Introduction
The hypothalamic-pituitary-gonadal axis is one of
the main systems regulating mammalian reproductive function,
including in humans (1).
Hypothalamic secretion of gonadotropin-releasing hormone (GnRH) can
stimulate the anterior pituitary gland to secrete gonadotropin,
which can act on the gonads to stimulate the synthesis of steroid
hormones, and promote sperm and egg production (2,3). In
the early 21st century, researchers identified two new
neuropeptides that could directly regulate the synthesis and
secretion of GnRH; gonadotropin-inhibitory hormone (GnIH) was
identified in 2000, and was revealed to inhibit the synthesis and
secretion of GnRH in the hypothalamus of mammals and birds by
acting on its receptor, neuropeptide FF receptor (NPFFR)1(4). Mammalian RFRP-1 and RFRP-3 are
homologs of poultry GnIH, and are key neuropeptides that regulate
the reproductive function in vertebrates (5). Whereas kisspeptin is a neuropeptide
encoded by the Kiss1 gene that was revealed to stimulate the
synthesis and secretion of GnRH by acting directly on the KISS1
receptor (KISS1R) of GnRH neurons (6,7).
Previous studies have shown that these two neuropeptides may act on
the reproductive axis via unilateral or bilateral linkage (8,9);
however, to the best of our knowledge, the specific mechanism has
not been clarified. It has been shown that RFRP-3 (mammalian
homolog of GnIH) may be involved in the inhibition of kisspeptin
via the calcium or protein kinase C-related signaling pathways
(10), but there is currently no
direct evidence that RFRP-3 can directly act on kisspeptin
neurons.
The association between RFRP-3 and kisspeptin, as
well as their role and mechanism in the hypothalamic-pituitary
reproductive axis, were investigated in the present study. An
ovariectomized estrogen-primed (OEP) rat model was first
established and RFRP-3 was microinjected into the lateral
ventricle. Co-localization of RFRP-3 and kisspeptin was assessed
using laser confocal microscopy. Direct binding between RFRP-3 and
kisspeptin was evaluated using surface plasmon resonance (SPR). The
results indicated that there may be a direct interaction between
RFRP-3 and kisspeptin neuropeptides in the hypothalamus of OEP
rats, providing experimental evidence for the further study of the
regulatory mechanism of the neuroendocrine reproductive axis.
Materials and methods
Animals
A total of 53 specific pathogen-free grade female
Sprague Dawley (SD) rats (age, 6-7 weeks; weight, 200±20 g) were
purchased from Beijing Huafukang Biotechnology Co., Ltd.
(certificate no. 11401300067446). The rats were fed in a
well-ventilated environment, at a constant temperature (22-25˚C),
humidity (50-54%) and a natural 12/12-h light cycle and free access
to food and water. All animal experiments were conducted according
to the ethical guidelines of Chengde Medical University and were
approved by the ethical review board of Chengde Medical University
(Chengde, China).
Animal treatment and grouping
The SD rats were fed adaptively for 1 week, then
bilateral oophorectomy was performed under sterile conditions, with
animals anesthetized with 0.5% sodium pentobarbital (40 mg/kg). On
day 15 following surgery, 17β-estradiol (0.005 mg/kg/day) was
subcutaneously injected into the abdomen. After 5 days of
continuous injection, the rats (n=48) were randomly divided into
four groups: The 60-min group (n=12); the 120-min group (n=12); the
240-min group (n=12); and the 360-min group (n=12). Each group
included a saline control subgroup (n=6) and a RFRP3 subgroup
(n=6). Under anesthesia with 0.5% sodium pentobarbital (40 mg/kg),
the RFRP3 subgroups were injected with 2 µg/µl freshly prepared
RFRP3 (Bachem AG) into the lateral ventricle at a dose of 16 µl/kg,
while the saline control subgroups were injected with an equal
volume of normal saline. At 60, 120, 240 and 360 min after
injection, the rats were anesthetized with an intraperitoneal
injection of 0.5% sodium pentobarbital (40 mg/kg), samples were
collected and rats were sacrificed by cervical dislocation. The
humane endpoints used to determine when animals should be
euthanized were reduced heart and respiration rates. Cardiac and
respiratory arrest was observed for 2-3 min to confirm animal
death, and this was defined by the lack of spontaneous breathing
for 2-3 min, without blink reflex.
Thionine staining
For rats that did not receive lateral ventricle
injection (n=5), after 500 ml 4% paraformaldehyde solution was
perfused to fix the tissues, the brain tissues were collected. The
brain tissues were fixed with 4% paraformaldehyde for 24 h, then
dehydrated in 30% sucrose solution for 48 h at 4˚C. The tissues
were embedded in paraffin and sliced into continuous 20-µm thick
sections-. The brain slices were marked in order and stored at
-20˚C for use. The brain slices with odd numbers were selected for
thionine staining. Briefly, at room temperature, the brain sections
were successively incubated with 100% ethanol twice for 3 min each
time, 95% ethanol for 3 min, 70% ethanol for 3 min, 50% ethanol for
3 min, ultrapure water for 2 min, 0.25% thionine solution for 3
min, ultrapure water for 2 sec, 50% ethanol for 3 min, 70% ethanol
+ 1% acetic acid for 5 sec, 95% ethanol for 3 min, 100% ethanol
twice for 3 min each time and xylene for 5 min. Finally, the
sections were mounted and the posterior medial nucleus of the
hypothalamus was determined under a light microscope
(magnification, x400).
