Introduction
Intracerebral hemorrhage (ICH) is the most
devastating subtype of stroke and exhibits a poor functional
prognosis and high mortality rate worldwide (1-3).
Clinically, patients with ICH, except those with severe symptoms
and surgical indications (Bleeding volume >30 ml; Glasgow Coma
index score ≥5), are usually administered conservative medical
treatments (4). Secondary brain
injury (SBI) following ICH serves an important role in the
deterioration of neurological function (5,6). The
mechanisms underlying ICH-induced SBI are complex and involve many
factors, such as destruction of the blood-brain barrier (BBB),
release of thrombin, toxicity from erythrocyte lysis and its
products, inflammatory reactions, immune-mediated damage and
excessive production of reactive oxygen species that are involved
in complement system (7). The
inflammatory response induced by ICH serves a key role in the
continuous development of SBI. For example, blood components
leaking into the brain lead to activation of immune cells, such as
microglia. This leads to the destruction of the BBB and
infiltration of peripheral blood leukocytes into the brain tissue,
resulting in the production of a large number of inflammatory
factors that induce brain edema, ultimately leading to dysfunction
and death of both neurons and glia (8-11).
Therefore, interventions that regulate ICH-induced inflammatory
responses are important for the treatment of ICH-induced brain
injury. Silent information regulator 1 (SIRT1), an
NAD+-dependent protein deacetylase, is involved in a
variety of disease processes, such as inflammation, cell death and
metabolism, by regulating targets via deacetylation (12). Evidence has shown that SIRT1 exerts
a neuroprotective effect following ICH (13). SIRT1 protects against ICH-induced
brain damage via inhibiting neuroinflammation by deacetylating
NF-κB/p65(14).
C1q/TNF-related proteins (CTRPs) are a newly
discovered and highly conserved family of adiponectin paralogs,
which comprises ≥15 members (CTRP1-15) (15). CTRPs are widely distributed and are
involved in regulating many physiological or pathological
processes, such as substance metabolism, vasodilation and
inflammatory reactions. CTRP3 is a member of this family that is
expressed by adipocytes, adipose stromal cells and other types of
cell and exhibits homology with the genomic structure of
adiponectin (16,17). Furthermore, CTRP3 serves an
important role in inflammation, metabolism, anti-apoptosis,
angiogenesis and cardioprotection (18). To the best of our knowledge,
however, at present, there is a lack of research regarding the
effects of CTRP3 on ICH. Thus, the aim of the present study was to
investigate the protective effects and mechanisms of CTRP3 in a rat
model of ICH.
Materials and methods
Animals
Adult male Sprague-Dawley (SD) rats (n=104; weight,
240±20 g; age, 12.5±0.1 weeks) were purchased from the Experimental
Animal Center of Southwest Medical University (Luzhou, China). All
animal studies were approved by the Biomedical Ethics Committee of
Southwest Medical University (approval no. 20210223-135). All
experimental procedures were in accordance with guidelines for the
care and use of laboratory animals of the National Institutes of
Health (19). Rats were placed in
standard cages and exposed to a 12/12-h light/dark cycle at a
constant room temperature of 24-26˚C and 60% indoor relative
humidity and were provided food and water ad libitum. For
euthanasia, rats were anesthetized by an intraperitoneal injection
of 3% pentobarbital sodium (30 mg/kg body weight). Once fully
anesthetized, rats were sacrificed by cervical dislocation and
death was confirmed by cessation of breathing and faded eye
color.
Experimental design
To investigate the effects of CTRP3 in an ICH model
established via autologous blood injection, rats were randomly
distributed into four groups: i) Sham surgery (Sham, n=33); ii) ICH
(n=31; 3 died); iii) ICH + null-vector control (Lenti.Null, n=33; 5
died) and iv) ICH + lentiviral CTRP3-overexpression (Lenti.CTRP3,
n=34; 5 died). Behavioral tests, measurement of brain water
content, evaluation of BBB permeability, western blotting and ELISA
were performed 3 days following ICH.
To determine whether the SIRT1 signaling pathway was
involved in CTRP3-induced neuroprotection, rats were randomized to
the following groups: ICH + Lenti.Null (n=8; 1 died), ICH +
Lenti.CTRP3 (n=22; 2 died), ICH + Lenti.CTRP3 + EX527 (SIRT1
inhibitor, n=22; 2 died) and ICH + vehicle (n=21; 1 died).
Lenti-CTRP3 was injected intraventricularly 14 days before ICH.
EX527 was injected intraventricularly 30 min before ICH. Modified
Garcia test, BBB integrity/permeability, brain edema and IL-1β, and
TNF-α levels were assessed 3 days after ICH.
ICH rat model
A rat model of striatal ICH was established by
injecting autologous blood into the rat right striatum, as
previously described (20). SD
rats were anesthetized by intraperitoneal injection of
pentobarbital sodium (66 mg/kg) (21). Then, rats were horizontally fixed
on the platform of a stereotaxic frame (David Kopf Instruments) in
the prone position. Autologous blood (50 µl), which was drawn from
the femoral artery, was injected into the striatum (0.2 mm
anterior, 3.0 mm laterally to the right of bregma and 5.8 mm deep
from the surface of the skull) via a microsyringe pump. Sham rats
were injected with an equal volume of saline. After the operation,
each rat was placed into a separate cage and body temperature
maintained at 37.0±0.5˚C before follow-up experiments were
conducted.
Injection of lentiviral CTRP3 gene and
administration of EX527
Lentivirus harboring CTRP3 and empty vector were
constructed by Shanghai GeneChem Co., Ltd. with green fluorescent
protein. Rats were administered 5 µl CTRP3 lentivirus
(1x109 titer units/ml, diluted 10X with Enhanced
Infection Solution). Enhanced Infection Solution (cat. no.
REVG002A; Shanghai GeneChem Co., Ltd.) was used to improve the
efficiency of virus infection. The lentivirus CTRP3 gene was
injected into the right ventricle (0.9 mm lateral to the sagittal
suture, 1.9 mm posterior to the coronal suture and 3.5 mm deep into
the cortex) 14 days before ICH, as previously reported (20). The Lenti.Null rats were subjected
to the same procedure. Then, EX527, a SIRT1 inhibitor (Selleck
Chemicals) or vehicle was also injected intraventricularly 1 h
before induction of ICH. The dose of EX527 (10 µg/5µl dissolved in
10% DMSO) was selected as previously described (22). The needle was withdrawn after 15
min injection. Each rat was then placed back into an individual
cage and provided food and water ad libitum.
