Introduction
Parkinson's disease (PD) is one of the most common
neurodegenerative diseases in the world (1,2). Its
pathogenesis is characterized by the progressive loss and
functional impairment of dopaminergic neurons in the substantia
nigra (SN) and striatum (3-5).
As the functions of dopaminergic neurons are gradually impaired,
the expression level of the rate-limiting enzyme tyrosine
hydroxylase (TH), which synthesizes levodopa in the SN and
striatum, will decrease or even disappear (6,7).
Existing therapeutic drugs, such as levodopa, can sometimes relieve
the symptoms of PD, but there is currently no therapy to prevent
the neurodegeneration of PD (8,9).
Studies have indicated that neuroinflammation is associated with
the neurodegenerative diseases of PD and is accompanied by a large
number of proinflammatory cytokines in the SN, such as TNF-α
(10) and IL-1β (11). Therefore, it is important to be
able to treat or prevent PD to prevent the degeneration of
dopaminergic neurons.
The microbiome-gut-brain axis, a hypothesis that has
been proposed in previous years, indicates that there is
bidirectional communication between the gut microbiota and the
brain, which includes the enteric and central nervous systems
(12). Studies have demonstrated
that an imbalance of the gut microbiota leads to changes in the
interaction between the host and microbes, including numerous
neurological diseases, such as depression and PD (13-15).
Clinical data have reported that the gut microbiota in patients
with PD is different compared with that in healthy individuals
(16,17). Therefore, it might be that the gut
microbiota is a potential target for PD treatment.
Faecal microbiota transplantation (FMT) is a therapy
that has received increased attention in the treatment of human
disease (18). Compared with
prebiotics or probiotics, FMT can quickly regulate the imbalanced
gut microbiota in a short amount of time. However, in the treatment
of PD, FMT has not received enough attention from clinicians and,
to the best of our knowledge, there is a lack of systematic
research. A recent study assessed the efficacy and safety of FMT in
PD, which indicated that FMT can relieve motor and non-motor
symptoms with acceptable safety in PD (19). However, the potential molecular
mechanism of FMT in the treatment of PD has not yet been
reported.
The present study evaluated the effect of FMT on
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced motor
function through a series of behavioural tests, such as the pole
test and open field test. At the same time, the protective effect
of FMT on intestinal inflammation and SN injury in PD mice was
evaluated and verified. From 16S ribosomal (r)RNA sequencing
analysis through the evaluation of the intestinal microbial
composition of PD mice after FMT implementation, the present study
explored the neuroprotective mechanism of the intestinal
flora-gut-brain axis on PD, which could provide potential for
reducing the pathogenesis of PD strategies.
Materials and methods
Animals and treatment
A total of 18 male C57BL/6 mice (age, 6-8 weeks;
weight, 18-22 g) were purchased from Zhejiang Academy of Medical
Sciences [License number: SCXK (Su) 2019-0002)]. All mice were kept
at 23 ± 2˚C with 12 h light/dark cycles with 55% relative humidity
and access to water and food ad libitum. All experimental
protocols were authorized by the Animal Ethical Committee of the
First Affiliated Hospital of Hainan Medical University (approval
no. D-2017027; Hainan, P.R. China). All mice except the control
group were administered an intraperitoneal injection of MPTP (20
mg/kg; MilliporeSigma). After 1 h, mice were administered an
intraperitoneal injection of probenecid (250 mg/kg;
MilliporeSigma), which enhances the toxic effect of MPTP. MPTP and
probenecid were injected every 3.5 days for a duration of 5 weeks,
and the mice were free to drink pure water. Mice in control group
were given normal saline at the same volume. During the whole
treatment period, changes in body weight, visible stool consistency
and faecal bleeding were assessed daily. The disease activity index
(DAI) was defined as the sum of the stool consistency index (0=
normal; 1= soft but still formed; 2= very soft; 3= diarrhoea),
faecal bleeding index (0= negative hemoccult, 1= positive
hemoccult; 2= blood traces in stool visible; 3= rectal bleeding)
and body weight loss index (0= ~1%; 1= between 1-5%; 2= between
5-10%; 3= between 10-15%; 3= >15%) (20).
Behavioural tests
To evaluate the impairment of motor function in the
MPTP-induced PD mouse model, a series of behavioural tests were
performed, including the rotarod (21), pole (22) and open field (23) tests.
Rotarod test
The mice underwent three consecutive trials and
rested for 30 min after each trial. The first time was the rotating
rod training. In the last two trials, the average waiting time for
falling from the rotating rod was used for analysis. The parameters
of the rotating tripod system include starting speed, acceleration
and maximum speed (1 rpm, acceleration 12 rpm/2 sec, 50 rpm).
Pole test
Each mouse was trained three times a day before the
test. On the day of the test, each mouse was tested twice, and the
average value was used for statistical analysis. The test was
performed after the end of MPTP administration. The climbing pole
was 50-cm high, 1-cm in diameter and had a 35-mm diameter ball at
the top. During the test, the mouse was placed on the ball, the
time mouse headed down was recorded as T-turn (turn time), and the
time to climb to the sole was recorded as T-LA (locomotor activity
time). If the mouse stayed on the ball for >30 sec, it was
guided to climb down the rod and T-turn was recorded as 30 sec.
Open field test
The square arena (40x40x40 cm) was divided into 16
equal-sized squares. Each mouse was placed in the centre of the
arena, acclimatized for 5 min and the number of crossings and
rearing were recorded. The mouse was placed into the surrounding
area, and the speed of the mouse's 10 min movement track was
observed and analysed.
Immunofluorescence
Mice were anaesthetised by being intraperitoneally
injected with sodium pentobarbital (100 mg/kg), after which the
mice were euthanised via cervical dislocation. SN samples were
collected from each mouse (3 per group). The samples were embedded
in 100% paraffin wax (melting point, 54-56˚C; 5 ml; cat no.
P100930; Shanghai Aladdin Biochemical Technology Co., Ltd.) at
55˚C, left at room temperature for 12 h and subsequently cut into
5-µm sections. The resulting sections were heated at 60˚C for 2 h.
After dewaxing with xylene (cat no. X112054; Shanghai Aladdin
Biochemical Technology Co., Ltd.) for 3 min at room temperature and
rehydration in a descending alcohol series with concentrations of
100, 95, 90, 80 and 70%, the sections were blocked with 5% foetal
bovine serum (cat. no. 086-150; Nanjing Wisent Biotechnology Co.,
Ltd.) at room temperature for 30 min. Sections were subsequently
incubated with arginase (cat. no. ab233548; Abcam; 1:2,000),
ionized calcium-binding adaptor molecule 1 (Iba-1; cat. no.
ab178846; Abcam; 1:2,000), inducible nitric oxide synthase (iNOS;
cat. no. AF0199; Affinity Biosciences, Ltd.; 1:200) and TH (cat.
no. ab137869; Abcam; 1:500) overnight at 4˚C. The sections were
washed 10 ml tris buffered saline + 1% Tween-20 (TBST) for three
times and incubated with Alexa Fluor 488 conjugated-donkey
Anti-Rabbit IgG H&L secondary antibody (cat. no. ab150073;
Abcam; 1:1,000) at room temperature for 30 min. Subsequently, the
samples were stained with DAPI (SouthernBiotech) at room
temperature for 10 min. The sections were sealed with glycerin and
observed under a fluorescence microscope (magnification, x400;
LSM710; Zeiss GmbH). Image Pro Plus 7.0 (Media Cybernetics, Inc.)
software was used to analyse the TH+, iNOS+
and Arginase+ cells in the immunofluorescence images.