Double immunofluorescence
labeling
For double immunofluorescence labeling, rats without
lateral ventricle injection (n=5) were used. According to the
results from thionine staining, the posterior medial nucleus
sections of the hypothalamus were selected. The sections were
washed with 0.02% PBS twice for 10 min each time, blocked with 5%
donkey serum (Beijing Solarbio Science & Technology Co., Ltd.)
for 2 h at 4˚C, washed with 0.01% PBS-Tween-20% (PBST) for 5 min,
and then incubated with goat anti-rat RFRP3 (1:25; cat. no.
sc-32380) and rabbit anti-rat kisspeptin (1:50; cat. no. sc-15400;
subtype of kisspeptin not available; both from Santa Cruz
Biotechnology, Inc.) polyclonal antibodies for 48 h at 4˚C. The
sections were washed with 0.01% PBST, incubated with the Alexa
Fluor® 568-labeled donkey anti-goat (cat. no. A11057)
and Alexa Fluor 488-labeled donkey anti-rabbit (1:500; cat. no.
A21206) secondary antibodies for RFRP-3 and kisspeptin,
respectively (both from Thermo Fisher Scientific, Inc.) for 2 h at
4˚C in the dark, then washed with 0.01% PBST again. DAPI (cat. no.
BS130A; Biosharp Life Sciences) was added and the samples were
incubated for 5 min at room temperature in the dark. After washing,
the sections were mounted and observed with a laser confocal
microscope (magnification, x400; TCS SP8X; Leica AG).
ELISA
At 60, 120, 240 and 360 min after injection with
RFRP-3, venous blood was collected from the rats of each group.
After centrifugation at (3,000 x g) for 15 min at 4˚C, the serum
was isolated. The concentrations of GnRH, follicle-stimulating
hormone (FSH) and luteinizing hormone (LH) in the rat serum were
determined with rat GnRH (cat. no. ml003038; Shanghai Enzyme-linked
Biotechnology Co., Ltd.), rat FSH (cat. no. E-EL-R0391C;
Elabscience Biotechnology, Inc.) and rat LH ELISA kits (cat. no.
E-EL-R0026C; Elabscience Biotechnology, Inc.).
Western blot analysis
The fresh hypothalamic tissues were separated and
the total proteins were extracted using RIPA lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd.). The BCA method was
used for protein quantification (Beijing Solarbio Science and
Technology Co., Ltd.). Proteins were separated via 12% SDS-PAGE (20
µg per lane) and transferred onto a PVDF membrane (MilliporeSigma).
After blocking with 5% skimmed milk for 2 h at room temperature,
rabbit anti-rat kisspeptin polyclonal (1:50; cat. no. sc-15400;
subtype of kisspeptin not available; Santa Cruz Biotechnology,
Inc.) and anti-GAPDH antibodies (1:8,000; cat. no. AP0063; Bioworld
Technology, Inc.) were added and the membranes were incubated
overnight at 4˚C. After washing with 2.5% TBS-Tween 20 three times,
the goat anti-rabbit IgG HRP-conjugated secondary antibody
(1:80,000; cat. no. E030120; Beijing Merida Technology Co., Ltd.)
was added. After incubation for 1 h at room temperature, the
membrane was washed three times and then analyzed using the
Chemiluminescence image analysis kit (Tanon Science &
Technology Co., Ltd.). Image J analysis software (version 1.46r;
National Institutes of Health) was used to analyze the average gray
value.
SPR
The interaction between kisspeptin and RFRP3 was
analyzed using a Biacore T200 SPR system (Cytiva). Kisspeptin (cat.
no. CFB170220017; Shanghai Qiang Yao Biotechnology Co., Ltd.) was
diluted to 20 µg/ml with sodium acetate buffer (pH 5.5) and coupled
to a CM5 sensor chip with a coupling amount of 5,720 response units
(RU). PBS-P (10 mM phosphate buffer with 2.7 mM KCl, 137 mM NaCl
and 0.05% surfactant P20, pH 4.5) was used as the running buffer
and RFRP3 (cat. no. H-5846; Bachem AG) was diluted to five
concentrations (3.125, 6.25, 12.5, 25 and 50 µM). Different
concentrations of RFRP-3 were injected into the detection channel
and the reference channel to perform the binding reaction at 4˚C
for 3 min. The experiment was performed by selecting the ‘Kinetics’
program. The samples were analyzed with an injection time of 60
sec, a flow rate of 15 µl/min and a dissociation time of 15 min.
After each concentration cycle, the chip was regenerated with
glycine-HCl (pH 2.0) at a concentration of 10 mM for 30 sec. After
the end of the cycle, the obtained curve was subjected to data
processing using Biacore Evaluation Software (T200 version 2.0;
Cytiva) to obtain the kinetic parameters.