Behavioral tests
Neurological function was evaluated by the Garcia,
beam walking and wire hanging test, as previously described
(20). The Garcia test consists of
six tests, including autonomous activity, limb activity symmetry,
table-edge forepaw extension, grab cage and sensory touch response
tests. Each test yields a score of 0-3; the minimum overall score
was 0 and the maximum overall score was 18. Lower scores are
indicative of more serious neuronal damage. In the beam walking
test (23), a square wood bar (590
x 25 mm) was placed 100 mm from the ground. Each rat was allowed to
walk on the balance beam. The rats were assessed to determine if
they could use their limbs symmetrically to reach the platform at
the end of the beam and given a score of 0-5 as follows: 0, fell
from the beam; 1, sat on the beam but did not walk; 2, jumped onto
the beam but did not move forward; 3, jumped onto the beam and
walked with contralateral hind limbs of lesion slipping down the
beam >50% of the time; 4, jumped onto the beam and walked with
contralateral hind limbs slipping down the beam <50% of the time
and 5, jumped onto the beam and walked without falling. Each rat's
performance was calculated as the mean score of three trials. In
the wire hanging test (24), the
rats were placed in the center of a 600 x 2 mm wire 50 cm from the
ground with a sponge pad underneath. Each rat was observed four
times for 30 sec each. The rats were scored based on the time they
were suspended from the wire and the position of the limbs as
follows: 0, fell <30 sec; 1, did not fall off <30 sec,
grasped firmly with both front paws; 2, did not fall <30 sec,
attempted to climb the wire; 3, did not fall <30 sec, held wire
with both front paws and one or both hind paws; 4, did not fall
<30 sec, clutched wire with all four limbs, wrapped tail around
the wire and 5, did not fall <30 sec, climbed to the end of the
wire. Rats were scored in a blinded manner.
Brain water content
Brain water content was measured as described
previously (25). The brain water
content in each group was measured by the dry-wet weight method to
evaluate brain edema following ICH. Brain water content was
calculated as follows: (Wet weight - dry weight)/wet weight x
100%.
BBB permeability
Evans blue (EB) is a commonly used dye indicator in
the laboratory. EB content in brain tissue is directly proportional
to the degree of BBB damage. Therefore, EB is often used as a
tracer to evaluate BBB integrity (26). At 3 days post-ICH, rats were
intravenously injected with 2% EB. Then, 3 h later, extravasated EB
in the brain was evaluated by spectrophotometry (Thermo Fisher
Scientific, Inc.) at 620 nm.
Western blotting
Total protein from the perihematoma area of the rat
striatum was extracted using ice-cold RIPA lysis buffer (Beyotime
Institute of Biotechnology) supplemented with proteinase and
phosphatase inhibitors. Protein concentration was determined by BCA
assay. Then, a total of 50 µg protein/lane was separated by 10%
SDS-PAGE and transferred onto PVDF membranes. The membranes were
blocked for 1.5 h at room temperature in 5% non-fat-milk TBST (0.1%
Tween-20) buffer and incubated overnight at 4˚C with a primary
rabbit anti-rat CTRP3 (1:500; ab36870, Abcam,), SIRT1 (1:500; cat.
no. BS64501, Bioworld Technology, Inc.), TNF-α (1:500; cat. no.
BS6000, Bioworld Technology, Inc.), IL-1β (1:500; cat. no. BS6067,
Bioworld Technology, Inc.) and β-actin (1:3,000; cat. no. AP0060,
Bioworld Technology, Inc.) antibody. The membrane was incubated
with secondary anti-rabbit antibody (1:4,000; horseradish
peroxidase-conjugated Goat Anti-Rabbit IgG, cat. no. D110058,
Sangon Biotech Co. Ltd.) for 1.5 h at room temperature. Images were
captured using MicroChemi (Bio-Rad Laboratories, Inc.) and analyzed
using ImageJ version 1.8.0 (National Institutes of Health)
software.
ELISA
At 3 days following ICH, rats were deeply
anesthetized and the perihematomal brain tissue homogenate was
harvested. The tissue was homogenized with the BioVision
fractionation kit (K256-100), centrifuged (1500 g, 4˚C, 30 min) and
serum collected from the supernatant. The levels of TNF-α and IL-1β
in peripheral blood were determined with ELISA kits (cat. nos.
EK0526 and EK0394, respectively; Boster Biological Technology),
according to the manufacturer's instructions.