The integrated optical density (IOD) of the positive points in each
of the images was recorded using the Image Pro Plus 7.0 software,
and then the value of the IOD was presented in the histogram.
Western blotting
Western blotting analysis was performed as described
in previous studies (20,24). SN samples were obtained from each
mouse (3 per group), rapidly dissected, rinsed in 1Χ PBS, and
homogenized in ice-cold homogenization buffer (Beyotime Institute
of Biotechnology). The samples were then analysed by western
blotting. The proteins were collected and centrifuged at 12,000 x g
for 15 min at 4˚C. A BCA protein assay kit (Beyotime Institute of
Biotechnology) was used to determine the protein concentration. The
samples (20 µg) were separated using 12% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis and transferred onto a
nitrocellulose membrane (Bio-Rad Laboratories, Inc.). The membranes
were incubated in a blocking solution containing 5% non-fat milk in
PBST with 1% Tween at room temperature for 1.5 h and then incubated
with the primary antibodies diluted in blocking solution
individually. The primary antibodies used were arginase (cat. no.
ab233548; 1:5,000; Abcam), GSK3β (cat. no. 21002; 1:200; Signalway
Antibody LLC), IL-1β (cat. no. ab5076; 1:1,000; Abcam), iNOS (cat.
no. AF0199; 1:2,000; Affinity Biosciences, Ltd.), phosphorylated
(p)-PTEN (cat. no. ab109454; 1:10,000; Abcam), PTEN (cat. no.
ab267787; 1:1,000; Abcam) and α-7 nicotinic acetylcholine receptor
(α7n AChR; cat. no. ab216485; 1:1,000; Abcam). After incubation
with the primary antibodies at 4˚C for 12 h, the membranes were
incubated with a horseradish peroxidase-conjugated goat anti-rabbit
IgG secondary antibody (cat. no. A0208; 1:10,000; Beyotime
Institute of Biotechnology) for 1 h at room temperature. The bound
antibodies were detected using a chemiluminescence system (ECL
Plus, Thermo Fisher Scientific, Inc.). GAPDH was used as an
internal control. Image Lab V 3.0 (Bio-Rad Laboratories, Inc.) and
Quantity One v4.62 (Bio-Rad Laboratories, Inc.) software were used
to quantified the immunoblotted bands.
Gut microbiota analysis
At the end of the pole test, mice were anaesthetised
via intraperitoneal injection with sodium pentobarbital (100
mg/kg), and were then euthanised using cervical dislocation. The
caecum content (0.5 g per sample) was quickly removed, and the gut
microbiota was analysed using next gene sequencing (25). 16S rRNA gene amplification was
evaluated using the Illumina MiSeq platform by a standardized
experimental procedure that provided by Illumina (Illumina, Inc.)
(26). Operational taxonomic units
were used for Chao1 α diversity and observed species value. The
characterization of differences was assessed using the
Kruskal-Wallis rank-sum test to detect features with different
abundances.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
After euthanasia, the colon tissues were collected
immediately. Total RNA was extracted from colon/SN tissues using
RNAiso Plus (cat. no. 9108; Takara Bio, Inc.) according to the
manufacturer's instructions. cDNA was generated from the total RNA
isolated above using a PrimeScript™ RT reagent kit with a gDNA
Eraser Maxima First-Strand cDNA Synthesis kit (cat. no. RR047A;
Takara Bio, Inc.) according to the manufacturer's instructions.
Expression levels of target mRNAs were quantified by RT-qPCR using
TB Green® Premix Ex Taq™ (cat. no. RR820A; Takara Bio,
Inc.) and the following primer pairs: IL-1β Forward,
5'-GAAATGCCACCTTTTGACAGTG-3', and reverse,
5'-ATCTTTTGGGGTCCGTCAACT-3'; IL-10 forward,
5'-TGCTATGCTGCCTGCTCTTA-3', and reverse,
5'-TCATTTCCGATAAGGCTTGG-3'; TGF-β forward,
5'-GCCTGAGTGGCTGTCTTTTGA-3', and reverse,
5'-GCTGAATCGAAAGCCCTGTATT-3'; TNF-α forward,
5'-GTGGAACTGGCAGAAGAG-3', and reverse, 5'-AATGAGAAGAGGCTGAGAC-3';
allograft inflammatory factor 1 (Iba1) forward,
5'-CAGACTGCCAGCCTAAGACA-3', and reverse,
5'-AGGAATTGCTTGTTGATCCC-3'; arginase forward,
5'-CTCCAAGCCAAAGTCCTTAGAG-3', and reverse,
5'-GGAGCTGTCATTAGGGACATCA-3'; GSK3β forward,
5'-ATGGCAGCAAGGTAACCACAG-3', and reverse,
5'-TCTCGGTTCTTAAATCGCTTGTC-3'); iNOS forward,
5'-GTTCTCAGCCCAACAATACAAGA-3', and reverse,
5'-GTGGACGGGTCGATGTCAC-3'; and GAPDH forward,
5'-CCAGTATGACTCCACTCACG-3', and reverse,
5'-GACTCCACGACATACTCAGC-3'. PCR was performed on an Step One plus
(Applied Biosystems) with the following thermocycling conditions:
Initial denaturation at 95˚C for 10 min followed by 40 cycles of
95˚C for 15 sec and 60˚C for 60 sec. Melt curves were recorded at
95˚C for 15 sec, 72˚C for 60 sec and 95˚C for 15 sec. The fold
difference in expression was calculated using the 2-∆∆Ct
method (27).
Transplantation of faecal
microbiota
Donor male C57BL/6 (n=6; age, 6-8 weeks; weight,
18-22 g) mice were intraperitoneally injected with sodium
pentobarbital (100 mg/kg), and then euthanised using cervical
dislocation. Fresh faecal pellets were collected from normal
healthy mice and diluted immediately with sterile PBS (1 faecal
pellet/ml). For each experiment, several faecal pellets from
different mice were resuspended together in PBS. Briefly, the
faeces were steeped in sterile PBS for ~15 min, shaken and then
centrifuged at 1,000 x g and 4˚C for 5 min. The suspension was
centrifuged at 8,000 x g and 4˚C for 5 min to obtain the total
microbiota and then washed twice in PBS. The final microbial
suspension was mixed with an equal volume of 40% sterile glycerol
to a final concentration of 20% and then stored at -80˚C (28). After MPTP (20 mg/kg) and probenecid
(250 mg/kg) were injected every 3.5 days for a duration of 5 weeks,
mice were randomly divided into the following three groups: The
control group, the MPTP group, and the MPTP + FMT group. For the
MPTP + FMT group, 200 µl/day of microbial suspension
(108 CFU/ml) was transplanted to gut microbiota-depleted
mice for 2 weeks, and the control and MPTP groups were administered
saline at a dose of 200 µl/day.
Histopathological scoring
At the end of the experiment, colon tissues were
collected and then placed into 70% ethanol at 4˚C for 24 h. Fixed
distal colon tissues were embedded in paraffin and cut into 5-mm
sections. Tissues were stained with haematoxylin and eosin
(H&E) using standard techniques as previously reported
(29).
