Introduction
Over the last two decades, there has been a dramatic
change in the epidemiology of Clostridium difficile
infection (CDI). A disease considered to be a side effect of
antibiotic use, easy to treat, is now associated with outbreaks,
with increased mortality and morbidity. The incidence of CDI in
hospitalized patients has increased dramatically with more than
300,000 reported cases per year, with an increased mortality rate.
In the USA, more than 12,800 deaths were reported in 2017 due to
this pathology, adding CDI to the list of diseases of the century
we live in (1).
The new epidemic of CDI has been defined by
community outbreaks affecting people considered to be at low risk
of developing the disease. Initially, until the year 2000, it was
considered that ribotype BI/NAP1/027 did not represent a risk of
toxicity until the Quebec epidemic. Subsequently, several US
hospitals reported outbreaks of CDI ribotype BI/NAP1/027, with
fulminant clinical symptoms (2).
The BI/NAP1/027 ribotype has certain unique characteristics that
could explain why the severity associated with this strain has
increased (3). From the ribotype
point of view, CDI is a real ‘chameleon’ of infectious pathology;
however, unfortunately, the overall power of knowledge about
circulating toxins in different sections in the Western part of
Romania today is an enigma due to the additional cost of the
specificity test.
Another essential problem in this pathology is the
fact that the C. difficile strain carries several mobile
genetic elements (4), which are
able to confer resistance genes to antibacterial medication. The
use of broad-spectrum and ultra-broad-spectrum antibiotics has
allowed the development of hypervirulent strains with a rate of
therapeutic failure, mortality and recurrence. Generally, most
circulating strains are sensitive to metronidazole and vancomycin,
although antimicrobial susceptibility testing has shown, in several
international studies (5), lower
susceptibility to metronidazole in infection with CD 027/BI/NAP1
strains, as well as with ribotypes 106 and 001. Albeit the current
protocols used in our country recommend this type of therapy in
case of recurrence (6); the
importance of ribotyping and targeted antibiotic therapy based on
the antibiogram is thus emphasized once again.
A study conducted in 2013-2014, a collaboration
among the Clinical Hospital for Infectious Diseases of Timisoara,
the Laboratory of Microbiology, Timisoara County Emergency Clinical
Hospital and the Discipline of Epidemiology, ‘Victor Babeș’
University of Medicine and Pharmacy of Timisoara, also demonstrated
the prevalence of CDI endemicity in the western part of Romania,
with an incidence of 20.57/15.70 per 1,000 patients discharged in
2013/2014 or 17.73/14.04 per 10,000 patients per day. As a result
of this research, the incidence of the pathology could be estimated
and the risk factors were investigated in relapses and cases with
fulminant symptoms (7).
The objectives of this study were to determine the
incidence of C. difficile infection within the past few
years, to monitor bacterial toxin by ribotyping, to test
circulating toxins, to correlate the ribotyping with the clinical
form of the disease and to correlate the treatment with ribotyping
and clinical form.
Materials and methods
Study population
We performed an observational retrospective study
regarding the incidence of CDI at ‘Victor Babeș’ Hospital of
Infectious Diseases and Pneumophtisiology of Timisoara, between
January 2016 and December 2017.
Upon admission, the patients signed the standardized
informed consent by which they consented to their data being used
for research purposes. The study was approved by the Ethics
Committee of ‘Victor Babeș’ Hospital of Infectious Diseases and
Pneumophtisiology of Timisoara.
‘Victor Babeș’ Hospital of Infectious Diseases and
Pneumophtisiology of Timisoara is composed of two sections of
infectious diseases, with a case rate of 2,076 cases in 2016 and
2,048 cases in 2017 (patients over 18 years of age).
Methods
We only included laboratory confirmed cases in
patients aged 18 or older, subdivided in 6 age groups, regardless
of sex, personal history, and probable infection source nosocomial
or community acquired.
We collected demographic data, the presence of
various comorbidities (malignancy, diabetes, chronic renal failure,
cardiac, pulmonary, mild/moderate/severe liver pathology, or
peripheral vascular, cerebrovascular, hematological diseases,
dementia, gastro-duodenal ulcer, presence of concomitant
infections); possible causes of immunosuppression during the last 2
months prior to the onset (chemotherapy, radiotherapy,
corticosteroids, chronic dialysis, surgery including the type of
intervention); other risk factors (inflammatory bowel disease,
colorectal cancer, previous exposure to antimicrobial agents,
enteral/parenteral nutrition); and clinical data.