Statistical analysis
All data are presented as the mean ± SEM. One-way
ANOVA followed by Tukey's post hoc test was used to compare results
among all groups. SPSS 19.0 (IBM Corp.) software was used to
perform all statistical analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
CTRP3 protects the brain from SBI in
ICH rats
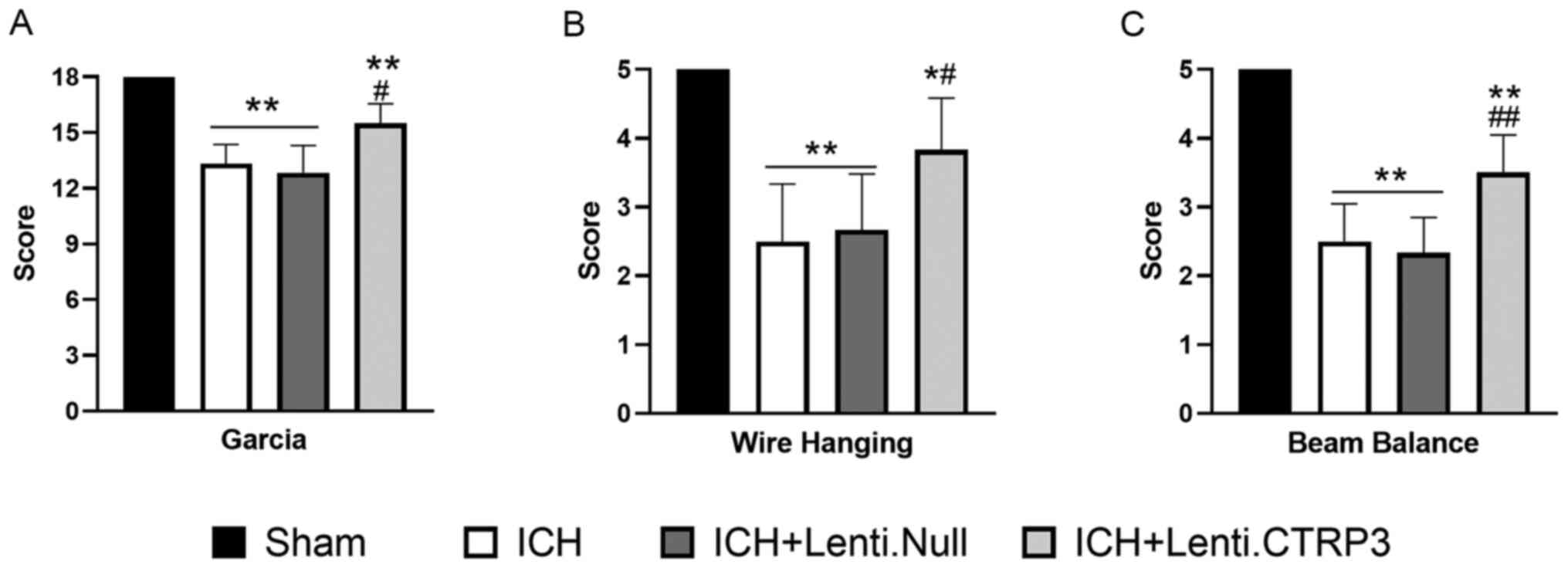
Modified Garcia, wire hang and beam balance test
were used to investigate the effect of CTRP3 on ICH-induced
neurological deficit. In the three tests, CTRP3 overexpression
mitigated neurological deficit at 3 days after the induction of ICH
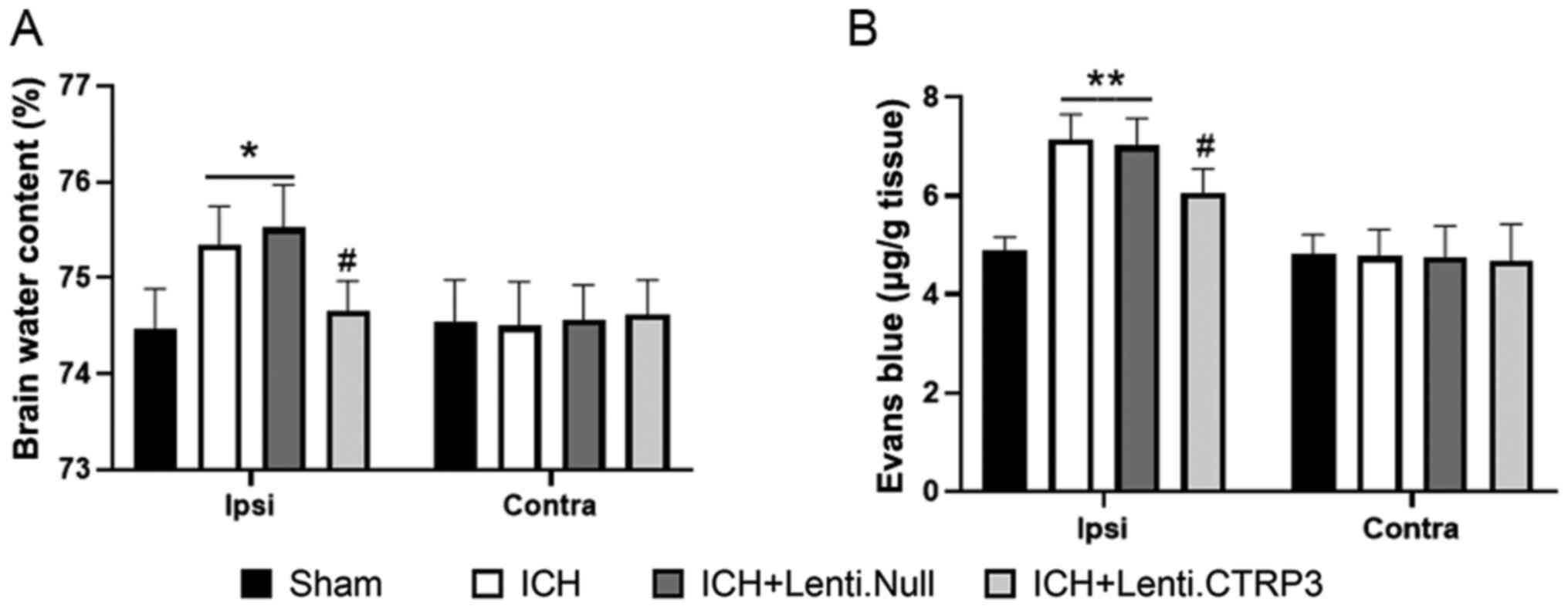
(Fig. 1A-C). Brain edema volume
was assessed by measuring brain water content. The brain water
content of the ipsilateral side, particularly in the striatum, was
significantly lower in the Lenti.CTRP3 group than in the Lenti.Null
group (Fig. 2A). BBB permeability
was assessed via EB extravasation in ICH rats. Significant
accumulation of EB was seen in the ipsilateral hemispheres of ICH
rats compared with that in sham rats (Fig. 2B). Treatment with CTRP3
significantly decreased extravasation in the ipsilateral hemisphere
compared with the Lenti.Null group at 3 days after ICH (Fig. 2B). These results suggested that
CTRP3 provided neuroprotection and attenuated SBI following
ICH.