Statistical analysis
Data are presented as the mean ± standard deviation
(unless otherwise shown) of at least three experiments. Differences
between experimental groups were analysed for statistical
significance using one-way analysis of variance, followed by the
Tukey's post hoc multiple comparison test with GraphPad Prism 7.0
software (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
FMT relieves motor dysfunction in the
MPTP-induced PD mouse model
MPTP is a commonly used drug to build an animal
model of PD (30). In the present
study, mice received intraperitoneal injections of MPTP (20 mg/kg)
and probenecid (250 mg/kg) in PBS every 3.5 days. To explore the
effect of MPTP on motor dysfunction, motor coordination was
examined using the rotarod test, bradykinesia was measured by the
pole test and motor performance was assessed by the open-field
test. In the rotarod test, MPTP significantly reduced the fall-off
latency compared with the control (Fig. 1A). However, FMT markedly increased
the fall-off latency compared with the MPTP group (Fig. 1A). Additionally, in the pole test,
the T-turn and T-LA values were both significantly increased in the
MPTP group compared with the control group (Fig. 1B and C); however, these values were decreased
in the MPTP + FMT group compared with the MPTP group, significantly
so for T-LA. Furthermore, in the open-field test (Fig. 1D), MPTP disturbed the motor
activity of mice compared with the control mice, as evidenced by a
marked decrease in the total travelled distance (Fig. 1E) and a significant decrease in
average velocity (Fig. 1F) and
movement time (Fig. 1G). FMT
protected the motor activity of mice, as evidenced by the markedly
increased total travelled distance (Fig. 1E) and movement time (Fig. 1G) and the significantly increased
average velocity (Fig. 1F)
compared with the MPTP group mice. These results indicated that FMT
might have positive effects in relieving motor dysfunction of
PD.
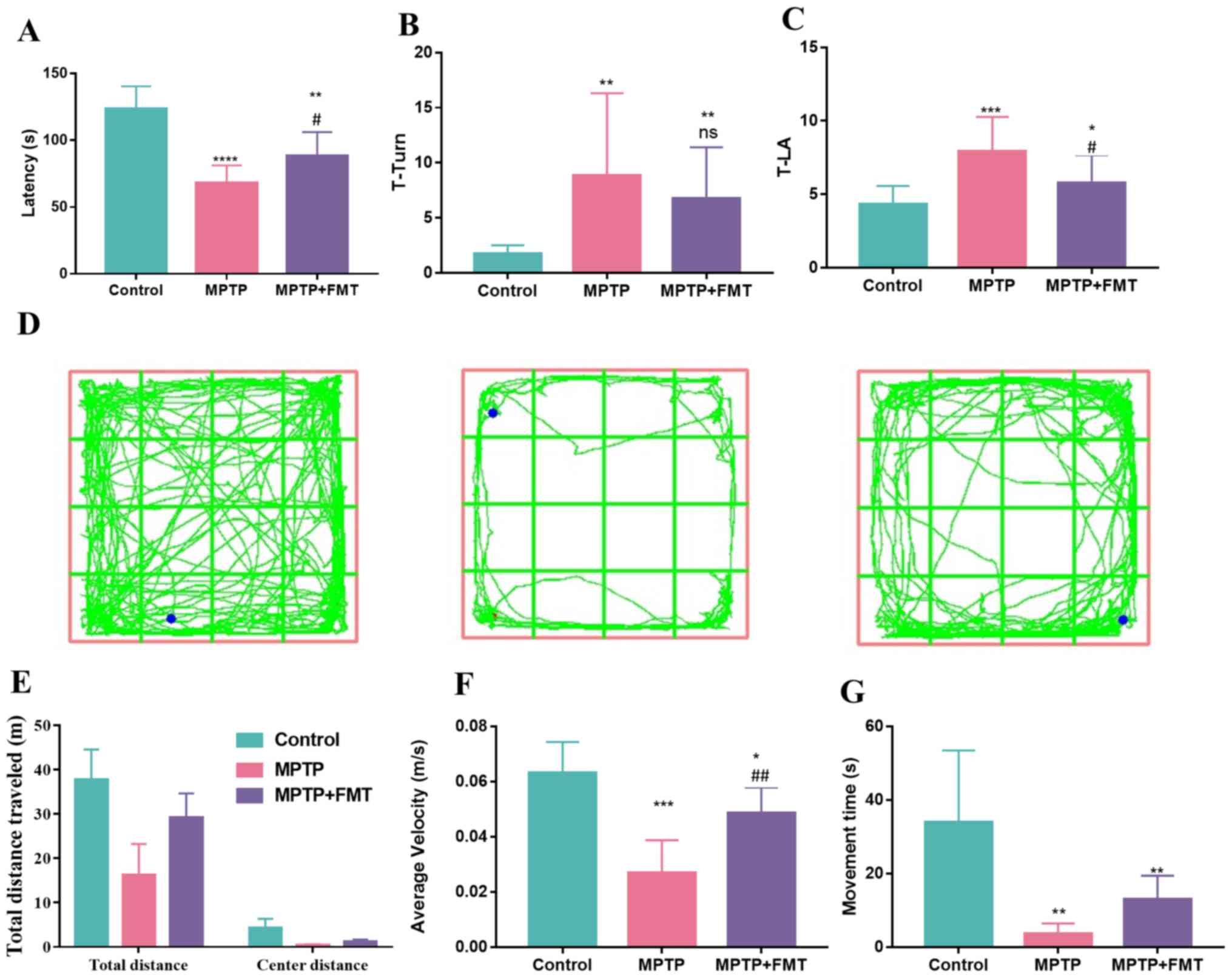 | Figure 1FMT exhibits protective effects in
the MPTP-induced Parkinson's disease mouse model. (A) Fall-off
latency measurement in rotarod test. (B) T-turn and (C) T-LA values
in pole test. (D) Mice motion trajectory, (E) total travelled, (F)
average velocity distance and (G) movement time in open field test.
n=3. *P<0.05, **P<0.01 and
***P<0.005 and ****P<0.001 vs. control;
#P<0.05, ##P<0.01 vs. MPTP. ns.
indicates no significance vs. MPTP. MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; FMT, faecal
microbiota transplantation; ns., no significance; T-LA, locomotor
activity time. |
FMT relieves chronic inflammation in
the colon of the MPTP-induced PD mouse model
Whether FMT could relieve chronic inflammation in
the colon of mice was next assessed, and the body weight and DAI
score were recorded during the present study. The results indicated
that the body weight did not show significant differences between
the MPTP and control groups (Fig.
2A); however, the DAI score was increased significantly in the
MPTP group (Fig. 2B). After
administration of FMT, the body weight was not significantly
different compared with that of the MPTP group (Fig. 2A), but the DAI score was
significantly decreased in the FMT group (Fig. 2B). Moreover, the gene expression
levels of IL-1β and IL-10 in colon tissue were analysed. The
results revealed that the expression of IL-1β was upregulated but
IL-10 was downregulated in the MPTP group compared with the control
(Fig. 2C and D). FMT significantly downregulated the
expression level of IL-1β and upregulated the gene expression level
of IL-10 compared with the MPTP group (Fig. 2C and D). When the mice were sacrificed, the
histological effects of MPTP were measured using H&E staining.
Mice in the MPTP group exhibited disruption of the epithelial
architecture (labelled using black arrows), crypt loss (labelled
using red arrows) and an increase in the number of inflammatory
cells (labelled using blue arrows; Fig. 2E). FMT prevented these signs of
intestinal inflammation, particularly for the crypt structure,
which was relatively intact and had a low number of inflammatory
cells (Fig. 2E).