Statistical analysis
Data analysis was performed using SPSS v19.0
software (IBM Corp.). The results are presented as the mean ±
standard deviation. Independent-samples t-tests were used to
analyze differences between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
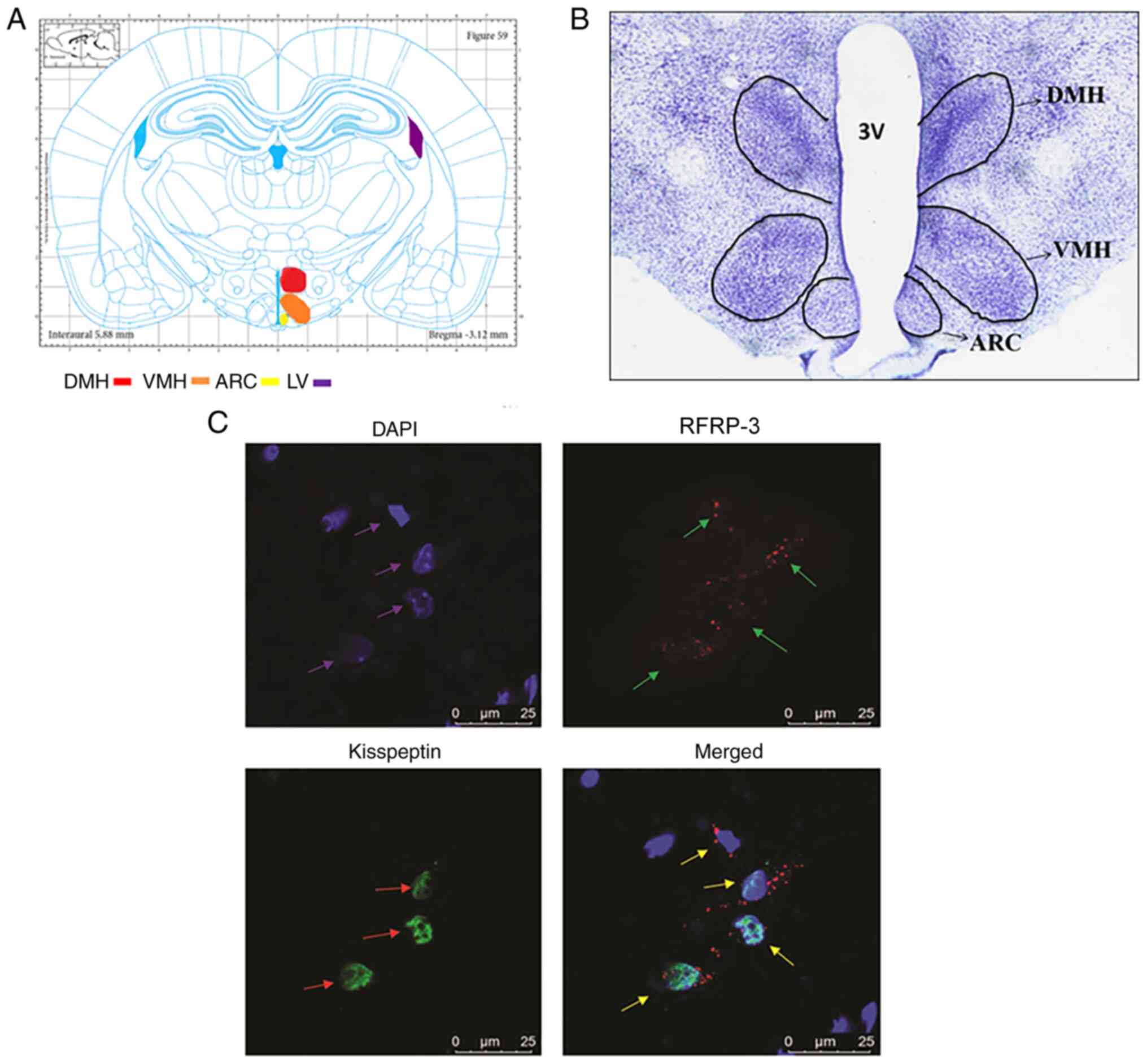
Kisspeptin and RFRP3 are co-expressed
in the nerve cells of the hypothalamus
To observe the localization of kisspeptin and RFRP-3
in the nerve cells of the hypothalamus, thionine staining and
immunofluorescence labeling was performed. After thionine staining,
the structures of the brain tissues in the rats were observed to be
clear and intact with a light blue color. The nerve cells were
densely distributed and neatly arranged with clear nucleoli;
regions such as the medial dorsomedial nucleus of the hypothalamus
(DMH), ventromedial nucleus of the hypothalamus (VMH) and arcuate
nucleus (ARC) were clearly visible (Fig. 1A and B).
Double-labeling immunofluorescence was used to
determine the expression and localization of RFRP3 and kisspeptin.
It was observed that RFRP3 and kisspeptin were co-expressed in the
neurons of the hypothalamus, as determined using laser confocal
microscopy (Fig. 1C). RFRP3 showed
red fluorescence, which was mainly expressed in the cytoplasm of
the nerve cells, whereas kisspeptin was labeled green and mainly
expressed in the nucleus. In addition, the nuclei were stained with
DAPI and showed blue fluorescence. These results indicated that
kisspeptin and RFRP-3 were co-expressed in the nerve cells of the
hypothalamus.
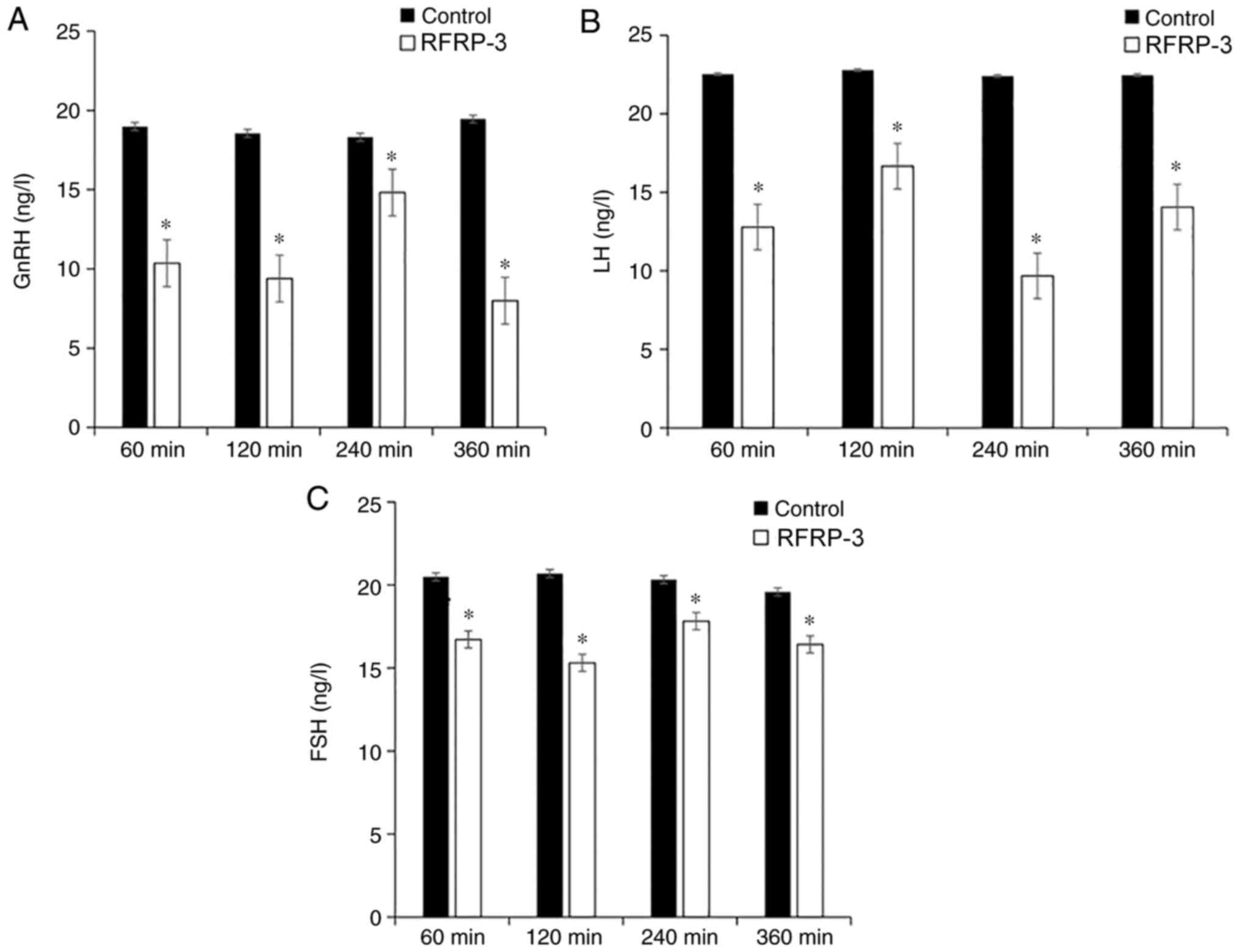
Reduction of GnRH, LH and FSH in the
serum by RFRP3
To determine the concentrations of GnRH, LH and FSH
in the serum, ELISA was performed (Fig.
2). The concentration of serum GnRH was 10.36±1.13, 9.39±1.15,
14.82±1.22 and 7.99±1.24 ng/l at 60, 120, 240 and 360 min after the
microinjection of RFRP3 into the lateral ventricle, respectively,
while the corresponding GnRH concentrations in the control groups
were 18.98±0.92, 18.55±0.91, 18.31±0.82 and 19.45±1.07 ng/l,