The stool samples were collected through regular
methods, using sterile recipients.
The etiology was confirmed by VIDAS®
C. difficile Toxin A & B (bioMérieux) test, an ELFA
(enzyme-linked fluorescent assay) that detects toxins A and B in
fresh stool samples with a sensitivity of 88.3% and a specificity
of 99.8%. Ribotyping was performed using C. difficile assay
polymerase chain reaction, which allowed the differentiation
between toxin B and binary toxin as well as the presumptive
detection of strain 027/NAP/B1 with a sensitivity of 93.49% and a
specificity of 94.02%.
Statistical analysis
Data analysis was performed using the Statistical
Package for Social Sciences (SPSS) v.25 (IBM Corp.). We presented
continuous variables as mean and standard deviation and categorical
variables as frequency and percentages. For the evaluation of the
potential connection between ribotype and clinical form of CDI, we
employed the χ2 test. In order to determine the
association between the treatment, clinical form and ribotype, a
regression model and the Hosmer-Lemeshow test was used. P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
Between January 2016 and December 2017, 210 patients
were hospitalized with a diagnosis of acute enterocolitis with
C. difficile. All patients tested showed C. difficile
toxin A/B positivity.
In 2016, 95 patients with the diagnosis of C.
difficile enterocolitis were hospitalized. If we refer to 2017,
we can see an increase in 20 hospitalized cases with the same
pathology, amounting to 115 reported cases (Table I).
 | Table IIncidence of Clostridium
difficile infection (CDI) at ‘Victor Babeș’ hospital of
infectious diseases and pneumophtisiology of Timisoara. |
Table I
Incidence of Clostridium
difficile infection (CDI) at ‘Victor Babeș’ hospital of
infectious diseases and pneumophtisiology of Timisoara.
| Year | Hospitalized
patients | Relapses | New cases | Total Clinic I +
Clinic II |
|---|
| 2016 | 95 | 8 | 87 | 2,076 |
| 2017 | 115 | 4 | 111 | 2,048 |
| Total | 210 | 12 | 198 | 4,124 |
The incidence of patients with C. difficile
in the period of 2016-2017 was Itotal=4.801164. In 2016,
the incidence was I2016=4.190751, and in 2017,
I2017=5.419922, there was an increase in the incidence
of CDI comparing the years 2016 and 2017.
In this study, we followed the evolution of 210
patients, aged between 28 and 90 years, mean age 67.99±17.50 years
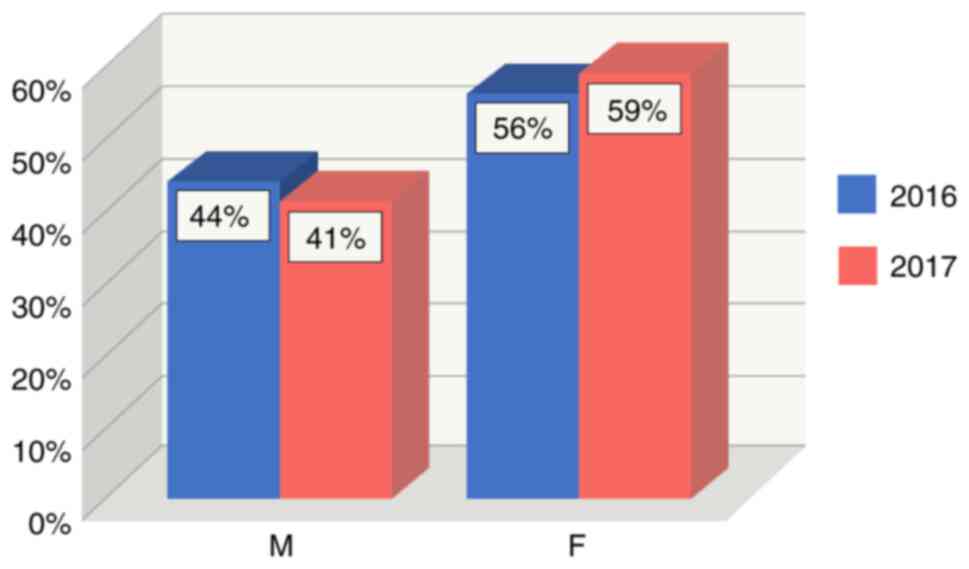
(P<0.0001). Regarding the distribution by sex, both in 2016 and
in 2017, the female sex predominated, with a total number of 121
cases, divided into 53 cases (56%), recorded in 2016 vs. 68 cases
(59%) in 2017 (P<0.0001). Conversely, the male sex maintained a
linear pattern with 42 cases (44%) reported in 2016 vs. 47 cases
(41%) in 2017 (P<0.0001) (Fig.
