Introduction
Remote ischaemic preconditioning (RIPC) is known to
protect the heart against myocardial ischaemia/reperfusion (I/R)
injury in numerous experimental and clinical settings (1-10),
but the relevant mechanisms remain poorly understood. However,
RIPC-associated cardio-protection may be mediated in part by the
release of effector extracellular particles (EPs), including
extracellular vesicles (EVs), lipoprotein particles and
ribonucleoprotein particles, that activate cardioprotective
pathways and lead to higher resistance of the heart to I/R injury
(8-10).
These particles carry non-coding RNAs, proteins and lipids that
mediate cellular responses through autocrine, paracrine and
endocrine mechanisms, and their composition and concentration vary
under different pathophysiological conditions, such as hypoxia and
radiation (10-13).
At present, it is unclear whether the composition, concentration
and function of plasma EPs change under RIPC conditions.
Hypoxia-inducible factor 1 (HIF-1) is a nuclear
transcription factor composed of the HIF-1α and HIF-1β subunits
that regulates the transcription of hundreds of genes (14,15).
While HIF-1β is constitutively expressed, HIF-1α is upregulated and
stabilized in response to hypoxia. Therefore, HIF-1 activity is
mainly dependent on the level of HIF-1α expression (14,15).
Over the past decade, HIF-1α has been established as a central
regulator of oxygen homeostasis; it regulates energy utilization,
oxidative stress, metabolism, cell survival and cell death through
the transcriptional activation of hundreds of target genes
(16). Hypoxia has been indicated
to induce cardiomyocyte and pulmonary arterial smooth muscle cell
proliferation in a HIF-1α-dependent manner (17). Emerging evidence in a previous study
has also demonstrated that HIF-1α mediated RIPC-associated
protection against myocardial injury by activating interleukin-10
(IL-10) gene transcription (18).
The vascular endothelium, especially in the heart,
could play a significant role in the RIPC-mediated mechanisms of
heart protection from I/R injury (19,20):
i) Humoral factors that are released into the circulatory systems
under RIPC stimulus may directly interact with endothelial cells
which directly or indirectly transfer the RIPC stimulus to the
heart; ii) endothelial cells are among the first cell types that
encounter hypoxia in the heart and respond to it; and iii)
endothelial dysfunction is a central reason for severe local and
systemic consequences of I/R injury. Moreover, endothelial changes
and vascular dysfunction serve critical roles in I/R injury
(19,20). These data indicated that the
improvement of endothelial function may be one possible explanation
for the protective effects of RIPC.
In the present study, healthy male volunteers were
subjected to a RIPC protocol with a 12-cm-wide cuff placed around
the upper nondominant arm (21,22). A
blood pressure cuff was alternatively inflated (up to 200 mmHg) for
5 min and deflated for the same duration for four successive cycles
(23,24) to induce RIPC. Laser Doppler blood
flow (LDF) measurements were performed to confirm successful
induction of transient upper limb ischaemia after RIPC treatment
(25). EPs were derived from
volunteers who did or did not undergo RIPC using an
ultracentrifugation-based method (26). Human umbilical vein endothelial
cells (HUVECs) were assigned to two groups: i) Group 1 was
preincubated for 24 h with EPs from volunteers after sham-RIPC,
then treated with H2O2 (1 mM; 6 h) mimicking
the in vivo conditions of I/R-induced oxidative stress
(27); and ii) group 2 was
preincubated for 24 h with EPs from volunteers after RIPC, then
treated with H2O2.
Moreover, a total of 32 8-week-old male Sprague
Dawley rats were used in the present study. A remote hind limb
preconditioning stimulus was delivered using a blood pressure cuff
attached at the inguinal level of the rat. The blood pressure cuff
was alternatively inflated (up to 150 mmHg) for 5 min, then
deflated for the same duration for four successive cycles to induce
conditioning of the tissue (23,24).
EPs were derived from rats that received RIPC or sham-RIPC and/or
cadmium (Cd) pre-treatment (28).
HUVECs were assigned to six groups: i) Group 1 was untreated; ii)
group 2 received only H2O2 treatment (1 mM; 6
h); iii) group 3 was preincubated for 24 h with EPs from rats
exposed to sham-RIPC, then treated with H2O2;
iv) group 4 was preincubated for 24 h with EPs from rats that
received an intraperitoneal injection of 1 mg/kg Cd (a
pharmacological inhibitor of HIF-1α in vivo) 180 min before
sham-RIPC then treated with H2O2; v) group 5
was preincubated for 24 h with EPs from rats exposed to RIPC, then
treated with H2O2; and vi) group 6 was
preincubated for 24 h with EPs from rats that received an
intraperitoneal injection of 1 mg/kg Cd 180 min before RIPC, then
treated with H2O2.
We hypothesized that EPs released during RIPC
preconditioning in volunteers or rats could contribute to
mitigating oxidative stress-induced damage, including cell
viability, cytotoxicity, apoptosis and necrosis in HUVECs, and that
these processes may further involve altered HIF-1α expression.
Materials and methods
RIPC models
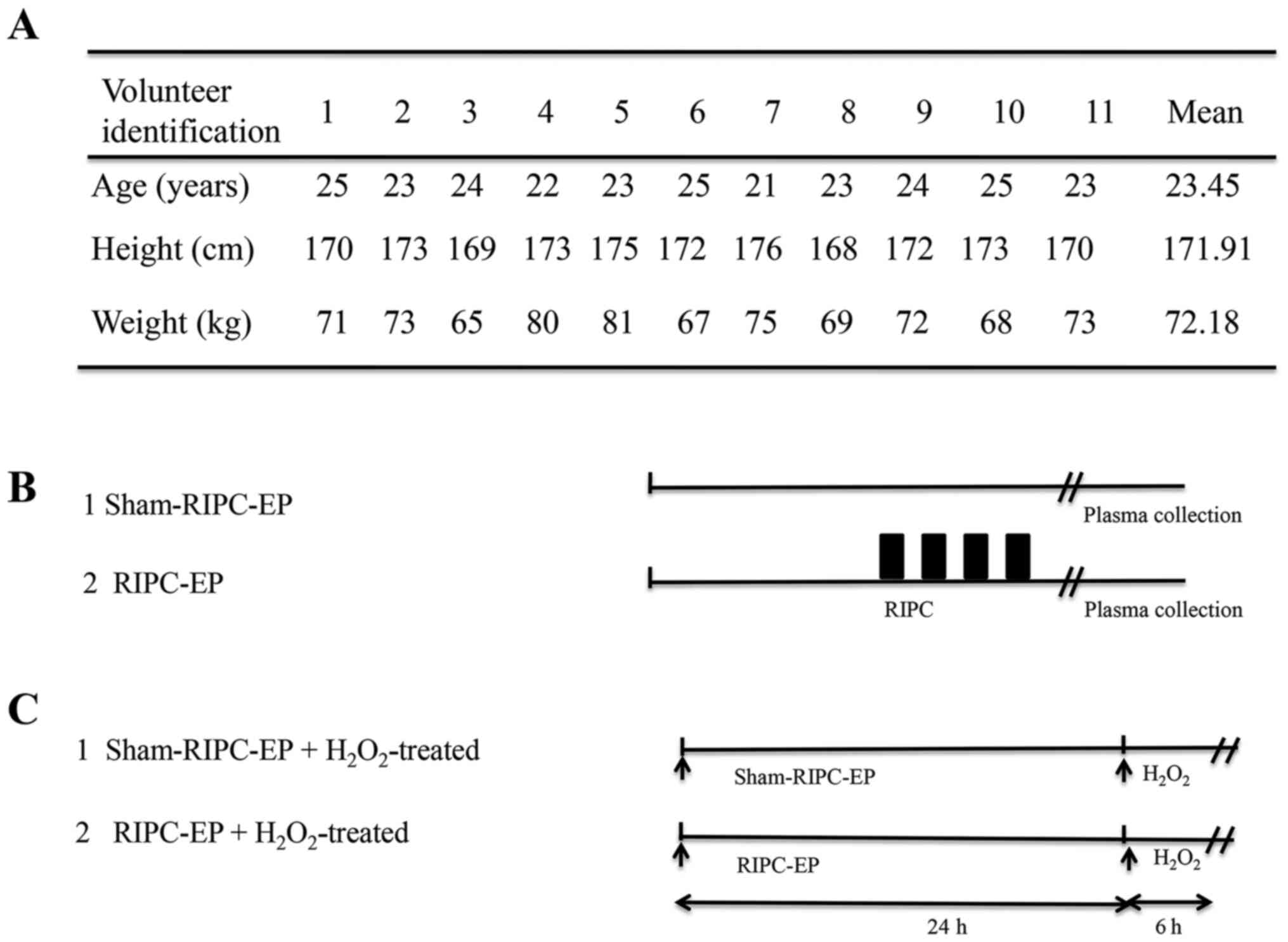
A total of 11 healthy male volunteers (mean age,
23.45 years; age range, 21-25 years; mean body mass index,
24.42±1.38 kg/m2) were recruited in the Second
Affiliated Hospital of Nanchang University (Nanchang, China)
between 18 and 20 July 2019 and examined in a
temperature-controlled laboratory (24-26˚C). The exclusion criteria
were as follows: i) Cardio-cerebro-vascular, pulmonary, liver,
kidney, infectious or immune diseases; ii) alcohol or drug abuse;
and iii) malignant tumours. The present study only included male
volunteers to avoid potential effects of oestrogens (21). The volunteers were subjected to a
RIPC protocol and treated with a 12-cm-wide cuff (OMRON Healthcare,
Inc.) placed around the upper nondominant arm (22). Six volunteers were subjected to RIPC
and five volunteers to sham-RIPC. To induce RIPC, the blood
pressure cuff was alternatively inflated (up to 200 mmHg) for 5 min
and deflated for the same duration for four successive cycles
(23,24). For volunteers subjected to
sham-RIPC, the cuff was put around the arm without adding pressure.
LDF measurements were performed to confirm successful induction of
transient upper limb ischaemia after RIPC treatment.