respectively. After the injection of RFRP3, the concentrations of
GnRH were significantly lower compared with in the control
subgroups (P<0.05; Fig. 2A).
The LH concentrations of the RFRP3 groups at the
four time points were 12.79±1.27, 16.67±1.18, 9.68±0.82 and
14.06±1.17 ng/l, while those in the control groups were 22.53±0.99,
22.79±0.76, 22.41±0.99 and 22.47±0.94 ng/l, respectively. The
concentrations of LH in the RFRP3 groups were significantly lower
compared with in the corresponding control groups (P<0.05;
Fig. 2B).
The FSH concentrations of the RFRP3 groups at the
four time points were 16.72±0.67, 15.31±1.12, 17.82±0.74 and
16.42±1.04 ng/l, while those in the saline control subgroups were
20.49±0.68, 20.69±0.75, 20.32±0.95 and 19.58±0.85 ng/l,
respectively. The concentrations of FSH in the RFRP3 groups were
significantly lower compared with in the corresponding control
groups (P<0.05; Fig. 2C). These
results indicated that microinjection of RFRP-3 into the lateral
ventricle inhibited the secretion of GnRH, LH and FSH in the serum
of OEP rats, and these three hormones exhibited similar trends at
the different time points.
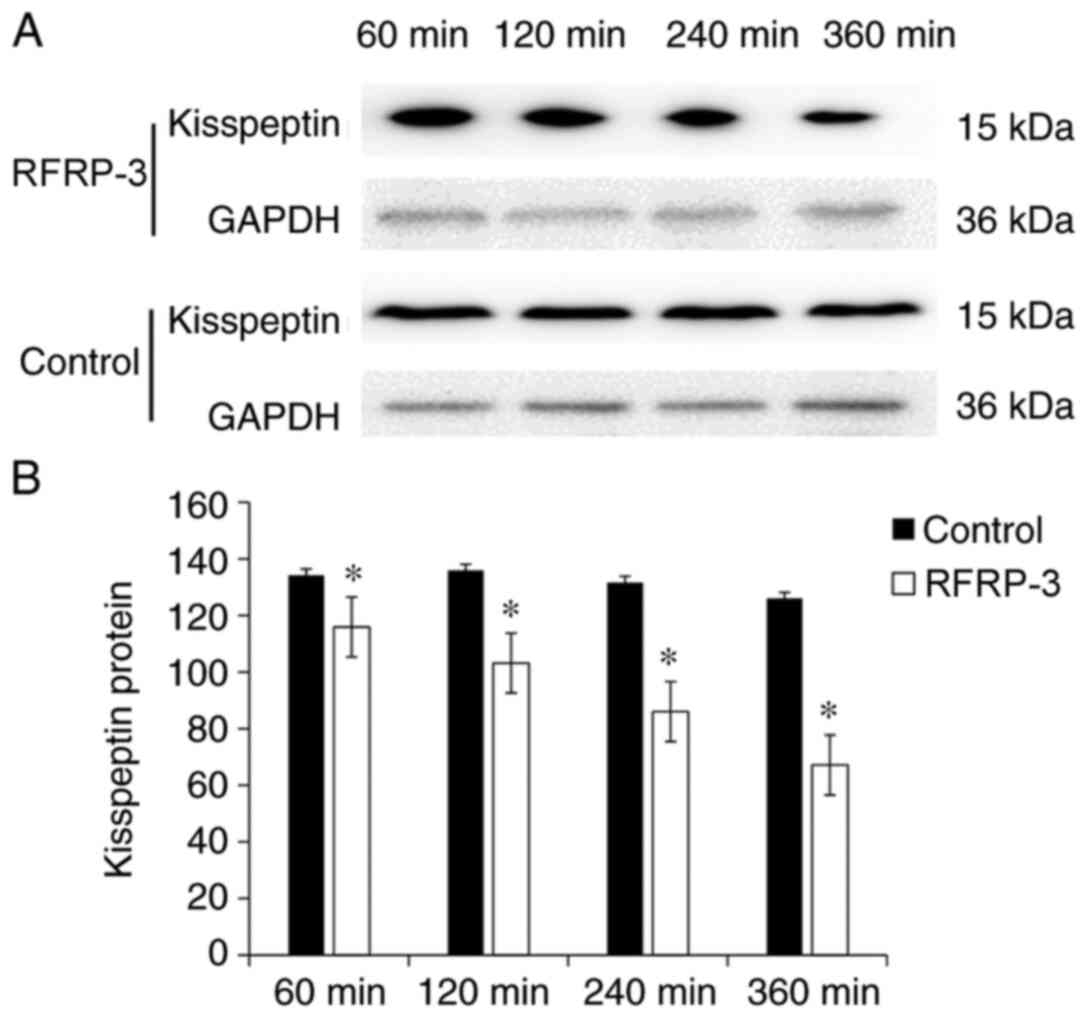
Inhibition of hypothalamic kisspeptin
by RFRP3
To demonstrate whether RFRP-3 had a regulatory
effect on kisspeptin in the hypothalamus, western blot analysis was
performed. The protein expression levels of kisspeptin were
presented as the gray value ratio of kisspeptin to GAPDH. At 60,
120, 240 and 360 min after the microinjection of RFRP3, the protein
expression levels of kisspeptin in the hypothalamus of the OEP rats
were 115.93±1.77, 103.17±1.85, 86.05±1.78 and 67.14±1.80,
respectively, while those in the corresponding control groups were
134.24±1.74, 135.94±1.74, 131.67±1.93 and 125.99±1.92, respectively
(Fig. 3). The expression levels of
kisspeptin in the hypothalamus of OEP rats after the RFRP3
microinjection were significantly different compared with in the
control groups (P<0.05; Fig. 3),
showing a time-dependent decrease.
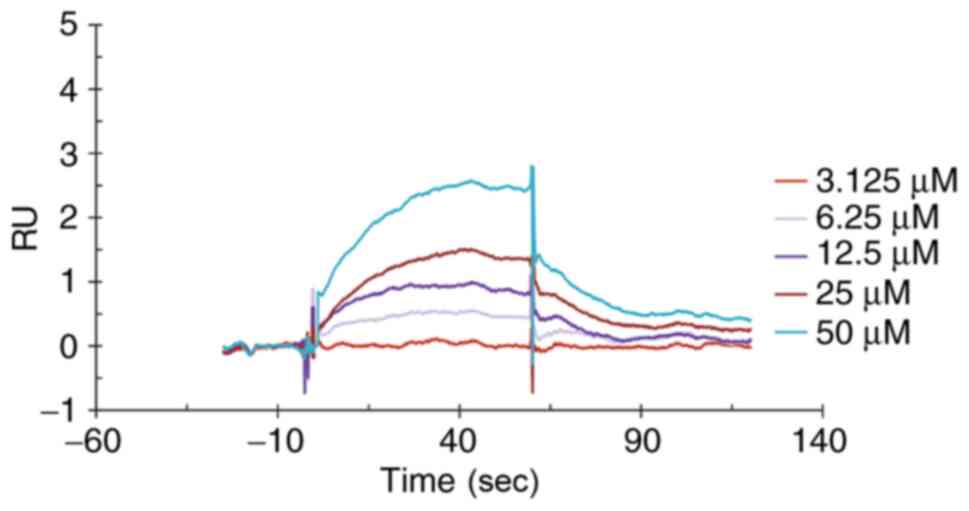