CTRP3 suppresses inflammatory
responses in ICH rats
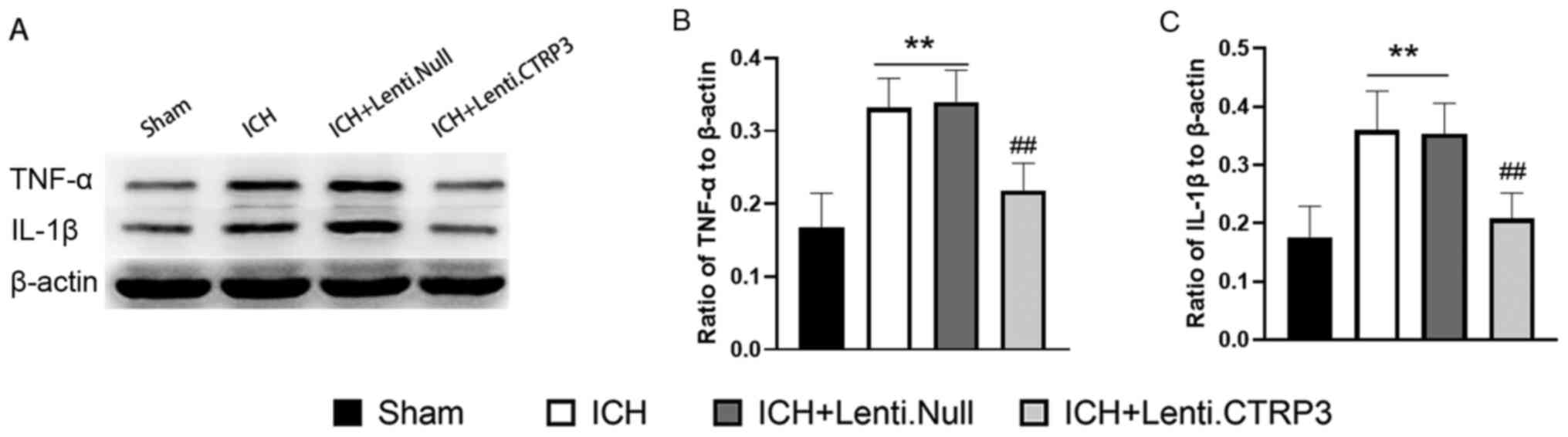
To investigate the function of CTRP3 in inflammatory
responses in ICH rats, levels of inflammatory-associated cytokines
were assessed in perihematomal brain tissue at 3 days after ICH via
western blotting. Expression levels of proinflammatory cytokines
TNF-α and IL-1β were significantly higher in the ICH group than in
the sham group (Fig. 3). In
addition, the Lenti.CTRP3 group exhibited significantly decreased
expression levels of these factors compared with those in the
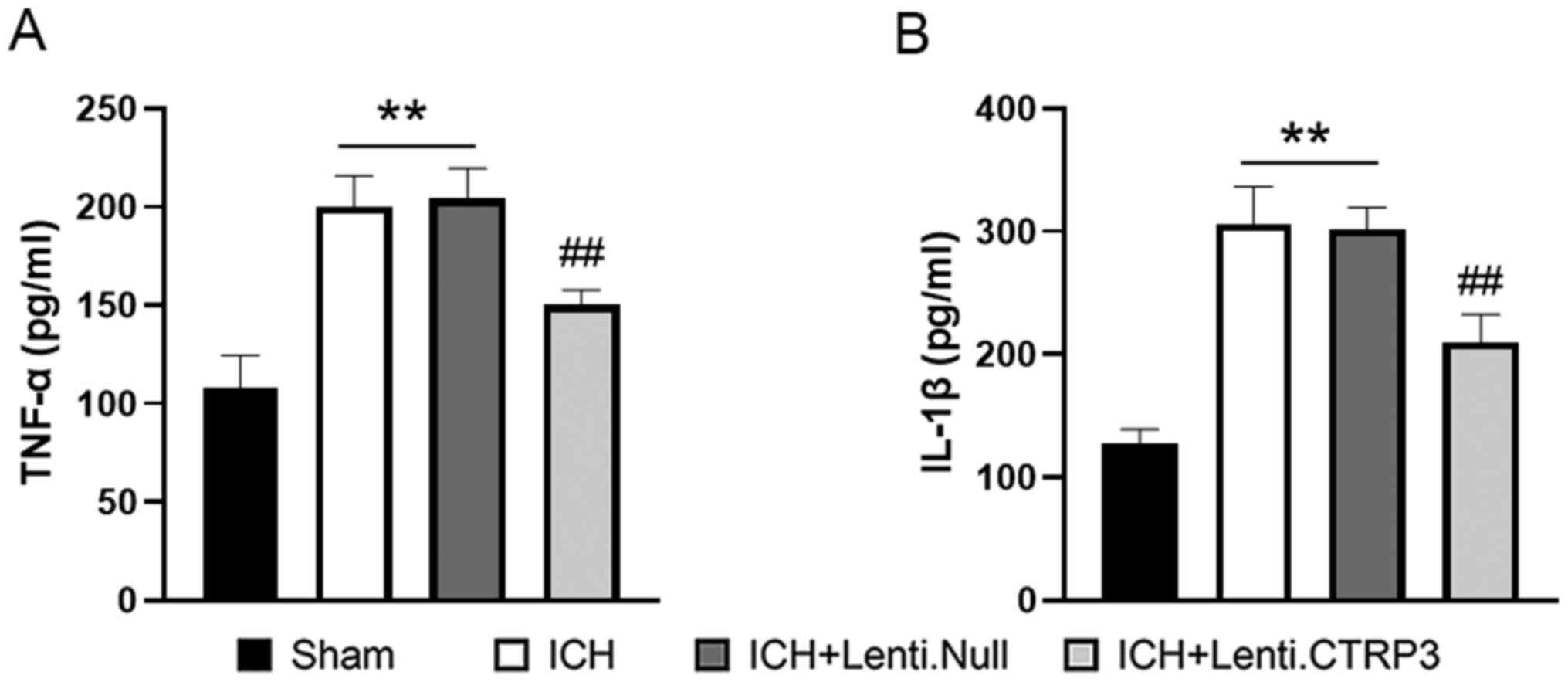
Lenti.Null group (Fig. 3). ELISA
revealed that the activity of TNF-α and IL-1β (Fig. 4A and B) was also decreased in the Lenti.CTRP3
group compared with the Lenti.Null group. Taken together, these
findings suggested that CTRP3 protected against inflammation
following ICH.