The blood flow in the upper limb was diminished
during RIPC compared to the sham-RIPC group, as measured using a
laser Doppler flowmeter (Omegaflo FLO-C1 Omegawave Laser Tissue
Blood Flow Meter; OMEGAWAVE, Inc.) (25). The probe for the blood flow (ML
type; OMEGAWAVE, Inc.) and the thermistor for the temperature
(TSD202F type; BIOPAC® Systems, Inc.) were attached to
the ventral surface of the distal phalanx of the middle finger
using surgical tape. The diameter and the penetration depth of the
LDF probes were 15 and 1.0 mm, respectively, and the diameter of
the skin temperature sensor was 9.8 mm. To reduce the risk of water
intrusion between the probes and the skin and the influence of the
medium temperature on the LDF measurement, the probes were covered
by a custom-made heat insulator. The thermistor was connected to an
amplifier (SKT100C type; BIOPAC® Systems, Inc.), and the
finger skin blood flow and temperature were recorded at 200 Hz
using a data acquisition and analysis software (MP150 software;
v3.4.3; BIOPAC® Systems, Inc.) (25).
The volunteer characteristics and study design are
presented in Fig. 1A and B. Written informed consent was obtained
from all participants before they entered the present study. The
present study was conducted in accordance with The Declaration of
Helsinki and was approved by the Ethics Committee of the Second
Affiliated Hospital of Nanchang University [approval no. SYXK(G)
2019-0007; Nanchang, China].
A total of 32 8-week-old male Sprague Dawley rats
(weight, 150-200 g) were used in the present study. The animals
were supplied by the Animal Research Department of Nanchang
University. Rats were kept under standard conditions at 22±2˚C,
with indoor sterile fresh air and a 12-h light-dark cycle with free
access to water and food. Humidity levels were between 45 and 55%.
The rats were anaesthetized with pentobarbital sodium (40 mg/kg;
intraperitoneal injection). A remote hind limb preconditioning
stimulus was delivered using a 1-cm-wide blood pressure cuff (OMRON
Healthcare, Inc.) attached at the inguinal level of the rat. The
blood pressure cuff was alternatively inflated (up to 150 mmHg) for
5 min, then deflated for the same duration for four successive
cycles to induce conditioning of the tissue (Fig. 2A) (23,24).
Using 3.5x magnifying surgical glasses, venous congestion was
observed during occlusion, which was rapidly followed by brisk
reactive hyperaemia during reperfusion. The body temperature was
maintained at 37˚C. The reproducibility and reliability of the
method of inducing rat lower-limb ischaemia has been verified via a
modified pulse oximetry protocol for use in rats (24). A total of 180 min before RIPC
stimulus, rats received a single intraperitoneal injection of 1
mg/kg Cd chloride (MilliporeSigma) dissolved in PBS in RIPC-EP
group (28).
After the study, the rats were anaesthetized by
isoflurane inhalation (3%) plus 1 l/min O2 and
euthanized by exsanguination. Rat limb muscle tissues and blood
were isolated from rats after sacrifice and were stored at
-20˚C.
All animal experiments were conducted in compliance
with the Guide for the Care and Use of Laboratory Animals published
by the US National Institutes of Health (NIH publication no. 85-23,
revised 1996) and were approved by the Ethics Committee of the
Second Affiliated Hospital of Nanchang University [approval no.
SYXK(G) 2019-0102; Nanchang, China].
Plasma collection, plasma preparation
and extracellular particle enrichment
Human volunteer (10 ml) and rat blood samples (10
ml) were collected immediately after sham-RIPC or RIPC into
K2EDTA tubes (BD Biosciences) and processed within 5 min
for plasma preparation. The blood samples were first centrifuged at
1,500 x g for 15 min at room temperature. The supernatants were
collected and transferred to nuclease-free tubes. EPs were enriched
using an ultracentrifugation-based method according to methods
described previously (26).
Briefly, 10 µl of 500 U/ml thrombin were added to 1 ml of plasma.
The solution was incubated for 5 min at room temperature and
centrifuged for 5 min at 2,000 x g at 4˚C. Subsequently, the plasma
was filtered using a 0.22-µm pore filter (Steradisc; Kurabo
Industries Ltd. Bio-Medical Department). Next, the filtrate was
ultracentrifuged at 100,000 x g for 70 min at 4˚C (Optima™ XE-90
ultracentrifuge with a swing rotor; cat. no. SW41Ti; Beckman
Coulter, Inc.). The cell-free plasma samples were mixed well with
ExoQuick™ Exosome Precipitation Solution (cat. no. EXOQ5A-1;
Shanghai Yeasen BioTechnologies Co., Ltd.). After the mixtures were
incubated at 4˚C for 30 min and centrifuged at 4˚C at 1,500 x g for
30 min, the obtained pellets were washed with PBS. Then, the EP
pellets were dissolved in 20 µl PBS and stored at -80˚C until
further use.
Extracellular particle
characterization by transmission electron microscopy (TEM)
TEM was used to observe exosome morphology (Hitachi
H-7100 microscope; Hitachi High-Technologies Corporation). For
exosome TEM observation, exosomes were fixed with 2.5%
glutaraldehyde at 4˚C overnight. After washing, the samples were
prepared by dropping 4 µl of exosome solution onto a formvar-coated
copper grid (Sigma-Aldrich; Merck KGaA) for 2 min at 25˚C,
negatively stained with aqueous phosphotungstic acid for 60 sec at
25˚C, and images were taken with a transmission electron microscope
at 80 kV (magnification, x500,000; Hitachi H-7100 microscope;
Hitachi High-Technologies Corporation). The images were observed
using Image-Pro Plus (v6.0; Media Cybernetics, Inc.).
Nanoparticle tracking analysis
(NTA)
Analysis of the EP size distribution was performed
using NanoSight NS300 (Malvern Instruments, Ltd.). The particles
were automatically tracked and sized based on their Brownian motion
and the diffusion coefficient. Resuspended EPs were diluted in 1 ml
sterile PBS. Sterile PBS samples were used to assess background.
The NTA measurement conditions were a temperature of 23.75±0.5˚C,
25 frames per sec and a measurement time of 60 sec. The detection
threshold was identical in all samples. Three recordings were
performed three times for each sample.
Cell culture and treatment
HUVECs were purchased from the American Type Culture
Collection (https://www.atcc.org/products/pcs-100-010; cat. no.
PCS-100-010) and cultured in DMEM (HyClone; Cytiva) supplemented
with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 mg/ml streptomycin in a humidified atmosphere
containing 5% CO2 at 37˚C. HUVECs were treated with
H2O2 (MilliporeSigma) at different
concentrations (0.1, 1 and 10 mM) for 6 h to induce cell apoptosis
and necrosis, thus mimicking the in vivo conditions of
I/R-induced oxidative stress (27).
Confirmation of EP transfer into
HUVECs with PKH26 dye
EPs precipitated from volunteer plasma after RIPC
were mixed with PKH26 Red Fluorescent Cell Linker kit for General
Cell Membrane Labeling (MilliporeSigma) for 4 min at 4˚C, following
the manufacturer's instructions. Subsequently, the reaction was
terminated by incubation with FBS for 5 min at 4˚C. The labelled
material was washed three times with PBS to remove the excess dye
and incubated with HUVECs grown to 70-80% density seeded on
six-well plates for 10 min at 25˚C. The cell nuclei were stained
with DAPI (cat. no. C0065; Beijing Solarbio Science &
Technology Co., Ltd.) for 10 min at 25˚C, and all stained sections
were viewed by confocal microscope (magnification, x500, Olympus
Corporation). The data were visualized and quantified using
Image-Pro Plus v6.0 (Media Cybernetics, Inc.).