Interaction between RFRP3 and
kisspeptin
To demonstrate whether RFRP-3 binds directly to
kisspeptin, SPR was performed. The binding dissociation curve
corresponding to each concentration is shown in Fig. 4. The obtained curve was subjected to
data processing using Biacore T200 evaluation software. The
obtained binding rate constant, Ka, was
5.775x103 M-1 sec-1, the
dissociation rate constant, Kd, was
3.467x10-2 sec-1 and the affinity constant,
KD, was 6.005x10-5 M. These results indicated
that RFRP-3 could bind directly to kisspeptin.
Discussion
GnRH is recognized as the only hypothalamic
neuropeptide that regulates gonadotropin secretion in vertebrates,
which in turn stimulates the secretion of LH and FSH from the
anterior pituitary (2,3). The present study showed that RFRP-3
could be co-expressed with kisspeptin in the nerve cells of the
hypothalamus and that RFRP-3 could directly bind to kisspeptin.
Microinjection of RFRP-3 into the lateral ventricle inhibited the
concentrations of GnRH, LH and FSH in the serum, and decreased the
protein expression levels of kisspeptin in the hypothalamus. In
2000, Tsutsui et al (4)
identified a new hypothalamic neuropeptide that could inhibit the
secretion of LH and FSH in the brain of Japanese quail. The
sequence of this natural peptide contains 12 amino acids
(SIKPSAYLPLRFa), with a RF amide peptide at the carboxy terminus,
which was called GnIH (4). Since
then, several studies (5,9-18)
have found that GnIH is expressed in poultry, fish, amphibians and
vertebrate mammals, including humans, and that the structure is
highly conserved. Mammalian RFRP-1 and RFRP-3 are homologs of
poultry GnIH, and are key neuropeptides that regulate the
reproductive function in vertebrates (5). In previous years, numerous studies
(14,19,20)
have found that GnIH could regulate the feeding behavior of
vertebrates, in addition to regulating reproduction. GnIH nerve
fibers in sheep could be projected to neuropeptide Y,
pro-opiomelanocortin, orexin and melanin-concentrating hormone
neurons, which all play important roles in regulating feeding.
