Introduction
Elderly patients with multiple comorbidities or
diseases often need repeated anesthesia for multiple treatment.
Sevoflurane is a volatile anesthetic that is widely applied in
medicine, due to its efficiency, reduced risk of airway irritation
compared with other inhaled anesthetics (for example, halothane and
desflurane) and quick induction (~2-5 min) and recovery periods
(~10-30 min); however, sevoflurane is potentially neurotoxic and
has been previously reported to be associated with postoperative
cognitive impairments in elderly individuals (1). A study previously performed on mice
at postnatal day 6 reported that sevoflurane can mediate
neurological damage and brain dysplasia by promoting oxidative
stress (2).
Docosahexaenoic acid (DHA) is an unsaturated fatty
acid and an important component of the neuronal cell membrane
(3). DHA serves numerous key roles
in signal transduction in neurons, preventing cytoskeletal protein
degradation and inhibiting oxidative stress and lipid peroxidation
(4). Aging is associated with
changes in the DHA content in the membranes of neurons in the
brain, which can contribute to memory impairment (5). Age-related decrease associated with
the DHA content in the hippocampus has previously been observed in
rat models of 3- and 13-month-old rats (6) in addition to 3-4-month and
24-25-month-old rats (7). The
present study aimed to investigate the effects of different DHA
doses on behavioral memory impairment induced by the repeated
administration of sevoflurane in aged rats. In addition, any
potential side effects as a result of repeated sevoflurane
anesthesia were also explored.
A previous study has demonstrated that DHA can
induce activation of nuclear factor erythroid-2-related factor 2
(Nrf2) and subsequently heme oxygenase 1 (HO-1) (8). The Nrf2/HO-1 signaling pathway is a
protection system that exists in a number of organs and is
activated in response to a number of different stressors, such as
radiation, UV light, air pollution and toxins (9). The Nrf2/HO-1 signaling pathway serves
numerous functions, including anti-oxidation and anti-inflammatory
responses, reduction of mitochondrial damage and regulation of cell
death (10-13).
A previous study has reported that Nrf2/HO-1 signaling can mediate
a neuroprotective role by delaying the occurrence of Alzheimer's
disease in a mouse model (14).
However, to the best of our knowledge, there is no evidence of the
role of this pathway after repeated anesthesia.
In the present study, an aged rat model of repeated
sevoflurane anesthesia was established to explore the effects of
DHA treatment on sevoflurane-induced behavioral memory impairments.
The aim of the present study was to provide a theoretical basis for
DHA treatment in improving behavioral memory impairment and its
underlying molecular mechanisms.
Materials and methods
Experimental animals
A total of 54 aged Sprague Dawley (SD) male rats
(Jinan Peng Yue Experimental Animal Breeding Co., Ltd.; production
license no. SCXK (LU) 20140007; age, 18 months; weight, 540±50 g)
were used in the present study. The housing conditions for the
animals were as follows: Room temperature, 20-26˚C with daily
temperature difference ≤4˚C; relative humidity 40-70%; and 12-h
light/dark cycles. During the experimental periods, all rats had
free access to food and water. Animal health was monitored twice a
day. No adverse effects of the treatment were observed during the
experiment. All experimental protocols were conducted according to
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (15) and were
approved by the Animal Protection and Use Committee of The
Affiliated Yantai Yuhuangding Hospital of Qingdao University
(Yantai, China).
Animal grouping, anesthesia and DHA
administration
The SD rats were randomly divided into the following
six groups (n=9): i) Blank control group (control); ii) sevoflurane
group (Sev; 2.5%; duration, 5 min); iii) DHA group (3 g/kg); iv)
Sev + DHA (0.3 g/kg) group, v) Sev + DHA (1 g/kg) group; and vi)
Sev + DHA (3 g/kg). Sevoflurane was purchased from Shanghai Hengrui
Pharmaceutical Co., Ltd. (cat. no. NMPN-H20070172) and DHA was
purchased from Rongcheng Baihe Biotechnology Co., Ltd.
Rats in the sevoflurane-induced groups were placed
in a custom-made transparent anesthesia box (clear tempered glass;
50x40x40 cm). A hole on one side of the box was made to allow for
an anesthesia machine (Drägerwerk AG) to be connected. The rats
were treated with 2.5% sevoflurane for 5 min and the heart rate
(HR), respiratory frequency (RF) and blood oxygen saturation (BOS)
of the animals were continuously examined using an
electrocardiogram monitor (Nordep, Ltd.) to detect brain damage
caused by hypoxia. When the rats became fully anaesthetized, they
were exposed to air for awakening. The animals were anesthetized
once a day for 10 consecutive days. The control group received no
sevoflurane treatment.
Rats in the DHA treatment groups received daily feed
supplemented with DHA on days 1-20 of the experiment. On days
11-20, rats in the Sev groups received daily inhalation of 2.5%
sevoflurane for 5 min for repeated anesthesia. Rats in the DHA
group received only DHA and were not anesthetized. The rats were
trained for the Morris water maze (MWM) experiment on days 16-20,
as described below. No drugs were administered on day 21 before
this test. The body weight of the rats in each group was recorded
on days 1, 7, 14 and 21. The experimental design of the present
study is presented in Fig. 1.