Study groups and experimental
protocol
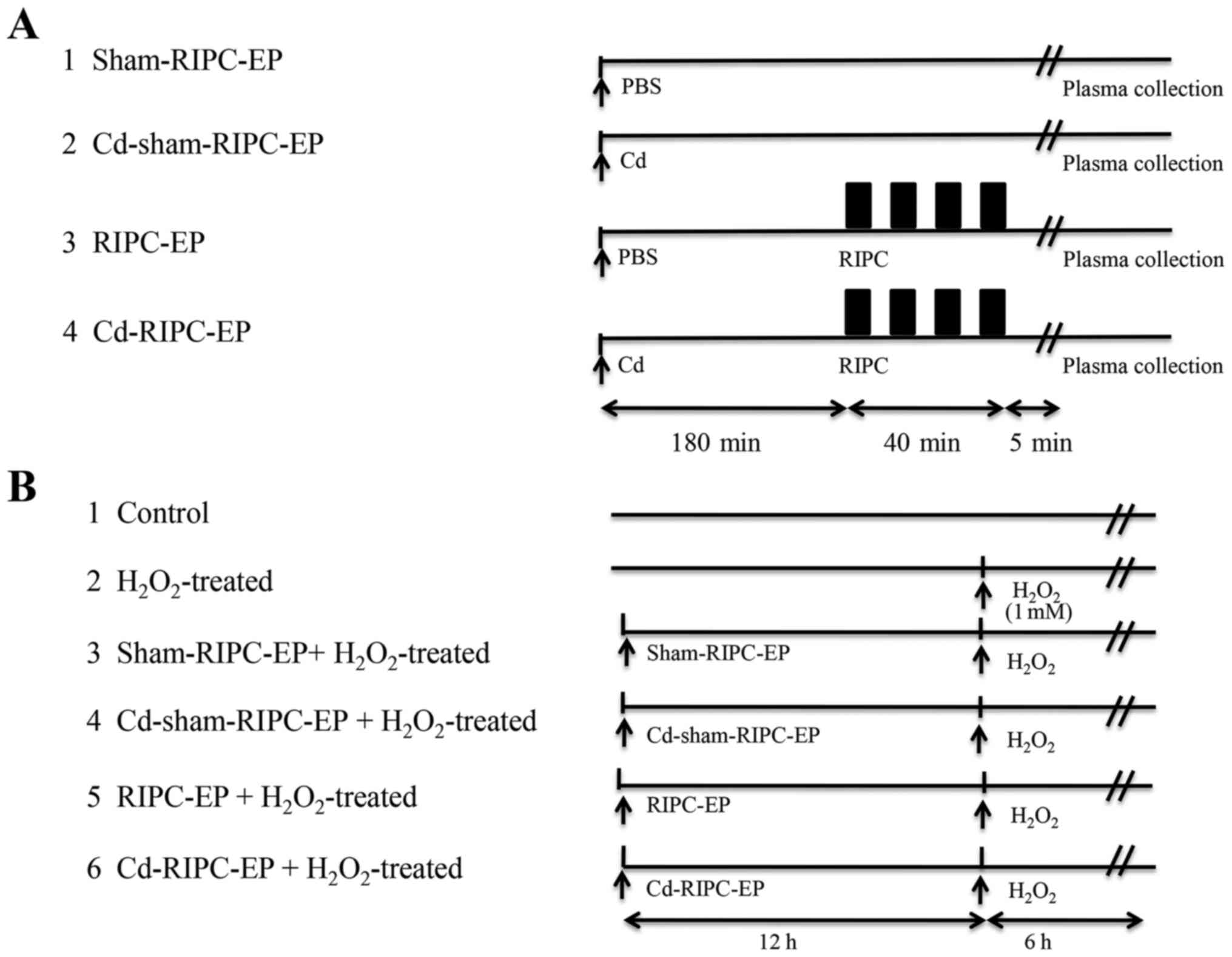
The present study was divided into two parts. In the
first part, EPs were derived from volunteers treated or non-treated
with RIPC. HUVECs were assigned to two groups: i) Group 1 included
HUVECs that were preincubated for 24 h with 4 µl EPs
(1x109 nanoparticles/ml) from volunteers after
sham-RIPC, then treated with H2O2 (1 mM; 6
h); ii) group 2 included HUVECs that were preincubated for 24 h
with 4 µl EPs (1x109 nanoparticles/ml) from volunteers
after RIPC, then treated with H2O2 (1 mM; 6
h). The study design is presented in Fig. 1C. In the second part, EPs were
derived from rats that did or did not receive RIPC and Cd
treatment. HUVECs were assigned to six groups: i) Group 1 (control)
were untreated cells; ii) group 2 received only
H2O2 treatment (1 mM; 6 h); iii) group 3 was
preincubated for 24 h with 4 µl EPs (1x109
nanoparticles/ml) from rats exposed to sham-RIPC, then treated with
H2O2 (1 mM; 6 h); iv) group 4 was
preincubated for 24 h with 4 µl EPs (1x109
nanoparticles/ml) from rats exposed to sham-RIPC that received an
intraperitoneal injection of 1 mg/kg Cd [MilliporeSigma; the dose
was based on the minimal dose required to enhance HIF-1α
degradation (29,30) by the proteasome (31) via an effect on the ubiquitin system
(32)] 180 min before sham-RIPC,
then treated with H2O2 (1 mM; 6 h); v) group
5 was preincubated for 24 h with 4 µl EPs (1x109
nanoparticles/ml) from rats exposed to RIPC, then treated with
H2O2 (1 mM; 6 h); and vi) group 6 was
preincubated for 24 h with 4 µl EPs (1x109
nanoparticles/ml) from rats exposed to RIPC that received an
intraperitoneal injection of 1 mg/kg Cd 180 min before RIPC, then
treated with H2O2 (1 mM; 6 h). The study
design is presented in Fig. 2A and
B.
In vitro lactate dehydrogenase (LDH)
and cell viability assays
HUVECs were exposed to 1 mM
H2O2 for 6 h in the presence or absence of
RIPC-EPs. LDH release, used as a marker of cell injury, was
quantified using a CytoTox-ONE™ Homogeneous Membrane Integrity
Assay (cat. no. G7890; Promega Corporation) according to the
manufacturer's protocol. Cell viability was determined by a Cell
Counting Kit-8 (CCK-8; cat. no. C0037; Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. HUVECs
were incubated with 10 µmol CCK-8 solution at 37˚C for 2 h. The
absorbance at 450 nm was measured using a microplate reader
(Bio-Rad Laboratories, Inc.). Cell viability was calculated based
on the relative optical density compared with that of untreated
controls.
Flow cytometry detection of apoptosis
and necrosis
After treatment with H2O2 or
EPs, cell apoptosis and necrosis were assayed using the FITC
Annexin V Apoptosis Detection kit (cat. no. KGA108; Nanjing KeyGen
Biotech Co., Ltd.) following the manufacturer's instructions.
Briefly, HUVECs were washed in PBS three times and resuspended in
400 µl of binding buffer with FITC Annexin-V and propidium iodide
(PI; 5 µl each). The cell suspension was incubated for 15 min at
room temperature in the dark, then analyzed by flow cytometry (BD
FACSCanto™ II; BD Biosciences) within 1 h. The indexes of apoptosis
and necrosis were calculated by the FlowJo software (v10.4.2; BD
Biosciences). The apoptosis index was expressed as the percentage
of total apoptotic cells, which included the percentage of early
apoptotic cells (Annexin V-positive and PI-negative) plus the
percentage of late apoptotic cells (Annexin V-positive and
PI-positive). The index of necrosis was expressed as the percentage
of necrotic cells (Annexin V-negative and PI-positive).
Western blot analysis
The characterization of the EP precipitates was
performed via western blotting, and the proteins were isolated from
cultured HUVECs or rat limb musculature tissue samples by lysis in
RIPA buffer containing protease inhibitors (MilliporeSigma). The
protein concentration was assessed using a BCA Protein Assay kit
(MilliporeSigma). Equal amounts of protein (30 µg) were separated
via 10% SDS-PAGE and transferred to PVDF membranes
(MilliporeSigma). The PVDF membranes were then blocked for 1 h at
room temperature in 5% non-fat dry milk and incubated overnight at
4˚C with primary antibodies [anti-β-tubulin (1:1,000; Abcam; cat.
no. ab210797), anti-CD31 (1:1,000; Abcam; cat. no. ab281583),
anti-CD63 (1:1,000; Abcam; cat. no. ab59479), anti-CD9 (1:1,000;
Abcam; cat. no. ab92726), anti-CD81 (1:1,000; Abcam; cat. no.
ab79559), anti-HIF-1α (1:1,500; Abcam; cat. no. ab1),
anti-caspase-3 (1:1,000; Cell Signaling Technology, Inc., cat. no.
9662) and anti-cleaved caspase-3 (1:1,000; Cell Signaling
Technology, Inc., cat. no. 9661)]. After washing with TBST (0.1%
Tween 20), immunoreactive bands were incubated with HRP-conjugated
Goat Anti-mouse IgG (H+L) secondary antibody (1:5,000; BA1051;
Wuhan Boster Biological Technology, Ltd.) or HRP-conjugated Goat
Anti-rabbit IgG (H+L) antibody (1:5,000; cat. no. BA1055; Wuhan
Boster Biological Technology, Ltd.) for 1 h at 25˚C. Immunoreactive
bands were visualized using enhanced chemiluminescence reagents
(ECL; Thermo Fisher Scientific, Inc.) with a ChemiDoc™ XRS+
luminescent image analyser (v4.0; Bio-Rad Laboratories, Inc.). The
results were normalized to those of β-tubulin.
ELISA-based measurement of plasma
HIF-1α activation
Blood samples of rats were collected immediately at
the end of the four cycles of 5-min exposures to RIPC or sham-RIPC
treatment. Nuclear extracts were obtained with a commercial kit
(Nuclear Extraction kit; cat. no. ab113474; Abcam), according to
the manufacturer's instructions. Activation of HIF-1α was
quantified by a DNA-binding TransAM® HIF-1 Transcription
Factor ELISA kit (cat. no. 47096; Active Motif, Inc.), according to
the manufacturer's protocol and based on the binding of activated
HIF-1α to an oligonucleotide containing a hypoxia response element
(5'-TACGTGCT-3') from the erythropoietin gene.
Statistical analysis
The data are presented as the mean ± SEM. The
D'Agostino and Pearson omnibus normality test was used for testing
data normality. Statistical analysis was performed with GraphPad
Prism 6.0 Software (GraphPad Software, Inc.). Unpaired Student's
t-test was used for comparing data between two groups. One-way
ANOVA was conducted followed by Tukey's post hoc test for
comparisons between >2 groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Circulating EPs are more abundant in
the plasma from RIPC-compared with sham-treated human subjects
LDF measurements were performed to confirm
successful induction of transient upper limb ischaemia via RIPC
treatment in volunteers, as measured using a laser Doppler
flowmeter (Fig. 3A and B). The blood flow in the upper limb was
diminished during RIPC and recovered after the blood pressure meter
deflated. The EV markers CD63, CD9 and CD81 (8,25)
appeared to be expressed more abundantly in plasma from volunteers
after RIPC compared with the sham-RIPC-EP group (Fig. 3C and D); this result was likely due to the
pressure-induced activation of platelets (12). An approximately spherical structure
was observed within the EV population using TEM, with a diameter of
~130 nm (Fig. 3E). NTA, an optical
method of detecting particles of ~90 nm in diameter or larger,
detected particles with a median size of just >100 nm in both
types of volunteer plasma (Fig.
3F). RIPC appeared to increase the total number of EPs in the
volunteer plasma, again likely due to platelet activation (Fig. 3G). In the present experiments, no
differences between the EPs from human or rat blood after ischaemia
were noticed in EV markers (CD63, CD9 and CD81), with similar
roughly spherical structure and size distribution (data not
shown).