Anjum et al (21) reported
that the administration of GnIH in mice increased food intake,
increased the expression levels of glucose transporter 4 and
increased the synthesis of triglycerides in adipose tissue; in
addition, GnIH reduced glucose uptake by downregulating the
expression levels of glucose transporter 8 and reduced testosterone
synthesis, suggesting that GnIH plays a role in fat accumulation,
in addition to negative regulation of testosterone synthesis.
Mammalian GnIH neurons are mainly found in the periventricular
nucleus (PeN) of the hypothalamus, the DMH, and the area between
the DMH and the VMH (22). GnIH
directly inhibits the secretion and release of GnRH via the GnIH
receptor, NPFFR1, on the GnRH neurons, thereby inhibiting the
synthesis and secretion of gonadotropin (5,14).
Since only some GnRH neurons express NPFFR1, GnIH may regulate the
GnRH axis via other interneurons, such as kisspeptin neurons.
Studies have shown that RFRP-3 nerve fibers in female mice could be
projected onto the anteroventral periventricular nucleus (AVPV) and
the PeN of the kisspeptin neurons (23), and GnIH could also bind to NPFFR1
expressed by interneurons, such as the kisspeptin neurons, to
inhibit the activity of these neurons, thereby inhibiting the
function of the GnRH neurons (5).
Kisspeptin and its receptor, KISS1R, have a
stimulating effect on reproductive function (6). Adolescent hypogonadism occurs in
humans and rodents lacking functional kisspeptin or the kisspeptin
receptor gene, which is characterized by low levels of
gonadotropins and sex hormones, hypogonadism and infertility
(24-27).
Administration of exogenous kisspeptin can effectively promote the
secretion of LH and FSH via a GnRH-dependent mechanism (26,28-31).
Studies have shown that most GnRH neurons overexpress kisspeptin,
and kisspeptin nerve fibers can be projected to GnRH neurons, thus
directly activating GnRH neurons (26,29,32).
Kisspeptin is a potent stimulator of GnRH release; however, little
is known regarding the upstream pathway that regulates the
synthesis and secretion of kisspeptin. Kisspeptin is mainly
expressed in AVPV, PeN and ARC in the hypothalamus of rodents
(28,33). Poling et al (34) studied the co-expression of RFRP-3
receptors (NPFFR1 and NPFFR2) in adult male and female hypothalamic
kisspeptin neurons. They found that most of the kisspeptin AVPV/PeN
neurons did not express RFRP-3 receptors, while some of the ARC
kisspeptin neurons expressed the RFRP-3 receptor, suggesting that
RFRP-3 may regulate kisspeptin neurons in specific regions of the
brain. By contrast, almost all RFRP neurons did not express the
kisspeptin receptor and no kisspeptin axon fibers were projected to
RFRP-3 neurons, further suggesting that kisspeptin neurons could
not directly interact with RFRP-3 neurons (34).
Considering that RFRP-3 could act on kisspeptin
neurons to regulate GnRH neurons, it was hypothesized that RFRP-3
is an upstream factor of kisspeptin neurons, which could negatively
regulate kisspeptin to inhibit the release of GnRH. There are
several types of kisspeptin; however, the specific type of
kisspeptin was not distinguished in the present study.
Co-expression of RFRP-3 and kisspeptin was found in nerve cells in
the hypothalamus of male SD rats using double immunofluorescence
labeling, which differs from previous reports that RFRP3 and
kisspeptin are expressed in different nerve cells (5,23,33,34).
This suggested that RFRP-3 may have a direct effect on kisspeptin
in the same nerve cell, which provides a prerequisite for the
direct interaction between RFRP-3 and kisspeptin. Since the
expression of RFRP-3 was relatively low and the fluorescence
brightness was also relatively weak, the co-localized expression
was weak. In future studies, how the proteins directly interact
will be investigated, for example using co-immunoprecipitation.
To further demonstrate that RFRP3 has a direct
regulatory effect on kisspeptin, RFRP3 was microinjected into the
lateral ventricle of OEP rats. The results showed that the
concentrations of serum GnRH from the OEP rats were significantly
decreased after 60, 120, 240 and 360 min following RFRP3
microinjection into the lateral ventricle, in which the serum GnRH
concentration at 360 min was the lowest. As previously described
(35), RFRP-3 is released in pulses
instead of continuously. Therefore, it was hypothesized that the
change in GnRH concentration was associated with the time-dependent
effect of RFRPRFRP-3. The LH and FSH concentrations of each RFRP3
group were also reduced compared with in the control group. These
results demonstrated that RFRP3 was successfully injected into the
lateral ventricle and inhibited gonadotropin secretion. RFRP3 also
inhibited the protein expression levels of kisspeptin in the
hypothalamus. The distribution and expression patterns of RFRP-3
nerve fibers and their receptors suggested that RFRP-3 may directly
act at the pituitary level. A previous study has shown that RFRP-3
could inhibit the secretion of GnRH via its receptor on GnRH
neurons, thus acting on the pituitary system to inhibit the
expression and secretion of gonadotropin, LH and FSH (36). The experimental results in the
present study also confirmed this statement. As kisspeptin has a
positive regulatory effect on GnRH, and the microinjection of
RFRP-3 into the lateral ventricle inhibited the secretion of GnRH,
it is hypothesized that RFRP-3 may indirectly inhibit the secretion
of GnRH via the intermediate link of kisspeptin, thereby inhibiting
reproductive function.
As RFRP-3 and kisspeptin can be co-expressed in the
same nerve cells in the hypothalamus of SD rats, the possibility
that the two proteins could directly interact to exert their
regulatory effects cannot be excluded. To verify this hypothesis,
SPR was performed to detect the binding between the recombinant rat
RFRP-3 and the kisspeptin proteins (37,38).