1).
Urban-rural distribution was found to have the same
increased incidence (59%) in urban areas (Utot) in 2016/2017, thus
reporting 124 cases out of the total (P=0.0091). Regarding the
rural environment (Rtot), it was found to have an equally
increasing trend, with a percentage of 41% of the cases (86
patients), explainable due to easy access to the urban health
system and the lack of territorial care systems (Table II).
 | Table IIDistribution by urban vs. rural origin
of the Clostridium difficile infection (CDI) cases for 2016
and 2017. |
Table II
Distribution by urban vs. rural origin
of the Clostridium difficile infection (CDI) cases for 2016
and 2017.
| Urban (U)
distribution | Utot= | 0.585714 | 59% | U2016= | 0.568421 | 57% | U2017= | 0.6 | 60% |
|---|
| Rural (R)
distribution | Rtot= | 0.414286 | 41% | R2016= | 0.431579 | 43% | R2017= | 0.4 | 40% |
The incidence of these infections was significantly
higher in the age group over 61 years (totaling over half of the
cases, P<0.0001), followed by adults (40-60 years, over 20% of
cases, P<0.0001). Although it was the prerogative of the study
to research the elderly in the period of the study, there were also
cases of young individuals under 40 (9.47% in 2016, 5.20% in 2017,
P<0.0001) (Table III).
 | Table IIIBreakdown of the incidence of
Clostridium difficile infection (CDI) by age group. |
Table III
Breakdown of the incidence of
Clostridium difficile infection (CDI) by age group.
| | Age group
(years) |
|---|
| Year | <40 (%) | (40-50) (%) | (50-60) (%) | (60-70) (%) | (70-80) (%) | (80-90) (%) |
|---|
| 2016 | 9.47 (9 cases) | 1.05 (1 case) | 11.58 (11 cases) | 17.90 (17 cases) | 42.10 (40 cases) | 17.90 (17 cases) |
| 2017 | 5.20 (6 cases) | 5.20 (6 cases) | 13.05 (15 cases) | 17.40 (20 cases) | 46.10 (53 cases) | 13.05 (15 cases) |
Because most hospital-acquired CDI cases occur in
elderly patients with an increased burden of chronic comorbidities
affecting multiple organs and systems (8), multimorbidity itself may play a
relevant role in defining CDI risk.
Comorbidity was well represented. Cardiovascular
diseases were found to be a primary comorbidity (over 60% of cases
reported in both years, P=0.0018), followed by renal pathology,
metabolic diseases (diabetes), chronic lung disease and dementia
(with a percentage of over 10% of cases in 2016, P<0.0001)
(Table IV).
 | Table IVComorbidities of the Clostridium
difficile infection (CDI) cases. |
Table IV
Comorbidities of the Clostridium
difficile infection (CDI) cases.