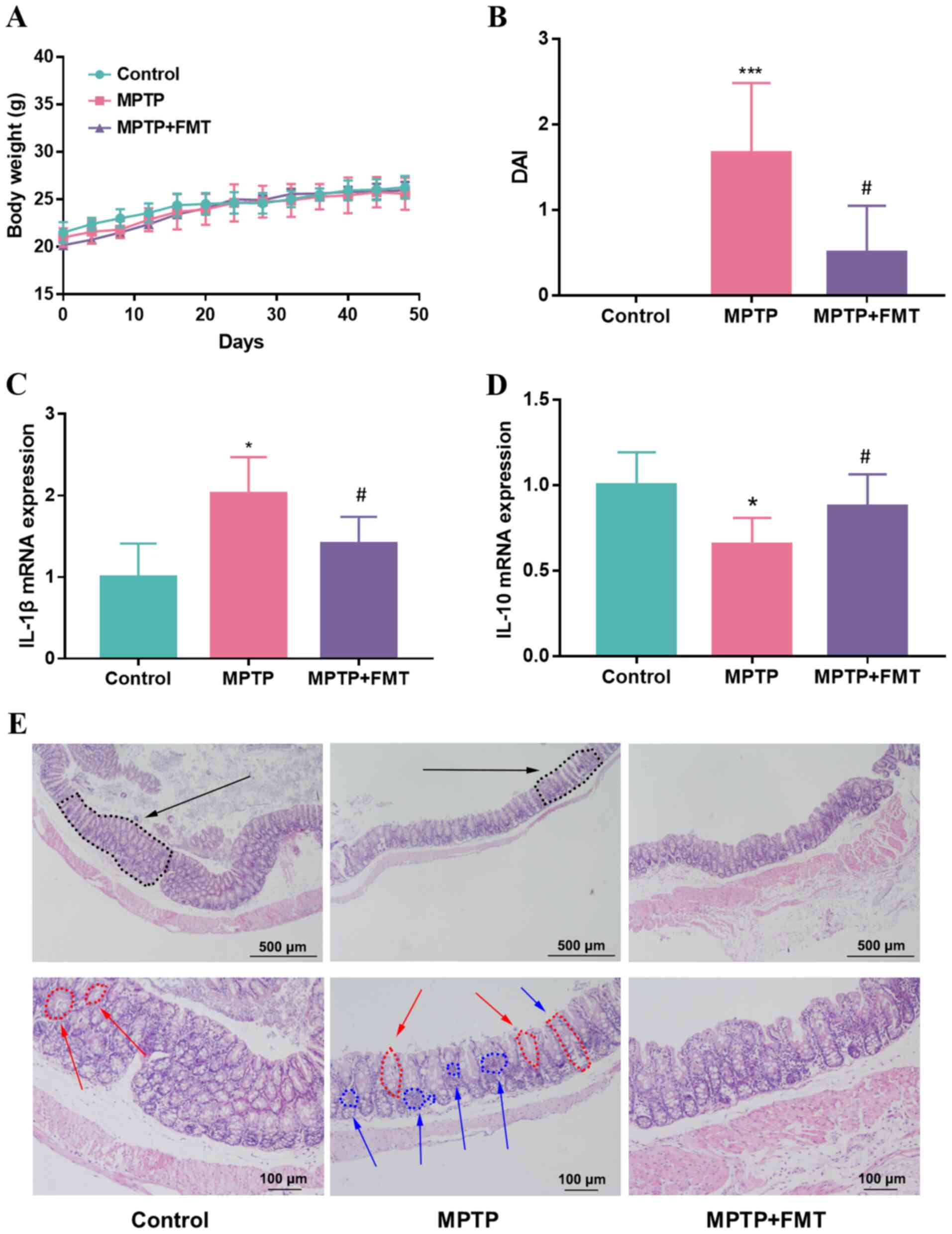 | Figure 2FMT relieves chronic inflammation in
the colon of the MPTP-induced Parkinson's disease mouse model. (A)
Mice body weight, (B) DAI score, The gene expression levels of (C)
IL-1β and (D) IL-10 in colon tissue. (E) Haematoxylin and eosin
staining of mice colon tissues. Black arrow, epithelial
architecture; red arrow, crypt; blue arrow, inflammatory cells.
n=3, scale bars represent 500 µm and 100 µm, respectively.
*P<0.05 and ***P<0.005 vs. control;
#P<0.05 vs. MPTP. MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; FMT, faecal
microbiota transplantation; DAI, disease activity index. |
FMT reconstructs the composition of
gut microbiota in the MPTP-induced PD mouse model
To assess whether FMT could reconstruct the
compositions of gut microbiota in the MPTP-induced PD mouse model,
faecal microbiota communities were profiled by next gene
sequencing. Based on 16S rRNA gene amplicon sequencing, as
demonstrated in Fig. 3A, the
composition of gut microbiota in control and MPTP groups were
different. The α diversity for all groups represented by Chao1
indexes is shown in Fig. 3B, which
indicated that MPTP significantly increased the richness of gut
microbiota at the phylum level. In addition, the observed species
were significantly increased in the MPTP group compared with the
control group (Fig. 3C). However,
compared with the MPTP group, there was no significant difference
in the FMT group. In further analysis, the bacterial composition of
Actinobacteria, Proteobacteria and Tenericutes were significantly
increased in the MPTP groups compared with that of the controls
(Fig. 3D-F). Moreover, compared
with the MPTP group, the bacterial composition of Proteobacteria
and Tenericutes was significantly decreased (Fig. 3D and F). In addition, the gut microbiota
differences between the control and MPTP groups at the genus level
are presented in Fig. 3G. The
results indicated that the compositions of Anaerostipes,
Bifidobacterium, Turicibacter, ASF356 and
Ruminococcus were significantly increased, but
Blautia was significantly decreased (Fig. 3H-M). In the FMT group, the gut
microbiota compositions of Anaerostipes,
Bifidobacterium, ASF356 and Ruminococcus were
significantly decreased, but Blautia was significantly
increased (Fig. 3H-M).