RFRP-3 showed a binding and dissociation process with kisspeptin,
which could be analyzed by kinetic methods. A small affinity value,
indicating strong binding between these two proteins, was observed
with binding rate, dissociation rate and affinity constants of
5.775x103 M -1s-1,
3.467x10-2 s-1 and 6.005x10-5 M,
respectively. The affinity of RFRP-3 to kisspeptin was in the range
of protein-protein binding strength (KD,
10-3-10-6 M), indicating that there may be a
specific direct binding between the two proteins. Kisspeptin
expression was higher in the nucleus, whereas RFRP-3 expression was
higher in the cytoplasm. In the preliminary experiments, single
staining for kisspeptin was performed and the results showed that
there was kisspeptin expression in the cytoplasm. The SPR results
showed direct interaction between RFRP-3 and kisspeptin; however,
further experiments will be performed to indicate how they
interact.
In conclusion, the present study demonstrated that
RFRP-3 could co-express with kisspeptin in nerve cells in the
hypothalamus, and RFRP-3 could directly bind to kisspeptin. RFRP-3
may regulate the hypothalamic-pituitary reproductive axis by
inhibiting the expression of the hypothalamic kisspeptin protein.
However, the factors affecting neuronal secretion of RFRP-3 or
kisspeptin require further research. The present study provides
evidence for further understanding the regulatory mechanism of
RFRP-3 and kisspeptin in reproductive function and provides
potential targets for the treatment of reproductive dysfunction,
which could aid in exploring regulation of reproduction.
Acknowledgements
The authors would like to thank Ms Lei Chen
(Graduate School, Chengde Medical University, Chengde, China) and
Ms Xiaochao Liu (Graduate School, Chengde Medical University,
Chengde, China) for their assistance with performing animal
treatments.
Funding
The present study was supported by the open project of Key
Laboratory of Family Planning and Eugenics of National Health and
Family Planning Commission (grant no. 201502), the Natural Science
Foundation of Hebei Province (grant no. H2013406115), the Plan
Project of Hebei Provincial Science and Technology Department
(grant no. 08276101D-20) and the Hebei Higher Education Research
Project (grant no. QN2015121).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YQ and SW conceived and designed the study, and
funded and supervised the study. LC, SY and LS performed the
experiments, collected and analyzed data, and drafted the
manuscript. MW, SG, and ZC participated performing
experiments/acquiring data and manuscript revision. LC, SY and LS
confirmed the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical Review
Board of Chengde Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iwasa T, Matsuzaki T, Yano K, Mayila Y and
Irahara M: The roles of kisspeptin and gonadotropin inhibitory
hormone in stress-induced reproductive disorders. Endocr J.
65:133–140. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Burgus R, Butcher M, Amoss M, Ling N,
Monahan M, Rivier J, Fellows R, Blackwell R, Vale W and Guillemin
R: Primary structure of the ovine hypothalamic luteinizing
hormone-releasing factor (LRF) (LH-hypothalamus-LRF-gas
chromatography-mass spectrometry-decapeptide-Edman degradation).
Proc Natl Acad Sci USA. 69:278–282. 1972.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Matsuo H, Baba Y, Nair RM, Arimura A and
Schally AV: Structure of the porcine LH- and FSH-releasing hormone.
I. The proposed amino acid sequence. Biochem Biophys Res Commun.
43(1334)1971.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tsutsui K, Saigoh E, Ukena K, Teranishi H,
Fujisawa Y, Kikuchi M, Ishii S and Sharp PJ: A novel avian
hypothalamic peptide inhibiting gonadotropin release. Biochem
Biophys Res Commun. 275:661–667. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tsutsui K and Ubuka T: GnIH control of
feeding and reproductive behaviors. Front Endocrinol (Lausanne).
7(170)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Roa J, Navarro VM and Tena-Sempere M:
Kisspeptins in reproductive biology: Consensus knowledge and recent
developments. Biol Reprod. 85:650–660. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Terasaka T, Otsuka F, Tsukamoto N,
Nakamura E, Inagaki K, Toma K, Ogura-Ochi K, Glidewell-Kenney C,
Lawson MA and Makino H: Mutual interaction of kisspeptin, estrogen
and bone morphogenetic protein-4 activity in GnRH regulation by
GT1-7 cells. Mol Cell Endocrinol. 381:8–15. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Leon S and Tena-Sempere M: Dissecting the
roles of gonadotropin-inhibitory hormone in mammals: Studies using
pharmacological tools and genetically modified mouse models. Front
Endocrinol (Lausanne). 6(189)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Talbi R, Laran-Chich MP, Magoul R, El
Ouezzani S and Simonneaux V: Kisspeptin and RFRP-3 differentially
regulate food intake and metabolic neuropeptides in the female
desert jerboa. Sci Rep. 6(36057)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Son YL, Ubuka T and Tsutsui K: Molecular
mechanisms of gonadotropin-inhibitory hormone (GnIH) actions in
target cells and regulation of GnIH expression. Front Endocrinol
(Lausanne). 10(110)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka
T, Mason AO, Inoue K, Ukena K, Tsutsui K and Silver R:
Identification and characterization of a gonadotropin-inhibitory
system in the brains of mammals. Proc Natl Acad Sci USA.
103:2410–2415. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ubuka T, Son YL and Tsutsui K: Molecular,
cellular, morphological, physiological and behavioral aspects of
gonadotropin-inhibitory hormone. Gen Comp Endocrinol. 227:27–50.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tsutsui K: How to contribute to the
progress of neuroendocrinology: New insights from discovering novel
neuropeptides and neurosteroids regulating pituitary and brain
functions. Gen Comp Endocrinol. 227:3–15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tsutsui K, Ubuka T, You LS, Bentley GE and
Kriegsfeld LJ: Contribution of GnIH research to the progress of
reproductive neuroendocrinology. Front Endocrinol (Lausanne).
6(179)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tsutsui K and Ukena K: Hypothalamic
LPXRF-amide peptides in vertebrates: Identification, localization
and hypophysiotropic activity. Peptides. 27:1121–1129.