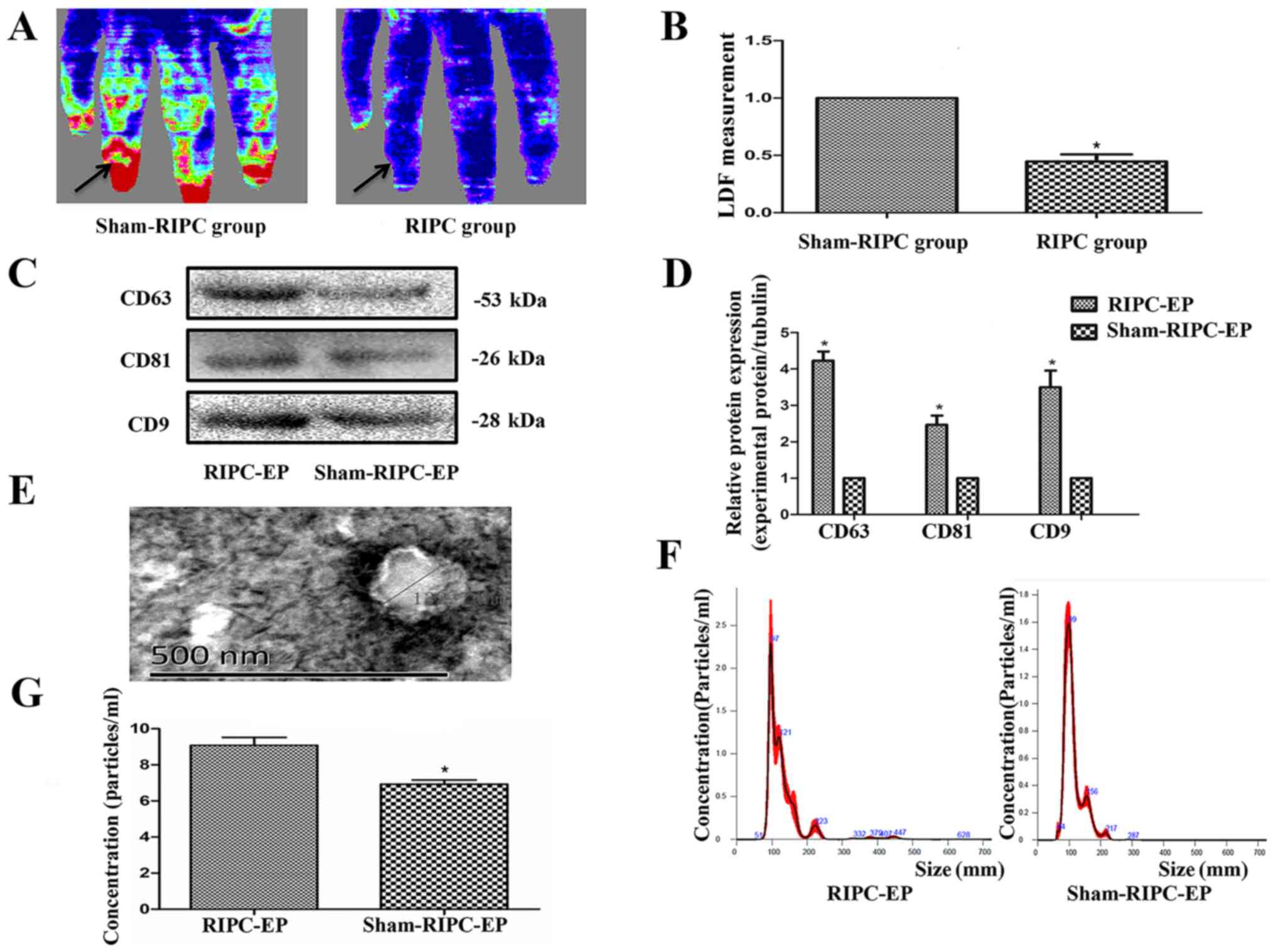 | Figure 3Establishment of the RIPC model and
characterization of plasma EPs. (A) LDF measurements were performed
to confirm successful induction of transient upper limb ischaemia
after RIPC treatment. The blood flow in the upper limb was
diminished during RIPC and recovered after the blood pressure meter
deflated. This was measured using a laser Doppler flowmeter
(Omegaflo FLO-C1 Omegawave Laser Tissue Blood Flow Meter;
OMEGAWAVE, Inc.). (B) Computer-assisted quantitative analysis
indicated a significant decrease in the flow rate after
pressurization. *P<0.05, the sham-RIPC group vs. the
RIPC group, n=4. (C) Western blot analysis demonstrated that the
protein expression levels of the EP markers CD63, CD9 and CD81
appeared to be higher in volunteer plasma after RIPC. (D) Western
blotting quantification based on three blots. *P<0.05
vs. the sham-RIPC-EP group, n=3. (E) Transmission electron
microscopy of purified exosomes from volunteers, n=3. Scale bar=500
nm. (F) Nanoparticle tracking analysis demonstrated the similar
variance in exosome size within the range of 50-150 nm (average,
108 nm) in RIPC-associated EPs and sham-RIPC-associated EPs derived
from equal volumes of volunteer plasma, n=3. (G) The concentration
of the RIPC-associated EPs was higher compared with that of the
sham-RIPC-associated EPs. *P<0.05 vs. the
sham-RIPC-EP group, n=4. RIPC, remote ischaemic preconditioning;
EP, extracellular particle; LDF, laser Doppler blood flow. |
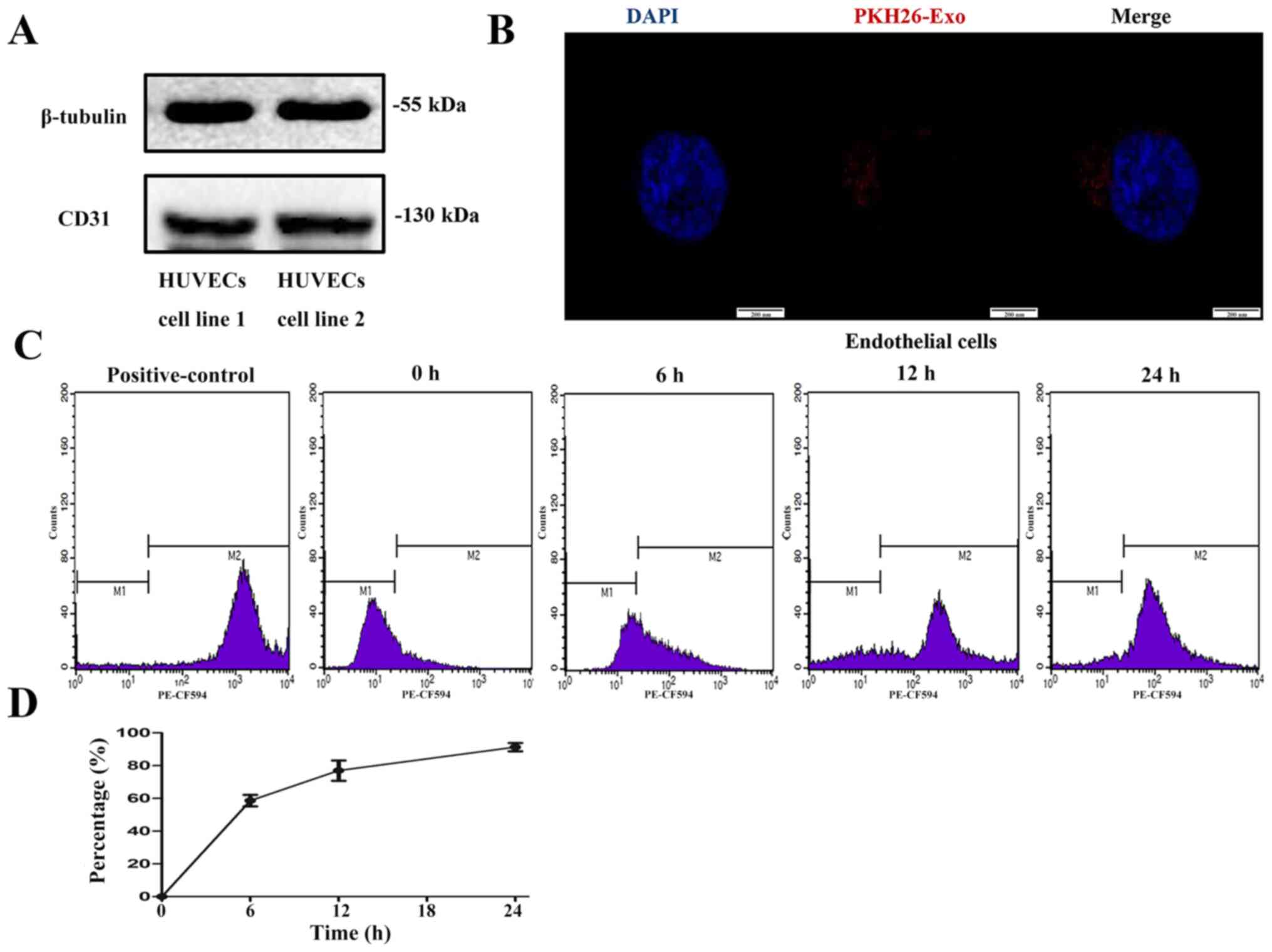
Exosome labelling and uptake by
HUVECs
Western blot analysis confirmed HUVEC expression of
CD31, a marker of endothelial cells (33) (Fig.
4A). To determine whether HUVECs could take up particles
labelled by a fluorescent dye, EPs from volunteers were firstly
labelled with PKH26, a fluorescent dye that stains EVs and other
EPs. After labelling, fluorescence was detected in the EP fraction.
When the HUVECs were incubated with the PKH26-labelled EPs,
fluorescence could be observed in the cytoplasm (Fig. 4B), which indicated that the dye had
been taken up by HUVECs. A total of ~88% of the cells incubated for
24 h were positive for the dye according to flow cytometry
(Fig. 4C and D).