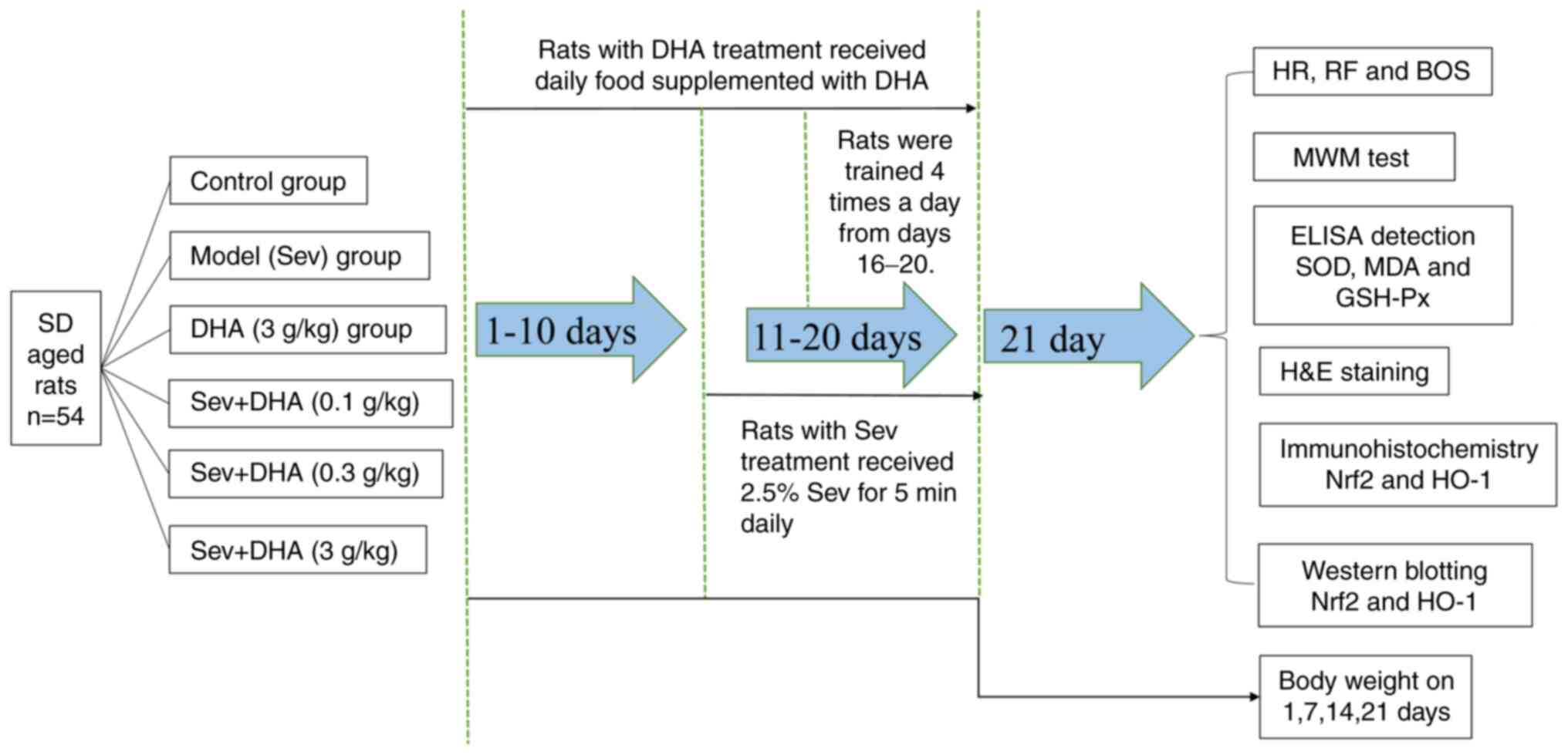 | Figure 1Integrated experimental design flow
chart. SD, Sprague Dawley; DHA, docosahexaenoic acid; Sev,
sevoflurane; HR, heart rate; RF, respiratory frequency; BOS, blood
oxygen saturation; MWM, Morris water maze; SOD, superoxide
dismutase; MDA, malondialdehyde; GSH-Px, glutathione peroxidase;
Nrf2, nuclear factor erythroid-2-related factor 2; HO-1, heme
oxygenase 1. |
MWM experiment
The MWM experiment was used to test the learning
capability of the rats by assessing their navigation abilities. MWM
(Shanghai Institute of Materia Medica, Chinese Academy of Sciences)
consisted of two parts: One round pool (diameter, 120 cm; height,
50 cm; stainless steel) and one movable platform. The platform was
located 2 cm below the water. The water temperature of the pool was
~25˚C. The pool surface was divided into four quadrants (named
quadrants 1, 2, 3 and 4). The time required for the rats to locate
the platform after being placed in the pool was then calculated and
the rats' swimming trajectories were automatically tracked using
the EthoVision XT 14 software (Noldus Information Technology BV).
This positioning navigation experiment recorded the time required
for the rats to locate the platform hidden under the water's
surface and examined the spatial orientation learning ability of
the rats.
All rats started the MWM test at the center of any
quadrant facing the pool wall. If the animal failed to locate the
platform in the pool within 120 sec, the time was recorded as 120
sec and the rat was then guided to the platform for 30 sec. The
rats were subsequently removed from the platform and wiped dry.
After the rats rested for 60 sec, they were trained again. Rats
were trained four times a day from days 16-20 for 5 consecutive
days. An average of the four training incubation periods was used
as the daily learning achievement of each rat and presented as the
escape latency.
On day 21, 24 h following the end of the MWM
positioning navigation experiment, the platform was withdrawn, and
the rats were placed in the water at the center of any quadrant
facing the wall. The rats were then permitted to swim for 120 sec,
where the time spent in the target quadrant and the number of
attempts to cross the platform were recorded.
H&E staining
After the behavioral tests were all completed on day
21, all animals were anesthetized via an intraperitoneal injection
of 1% pentobarbital sodium (40 mg/kg) and sacrificed via cervical
dislocation. Brain tissues were carefully extracted and washed with
cold normal saline. The left brain tissue was used for
histopathological examination. Tissue was fixed in 10% formalin for
24 h at room temperature. Subsequently, samples were embedded in
paraffin and cut into 4 µm-thick sections. The paraffin sections
were dewaxed using xylene, dehydrated using an ethanol descending
gradient and incubated with hematoxylin for 10 min and eosin for 5
min at room temperature (Beijing Solarbio Science & Technology
Co., Ltd.) before being rinsed with distilled water for 30 sec. The
sections were soaked in 95% ethanol twice for 1 min and placed in
xylene three times for 5 min before Permount™ mounting medium
(Thermo Fisher Scientific, Inc.) was applied. Pathological changes
were imaged using an optical microscope (magnification, x100;
Olympus Corporation).
ELISA
The right brain tissue was collected from each group
of rats, 4˚C pre-cooled normal saline was used obtain brain tissue
homogenate at 1:10 (volume/volume). Tissue homogenate was
centrifuged at 12,000 x g for 30 min at 4˚C. The supernatant was
collected before superoxide dismutase (SOD; cat. no. A001-3-2),
malondialdehyde (MDA; cat. no. A003-1-1) and glutathione peroxidase
(GSH-Px; cat. no. A005-1-2) levels were detected using assay kits
(Nanjing Jiancheng Bioengineering Institute), according to the
manufacturer's protocol.
Immunohistochemistry
The left brain tissues were fixed in 10% formalin
for 24 h at room temperature then embedded in paraffin and
sectioned (5-µm). The sections were deparaffinized twice using
xylene and rehydrated in a descending ethanol gradient. Endogenous
peroxidase was inhibited by incubating the sections for 30 min with
3% H2O2 at 37˚C. Antigen retrieval was
performed using a 0.01 M citrate buffer at 95˚C for 10 min. The
sections were subsequently blocked for 20 min in 5% BSA (Beijing
Solarbio Science & Technology Co., Ltd.) at 37˚C, and incubated
using a primary anti-HO-1 antibody (1:100; cat. no. ab13243; Abcam)
and an anti-Nrf2 antibody (1:100; cat. no. ab31163; Abcam)
overnight at 4˚C. After rewarming, sections were incubated with an
HRP-conjugated goat anti-rabbit IgG secondary antibody (1:1,000;
cat. no. ab6721; Abcam) at 37˚C for 1 h. Sections were visualized
using 3,3' diaminobenzidine (DAB) as the chromogen (Beijing
Solarbio Science & Technology Co., Ltd.), then counterstained
with hematoxylin for 1 min and washed in running water two times (3
min each) at room temperature. Subsequently, sections were
dehydrated with ethanol of gradient concentration, cleared in
xylene and placed onto a coverslip in Permount™ mounting medium
(Thermo Fisher Scientific, Inc.). The samples were imaged using an
optical microscope (magnification, x200; Olympus Corporation) with
five fields randomly selected view. Protein expression levels were
quantified using Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc.). The data were expressed as the percentage of
positive cells/total number of cells counted.