2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ubuka T, Morgan K, Pawson AJ, Osugi T,
Chowdhury VS, Minakata H, Tsutsui K, Millar RP and Bentley GE:
Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the
cognate receptor, GPR147 in the human hypothalamic pituitary axis.
PLoS One. 4(e8400)2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ukena K, Iwakoshi E, Minakata H and
Tsutsui K: A novel rat hypothalamic RFamide-related peptide
identified by immunoaffinity chromatography and mass spectrometry.
FEBS Lett. 512:255–258. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ukena K and Tsutsui K: A new member of the
hypothalamic RF-amide peptide family, LPXRF-amide peptides:
Structure, localization, and function. Mass Spectrom Rev.
24:469–486. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Clarke IJ, Smith JT, Henry BA, Oldfield
BJ, Stefanidis A, Millar RP, Sari IP, Chng K, Fabre-Nys C, Caraty
A, et al: Gonadotropin-inhibitory hormone is a hypothalamic peptide
that provides a molecular switch between reproduction and feeding.
Neuroendocrinology. 95:305–316. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kriegsfeld LJ, Ubuka T, Bentley GE and
Tsutsui K: Seasonal control of gonadotropin-inhibitory hormone
(GnIH) in birds and mammals. Front Neuroendocrinol. 37:65–75.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Anjum S, Krishna A and Tsutsui K: Possible
role of GnIH as a mediator between adiposity and impaired
testicular function. Front Endocrinol (Lausanne).
7(6)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ubuka T, Inoue K, Fukuda Y, Mizuno T,
Ukena K, Kriegsfeld LJ and Tsutsui K: Identification, expression,
and physiological functions of Siberian hamster
gonadotropin-inhibitory hormone. Endocrinology. 153:373–385.
2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rizwan MZ, Poling MC, Corr M, Cornes PA,
Augustine RA, Quennell JH, Kauffman AS and Anderson GM:
RFamide-related peptide-3 receptor gene expression in GnRH and
kisspeptin neurons and GnRH-dependent mechanism of action.
Endocrinology. 153:3770–3779. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
De Roux N, Genin E, Carel JC, Matsuda F,
Chaussain JL and Milgrom E: Hypogonadotropic hypogonadism due to
loss of function of the KiSS1-derived peptide receptor GPR54. Proc
Natl Acad Sci USA. 100:10972–10976. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Funes S, Hedrick JA, Vassileva G,
Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ and
Gustafson EL: The KiSS-1 receptor GPR54 is essential for the
development of the murine reproductive system. Biochem Biophys Res
Commun. 312:1357–1363. 2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Messager S, Chatzidaki EE, Ma D, Hendrick
AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB,
et al: Kisspeptin directly stimulates gonadotropin-releasing
hormone release via G protein-coupled receptor 54. Proc Natl Acad
Sci USA. 102:1761–1766. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Seminara SB, Messager S, Chatzidaki EE,
Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W,
Schwinof KM, Hendrick AG, et al: The GPR54 gene as a regulator of
puberty. N Engl J Med. 349:1614–1627. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gottsch ML, Cunningham MJ, Smith JT, Popa
SM, Acohido BV, Crowley WF, Seminara S, Clifton DK and Steiner RA:
A role for kisspeptins in the regulation of gonadotropin secretion
in the mouse. Endocrinology. 145:4073–4077. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Irwig MS, Fraley GS, Smith JT, Acohido BV,
Popa SM, Cunningham MJ, Gottsch ML, Clifton DK and Steiner RA:
Kisspeptin activation of gonadotropin releasing hormone neurons and
regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology.
80:264–272. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kinoshita M, Tsukamura H, Adachi S, Matsui
H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H
and Maeda K: Involvement of central metastin in the regulation of
preovulatory luteinizing hormone surge and estrous cyclicity in
female rats. Endocrinology. 146:4431–4436. 2005.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Navarro VM, Castellano JM,
Fernandez-Fernández R, Tovar S, Roa J, Mayen A, Barreiro ML,
Casanueva FF, Aguilar E, Dieguez C, et al: Effects of KiSS-1
peptide, the natural ligand of GPR54, on follicle-stimulating
hormone secretion in the rat. Endocrinology. 146:1689–1697.
2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yeo SH and Herbison AE: Projections of
arcuate nucleus and rostral periventricular kisspeptin neurons in
the adult female mouse brain. Endocrinology. 152:2387–2399.
2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Clarkson J and Herbison AE: Postnatal
development of kisspeptin neurons in mouse hypothalamus; sexual
dimorphism and projections to gonadotropin-releasing hormone
neurons. Endocrinology. 147:5817–5825. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Poling MC, Quennell JH, Anderson GM and
Kauffman AS: Kisspeptin neurones do not directly signal to RFRP-3
neurones but RFRP-3 may directly modulate a subset of hypothalamic
kisspeptin cells in mice. J Neuroendocrinol. 25:876–886.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Smith JT, Young IR, Veldhuis JD and Clarke
IJ: Gonadotropin-inhibitory hormone (GnIH) secretion into the ovine
hypophyseal portal system. Endocrinology. 153:3368–3375.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sari IP, Rao A, Smith JT, Tilbrook AJ and
Clarke IJ: Effect of RF-amide-related peptide-3 on luteinizing
hormone and follicle-stimulating hormone synthesis and secretion in
ovine pituitary gonadotropes. Endocrinology. 150:5549–5556.
2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhao Y, Tong RJ, Xia F and Peng Y: Current
status of optical fiber biosensor based on surface plasmon
resonance. Biosens Bioelectron. 142(111505)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xue JJ, Bai Y and Liu HW: Hybrid methods
of surface plasmon resonance coupled to mass spectrometry for
biomolecular interaction analysis. Anal Bioanal Chem.
411:3721–3729. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Paxinos G and Watson C: The Rat Brain in
Stereotaxic Coordinates. 6th edition. Elsevier, Amsterdam, Boston,
MA, 2009.
|