| | Year |
|---|
| Comorbidities | 2016 (%) | 2017 (%) | Total (%) |
|---|
| Acute renal
pathology | 17.89 (17 cases) | 29.57 (34 cases) | 47.46 (51 cases) |
| Vascular cerebral
pathology | 10.53 (10 cases) | 4.35 (5 cases) | 14.88 (15 cases) |
| Malignant
pathology | 10.53 (10 cases) | 10.43 (12 cases) | 20.96 (22 cases) |
| Diabetes | 28.42 (27
cases) | 21.74 (25
cases) | 50.16 (52
cases) |
| Chronic kidney
failure | 14.74 (14
cases) | 28.70 (33
cases) | 43.44 (47
cases) |
| Chronic heart
disease | 60.00 (57
cases) | 65.22 (75
cases) | 125.22 (132
cases) |
| Peripheral vascular
disease | 5.26 (5 cases) | 5.22 (6 cases) | 10.48 (11
cases) |
| Chronic lung
disease | 13.68 (13
cases) | 6.96 (8 cases) | 20.64 (21
cases) |
| Medium/severe
hepatic pathology | 7.37 (7 cases) | 9.57 (11
cases) | 16.94 (18
cases) |
| Dementia | 12.63 (12
cases) | 8.70 (10
cases) | 21.33 (22
cases) |
Because elderly patients are currently the ones who
most frequently develop CDI, further studies are needed in order to
explore the association between this infection and the areas of
comorbidity, deficiencies and polymedication, which are intrinsic
features of hospital-admitted geriatric patients.
The comorbidity score, Cumulative Illness Rating
Scale (CIRS) can be a useful tool in CDI to estimate this
additional risk, especially in patients undergoing long-term
antibiotic treatment (9).
Until recently, septic shock was considered to be
composed of three components: systemic hypotension, tissue
hypoperfusion associated with organ dysfunction and hyperlactatemia
(10,11).
According to the new definition, septic shock can be
diagnosed in two conditions. The first condition is persistent
hypotension after fluid correction; it requires vasopressors to
maintain mean blood pressure >65 mmHg (12). The second condition is the level of
serum lactate >2 mmol/l. Since heart rate, respiratory rate and
other laboratory data are not included, the diagnosis and
recognition of septic shock have become simplified (13). This very new definition implies that
elevated serum lactate levels may represent tissue hypoperfusion
associated with signs of organ dysfunction in critically ill
patients (14). In addition, it
should be noted that the serum lactate level was decreased from 4
to 2 mmol/l.
The level of serum lactate as a clinical tool was
described about half a century ago by Broder and Weil (15). At that time, a serum lactate level
>4 mmol/l was associated with shock. Because serum lactate
levels have been reduced to 2 mmol/l, serum lactate levels are a
more sensitive marker for septic shock.
Lactate is an important source of energy, especially
during diet (when the body has no other resources). Therefore, when
lactate is not produced, humans cannot survive. Lactate also
contributes to the acidic environment by converting to lactic acid.
Next, lactate is converted to bicarbonate, and it becomes a major
source of alkalization of the body under normal conditions. Lactate
of 1.4-1.5 mmol/day consists of the reduction of pyruvate, which is
largely generated by anaerobic glycolysis. In tissue hypoxia,
lactate is overproduced by increasing anaerobic glycolysis
(16). Usually, lactic acid
clearance occurs in the liver (60%), followed by the kidneys (30%)
and to a lesser extent by other organs (heart and skeletal
muscle).
Lactic acid clearance cannot exceed lactate
production and can be altered during the critical state. Septic
shock with liver dysfunction and acute kidney damage increase
lactate levels due to decreased clearance.
Some patients recovering from septic shock have
normal serum lactate values, although vasopressors are still needed
to maintain an average blood pressure of 65 mmHg or higher
(17). In addition, low or
normalized lactate levels are important signs of recovery from
septic shock.
Within the case study in hospitalized patients in
the period of 2016-2017, an increased prevalence of lactic acid was
observed. A total of 105 cases (prevalence 0.5) showed a lactic
acid value between 2-5 mmol/l. Nine cases (prevalence 0.04) out of
the 201 presented a lactic acid with a value >5 mmol/l (Table V).
 | Table VLactic acid levels in the
Clostridium difficile infection (CDI) cases. |
Table V
Lactic acid levels in the
Clostridium difficile infection (CDI) cases.
| | Lactic acid |
|---|
| Year | <2 mmol/l | 2-5 mmol/l | >5 mmol/l |
|---|
| 2016 | 0.463157895 | 0.515789474 | 0.021052632 |
| 2017 | 0.452173913 | 0.486956522 | 0.060869565 |
| Total | 0.457142857 | 0.500000000 | 0.042857143 |
If we correlate the ribotype with the clinical form
of the disease by applying the χ2 test to see the
association of the two variables, we obtained χ2=5.9
with n=2 degrees of freedom, and P-value=0.0522>0.05, which is
why no conclusion can be drawn regarding the association of the two
variables without the Cramer's correction V=0.167, which indicates
a weak association. In conclusion, it can be stated that the
severity of the disease depends on the form of the ribotype
(Table VI).