Western blotting
Total protein was extracted from the right brain
tissue using RIPA lysis buffer containing proteinase inhibitor
cocktail (Beyotime Institute of Biotechnology). Total protein
concentration was quantified using a BCA assay and total protein
(50 µg protein/lane) was separated using SDS-PAGE on a 10% gel. The
separated proteins were transferred onto PVDF membranes
(MilliporeSigma). The membranes were blocked using 5% skimmed milk
at 4˚C overnight and subsequently incubated with the primary
anti-Nrf2 (1:1,000; cat. no. ab137550; Abcam), anti-HO-1 (1:2,000;
cat. no. ab13243; Abcam) and anti-β-actin antibody (1:2,000; cat.
no. ab8227; Abcam) at 4˚C overnight. Following primary incubation,
the membranes were incubated with the secondary antibody,
HRP-labeled sheep anti rabbit IgG (1:5,000; cat. no. ab97095;
Abcam) at 37˚C for 1 h. Protein bands were visualized using an ECL
Chemiluminescence System (Thermo Fisher Scientific, Inc.). Protein
expression levels were normalized to β-actin and were
semi-quantified using ImageJ software version 1.46 (National
Institutes of Health).
Statistical analysis
All experimental data were analyzed using SPSS 20.0
(IBM Corp.) and are presented as the mean ± standard deviation.
One-way ANOVA was used to perform statistical comparisons among ≥3
groups followed by Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of sevoflurane and DHA on the
body weight, HR, RF and BOS of rats
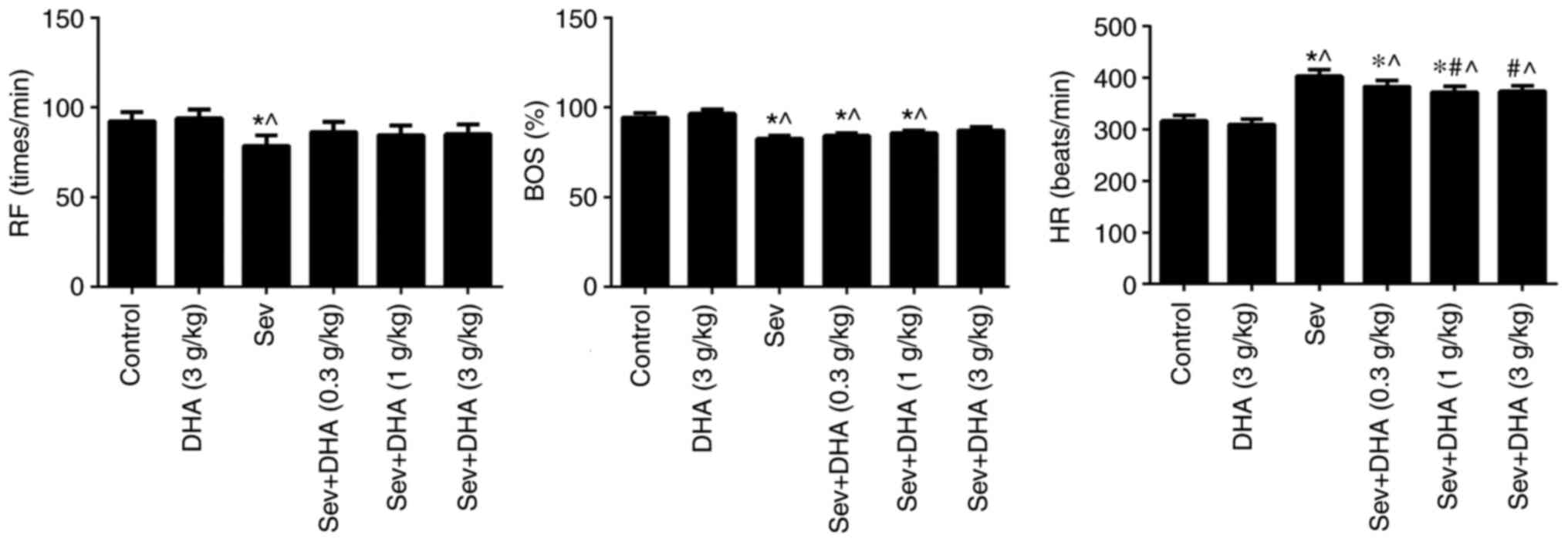
The body weight of rats in each treatment group
increased gradually over 21 days The body weight of the rats in the
Sev group increased at a markedly slower rate (Table I). On day 21, the body weight of
the rats in the Sev group was significantly lower compared with
that in the control and DHA groups.
 | Table IChanges in rat body weight
(n=9/group). |
Table I
Changes in rat body weight
(n=9/group).
| | Weight, g |
|---|
| Group | Day 1 | Day 7 | Day 14 | Day 21 |
|---|
| Control | 584.52±21.16 | 598.62±22.98 | 613.62±21.08 | 627.62±23.17 |
| DHA (3 g/kg) | 585.43±20.32 | 601.17±20.34 | 617.34±21.67 | 633.19±24.58 |
| Sev | 583.26±19.74 | 597.13±20.69 | 603.41±22.12 |
608.41±23.36a,b |
| Sev + DHA (0.3
g/kg) | 582.31±19.42 | 598.43±19.86 | 606.51±20.82 |
614.12±21.91b |
| Sev + DHA (1
g/kg) | 584.65±20.97 | 599.49±21.06 | 608.27±21.94 | 616.39±22.63 |
| Sev + DHA (3
g/kg) | 583.36±20.09 | 599.78±21.43 | 611.03±22.06 | 619.56±23.74 |
The RF in the Sev group was significantly reduced
compared with those in the control and DHA groups (Fig. 2). The BOS in the Sev + DHA (3 g/kg)
group was not significantly reduced compared with control and DHA
groups. The HR in the Sev, Sev + DHA (0.3 g/kg), Sev + DHA (1 g/kg)
and Sev + DHA (3 g/kg) groups were significantly increased compared
with that in the control or DHA groups. The HR in Sev + DHA (1
g/kg) and Sev + DHA (3 g/kg) groups were significantly decreased
compared with the Sev group. No significant differences were seen
between the control and DHA groups (Fig. 2).