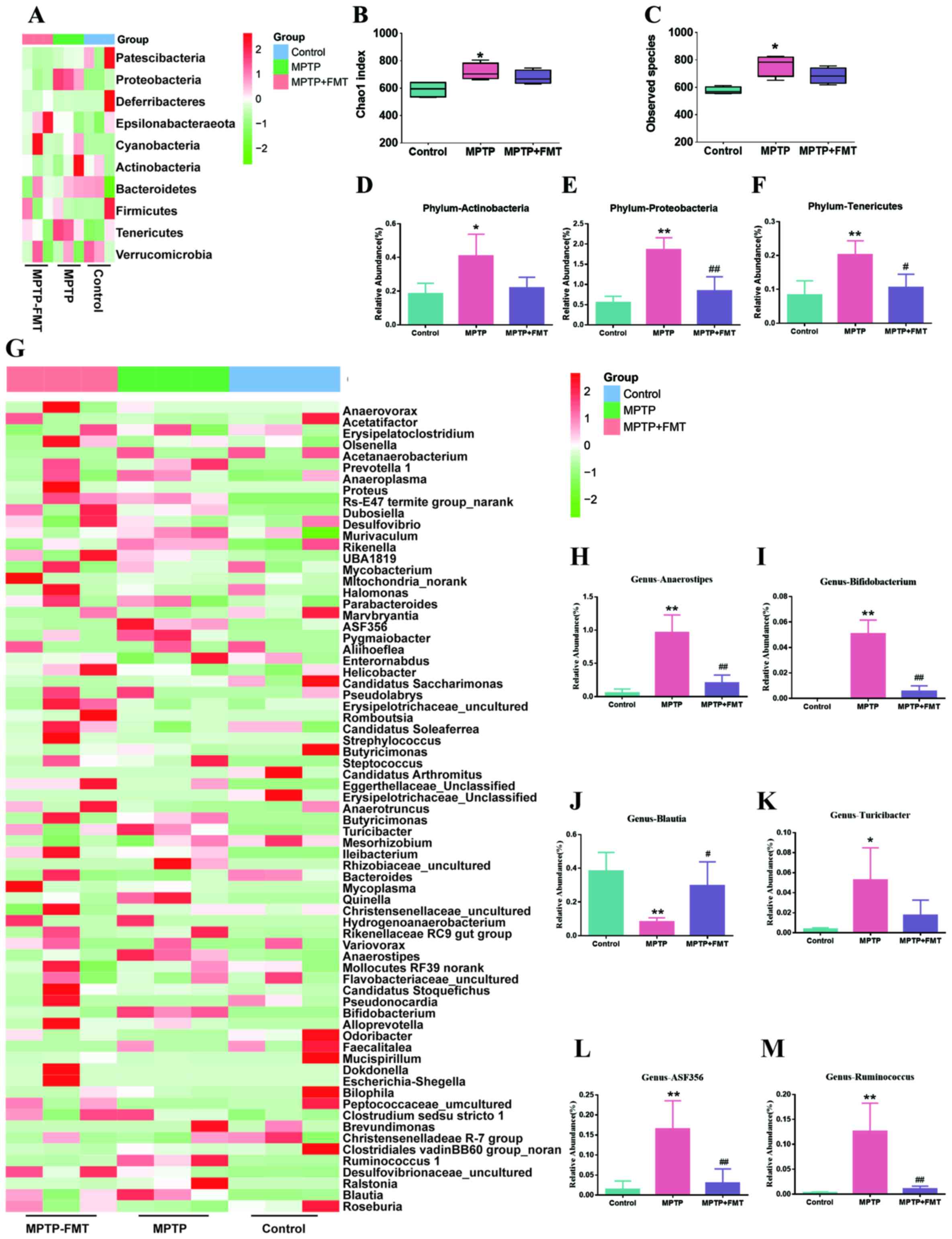 | Figure 3FMT reconstructs the compositions of
gut microbiota in the MPTP-induced Parkinson's disease mouse model.
(A) Heatmap of gut microbiota composition at the phylum level. Red,
high expression; green, low expression. (B) Shannon index of
microbiota diversity and (C) observed species. The relative
abundance of bacterial of (D) Actinobacteria, (E) Proteobacteria
and (F) Tenericutes at phylum level. (G) Heatmap of gut microbiota
composition at genus level. The relative abundance of bacterial of
(H) Anaerostipes, (I) Bifidobacterium, (J)
Blautia, (K) Turicibacter, (L) ASF356 and (M)
Ruminococcus. *P<0.05 and
**P<0.01 vs. control; #P<0.05 and
##P<0.01 vs. MPTP. MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; FMT, faecal
microbiota transplantation. |
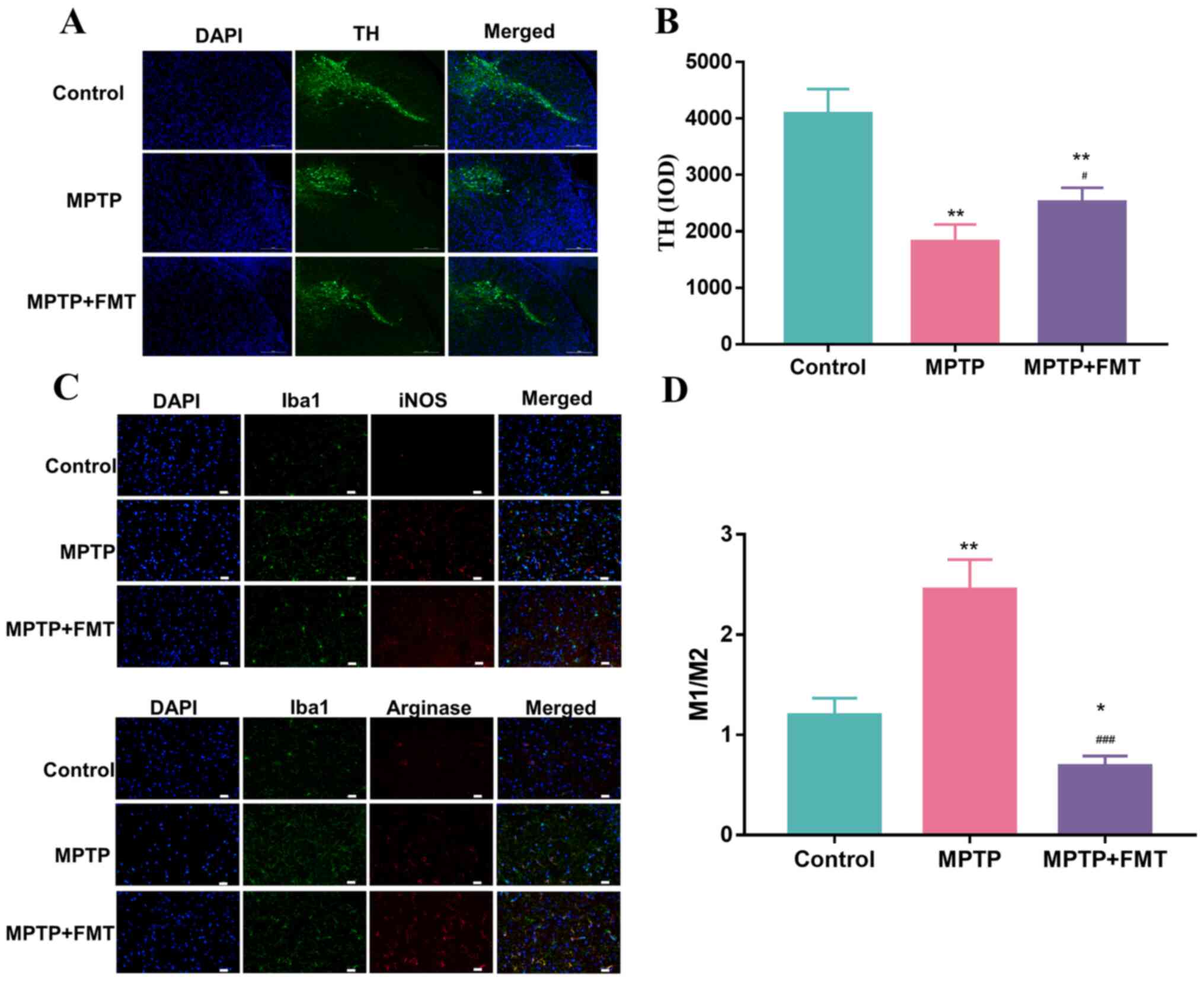
FMT protects against neuronal damage
in the MPTP-induced PD mouse model
To confirm the impact of FMT on the protection of
the SN, the expression levels of TH, iNOS and arginase were
measured using immunofluorescence. As presented in Fig. 4A and B, the expression levels of TH was
significantly decreased. These results indicated that MPTP has a
positive effect on damage to the SN. After treatment with FMT, the
expression level of TH was significantly increased. In addition,
the Iba1+ iNOS+ cells (representing M1 cells)
and Iba1+ Arginase+ cells (representing M2
cells) were analysed by immunofluorescence and are presented in
Fig. 4C. The ratio of the number
of positive cells per 0.1 mm2 of M1 and M2 cells are
presented in Fig. 4D, which
revealed that the number of M1 cells was significantly upregulated
in the MPTP group compared with the control. After treatment with
FMT, it was significantly downregulated compared with the MPTP and
control groups. These results indicated that FMT could have a
positive effect on the protection of neuronal damage.