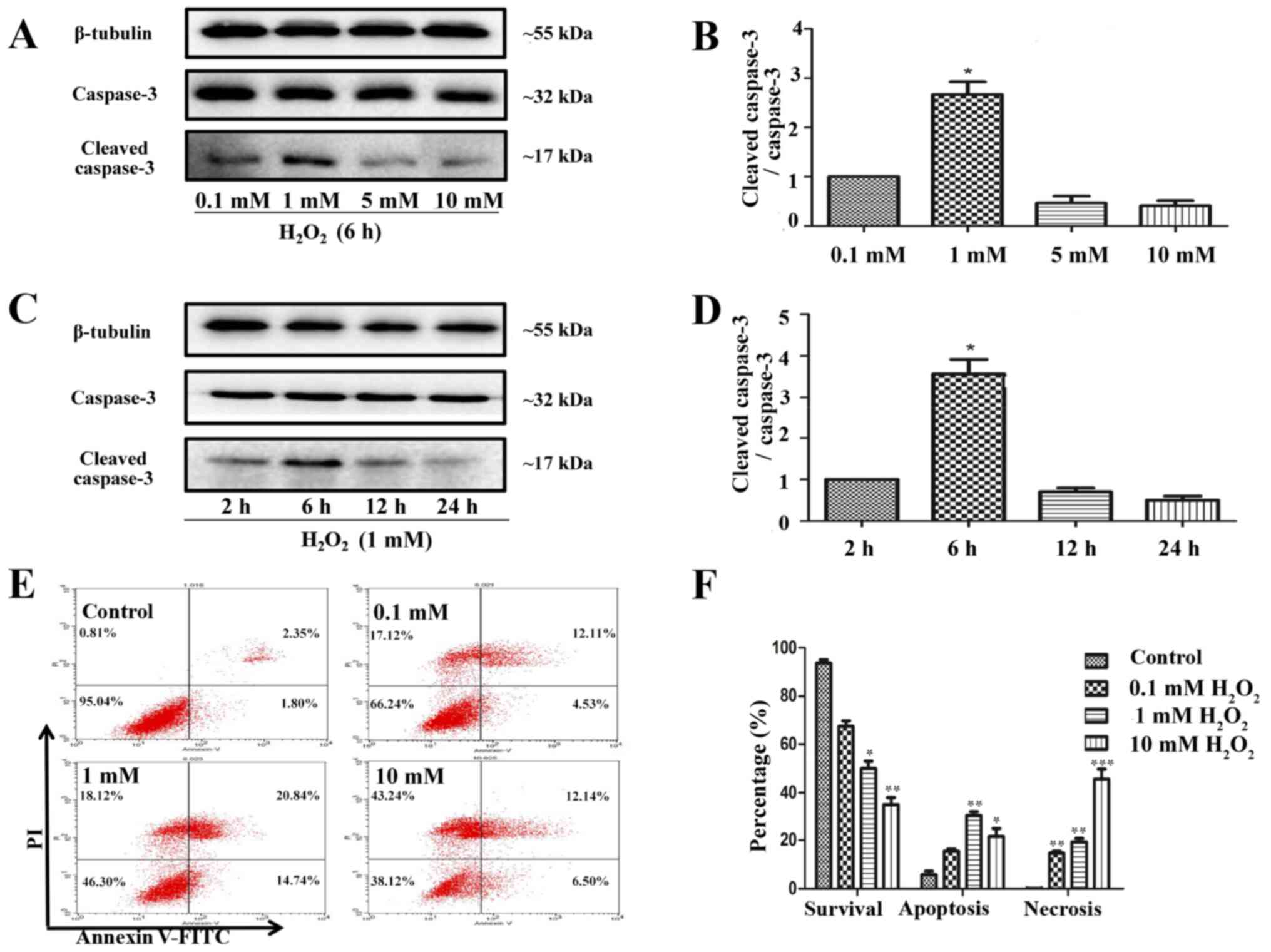
HUVECs treated with
H2O2 to model in vivo I/R conditions
Cells are commonly treated with
H2O2 to mimic I/R injury in in vitro
experiments (26). In the present
study, HUVEC treatment with 1 mM H2O2 for 6 h
significantly triggered apoptosis (Fig.
5A-D), as indicated by the increase in cleaved-caspase-3
expression, whereas 10 mM H2O2 for 6 h
preferentially caused necrosis (Fig.
5E and F). Therefore, 1 mM
H2O2 treatment was selected for 6 h to mimic
I/R injury.
RIPC decreases
H2O2-induced damage in HUVECs
To examine the effects of RIPC-EPs on HUVECs treated
with H2O2, HUVECs were incubated for 24 h
with 4 µl EPs (1x109 nanoparticles/ml) from the plasma
of volunteers treated with sham-RIPC or RIPC, then treated with
H2O2 (1 mM; 6 h). Compared with sham-RIPC
EPs, RIPC-EPs increased cell viability and reduced cytotoxicity in
HUVECs (Fig. 6A and B). Flow cytometry results also suggested
that RIPC-EPs alleviated H2O2-induced
apoptosis and necrosis in HUVECs compared with sham-RIPC-EPs
(Fig. 6C and D), accompanied by a reduced
cleaved-caspase-3 to caspase-3 ratio (Fig. 6E and F). The present results indicated a
protective effect of RIPC-EPs against
H2O2-induced cell damage in HUVECs.
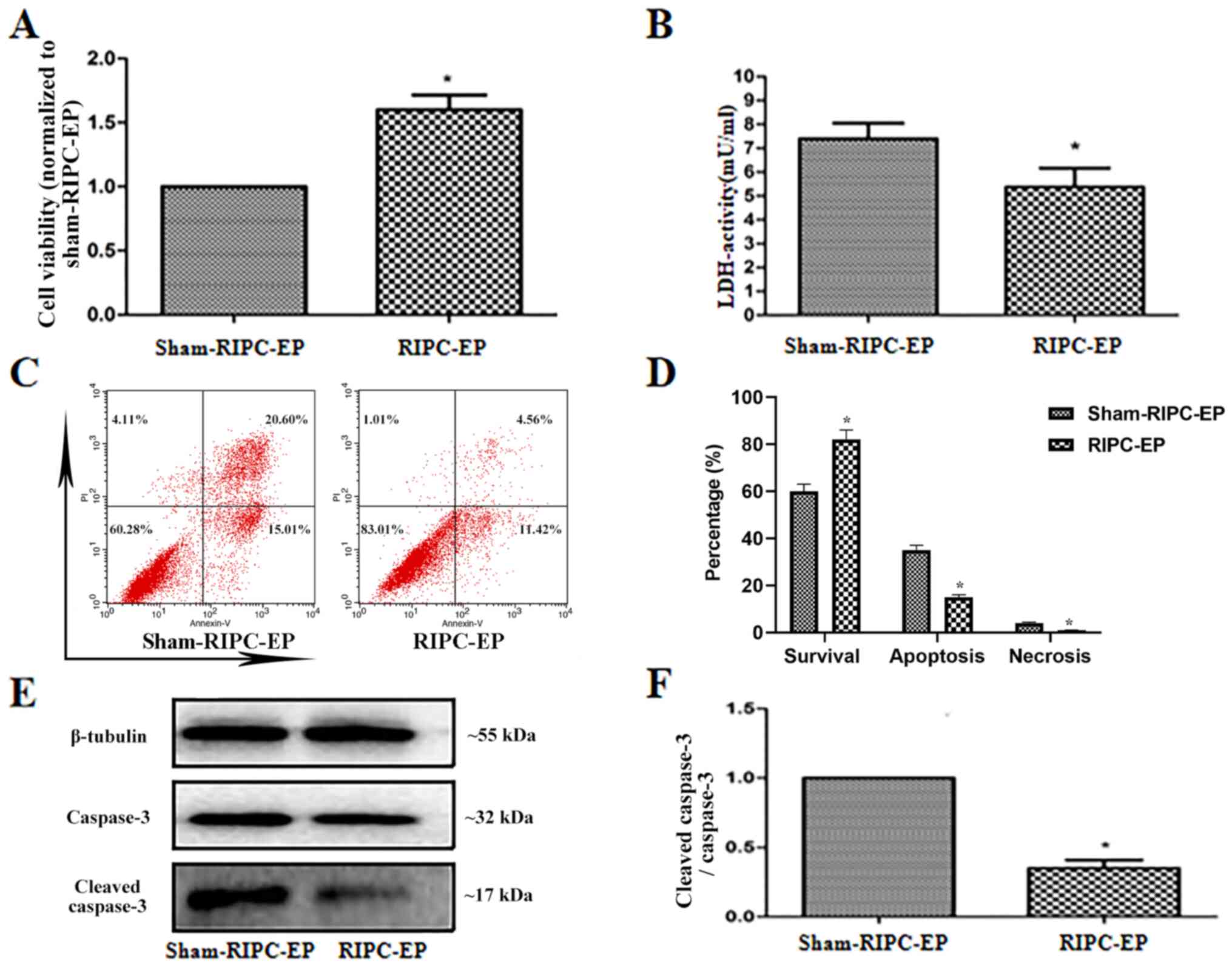 | Figure 6EPs induced by RIPC reduce
H2O2-induced damage in HUVECs. (A) HUVECs
were preincubated for 24 h with EPs from volunteers after RIPC,
then treated with H2O2 (1 mM; 6 h).
RIPC-associated EPs were observed to enhance cell viability
compared with sham-RIPC-associated EPs. *P<0.05 vs.
the sham-RIPC-EP group, n=3. (B) Relative LDH activities in the
culture media of HUVECs in the various groups. Cytotoxicity was
significantly reduced by incubation for 24 h with RIPC-associated
EPs compared with sham-RIPC-associated EPs. *P<0.05
vs. the sham-RIPC-EP group, n=3. (C and D) Apoptosis and necrosis
in HUVECs that that preincubated for 24 h with EPs from human
volunteers who did or did not receive RIPC, then treated with
H2O2 (1 mM; 6 h) were analysed by flow
cytometry using Annexin V/PI assay. *P<0.05 vs. the
sham-RIPC-EP group, n=4. (E) HUVECs were preincubated for 24 h with
EPs from volunteers after RIPC, then treated with
H2O2 (1 mM; 6 h). Western blot analysis of
the caspase-3 and cleaved caspase-3 expression levels. Tubulin was
used as an internal control. (F) Quantification of western blots
based on three blots. *P<0.05 vs. the sham-RIPC-EP
group, n=3. LDH, lactate dehydrogenase; RIPC, remote ischaemic
preconditioning; HUVECs, human umbilical vein endothelial cells;
EP, extracellular particle; PI, propidium iodide. |
Role of HIF-1α in the protective
effect of EPs
To elucidate whether HIF-1α was involved in the
protective effects of RIPC, western blot analysis was performed.
RIPC significantly induced increased expression of HIF-1α in the
rat limb musculature (Fig. 7A and
B). Compared with the sham-RIPC
group, HIF-1α molecular mass increased in samples from the groups
treated with RIPC. The present result demonstrated that RIPC could
be associated with increased levels of HIF-1α and a
post-translational modification may have occurred (such as
hydroxylation, ubiquitination, acetylation, phosphorylation or
methylation) in the HIF-1α protein after RIPC treatment. When the
rats received an intraperitoneal injection of 1 mg/kg Cd before
RIPC, the RIPC-induced increased expression of HIF-1α in the rat
limbs was partially counteracted. Cd treatment alone did not seem
to have any effect on HIF-1α activation (Fig. 7C and D). In addition, a significant decrease in
the EP levels from the plasma of rats that received an
intraperitoneal injection of Cd before RIPC was observed compared
with that in the rats that received RIPC treatment alone (Fig. 7E and F). There was no statistically significant
difference in the expression of HIF-1α in the plasma of rats that
did or did not receive RIPC (Fig.