DHA improves spatial learning in rats
following repeated sevoflurane treatment
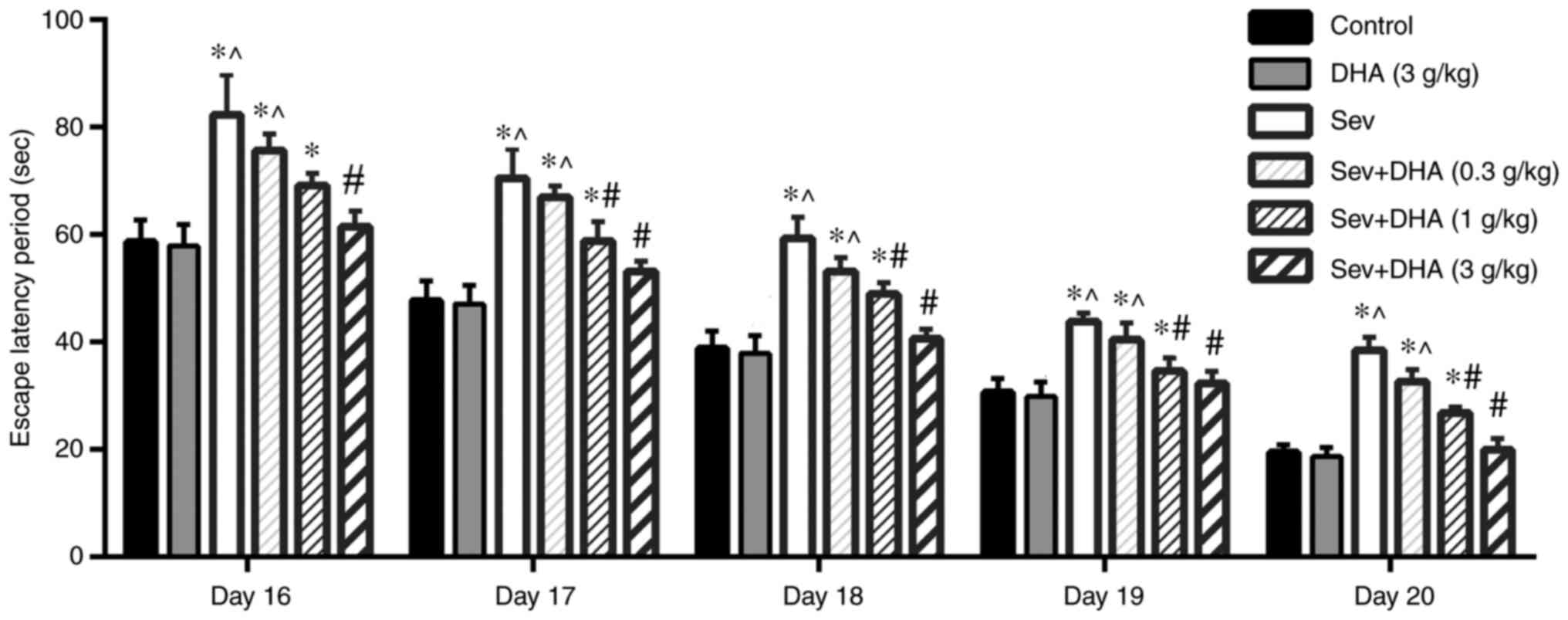
Compared with that in the control and DHA groups,
the escape latency period of rats in the Sev group was
significantly increased from 16-20 days (Fig. 3). Compared with that in the Sev
group, the escape latency of rats in the Sev + DHA (0.3 g/kg), Sev
+ DHA (1 g/kg) and Sev + DHA (3 g/kg) groups was decreased, where
DHA exhibited a dose-dependent effect. Sev + DHA (1 g/kg) group was
significantly decreased compared with the Sev group from 17-20
days. The effect mediated by the Sev + DHA (3 g/kg) group was
demonstrated to be significantly different compared with that in
the Sev group (Fig. 3).
DHA improves memory in rats following
repeated sevoflurane treatment
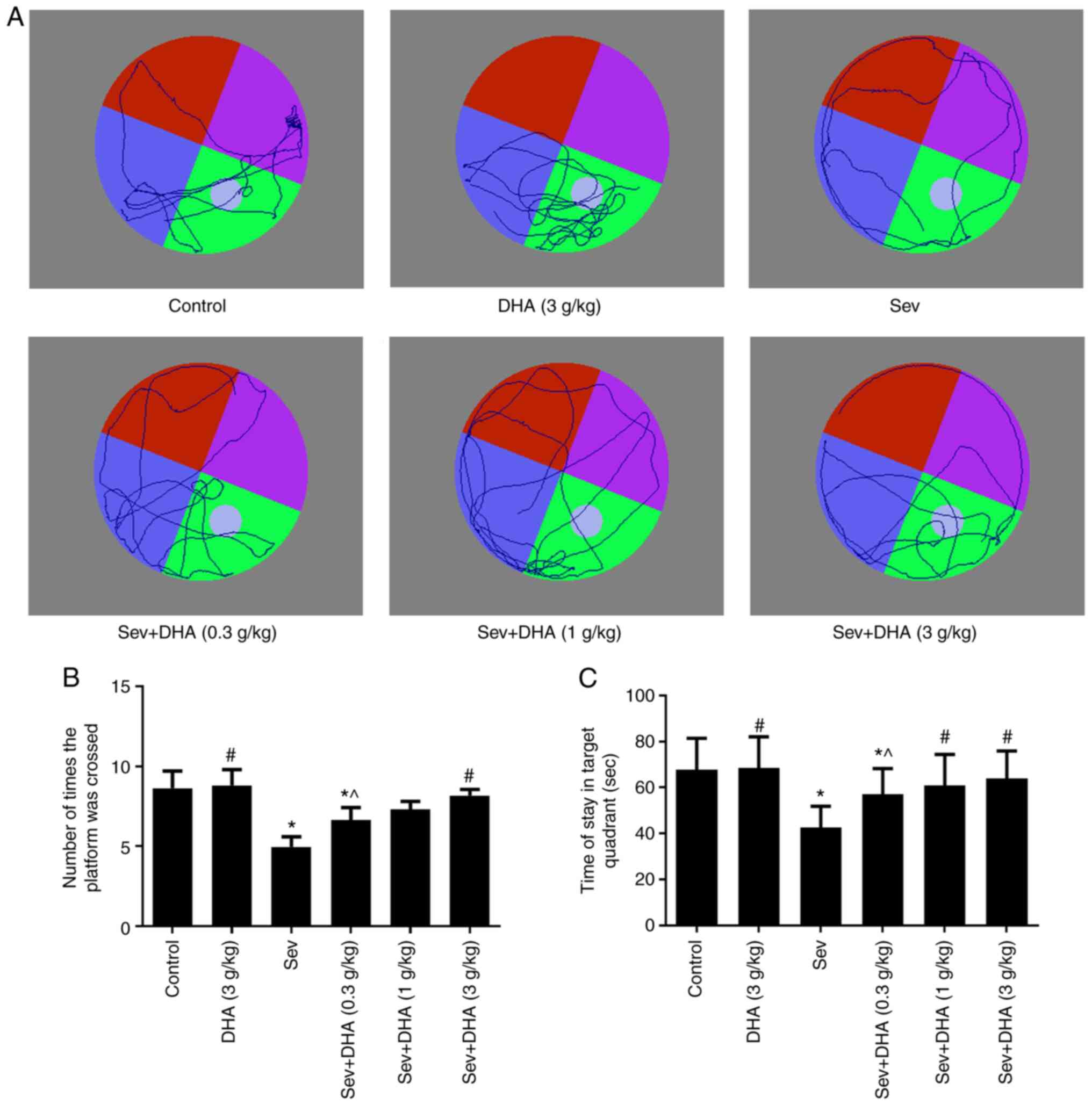
Compared with that in the control and DHA groups,
the number of times the rats crossed the platform and the time rats
stayed in the target quadrant in the Sev group were significantly
decreased following sevoflurane (Fig.
4A-C). Compared with that in the Sev group, the number of times
the rats crossed the platform and the time rats stayed in the
target quadrant in the Sev + DHA (0.3 g/kg), Sev + DHA (1 g/kg) and
Sev + DHA (3 g/kg) groups were markedly increased. In particular,
the effects exerted by the Sev + DHA (1 g/kg) and Sev + DHA (3
g/kg) groups were statistically significant compared with those in
the Sev group (Fig. 4B and
C).