CTRP3 activates the SIRT1 signaling
pathway in ICH rats
To investigate the effect of CTRP3 in an ICH model,
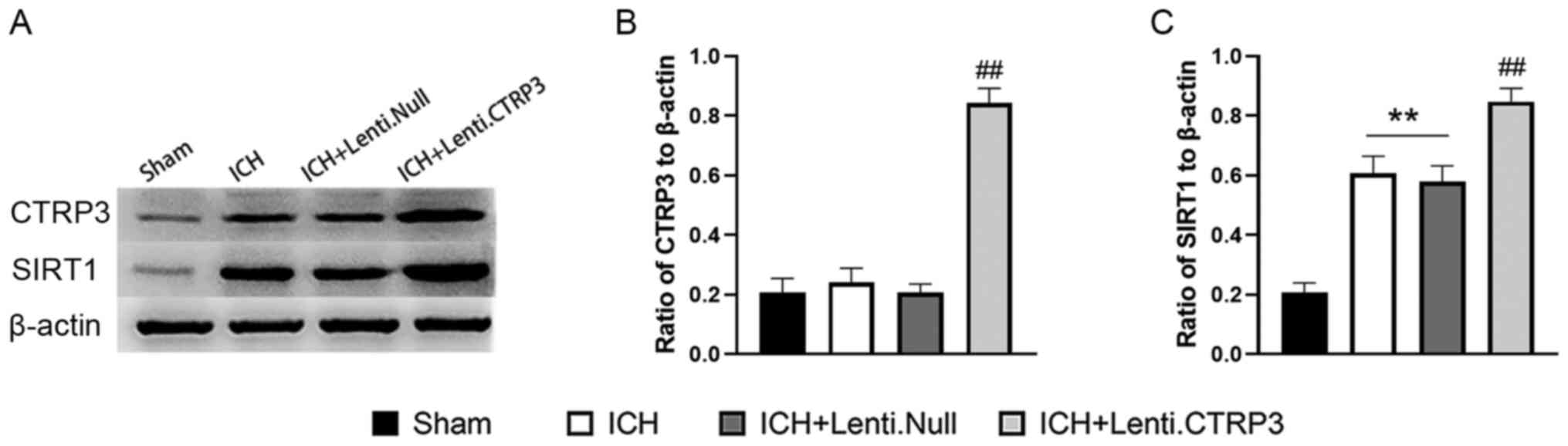
CTRP3 was successfully overexpressed in rats (Fig. 5A). To determine the mechanisms
responsible for CTRP3-mediated neuroprotection at the molecular
level, SIRT1 protein expression was measured in perihematomal brain
tissue 3 days after ICH. Compared with the sham group, SIRT1 was
increased in the ICH group and CTRP3 overexpression induced a
further increase in expression of SIRT1 compared with that in
Lenti.Null group at 3 days post-ICH (Fig. 5B and C).
Neuroprotective effects of CTRP3 are
blocked by SIRT1 inhibition
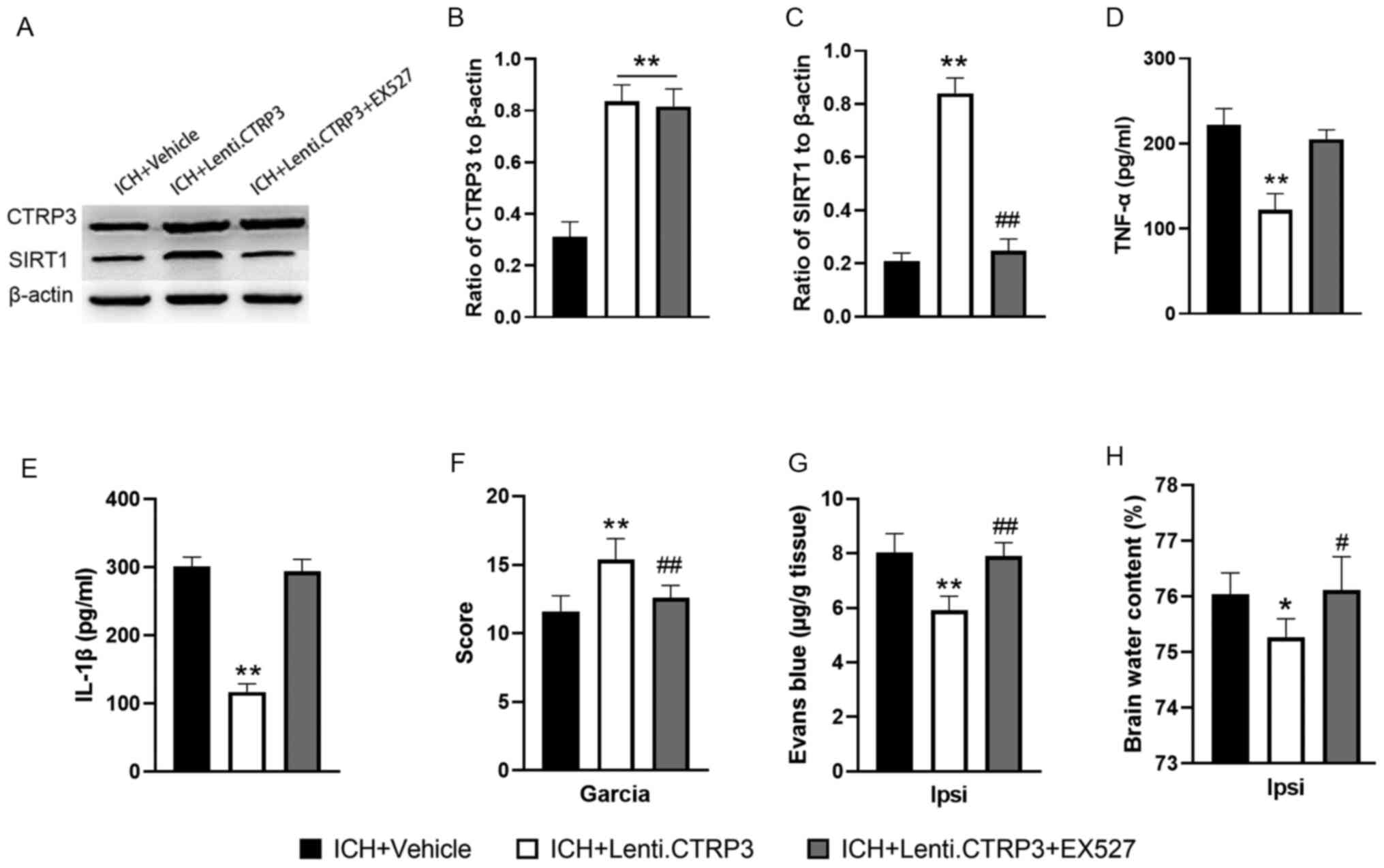
Activation of SIRT1 occurred following CTRP3
overexpression (Fig. 6A-C) and
inhibition of SIRT1 with EX527 abolished CTRP3-induced SIRT1
upregulation (Fig. 6A-C). Next,
the effect of the SIRT1 signal pathway in CTRP3-mediated
suppression of ICH-induced inflammatory response was investigated.
