternal complications and the associated risks of
intrauterine growth restriction, premature rupture of membranes,
abruptio placentae and intrauterine fetal death (32,33).
The justification in adding enoxaparin to the treatment of the
patient was the existence of associated pathologies: Peripheral
venous insufficiency with a high risk of thrombosis in the context
of pregnancy and thrombocytosis, as well as an advanced maternal
age. Thus, it can be confirmed that better results are obtained
when using cytoreductive treatment and low-dose aspirin; the use of
anti-coagulant treatment should be individualized and recommended
whenever needed.
In conclusion, pregnancy by definition generates a
hypercoagulability status; PV represents a procoagulant factor due
to the elevated thrombocyte count, and due to a high risk of
thrombosis, thrombophilia screening is recommended, particularly in
PV, for each and every pregnant women or women who are aiming at
conceiving. In addition, the active implication and management of
this pathology is required in order to improve live births.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
REB conceived the article following the successful
management of the presented case. BMM and ID performed the
literature search and wrote the manuscript. TAG, FF, OM and CG
contributed to the literature review. ENM conducted the follow-up
of the patient. REB, ENM, CG and FF collected, assembled and
interpreted the data, and revised the manuscript critically for
important intellectual content. REB and BMM confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the World Medical Association Declaration of Helsinki and was
approved by the Ethical Board of the ‘Life Memorial Hospital’
Bucharest, Romania.
Patient consent for publication
The patient provided informed consent for
publication of the present case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stuart BJ and Viera AJ: Polycythemia vera.
Am Fam Physician. 69:2139–2144. 2004.PubMed/NCBI
|
|
2
|
Robinson S, Bewley S, Hunt BJ, Radia DH
and Harrison CN: The management and outcome of 18 pregnancies in
women with polycythemia vera. Haematologica. 90:1477–1483.
2005.PubMed/NCBI
|
|
3
|
Passamonti F, Malabarba L, Orlandi E,
BaratèC Canevari A, Brusamolino E, Bonfichi M, Arcaini L, Caberlon
S, Pascutto C and Lazzarino M: Polycythemia vera in young patients:
A study on the long-term risk of thrombosis, myelofibrosis and
leukemia. Haematologica. 88:13–18. 2003.PubMed/NCBI
|
|
4
|
Teofili L, Giona F, Martini M, Cenci T,
Guidi F, Torti L, Palumbo G, Amendola A, Foà R and Larocca LM:
Markers of myeloproliferative diseases in childhood polycythemia
vera and essential thrombocythemia. J Clin Oncol. 25:1048–1053.
2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tefferi A, Rumi E, Finazzi G, Gisslinger
H, Vannucchi AM, Rodeghiero F, Randi ML, Vaidya R, Cazzola M,
Rambaldi A, et al: Survival and prognosis among 1545 patients with
contemporary polycythemia vera: An international study. Leukemia.
27:1874–1881. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Anía BJ, Suman VJ, Sobell JL, Codd MB,
Silverstein MN and Melton LJ III: Trends in the incidence of
polycythemia vera among olmsted county, Minnesota residents,
1935-1989. Am J Hematol. 47:89–93. 1994.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Johansson P: Epidemiology of the
myeloproliferative disorders polycythemia vera and essential
thrombocythemia. Semin Thromb Hemost. 32:171–173. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chievitz E and Thiede T: Complications and
causes of death in polycythaemia vera. Acta Med Scand. 172:513–523.
1962.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tefferi A, Guglielmelli P, Larson DR,
Finke C, Wassie EA, Pieri L, Gangat N, Fjerza R, Belachew AA, Lasho
TL, et al: Long-term survival and blast transformation in
molecularly annotated essential thrombocythemia, polycythemia vera,
and myelofibrosis. Blood. 124:2507–2513. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Campbell PJ and Green AR: The
myeloproliferative disorders. N Engl J Med. 355:2452–2466.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schafer AI: Molecular basis of the
diagnosis and treatment of polycythemia vera and essential
thrombocythemia. Blood. 107:4214–4222. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kralovics R, Buser AS, Teo SS, Coers J,
Tichelli A, van der Maas AP and Skoda RC: Comparison of molecular
markers in a cohort of patients with chronic myeloproliferative
disorders. Blood. 102:1869–1871. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kralovics R, Guan Y and Prchal JT:
Acquired uniparental disomy of chromosome 9p is a frequent stem
cell defect in polycythemia vera. Exp Hematol. 30:229–236.