7G).
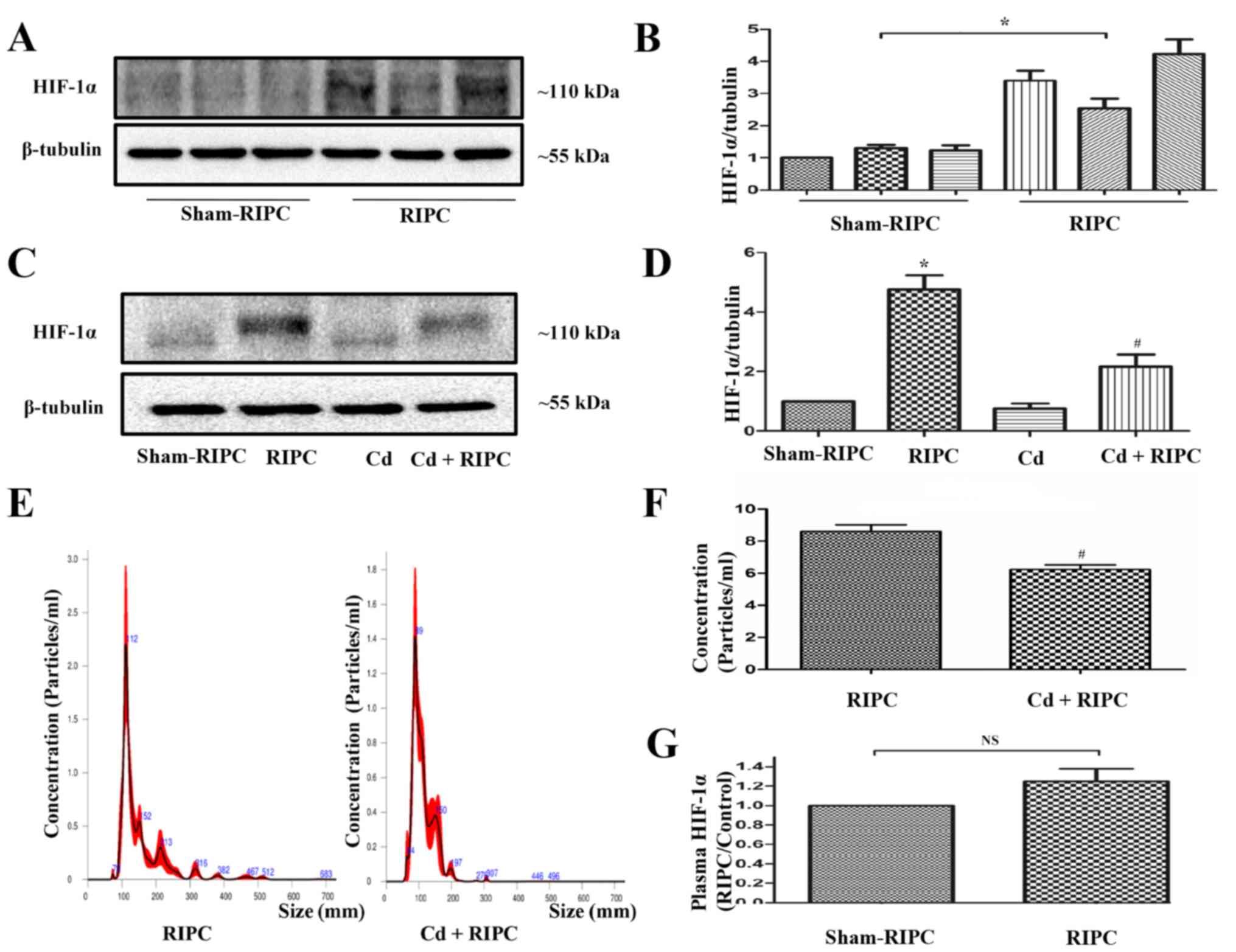 | Figure 7RIPC-induced HIF-1α activation in rat
limbs is inhibited by Cd. (A) Representative immunoblots of HIF-1α
expression in the limbs of rats that received a RIPC stimulus. The
control animals were not subjected to RIPC. (B) Quantification of
western blots based on three blots. *P<0.05 vs. the
sham-RIPC group, n=3. (C) Representative immunoblots of HIF-1α
expression in the limbs of rats that did or did not receive a RIPC
stimulus with or without an intraperitoneal injection of 1 mg/kg Cd
180 min before RIPC. Cd pre-treatment could counteract the
RIPC-induced HIF-1α activation in rat limbs. The control animals
did not undergo RIPC. (D) Quantification of western blots based on
four blots. *P<0.05 vs. the sham-RIPC group;
#P<0.05 vs. the RIPC group, n=4. (E) NTA demonstrated
the size distributions of RIPC-associated and Cd-RIPC-associated
exosomes, which were derived from the same volume of volunteer
plasma, n=3. (F) NTA demonstrated a significant decrease in the
levels of EPs in the plasma of rats that received an
intraperitoneal injection of 1 mg/kg Cd 180 min before RIPC
compared with those in rats that received RIPC treatment alone.
#P<0.05 vs. the RIPC group, n=4. (G) Expression of
HIF-1α in plasma detected by ELISA. There was no statistically
significant difference in the expression of HIF-1α in the plasma of
rats with or without RIPC. n=4. NTA, nanoparticle tracking
analysis; RIPC, remote ischaemic preconditioning; EP, extracellular
particle; HIF-1α, hypoxia-inducible factor 1-α; Cd, cadmium; NS,
non-significant. |
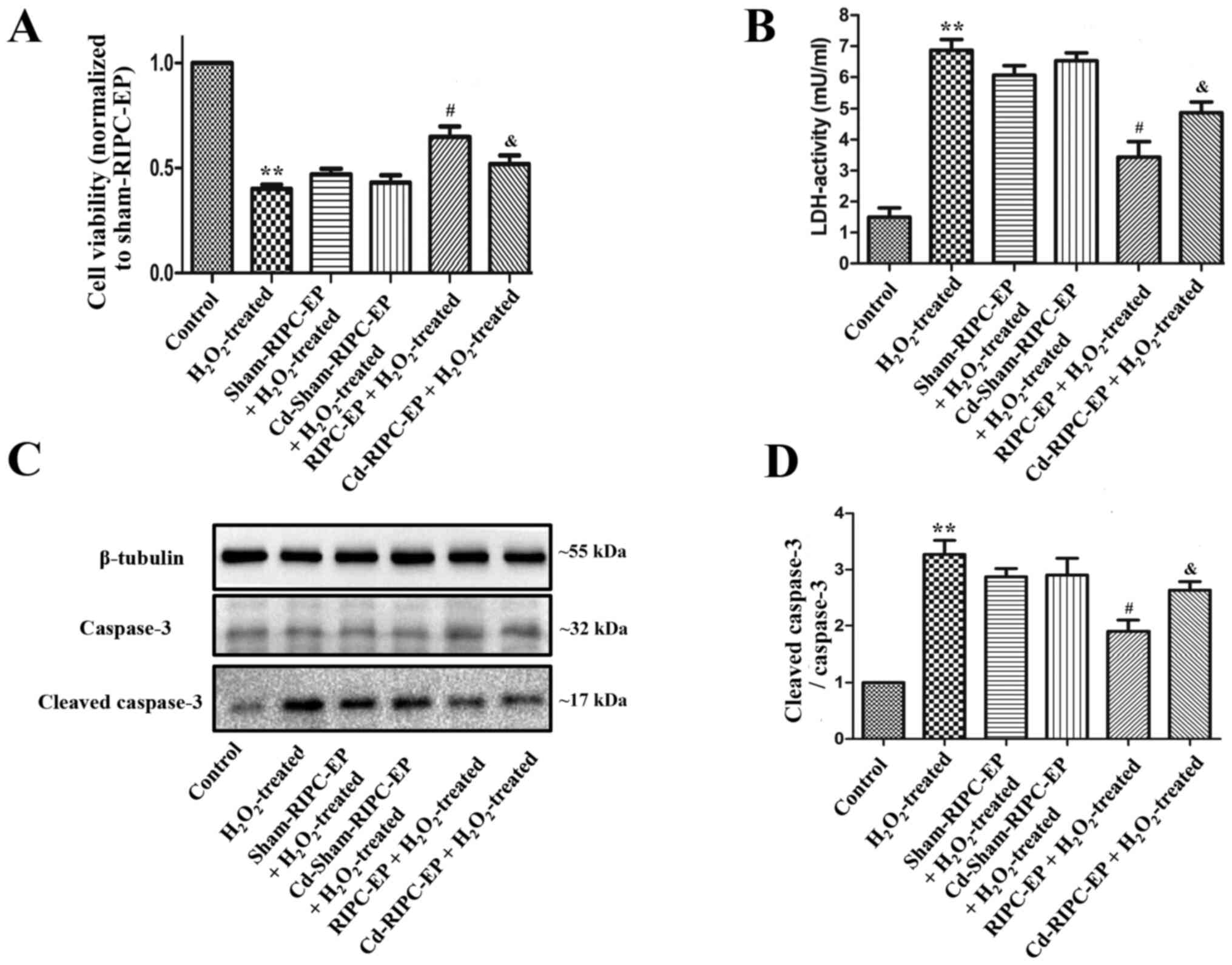
Finally, plasma was collected from the different
groups presented in Fig. 2A to
assess the effects of the corresponding EPs on
H2O2-induced cell damage in HUVECs. Compared
with the control group, HUVECs treated with 1 mM
H2O2 for 6 h had a significantly decreased
cell viability, increased LDH cytotoxicity and the ratio of
cleaved-caspase-3 to caspase-3 (Fig.