SIRT1-induced suppression of inflammatory responses was abrogated
by EX527 treatment, which manifested as increased expression levels
of TNF-α and IL-1β (Fig. 6D and
E). Furthermore, EX527 blocked the
neuroprotective effects of Lenti.CTRP3 (Fig. 6F-H). These results indicated that
SIRT1 inhibition abolished the protective effects of CTRP3 in
vivo.
Discussion
SBI is characterized as hematomal expansion caused
by neuroinflammation that contributes to exacerbating functional
outcomes following ICH (27,28).
The present study focused on CTRP3 as an approach to mitigate
ICH-induced brain injury and dysfunction. The present results
suggested that CTRP3 significantly alleviated neurobehavioral
deficit, ICH-induced brain edema and breakdown of the BBB.
Moreover, exogenous CTRP3 overexpression activated the SIRT1
signaling pathway, suggesting that the SIRT1 signaling pathway
represents a potential mechanism by which CTRP3 protects against
ICH. Furthermore, CTRP3 suppressed ICH-induced inflammatory
responses in vivo. Collectively, the present findings
provided a novel approach for the treatment of ICH-induced SBI.
Inflammation is one of the key processes underlying
ICH-induced brain injury (29,30).
A previous study suggested that the mechanisms underlying brain
edema formation are dependent on disruption of BBB integrity
(31). Inflammation leads to
increased vascular permeability and, ultimately, BBB damage
(32-34).
In the present study, CTRP3 overexpression resulted in maintenance
of BBB integrity following ICH. ICH activates NF-κB, thus promoting
release of inflammatory cytokines and ultimately leading to
neurological dysfunction (34,35).
TNF-α and IL-1β are the most studied cytokines in ICH (36-38).
Previous studies have shown that these two factors are increased
significantly following ICH and are associated with brain edema
(39,40). The present study investigated
whether CTRP3 inhibits ICH-induced neuroinflammation and found that
CTRP3 decreased TNF-α and Il-1β levels. However, the pathway by
which CTRP3 exerts its anti-inflammatory effects remains unclear.
SIRT1 suppresses expression of NF-κB, which is the upstream
regulator of TNF-α and IL-1β (41). In the present study, a
SIRT1-specific inhibitor partially abolished CTRP3-mediated
downregulation of TNF-α and IL-1β in ICH rats, implying a key role
of SIRT1 as an anti-inflammatory effector of CTRP3.
SIRT1 is a member of the class III group of histone
deacetylases and is activated in response to various types of
cellular stressor, such as inflammation, cell death and metabolism.
Accumulating evidence has indicated the role of SIRT1 in
neurobehavioral deficit; activation of SIRT1 exhibits
neuroprotective effects on cerebral ischemia or hemorrhagic stroke
(42,43). Zhou et al (43) reported that SIRT1 protein levels
are slightly increased following ICH. EX527 is a SIRT1 specific
inhibitor; it significantly inhibits SIRT1 activity in vitro
but has no effect on SIRT1 mRNA and protein expression. In
vivo, EX527 not only inhibits the activity of SIRT1, but also
downregulates expression of SIRT1 protein (44). Consistent with the aforementioned
studies, here SIRT1 protein levels were increased following ICH in
rats. Moreover, activating SIRT1 by CTRP3 in vivo attenuated
ICH-induced brain dysfunction and inhibition of SIRT1 abolished the
protective effect provided by CTRP3 in ICH rats. These findings
indicated that CTRP3-mediated amelioration of neurobehavioral
deficit may be at least partly mediated by SIRT1.
As a novel adipokine, little is known about
CTRP3-associated signaling pathways. The present study demonstrated
that SIRT1 inhibition partially abolished CTRP3-mediated
neuroprotection in ICH rats, implying that CTRP3 may suppress
ICH-induced SBI via activating SIRT1. These findings reveal the
complexity of signaling pathways regulated by CTRP3. Further
studies are needed to advance understanding of the precise
mechanisms by which CTRP3 exerts its biological effects.
In conclusion, the present study demonstrated that
CTRP3 prevented the aggravation of brain edema and neuronal damage
following ICH in vivo. CTRP3 inhibited inflammation via the
SIRT1 pathway and may have protected the BBB, as well as neurons
and glia, against SBI following ICH. Thus, the present findings
suggested that CTRP3 may be an effective therapeutic candidate for
the treatment of SBI following ICH.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from Natural
Science Foundation of the Sichuan Provincial Education Office
(grant no. 18ZA0529),Science Foundation of Southwest Medical
University (grant no. 0903-00030982), The Science Foundation of the
Affiliated Hospital of Southwest Medical University (grant no.
2017-PT-7) and The Doctoral Scientific Research Start-up Fund
Project of the Affiliated Hospital of Southwest Medical University
(grant no. 18058).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and SW confirm the authenticity of all the raw
data. YW and SW designed the research and performed the analysis.
JW assisted in study design. BY, CH, XT, HY and YL performed
experiments and analyzed data. YW and SW drafted and revised the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures were in accordance with
the guidelines for the care and use of laboratory animals of the
National Institutes of Health. All animal studies were approved by
the Biomedical Ethics Committee of Southwest Medical University
(approval no. 20210223-135).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gunda B, Böjti P and Kozák LR: Hyperacute
Spontaneous Intracerebral Hemorrhage During Computed Tomography
Scanning. JAMA Neurol. 78:365–366. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cho S, Rehni AK and Dave KR: Tobacco Use:
A Major Risk Factor of Intracerebral Hemorrhage. J Stroke.