Histopathological examination of the
rat hippocampus following repeated sevoflurane and DHA
treatment
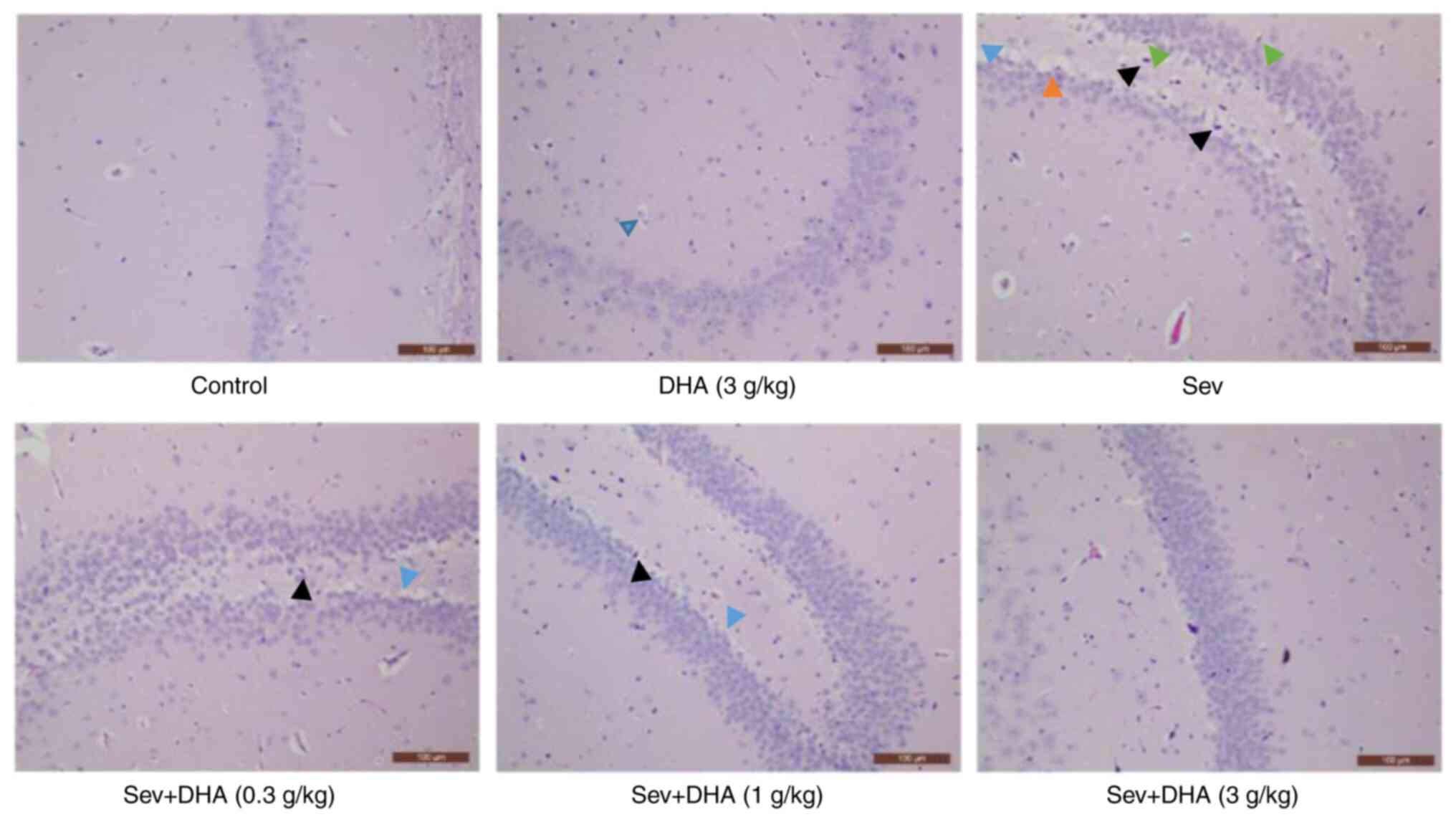
Hippocampus section images in the control and DHA (3
g/kg) groups were observed to be normal, whereas tissues from rats
in the Sev, Sev + DHA (0.3 g/kg), Sev + DHA (1 g/kg) and Sev + DHA
(3 g/kg) groups all exhibited pathological changes in the
hippocampus (Fig. 5). Pathological
changes observed in the Sev group included the disordered
arrangement of neurons, deep staining of neuronal nucleus pyknosis,
cell edema and microglia foaming, with certain areas exhibiting a
small amount of cell necrosis. These aforementioned pathological
changes in the Sev + DHA (0.3 g/kg) and Sev + DHA (1 g/kg) groups
appeared to be slightly reduced compared with those in the Sev
group. However, neurons in these two DHA groups also exhibited a
degree of disordered arrangement, deep staining of the neuronal
nucleus pyknosis and cell edema. The pathological changes in the
Sev + DHA (3 g/kg) group appeared to have been alleviated compared
with those in the Sev + DHA (0.3 g/kg) and Sev + DHA (1 g/kg)
groups.
Effects of repeated sevoflurane
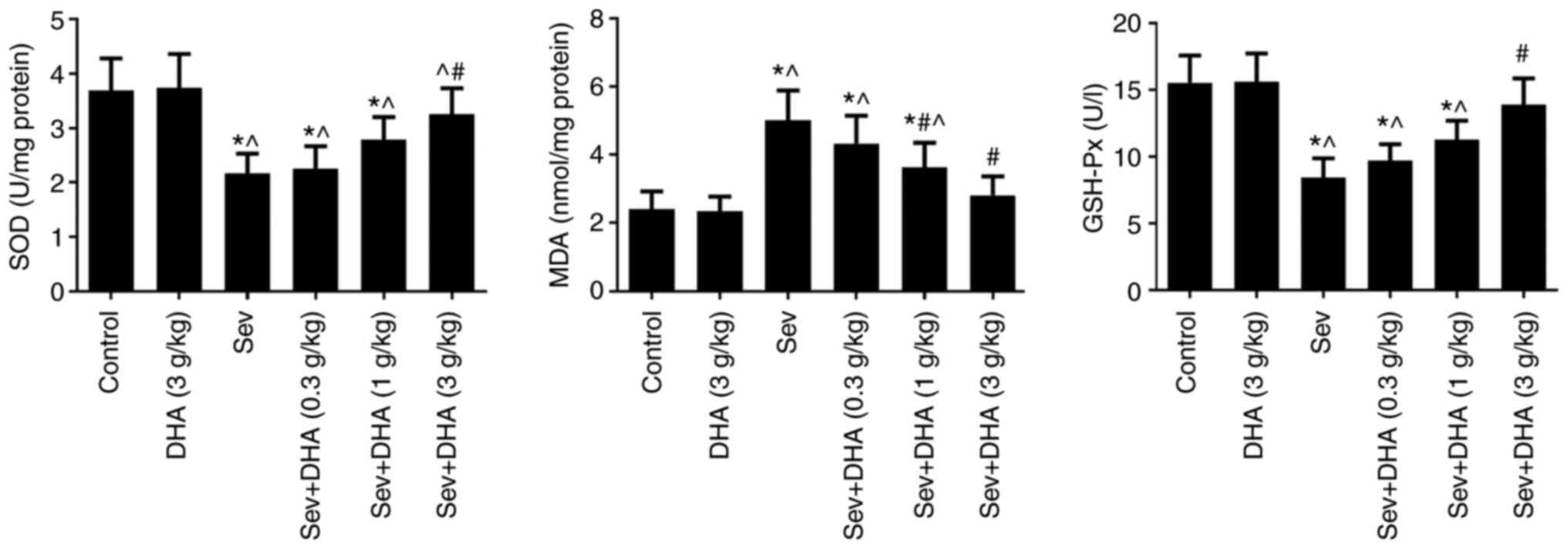
treatment and DHA on SOD, MDA and GSH-Px levels
SOD and GSH-Px levels were significantly decreased
in the Sev group compared with those in the control and DHA groups
(Fig. 6). DHA treatment resulted
in marked increases in SOD and GSH-Px levels in rats exposed to
repeated sevoflurane anesthesia, where there was a significant
difference between Sev and Sev + DHA (3 g/kg) groups (Fig. 6). No significant differences in
SOD, GSH-Px or MDA levels were observed between the control and DHA
groups. In the Sev group, MDA levels were significantly increased
compared with those in the control and DHA groups (Fig. 6). DHA treatment resulted in a
marked decreases in MDA levels in rat brain samples following
exposure to repeated sevoflurane anesthesia, where significant
differences were observed between the Sev group and the Sev + DHA
(1 g/kg) or Sev + DHA (3 g/kg) groups (Fig. 6). These results suggested that DHA
treatment may reduce oxidative stress caused by repeated
sevoflurane anesthesia.