FMT suppresses inflammation in the SN
in the MPTP-induced PD mouse model
To verify whether FMT could suppress inflammation in
the SN, the expression levels of inflammatory cytokines in the SN
were detected. The protein expression levels of arginase, GSK3β,
IL-1β, iNOS and p-PTEN were significantly upregulated in the MPTP
group compared with the control (Fig.
5A-F), while the protein expression level of α7n AchR was
downregulated (Fig. 5G). In the
FMT group, the protein expression levels of GSK3β, IL-1β, iNOS and
p-PTEN were significantly downregulated compared with the MPTP
group (Fig. 5C-F), while the
protein expression levels of α7n AchR (Fig. 5G) and arginase (Fig. 5B) were significantly upregulated.
In addition, the gene expression levels of arginase, GSK3β, IL-1β
and iNOS were consistent with the protein levels (Fig. 5H-K). Furthermore, the gene
expression levels of TNF-α (Fig.
5L) were upregulated in the MPTP group compared with the
control, but IL-10 and TGF-β were downregulated (Fig. 5M and N).
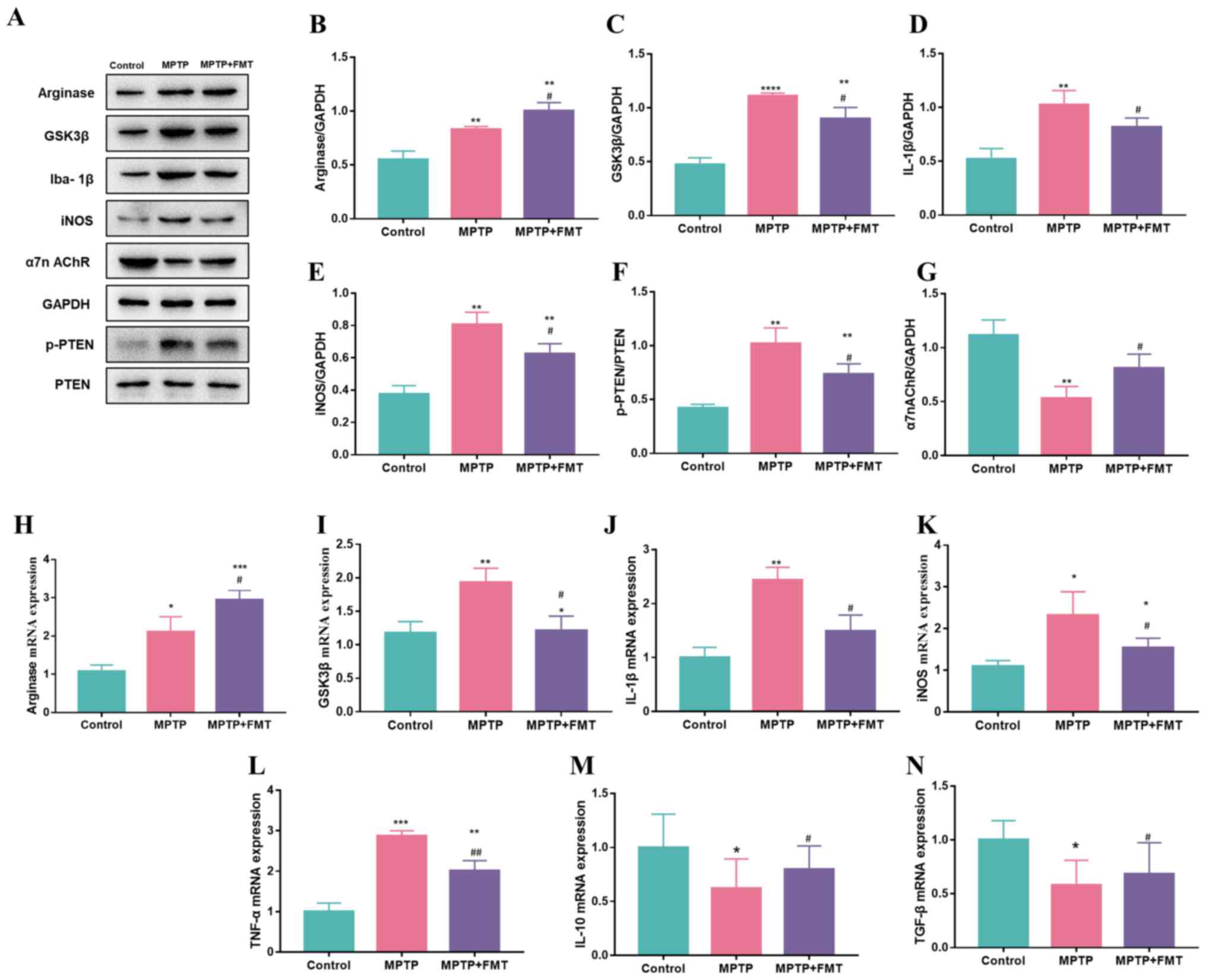 | Figure 5FMT suppresses inflammation in the
substantia nigra in an MPTP-induced Parkinson's disease mouse
model. (A) Western blotting bands of Arginase, GSK3β, IL-1β, iNOS,
α7n AchR and p-PTEN. Western blotting bands statistical
quantification of (B) Arginase, (C) GSK3β, (D) IL-1β, (E) iNOS, (F)
p-PTEN and (G) α7n AchR. Gene expression levels of (H) Arginase,
(I) GSK3β, (J) IL-1β, (K) iNOS, (L) TNF-α, (M) IL-1β, and (N)
TGF-β. n=3. *P<0.05, **P<0.01,
***P<0.005 and ****P<0.001 vs. control;
#P<0.05 and ##P<0.01 vs. MPTP. MPTP,
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; FMT, faecal
microbiota transplantation; Iba1, allograft inflammatory factor 1;
iNOS, inducible nitric oxide synthase; α7n AchR, α-7 nicotinic
acetylcholine receptor; p-, phosphorylated. |
Discussion
Recent studies have demonstrated that the gut
microbial composition is disordered in patients with PD (31-33),
and the data of the present study validated the difference in the
composition of the intestinal flora in the mouse PD model. Notably,
the present study revealed that the destruction of intestinal
microbes in PD mice involved an increase in the phyla
Actinobacteria, Proteobacteria and Tenericutes, which is consistent
with the results observed in patients with PD (34,35).
It is known that the increase in Tenericutes may be the result of
intestinal inflammation (36). In
particular, the abundance of Actinobacteria and Proteobacteria has
been indicated to be positively correlated with the severity of
postural instability and gait difficulty in PD (37). These results indicate that specific
intestinal microbial disorders may be associated with the
pathogenesis and clinical manifestations of PD.
The present study revealed that reducing intestinal
microbial malnutrition through FMT had a protective effect on PD.
FMT is a treatment method based on the relationship between the
intestinal flora and the host (19,38,39).
It involves transplanting the intestinal microbes from healthy
donors to patients with PD to reform the composition of the
intestinal flora of the patients and achieve the therapeutic effect
(34,40). Notably, in the present study, FMT
not only alleviated the dyskinesia of PD mice, but also improved
the main microbial populations of PD mice, such as
Anaerostipes, Bifidobacterium, ASF356 and
Ruminococcus.