 | Table VIAssociation between disease form and
ribotype of the Clostridium difficile infection (CDI)
cases. |
Table VI
Association between disease form and
ribotype of the Clostridium difficile infection (CDI)
cases.
| Disease
form/Ribotype | Mild form | Average form | Severe form | Total |
|---|
| 0.27 | 16 | 8 | 4 | 28 |
| Other | 130 | 45 | 7 | 182 |
| Total | 146 | 53 | 11 | 210 |
In terms of treatment, metronidazole in the form of
oral tablets should be limited only to mild cases of the disease.
Oral vancomycin remains the recommended treatment for mild/moderate
forms that do not respond to metronidazole treatment. Repeated
courses of metronidazole should be avoided due to the increased
risk of potentially irreversible cumulative neurotoxicity.
Although metronidazole may be associated with more
common side effects and there has been a significant increase in
treatment failure (especially in patients infected with emerging
strain 027/BI/NAP1), oral metronidazole 500 three times a day for
10 days can be used to treat mild to moderate cases of CDI.
In current IDSA guidelines, metronidazole is used
only in mild cases in patients without comorbidities or the risk of
recurrence, as access to vancomycin or fidaxomicin is limited.
In a study published in 2015, a meta-analysis and
systematic review was performed, comparing the efficacy and safety
of metronidazole monotherapy with vancomycin monotherapy and
combination therapy in patients with CDI. No statistically
significant differences were found in the clinical cure rate
between metronidazole and vancomycin for mild CDI form (4) or between monotherapy and combination
therapy for CDI; however, the clinical cure rate was lower for
metronidazole than for vancomycin for severe CDI.
In the present study, applying the χ2
test to see the association of the two variables (ribotype and
treatment), we obtained χ2=1.55 with n=2 degrees of
freedom, and P=0.46>0.05, so the two variables are independent.
The conclusion being that there is no association between the two
variables (Table VII).
 | Table VIIAssociation between ribotype and
treatment of the Clostridium difficile infection (CDI)
cases. |
Table VII
Association between ribotype and
treatment of the Clostridium difficile infection (CDI)
cases.
|
Treatment/Ribotype | Metronidazole | Vancomycine | Metronidazole +
vancomycine | Total |
|---|
| 0.27 | 15 | 8 | 5 | 28 |
| Other | 115 | 34 | 33 | 182 |
| Total | 130 | 42 | 38 | 210 |
In order to determine the association between the 3
variables (treatment, clinical form and ribotype), the regression
model was used, resulting in: logit(p)=-2.34-0.33x treatment +0.68x
form.
The Hosmer-Lemeshow test shows that the logistics
model is the right one (P=0.6859>0.05) and that 86.67% of the
cases were accurately predicted (Table VIII).
 | Table VIIIAssociation of the ribotype with the
clinical form of disease and treatment of the Clostridium
difficile infection (CDI) cases. |
Table VIII
Association of the ribotype with the
clinical form of disease and treatment of the Clostridium
difficile infection (CDI) cases.
| | Ribotype=0 | Ribotype=1 |
|---|
| Form/Treatment | Metronidazole | Vancomycine | Metronidazole +
vancomycine | Metronidazole | Vancomycine | Metronidazole +
vancomycine |
|---|
| Mild | 95 | 21 | 14 | 13 | 2 | 0 |
| Average | 18 | 10 | 17 | 1 | 5 | 2 |
| Severe | 2 | 3 | 2 | 2 | 1 | 2 |
Discussion
The etiology of infectious diarrhea is very varied,
involving numerous pathogenic germs of bacterial, viral, parasitic,
and fungal origin. The higher frequency represented by intestinal
bacteriosis in the official statistics in our country does not
represent the real incidence of the etiologies involved, but rather
the concerns and limits of the activity of the specialized
laboratory, especially for determining these bacterial
etiologies.