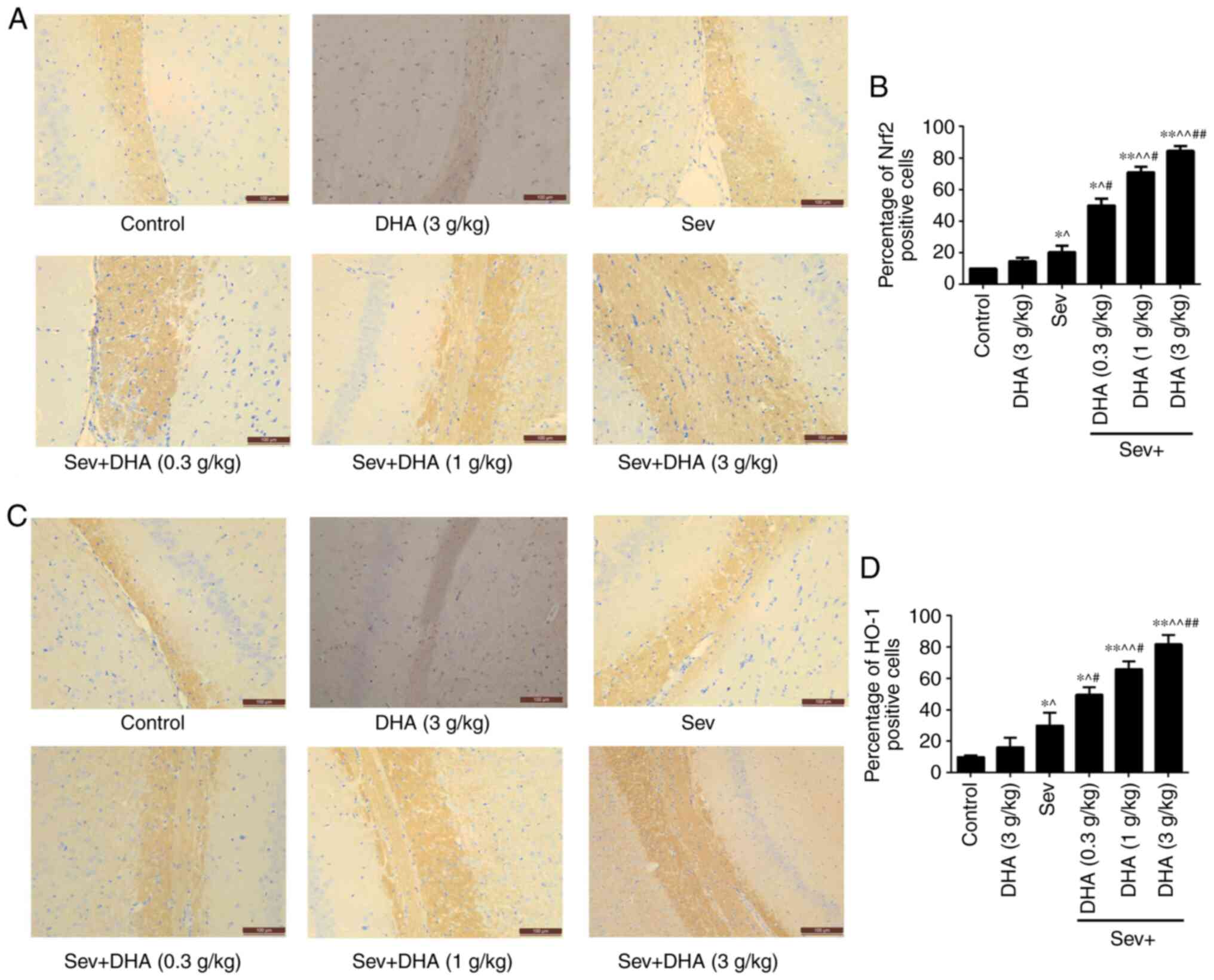
Effects of sevoflurane and DHA
treatment on Nrf2 and HO-1 protein expression
The background color of immunohistochemical staining
is uniform and does not affect the cell count. The cell count was
calculated based on the strong staining of the brown parts. The
results of immunohistochemical staining revealed that compared with
that in the control and DHA groups, Nrf2 and HO-1 staining in the
Sev and Sev + DHA treatment groups increased in a DHA
dose-dependent manner. Compared with the Sev group, Nrf2 and HO-1
staining in Sev + DHA treatment groups significantly increased.
Staining in the Sev + DHA (3 g/kg) group was the highest (Fig. 7).