2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kralovics R, Stockton DW and Prchal JT:
Clonal hematopoiesis in familial polycythemia vera suggests the
involvement of multiple mutational events in the early pathogenesis
of the disease. Blood. 102:3793–3796. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Levy DE and Darnell JE Jr: Stats:
Transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Neubauer H, Cumano A, Müller M, Wu H,
Huffstadt U and Pfeffer K: Jak2 deficiency defines an essential
developmental checkpoint in definitive hematopoiesis. Cell.
93:397–409. 1998.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Scott LM, Tong W, Levine RL, Scott MA,
Beer PA, Stratton MR, Futreal PA, Erber WN, McMullin MF, Harrison
CN, et al: JAK2 exon 12 mutations in polycythemia vera and
idiopathic erythrocytosis. N Engl J Med. 356:459–468.
2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Scott LM: The JAK2 exon 12 mutations: A
comprehensive review. Am J Hematol. 86:668–676. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ferguson JE II, Ueland K and Aronson WJ:
Polycythemia rubra vera and pregnancy. Obstet Gynecol. 62 (Suppl
3):16S–20S. 1983.PubMed/NCBI
|
|
20
|
Barbui T, Barosi G, Birgegard G, Cervantes
F, Finazzi G, Griesshammer M, Harrison C, Hasselbalch HC, Hehlmann
R, Hoffman R, et al: Philadelphia-negative classical
myeloproliferative neoplasms: Critical concepts and management
recommendations from European LeukemiaNet. J Clin Oncol.
29:761–770. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Harrison C: Pregnancy and its management
in the Philadelphia negative myeloproliferative diseases. Br J
Haematol. 129:293–306. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Landolfi R, Marchioli R, Kutti J,
Gisslinger H, Tognoni G, Patrono C and Barbui T: European
Collaboration on Low-Dose Aspirin in Polycythemia Vera
Investigators. Efficacy and safety of low-dose aspirin in
polycythemia vera. N Engl J Med. 350:114–124. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Barbui T, Thiele J, Gisslinger H,
Kvasnicka HM, Vannucchi AM, Guglielmelli P, Orazi A and Tefferi A:
The 2016 WHO classification and diagnostic criteria for
myeloproliferative neoplasms: Document summary and in-depth
discussion. Blood Cancer J. 8(15)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bohîlţea RE, Cîrstoiu MM, Ionescu CA,
Niculescu-Mizil E, Vlădăreanu AM, Voican I, Dimitriu M and Turcan
N: Primary myelofibrosis and pregnancy outcomes after low
molecular-weight heparin administration: A case report and
literature review. Medicine (Baltimore). 96(e8735)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Thiele J, Kvasnicka HM and Diehl V:
Initial (latent) polycythemia vera with thrombocytosis mimicking
essential thrombocythemia. Acta Haematol. 113:213–219.
2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Beauverd Y, Radia D, Cargo C, Knapper S,
Drummond M, Pillai A, Harrison C and Robinson S: Pegylated
interferon alpha-2a for essential thrombocythemia during pregnancy:
Outcome and safety. A case series. Haematologica. 101:e182–e184.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Finazzi G and Barbui T: How I treat
patients with polycythemia vera. Blood. 109:5104–5111.
2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fruchtman SM, Mack K, Kaplan ME, Peterson
P, Berk PD and Wasserman LR: From efficacy to safety: A
polycythemia vera study group report on hydroxyurea in patients
with polycythemia vera. Semin Hematol. 34:17–23. 1997.PubMed/NCBI
|
|
30
|
Bertozzi I, Rumi E, Cavalloni C, Cazzola
M, Fabris F and Randi ML: Pregnancy outcome and management of 25
pregnancies in women with polycythemia vera. Am J Hematol.
93:E234–E235. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yazdani Brojeni P, Matok I, Garcia
Bournissen F and Koren G: A systematic review of the fetal safety
of interferon alpha. Reprod Toxicol. 33:265–268. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bohiltea RE, Cirstoiu MM, Turcan N, Stoian
AP, Zugravu CA, Munteanu O, Arsene LV, Oana B, Neacsu A and
Furtunescu F: Inherited thrombophilia is significantly associated
with severe preeclampsia. Exp Ther Med. 21:261–267. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Grigoriu C, Anca AF, Grigoras M, Cezar C,
Lungu A and Horhoianu V: Premature birth and systemic inflammatory
response syndrome. Gineco Ro. 4:80–83. 2008.
|