8A-D). Compared with the sham-RIPC-EP group, Cd-sham-RIPC-EP
did not influence the cell viability and LDH cytotoxicity of HUVECs
treated with 1 mM H2O2 for 6 h, while RIPC-EP
treatment increased cell viability and attenuated LDH cytotoxicity
of HUVECs treated with 1 mM H2O2 for 6 h,
while Cd preconditioning partially counteracted the protective
effect of RIPC-EPs (Fig. 8A and
B). Compared with the sham-RIPC-EP
group, Cd-sham-RIPC-EP did not influence the ratio of
cleaved-caspase-3 to caspase-3 of HUVECs treated with 1 mM
H2O2 for 6 h. RIPC-EP treatment attenuated
the ratio of cleaved-caspase-3 to caspase-3 in HUVECs treated with
1 mM H2O2 for 6 h, while Cd preconditioning
partially counteracted the anti-apoptosis effect of RIPC-EPs
(Fig. 8C and D). The present results suggested that
HIF-1α may contribute to the effects of RIPC.
Discussion
The main findings of the current study were as
follows: i) EPs precipitated from human plasma after RIPC may
contribute to reducing H2O2-induced damage to
HUVECs in vitro; and ii) the expression of HIF-1α in the rat
limbs is increased during RIPC and may contribute to the protective
effects of RIPC.
Recently, RIPC has emerged as an effective strategy
for alleviating myocardial I/R injury (34,35).
The ability to use transient limb ischaemia as a RIPC stimulus has
facilitated its application from bench to bedside in various
clinical settings (4-6,36-40).
Although the exact mechanisms of RIPC are not precisely known, the
importance of neural or humoral mediators in RIPC-mediated
myocardial protection of cells and organs has been emphasized in
previous studies (8-10,41,42);
such mediators include stromal derived factor-1α (43), nitrite (44), apolipoprotein A1(45), IL-10(46) and microRNA-144(47), and may be present within EVs or
other EPs (9-13).
In the present study, EPs from the plasma of healthy
volunteers treated with sham-RIPC or RIPC were added to HUVEC
cultures for 24 h before H2O2 stimulation.
Since age, oestrogen levels, comorbidities and other factors may
influence the protective potential of ischaemic conditioning
(48-52),
EPs were only collected from healthy young males. Furthermore,
Abete et al (48) reported
that the cytoprotective effect of plasma from RIPC-treated
volunteers did not last >60 min after RIPC. Therefore, in the
present study, EPs were collected from plasma directly after RIPC.
Furthermore, Cd is an effective pharmacological HIF-1α inhibitor
(28,29,30).
Cd pre-treatment could counteract RIPC-induced HIF-1α activation in
rat limbs (30), resulting in loss
of myocardial HIF-1α activation and hypoxic preconditioning in rat
hearts (28), and abolish the
beneficial effects on both reduced myocardial infarction size and
increased coronary flow in rats (29). Kalakech et al (30) revealed that Cd treatment alone (1
mg/kg Cd for 220 min before coronary occlusion) had no influence on
infarct size in wild-type mice and HIF-1α heterozygous mice.
Belaidi et al (28,29) also reported that Cd treatment alone
(1 mg/kg) had no influence on HIF-1α activation, haemodynamic
parameters, infarct size or coronary flow in rats. Similarly, in
the present experimental protocol, Cd treatment alone (1 mg/kg) had
no effect on HIF-1α activation. Compared with HUVECs treated with
EPs from the blood of rats not receiving intraperitoneal injections
of Cd, HUVECs treated with EPs from the blood of rats receiving
intraperitoneal injections of Cd displayed no differences in cell
viability, LDH cytotoxicity or cleaved-caspase-3/caspase-3 ratio.
Therefore, EPs from the blood of animals receiving intraperitoneal
injections of Cd did not contain Cd, which does not interfere with
the viability of HUVECs.
A previous study has indicated that HIF-1α mediated
the protective effect of RIPC against myocardial I/R by activating
IL-10 gene transcription (18). In
another study, right atrial tissues were collected from patients
subjected to RIPC or sham treatment before cardiopulmonary bypass
surgery. The results indicated that the patients subjected to RIPC
exhibited reduced troponin T serum levels during the 48 h after
surgery, and increased HIF-1α levels were observed in the atrial
samples (53). The present results
also demonstrated that HIF-1α served an important role in EP
production after RIPC. Together, these results demonstrated that
RIPC could be associated with increased levels of HIF-1α. However,
the exact mechanism via which HIF-1 regulates EP biogenesis and
secretion after RIPC is unclear. In previous research, HIF-1 has
been reported to mediate the induction of Rab20 and Rab22 (54,55),
which may be involved in exosome formation and secretion (12,56).
In the present experiments, the molecular weight of HIF-1α was
observed to be slightly increased in the RIPC and Cd + RIPC groups
compared with the control and Cd groups. We hypothesize that a
post-translational modification may have occurred (such as
hydroxylation, ubiquitination, acetylation, phosphorylation and
methylation) in the HIF-1α protein after RIPC treatment (57,58).
The current investigation presents certain
limitations. Firstly, the present study did not propose a detailed
mechanism via which RIPC-EPs protected HUVECs against oxidative
stress injury. Secondly, the present study did not establish a
knockout model of the HIF-1α gene in HUVECs to provide more direct
evidence that HIF-1α regulated EPs after RIPC. Moreover, as
aforementioned, the changes in the EP levels that were suggested by
the present data could be attributed to platelet activation during
RIPC, and it is possible that these processes also influence the
outcomes described in the current study.
In conclusion, the results of the current study
suggested that HIF-1α and plasma particular matter may contribute
to the effects of RIPC on oxidative stress injury in HUVECs.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the National Natural
Science Foundation of China (grant nos. 8156020189, 81560049,
81560051 and 82060063) and Natural Science Foundation of Jiangxi
province (grant nos. 20161BAB205256 and 20202BABL216004).
Availability of data and materials
The datasets used and/or analysed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
MW and FH participated in the study design,
contributed to exosomes collection and cell experiments, performed
the data and statistical analysis, and drafted the manuscript. ZG
and CH participated in clinical data acquisition, contributed to
data analysis and editing of the manuscript. XSC participated in
the whole study design, contributed to quality control of data and
images and editing and review of the manuscript. FH and XSC confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The experiments involving human subjects were based
on The Declaration of Helsinki and the European Declaration of
Human Rights. The present study was approved by the Ethics
Committee of the Second Affiliated Hospital of Nanchang University
[Nanchang, China; approval no. SYXK(G) 2019-0007]. All animal
experiments were conducted in compliance with the National
Institutes of Health policies in the Guide for the Care and Use of
Laboratory Animals and were approved by the Ethics Committee of the
Second Affiliated Hospital of Nanchang University [Nanchang, China;
approval no. SYXK(G) 2019-0102]. Written informed consents were
obtained from all healthy male volunteers.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hausenloy DJ and Yellon DM: Remote
ischaemic preconditioning: Underlying mechanisms and clinical
application. Cardiovasc Res. 79:377–386. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gho BC, Schoemaker RG, van den Doel MA,
Duncker DJ and Verdouw PD: Myocardial protection by brief ischemia
in noncardiac tissue. Circulation. 94:2193–2200. 1996.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Heusch G, Musiolik J, Kottenberg E, Peters
J, Jakob H and Thielmann M: STAT5 activation and cardioprotection
by remote ischemic preconditioning in humans: Short communication.
Circ Res. 110:111–115. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kttenberg E, Thielmann M, Bergmann L,
Heine T, Jakob H, Heusch G and Peters J: Protection by remote
ischemic preconditioning during coronary artery bypass graft
surgery with isoflurane but not propofol-a clinical trial. Acta
Anaesthesiol Scand. 56:30–38. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ali ZA, Callaghan CJ, Lim E, Ali AA,
Nouraei SA, Akthar AM, Boyle JR, Varty K, Kharbanda RK, Dutka DP
and Gaunt ME: Remote ischemic preconditioning reduces myocardial
and renal injury after elective abdominal aortic aneurysm repair: A
randomized controlled trial. Circulation. 116 (Suppl 11):I98–I105.
2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kharbanda RK, Nielsen TT and Redington AN:
Translation of remote ischaemic preconditioning into clinical
practice. Lancet. 374:1557–1565. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bøtker HE, Lassen TR and Jespersen NR:
Clinical translation of myocardial conditioning. Am J Physiol Heart
Circ Physiol. 314:H1225–H1252. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abel F, Murke F, Gaida M, Garnier N,
Ochsenfarth C, Theiss C, Thielmann M, Kleinbongard P, Giebel B,
Peters J and Frey UH: Extracellular vesicles isolated from patients
undergoing remote ischemic preconditioning decrease hypoxia-evoked
apoptosis of cardiomyoblasts after isoflurane but not propofol
exposure. PLoS One. 15(e0228948)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Frey UH, Klaassen M, Ochsenfarth C, Murke
F, Thielmann M, Kottenberg E, Kleinbongard P, Klenke S, Engler A,
Heusch G, et al: Remote ischaemic preconditioning increases serum
extracellular vesicle concentrations with altered micro-RNA
signature in CABG patients. Acta Anaesthesiol Scand. 63:483–492.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bartekova M, Jelemensky M and Dhalla NS:
Emerging role of non-coding RNAs and extracellular vesicles in
cardioprotection by remote ischemic conditioning of the heart. Rev
Cardiovasc Med. 20:59–71. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ratajczak J, Wysoczynski M, Hayek F,
Janowska-Wieczorek A and Ratajczak MZ: Membrane-derived
microvesicles: Important and underappreciated mediators of
cell-to-cell communication. Leukemia. 20:1487–1495. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Semenza GL: A compendium of proteins that
interact with HIF-1α. Exp Cell Res. 356:128–135. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Aga M, Kondo S, Wakisaka N, Moriyama-Kita
M, Endo K, Nakanishi Y, Murono S, Sugimoto H, Ueno T and Yoshizaki
T: Siah-1 is associated with expression of hypoxia-inducible
factor-1α in oral squamous cell carcinoma. Auris Nasus Larynx.
44:213–219. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kosaka N, Iguchi H, Hagiwara K, Yoshioka
Y, Takeshita F and Ochiya T: Neutral sphingomyelinase 2
(nSMase2)-dependent exosomal transfer of angiogenic microRNAs
regulate cancer cell metastasis. J Biol Chem. 288:10849–10859.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hubbi ME and Semenza GL: Regulation of
cell proliferation by hypoxia-inducible factors. Am J Physiol Cell
Physiol. 309:C775–C782. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cai Z, Luo W, Zhan H and Semenza GL:
Hypoxia-inducible factor 1 is required for remote ischemic
preconditioning of the heart. Proc Natl Acad Sci USA.