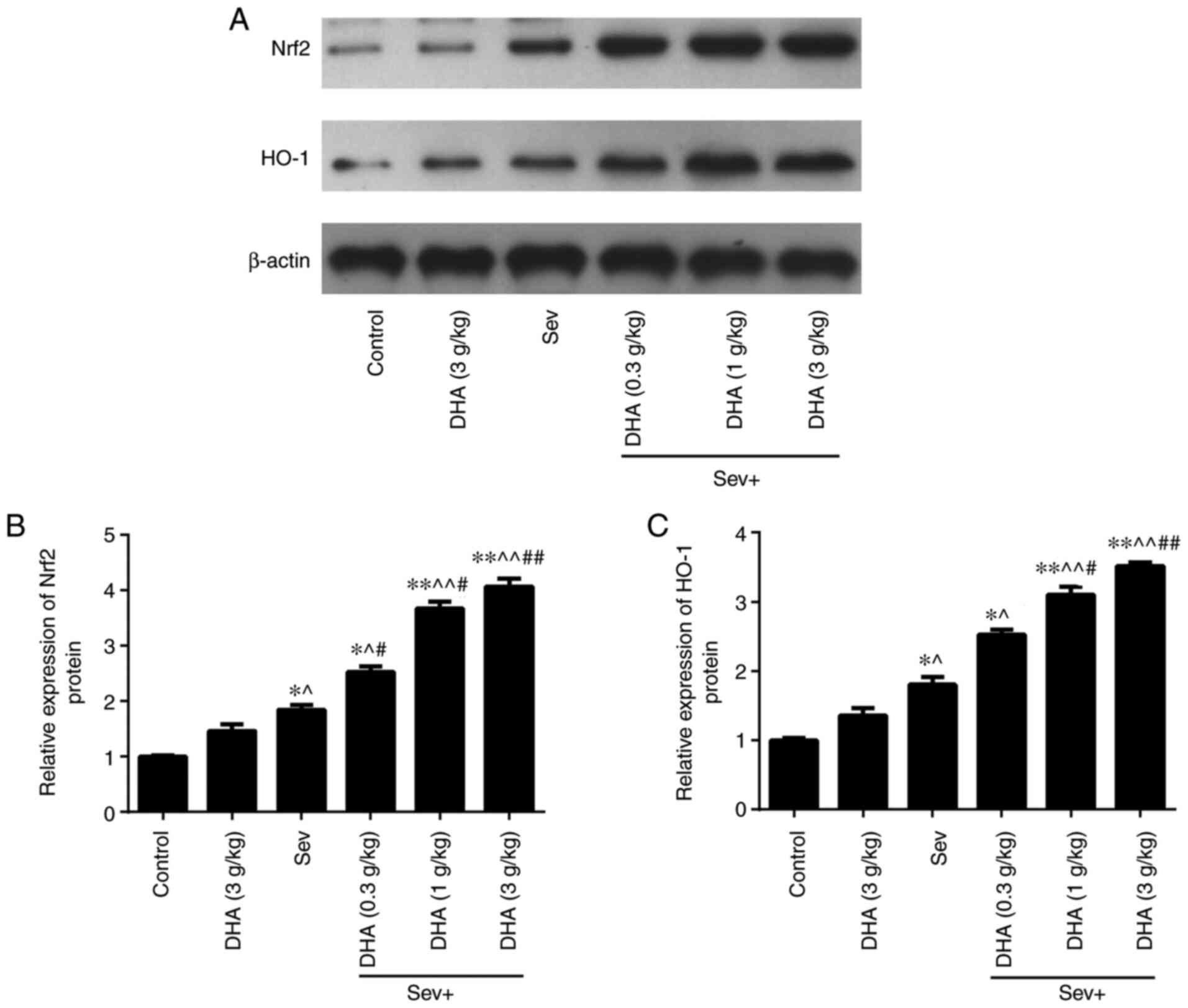
Compared with those in the control and DHA groups,
the protein expression levels of Nrf2 and HO-1 in the Sev group
were significantly increased (Fig.
8). The Nrf2 and HO-1 protein expression levels in the Sev +
DHA treatment groups were increased further compared with those in
the Sev group. In the Sev + DHA treatment groups, the Nrf2 and HO-1
protein expression levels were significantly increased compared
with those in the Sev group (Fig.
8). These results suggested that DHA may alleviate damage
caused by repeated sevoflurane anesthesia on the brain tissue of
rats by increasing the Nrf2 and HO-1 protein expression levels.
Discussion
In the present study, following repeated exposure to
sevoflurane anesthesia, rats were observed to exhibit decreased
spatial learning capabilities, aimless movement, reduced memory
capacity, required more time for space exploration and crossing the
platform. A previous study demonstrated that long-term exposure to
sevoflurane (2.5% for 30 min) can lead to neurodevelopmental
disorders, impaired learning and memory in 7-day-old and 15-day-old
rats (16). Furthermore, a
clinical study in elderly individuals has also reported that
exposure to sevoflurane is associated with a decline in
postoperative cognitive function (17). The neurotoxicity of sevoflurane is
time- and dose-dependent (18).
Another study previously demonstrated that exposure of pregnant
rats to sevoflurane can lead to brain damage in the neonatal
offspring within 2 weeks of birth (19). In laboratory animals (rat and
mouse) exposed to sevoflurane during the peak period of
neurodevelopment, synaptic plasticity and long-term potentiation
were indicated to be affected, as learning and memory capabilities
were decreased (20,21). In addition, a previous study
presented that sevoflurane repeated exposure (2 h daily for 5
consecutive days) can damage the learning and memory of 16-18
months old male rats (22). In the
present study, behavioral experiments in aged rats revealed that
the learning and memory abilities of those in sevoflurane-exposed
groups were lower compared with those in the control group. The
results of the present study are consistent with those reported by
the previous studies aforementioned.
DHA is a structural plasma membrane component that
is important for normal brain function and can be readily obtained
from fish oil (23). DHA is
therefore applied as a health product or nutritional supplement
(23). A previous study reported
that the incidence of neurodegenerative diseases is lower in
populations that adopt a Mediterranean diet, which may be
associated with the long-term intake of foods with a high DHA
content (24). In previous
studies, DHA and/or eicosapentaenoic acid supplements were reported
to improve cognitive function and protect against neuroinflammation
and oxidative stress in rodents (25,26).
In the present study, following the administration of different
doses of DHA in aged rats exposed to repeated sevoflurane
anesthesia, spatial exploration and navigational abilities were
ameliorated in a dose-dependent manner. These results suggested
that DHA may effectively reverse spatial learning and memory
impairments induced by repeated sevoflurane anesthesia.
Histopathological examination of the rat brain tissues also
revealed that DHA can prevent brain damage to alleviate cognitive
impairment in rats exposed to repeated sevoflurane anesthesia.
Furthermore, the effect of DHA was enhanced in a dose-dependent
manner. These results suggested that DHA may exert protective
effects against learning and memory impairment induced by repeated
sevoflurane anesthesia in aged rats.
Activation of the Nrf2/HO-1 signaling pathway serves
an important role in ameliorating brain injury and is key to the
anti-oxidative stress response in the body (27). Nrf2 nuclear translocation is
important for HO-1 activation (28). In the present study, SOD, GSH-Px
and MDA levels were detected in rat brain tissues. The results
demonstrated that DHA ameliorated oxidative stress induced by
repeated sevoflurane anesthesia in aged rats. Immunohistochemistry
and western blotting were used to verify whether the DHA-induced
anti-oxidative stress effects were mediated through the Nrf2/HO-1
signaling pathway. The results demonstrated that Nrf2 and HO-1
protein expression was increased in each of the DHA dose groups
compared with that in the Sev group in a dose-dependent manner.
Furthermore, Nrf2 and HO-1 protein expression levels in the Sev
group were also increased compared with control and DHA group. A
previous study indicated that activation of the Nrf2/HO-1 signaling
pathway is the main mechanism of cellular defense against oxidative
stress (29). It can therefore be
hypothesized that the increased Nrf2 and HO-1 protein expression
levels may be induced as a cellular defense mechanism. However,
this was not explored further in the present study. Therefore, in
future studies, experiments will be required to verify the
molecular mechanism through which DHA mediates its effects in the
repeated sevoflurane anesthesia model.
In conclusion, the results of the present study
indicated that DHA exhibited protective effects against learning
and memory impairment in aged rats, which was induced by repeated
sevoflurane anesthesia. The increased Nrf2 and HO-1 protein
expression levels suggested that the mechanism may associated with
the Nrf2/HO-1 signaling pathway.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Science and
Technology Project of Yantai City (grant no. 2016WS009).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
MT and XLZ designed the study. MT, YXW and DGL
performed the experiments. MT, YXW and DGL performed data analysis,
interpreted the data and acquired samples. MT, YXW and XLZ
contributed to pathological analysis. MT and XLZ confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by Animal Protection
and Use Committee of The Affiliated Yantai Yuhuangding Hospital of
Qingdao University (Yantai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gross AF and Stern TA: Neuropsychiatric
conditions associated with anesthesia exposure. Psychosomatics.