Results of a previous study revealed that TH is a
key enzyme in the biosynthetic pathway of dopamine (DA) (41). PD is a neurodegenerative disease
caused by severely insufficient DA in the SN striatum (42). Upregulated expression of TH in the
SN has a positive effect on alleviating the symptoms of PD
(43). The current study
demonstrated that FMT treatment could increase the expression of TH
in the SN of mice with PD. Microglia, which were immune cells
reside in the central nervous system, are the main participants of
the inflammatory process. Therefore, moderate intervention in
microglial activation may delay the progression of PD pathology
(44,45). As a result of the different
surrounding microenvironments, microglia exhibit two different
types of polarized phenotypes: Classic activation M1-like can
promote inflammation and is associated with neurotoxicity, and
choose activated M2-like is anti-inflammatory and can repair damage
(46,47). The present study data revealed that
FMT treatment promoted the M2 polarization of microglia, and
downregulated the expression of inflammatory factors, such as
GSK3β, IL-1β, iNOS and TNF-α.
The interaction between the brain and the intestines
affects the behaviour of the host, and this effect mainly occurs
through the sympathetic nerve, parasympathetic nerve branch of the
autonomic nervous system and neuroendocrine nervous system
(48). By activating the Toll-like
receptor 4/NF-κB signalling pathway in the intestine to release
proinflammatory factors, such as TNF-α, it may induce the
activation of intestinal immune cells, thereby releasing some
inflammatory factors. Subsequently, glial cells enter the brain
through the blood-brain barrier and induce neuroinflammation
(49,50).
In summary, the present study provided evidence that
FMT could reverse intestinal microbial dysfunction and lead to
neuroprotection. However, whether this process reduces the activity
of the TNF-α, IL-1β and TGF-β pathways in the intestine should be
further studied. In addition, results of the present study revealed
that treatment with FMT altered the expression levels of arginase,
GSK3β, IL-1β, iNOS, p-PTEN and α7n AchR; however, as inhibition,
knockout or knockdown experiments were not explored, whether these
changes may be attributed to FMT treatment require further
investigation. Furthermore, the gene expression levels of TNF-α,
IL-1β, IL-10 and TGF-β were closely associated with FMT therapeutic
effects, but whether these changes were caused by FMT treatment
should be further discussed. Future research should look for other
molecular markers that can be used in conjunction with information
on intestinal microbial malnutrition, laying the foundation for the
clinical promotion of FMT.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Hainan Provincial
Natural Science Foundation of China (grant no. 818NQ316).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
TZ and XC conceived the study and performed the
methodology and investigation. TW performed the FMT experiments,
analysed the gut microbiota data and wrote the original draft
preparation. ZZ performed the western blot experiments, analysed
the data, and reviewed and edited the manuscript. ZC designed the
experiments. All authors have read and approved the final
manuscript. TZ and ZC confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The research was approved by the Ethics Committee of
the First Affiliated Hospital of Hainan Medical University
(approval no. D-2017027).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Takahashi J: Clinical trial for
Parkinson's disease gets a green light in the US. Cell Stem Cell.
28:182–183. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ding XB, Wang XX, Xia DH, Liu H, Tian HY,
Fu Y, Chen YK, Qin C, Wang JQ, Xiang Z, et al: Impaired meningeal
lymphatic drainage in patients with idiopathic Parkinson's disease.
Nat Med. 27:411–418. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang J, Perry G, Smith MA, Robertson D,
Olson SJ, Graham DG and Montine TJ: Parkinson's disease is
associated with oxidative damage to cytoplasmic DNA and RNA in
substantia nigra neurons. Am J Pathol. 154:1423–1429.
1999.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Waters CM, Peck R, Rossor M, Reynolds GP
and Hunt SP: Immunocytochemical studies on the basal ganglia and
substantia nigra in Parkinson's disease and Huntington's chorea.
Neuroscience. 25:419–438. 1988.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Crowther RA, Daniel SE and Goedert M:
Characterisation of isolated α-synuclein filaments from substantia
nigra of Parkinson's disease brain. Neurosci Lett. 292:128–130.
2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bhattarai Y, Si J, Pu M, Ross OA, McLean
PJ, Till L, Moor W, Grover M, Kandimalla KK, Margolis KG, et al:
Role of gut microbiota in regulating gastrointestinal dysfunction
and motor symptoms in a mouse model of Parkinson's disease. Gut
Microbes. 13(1866974)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhong XH, Haycock JW, Shannak K,
Robitaille Y, Fratkin J, Koeppen AH, Hornykiewicz O and Kish SJ:
Striatal dihydroxyphenylalanine decarboxylase and tyrosine
hydroxylase protein in idiopathic Parkinson's disease and
dominantly inherited olivopontocerebellar atrophy. Mov Disord.
10:10–17. 1995.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhong R, Chen Q, Zhang X, Li M and Lin W:
Helicobacter pylori infection is associated with a poor response to
levodopa in patients with Parkinson's disease: A systematic review
and meta-analysis. J Neurol: Feb 22, 2021 (Epub ahead of
print).
|
|
9
|
Schaeffer E, Vaterrodt T, Zaunbrecher L,
Liepelt-Scarfone I, Emmert K, Roeben B, Elshehabi M, Hansen C,
Becker S, Nussbaum S, et al: Effects of Levodopa on quality of
sleep and nocturnal movements in Parkinson's Disease. J Neurol.
268:2506–2514. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Anwer S, Branchard E, Dan Q, Dan A and
Szaszi K: Tumor necrosis factor-α induces claudin-3 upregulation in
kidney tubular epithelial cells through NFκB and CREB1. Am J
Physiol Cell Physiol. 320:C495–C508. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Corbo RM, Businaro R and Scarabino D:
Leukocyte telomere length and plasma interleukin-1β and
interleukin-18 levels in mild cognitive impairment and Alzheimer's
disease: New biomarkers for diagnosis and disease progression?
Neural Regen Res. 16:1397–1398. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Carabotti M, Scirocco A, Maselli MA and
Severi C: The gut-brain axis: Interactions between enteric
microbiota, central and enteric nervous systems. Ann Gastroenterol.
28:203–209. 2015.PubMed/NCBI
|
|
13
|
Cryan JF, O'Riordan KJ, Sandhu K, Peterson
V and Dinan TG: The gut microbiome in neurological disorders.
Lancet Neurol. 19:179–194. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shen L: Gut, oral and nasal microbiota and
Parkinson's disease. Microb Cell Fact. 19(50)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moustafa SA, Mohamed S, Dawood A, Azar J,
Elmorsy E, Rizk NAM and Salama M: Gut brain axis: An insight into
microbiota role in Parkinson's disease. Metab Brain Dis.
36:1545–1557. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Heintz-Buschart A, Pandey U, Wicke T,
Sixel-Döring F, Janzen A, Sittig-Wiegand E, Trenkwalder C, Oertel
WH, Mollenhauer B and Wilmes P: The nasal and gut microbiome in
Parkinson's disease and idiopathic rapid eye movement sleep
behavior disorder. Mov Disord. 33:88–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cirstea MS, Yu AC, Golz E, Sundvick K,
Kliger D, Radisavljevic N, Foulger LH, Mackenzie M, Huan T, Finlay
BB, et al: Microbiota composition and metabolism are associated
with gut function in Parkinson's disease. Movement Disord.
35:1208–1217. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang F, Cui B, He X, Nie Y, Wu K and Fan
D: FMT-standardization Study Group. Microbiota transplantation:
concept, methodology and strategy for its modernization. Protein
Cell. 9:462–473. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xue LJ, Yang XZ, Tong Q, Shen P, Ma SJ, Wu
SN, Zheng JL and Wang HG: Fecal microbiota transplantation therapy
for Parkinson's disease: A preliminary study. Medicine (Baltimore).
99(e22035)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zha Z, Lv Y, Tang H, Li T, Miao Y, Cheng
J, Wang G, Tan Y, Zhu Y, Xing X, et al: An orally administered
butyrate-releasing xylan derivative reduces inflammation in dextran
sulphate sodium-induced murine colitis. Int J Biol Macromol.