23:37–50. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wu YC, Ding Z, Wu J, Wang YY, Zhang SC,
Wen Y, Dong WY and Zhang QY: Increased glycemic variability
associated with a poor 30-day functional outcome in acute
intracerebral hemorrhage. J Neurosurg. 129:861–869. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kuramatsu JB, Biffi A, Gerner ST, Sembill
JA, Sprügel MI, Leasure A, Sansing L, Matouk C, Falcone GJ, Endres
M, et al: Association of surgical hematoma evacuation vs
conservative treatment with functional outcome in patients with
cerebellar intracerebral hemorrhage. JAMA. 322:1392–1403.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sugiyama T, Imai T, Nakamura S, Yamauchi
K, Sawada S, Shimazawa M and Hara H: A novel Nrf2 activator, RS9,
attenuates secondary brain injury after intracerebral hemorrhage in
sub-acute phase. Brain Res. 1701:137–145. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Atangana E, Schneider UC, Blecharz K,
Magrini S, Wagner J, Nieminen-Kelhä M, Kremenetskaia I, Heppner FL,
Engelhardt B and Vajkoczy P: Intravascular Inflammation Triggers
Intracerebral Activated Microglia and Contributes to Secondary
Brain Injury After Experimental Subarachnoid Hemorrhage (eSAH).
Transl Stroke Res. 8:144–156. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang Z, Zhou F, Dou Y, Tian X, Liu C, Li
H, Shen H and Chen G: Melatonin Alleviates Intracerebral
Hemorrhage-Induced Secondary Brain Injury in Rats via Suppressing
Apoptosis, Inflammation, Oxidative Stress, DNA Damage, and
Mitochondria Injury. Transl Stroke Res. 9:74–91. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Walsh KB, Zhang X, Zhu X, Wohleb E, Woo D,
Lu L and Adeoye O: Intracerebral Hemorrhage Induces Inflammatory
Gene Expression in Peripheral Blood: Global Transcriptional
Profiling in Intracerebral Hemorrhage Patients. DNA Cell Biol.
38:660–669. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang J and Doré S: Inflammation after
intracerebral hemorrhage. J Cereb Blood Flow Metab. 27:894–908.
2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tschoe C, Bushnell CD, Duncan PW,
Alexander-Miller MA and Wolfe SQ: Neuroinflammation after
Intracerebral Hemorrhage and Potential Therapeutic Targets. J
Stroke. 22:29–46. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhu H, Wang Z, Yu J, Yang X, He F, Liu Z,
Che F, Chen X, Ren H, Hong M and Wang J: . : Role and mechanisms of
cytokines in the secondary brain injury after intracerebral
hemorrhage. Prog Neurobiol. 178(101610)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gao K, Niu J and Dang X: Neuroprotection
of melatonin on spinal cord injury by activating autophagy and
inhibiting apoptosis via SIRT1/AMPK signaling pathway. Biotechnol
Lett. 42:2059–2069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vellimana AK, Aum DJ, Diwan D, Clarke JV,
Nelson JW, Lawrence M, Han BH, Gidday JM and Zipfel GJ: SIRT1
mediates hypoxic preconditioning induced attenuation of
neurovascular dysfunction following subarachnoid hemorrhage. Exp
Neurol. 334(113484)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Deng HJ, Zhou CH, Huang LT, Wen LB, Zhou
ML and Wang CX: Activation of silent information regulator 1 exerts
a neuroprotective effect after intracerebral hemorrhage by
deacetylating NF-κB/p65. J Neurochem. 157:574–585. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ahima RS, Qi Y, Singhal NS, Jackson MB and
Scherer PE: Brain adipocytokine action and metabolic regulation.
Diabetes. 55 (Suppl 2):S145–S154. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schmid A, Gehl J, Thomalla M, Hochberg A,
Kreiss A, Patz M, Karrasch T and Schäffler A: Downregulation of
CTRP-3 by Weight Loss In Vivo and by Bile Acids and Incretins in
Adipocytes In Vitro. Int J Mol Sci. 21(8168)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Liu Y, Li LN, Guo S, Zhao XY, Liu YZ,
Liang C, Tu S, Wang D, Li L, Dong JZ, et al: Melatonin improves
cardiac function in a mouse model of heart failure with preserved
ejection fraction. Redox Biol. 18:211–221. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yuan YP, Ma ZG, Zhang X, Xu SC, Zeng XF,
Yang Z, Deng W and Tang QZ: CTRP3 protected against
doxorubicin-induced cardiac dysfunction, inflammation and cell
death via activation of Sirt1. J Mol Cell Cardiol. 114:38–47.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the care and use of laboratory animals: 8th
edition. The National Academies Press, Washington, DC, 2011.
|
|
20
|
Wang S, Zhou Y, Yang B, Li L, Yu S, Chen
Y, Zhu J and Zhao Y: C1q/Tumor Necrosis Factor-Related Protein-3
Attenuates Brain Injury after Intracerebral Hemorrhage via
AMPK-Dependent Pathway in Rat. Front Cell Neurosci.
10(237)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Berg T: Kv7(KCNQ)-K+-Channels Influence
Total Peripheral Resistance in Female but Not Male Rats, and Hamper
Catecholamine Release in Hypertensive Rats of Both Sexes. Front
Physiol. 9(117)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu SP, Huang L, Flores J, Ding Y, Li P,
Peng J, Zuo G, Zhang JH, Lu J and Tang JP: Secukinumab attenuates
reactive astrogliosis via IL-17RA/(C/EBPβ)/SIRT1 pathway in a rat
model of germinal matrix hemorrhage. CNS Neurosci Ther.
25:1151–1161. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yue X, Liu L, Yan H, Gui Y, Zhao J and
Zhang P: Intracerebral Hemorrhage Induced Brain Injury Is Mediated
by the Interleukin-12 Receptor in Rats. Neuropsychiatr Dis Treat.