The endemic nature of CDI reported in recent years
cannot exclude the presence of deficiencies in the development of
the epidemiological surveillance process of the hospital. In the
presence of diseases with potential to induce secondary
immunodeficiencies, the appropriate therapeutic act for the disease
in question must be accompanied by the application of measures
aimed at limiting nosocomial infections, in our case of
Clostridium infection.
A study published in 2015 reported the cases of CDIs
in our hospital. The incidence of infections in 2013-2014 was
20.57/15.70 per 1,000 patients discharged. If we compare it with
current data, we can see an increase in the incidence of about 2%
per hospitalized patient. This seeks to highlight once again the
importance of the emergence of C. difficile infections
(7).
Age is considered a primary risk factor for common
forms of CDI (especially in young people, there are fewer
relapses), but also for severe forms.
A feature that deserves a more detailed analysis is
the role of general health condition, including dietary deficiency
(hypoproteinemia) and the risk of CDI. Dietary deficiency
(hypoproteinemia), the expression of biological aging, increases
susceptibility to a variety of adverse events. Deficiency also
results in increased exposure to health systems, including
encounters with emergency departments, hospitalization and
institutionalization, which has led to increased exposure to
antibiotics, the most important risk factor for CDI.
The clinical picture of CDI causes a wide range of
clinical manifestations, ranging from asymptomatic colonization of
the gastrointestinal tract to diarrhea and colitis, which has the
potential to progress to severe forms with sepsis, hemodynamic
instability, and toxic dilation of the colon. This evolutionary
method is associated with an increased risk of spontaneous
perforation. A review of existing observational data has shown that
3% of patients with CDI will progress to fulminant colitis, which
is associated with an 80% mortality rate.
Knowing the many factors that can contribute to the
increased incidence of C. difficile infections in hospitals,
it is necessary to intensify active surveillance, to promote the
proper use of protective equipment and disinfection techniques, to
ensure isolation spaces, to conduct visitor traffic control, to
review antibiotic use policy, and to monitor measures undertaken in
the case of ‘serial’ diseases.
Acknowledgements
Professional editing, linguistic and technical
assistance performed by Irina Radu, Individual Service
Provider.
Funding
No funding was received.
Availability of data and materials
All data and materials supporting the results of the
present study are available in the published article.
Authors' contributions
ARM and ML designed the study. VL, RL, VM, NN, CD
and CO consulted the literature and collected the bibliographical
data. ARM and ML wrote the paper. TGC and VL reviewed the data and
edited the manuscript. All authors read and approved the final
manuscript for publication.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
‘Victor Babeș’ Hospital of Infectious Diseases and
Pneumophtisiology of Timisoara, Romania (approval number
4536/2021).
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
Authors' information
ARM: MD PhD student, Infectious Disease Physician at
Clinical ‘Dr. Victor Babeș’ Hospital for Infectious Diseases,
Timisoara, Romania; PhD student at ‘Victor Babeș’ University of
Medicine and Pharmacy. VL: MD PhD, Senior Lecturer of Infectious
Diseases, Assistant Professor at the Infectious Diseases II
Department, Member of the Advisory Commission of Infectious
Diseases of the Ministry of Health, Romania. RL: MD PhD, Senior
Lecturer of Infectious Diseases, Assistant Professor at the
Infectious Diseases II Department of ‘Victor Babeş’ University of
Medicine and Pharmacy, Timisoara, Romania. VM: MD PhD, Senior
Lecturer of Infectious Diseases, Assistant Professor at the
Infectious Diseases II Department of ‘Victor Babeş’ University of
Medicine and Pharmacy, Timisoara, Romania. NN: MD PhD, Senior
Lecturer of Infectious Diseases, Assistant Professor at the
Infectious Diseases II Department of ‘Victor Babeş’ University of
Medicine and Pharmacy, Timisoara, Romania. TGC: MD PhD student,
Infectious Disease Physician, Clinical Hospital for Infectious
Diseases ‘Dr. Victor Babeș’ Timisoara, Romania, PhD student at
‘Victor Babeş’ University of Medicine and Pharmacy. CD: CF PhD,
Professor at the Toxicology and Drug Industry Department of ‘Victor
Babeş’ University of Medicine and Pharmacy CO: MD PhD, Professor at
the Pneumology Department of ‘Victor Babeş’ University of Medicine
and Pharmacy. ML: MD PhD, Professor at the Microbiology Department
of ‘Victor Babeş’ University of Medicine and Pharmacy.