110:17462–17467. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Seal JB and Gewertz BL: Vascular
dysfunction in ischemia/reperfusion injury. Ann Vasc Surg.
19:572–584. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Michiels C: Endothelial cell functions. J
Cell Physiol. 196:430–443. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pitcher JM, Wang M, Tsai BM, Kher A,
Turrentine MW, Brown JW and Meldrum DR: Preconditioning: Gender
effects. J Surg Res. 129:202–220. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kharbanda RK, Peters M, Walton B,
Kattenhorn M, Mullen M, Klein N, Vallance P, Deanfield J and
MacAllister R: Ischemic preconditioning prevents endothelial injury
and systemic neutrophil activation during ischemia-reperfusion in
humans in vivo. Circulation. 103:1624–1630. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Randhawa PK and Jaggi AS: Exploring the
putative role of TRPV1-dependent CGRP release in remote
hind preconditioning-induced cardioprotection. Cardiovasc Ther.
35:2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yamaguchi T, Izumi Y, Nakamura Y, Yamazaki
T, Shiota M, Sano S, Tanaka M, Osada-Oka M, Shimada K, Miura K, et
al: Repeated remote ischemic conditioning attenuates left
ventricular remodeling via exosome-mediated intercellular
communication on chronic heart failure after myocardial infarction.
Int J Cardiol. 178:239–246. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sera T, Kohno T, Nakashima Y, Uesugi M and
Kudo S: Low-frequency oscillations of finger skin blood flow during
the initial stage of cold-induced vasodilation at different air
temperatures. J Physiol Anthropol. 39(37)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yang Y, Li Y, Chen X, Cheng X, Liao Y and
Yu X: Exosomal transfer of miR-30a between cardiomyocytes regulates
autophagy after hypoxia. J Mol Med (Berl). 94:711–724.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jiang F, Xu XR, Li WM, Xia K, Wang LF and
Yang XC: Monotropein alleviates H2O2-induced inflammation,
oxidative stress and apoptosis via NF-κB/AP-1 signaling. Mol Med
Rep. 22:4828–4836. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Belaidi E, Beguin PC, Levy P, Ribuot C and
Godin-Ribuot D: Prevention of HIF-1 activation and iNOS gene
targeting by low-dose cadmium results in loss of myocardial hypoxic
preconditioning in the rat. Am J Physiol Heart Circ Physiol.
294:H901–H908. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Belaidi E, Beguin PC, Levy P, Ribuot C and
Godin-Ribuot D: Delayed myocardial preconditioning induced by
cobalt chloride in the rat: HIF-1α and iNOS involvement. Fundam
Clin Pharmacol. 26:454–462. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kalakech H, Tamareille S, Pons S,
Godin-Ribuot D, Carmeliet P, Furber A, Martin V, Berdeaux A, Ghaleh
B and Prunier F: Role of hypoxia inducible factor-1α in remote limb
ischemic preconditioning. J Mol Cell Cardiol. 65:98–104.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chun YS, Choi E, Kim GT, Choi H, Kim CH,
Lee MJ, Kim MS and Park JW: Cadmium blocks hypoxia-inducible factor
(HIF)-1-mediated response to hypoxia by stimulating the
proteasome-dependent degradation of HIF-1alpha. Eur J Biochem.
267:4198–4204. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jungmann J, Reins HA, Schobert C and
Jentsch S: Resistance to cadmium mediated by ubiquitin-dependent
proteolysis. Nature. 361:369–371. 1993.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu L and Shi GP: CD31: Beyond a marker
for endothelial cells. Cardiovasc Res. 94:3–5. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sanada S, Komuro I and Kitakaze M:
Pathophysiology of myocardial reperfusion injury: Preconditioning,
postconditioning, and translational aspects of protective measures.
Am J Physiol Heart Circ Physiol. 301:H1723–H1741. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang X, Zhu H, Zhang X, Liu Y, Chen J,
Medvedovic M, Li H, Weiss MJ, Ren X and Fan GC: Loss of the
miR-144/451 cluster impairs ischaemic preconditioning-mediated
cardioprotection by targeting Rac-1. Cardiovasc Res. 94:379–390.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wagner R, Piler P, Bedanova H, Adamek P,
Grodecka L and Freiberger T: Myocardial injury is decreased by late
remote ischaemic preconditioning and aggravated by tramadol in
patients undergoing cardiac surgery: A randomised controlled trial.
Interact Cardiovasc Thorac Surg. 11:758–762. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cheung MM, Kharbanda RK, Konstantinov IE,
Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van
Arsdell GS and Redington AN: Randomized controlled trial of the
effects of remote ischemic preconditioning on children undergoing
cardiac surgery: First clinical application in humans. J Am Coll
Cardiol. 47:2277–2282. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hausenloy DJ and Yellon DM: The
therapeutic potential of ischemic conditioning: An update. Nat Rev
Cardiol. 8:619–629. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Thielmann M, Wendt D, Tsagakis K, Price V,
Dohle DS, Pasa S and Kottenberg E: Remote ischemic preconditioning:
The surgeon's perspective. J Cardiovasc Med (Hagerstown).
14:187–192. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Veighey K and Macallister RJ: Clinical
applications of remote ischemic preconditioning. Cardiol Res Pract.
2012(620681)2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pickard JM, Davidson SM, Hausenloy DJ and
Yellon DM: Co-dependence of the neural and humoral pathways in the
mechanism of remote ischemic conditioning. Basic Res Cardiol.
111(50)2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Oba T, Yasukawa H, Nagata T, Kyogoku S,
Minami T, Nishihara M, Ohshima H, Mawatari K, Nohara S, Takahashi
J, et al: Renal nerve-mediated erythropoietin release confers
cardioprotection during remote ischemic preconditioning. Circ J.
79:1557–1567. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bromage DI, Davidson SM and Yellon DM:
Stromal derived factor 1α: a chemokine that delivers a two-pronged
defence of the myocardium. Pharmacol Ther. 143:305–315.
2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rassaf T, Totzeck M, Hendgen-Cotta UB,
Shiva S, Heusch G and Kelm M: Circulating nitrite contributes to
cardioprotection by remote ischemic preconditioning. Circ Res.
114:1601–1610. 2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Hibert P, Prunier-Mirebeau D, Beseme O,
Chwastyniak M, Tamareille S, Lamon D, Furber A, Pinet F and Prunier
F: Apolipoprotein A-I is a potential mediator of remote ischemic
preconditioning. PLoS One. 8(e77211)2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cai ZP, Parajuli N, Zheng X and Becker L:
Remote ischemic preconditioning confers late protection against
myocardial ischemia-reperfusion injury in mice by upregulating
interleukin-10. Basic Res Cardiol. 107(277)2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li J, Rohailla S, Gelber N, Rutka J, Sabah
N, Gladstone RA, Wei C, Hu P, Kharbanda RK and Redington AN:
MicroRNA-144 is a circulating effector of remote ischemic
preconditioning. Basic Res Cardiol. 109(423)2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Abete P, Ferrara N, Cacciatore F, Madrid
A, Bianco S, Calabrese C, Napoli C, Scognamiglio P, Bollella O,
Cioppa A, et al: Angina-induced protection against myocardial
infarction in adult and elderly patients: A loss of preconditioning
mechanism in the aging heart? J Am Coll Cardiol. 30:947–954.
1997.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ebrahim Z, Yellon DM and Baxter GF:
Ischemic preconditioning is lost in aging hypertensive rat heart:
Independent effects of aging and longstanding hypertension. Exp
Gerontol. 42:807–814. 2007.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ferdinandy P, Schulz R and Baxter GF:
Interaction of cardiovascular risk factors with myocardial
ischemia/reperfusion injury, preconditioning, and postconditioning.
Pharmacol Rev. 59:418–458. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Babiker F, Al-Jarallah A and Al-Awadi M:
Effects of cardiac hypertrophy, diabetes, aging, and pregnancy on
the cardioprotective effects of postconditioning in male and female
rats. Cardiol Res Pract. 2019(3403959)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Weber NC, Riedemann I, Smit KF, Zitta K,
van de Vondervoort D, Zuurbier CJ, Hollmann MW, Preckel B and
Albrecht M: Plasma from human volunteers subjected to remote
ischemic preconditioning protects human endothelial cells from
hypoxia-induced cell damage. Basic Res Cardiol.
110(17)2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Albrecht M, Zitta K, Bein B, Wennemuth G,
Broch O, Renner J, Schuett T, Lauer F, Maahs D, Hummitzsch L, et
al: Remote ischemic preconditioning regulates HIF-1α levels,
apoptosis and inflammation in heart tissue of cardiosurgical
patients: A pilot experimental study. Basic Res Cardiol.
108(314)2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hackenbeck T, Huber R, Schietke R, Knaup
KX, Monti J, Wu X, Klanke B, Frey B, Gaipl U, Wullich B, et al: The
GTPase RAB20 is a HIF target with mitochondrial localization
mediating apoptosis in hypoxia. Biochim Biophys Acta. 1813:1–13.
2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wang T, Gilkes DM, Takano N, Xiang L, Luo
W, Bishop CJ, Chaturvedi P, Green JJ and Semenza GL:
Hypoxia-inducible factors and RAB22A mediate formation of
microvesicles that stimulate breast cancer invasion and metastasis.
Proc Natl Acad Sci USA. 111:E3234–E3242. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ostrowski M, Carmo NB, Krumeich S, Fanget
I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et
al: Rab27a and Rab27b control different steps of the exosome
secretion pathway. Nat Cell Biol. 12:19–30. 2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bao L, Chen Y, Lai HT, Wu SY, Wang JE,
Hatanpaa KJ, Raisanen JM, Fontenot M, Lega B, Chiang CM, et al:
Methylation of hypoxia-inducible factor (HIF)-1α by G9a/GLP
inhibits HIF-1 transcriptional activity and cell migration. Nucleic
Acids Res. 46:6576–6591. 2018.PubMed/NCBI View Article : Google Scholar
|