16:891–900. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang S, Yao Q, Yu W, Wang JQ, Huang CG, Li
D and Yang B: Adiponectin reduces brain injury after intracerebral
hemorrhage by reducing NLRP3 inflammasome expression. Int J
Neurosci. 130:301–308. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Xie L, Wang Y and Chen Z: EGR1 knockdown
alleviates the cerebral injury in rats with intracerebral
hemorrhage (ICH) via STAT3/NF-κB pathway by reducing RXRα
acetylation level. Neuroscience: Feb 16, 2021 (Epub ahead of
print).
|
|
26
|
Zhao H, Zhang K, Tang R, Meng H, Zou Y, Wu
P, Hu R, Liu X, Feng H and Chen Y: Trpv4 blockade preserves the
blood–brain barrier by inhibiting stress fiber formation in a rat
model of intracerebral hemorrhage. Front Mol Neurosci.
11(97)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
James ML, Komisarow JM, Wang H and
Laskowitz DT: Therapeutic Development of Apolipoprotein E Mimetics
for Acute Brain Injury: Augmenting Endogenous Responses to Reduce
Secondary Injury. Neurotherapeutics. 17:475–483. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mazzeo AT, Filippini C, Rosato R, Fanelli
V, Assenzio B, Piper I, Howells T, Mastromauro I, Berardino M,
Ducati A, et al: Multivariate projection method to investigate
inflammation associated with secondary insults and outcome after
human traumatic brain injury: A pilot study. J Neuroinflammation.
13:157. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xiao H, Chen H, Jiang R, Zhang L, Wang L,
Gan H, Jiang N, Zhao J, Zhai X and Liang P: NLRP6 contributes to
inflammation and brain injury following intracerebral haemorrhage
by activating autophagy. J Mol Med (Berl). 98:1319–1331.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li Z, He Q, Zhai X, You Y, Li L, Hou Y, He
F, Zhao Y and Zhao J: Foxo1-mediated inflammatory response after
cerebral hemorrhage in rats. Neurosci Lett. 629:131–136.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhu J, Li X, Yin J, Hu Y, Gu Y and Pan S:
Glycocalyx degradation leads to blood-brain barrier dysfunction and
brain edema after asphyxia cardiac arrest in rats. J Cereb Blood
Flow Metab. 38:1979–1992. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhao F, Deng J, Xu X, Cao F, Lu K, Li D,
Cheng X, Wang X and Zhao Y: Aquaporin-4 deletion ameliorates
hypoglycemia-induced BBB permeability by inhibiting inflammatory
responses. J Neuroinflammation. 15(157)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Varatharaj A and Galea I: The blood-brain
barrier in systemic inflammation. Brain Behav Immun. 60:1–12.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhou F, Jiang Z, Yang B and Hu Z: Magnolol
exhibits anti-inflammatory and neuroprotective effects in a rat
model of intracerebral haemorrhage. Brain Behav Immun. 77:161–167.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wu CH, Chen CC, Lai CY, Hung TH, Lin CC,
Chao M and Chen SF: Treatment with TO901317, a synthetic liver X
receptor agonist, reduces brain damage and attenuates
neuroinflammation in experimental intracerebral hemorrhage. J
Neuroinflammation. 13(62)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shang Y, Dai S, Chen X, Wen W and Liu X:
MicroRNA-93 regulates the neurological function, cerebral edema and
neuronal apoptosis of rats with intracerebral hemorrhage through
TLR4/NF-κB signaling pathway. Cell Cycle. 18:3160–3176.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen S, Peng J, Sherchan P, Ma Y, Xiang S,
Yan F, Zhao H, Jiang Y, Wang N, Zhang JH, et al: TREM2 activation
attenuates neuroinflammation and neuronal apoptosis via PI3K/Akt
pathway after intracerebral hemorrhage in mice. J
Neuroinflammation. 17(168)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Shi SX, Li YJ, Shi K, Wood K, Ducruet AF
and Liu Q: IL (Interleukin)-15 Bridges Astrocyte-Microglia
Crosstalk and Exacerbates Brain Injury Following Intracerebral
Hemorrhage. Stroke. 51:967–974. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen S, Zhao L, Sherchan P, Ding Y, Yu J,
Nowrangi D, Tang J, Xia Y and Zhang JH: Activation of melanocortin
receptor 4 with RO27-3225 attenuates neuroinflammation through
AMPK/JNK/p38 MAPK pathway after intracerebral hemorrhage in mice. J
Neuroinflammation. 15(106)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gong Y, Wu M, Shen J, Tang J, Li J, Xu J,
Dang B and Chen G: Inhibition of the NKCC1/NF-κB Signaling Pathway
Decreases Inflammation and Improves Brain Edema and Nerve Cell
Apoptosis in an SBI Rat Model. Front Mol Neurosci.
14(641993)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Han S, Li Z, Han F, Jia Y, Qi L, Wu G, Cai
W, Xu Y, Li C, Zhang W and Hu D: ROR alpha protects against
LPS-induced inflammation by down-regulating SIRT1/NF-kappaB
pathway, Arch Biochem. Biophy. 668:1–8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hu Q, Manaenko A, Bian H, Guo Z, Huang JL,
Guo ZN, Yang P, Tang J and Zhang JH: Hyperbaric Oxygen Reduces
Infarction Volume and Hemorrhagic Transformation Through
ATP/NAD+/Sirt1 Pathway in Hyperglycemic Middle Cerebral Artery
Occlusion Rats. Stroke. 48:1655–1664. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhou Y, Wang S, Li Y, Yu S and Zhao Y:
SIRT1/PGC-1α Signaling Promotes Mitochondrial Functional Recovery
and Reduces Apoptosis after Intracerebral Hemorrhage in Rats. Front
Mol Neurosci. 10(443)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shi YH, Zhang XL, Ying PJ, Wu ZQ, Lin LL,
Chen W, Zheng GQ and Zhu WZ: Neuroprotective Effect of
Astragaloside IV on Cerebral Ischemia/Reperfusion Injury Rats
Through Sirt1/Mapt Pathway. Front Pharmacol.
12(639898)2021.PubMed/NCBI View Article : Google Scholar
|