References
|
1
|
CDC: Antibiotic Resistance Threats in the
United States 2019. CDC. 2019. Updated December 2019. http://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf.
Accessed September 30, 2020.
|
|
2
|
Culligan EP and Sleator RD: Advances in
the microbiome: Applications to Clostridium difficile
infection. J Clin Med. 5(83)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Guh AY and Kutty PK: Clostridioides
difficile infection. Ann Intern Med. 169:ITC49–ITC64.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
McDonald LC, Gerding DN, Johnson S, Bakken
JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly
C, et al: Clinical practice guidelines for Clostridium
difficile infection in adults and children: 2017 update by the
infectious diseases society of America (IDSA) and society for
healthcare epidemiology of America (SHEA). Clin Infect Dis.
66:e1–e48. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jump RLP and Donskey CJ: Clostridium
difficile in the long-term care facility: Prevention and
management. Curr Geriatr Rep. 4:60–69. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Willems RPJ, van Dijk K, Ket JCF and
Vandenbroucke-Grauls CMJE: Evaluation of the association between
gastric acid suppression and risk of intestinal colonization with
multidrug-resistant microorganisms: A systematic review and
meta-analysis. JAMA Intern Med. 180:561–571. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Laza R, Jurac R, Crişan A, Lăzureanu V,
Licker M, Popovici ED and Bădiţoiu LM: Clostridium difficile
in western Romania: Unfavourable outcome predictors in a hospital
for infectious diseases. BMC Infect Dis. 15(141)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Song JH and Kim YS: Recurrent
Clostridium difficile infection: Risk factors, treatment,
and prevention. Gut Liver. 13:16–24. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Knafl D, Vossen MG, Gerges C, Lobmeyr E,
Karolyi M, Wagner L and Thalhammer F: Hypoalbuminemia as predictor
of recurrence of Clostridium difficile infection. Wien Klin
Wochenschr. 131:68–74. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wei Y, Yang F, Wu Q, Gao J, Liu W, Liu C,
Guo X, Suwal S, Kou Y, Zhang B, et al: Protective effects of
bifidobacterial strains against toxigenic Clostridium
difficile. Front Microbiol. 9(888)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maisa A, Ross G, Verlander NQ, Fairley D,
Bradley DT and Patterson L: Comparing the epidemiology of
community- and hospital-associated Clostridium difficile
infections in Northern Ireland, 2012-2016: A population data
linkage and case-case study. Epidemiol Infect.
147(e141)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dharbhamulla N, Abdelhady A, Domadia M,
Patel S, Gaughan J and Roy S: Risk factors associated with
recurrent Clostridium difficile infection. J Clin Med Res.
11:1–6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Appaneal HJ, Caffrey AR, Beganovic M,
Avramovic S and LaPlante KL: Predictors of clostridioides difficile
recurrence across a national cohort of veterans in outpatient,
acute, and long-term care settings. Am J Health Syst Pharm.
76:581–590. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chalhoub V, Kallab R, El Hajj A, Hachem K
and Yazbeck P: Septic shock due to Clostridium tertium in an
immunocompetent patient following colitis without inflammatory
bowel disease. Anaesth Crit Care Pain Med. 35:167–168.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Linsenmeyer K, O'Brien W, Brecher SM,
Strymish J, Rochman A, Itani K and Gupta K: Clostridium
difficile screening for colonization during an outbreak
setting. Clin Infect Dis. 67:1912–1914. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Magill SS, Edwards JR, Bamberg W, Beldavs
ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod
L, Nadle J, et al: Multistate point-prevalence survey of health
care-associated infections. N Engl J Med. 370:1198–1208.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Di Bella S, Ascenzi P, Siarakas S,
Petrosillo N and di Masi A: Clostridium difficile toxins A
and B: Insights into pathogenic properties and extraintestinal
effects. Toxins (Basel). 8(134)2016.PubMed/NCBI View Article : Google Scholar
|