156:1217–1233. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang Y, Wei N and Li X: Preclinical
evidence and possible mechanisms of baicalein for rats and mice
with Parkinson's disease: A systematic review and meta-analysis.
Front Aging Neurosci. 12(277)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guo HM, Zhou ZY, Huang YQ, Li X and Wang
XJ: Investigation of the roles of dysbindin-1 and SATB2 in the
progression of Parkinson's disease. Eur Rev Med Pharmacol Sci.
23:7510–7516. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li L, Chen Y, Tang J and Yuan L:
Evaluation of efficiency omega 3 fatty acid improves the
behavioural phenotype and protects against oxidative stress against
MPP+ induces Parkinson's disease in mice. Pak J Pharm
Sci. 34:861–867. 2021.PubMed/NCBI
|
|
24
|
Koentjoro B, Park J-S and Sue CM: Parkin
western blotting is useful for identification of patients with
Parkin-related Parkinson's disease. J Neurol Neurosurg Psychiatry.
85:1436–1437. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Oluwole OG, Kuivaniemi H, Abrahams S,
Haylett WL, Vorster AA, van Heerden CJ, Kenyon CP, Tabb DL, Fawale
MB, Sunmonu TA, et al: Targeted next-generation sequencing
identifies novel variants in candidate genes for Parkinson’s
disease in Black South African and Nigerian patients. BMC Med
Genet. 21(23)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Illumina Inc: 16S Metagenomic Sequencing
Library Preparation, 2013. https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf.
Accessed, April 15, 2020.
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hamilton MJ, Weingarden AR, Sadowsky MJ
and Khoruts A: Standardized frozen preparation for transplantation
of fecal microbiota for recurrent Clostridium difficile infection.
Am J Gastroenterol. 107:761–767. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wlodarska M, Thaiss CA, Nowarski R,
Henao-Mejia J, Zhang JP, Brown EM, Frankel G, Levy M, Katz MN,
Philbrick WM, et al: NLRP6 inflammasome orchestrates the colonic
host-microbial interface by regulating goblet cell mucus secretion.
Cell. 156:1045–1059. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang L, Zhang L, Li Y, Li L, Melchiorsen
JU, Rosenkilde M and Hölscher C: The novel dual GLP-1/GIP receptor
agonist DA-CH5 is superior to single GLP-1 receptor agonists in the
MPTP model of Parkinson's disease. J Parkinsons Dis. 10:523–542.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li W, Wu X, Hu X, Wang T, Liang S, Duan Y,
Jin F and Qin B: Structural changes of gut microbiota in
Parkinson's disease and its correlation with clinical features. Sci
China Life Sci. 60:1223–1233. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bedarf JR, Hildebrand F, Coelho LP,
Sunagawa S, Bahram M, Goeser F, Bork P and Wüllner U: Functional
implications of microbial and viral gut metagenome changes in early
stage L-DOPA-nave Parkinson's disease patients. Genome Med.
9(39)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hill-Burns EM, Debelius JW, Morton JT,
Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E,
Zabetian CP, et al: Parkinson's disease and Parkinson's disease
medications have distinct signatures of the gut microbiome. Mov
Disord. 32:739–749. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhou ZL, Jia XB, Sun MF, Zhu YL, Qiao CM,
Zhang BP, Zhao LP, Yang Q, Cui C, Chen X, et al: Neuroprotection of
fasting mimicking diet on MPTP-induced Parkinson's disease mice via
gut microbiota and metabolites. Neurotherapeutics. 16:741–760.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sun B-L, Li W-W, Wang J, Xu YL, Sun HL,
Tian DY, Wang YJ and Yao XQ: Gut microbiota alteration and its time
course in a tauopathy mouse model. J Alzheimers Dis. 70:399–412.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nagalingam NA, Kao JY and Young VB:
Microbial ecology of the murine gut associated with the development
of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis.
17:917–926. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Scheperjans F, Aho V, Pereira PAB,
Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S,
Eerola-Rautio J, Pohja M, et al: Gut microbiota are related to
Parkinson's disease and clinical phenotype. Mov Disord. 30:350–358.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Huang H, Xu H, Luo Q, He J, Li M, Chen H,
Tang W, Nie Y and Zhou Y: Fecal microbiota transplantation to treat
Parkinson's disease with constipation: A case report. Medicine
(Baltimore). 98(e16163)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dutta SK, Verma S, Jain V, Surapaneni BK,
Vinayek R, Phillips L and Nair PP: Parkinson's disease: The
emerging role of gut dysbiosis, antibiotics, probiotics, and fecal
microbiota transplantation. J Neurogastroenterol Motil. 25:363–376.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lubomski M, Tan AH, Lim SY, Holmes AJ,
Davis RL and Sue CM: Parkinson's disease and the gastrointestinal
microbiome. J Neurol. 267:2507–2523. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Haavik J and Toska K: Tyrosine hydroxylase
and Parkinson's disease. Mol Neurobiol. 16:285–309. 1998.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kitamura Y, Sakanashi M, Ozawa A, Saeki Y,
Nakamura A, Hara Y, Saeki KI and Arimoto-Kobayashi S: Protective
effect of Actinidia arguta in MPTP-induced Parkinson's disease
model mice. Biochem Biophys Res Commun. 555:154–159.
2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
da Silva WAB, Ferreira Oliveira K,
Caroline Vitorino L, Ferreira Romão L, Allodi S and Lourenço Correa
C: Physical exercise increases the production of tyrosine
hydroxylase and CDNF in the spinal cord of a Parkinson's disease
mouse model. Neurosci Lett. 760(136089)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang Y, Li M, Song M, Xu X, Xiong J, Yang
X, Tan J and Bai Y: Expression of OX40 ligand in microglia
activated by IFN-gamma sustains a protective CD4+ T-cell
response in vitro. Cell Immunol. 251:86–92. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Utz SG, See P, Mildenberger W, Thion MS,
Silvin A, Lutz M, Ingelfinger F, Rayan NA, Lelios I, Buttgereit A,
et al: Early fate defines microglia and non-parenchymal brain
macrophage development. Cell. 181:557–573.e18. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kam TI, Hinkle JT, Dawson TM and Dawson
VL: Microglia and astrocyte dysfunction in parkinson's disease.
Neurobiol Dis. 144(105028)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Joers V, Masilamoni G, Kempf D, Weiss AR,
Rotterman TM, Murray B, Yalcin-Cakmakli G, Voll RJ, Goodman MM,
Howell L, et al: Microglia, inflammation and gut microbiota
responses in a progressive monkey model of Parkinson's disease: A
case series. Neurobiol Dis. 144(105027)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hefazi M, Patnaik MM, Hogan WJ, Litzow MR,
Pardi DS and Khanna S: Safety and efficacy of fecal microbiota
transplant for recurrent clostridium difficile infection in
patients with cancer treated with cytotoxic chemotherapy: A
single-institution retrospective case series. Mayo Clin Proc.
92:1617–1624. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Porcu F, Rubio A, Pérez-Hernández M and
Gutiérrez-López MD: Effect of intensive and repeated use of ethanol
on the integrity of blood-brain barrier in mouse brain. Role of
toll-like receptor 4 (TLR4). Basic Clin Pharmacol Toxicol.
115(41)2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Cao L, Liu C, Wang F and Wang H: SIRT1
negatively regulates amyloid-beta-induced inflammation via the
NF-κB pathway. Braz J Med Biol Res. 46:659–669. 2013.PubMed/NCBI View Article : Google Scholar
|