55:21–28. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yufune S, Satoh Y, Akai R, Yoshinaga Y,
Kobayashi Y, Endo S and Kazama T: Suppression of ERK phosphor
rylation through oxidative stress is involved in the mechanism
under lying sevoflurane-induced toxicity in the developing brain.
Sci Rep. 6(21859)2016.
|
|
3
|
Kabuto H, Amakawa M, Mankura M, Yamanushi
TT and Mori A: Docosahexaenoic acid ethyl ester enhances
6-hydroxydopamine-induced neuronal damage by induction of lipid
peroxidation in mouse striatum. Neurochem Res. 34:1299–1303.
2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bazan NG: Neuroprotectin D1 (NPD1): A
DHA-derived mediator that protects brain and retina against cell
injury-induced oxidative stress. Brain Pathol. 15:159–166.
2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hartmann T, van Wijk N, Wurtman RJ,
Rikkert MG, Sijben JW, Soininen H, Vellas B and Scheltens P: A
nutritional approach to ameliorate altered phospholipid metabolism
in Alzheimer's disease. J Alzheimers Dis. 41:715–717.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Létondor A, Buaud B, Vaysse C, Fonseca L,
Herrouin C, Servat B, Layé S, Pallet V and Alfos S: Erythrocyte DHA
level as a biomarker of DHA status in specific brain regions of n-3
long-chain PUFA-supplemented aged rats. Br J Nutr. 112:1805–1818.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dyall SC, Michael GJ, Whelpton R, Scott AG
and Michael-Titus AT: Dietary enrichment with omega-3
polyunsaturated fatty acids reverses age-related decreases in the
GluR2 and NR2B glutamate receptor subunits in rat forebrain.
Neurobiol Aging. 28:424–439. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Favrelière S, Perault MC, Huguet F, De
Javel D, Bertrand N, Piriou A and Durand G: DHA-enriched
phospholipid diets modulate age-related alterations in rat
hippocampus. Neurobiol Aging. 24:233–243. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bang HY, Park SA, Saeidi S, Na HK and Surh
YJ: Docosahexaenoic acid induces expression of heme oxygenase-1 and
NAD(P)H: Quinone oxidoreductase through activation of Nrf2 in human
mammary epithelial cells. Molecules. 22(969)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Loboda A, Damulewicz M, Pyza E, Jozkowicz
A and Dulak J: Role of Nrf2/HO-1 system in development, oxidative
stress response and diseases: An evolutionarily conserved
mechanism. Cell Mol Life Sci. 73:3221–3247. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Konrad FM, Knausberg U, Höne R, Ngamsri KC
and Reutershan J: Tissue heme oxygenase-1 exerts anti-inflammatory
effects on LPS-induced pulmonary inflammation. Mucosal Immunol.
9:98–111. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang LL, Yu QL, Han L, Ma XL, Song RD,
Zhao SN and Zhang WH: Study on the effect of reactive oxygen
species-mediated oxidative stress on the activation of
mitochondrial apoptosis and the tenderness of yak meat. Food Chem.
244:394–402. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Piantadosi CA, Carraway MS, Babiker A and
Suliman HB: Heme oxygenase-1 regulates cardiac mitochondrial
biogenesis via Nrf2-mediated transcriptional control of nuclear
respiratory factor-1. Circ Res. 103:1232–1240. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cui Y, Ma S, Zhang C, Li D, Yang B, Lv P,
Xing Q, Huang T, Yang GL, Cao W and Guan F: Pharmacological
activation of the Nrf2 pathway by 3H-1, 2-dithiole-3-thione is
neuroprotective in a mouse model of Alzheimer disease. Behav Brain
Res. 336:219–226. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
National Institutes of Health (NIH): Guide
for the Care and Use of Laboratory Animals. National Academies
Press, Washington, DC, 1996.
|
|
16
|
Qiu L, Zhu C, Bodogan T, Gómez-Galán M,
Zhang Y, Zhou K, Li T, Xu G, Blomgren K, Eriksson LI, et al: Acute
and long-term effects of brief sevoflurane anesthesia during the
early postnatal period in rats. Toxicol Sci. 149:121–133.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Micha G, Tzimas P, Zalonis I, Kotsis K,
Papdopoulos G and Arnaoutoglou E: Propofol vs Sevoflurane
anaesthesia on postoperative cognitive dysfunction in the elderly.
A randomized controlled trial. Acta Anaesthesiol Belg. 67:129–137.
2016.PubMed/NCBI
|
|
18
|
Qi J, Wang W, Lu H, Wang Y and Li Z: The
role of Bag2 in neurotoxicity induced by the anesthetic
sevoflurane. J Cell Biochem: doi: 10.1002/jcb.28029, 2018.
|
|
19
|
Zhang Y, Wu Z, Li X, Wan Y, Zhang Y and
Zhao P: Maternal sevoflurane exposure affects differentiation of
hippocampal neural stem cells by regulating miR-410-3p and ATN1.
Stem Cell Res Ther. 11(423)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Drobish JK, Gan ZS, Cornfeld AD and
Eckenhoff MF: From the cover: Volatile anesthetics transiently
disrupt neuronal development in neonatal rats. Toxicol Sci.
154:309–319. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhao Y, Chen K and Shen X: Environmental
enrichment attenuated sevoflurane-induced neurotoxicity through the
PPAR-γ signaling pathway. Biomed Res Int.
2015(107149)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guo S, Liu L, Wang C, Jiang Q, Dong Y and
Tian Y: Repeated expose to sevoflurane impairs the learning and
memory of older male rats. Life Sci. 192:75–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Calder PC and Yaqoob P: Omega-3
polyunsaturated fatty acids and human health outcomes. Biofactors.
35:266–272. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Ho CF, Bon CP, Ng YK, Herr DR, Wu JS, Lin
TN and Ong WY: Expression of DHA-Metabolizing Enzyme Alox15 is
regulated by selective histone acetylation in neuroblastoma cells.
Neurochem Res. 43:540–555. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Valentini KJ, Pickens CA, Wiesinger JA and
Fenton JI: The effect of fish oil supplementation on brain DHA and
EPA content and fatty acid pro file in mice. Int J Food Sci Nutr.
69:705–717. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Butler MJ, Deems NP, Muscat S, Belury MA
and Barrientos RM: Dietary DHA prevents cognitive impairment and
inflammatory gene expression in aged males rats fed a diet enriched
with refinedcarbohydrates. Brain Behav Immun. 98:198–209.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shu L, Wang C, Wang J, Zhang Y, Zhang X,
Yang Y, Zhuo J and Liu J: The neuroprotection of hypoxic
preconditioning on rat brain against traumatic brain injury by
up-regulated transcription factor Nrf2 and HO-1 expression.
Neurosci Lett. 611:74–80. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Z, Zhang H, Sun X and Ren L: The
protective role of vitamin D3 in a murine model of asthma via the
suppression of TGF-β/Smad signaling and activation of the Nrf2/HO-1
pathway. Mol Med Rep. 14:2389–2396. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 5:13291–13295.
2009.PubMed/NCBI View Article : Google Scholar
|